Introduction
Osteoporosis is a skeletal disease, which has the
characteristics of decreased bone mass and deterioration of bone
tissue (1). Increased bone
fragility and fracture risk in patients with osteoporosis leads to
disability, diminution of quality of life and premature death. In
addition, the ever-rising population of the elderly is leading to a
socioeconomic burden estimated to be $25.3 billion each year
(2).
There are many reasons for the loss of bone,
including genetics, age, nutrition and lifestyle (3). In particular, the deficiency of sex
hormones is closely associated with the increased activity of
osteoclasts and loss of bone (4).
The homeostasis of the bone matrix is maintained between the
formation of the bone matrix by osteoblasts and bone resorption by
osteoclasts (5). With age, the
homeostatic balance of the bone shifts towards osteoclasts
(6). Because estrogen has a role
promoting apoptosis of osteoclasts and is involved in the
differentiation of osteoblasts, its deficiency leads to greater
osteoporotic changes in menopausal women (7). Therefore, the prevalence of
osteoporosis is higher in women aged 50 years and older (8).
Currently, the treatments for osteoporosis include
hormone replacement therapy (HRT), bisphosphonates, recombinant
human parathyroid hormone (PTH) and supplements of Calcium and
Vitamin D (9). Due to the
critical roles of sex hormones in osteoporosis, HRT used to be the
primary treatment for osteoporosis. However, no net benefits were
observed between fracture risk reduction and burden to other parts
of the body according to the results from the Women's Health
Initiative, in which increased risk of stroke, coronary heart
disease, pulmonary embolism and invasive breast cancer was the
outcome (10). Calcium and
Vitamin D supplements were the conventional first step to
osteoporosis; however, the evidence was deemed insufficient by the
US Preventive Task Force in 2013 to assess their benefits and/or
potential harm (11). In
addition, bisphosphonate is reported to inhibit bone resorption by
osteoclasts. However, there is controversy over the use of
bisphosphonates in the treatment of osteoporosis, especially due to
incidences of unexpected serious adverse events, such as
osteonecrosis of the jaw and atypical fracture of the femur
(12). In summary, the effects of
the current treatments for osteoporosis are beneficial in some and
deleterious in others (13),
requiring further studies. Traditional herbal medications
exhibiting therapeutic effects against osteoporosis in both
clinical and experimental conditions could be used as novel
treatments.
The rhizome of Anemarrhena asphodeloides
Bunge (Liliaceae) has been traditionally used in Asia for its
antipyretic, diuretic, sedative, and antitussive effects (14). The steroidal saponins from A.
asphodeloides Bunge exhibit anti-osteoporotic effects by
increasing bone formation in ovariectomized (OVX) rats (15). Bu-Shen-Ning-Xin Decoction, a
Traditional Chinese Medicine formula containing A.
asphodeloides and seven other herbs has been demonstrated to
have some positive effects in suppressing osteoclast
differentiation (16).
Nevertheless, the efficacy of A. asphodeloides on the
amelioration of osteoporosis and its mechanisms of action remain
unclear. In the present study, the effects of an A.
asphodeloides Bunge extract on osteoporotic indexes were
assessed and its mechanisms on osteoclast and osteoblast remodeling
were determined.
Materials and methods
Sample preparation
The rhizome of A. asphodeloides was purchased
from Jung-do Herb (Seoul, Korea). Fifty grams of the herb were
soaked in 500 ml of 70% ethanol for 24 h. The solvent was
separated, evaporated and vacuum dried using a freeze-dryer to
obtain the extracted powder. The extract of A. asphodeloides
Bunge (termed here AAB) was 15.11 g, indicating that the yield was
30.22%. A voucher specimen (OP-AAB70) was deposited at our
laboratory.
Animal experiments
Six-week-old female ICR strain mice were provided by
RAONBIO Inc. (Yongin, Korea) and adapted for 1 week prior to the
experiment. The mice were housed at a temperature of 20±2°C and a
humidity of 50±5% under a 12‑h light/dark cycle. The experimental
protocols were approved by the Institutional Animal Ethics
Committee of Kyung Hee University in Korea [approval no.
KHUASP(SE)-15-093].
