Introduction
Colon cancer is the third most common malignancy and
the fourth leading cause of cancer-related deaths worldwide
(1,2). In China, colon cancer is the 4-5th
most commonly diagnosed type of cancer in men and women. Despite
improvements in colon cancer diagnosis and therapy, many patients
are still diagnosed at late stages, and the 5-year survival rate is
only ~30%. Owing to its aggressive nature and poor response to
chemotherapy, colon cancer remains a challenging disease to treat.
Therefore, the identification and development of more effective
drugs to prevent or treat colon cancer is urgently needed.
Over recent years, there has been increasing
interest in natural compounds derived from plants that show
potential preventative effects against cancer development but have
minor side effects on normal cells or organs (3). Resveratrol
(3,5,4’-trihydroxystilbene) is a natural compound derived from
plants that can be found in large amounts in the skin of grapes and
tomatoes as well as in red wine (4). It can also be extracted from the
traditional Chinese plant Polygonum cuspidatum(5). Resveratrol has exhibited promising
anticancer activity against breast cancer, ovarian cancer,
leukemia, prostate cancer, liver cancer, melanoma, head and neck
squamous cell carcinoma, thyroid cancer, and bladder cancer, via
regulating cancer-related gene expression or cancer-associated
signaling pathways (5-10). The anticarcinogenic action of
resveratrol is associated to its ability to neutralize reactive
oxygen species (ROS) and to modulate cellular processes, such as
apoptosis and cancer cell proliferation and differentiation
(11). Resveratrol can regulate
the levels of ROS. Several papers have demonstrated that
resveratrol increases ROS levels via extracellular signal-regulated
kinase (ERK)1/2 inhibition and mitogen-activated protein kinase
(MAPK) activation to induce human diffuse large B cell lymphoma
cell apoptosis in vivo and in vitro(12). Resveratrol also regulates the
activation of the mitochondrial enzyme system and the caspase
cascade; upregulates the expression of cyclin-dependent kinase
inhibitors, tumor suppressor genes, and death-induced cytokines and
their receptors; and downregulates the expression of survival
proteins associated with the development of chemoresistance,
including survivin, cellular FLICE inhibitory protein (cFLIP),
cellular inhibitor of apoptosis proteins (cIAPs), and
antiapop-totic proteins BCL2 apoptosis regulator (Bcl2) and BCL2
extra-large (Bcl-XL). Resveratrol activates AMP-activated protein
kinase (AMPK) and inhibits the MAPK and phosphoinositide 3-kinase
(PI3K)/AKT serine/threonine kinase (AKT) signaling pathways
(13). It has also been reported
that during the cancer promotion phase, resveratrol reduces the
transcription and activity of cytochrome p450 (14). Several reports have illustrated
that resveratrol inhibits the proliferation of colon cancer cells,
induces colon cancer cell apoptosis and G2 phase arrest
and modulates gene expression through its effects on p21 and BCL2
associated X (Bax) (10,15-17). However, the exact molecular
mechanism requires further investigation.
AKT is a proto-oncogene belonging to the
serine/threonine kinase family that regulates a large number of
downstream mediators and ultimately controls critical cell survival
and metabolic processes (18). In
addition, it promotes cell cycle progression and inhibits
apoptosis. AKT is highly activated in 60-70% of human colon cancers
(19). The AKT signaling pathway
is upregulated in numerous cancer types and is involved in cancer
cell proliferation, survival, and metabolism (20). Two FDA-approved anticancer drugs,
everolimus and temsirolimus, have employed targeted inhibition of
AKT in the clinic and are currently in early clinical trials for
various types of cancer (21,22). Targeting AKT signaling, from the
perspective of finding novel molecular targets for cancer therapy,
has resulted in the discovery of candidates and their therapeutic
inhibitors in crucial pathways. MK-2206, an AKT kinase inhibitor,
has shown promising preclinical anticancer activity (23,24) and entered phase II clinical trials
for metastatic breast cancer and colorectal cancer in 2017. The
crucial roles of AKT kinase have rendered it an attractive target
for developing therapeutic cancer drugs (25). It has been reported that the AKT1
interaction with N-ribosyldihydronicotinamide-quinone reductase
(NQO2) is a target of resveratrol (26).
