Introduction
Cataracts affect millions of patients worldwide, a
significant proportion of whom achieve good visual restoration
through surgical intervention. However, remnant lens epithelial
cells (LECs) may proliferate, transdifferentiate and undergo a
wound healing response driven by continuous pathological factors
caused by surgery, including cascade reactions. The residual LECs
migrate towards the posterior capsule, proliferate abnormally and
secrete extracellular matrix (ECM), causing obscuration of the
central visual axis and a secondary loss of vision, referred to as
posterior capsule opacification (PCO) (1,2). A
thorough mechanistic understanding of PCO is crucial for the
prevention and identification of effective treatment options for
this condition.
A variety of structural and signaling proteins have
been demonstrated to facilitate PCO development: Transforming
growth factor (TGF)-β expression is increased in response to injury
(3) and it has been demonstrated
that TGF-β not only induces epithelial-to-mesenchymal transition
(EMT) in LECs, but also regulates cell migration, which are each
considered key events in the initiation of PCO (4). In addition to the canonical Mothers
against decapentaplegic signaling pathway, which mediates essential
functions of TGF-β, non-canonical signaling pathways also exist and
are involved in cell type- or process-specific events (5). Accumulating evidence indicates the
presence of crosstalk between growth factors and adhesive signaling
pathways. Firstly, TGF-β may regulate integrin signaling through
physical interaction between TGF-β receptors (TGF-βR) and integrins
(6), stable interactions between
the Type II TGF-β receptor (TβRII) and α5β1 integrin have also been
described in rapid fibrillogenesis (7), and the association of integrin αvβ3
with TGF-βR has been suggested to enhance TGF-β-induced invasion of
breast cancer cells and contribute to abnormal wound healing in
lung fibroblasts (8,9). Secondly, TGF-β may upregulate
integrin expression: TGF-β signaling increased α5β1 integrin
expression in keratinocytes during wound healing and promoted
carcinoma cell migration (10).
Finally, TGF-β may indirectly regulate the integrin signaling
pathway by modulating the ECM: TGF-β signaling exerts critical
effects on the expression of genes encoding ECM components
(6). In response to TGF-β
stimulation, fibronectin is produced and assembled into fibers that
are connected with the terminal portion of α-smooth muscle
actin-positive stress fibers through focal adhesion (11). TGF-β1-induced phosphorylation of
focal adhesion kinase (FAK) only occurs when cells adhere to
fibronectin secreted by TGF-β-stimulated cells (12). Following cataract surgery,
increased levels of active TGFβ2 are present in the aqueous humor
(13,14) and induce aberrant expression of
ECM proteins, including fibronectin (15). While it has been hypothesized that
the microenvironment in certain disease states may alter integrin
function and facilitate PCO development (16), the underlying mechanism remains
unknown.
FAK serves a key role in normal cell migration and
is implicated in the metastasis of a wide variety of human cancer
cells, including hepatocellular carcinoma cells (17), GS-Tg microglia (18), glioblastoma cells (19) and breast cancer cells (20). Upon extracellular stimuli, FAK is
activated by phosphorylation and initiates a signaling cascade that
promotes cell migration (21).
Previous studies have demonstrated that FAK is required for
TGF-β-induced EMT in hepatocytes and lung fibroblasts (12,22). The downregulation of FAK abrogates
platelet-derived growth factor-BB-stimulated cell migration and
cell motility toward fibronectin and collagen (23). However, at present, the role of
FAK in TGF-β2-induced human LEC migration in PCO has not been
investigated.
Integrin signaling mediates important functions of
TGF-β, including cell adhesion and migration (24). The aim of the present study was to
investigate the crosstalk between integrins and TGF-β2 signaling,
and the role of FAK in the context of PCO, in order to determine
whether TGF-β2 interacts with integrin/FAK by regulating
fibronectin expression, and whether inhibition of FAK activity
decreases TGF-β2-enhanced cell migration in vitro. The
efficacy of FAK inhibition in improving the symptoms of PCO was
also investigated in a rabbit model. The present results
demonstrate that TGF-β2 promotes the migration of lens epithelial
cells through the TGF-β2/fibronectin/integrin/FAK axis and
inhibition of FAK activity decreases TGF-β2-mediate cell migration;
thus improving the symptoms of PCO in a rabbit model.
Materials and methods
Cell culture and reagents
HLE-B3 cells were obtained from American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultured in
Eagle's Minimum Essential Medium (EMEM; ATCC) supplemented with 20%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 1% penicillin-streptomycin. The cells were cultured at
37°C in a humidified atmosphere containing 5% CO2, and
the culture medium was changed every 2 days. Cells were used
between passages 2 and 8 for all experiments. Recombinant human
TGF-β2 was purchased from PeproTech, Inc. (Rocky Hill, NJ, USA) and
FAK inhibitor-1,2,4,5-tetraaminobenzene tetra hydrochloride which
prevents FAK autophosphorylation at Tyr397, was obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). The α5β1-integrin
neutralizing antibody was purchased from EMD Millipore (Billerica,
MA, USA).
Cell treatment
HLE-B3 cells were treated with different doses (0,
0.1, 0.5, 1, 5 and 10 ng/ml) of TGF-β2 for 48 h at 37°C, or for
various times (0, 24, 48, 72 h) at a concentration of 10 ng/ml.
HLE-B3 cells were otherwise seeded on fibronectin- (Advanced
Biomatrix, Inc., San Diego, CA, USA; cat. no. 0505; 1:10),
collagen- (Advanced Biomatrix, Inc.; cat. no. 5007; 1:30) or
polylysine-coated surfaces (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany; cat. no. P4707, 1:10) for 1 h at 37°C; and were then
treated with 1,2,4,5-tetraaminobenzene tetra-hydrochloride (1
µM, 2 µM) for 12 h at 37°C prior to TGF-β2 (10 ng/ml)
for an additional 48 h.
Western blot analysis
Following treatment, cell culture medium was
removed, the HLE-B3 cells were washed and whole-cell lysates were
harvested by 1X loading buffer (diluted from 2X Laemmli sample
buffer; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Bicinchoninic acid assay was used to quantify the protein
concentration and 20 µg per lane was loaded to 8% SDS-PAGE
gels and then electrotransferred onto polyvinylidene fluoride
membranes. The membranes were blocked with 5% bovine serum albumin
(BSA; Thermo Fisher Scientific, Inc.) for 1 h at room temperature,
and then incubated with primary antibodies overnight at 4°C.
Primary antibodies against fibronectin (Santa Cruz Biotechnology,
Inc.; cat. no. Sc-9068), phosphorylated FAK (Cell Signaling
Technology, Inc., Danvers, MA, USA; cat. no. 3283s), FAK (Cell
Signaling Technology, Inc.; cat. no. 13009) and β-actin
(Sigma-Aldrich; Merck KGaA; cat. no. A2066) were diluted in TBS
with 0.1% Tween-20 (TBST) at a dilution of 1:1,000. Following
washing, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (Sigma-Aldrich; Merck
KGaA; cat. no. AP156P; 1:1,000) at room temperature for 1 h. The
results of the western blot analysis were visualized using enhanced
chemiluminescence substrate solution (Genshare Biological, Shaanxi,
China) and the protein expression levels were measured using
densitometry with ImageJ 1.50i software (National Institutes of
Health, Bethesda, MD, USA).