A total of 28 mice (n=7) were under the anesthetic
Zoletil (Virbac Lab, Carros cedex, France). A total of 21 mice were
surgically OVX, while seven normal mice in the sham group were
subjected to sham surgery. To recover and induce post-menopausal
osteoporosis, all mice were left for 7 weeks. After that, the mice
were divided into 4 groups: Sham, sham-operated mice receiving
daily oral PBS as the normal control; OVX, OVX mice receiving daily
oral PBS as the negative control; E2, OVX mice receiving
intraperitoneal injection of 17β-estradiol (E2) as the positive
control; AAB, OVX mice receiving orally AAB. The Sham and OVX
groups were administrated orally with 100 µl of vehicle PBS
daily. The experimental AAB group was administrated orally with 100
mg/kg of AAB daily. This dose of AAB was selected based on previous
references regarding the effect of the medicinal herb on
osteoporosis (17-19). The E2 group was intraperitoneally
injected with 10 µg/kg E2 daily. All treatments were
continued daily for 4 weeks. Subsequently, all the mice were
sacrificed.
Measurement of bone mineral density (BMD)
and bone mineral content (BMC)
The proximal femurs were excised and fixed in 10%
neutralized formalin for 18 h. To analyze the BMD and BMC, the
connective tissue was cleanly detached from the femurs. The
collected bone tissues were scanned by dual-energy X-ray
absorptiometry with the InAlyzer instrument (MEDIKORS, Seoul,
Korea).
Cell culture
Murine macrophage-like Raw 264.7 cells (Korean Cell
Line Bank, Seoul, Korea) were grown in Dulbecco's Modified Eagle's
Medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% heat inactivated fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100 units/ml
penicillin until confluence. The cells were incubated at 37°C under
an atmosphere of 5% CO2 in a 100 mm culture dish. All
cells were passaged no more than 10 times.
Cell viability assay
To assess the cell cytotoxicity of AAB, a cell
viability assay was performed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
method. Raw 264.7 cells were seeded in 96-well plates. Each well
was treated with various concentrations of AAB (0.1, 1 and 10
µg/ml) suspended in DMEM culture medium for 24, 48 and 72 h.
Then, 2 mg/ml of MTT solution was added to form formazan crystals.
Following incubation, dimethyl sulfoxide was added and cell
viability was measured at an absorbance of 570 nm using a
microplate reading instrument (BioTek Instruments, Inc., Winooski,
VT, USA). Cell viability was calculated as a % relative to
untreated cells. The experiments were performed three independent
times for reproducibility.
Osteoclast differentiation in vitro
To differentiate from macrophage-like cells to
osteoclasts, Raw 264.7 cells were cultured with 100 ng/ml receptor
activator of nuclear factor κB ligand (RANKL) in α-minimal
essential medium supplemented with 10% heat inactivated FBS for 7
days. ABB (0.1, 1 and 10 µg/ml) was added into the
differentiation media during those 7 days. Fresh differentiation
media containing RANKL and/or AAB was replaced on day 3. The cells
were fixed with 10% neutralized formalin and stained with acid
phosphatase, leukocyte kit (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) according to the manufacturer's protocol. The total
tartrate-resistant acid phosphatase (TRAP) activity was measured at
an absorbance of 405 nm using a microplate-reading instrument. The
experiments were performed three independent times for
reproducibility.
ELISA
Osteoclasts treated with or without AAB were
cultured with RANKL for 7 days, as aforementioned. The supernatants
were collected and cleared by centrifugation at 27,000 x g for 10
min. The concentrations of interleukin (IL)-6 and tumor necrosis
factor (TNF)-α were determined by ELISA kits (BD Biosciences, San
Jose, CA, USA; cat. nos. 555240 for IL-6 and 555268 for TNF-α,
respectively) according to the manufacturer's instructions.
Cytokine levels were estimated at an absorbance of 450 nm using a
microplate-reading instrument.
Western blot analysis
Osteoclasts treated with or without AAB (0.1, 1 and
10 µg/ml) were cultured with RANKL for 7 days, as
aforementioned. Total protein lysates were extracted from the
cultured cells with commercial lysis buffers.
Radioimmunoprecipitation assay buffer (Tech & Innovation,
Gangwon, Korea) was used for total protein extraction and a
ReadyPrep protein extraction kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used for cytoplasmic and nuclear protein
extraction. All procedures for protein extraction were performed
according to the manufacturer's instructions. The obtained cell
lysates were used to determine the concentration of protein by
Bradford assay. Protein samples (20 µg) were subjected to
SDS-PAGE on a 10% gel and transferred to polyvinylidene fluoride
membranes. To block nonspecific sites, the membrane was incubated
with 5% bovine serum albumin (Bio-Rad, Laboratories, Inc.) in a
mixture of TBS and Tween 20 (TBS-T). Primary antibodies (Cell
Signaling Technology, Inc., Danvers, MA, USA), against β-actin
(cat. no. 3700), nuclear factor (NF)-κB (cat. no. 8242), NF-κB
inhibitor α (IκB-α; cat. no. 4812), phosphorylated (p)-IκB-α (cat.
no. 5209), Fos proto-oncogene (c-fos; cat. no. 4384) and LaminB
(cat. no. 12255), diluted 1:1,000 in TBS-T, and extracellular
signal-regulated kinase (ERK; cat. no. 4695), p-ERK (cat. no.