Signal transducer and activator of transcription
(STAT)3 is an important transcription factor that translocates to
the nucleus to regulate the expression of essential pro-invasive
factors, such as matrix metallopeptidases, heat shock protein
(HSP)70 and HSP90, and it is also downstream of various tyrosine
kinase receptor signaling pathways that are involved in
angiogenesis (27). Several
reports have focused on the role of AKT phosphorylation and STAT3
translocation. Resveratrol can inhibit cell proliferation and
promote cell apoptosis via the STAT3 signaling pathway (28,29).
The present study investigated the anticancer
activity of resveratrol in colon cancer cells. The results
demonstrated that resveratrol inhibited cell growth of colorectal
cancer by inhibiting AKT and its downstream signaling targets, and
that AKT served as an upstream regulator of STAT3.
Materials and methods
Reagents
Resveratrol was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Antibodies against phosphorylated (p-)
STAT3 (Tyr705; cat. no. 9145), STAT3 (cat. no. 9139), p53 (cat. no.
48818), Bcl2 (cat. no. 15071), Bax (cat. no. 5023), AKT1 (cat. no.
75692), AKT2 (cat. no. 2964), cyclin D1 (cat. no. 2978) and cyclin
E2 (cat. no. 4132) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). An AKT1/2 (cat. no. sc-1619) antibody was
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Active AKT1/2 recombinant protein was purchased from SignalChem
(Richmond, BC, USA). RPMI-1640, fetal bovine serum (FBS) and Basal
Medium Eagle (BME) were obtained from Biological Industries
(Kibbutz Beit-Haemek, Israel). F-12K medium was purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture
The human colon cancer cell lines DLD1 and HCT15
were purchased from the American Type Culture Collection (Manassas,
VA, USA). The human colonic epithelial cell (HCEC) line was kindly
provided by Dr Jerry W Shay, University of Texas Southwestern
Medical Center, Dallas, Texas (30). DLD1 and HCT15 cells were cultured
in RPMI-1640 medium/10% FBS. HCECs were cultured in F-12K
medium/10% FBS. All cells were cytogenetically tested and
authenticated prior to freezing and were cultured with antibiotics
at 37°C in a 5% CO2 incubator for a maximum of 10
passages.
MTS assay
Cells at a density of 1×103 or
1×105 per well were seeded in 96-well plates in a final
volume of 100 μl per well for analysis of proliferation or
cytotoxicity, respectively. Cells were cultured overnight and were
then treated with different concentrations of resveratrol and
incubated for various times, as indicated. Cell Titer96 Aqueous MTS
reagent (20 μl; Promega Corporation, Madison, WI, USA) was
added to each well and the cells were incubated for an additional 1
h at 37°C. The absorbance was then measured at 492 nm with a
spectrophotometer (Multiskan; Thermo Fisher Scientific, Inc.).
Anchorage-independent cell growth
assay
DLD1 or HCT15 cells (8×103 per well) were
suspended in 1 ml of BME/10% FBS, and 0.33% agar with various
concentrations of resveratrol and plated on a layer of solidified
BME, 10% FBS, and 0.5% agar with the same concentration of
resveratrol as the suspension. The cultures were maintained at 37°C
in an incubator with 5% CO2 for 1-2 weeks. The colonies
were photographed and counted with Image-Pro Plus software (v.6.2;
Media Cybernetics, Rockville, MD, USA).
Flow cytometry for analysis of apoptosis
and cell cycle progression
DLD1 and HCT15 cells were seeded in 6-well plates
and treated with different concentrations of resveratrol (10, 20,
30 or 40 μM) for 72 h. The cells were harvested and stained
with Annexin V and propidium iodide prior to flow cytometry
analysis, data were analyzed by CellQuest Pro 6.0 (both from BD
Biosciences, San Jose, CA, USA). For cell cycle detection, cells
were fixed with ice-cold 70% ethanol overnight at −20°C. The cells
were stained with propidium iodide, the cell cycle phase was
determined by flow cytometry and data were analyzed by ModFit LTÔ
4.0.5 (Verity Software House, Inc., Topsham, ME, USA).