Flow cytometry analysis
The HLE-B3 cells were collected from the culture
following treatment with TGF-β2 (10 ng/ml) for different time
intervals (0, 24, 48 and 72 h), resuspended at a concentration of
4x106 cells/ml in complete medium and washed three times
with washing buffer (PBS containing 2% BSA and 0.05%
NaN3). Subsequently, the cells were blocked in 100
µl blocking buffer [1X PBS with 2% fetal bovine serum and
1:10,000 IgG (Thermo Fisher Scientific, Inc.; cat. no.
NB410280885)] for 15 min. The α5β1-integrin neutralizing primary
antibody (cat. no. MAB 1969; 1:100) was added and incubated on ice
(4°C) for 1 h. Subsequent to washing twice, the cells were
resuspended with an Alexa Fluor-conjugated goat anti-mouse
secondary antibody (Thermo Fisher Scientific, Inc.; cat. no.
A28175; 1:400) for 30 min on ice. The cells were washed twice with
PBS and fixed at room temperature in 500 µl 4% formaldehyde
for 30 min prior to analysis via flow cytometry on a CytoFLEX
system (Beckman Coulter, Inc., Brea, CA, USA).
Wound healing assay
Cell culture dishes were coated with fibronectin (50
µg/ml) at 4°C overnight. To create a cell-free gap, the
Ibidi Culture-Insert from Ibidi GmbH (Martinsried, Germany) was
used according to the manufacturer's protocol, as the regular
scratch method was hypothesized to disrupt the fibronectin on the
dish surface. HLE-B3 cells were incubated with
arginylglycylaspartic acid (RGD) peptide (50 µg/ml) or the
aforementioned α5β1-integrin neutralizing antibody (1:100) for 1 h.
An HLE-B3 cell suspension (70 µl) at 3x105
cells/ml was seeded in the designated areas and then cultured at
37°C for 24 h to form a confluent layer. Following gentle removal
of the Culture-Insert Well, non-adherent cells were washed away by
PBS and cell-free medium (2 ml) was added. The cells were incubated
at 37°C for an additional 24 h and images were captured at the
indicated times (0, 6, 12 and 24 h). The wound area was analyzed
using ImageJ 1.50i software.
Small interfering RNA (siRNA) knockdown
and cell migration assay
Fibronectin siRNAs were obtained from Shanghai
GenePharma Co., Ltd. (Shanghai, China). Briefly, 1.5x105
HLE-B3 cells were seeded into 6-well plates, and transfected 24 h
later with 1.25 µl fibronectin siRNAs (Sangon Biotech Co.,
Ltd., Shanghjai, China; 20 µm) for 48 h using RNAiMAX
transfection reagents (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The sequence of fibronectin siRNA was
5′-GCA CAA CUU CGA AUU AUG ATT-3′, and that of the non-specific
scrambled siRNA (niR16) of a similar length was 5′-AAU AUU GGC GUU
AAG AUU CUA-3′. Following siRNA trans-fection, HLE-B3 cells were
exposed to TGF-β2 (10 ng/ml) for 48 h prior to seeding onto Costar
Transwell permeable chambers (Corning Incorporated, Corning, NY,
USA) with an 8.0 µm polycarbonate membrane pore size in
24-well plates. Complete EMEM (1 ml) was placed in the basolateral
chamber, and 200 µl cell resuspension in serum-free medium
was placed in the upper chamber. Cells were incubated at 37°C for
an additional 24 h and fixed at room temperature with 4%
paraformaldehyde for 10 min. The cells were subsequently stained
with 0.5% crystal violet solution for 5 min at room temperature and
then washed with PBS 3 times. Light microscopy images were captured
at a magnification of x20. The total number of cells that had
migrated to the lower side of the membrane was quantified.
In vivo animal cataract surgery
model
A total of 16 male Chinese white rabbits (provided
by Animal Experimentation Center Affiliated to the Medical School
of Xi'an Jiaotong University, Xi'an, China; maintained at 25°C with
0.04% CO2 and food and water provided ad
libitum), aged 3 months and weighing 2.0±0.2 kg, were used in
the present study. All animal experiments complied with the ARRIVE
guidelines (25) and the 2013
AVMA Guidelines for the Euthanasia of Animals (26) and were approved by the Animal
Experimentation Center Affiliated to the Medical School of Xi'an
Jiaotong University. Pre-examination was conducted on the rabbits
under a slit lamp to ensure that they were eye disease-free.
Phacoemulsification and surgery were performed on the right eye of
each rabbit by the same senior surgeon who was blinded to the
treatment groups. The animals in the control (n=8) and experimental
groups (n=8) were administered topical steroid drops containing
3.5% lidocaine hydrochloride ophthalmic solution (Akorn, Inc., Lake
Forest, IL, USA) to control postoperative inflammation and daily
subconjunctival injection of abstractum: Epinephrine and
cyclopentolate hydrochloride (Tianjin Jinyao Amino Acid Co., Ltd.,
Tianjin, China; 1:1) to maintain pupil dilation. As there was a
high circulation rate in the aqueous humor and, therefore, the drug
was rapidly diluted by intraocular injection. Daily drug delivery
was performed by subconjunctival injection of 10 µl dimethyl
sulfoxide (DMSO) in the control group and 10 µl
1,2,4,5-tetraaminobenzene tetrahydrochlo-ride (10 µM) in the
treatment group for 60 days. Anterior chamber inflammatory reaction
grading was conducted as previously described (27). PCO development grading was
performed by slit lamp microscopy (clinical scoring was based on
the combination of the area and severity of the opacity (28) and evaluation via EPCO2000
posterior capsule opacification software (http://www.epco2000.de/) (27). Images were captured using
retroillumination. All the PCO images were graded by an experienced
ophthalmologist twice, with an interval of 1 week. The rabbits were
euthanized on the 60th day.
Surgical procedures
All surgical procedures were performed under general
anesthesia by injecting sodium pentobarbital (30 mg/kg
intravenously) from the ear base and topical anesthesia by
administering 3.5% lidocaine hydrochloride ophthalmic solution to
the eye surface. Pupil dilation was achieved by application of 1%
cyclopentolate hydrochloride 30 min prior to surgery, and the
eyelids were retracted with a wire lid speculum. A 3.0 mm blade was
used to make a small corneal incision, and sodium hyaluronate was
injected into the anterior chamber to fill the opening and protect
the corneal endothelium. Continuous curvilinear capsulorhexis was
then performed, and cortical materials and the nuclei were removed
by phacoemulsification. Repeated irrigation and aspiration were
performed to remove all cortical materials, which was followed by
implantation of an intraocular lens (Oculens; Rafi Systems Inc.,
Diamond Bar, CA, USA) in the capsular bag.