4370), c-Jun N-terminal kinase (JNK; cat. no. 9252), p-JNK (cat.
no. 9251), p38 (cat. no. 9212) and p-p38 (cat. no. 9211), diluted
1:1,500 in TBS-T, were incubated with the membranes at 4°C
overnight. Anti‑rabbit and anti-mouse horseradish peroxidase
(HRP)-conjugated secondary antibodies diluted 1:4,000 in TBS-T
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat. nos. sc-2357
and sc-516102, respectively) were used to bind to the primary
antibody. After incubation for 2 h, enhanced chemiluminescence
detection reagent (Amersham; GE Healthcare, Chicago, IL, USA) was
added to visualize the protein bands. β-actin was used as an
internal loading control for c-fos. LaminB was used as an internal
reference protein for nuclear NF-κB. The total level of each total
IκB-α and ERK was used as internal loading controls for
phosphorylated forms. The band density was quantified with ImageJ
(National Institutes of Health, Bethesda, MD, USA) (20). The experiments were performed
three times independently for reproducibility.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Significance was determined by one-way analysis of
variance, followed by Turkey's multiple comparison tests using
GraphPad Prism 5 software (version 5.0; GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
AAB reverses the decrease of BMD and BMC
in the osteoporotic femur
In the osteoporotic femurs, there were significant
decreases of the BMD and BMC levels compared with normal femurs
(P<0.001; Fig. 1). In terms of
the BMD, there was a 20.3% decrease in the animals of the OVX group
compared with the sham group (Sham, 0.068±0.005 g/cm2;
OVX, 0.054±0.001 g/cm2). The femurs of the AAB-treated
mice exhibited a 12.7% recovery of the BMD compared with the
OVX-induced osteoporotic femurs (E2, 0.061±0.001 g/cm2;
AAB, 0.061±0.002 g/cm2). The BMC level in the OVX group
was ~18.2% decreased compared with the sham group (Sham,
0.0427±0.0009 g; OVX, 0.0349±0.0007 g). AAB treatment significantly
ameliorated the decrease of the BMC, to levels similar to the
positive control group (E2, 0.0365±0.0006 g; AAB, 0.0359±0.0010
g).
AAB inhibits the formation of osteoclasts
in RANKL‑ stimulated Raw 264.7 cells
RANKL stimulation resulted in a significant
induction of osteoclast formation activity in Raw 264.7 cells.
TRAP-positive multinucleated cells were increased by 38.8% in the
RANKL-stimulated cells compared with control cells (Fig. 2A). However, AAB treatment
exhibited inhibitory effects on osteoclastogenesis. AAB treatment
(0.1, 1 and 10 µg/ml) decreased the formation of osteoclasts
in a dose-dependent manner, by 4.9, 11.2 and 39.4%, respectively,
compared to the RANKL-treated cells (Fig. 2A). In addition, AAB showed no
cytotoxicity at all the concentrations tested (0.1, 1 and 10
µg/ml) in the Raw 264.7 cells (Fig. 2B), indicating that AAB inhibited
osteoclastogenesis without any toxic effects on the cells.
AAB inhibits the RANKL‑induced TNF‑α and
IL‑6 production
RANKL-induced osteoclasts exhibited an obvious
increase of pro‑inflammatory cytokines related with
osteoclastogenesis. The levels of TNF-α were increased by
~23.2-fold following RANKL stimulation in the Raw 264.7 cells
(untreated cells, 3.16±0.52 pg/ml; RANKL-treated cells, 73.26±12.58
pg/ml; Fig. 3). In terms of the
IL-6 levels, there was a ~13.3-fold increase in the RANKL-induced
osteoclasts (untreated cells, 0.66±0.14 pg/ml; RANKL-treated cells,
8.79±1.29 pg/ml; Fig. 3). AAB
treatment dose-dependently decreased both the TNF-α and IL-6 levels
(Fig. 3). Compared with cells
treated with RANKL alone, the 0.1, 1 and 10 µg/ml
AAB-treated osteoclasts exhibited a significant inhibition of TNF-α
(37.31±2.26, 16.85±1.11 and 6.97±0.32 pg/ml, respectively). In
addition, RANKL-induced IL-6 production in Raw 264.7 cells was
significantly reduced by AAB at the 0.1, 1 and 10 µg/m
concentrations (6.19±1.25, 4.50±0.64 and 3.55±0.39 pg/ml,
respectively).
