Introduction
Intracranial aneurysm (IA) is a common
cerebrovascular disease, and ruptured IA remains life-threatening.
With the improvement of surgical and intervention techniques, the
treatment of IA has achieved superior results (1-3).
However, difficulties still exist in the treatment of complex IAs
(4-7). In the treatment of complex IA that
is complicated by severe atherosclerosis, surgical clipping may
lead to fatal bleeding, causing the failed blockage of the internal
carotid artery temporarily. The passage of the guide wire through
the internal carotid artery remains difficult to be passed and
thus, the embolization cannot be completed. This type of aneurysm
requires novel technical treatment; however, to date, and at least
to the best of our knowledge, no practical reports of drug
treatment for IA are available. Thus, the understanding of the
biological characteristics of IA development and the identification
of molecular markers that can help pinpoint people at high risk are
crucial to the development of novel therapeutic strategies.
It is now widely accepted that oxygen free radicals
contribute to the pathogenesis of ischemic cerebrovascular disease
(8-10). The nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase system is an important
enzymatic source of oxygen free radicals (11). p22phox is an important
component of both phagocytic and nonphagocytic NADPH oxidase
(12-14).
It has been demonstrated that the NADPH oxidase
system is involved in the pathogenesis of atherosclerotic vascular
disease (15). However, previous
studies have mainly focused on the association of
p22phox in abdominal aortic aneurysm, thoracic aortic
aneurysm and ischemic disease (16-18), while fewer scholars have
investigated the association between p22phox single
nucleotide polymorphisms (SNPs) and IA (19).
A few scholars have emphasized the role of oxidative
stress in abdominal aortic aneurysmal (AAA), suggesting that
antioxidant therapy may be a novel therapeutic strategy with which
to delay AAA progression (20).
Some antioxidant treatments, such as apocynin and vitamin E
(21,22) have been reported to prevent the
formation of AAA. However, some other researchers have presented
significantly lower plasmatic levels of vitamin A and vitamin E in
patients suffering from subarachnoid hemorrhage (SAH) than in the
controls, speculating that the NADPH oxidase system may influence
the rupture of IA (23).
Theoretically, IA formation can be inhibited by
antioxidants. In the clinical setting, although low circulating
vitamins, such as B6/C/D/E (not B12) levels are associated with the
presence of AAA, the supplementation of vitamins B6/B12/E may not
reduce the incidence of AAA (24). Previous studies have demonstrated
that matrix metalloproteinase (MMP)-2 and MMP-9 are required for
the formation of AAA (25,26)
and that reactive oxygen species (ROS) induce the apoptosis of
vascular smooth muscle cells (VSMCs) (27). Edaravone is a widely used free
radical scavenger in patients with acute cerebral infarction, and
its administration has been shown to inhibit the production of ROS,
preventing aneurysm formation and expansion in a rat model of AAA
(28). The free-radical
scavenger, edaravone, may thus be an effective pharmaceutical agent
for use in the treatment of IA in clinical practice.
The association between p22phox and the
occurrence and development of IA has not been reported to date, at
least to the best of our knowledge, and there is still no ideal
pharmaceutical treatment for IA. We hypothesized the active
participation of p22phox-214T/C gene (rs4673)
polymorphisms in IA formation and the suppressive potential of
edaravone against IA formation. Hence, this study aimed to explore
the role and mechanisms of action of p22phox in the
pathogenesis of IA, and to examine the inhibitory effects on
oxidative stress and the curative effects in an effort to provide a
novel strategy for the treatment of IA.
Materials and methods
Study population
This case-control study was approved by the Research
Ethics Committee of Taihe Hospital, Hubei University of Medicine,
Shiyan, China. A total of 192 patients (77 males and 115 females;
age range, 15-76 years; median age, 54±6.6 years) with IA and 112
healthy volunteers (61 males and 51 females; age range, 14-65
years; median age, 45±4.8 years) from Taihe Hospital (between
March, 2013 to October, 2016) participated in this study. The
specific inclusion criteria and personnel structure were consistent
with those previously reported (29). Informed consent was obtained from
all the patients. All specimens were handled and made anonymous
according to the ethical and legal standards.
Amplification of the
p22phox-214T/C (rs4673) locus and DNA sequencing
According to previous reports (30-32), venous blood was collected from
patients with IA on an empty stomach and from healthy volunteers
and was used for the isolation of genomic DNA according to the kit
instructions (MBI Fermentas, Burlington, ON, Canada). The following
primers were used: 5′-TGC TTG TGG GTA AAC CAA GG-3′ and 5′-GGA AAA
ACA CTG AGG TAA GTG-3′. The amplification conditions were
consistent with those of a previous study (29).
Analysis of NADPH oxidase expression by
RT-PCR
According to a previous study (18), total RNA was randomly extracted
from the vein samples of 3 patients with IA and from 4 anonymous
blood donors using regular TRIzol reagent (15596-026, Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The concentrations, as well as
purity were assayed using an ultraviolet spectrometer (NanoDrop
One, Thermo Fisher Scientific, Waltham, MA, USA). A reverse
transcription kit (KR116, Tiangen Biotech, Beijing, China) was used
for cDNA synthesis, and the primer sequences were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China) according to the
sequences of GenBank: 5′-GTT TTG AAA GCT ACC GCA GAA C-3′, and
5′-GGA ACC TTT TGT CTT CCT G AT G-3′. GAPDH was used as an internal
control. According to the sequences of GenBank: 5′-CCA TGT TCG TCA
TGG GTG TGA ACC A-3′, and 5′-GCC AGT AGA GGC AGG GAT GTT C-3′. The
reaction system was set consistent with that of a previous study
(18).
Animals
This study was approved and conducted according to
the guidelines set by the Experimental Animal Center of the Hubei
University of Medicine (Shiyan, China). All animal experiments were
performed in the Experimental Animal Center according to the
protocols approved by the Animal Ethics Committee of the Hubei
University of Medicine. Adult male Japanese white rabbits
(weighing, 2.32±0.25 kg; >5 months of age, ordinary food
rearing) were provided by the Experimental Animal Center of Hubei
University of Medicine. Those rabbits were randomly divided into 3
experimental groups as follows: The edaravone group (edaravone 2
mg/kg/day, n=18), the control group (saline, 4 ml/day, n=18) and
the normal group (normal rabbits, n=10). Edaravone (Simcere
Pharmaceutical Co., Ltd., Nanjing, China) or saline (0.9% NaCl,
Taihe Hospital, Shiyan, China) was intravenously injected into the
mice twice daily, beginning from 7 days after aneurysm preparation
to 21 days. Edaravone was intravenously administered for 14 days
twice daily respectively. The dose and the route of administration
of edaravone were according to previous reports (28,33).