Immunofluorescence
The lens capsule was dissected from the
post-operative rabbits and then fixed at room temperature for 2 h
in 4% paraformaldehyde. Lens were then incubated in 20% sucrose
overnight at 4°C and frozen in Optimal Cutting Temperature compound
(Thermo Fisher Scientific, Inc.). Cryosections (10 µm) were
then produced for an immuno-fluorescence assay. For this assay,
sections were blocked at room temperature for 1 h using blocking
solution (2% goat serum, 1% BSA and 0.25% Triton X-100). Primary
antibodies including anti-fibronectin antibody (Sigma-Aldrich;
Merck KGaA; cat. no. F0791) and anti-TGF-β antibody (Abcam,
Cambridge, UK; cat. no. ab113670) were used, and fluorescein
isothiocyanate-conjugated goat anti-mouse antibody (Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA; cat. no.
115096146; 1:500) and Alexa Fluor® 647-conjugated goat
anti-rabbit antibody (Jackson ImmunoResearch. Inc. PA, USA; cat.
no. 111606003, 1:500) for were applied as secondary antibodies for
fibronextin and TGF-β, respectively. Sections were then mounted
with Vectasheild mounting media with DAPI and examined using a
Zeiss confocal microscope (Zeiss AG, Oberkochen, Germany) at a
magnification of x10.
Statistical analysis
The data are presented as the mean ± standard error
of the mean of at least three repeats. A one-way analysis of
variance followed by Least Significant Difference post-hoc-test was
used to compare mean difference among multiple groups. The mean
differences from two groups were analyzed by a paired Student's
t-test. All statistical analyses were conducted using GraphPad
Prism 6.0c software (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
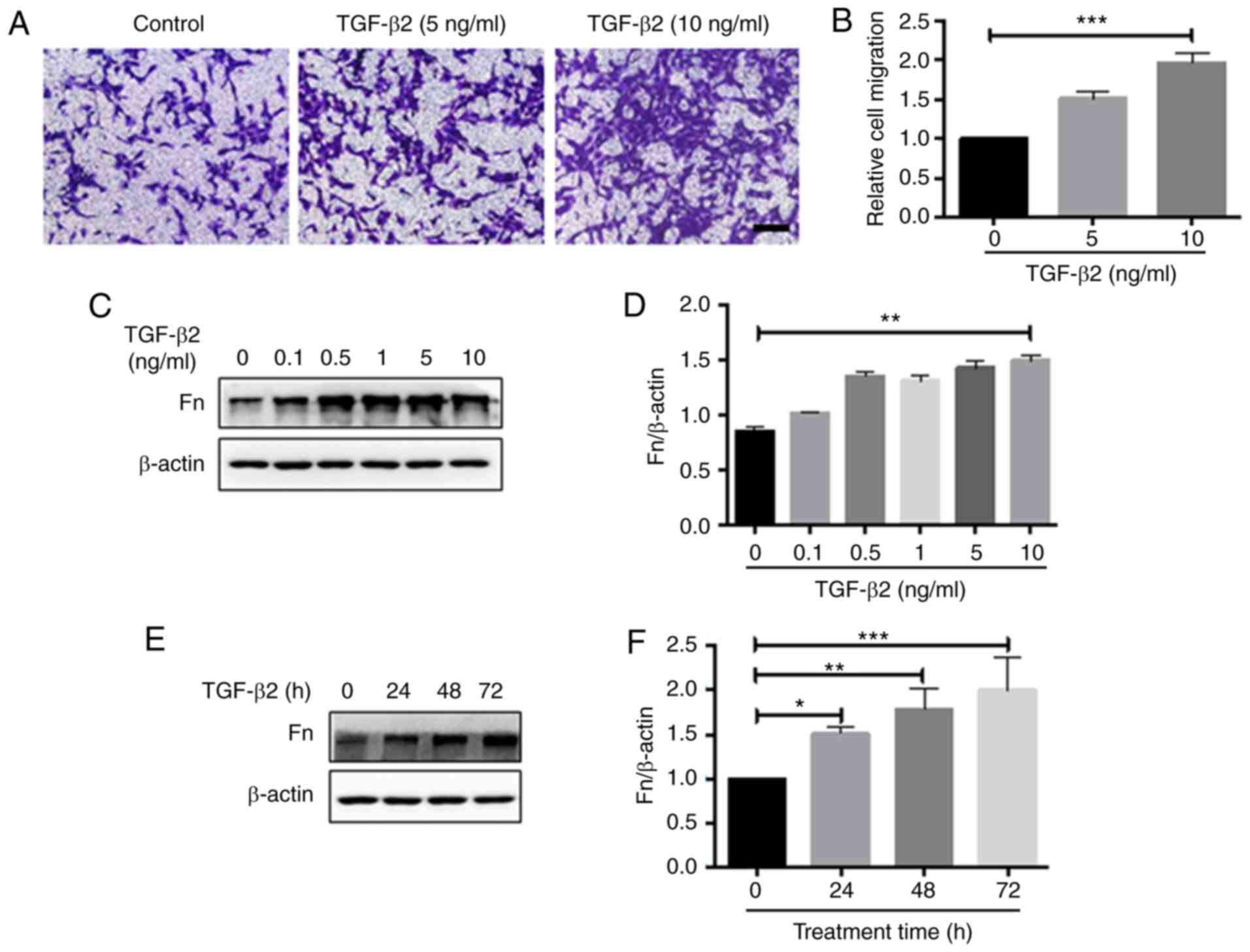
TGF-β2 promotes the migration of LECs and
enhances fibronectin expression
Migration of LECs to the posterior pole of the lens
capsule is an essential step during the development of PCO
(29). TGF-β2 has been
demonstrated to be a pro-migratory factor for a number of cell
types (30). TGF-β2 has also been
used to treat human lens epithelial cells to promote
epithelial-mesenchymal transition and PCO progression (31). In the present study, HLE-B3 cells
were treated with different doses (0, 5 and 10 ng/ml) of TGF-β2 for
48 h and cell migration was analyzed with the Transwell assay.
TGF-β2 was demonstrated to promote HLE-B3 cell migration in a
dose-dependent manner (Fig. 1A and
B). The expression of fibronectin, an ECM component implicated
in cell migration, was also analyzed by western blot analysis.
TGF-β2 induced fibronectin expression dose- and time-dependently
(Fig. 1C-F). Therefore, TGF-β2
increased the migration capacity of HLE-B3 cells and induced the
expression of fibronectin.