AAB inhibits the RANKL‑induced NF‑κB
nuclear translocation
Based on a previous report (21), treatment with RANKL induces NF-κB
translocation into the nucleus and IκB-α phosphorylation in the
cytoplasm. In the present study, AAB treatment significantly
inhibited the RANKL‑induced NF‑κB translocation into the nucleus
(Fig. 4). In addition, p-IκB-α
protein expression was significantly decreased following AAB
treatment compared with the RANKL alone-treated cells (Fig. 4).
AAB inhibits the RANKL‑induced
osteoclast‑specific transcription factor expression
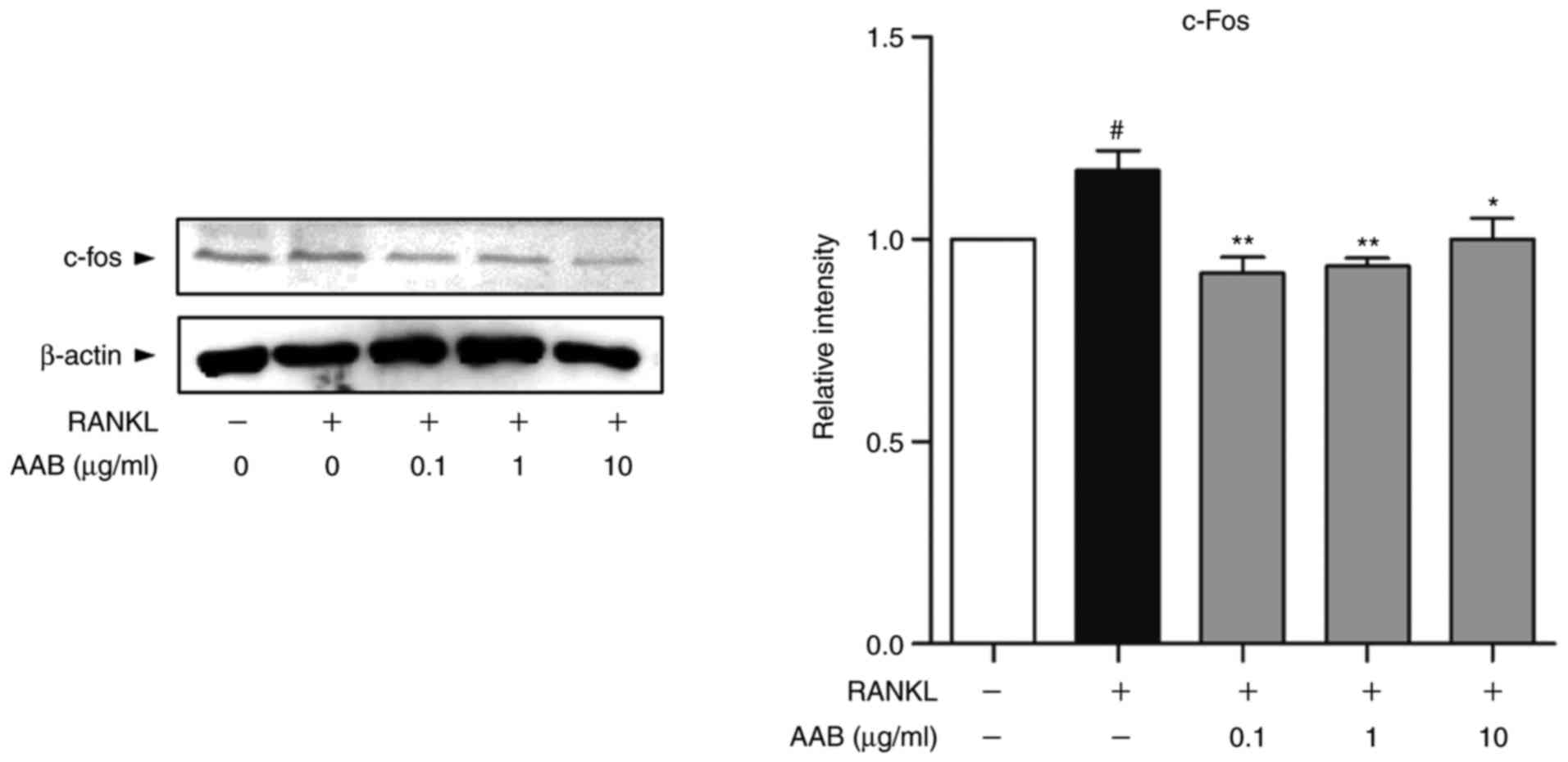
As presented in Fig.