Aneurysm creation
The animals were sedated by an auricular vein
intravenous injection of 3% pentobarbital sodium (30 mg/kg). For
the rabbits in the edaravone group and the control group, the
middle of the right common carotid artery (RCCA), approximately 2
cm long, was temporarily occluded with two aneurysm clips.
Subsequently, 20 units of pancreatic elastase was injected into the
clipped RCCA, and pancreatic elastase leakage was prevented. In
order to minimize post-operative infection in the rabbits with
aneurysm, the animals were administered penicillin (North China
Pharmaceutical Group Co., Ltd., Shijiazhuang, China)
intramuscularly at 0.1 million units per kilogram for 7 days after
surgery. The specific surgical procedures and subsequent infection
prevention measures were as previously described (34).
ELISA
The plasma from 10 random patients with IA and 10
donors using EDTA as an anticoagulant was collected. The samples
were centrifuged for 15 min at 1,000 × g at 2-8°C within 30 min of
collection. The plasma was then removed and assayed immediately or
sampled in aliquots at −20°C. The detection of NADPH oxidase in
plasma was achieved by ELISA following the manufacturer's
instructions (15272, Amplite Colorimetric NADPH Assay kit; AAT
Bioquest, Sunnyvale, CA, USA). Each sample was assayed in
triplicate. The plasma of rabbits was also collected using use EDTA
as an anticoagulant. The detection of NADPH, MMP-2 and MMP-9 in the
plasma was achieved by ELISA according to the manufacturer's
instructions (15272, Amplite Colorimetric NADPH Assay kit; AAT
Bioquest, Sunnyvale, CA, USA, E-EL-RB1540c, rabbit MMP-2 ELISA kit
and E-EL-RB1997c, rabbit MMP-9 ELISA kit; Elabscience, Wuhan,
China). Each sample was assayed in triplicate.
Histological analysis of elastase-induced
aneurysms
The experimental animals were sacrificed after 3
weeks. Each rabbit was humanely euthanized through transcardial
perfusion while under deep anesthesia [auricular vein intravenous
injection of 3% pentobarbital sodium (30 mg/kg)]. The diameter of
the elastase-induced aneurysms was measured, and the aneurysms or
the common carotid artery samples were fixed in 4% paraformaldehyde
and then embedded in paraffin for histopathological examination.
The sections (5-µm-thick) were cut and mounted on saline-coated
slides. The slides were stained with hematoxylin and eosin
(H&E) and CD34 (ZM-0046, 1:200, ZSGB-BIO, Beijing, China) for
histopathological and immunohistochemical analyses using standard
techniques and reagents. The sample was exposed to an
immunospecific primary antibody diluted in 5% goat serum overnight
at 4°C, then to a goat-anti-mouse secondary antibody (PV-6002,
ZSGB-BIO) for 1 h at room temperature (35,36). Measurements were performed with an
optical microscope (DP73; Olympus, Tokyo, Japan; ×4 to ×40
magnification) that was attached to a video camera and connected to
a computer equipped with image analysis software (cellSens Standard
software, Olympus).
In order to determine whether the rabbit aneurysm
models were successfully modeled, we selected typical human
aneurysm specimens as a control group. The control group was a
specimen of a large middle cerebral artery aneurysm in a
53-year-old female hospitalized in May, 2017 and a renal artery
specimen of a 50-year-old male renal cell carcinoma admitted to the
hospital at the same time. Informed consent was obtained from all
the patients. CD34 antibody (ZA-0550, 1:200, ZSGB-BIO) was used for
histopathological and immunohistochemical analysis using standard
techniques and reagents. The sample was exposed to an
immunospecific primary antibody diluted in 5% goat serum overnight
at 4°C, then to a goat-anti-rabbit secondary antibody (PV-6001,
ZSGB-BIO) for 1 h at room temperature. All specimens were handled
and made anonymous according to the ethical and legal standards.
This case-control study was approved by the Research Ethics
Committee of Taihe Hospital, Hubei University of Medicine.
CD34 is a known marker of circulating progenitor
cells. Studies have examined the role of CD34 cells in AAA and
peripheral vascular disease (PVD) (37,38). The number of CD34-positive cells
was counted using an optical microscope (DP73; Olympus) within 3
separate fields.
Study course and statistical methods
SPSS software version 17.0 for Windows (SPSS Inc.,
Chicago, IL, USA) and GraphPad Prism software 6.07 for Windows
(GraphPad Software, Inc., La Jolla, CA, USA) were used for
statistical analysis. Data are presented as the means ± standard
deviation (SD). A P-value <0.05 was considered to indicate a
statistically significant difference. Deviation from the
Hardy-Weinberg equilibrium was evaluated by comparing the observed
and expected genotype frequencies by an exact goodness-of-fit test
separately in the IA and control groups. The influencing factors of
IA, genotype and allele frequencies were analyzed using the
χ2 test. Differences in the ELISA results were compared
using the t-test (for 2 groups). One-way ANOVA was performed to
compare the multiple independent variables (3 or more groups). Post
hoc adjusted comparisons were performed timely with Bonferroni
correction and were considered significant at P<0.05.
Results
Increased IA formation with
p22phox-214T>C single nucleotide variation
Genotype distributions were consistent with the
Hardy-Weinberg equilibrium in both the patient and control groups.
The genotype and allele counts in patients and controls, as well as
the χ2 and P-value are presented below. The frequencies
of p22phox-214T>C (rs4673) genotypes in the patients
with IA differed significantly from those of the control group
(P<0.001). In the IA group, the p22phox-214T>C
allele frequencies were 86.9% (166 IA patients with the C allele
divided by 191 total IA patients), while they were 23.21% (26
healthy volunteers with the C allele divided by 112 total healthy
volunteers) in the control group. The differences between the
frequencies of the allele in patients with IA and the controls were
statistically significant. Our data indicated that the C allele
single nucleotide variation increased IA formation in the Chinese
population examined (Table I).