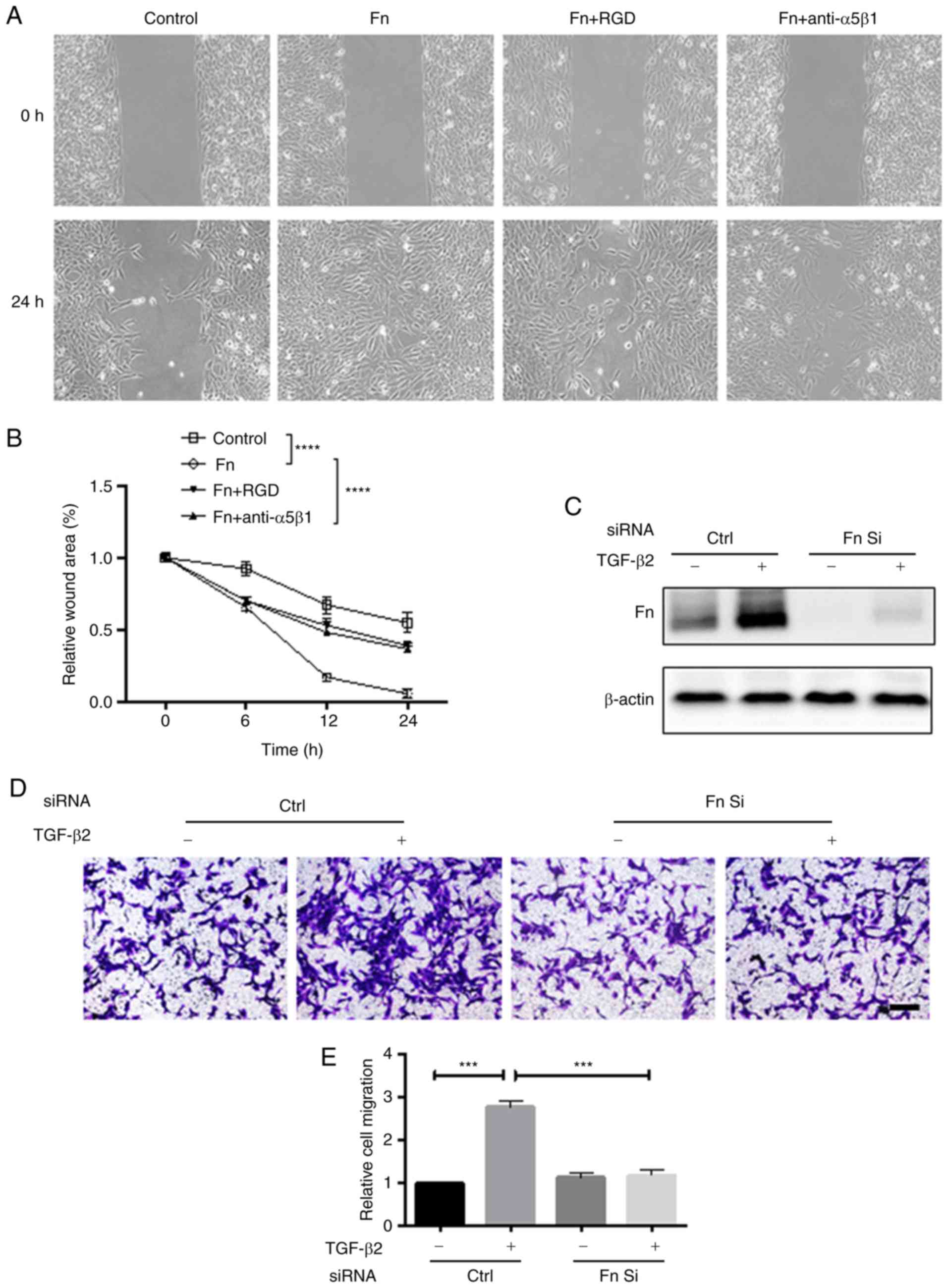
Fibronectin is required for the
pro-migration effect of TGF-β2 on HLE-B3 cells
TGF-β2-enhanced migration of HLE-B3 cells is
associated with upregulated fibronectin expression (32). Therefore, the present study aimed
to investigate whether fibronectin mediated the pro-migration
function of TGF-β2. The role of fibronectin in cell migration was
first examined by wound healing assay. The rate of wound closure
was significantly increased in the presence of fibronectin
(Fig. 2A and B). Additionally,
disruption of the binding of fibronectin to its receptor,
α5β1-integrin, with the RGD peptide or α5β1-integrin neutralizing
antibody, inhibited the migration-promoting effect of fibronectin,
indicating that fibronectin serves an important role in the
migration of HLE-B3 cells. It was then determined whether
TGF-β2-induced cell migration is dependent on the upregulation of
fibronectin. siRNA was used to knock down fibronectin expression in
HLE-B3 cells. As demonstrated in Fig.
2C, siRNA effectively depleted basal and TGF-β2-induced
expression of fibronectin. Notably, fibronectin knockdown almost
completely inhibited TGF-β2-induced cell migration (Fig. 2D and E), confirming that this
effect of TGF-β2 is dependent on the upregulation of
fibronectin.
FAK is the downstream effector of the
TGF-β2/fibronectin axis
FAK is required for the signaling cascade initiated
by the interaction between integrins and ECM proteins, which also
promotes cell migration (21). To
investigate whether FAK was the downstream effector of the
TGF-β2/fibronectin axis, HLE-B3 cells were first treated with
different doses (0, 0.1, 0.5, 1, 5 and 10 ng/ml) of TGF-β2 and FAK
activity was analyzed by western blot analysis. TGF-β2
dose-dependently activated FAK, as reflected by the phosphorylation
of FAK at Y397 after 48 h of treatment (Fig. 3A and B). Notably, the activation
of FAK by TGF-β2 was a delayed event, as the increase in FAK
phosphorylation was not detected before 24 h of treatment (data not
shown). To assess whether FAK activity is required for the
pro-migration effect of TGF-β2, FAK activity was blocked using the
FAK inhibitor, 1,2,4,5-tetraaminobenzene tetrahydrochloride.
Treatment with FAK inhibitor at a non-toxic dose (1-2 µM)
efficiently inhibited FAK Y397 phosphorylation and, notably,
decreased the migration capacity of HLE-B3 cells (Fig. 3C-E). These results indicated that
FAK serves as a downstream target of TGF-β2 to promote cell
migration. As FAK signaling is activated upon the binding of ECM
proteins with their cell surface binding partners, integrins, it
was hypoth-esized that the TGF-β2-induced expression of fibronectin
may participate in the activation of FAK. Indeed, HLE-B3 cells
seeded on the culture surface coated with fibronectin exhibited
markedly increased levels of FAK phosphorylation compared with
cells seeded on collagen- or polylysine-coated surfaces (Fig. 3F and G). Conversely, knockdown of
fibronectin significantly decreased the activation of FAK by TGF-β2
(Fig. 3H-I). Taken together,
these results confirmed the presence of a TGF-β2/fibronectin/FAK
signaling axis in the migration regulatory network of the HLE-B3
cells.
 | Figure 3FAK is the downstream effector of the
TGF-β2/Fn axis. (A) HLE-B3 cells were treated with TGF-β2 at the
indicated concentrations for 48 h, and examined using western blot
analysis. (B) Quantification of the western blot analysis results.