5, the protein expression levels of c-fos were upregulated in
response to RANKL stimulation compared with untreated cells. AAB
co‑treatment in the presence of RANKL significantly reduced the
expression of c-fos in the Raw 264.7 cells (Fig. 5).
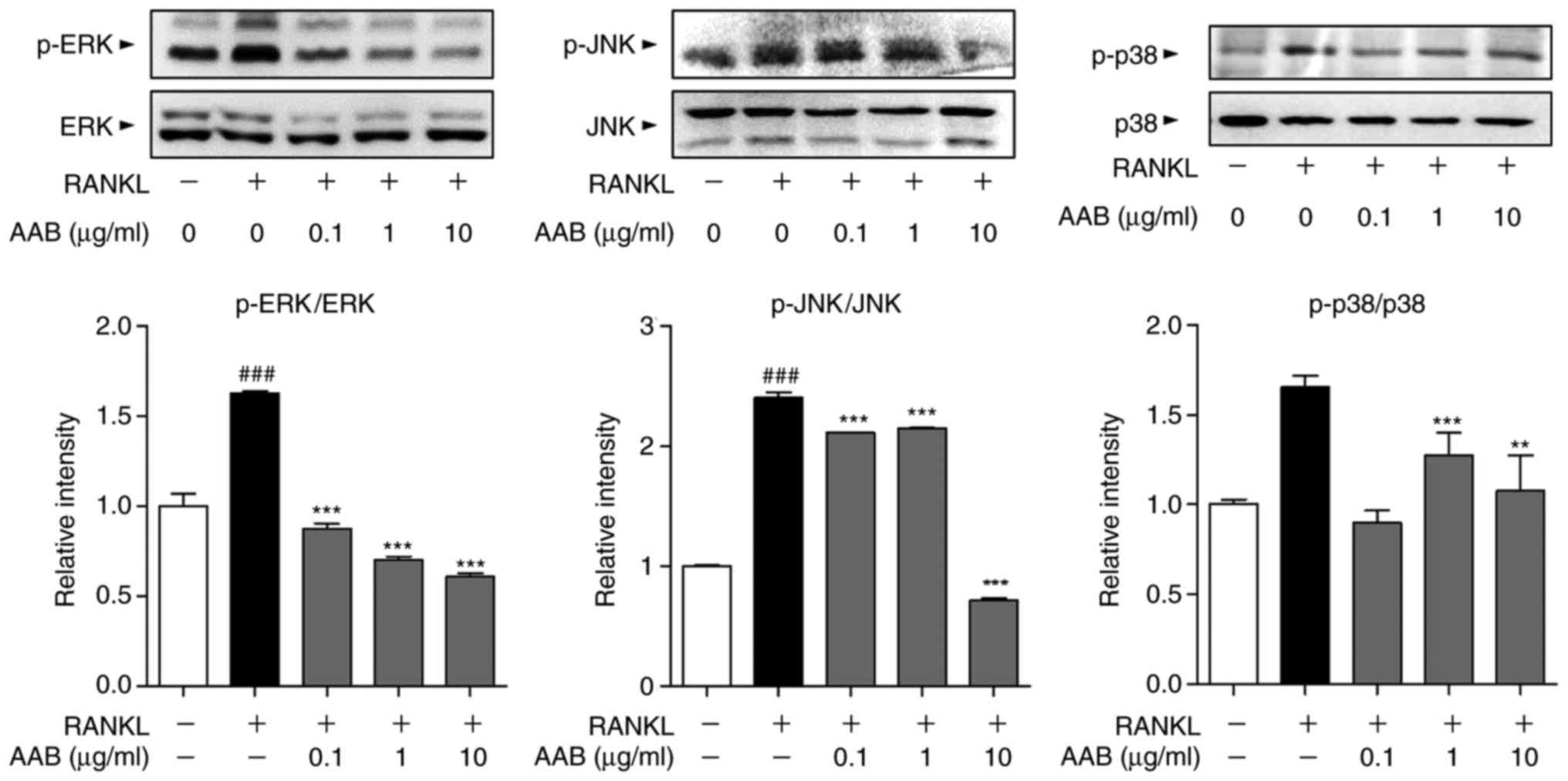
AAB inhibits the RANKL‑induced
mitogen‑activated protein kinase (MAPK) pathway activation
The phosphorylation status of the MAPKs, including
ERK, JNK and p38, was assayed in the RANKL-induced osteoclasts by
western blot analysis. RANKL stimulation resulted in significant
increases in the phosphorylated levels of all three MAPKs, compared
with untreated cells (Fig. 6).