Statistical analysis revealed that the C allele was not associated
with the IA number (single or multiple) and IA position (anterior
or posterior circulation), suggesting no statistical
significance.
 | Table IAllele frequencies of
p22phox-214T>C gene polymorphism in patients with IA
and controls. |
Table I
Allele frequencies of
p22phox-214T>C gene polymorphism in patients with IA
and controls.
| No. | T allele | C allele | P-value |
|---|
| Total numbers | 303 | 101 | 202 | |
| Controls | 112 | 86 | 26 | |
| Aneurysmal SAH | 191 | 25 | 166 | <0.001 |
| IA single | 147 | 8 | 139 | |
| IA multiple | 44 | 3 | 41 | 0.731 |
| IA anterior | 177 | 9 | 170 | |
| IA posterior | 13 | 2 | 9 | 0.070 |
| IA
anterior+posterior | 1 | 0 | 1 | 0.818 |
Increased NADPH oxidase content in the
plasma of patients with IA and in an animal model of
elastase-induced aneurysm
p22phox is a subunit of NADPH oxidase
that is expressed in VSMCs and serves as an important component of
super-oxide-generating vascular NADPH oxidase (12). According to the
p22phox-214T>C single nucleotide variation result,
the mRNA expression of NADPH oxidase in the IA group was
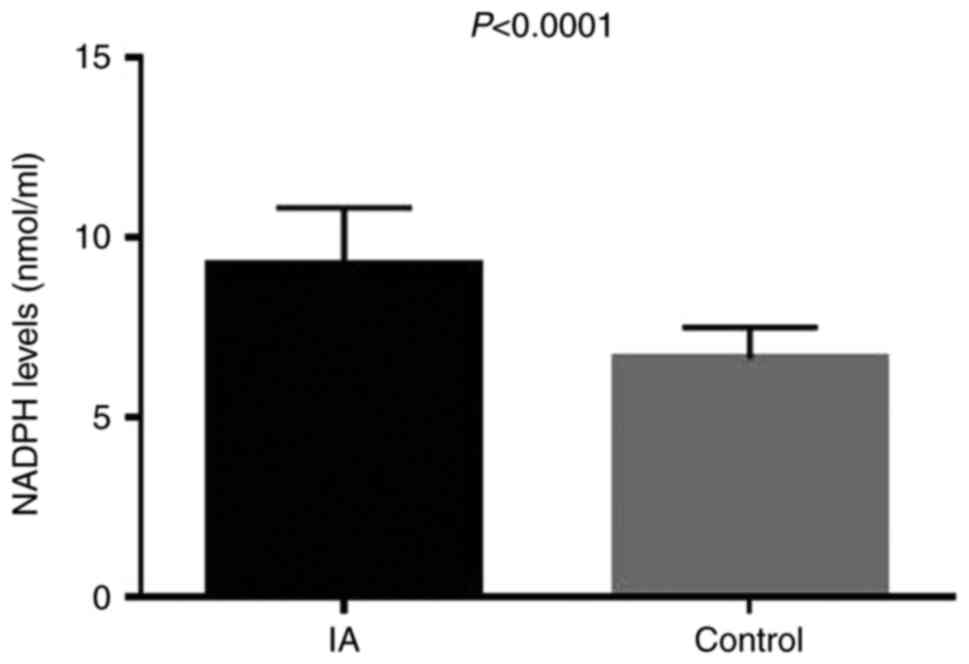
significantly higher than that in the control group (Fig. 1). The results of ELISA
demonstrated that the serum level of NADPH oxidase in the IA group
was significantly higher compared with that in the control group
(P<0.0001; Fig. 2).
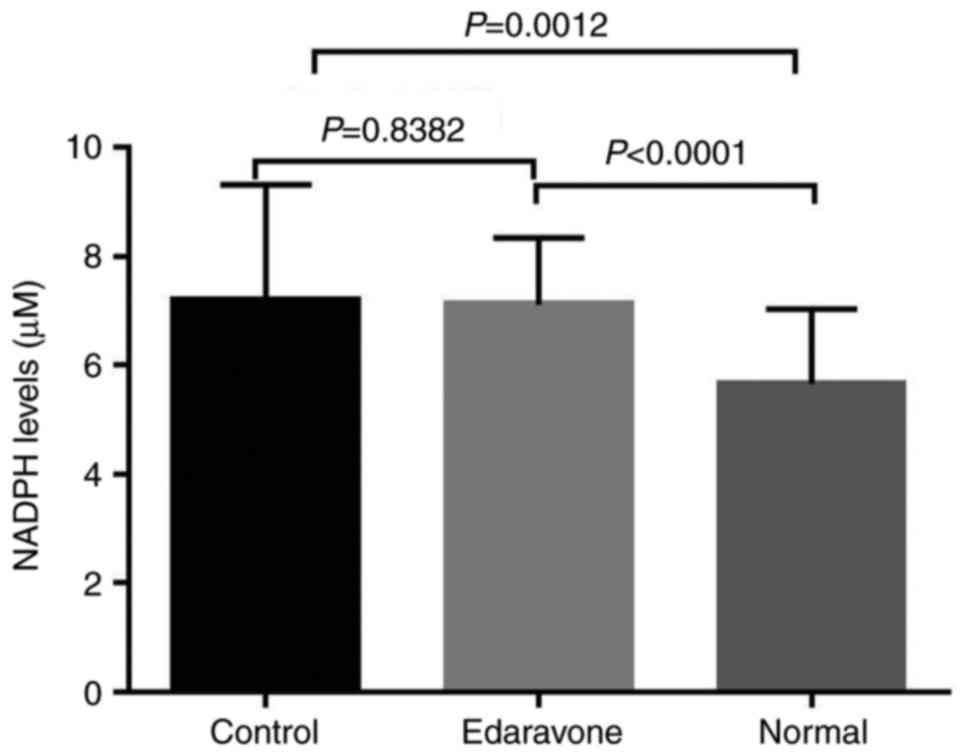
In the animal model of elastase-induced aneurysm,
the results revealed that the serum levels of NADPH oxidase in the
control group were significantly higher compared with those in the
normal group, as shown by ELISA (P<0.05). However, no
significant difference was observed between the edaravone group and
the control group (P=0.8382; Fig.
3). These results suggest that NADPH oxidase contributes to the
pathogenesis of IA and that drug therapy for IA warrants further
investigation.
Suppression of aneurysm development due
to the inhibition of ROS by edaravone in an animal model of
elastase-induced aneurysm
In the model of elastase-induced aneurysm, the
success rate of the model in the edaravone group was 62.5%, which
was lower than that in the control group (82.3%), and the diameter
of the aneurysm was smaller than that of the control group
(3.26±0.13 mm vs. 3.85±0.07 mm). In the edaravone group, there were
2 rabbits with peripheral ulcers following the injection of
edaravone into the auricular vein, and there was 1 rabbit with a
combination of ulcers around the incision site. However, in the
control group, the wound ulcer rate was higher than that in the
edaravone group (Table II). As
shown in Table II, there was 1
intraoperative death in the control group, and in the edavarone
group, there were 2 intraoperative deaths. Thus, in total, 3
rabbits died during surgery. This was due to the anesthesia or the
loss of blood. Our aneurysm modeling results are consistent with
those of previous studies. In a previous study, there were 121
(17%) deaths among 700 subjects. In that study, it was determined
that the causes of death were related to the anesthesia, device
failure, failure to thrive (FTT), etc. (39). The forms of aneurysm in the
edaravone group and the control group were very similar, although
the size of the fusiform aneurysms differed (Fig. 4).