Western blot analysis
DLD1 and HCT15 cells were treated with various
concentrations of resveratrol for 72 h and were then lysed with
RIPA buffer (50 mM Tris base, 1% NP-40, 0.25% sodium deoxycholate,
150 mM NaCl, 1 mM EDTA and 0.1% SDS; dissolved in 400 ml water and
adjusted to pH 7.4) supplemented with 1 mM PMSF. Protein
concentration was determined with a bicinchoninic acid protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein
was loaded (50 μg/lane) and separated by 10% SDS-PAGE and
then transferred onto polyvinylidene difluoride (PVDF) membranes
(EMD Millipore Corp., Burlington, MA, USA). The membranes were
blocked with 5% non-fat milk for 1 h at room temperature. The blots
were then probed with the appropriate primary antibodies (1:1,000)
overnight at 4°C and incubated with a horseradish peroxidase
(HRP)-conjugated secondary antibody (1:3,000; cat. no. SC-2005;
Santa Cruz Biotechnology, Inc.) at room temperature for 1 h.
Protein bands were visualized with a chemiluminescence reagent (GE
Healthcare Biosciences, Pittsburgh, PA, USA).
Computational docking model
To further confirm that resveratrol can bind AKT1
and AKT2, a in silico docking assay was performed using the
Schrödinger Suite 2017 software program (Schrödinger LLC,
Cambridge, MA, USA). AKT1 (PDB ID, 3OCB) and AKT2 (PDB ID, 3D0E)
crystal structures were first obtained from the Protein Data Bank
and were then prepared under the standard procedures of the Protein
Preparation Wizard (Schrödinger Suite 2017). Hydrogen atoms were
added consistent with a pH of 7, and all water molecules were
removed. The ATP-binding site-based receptor grid of AKT1/2 was
generated for docking. Resveratrol was prepared for docking with
the default parameters using the LigPrep program. Then, the docking
of resveratrol with AKT1 and AKT2 was accomplished with the default
parameters under the extra precision mode of the program Glide.
Finally, the best-docked representative structures were
obtained.
In vitro pull-down assay
Resveratrol-Sepharose 4B beads (Amersham Pharmacia
Biotech; GE Healthcare, Chicago, IL, USA) were prepared following
the manufacturer’s instructions. DLD1 and HCT15 cell lysates (500
μg) were incubated with Resveratrol-Sepharose 4B beads with
rocking overnight at 4°C. Following incubation, the beads were
washed 3 times with buffer (50 mM Tris-HCl pH 7.5, 5 mM EDTA, 150
mM NaCl, 1 mM dithiothreitol, 0.01% NP-40 and 0.2 mM PMSF). Bound
proteins were analyzed by western blotting.
Preparation of AKT1/2 knockdown
cells
The viral vectors and the packaging vectors (pMD2G,
psPAX2, Mock, shAKT1 and shAKT2) were obtained from Sigma-Aldrich
(Merck KGaA). Several shRNA sequences targeting AKT1 and AKT2 were
tested. Then, all plasmids were transfected into 293T cells, and
viral supernatant fractions were collected after 48 h. DLD1 and
HCT15 cells were infected with mock or shAKT1 and shAKT2 virus
particles for 24 h. Cells were selected with puromycin to obtain
both AKT1- and AKT2-silenced cell lines (shAKT1/2). The appropriate
experiments were performed with these cells until the control cells
(without infection) completely died (usually 2-3 days) in the
puromycin medium.