AAB treatment significantly reversed the MAPK activation, by
decreasing the protein expression levels of phosphorylated ERK, JNK
and p38 compared with the RANKL alone-treated cells (Fig. 6).
Discussion
Bone loss and the destruction of bone structure are
the crucial hallmarks of osteoporosis. Osteoporotic patients have a
lower BMD and BMC compared with healthy individuals. The WHO
defines osteoporosis as a BMD <2.5 standard deviations from the
average value (22). BMD is not
the only the diagnostic criterion, but it also serves as valuable
information to predict and prevent fractures in both genders
(23). In addition, a low BMD has
been reported to be associated with higher risk for almost every
type of fracture (24). Several
studies have demonstrated that OVX surgery results in a decreased
BMD and BMC in a rodent model, and therefore the OVX rodent is a
widely used model for postmenopausal loss of bone (25). In the present study, AAB was
demonstrated to exhibit positive effects on preventing bone loss in
a mouse model of osteoporosis, by ameliorating the BMD and BMC of
the femurs.
The development and maintenance of bone are
regulated by the continuous remodeling of the bone coordinated by
the actions between osteoclasts and osteoblasts (26). Aging increases the
stromal/osteoblast cell induced osteoclast genesis and expands its
pool of precursors (27).
Therefore, it promotes bone absorption and decreases bone
formation. To explain the mechanisms underlying the
anti-osteoporotic effects of AAB, the effects of AAB on bone
metabolic cells including osteoclasts and osteoblasts were
investigated.
Osteoclasts are capable of not only absorbing bone,
but also making intimate connections with the bone-generators, such
as osteoblasts, and cells from the immune system (28). Osteoclasts are specialized,
multinucleated cells originating from the monocyte and macrophage
lineage. They adhere to the bone matrix and degrade it through
secretion of acids and enzymes (29). During osteoclastogenesis, there
are two factors that are required, RANKL and the colony-stimulating
factor-1 (30). The most central
and critical regulator of osteoclasts is the RANKL/RANK pathway
with its decoy receptor OPG. The binding of RANKL to RANK leads to
osteoclast activation and differentiation through multiple
signaling pathways (31). Mature
osteoclasts can be observed by staining for their histochemical
marker TRAP (32). In the present
study, cells treated with AAB had significantly suppressed TRAP
activity in a dose-dependent manner, suggesting that AAB inhibited
the RANKL-induced osteoclast formation from its precursors without
any cytotoxicity.
During the differentiation of osteoclasts, the
inflammatory cytokine TNF-α mediates the stimulation of the RANK by
its ligand through an autocrine mechanism (33). TNF-α also stimulates the
production of IL-6 in osteoblasts, which has synergistic effects in
the bone resorption activity with TNF-α, regardless of OPG
(34). The current results
demonstrated that the production of TNF-α and IL-6 were reduced
following AAB treatment in a dose-dependent manner, indicating that
AAB may have inhibitory effects on inflammatory cytokines during
osteoclast differentiation.
NF-κB is a transcription factor with pleiotropic
features involved in the formation, action and survival of
osteoclasts (35). The regulation
of NF-κB is critically related to the IκB-α kinase complex, with
phosphorylation of IκB-α being a central factor in the activity of
NF-κB (36). The degradation of
IκB-α releases NF-κB, activating its translocation into the nucleus
(37). In the present study, AAB
was demonstrated to inhibit NF-κB activation and IκB-α
phosphorylation in RANKL-induced osteoclasts. The RANK signaling
pathway also involves the MAPK cascades, ERK, JNK and p38 (38). The MAPK cascades together promote
osteoclast activation. The MAPKs are also associated with
osteoclast formation through activation of the transcription factor
AP-1 complex, which contains Fos and Jun (39). c-fos serves as the critical switch
component in control of osteoclast differentiation from its
progenitor (40). In the present
study, AAB was demonstrated to have inhibitory effects on c-fos and
the MAPKs, indicating that osteoclastogenesis is the target of the
ameliorative effects of AAB in osteoporosis by regulating related
factors, such as cytokines, NF-κB, MAPKs and c-fos.
In summary, AAB had ameliorative effects on
osteoporosis in vivo and in vitro. In vivo,
AAB treatment improved BMD and BMC levels, while in vitro,
AAB treatment inhibited several osteoclastogenic markers, including
TNF-α, IL-6, NF-κB, MAPKs and c-fos. However, the potential single
ingredient or combination of phytochemicals of AAB involved in its
inhibitory effect on osteoclastogenesis remains unknown. Further
investigations at mechanistic and preclinical levels may provide
useful additional insight for the development and optimization of
advanced treatments for osteoporosis. The present findings suggest
that AAB may be a novel candidate for the treatment of osteoporosis
by inhibiting the formation of osteoclasts.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea funded by the Korea government (grant
no. NRF-2017R1A6A3A11032500).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JSL, MHK and WMY contributed to the study design.