 | Table IIResults obtained from the rabbit
model of elastase-induced aneurysms. |
Table II
Results obtained from the rabbit
model of elastase-induced aneurysms.
| Group | Control | Edaravone |
|---|
| Total numbers | 18 | 18 |
| Intraoperative
death | 1 | 2 |
| Postoperative
death | 0 | 0 |
| Aneurysm | 14 | 10 |
| Aneurysm rate | 82.3% | 62.5% |
| Wound ulcer
rate | 35.3% | 12.5% |
| Ear vein | 5 | 2 |
| Operative
incision | 2 | 1 |
| Ear vein +
incision | 1 | 1 |
| Mean diameter
(mm) | 3.85±0.07 | 3.26±0.13 |
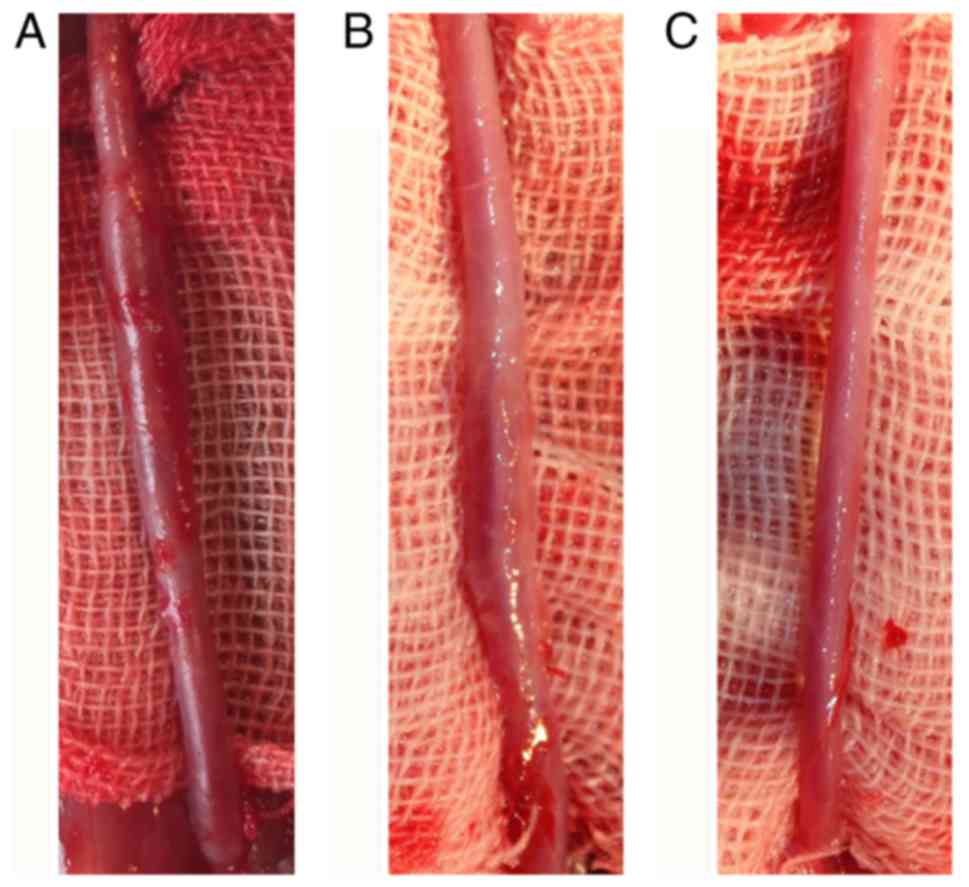
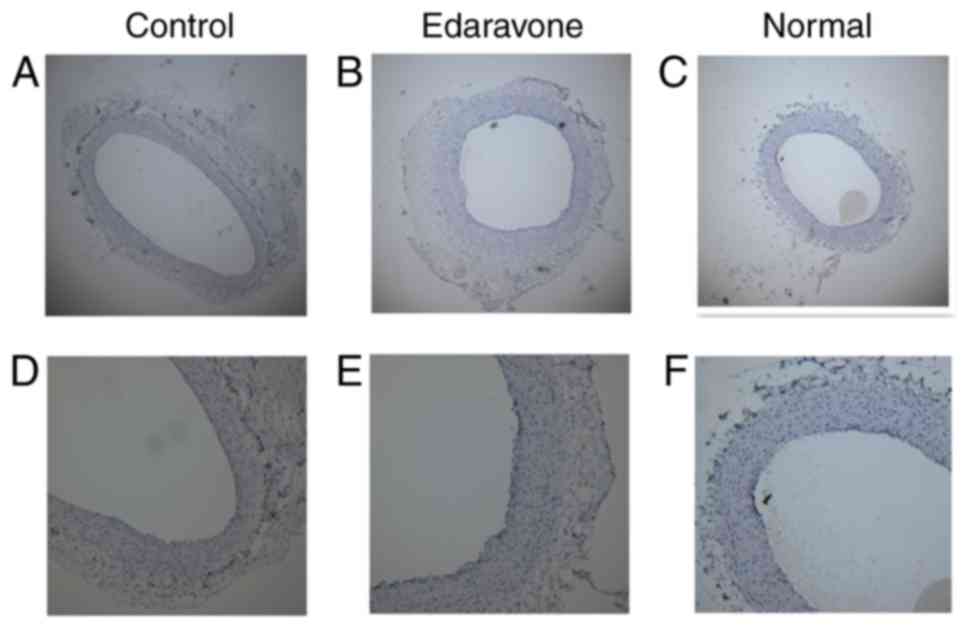
General views of fusiform aneurysm at day 21 or the
maximum diameter of the common carotid artery differed
significantly among the 3 groups. The mean diameter demonstrated
the average value of the maximal fusiform aneurysm diameter at day
21 or the maximum diameter of the common carotid artery in normal
rabbits, suggesting significant differences among the 3 groups
(P<0.05; Fig. 5). The fusiform
aneurysms in the control group were larger in size and more typical
than those in the edaravone group. The histologic findings from
H&E staining confirmed the general views. The vascular smooth
muscle of the control group was fractured and the distribution
remained inhomogeneous, which conforms to the morphological
characteristics of the aneurysm. The vascular smooth muscle of the
edaravone group was similar to the characteristics of the normal
vascular intima (Fig. 6).