The HLE-B3 cells were then treated with 1,2,4,5-tetraaminoben-zene
tetrahydrochloride for 12 h prior to TGF-β2 (10 ng/ml) for an
additional 48 h, and then examined by (C) western blot analysis and
(D) Transwell assays. Scale bar=100 µm. (E) Quantification
of the Transwell assay results. (F) The HLE-B3 cells were seeded on
culture surfaces coated with Fn (50 µg/ml), Poly-L or Col
(50 µg/ml) for 1 h and then collected for western blot
analysis to investigate the levels of p-FAK. (G) Quantification of
the western blot analysis results. (H) Western blot analysis
indicated that knockdown of Fn by siRNA decreased the activation of
FAK by TGF-β2. (I) Quantification of the western blot analysis
results. A one-way analysis of variance followed by a Least
Significant Difference post-hoc test was used to assess mean
differences from multiple groups. Data from three independent
experiments are presented as means ± standard error of the mean.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. FAK, focal
adhesion kinase; TGF-β2, transforming growth factor-β2; HLE, human
lens epithelial cells; p, phosphorylated; Fn, fibronectin; FAKi,
FAK inhibitor; Col, collagen; Poly-L, polylysine; siRNA, small
interfering RNA. |
Integrin α5β1 mediates FAK activation by
fibronectin in HLE-B3 cells
Integrin α5β1 is a major binding partner and signal
transducer of fibronectin in several types of cells (33). Flow cytometry was used to
investigate the expression of α5β1-integrin in HLE-B3 cells.
Integrin α5β1 is highly expressed on the surface of HLE-B3 cells,
and treatment with TGF-β2 for various time intervals (24-72 h) did
not affect the levels of α5β1 integrin on the cell surface
(Fig. 4A). To determine the
significance of α5β1 integrin in fibronectin-induced
phosphorylation of FAK, HLE-B3 cells were pretreated with an α5β1
integrin neutralizing antibody for 1 h prior to seeding on a
culture surface coated with fibronectin. The western blot analysis
results demonstrated that inactivation of α5β1 integrin inhibited
fibronectin-induced phosphorylation of FAK at Y397 (Fig. 4B and C). Therefore, while TGF-β2
does not affect the surface expression of α5β1 integrins in HLE-B3
cells, these integrins are mediators of fibronectin-dependent
activation of FAK.
Inhibition of FAK activity attenuates PCO
development in vivo
Considering the key role of FAK in TGF-β2-enhanced
cell migration, the present study aimed to investigate whether the
inhibition of FAK activity prevented or delayed the development of
PCO in a rabbit model. Cataract surgery was performed to generate
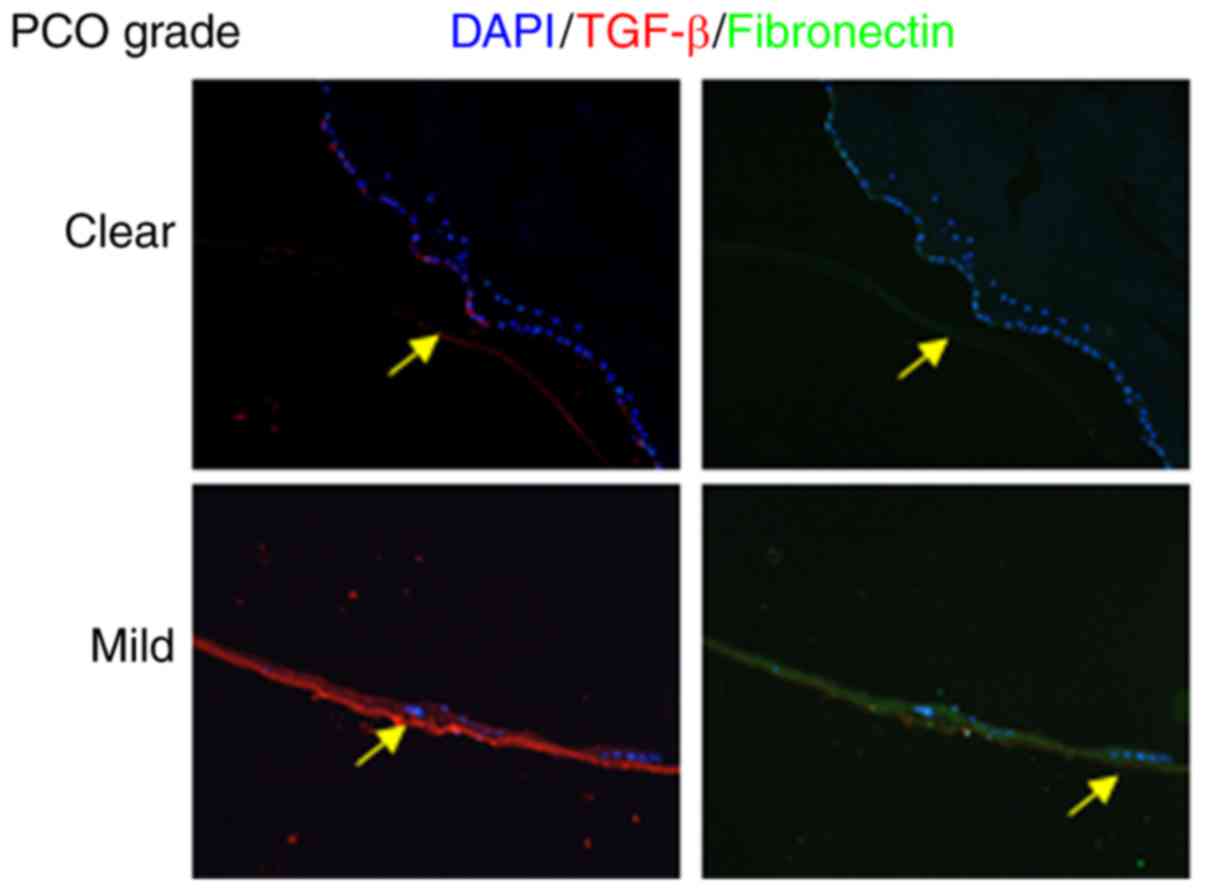
PCO models. To confirm the upregulation of TGF-β and fibronectin in
the rabbit model, immunofluorescence staining was performed, and
clearly demonstrated increased TGF-β and fibronectin levels in the
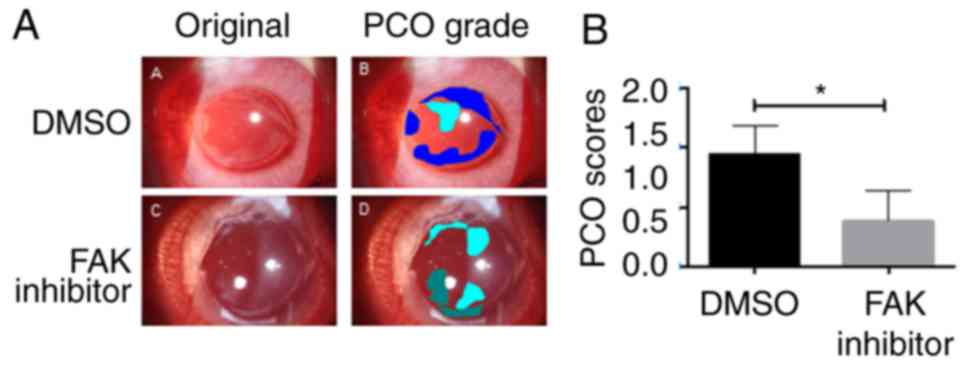
PCO mild group but not in the clear group (Fig. 5). Subsequent to establishing the
PCO model, rabbit eyes were subconjunctivally injected with FAK
inhibitor or DMSO on a daily basis, and the in vivo toxicity
and the effect on PCO development were monitored. Anterior chamber
inflammatory reaction grading results are summarized in Table I. The results of PCO images
grading by experienced ophthalmologist are presented in Table II. Notably, DMSO-treated eyes
developed PCO, as determined by opacification of the posterior
capsule on day 60, while FAK inhibitor-treated eyes exhibited
markedly less severe symptoms (Fig.