JSL, MHK and HL performed experiments and analyzed data. JSL, MHK
and WMY drafted the manuscript. WMY supervised the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Experimental protocols involving animals were
approved by the Institutional Animal Ethics Committee of Kyung Hee
University in Korea [approval no. KHUASP(SE)-15-093].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Consensus development conference:
Prophylaxis and treatment of osteoporosis. Am J Med. 90:107–110.
1991. View Article : Google Scholar
|
|
2
|
Burge R, Dawson-Hughes B, Solomon DH, Wong
JB, King A and Tosteson A: Incidence and economic burden of
osteoporosis-related fractures in the United States, 2005-2025. J
Bone Miner Res. 22:465–475. 2007. View Article : Google Scholar
|
|
3
|
Pietschmann P, Rauner M, Sipos W and
Kerschan-Schindl K: Osteoporosis: An age-related and
gender-specific disease-a mini-review. Gerontology. 55:3–12. 2009.
View Article : Google Scholar
|
|
4
|
El Maataoui A, El Maghraoui A, Biaz A,
Elmachtani SI, Dami A, Bouhsain S, Mounach A, Chabraoui L and
Ouzzif Z: Relationships between vertebral fractures, sex hormones
and vitamin D in Moroccan postmenopausal women: A cross sectional
study. BMC Womens Health. 15:412015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumoto Y, Otsuka F, Takano-Narazaki M,
Katsuyama T, Nakamura E, Tsukamoto N, Inagaki K, Sada KE and Makino
H: Estrogen facilitates osteoblast differentiation by upregulating
bone morphogenetic protein-4 signaling. Steroids. 78:513–520. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelley GA, Kelley KS and Kohrt WM: Effects
of ground and joint reaction force exercise on lumbar spine and
femoral neck bone mineral density in postmenopausal women: A
meta-analysis of randomized controlled trials. BMC Musculoskelet
Disord. 13:1772012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamrick I, Schrager S and Nye AM:
Treatment of osteoporosis: Current state of the art. Wien Med
Wochenschr. 165:54–64. 2015. View Article : Google Scholar
|
|
10
|
Jackson RD, Wactawski-Wende J, LaCroix AZ,
Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA,
Ko MG, et al: Effects of conjugated equine estrogen on risk of
fractures and BMD in postmenopausal women with hysterectomy:
Results from the women's health initiative randomized trial. J Bone
Miner Res. 21:817–828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moyer VA; U.S. Preventive Services Task
Force: Vitamin D and calcium supplementation to prevent fractures
in adults: U.S. Preventive Services Task Force recommendation
statement. Ann Intern Med. 158:691–696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansen PJ, Knitschke M, Draenert FG, Irle
S and Neff A: Incidence of bisphosphonate-related osteonecrosis of
the jaws (BRONJ) in patients taking bisphosphonates for
osteoporosis treatment-a grossly underestimated risk? Clin Oral
Investig. 17:1829–1837. 2013. View Article : Google Scholar
|
|
13
|
Body JJ, Bergmann P, Boonen S, Devogelaer
JP, Gielen E, Goemaere S, Kaufman JM, Rozenberg S and Reginster JY:
Extraskeletal benefits and risks of calcium, vitamin D and
anti-osteoporosis medications. Osteoporos Int. 23(Suppl 1): S1–S23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HA, Castro-Aceituno V, Abbai R, Moon
SS, Kim YJ, Simu SY and Yang DC: Rhizome of Anemarrhena
asphodeloides as mediators of the eco-friendly synthesis of silver
and gold spherical, face-centred cubic nanocrystals and its
anti-migratory and cytotoxic potential in normal and cancer cell
lines. Artif Cells Nanomed Biotechnol. 1–10. 2018.
|
|
15
|
Nian H, Qin LP, Chen WS, Zhang QY, Zheng
HC and Wang Y: Protective effect of steroidal saponins from rhizome
of Anemarrhena asphodeloides on ovariectomy-induced bone loss in
rats. Acta Pharmacol Sin. 27:728–734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gui Y, Qiu X, Xu Y, Li D and Wang L:
Bu-Shen-Ning-Xin decoction suppresses osteoclastogenesis via
increasing dehydro-epiandrosterone to prevent postmenopausal
osteoporosis. Biosci Trends. 9:169–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He CC, Hui RR, Tezuka Y, Kadota S and Li
JX: Osteoprotective effect of extract from Achyranthes bidentata in
ovariectomized rats. J Ethnopharmacol. 127:229–234. 2010.