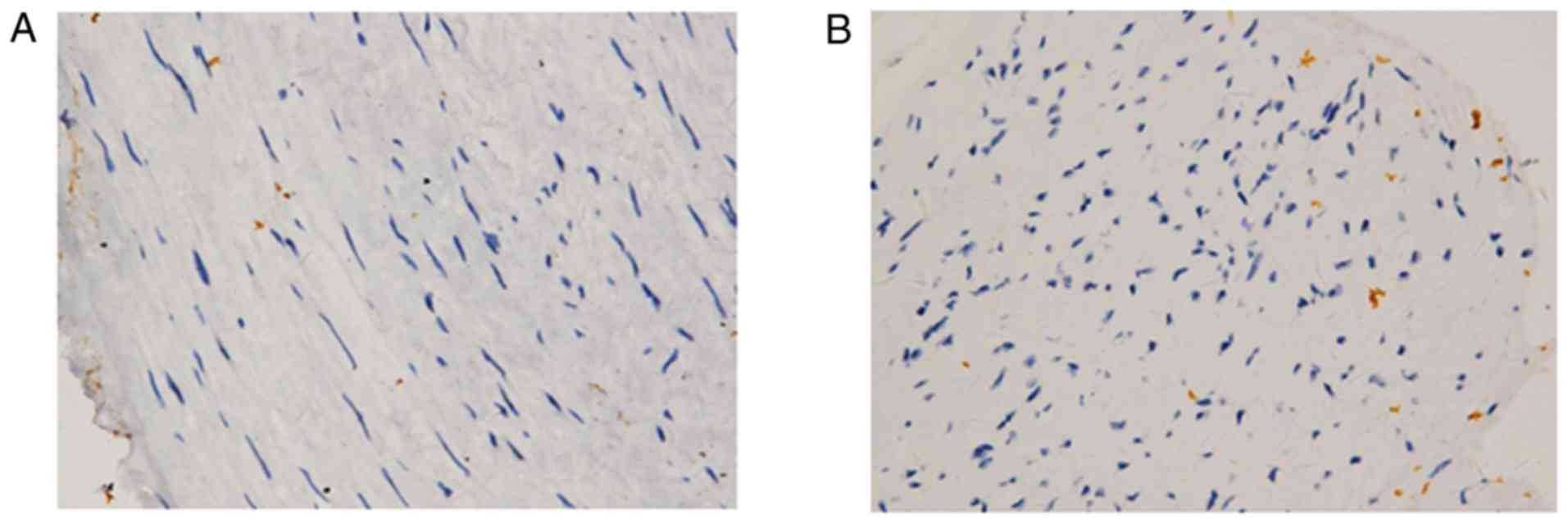
Cell apoptosis and inflammatory cell infiltration
are closely related to oxidative stress. The patients with IA had a
higher proportion of CD34+ cells than the patients with
renal cell carcinoma in our control group (Fig. 7). However, there were no obvious
positive findings observed in the 3 groups of tissue with CD34
staining, although the internal diameter of the 3 groups exhibited
similar results with the H&E findings (Fig. 8).
Effects of edaravone on MMP-2 and MMP-9
expression
Since aneurysmal degeneration is involved in the
destructive remodeling of the connective tissue of the aortic wall,
this structure is associated with the excessive production of local
matrix-degrading proteases and chronic inflammation (40); thus, in this study, we evaluated
the expression of MMP-2 and MMP-9 in the serum of rabbits with
elastase-induced aneurysm at day 21.
The results revealed that the serum levels of MMP-9
in the edaravone group were significantly lower compared with those
in the control group, as shown by ELISA (P<0.05). However, as
regards the serum levels of MMP-2, no significant differences were
observed between the edaravone group and the control group
(P=0.7544; Fig. 9).
Discussion
Although many researchers believe that IA is caused
by the interaction of a number of factors, such as genetic factors
and environmental factors (41-44), little is known about its specific
biological mechanisms. Genetic studies on IA have indicated that
the onset of IA is associated with a few specific single nucleotide
mutations, such as the tumor necrosis factor (TNF-α) gene
(45), the interleukin
(IL)-6 gene (46),
the IL-12 gene (47), and
the NADPH oxidase p22phox gene (48,49), which suggests that there is a
positive association between susceptibility genes and IA. The
genetic background of these findings however, remains unclear.
Currently, treatment options for complex IA include conservative
observations, microsurgical clipping and endovascular embolization.
Currently, there are no other effective options for the treatment
of IA. Very little is known about the mechanisms of IA formation
and progression. We have only a restricted knowledge regarding the
mechanisms of IA formation and progression, which is the main
reason for the difficulties in achieving breakthroughs in current
complex IA treatment situation.
With the development of molecular biotechnology and
the increasing understanding of IA, the importance of genetic
factors related to IA development has gained increasing attention,
and the family genetic predisposition of IA in different races and
different populations has been confirmed (20). p22phox is an important
component of the NADPH oxidase system and is closely related to the
development of atherosclerosis and ischemic cerebrovascular
disease. In a previous study, in a UK population group, a
significant correlation was demonstrated between the 214C>T
polymorphism and cardiovascular disease (CVD) (30). However, some studies provide
evidence that these polymorphisms are not associated with the
occurrence of IA in Caucasians (50). In this study, we found that the
p22phox-214T/C was associated with IA in a Chinese
population. In the IA group, the mRNA expression of NADPH oxidase
was significantly higher than that of the control group. The plasma
NADPH oxidase levels were also significantly higher in the IA group
than in the control group (P<0.001). The high expression of
NADPH oxidase is controlled by the p22phox polymorphism.
However, through statistical analysis, we found that the C allele
was not associated with the IA number (single or multiple) or IA
position (anterior or posterior circulation). These conflicting
results may be explained by ethnic-related differences, in addition
to the differences in study design, and the selection and size of
the study samples.
For clinical applications, as IA is always found in
a pre-existing form, it must be regressed by medical treatment. In
this study, we clarified the key role of ROS in the formation of
IA. ROS are the major mediators of various inflammatory cascades
and are associated with the onset of various diseases, including
arteriosclerosis and AAA (51,52).
The role of ROS activation by nuclear factor (NF)-κB
in the aneurysm wall in the formation of IA plays a key role. It
has been demonstrated that the c-Jun N-terminal kinase inhibitor,
SP600125, can promote the degradation of aneurysms in a model of
AAA (53); however, the drug has
never been used in the clinical. Clinically, vitamin E
supplementation may not reduce the incidence of AAA (54), and thus these drugs may not be a
suitable choice for clinical use. Edaravone has been shown to
effectively inhibit the activation of NF-κB through the ROS system,
resulting in decreased expression of MCP-1, VCAM-1 and MMP-2
(25). The inhibition of MCP-1
and VCAM-1 has been shown to significantly prevent macrophage
infiltration (25). These
findings indicate that edaravone can improve the oxidative
degeneration of the aortic wall and prevent the development of
aneurysms. We thus hypothesized that edaravone, as an oxygen free
radical scavenger, may have the potential to inhibit the growth of
aneurysms.