6). These results suggested that inhibition of FAK activity may
attenuate PCO development in vivo.
 | Table IAnterior chamber inflammatory
reaction grading following surgery. |
Table I
Anterior chamber inflammatory
reaction grading following surgery.
| Treatments
|
|---|
| Dimethyl sulfoxide,
n
| Focal adhesion
kinase inhibitor, n
|
|---|
| Grades | Day 1 | Day 7 | Day 14 | Day 1 | Day 7 | D ay 14 |
|---|
| None | 0 | 0 | 6 | 0 | 1 | 7 |
| Mild | 1 | 3 | 1 | 0 | 3 | 1 |
| Moderate | 2 | 4 | 1 | 1 | 4 | 0 |
| Severe | 5 | 1 | 0 | 7 | 0 | 0 |
 | Table IIPosterior capsule opacification grade
at day 60 following surgery. |
Table II
Posterior capsule opacification grade
at day 60 following surgery.
| Treatment
|
|---|
| Grade | Dimethyl
sulfoxide | Focal adhesion
kinase inhibitor |
|---|
| Clear | 0 | 0 |
| Minimal | 1 | 3 |
| Mild | 1 | 3 |
| Moderate | 2 | 2 |
| Severe | 4 | 0 |
Discussion
LECs, functionally coupled to one another, regulate
the majority of the homeostatic functions of the lens (34,35). Following cataract surgery residual
aberrant epithelial cells in injured tissues trigger a dysregulated
repair process, characterized by cell migration towards the
posterior capsule and ECM deposition, which results in secondary
visual loss (36). TGF-β2 is
considered the most important cytokine responsible for this ectopic
wound healing process (37). It
was previously demonstrated that TGF-β2 even participates in
non-canonical pathways to amplify and accelerate PCO formation
(38). Studies in humans and
rodents demonstrate that TGF-β levels become increased in the
aqueous humor in response to trauma (39,40). In addition, it was identified that
short intervals of exposure of TGF-β2 may result in long-term
changes to lens epithelial cells and their underlying matrix
(41). Concurrently, CAT-152 (a
specific anti-TGF-β2 human antibody) is capable of suppressing the
actions of TGF-β2. A single application of an antibody similar to
this at the time of surgery may therefore confer long-term
protective effects to prevent sustained TGF-β2 actions in PCO
(41). A previous study also
indicated that anti-fibronectin antibodies may inhibit migration of
LEC in patients (32).
Combinational therapy that inhibits integrins and mitogenic growth
factors appears to be promising for the prevention of PCO (42). In summary, the data from the
present study indicated that, in the pathogenesis of PCO,
fibronectin was an essential mediator in the crosstalk between
integrins and TGF-β signaling by the activation of FAK, and
inhibition of FAK activity markedly decreased TGF-β-induced cell
migration and PCO formation in vitro and in vivo.
Combinatorial signaling involving integrin and TGF
receptors has been well established in fibrotic diseases and tumor
metastasis (43-45); however, whether they cooperate in
the development of PCO and how they communicate remains unclear. In
the present study, it was demonstrated that TGF-β2 activated FAK
phosphorylation and promoted human LEC migration in a
dose-dependent manner. Inhibition of FAK activity by
1,2,4,5-tetraaminobenzene tetrahydrochloride, a selective FAK
inhibitor, abrogated TGF-β2-induced cell migration. This suggested
that FAK signaling may be involved in TGF-β2-mediated disorders.
Notably, FAK activation occurred in a delayed and cell
adhesion-dependent manner: Only when human LECs were treated with
various concentrations of TGF-β2 for 48 h was the phosphorylation
of FAK detected and increased dose-dependently. However,
fibronectin may increase the FAK activity as soon as the cells
adhere. This indicates that fibronectin may serve a mediating role
in the crosstalk between integrins and TGF-β signaling. A previous
study identified that secreted fibronectin assisted ovarian cancer
OvCa cells in establishing an initial metastatic colony (46). Secretion and polymerization of
fibronectin in the ECM is required for promoting cell migration.
This may explain why the activation of FAK was relatively delayed
in the present study. Downregulation of fibronectin attenuated cell
migration in gastric cancer (GC) cells (47). Furthermore, in late-stage human
colorectal cancer, fibronectin depletion significantly inhibited
the migration of shARNT cells (48). In the present study, inhibition of
fibronectin function by the RGD peptide attenuated cell adhesion
and motility. Similarly, knockdown of fibronectin abolished
TGF-β2-induced FAK activation and cell migration. These results are
consistent with our hypothesis that the increased synthesis of ECM
proteins by TGF-β2 stimulation may also be involved in and amplify
TGF-β2-induced dysregulation. Integrin α5β1 is a specific receptor
for fibronectin that is highly expressed on human LECs. A previous
study in human mesangial cells demonstrated that TGF-β regulates
integrins by increasing their expression level or upregulating
their activity (49). In the
present study, α5β1-integrin was not upregulated following TGF-β2
treatment, but blocking the receptor with a neutralizing antibody
significantly inhibited FAK activation by fibronectin. These
results suggested that TGF-β regulated integrin signaling by
increasing the expression of its ligand, rather than the receptor
itself.
Previously, FAK has been identified as a major drug
target, and FAK-targeting inhibitors have entered clinical trials
in a wide range of human cancer types (50). The present study provided in
vitro evidence that the inhibition of FAK activity abrogated
TGF-β2-induced cell migration in a dose-dependent manner. An in
vivo rabbit model also demonstrated that daily subconjunctival
injection of FAK inhibitor decreased PCO formation and limited its
severity. A recent study indicated that deletion of Integrin β-1
triggers stress response and cell death (51). Inhibition of FAK is likely to
exert a similar effect of deletion of Integrin β-1. It may
therefore by hypothesized that, compared with DMSO treated rabbit
groups, LECs in FAK inhibitor (1,2,4,5-tetraaminobenzene tetra
hydrochloride) treated rabbits may undergo increased apoptosis and
decreased migration. More detailed studies are required to address
this. In summary, the data of the present study established the
central role of the TGF-β2/fibronectin/integrin/FAK axis in the
regulation of LEC migration, and provided novel insight into the
potential application of FAK inhibitors in the prevention/treatment
of PCO.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation of China (grant no. 81470614).