View Article : Google Scholar
|
|
18
|
Lee H, Kim MH, Choi YY, Hong J and Yang
WM: Effects of Cynanchum wilfordii on osteoporosis with inhibition
of bone resorption and induction of bone formation. Mol Med Rep.
17:3758–3762. 2018.
|
|
19
|
Jeon EJ, Lee DH, Kim YJ, Ahn J, Kim MJ,
Hwang JT, Hur J, Kim M, Jang YJ, Ha TY, et al: Effects of yuja peel
extract and its flavanones on osteopenia in ovariectomized rats and
osteoblast differentiation. Mol Nutr Food Res. 60:2587–2601. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boyce BF, Xiu Y, Li J, Xing L and Yao Z:
NF-κB-mediated regulation of osteoclastogenesis. Endocrinol Metabol
(Seoul). 30:35–44. 2015. View Article : Google Scholar
|
|
22
|
Kanis JA, Melton LJ III, Christiansen C,
Johnston CC and Khaltaev N: The diagnosis of osteoporosis. J Bone
Miner Res. 9:1137–1141. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnell O, Kanis JA, Oden A, Johansson H,
De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D,
et al: Predictive value of BMD for hip and other fractures. J Bone
Miner Res. 20:1185–1194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stone KL, Seeley DG, Lui LY, Cauley JA,
Ensrud K, Browner WS, Nevitt MC and Cummings SR;
OsteoporoticFractures Research Group: BMD at multiple sites and
risk of fracture of multiple types: Long-term results from the
Study of Osteoporotic Fractures. J Bone Miner Res. 18:1947–1954.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim MH, Choi YY, Han JM, Lee HS, Hong SB,
Lee SG and Yang WM: Ameliorative effects of Schizandra chinensis on
osteoporosis via activation of estrogen receptor (ER)-α/-β. Food
Funct. 5:1594–1601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sims NA and Gooi JH: Bone remodeling:
Multiple cellular interactions required for coupling of bone
formation and resorption. Semin Cell Dev Biol. 19:444–451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao JJ, Wronski TJ, Iwaniec U, Phleger L,
Kurimoto P, Boudignon B and Halloran BP: Aging increases
stromal/osteo-blastic cell-induced osteoclastogenesis and alters
the osteoclast precursor pool in the mouse. J Bone Miner Res.
20:1659–1668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Charles JF and Aliprantis AO: Osteoclasts:
More than ‘bone eaters’. Trends Mol Med. 20:449–459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A,
et al: Osteoclast differentiation factor is a ligand for
osteoprote-gerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar
|
|
31
|
Motiur Rahman M, Takeshita S, Matsuoka K,
Kaneko K, Naoe Y, Sakaue-Sawano A, Miyawaki A and Ikeda K:
Proliferation-coupled osteoclast differentiation by RANKL: Cell
density as a determinant of osteoclast formation. Bone. 81:392–399.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hayman AR: Tartrate-resistant acid
phosphatase (TRAP) and the osteoclast/immune cell dichotomy.
Autoimmunity. 41:218–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zou W, Hakim I, Tschoep K, Endres S and
Bar-Shavit Z: Tumor necrosis factor-alpha mediates RANK ligand
stimulation of osteoclast differentiation by an autocrine
mechanism. J Cell Biochem. 83:70–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yokota K, Sato K, Miyazaki T, Kitaura H,
Kayama H, Miyoshi F, Araki Y, Akiyama Y, Takeda K and Mimura T:
Combination of tumor necrosis factor α and interleukin-6 induces
mouse osteoclast-like cells with bone resorption activity both in
vitro and in vivo. Arthritis Rheumatol. 66:121–129. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soysa NS and Alles N: NF-kappaB functions
in osteoclasts. Biochem Biophys Res Commun. 378:1–5. 2009.
View Article : Google Scholar
|
|
36
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar
|
|
38
|
Lee ZH and Kim HH: Signal transduction by
receptor activator of nuclear factor kappa B in osteoclasts.
Biochem Biophys Res Commun. 305:211–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wagner EF and Eferl R: Fos/AP-1 proteins
in bone and the immune system. Immunol Rev. 208:126–140. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang ZQ, Ovitt C, Grigoriadis AE,
Möhle-Steinlein U, Rüther U and Wagner EF: Bone and haematopoietic
defects in mice lacking c-fos. Nature. 360:741–745. 1992.
View Article : Google Scholar : PubMed/NCBI
|