In this study, in our model of elastase-induced
aneurysm, after observing the development of aneurysms, treatment
with edaravone began 7 days after the aneurysm was observed.
Edaravone at 2 mg/kg/day, twice daily, effectively reduced the
success rate of large auricular aneurysm models and the wound
healing rate. In the edaravone group, there were 2 rabbits with
peripheral ulcers following the injection of edaravone into the
auricular vein, and there was 1 rabbit with a combination of ulcers
around the incision site. However, in the control group, there are
5 rabbits with peripheral ulcers following the injection of saline
into the auricular vein, and 2 rabbits with ulcers around the
incision site, resulting in a higher wound ulcer rate than the
edaravone group. In theory, the expression of NADPH oxidase should
be significantly altered following oxidative treatment with
edaravone; however, the actual results are shown in Fig. 3. There is a significant difference
in the NADPH oxidase levels between the edaravone group and the
normal group; however, there was no significant difference between
the edaravone group and the aneurysm control group. The diameter of
the aneurysms in the edaravone group was lower than that of those
in the control group (3.26±0.13 mm vs. 3.85±0.07 mm), and the
expression of MMP-9 was lower in the edaravone group than in the
control group (P<0.0001). The major finding of this study was
that edaravone was beneficial for wound healing and suppressed the
development of aneurysms. Effective IA drug therapy may have
significant benefits for many patients with complex IA. The results
of this study provide new insight into the mechanisms of IA
formation.
However, this study has some limitations. Firstly,
we only analyzed some of the genes involved. In addition to
p22phox, genetic variations in other components of the
NADPH oxidase complex may also result in alterations in superoxide
in the blood vessel wall and, as a consequence, aneurysm formation.
Secondly, in this study, we used only one dose of edaravone. In
order to evaluate the dose-dependent effects of edaravone on IA
prevention, it is necessary to further examine the effects of
edaravone on IA at other concentrations. Thirdly, there is no
better method to accurately measure the diameter of aneurysms and
blood vessels. The measurement results from our direct method are
prone to certain errors. Fourthly, in theory, MMP-2 and MMP-9 are
required for aneurysm formation, and edaravone can reduce the
expression of MMP-2 and MMP-9, thereby preventing the development
of aneurysms (26,27). However, in our actual study, MMP-9
was inhibited; however, MMP-2 expression did not differt
significantly between the edaravone group and the control group
(P=0.7544). Fifthly, CD34 is an important pro-inflammatory and
pro-angiogenic cell in chronic inflammatory vascular diseases.
Previous studies have indicated that CD34 cells are closely related
to the development of aneurysms. Patients with AAA have a higher
proportion of CD34+ cells than patients with peripheral
vascular disease (PVD) (55,56). However, in our study,
CD34+ cells were difficult to find. The abnormal
expression of MMP-2 and the low expression of CD34 cells may be
related to the short time of our aneurysm modeling. In future
studies, long-term and large-scale aneurysm model samples are
required to arrive at more accurate conclusions.
In addition, IA as a complex disease affected by
multiple genes, is also greatly affected by environmental factors,
which has brought difficulties to molecular biology research. At
present, researchers have conducted a large number of basic and
clinical studies on the NADPH oxidase p22phox. The
results of this study, indicate that the p22phox
gene polymorphism is associated with vascular disease, at least in
Chinese populations. ROS was inhibited and the potential of
edaravone to inhibit the formation of IA was shown. These results
may provide new strategies for the treatment of IA.
Funding
The study was supported by the Research Project of
Taihe hospital (no. 2015JJXM08), the Research Project of Taihe
hospital (no. 2016JJXM067), the Natural Science Foundation of Hubei
Province (no. 2017CFC882) and the Natural Science Foundation of
Hubei Province (no. 2014CFB314).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JH, JL and QC made substantial contributions to the
conception and design of the present study. JH, HW, CW, RL, AL, YZ
and ZF performed the experiments. JH wrote the manuscript. HW and
RL edited and revised the manuscript critically for important
intellectual content. All authors have read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
This case-control study was approved by the Research
Ethics Committee of Taihe Hospital, Hubei University of Medicine,
Shiyan, China. Informed consent was obtained from all the patients.
The animal experiments were approved and conducted according to the
guidelines set by the Experimental Animal Center of the Hubei
University of Medicine (Shiyan, China). All animal experiments were
performed in the Experimental Animal Center according to the
protocols approved by the Animal Ethics Committee of the Hubei
University of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Etminan N and Macdonald RL: Management of
aneurysmal subarachnoid hemorrhage. Handb Clin Neurol. 140:195–228.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Safavi-Abbasi S, Kalani MYS, Frock B, Sun
H, Yagmurlu K, Moron F, Snyder LA, Hlubek RJ, Zabramski JM, Nakaji
P and Spetzler RF: Techniques and outcomes of microsurgical
management of ruptured and unruptured fusiform cerebral aneurysms.
J Neurosurg. 127:1353–1360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Safavi-Abbasi S, Moron F, Sun H, Wilson C,
Frock B, Oppenlander ME, Xu DS, Ghafil C, Zabramski JM, Spetzler RF
and Nakaji P: Techniques and outcomes of Goretex Clip-wrapping of
ruptured and unruptured cerebral aneurysms. World Neurosurgery.
90:281–290. 2016. View Article : Google Scholar
|
|
4
|
Ganesh Kumar N, Ladner TR, Kahn IS,
Zuckerman SL, Baker CB, Skaletsky M, Cushing D, Sanborn MR, Mocco J
and Ecker RD: Parent vessel occlusion for treatment of cerebral
aneurysms: Is there still an indication? A series of 17 patients. J
Neurol Sci. 372:250–255. 2017. View Article : Google Scholar
|
|
5
|
Lawton MT, Abla AA, Rutledge WC, Benet A,
Zador Z, Rayz VL, Saloner D and Halbach VV: Bypass surgery for the
treatment of dolichoectatic basilar trunk aneurysms: A work in
progress. Neurosurgery. 79:83–99. 2016. View Article : Google Scholar :
|
|
6
|
Lee K, Park H, Park I, Park SQ, Kwon OK
and Han J: Y-configuration Stent-assisted Coil Embolization for
Wide-necked intracranial bifurcation aneurysms. J Cerebrovasc
Endovasc Neurosurg. 18:355–362. 2016. View Article : Google Scholar
|
|
7
|
Tan J, Ndoro S, Okafo U, Garrahy A, Agha A
and Rawluk D: Delayed recovery of adipsic diabetes insipidus (ADI)
caused by elective clipping of anterior communicating artery and
left middle cerebral artery aneurysms. N Z Med J. 129:86–90.