Availability of data and materials
All data analyzed during this study are included in
this published article.
Authors' contributions
JLiu, DX, JLi, YS and CP designed the study. JLiu,
NG, CL and RJ conducted the experiments; RJ and BM contributed to
set up EPCO2000 software; JLiu, RJ, BW and CL analyzed the data;
and JLiu and YS wrote the manuscript.
Ethics approval and consent to
participate
All animal experiments complied with the ARRIVE
guidelines and the 2013 AVMA Guidelines for the Euthanasia of
Animals and were approved by the Animal Experimentation Center
Affiliated to the Medical School of Xi'an Jiaotong University. All
authors read and approved the final manuscript.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
HLE-B3
|
human lens epithelial cells
|
|
FN
|
fibronectin
|
|
FAK
|
focal adhesion kinase
|
|
PCO
|
posterior capsule opacification
|
|
TGF-β
|
transforming growth factor-β
|
|
RGD
|
arginylglycylaspartic acid
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
References
|
1
|
Wormstone IM and Eldred JA: Experimental
models for posterior capsule opacification research. Exp Eye Res.
142:2–12. 2016. View Article : Google Scholar
|
|
2
|
Nibourg LM, Gelens E, Kuijer R, Hooymans
JM, van Kooten TG and Koopmans SA: Prevention of posterior capsular
opacification. Exp Eye Res. 136:100–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kane CJ, Hebda PA, Mansbridge JN and
Hanawalt PC: Direct evidence for spatial and temporal regulation of
transforming growth factor beta 1 expression during cutaneous wound
healing. J Cell Physiol. 148:157–173. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan X, Zhu Y, Chen C, Chen X, Qin Y, Qu B,
Luo L, Lin H, Wu M, Chen W and Liu Y: Sprouty2 suppresses
epithelial-mesenchymal transition of human lens epithelial cells
through blockade of Smad2 and ERK1/2 pathways. PLoS One.
11:e01592752016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leask A: Potential therapeutic targets for
cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF,
partners in fibroblast activation. Circ Res. 106:1675–1680. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Munger JS and Sheppard D: Cross talk among
TGF-β signaling pathways, integrins, and the extracellular matrix.
Cold Spring Harb Perspect Biol. 3:a0050172011. View Article : Google Scholar
|
|
7
|
Varadaraj A, Jenkins LM, Singh P, Chanda
A, Snider J, Lee N, Amsalem-Zafran AR, Ehrlich M, Henis YI and
Mythreye K: TGF-β triggers rapid fibrillogenesis via a novel
TβRII-dependent fibronectin-trafficking mechanism. Mol Biol Cell.
28:1195–1207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scaffidi AK, Petrovic N, Moodley YP,
Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ and
Knight DA: Alpha(v)beta(3) Integrin interacts with the transforming
growth factor beta (TGFbeta) type II receptor to potentiate the
proliferative effects of TGFbeta1 in living human lung fibroblasts.
J Biol Chem. 279:37726–37733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galliher AJ and Schiemann WP: Beta 3
integrin and Src facilitate transforming growth factor-beta
mediated induction of epithelial-mesenchymal transition in mammary
epithelial cells. Breast Cancer Res. 8:R422006. View Article : Google Scholar
|
|
10
|
Margadant C and Sonnenberg A:
Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing.
EMBO Rep. 11:97–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leask A: Focal adhesion kinase: A key
mediator of transforming growth factor beta signaling in
fibroblasts. Adv Wound Care (New Rochelle). 2:247–249. 2013.
View Article : Google Scholar
|
|
12
|
Thannickal VJ, Lee DY, White ES, Cui Z,
Larios JM, Chacon R, Horowitz JC, Day RM and Thomas PE:
Myofibroblast differentiation by transforming growth factor-beta1
is dependent on cell adhesion and integrin signaling via focal
adhesion kinase. J Biol Chem. 278:12384–12389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wormstone IM: Posterior capsule
opacification: A cell biological perspective. Exp Eye Res.
74:337–347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wormstone IM, Tamiya S, Anderson I and
Duncan G: TGF-beta2-induced matrix modification and cell
transdifferen-tiation in the human lens capsular bag. Invest
Ophthalmol Vis Sci. 43:2301–2308. 2002.PubMed/NCBI
|
|
15
|
Dawes L, Elliott R, Reddan J, Wormstone Y
and Wormstone I: Oligonucleotide microarray analysis of human lens
epithelial cells: TGFbeta regulated gene expression. Mol Vis.
13:1181–1197. 2007.PubMed/NCBI
|
|
16
|
Walker J and Menko AS: Integrins in lens
development and disease. Exp Eye Res. 88:216–225. 2009. View Article : Google Scholar :
|
|
17
|
Niwa Y, Kanda H, Shikauchi Y, Saiura A,
Matsubara K, Kitagawa T, Yamamoto J, Kubo T and Yoshikawa H:
Methylation silencing of SOCS-3 promotes cell growth and migration
by enhancing JAK/STAT and FAK signalings in human hepato-cellular
carcinoma. Oncogene. 24:6406–6417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi I, Kim B, Byun JW, Baik SH, Huh YH,
Kim JH, Mook-Jung I, Song WK, Shin JH, Seo H, et al: LRRK2 G2019S
mutation attenuates microglial motility by inhibiting focal
adhesion kinase. Nat Commun. 6:82552005. View Article : Google Scholar
|
|
19
|
Hueng DY, Hsieh CH, Cheng YC, Tsai WC and
Chen Y: Cordycepin inhibits migration of human glioblastoma cells
by affecting lysosomal degradation and protein phosphatase
activation. J Nutr Biochem. 41:109–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Payne SL, Fogelgren B, Hess AR, Seftor EA,
Wiley EL, Fong SF, Csiszar K, Hendrix MJ and Kirschmann DA: Lysyl
oxidase regulates breast cancer cell migration and adhesion through
a hydrogen peroxide-mediated mechanism. Cancer Res. 65:11429–11436.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao XK, Cheng Y, Cheng ML, Yu L, Mu M, Li
H, Liu Y, Zhang B, Yao Y, Guo H, et al: Focal adhesion kinase
regulates fibroblast migration via integrin beta-1 and plays a
central role in fibrosis. Sci Rep. 6:192762016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cicchini C, Laudadio I, Citarella F,
Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L and
Tripodi M: TGFbeta-induced EMT requires focal adhesion kinase (FAK)
signaling. Exp Cell Res. 314:143–152. 2008. View Article : Google Scholar
|
|
23
|
Cai GQ, Zheng A, Tang Q, White ES, Chou
CF, Gladson CL, Olman MA and Ding Q: Downregulation of FAK-related
non-kinase mediates the migratory phenotype of human fibrotic lung
fibroblasts. Exp Cell Res. 316:1600–1609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang SE, Xiang B, Zent R, Quaranta V,
Pozzi A and Arteaga CL: Transforming growth factor beta induces
clustering of HER2 and integrins by activating Src-focal adhesion
kinase and receptor association to the cytoskeleton. Cancer Res.