2016.PubMed/NCBI
|
|
8
|
Imaizumi S, Woolworth V, Fishman RA and
Chan PH: Liposome-entrapped superoxide dismutase reduces cerebral
infarction in cerebral ischemia in rats. Stroke. 21:1312–1317.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
del Zoppo GJ, Schmid-Schonbein GW, Mori E,
Copeland BR and Chang CM: Polymorphonuclear leukocytes occlude
capillaries following middle cerebral artery occlusion and
reperfusion in baboons. Stroke. 22:1276–1283. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walder CE, Green SP, Darbonne WC, Mathias
J, Rae J, Dinauer MC, Curnutte JT and Thomas GR: Ischemic stroke
injury is reduced in mice lacking a functional NADPH oxidase.
Stroke. 28:2252–2258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dinauer MC: The respiratory burst oxidase
and the molecular genetics of chronic granulomatous disease. Crit
Rev Clin Lab Sci. 30:329–369. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ushio-Fukai M, Zafari AM, Fukui T,
Ishizaka N and Griendling KK: p22phox is a critical component of
the superoxide-generating NADH/NADPH oxidase system and regulates
angiotensin II-induced hypertrophy in vascular smooth muscle cells.
J Biol Chem. 271:23317–23321. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tada Y, Kitazato KT, Tamura T, Yagi K,
Shimada K, Kinouchi T, Satomi J and Nagahiro S: Role of
mineralocorticoid receptor on experimental cerebral aneurysms in
rats. Hypertension. 54:552–557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Griendling KK, Minieri CA, Ollerenshaw JD
and Alexander RW: Angiotensin II stimulates NADH and NADPH oxidase
activity in cultured vascular smooth muscle cells. Circ Res.
74:1141–1148. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cahilly C, Ballantyne CM, Lim DS, Gotto A
and Marian AJ: A variant of p22(phox), involved in generation of
reactive oxygen species in the vessel wall, is associated with
progression of coronary atherosclerosis. Circ Res. 86:391–395.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito D, Murata M, Watanabe K, Yoshida T,
Saito I, Tanahashi N and Fukuuchi Y: C242T polymorphism of NADPH
oxidase p22 PHOX gene and ischemic cerebrovascular disease in the
Japanese population. Stroke. 31:936–939. 2000. View Article : Google Scholar
|
|
17
|
Xia Y, Xia H, Chen D, Liao Z and Yan Y:
Mechanisms of autophagy and apoptosis mediated by JAK2 signaling
pathway after spinal cord injury of rats. Exp Ther Med.
14:1589–1593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belsley SJ and Tilson MD: Two decades of
research on etiology and genetic factors in the abdominal aortic
aneurysm (AAA)-with a glimpse into the 21st century. Acta Chir
Belg. 103:187–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Absi TS, Sundt TM III, Tung WS, Moon M,
Lee JK, Damiano RR Jr and Thompson RW: Altered patterns of gene
expression distinguishing ascending aortic aneurysms from abdominal
aortic aneurysms: Complementary DNA expression profiling in the
molecular characterization of aortic disease. J Thorac Cardiovasc
Surg. 126:344–357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pincemail J, Defraigne JO, Cheramy-Bien
JP, Dardenne N, Donneau AF, Albert A, Labropoulos N and Sakalihasan
N: On the potential increase of the oxidative stress status in
patients with abdominal aortic aneurysm. Redox Rep. 17:139–144.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gavrila D, Li WG, McCormick ML, Thomas M,
Daugherty A, Cassis LA, Miller FJ Jr, Oberley LW, Dellsperger KC
and Weintraub NL: Vitamin E inhibits abdominal aortic aneurysm
formation in angiotensin II-infused apolipoprotein E-deficient
mice. Arterioscler Thromb Vasc Biol. 25:1671–1677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong W, Mactaggart J, Knispel R, Worth J,
Zhu Z, Li Y, Sun Y, Baxter BT and Johanning J: Inhibition of
reactive oxygen species attenuates aneurysm formation in a murine
model. Atherosclerosis. 202:128–134. 2009. View Article : Google Scholar :
|
|
23
|
Marzatico F, Gaetani P, Tartara F,
Bertorelli L, Feletti F, Adinolfi D, Tancioni F and Rodriguez y
Baena R: Antioxidant status and alpha1-antiproteinase activity in
subarachnoid hemorrhage patients. Life Sci. 63:821–826. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takagi H and Umemoto T; lice
(All-Literature Investigation of Cardiovascular Evidence) group:
Vitamins and abdominal aortic aneurysm. Int Angiol. 36:21–30.
2017.
|
|
25
|
Aoki T, Nishimura M, Kataoka H, Ishibashi
R, Nozaki K and Hashimoto N: Reactive oxygen species modulate
growth of cerebral aneurysms: A study using the free radical
scavenger edaravone and p47phox(−/−) mice. Lab Invest. 89:730–741.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Longo GM, Xiong W, Greiner TC, Zhao Y,
Fiotti N and Baxter BT: Matrix metalloproteinases 2 and 9 work in
concert to produce aortic aneurysms. J Clin Invest. 110:625–632.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park WH: Exogenous
H2O2 induces growth inhibition and cell death
of human pulmonary artery smooth muscle cells via glutathione
depletion. Mol Med Rep. 14:936–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morimoto K, Hasegawa T, Tanaka A, Wulan B,
Yu J, Morimoto N, Okita Y and Okada K: Free-radical scavenger
edaravone inhibits both formation and development of abdominal
aortic aneurysm in rats. J Vasc Surg. 55:1749–1758. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu J, Luo J, Wang H, Wang C, Sun X, Li A,
Zhou Y, Liu Y and Chen Q: Association of TNF-α-3959T/C Gene
polymorphisms in the Chinese population with intracranial
aneurysms. J Mol Neurosci. 63:349–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meijles DN, Fan LM, Ghazaly MM, Howlin B,
Krönke M, Brooks G and Li JM: p22phox C242T Single-nucleotide
polymorphism inhibits inflammatory oxidative damage to endothelial
cells and vessels. Circulation. 133:2391–2403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Najafi M, Alipoor B, Shabani M,
Amirfarhangi A and Ghasemi H: Association between rs4673 (C/T) and
rs13306294 (A/G) haplotypes of NAD(P)H oxidase p22phox gene and
severity of stenosis in coronary arteries. Gene. 499:213–217. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun J, Wen M, Wang Y, Liu D, Ying W and
Wang X: The three CYBA variants (rs4673, rs1049254 and rs1049255)
are benign: New evidence from a patient with CGD. BMC Med Genet.