69:475–482. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. J Pharmacol
Pharmacother. 1:94–99. 2010. View Article : Google Scholar
|
|
26
|
Knesl O, Hart BL, Fine AH, Cooper L,
Patterson-Kane E, Houlihan KE and Anthony R: Veterinarians and
humane endings: When is it the right time to euthanize a companion
Animal? Front Vet Sci. 4:452017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma B, Yang L, Jing R, Liu J, Quan Y, Hui
Q, Li J, Qin L and Pei C: Effects of Interleukin-6 on posterior
capsular opacification. Exp Eye Res. 172:94–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Camparini M, Macaluso C, Reggiani L and
Maraini G: Retroillumination versus reflected-light images in the
photographic assessment of posterior capsule opacification. Invest
Ophthalmol Vis Sci. 41:3074–3079. 2000.PubMed/NCBI
|
|
29
|
Raj SM, Vasavada AR, Johar SK and Vasavada
VA and Vasavada VA: Post-operative capsular opacification: A
review. Int J Biomed Sci. 3:237–250. 2007.PubMed/NCBI
|
|
30
|
Bainbridge P: Wound healing and the role
of fibroblasts. J Wound Care. 22:407–408. 410–412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo R, Meng Q, Guo H, Xiao L, Yang X, Cui
Y and Huang Y: TGF-β2 induces epithelial-mesenchymal transition in
cultured human lens epithelial cells through activation of the
PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 13:1105–1110. 2016.
View Article : Google Scholar
|
|
32
|
Tiwari A, Kumar R, Ram J, Sharma M and
Luthra-Guptasarma M: Control of fibrotic changes through the
synergistic effects of anti-fibronectin antibody and an RGDS-tagged
form of the same antibody. Sci Rep. 6:308722016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schaffner F, Ray AM and Dontenwill M:
Integrin α5β1, the fibro-nectin receptor, as a pertinent
therapeutic target in solid tumors. Cancers (Basel). 5:27–47. 2013.
View Article : Google Scholar
|
|
34
|
Rae J and Kuszak J: The electrical
coupling of epithelium and fibers in the frog lens. Exp Eye Res.
36:317–326. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Candia OA: Electrolyte and fluid transport
across corneal, conjunctival and lens epithelia. Exp Eye Res.
78:527–535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bleaken BM, Menko AS and Walker JL: Cells
activated for wound repair have the potential to direct collective
invasion of an epithelium. Mol Biol Cell. 27:451–465. 2016.
View Article : Google Scholar :
|
|
37
|
de Iongh RU, Wederell E, Lovicu F and
McAvoy J: Transforming growth factor-beta-induced
epithelial-mesenchymal transition in the lens: A model for cataract
formation. Cells Tissues Organs. 179:43–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng F, Li J, Yang X, Yuan X and Tang X:
Role of Smad3 signaling in the epithelial-mesenchymal transition of
the lens epithelium following injury. Int J Mol Med. 42:851–860.
2018.PubMed/NCBI
|
|
39
|
Schlötzer-Schrehardt U, Zenkel M, Küchle
M, Sakai LY and Naumann GO: Role of transforming growth
factor-beta1 and its latent form binding protein in
pseudoexfoliation syndrome. Exp Eye Res. 73:765–780. 2001.
View Article : Google Scholar
|
|
40
|
Ohta K, Yamagami S, Taylor AW and
Streilein JW: IL-6 antagonizes TGF-beta and abolishes immune
privilege in eyes with endotoxin-induced uveitis. Invest Ophthalmol
Vis Sci. 41:2591–2599. 2000.PubMed/NCBI
|
|
41
|
Wormstone IM, Anderson IK, Eldred JA,
Dawes LJ and Duncan G: Short-term exposure to transforming growth
factor beta induces long-term fibrotic responses. Exp Eye Res.
83:1238–1245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marcantonio J and Reddan J: TGFbeta 2
influences alpha5-beta1 integrin distribution in human lens cells.
Exp Eye Res. 79:437–442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang C, Zeisberg M, Mosterman B, Sudhakar
A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N and Kalluri R:
Liver fibrosis: Insights into migration of hepatic stellate cells
in response to extracellular matrix and growth factors.
Gastroenterology. 124:147–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Parvani JG, Galliher-Beckley AJ, Schiemann
BJ and Schiemann WP: Targeted inactivation of β1 integrin induces
β3 integrin switching, which drives breast cancer metastasis by
TGF-β. Mol Biol Cell. 24:3449–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Salvo E, Garasa S, Dotor J, Morales X,
Peláez R, Altevogt P and Rouzaut A: Combined targeting of TGF-β1
and integrin β3 impairs lymph node metastasis in a mouse model of
non-small-cell lung cancer. Mol Cancer. 13:1122014. View Article : Google Scholar
|
|
46
|
Kenny HA, Chiang CY, White EA, Schryver
EM, Habis M, Romero IL, Ladanyi A, Penicka CV, George J, Matlin K,
et al: Mesothelial cells promote early ovarian cancer metastasis
through fibronectin secretion. J Clin Invest. 124:4614–4628. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoshino H, Enokida H, Itesako T, Tatarano
S, Kinoshita T, Fuse M, Kojima S, Nakagawa M and Seki N:
Epithelial-mesenchymal transition-related microRNA-200s regulate
molecular targets and pathways in renal cell carcinoma. J Hum
Genet. 58:508–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang CR, Lee CT, Chang KY, Chang WC, Liu
YW, Lee JC and Chen BK: Down-regulation of ARNT promotes cancer
metastasis by activating the fibronectin/integrin β1/FAK axis.
Oncotarget. 6:11530–11546. 2015.PubMed/NCBI
|
|
49
|
Weston BS, Wahab NA and Mason RM: CTGF
mediates TGF-beta-induced fibronectin matrix deposition by
upregulating active alpha5beta1 integrin in human mesangial cells.
J Am Soc Nephrol. 14:601–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Golubovskaya VM: Targeting FAK in human
cancer: From finding to first clinical trials. Front Biosci
(Landmark Ed). 19:687–706. 2014. View
Article : Google Scholar
|
|
51
|
Wang Y, Terrell AM, Riggio BA, Anand D,
Lachke SA and Duncan MK: β1-integrin deletion from the lens
activates cellular stress responses leading to apoptosis and
fibrosis. Invest Ophthalmol Vis Sci. 58:3896–3922. 2017. View Article : Google Scholar : PubMed/NCBI
|