18:1272017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watanabe S, Nitta N, Sonoda A, Nitta-Seko
A, Ohta S, Tsuchiya K, Otani H, Tomozawa Y, Nagatani Y, Mukaisho K,
et al: Inhibition of fibrosis and inflammation by triple therapy
with pirfenidone, edaravone and erythropoietin in rabbits with
drug-induced lung injury: Comparison of CT imaging and pathological
findings. Exp Ther Med. 6:1096–1100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Ma C, Xu N, Xu K, Wang H, Yu J, Li
Y, Wang K, Wang X and Luo Q: An improved elastase-based method to
create a saccular aneurysm rabbit model. Br J Neurosurg.
27:779–782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang ZZ, Liu XT, Ma CY, He C, Li XY, Hou
CL, Cheng ZS and Xia GY: Detection of atherosclerotic plaques in
the rabbit aorta using ultrasound microbubbles conjugated to
interleukin-18 antibodies. Med Sci Monit. 23:5446–5454. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rao J, Brown BN, Weinbaum JS, Ofstun EL,
Makaroun MS, Humphrey JD and Vorp DA: Distinct macrophage phenotype
and collagen organization within the intraluminal thrombus of
abdominal aortic aneurysm. J Vasc Surg. 62:585–593. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Van Spyk EN, Chun KC, Samadzadeh KM,
Peters JH and Lee ES: Increased levels of CD34+ cells
are associated in patients with abdominal aortic aneurysms compared
with patients with peripheral vascular disease. J Surg Res.
184:638–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang C, Feng H, Deng BQ, Li ZF, Huang QH,
Zhao W, Zhao WY, Yang PF, Xu Y, Zhao R and Liu JM: Decreased levels
and function of circulating endothelial progenitor cells in
unruptured intracranial saccular aneurysm patients. Neurol Sci.
35:23–28. 2014. View Article : Google Scholar
|
|
39
|
Lewis DA, Ding YH, Dai D, Kadirvel R,
Danielson MA, Cloft HJ and Kallmes DF: Morbidity and mortality
associated with creation of elastase-induced saccular aneurysms in
a rabbit model. AJNR Am J Neuroradiol. 30:91–94. 2009. View Article : Google Scholar :
|
|
40
|
Pyo R, Lee JK, Shipley JM, Curci JA, Mao
D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM and Thompson RW:
Targeted gene disruption of matrix metalloproteinase-9 (gelatinase
B) suppresses development of experimental abdominal aortic
aneurysms. J Clin Invest. 105:1641–1649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang X, Li J, Fang Y, Zhang Z, Jin D, Chen
X, Zhao Y, Li M, Huan L, Kent TA, et al: Rho guanine nucleotide
exchange factor ARHGEF17 Is a risk gene for intracranial aneurysms.
Circ Genom Precis Med. 11:e0020992018.
|
|
42
|
Yamada Y, Kato K, Oguri M, Horibe H,
Fujimaki T, Yasukochi Y, Takeuchi I and Sakuma J: Identification of
nine genes as novel susceptibility loci for early-onset ischemic
stroke, intracerebral hemorrhage, or subarachnoid hemorrhage.
Biomed Rep. 9:8–20. 2018.PubMed/NCBI
|
|
43
|
van Donkelaar CE, Potgieser ARE, Groen H,
Foumani M, Abdulrahman H, Sluijter R, van Dijk JMC and Groen RJM:
Atmospheric pressure variation is a delayed trigger for aneurysmal
subarachnoid hemorrhage. World Neurosurg. 112:e783–e790. 2018.
View Article : Google Scholar
|
|
44
|
Patrice T, Rozec B, Desal H and Blanloeil
Y: Oceanic meteorological conditions influence incidence of
aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis.
26:1573–1581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fontanella M, Rainero I, Gallone S, Rubino
E, Fenoglio P, Valfre W, Garbossa D, Carlino C, Ducati A and
Pinessi L: Tumor necrosis factor-alpha gene and cerebral aneurysms.
Neurosurgery. 60:668–673. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang G, Tu Y, Feng W, Huang L, Li M and
Qi S: Association of interleukin-6-572G/C gene polymorphisms in the
Cantonese population with intracranial aneurysms. J Neurol Sci.
306:94–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li LJ, Pan XM, Sima X, Li ZH, Zhang LS,
Sun H, Zhu Y, Liang WB, Gao LB and Zhang L: Interactions of
interleukin-12A and interleukin-12B polymorphisms on the risk of
intracranial aneurysm. Mol Biol Rep. 39:11217–11223. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sutliff RL, Hilenski LL, Amanso AM,
Parastatidis I, Dikalova AE, Hansen L, Datla SR, Long JS, El-Ali
AM, Joseph G, et al: Polymerase delta interacting protein 2
sustains vascular structure and function. Arterioscler Thromb Vasc
Biol. 33:2154–2161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tamura T, Jamous MA, Kitazato KT, Yagi K,
Tada Y, Uno M and Nagahiro S: Endothelial damage due to impaired
nitric oxide bioavailability triggers cerebral aneurysm formation
in female rats. J Hypertens. 27:1284–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Krex D, Ziegler A, Konig IR, Schackert HK
and Schackert G: Polymorphisms of the NADPH oxidase P22PHOX gene in
a Caucasian population with intracranial aneurysms. Cerebrovasc
Dis. 16:363–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dalman RL: Oxidative stress and abdominal
aneurysms: How aortic hemodynamic conditions may influence AAA
disease. Cardiovasc Surg. 11:417–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pincemail J, Defraigne JO, Courtois A,
Albert A, Cheramy-Bien JP and Sakalihasan N: Abdominal aorta
aneurysm (AAA): Is there a role for prevention and therapy using
antioxidants? Curr Drug Targets. Sep 18–2017.Epub ahead of
print.
|
|
53
|
Yoshimura K, Aoki H, Ikeda Y, Furutani A,
Hamano K and Matsuzaki M: Regression of abdominal aortic aneurysm
by inhibition of c-Jun N-terminal kinase in mice. Ann NY Acad Sci.
1085:74–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tornwall ME, Virtamo J, Haukka JK, Albanes
D and Huttunen JK: Alpha-tocopherol (vitamin E) and beta-carotene
supplementation does not affect the risk for large abdominal aortic
aneurysm in a controlled trial. Atherosclerosis. 157:167–173. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ollikainen E, Tulamo R, Frösen J, Lehti S,
Honkanen P, Hernesniemi J, Niemela M and Kovanen PT: Mast cells,
neovascularization, and microhemorrhages are associated with
saccular intracranial artery aneurysm wall remodeling. J
Neuropathol Exp Neurol. 73:855–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rodella LF, Rezzani R, Bonomini F, Peroni
M, Cocchi MA, Hirtler L and Bonardelli S: Abdominal aortic aneurysm
and histological, clinical, radiological correlation. Acta
Histochem. 118:256–262. 2016. View Article : Google Scholar : PubMed/NCBI
|