Statistical analysis
All quantitative data are presented as the mean
values ± standard deviation. Each experiment was repeated at least
three times. Data were analyzed with SPSS 19.0 software (IBM
Corporation, Armonk, NY, USA). Statistically significant
differences were determined using one-way analysis of variance, and
multiple comparisons between groups were conducted using the
Dunnett’s test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Resveratrol suppresses the cell
proliferation and colony growth of colon cancers
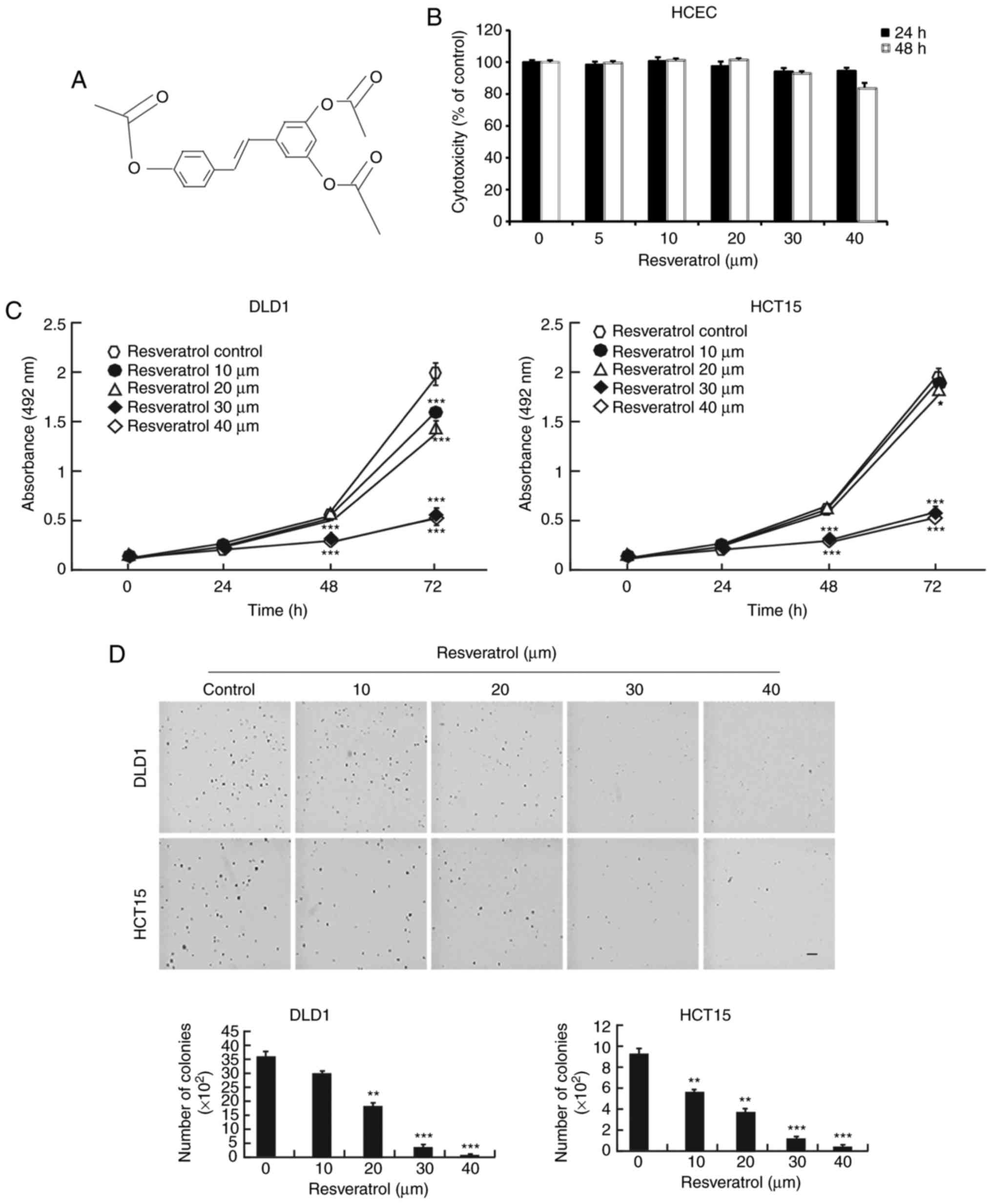
The chemical structure of resveratrol is shown in
Fig. 1A. Resveratrol is a natural
compound and has few side effects, giving it an important
advantage. First, normal HCECs were treated with resveratrol. The
results demonstrated that resveratrol had no toxicity in the normal
HCECs until the 40 μM concentration (Fig. 1B). However, resveratrol
significantly decreased the growth of the DLD1 and HCT15 colon
cancer cells when they were exposed to different concentrations
(Fig. 1C). Furthermore, colony
growth was also inhibited by treatment with resveratrol in a
dose-dependent manner (Fig. 1D).
These results demonstrated that resveratrol might be a potential
treatment for colon cancer.
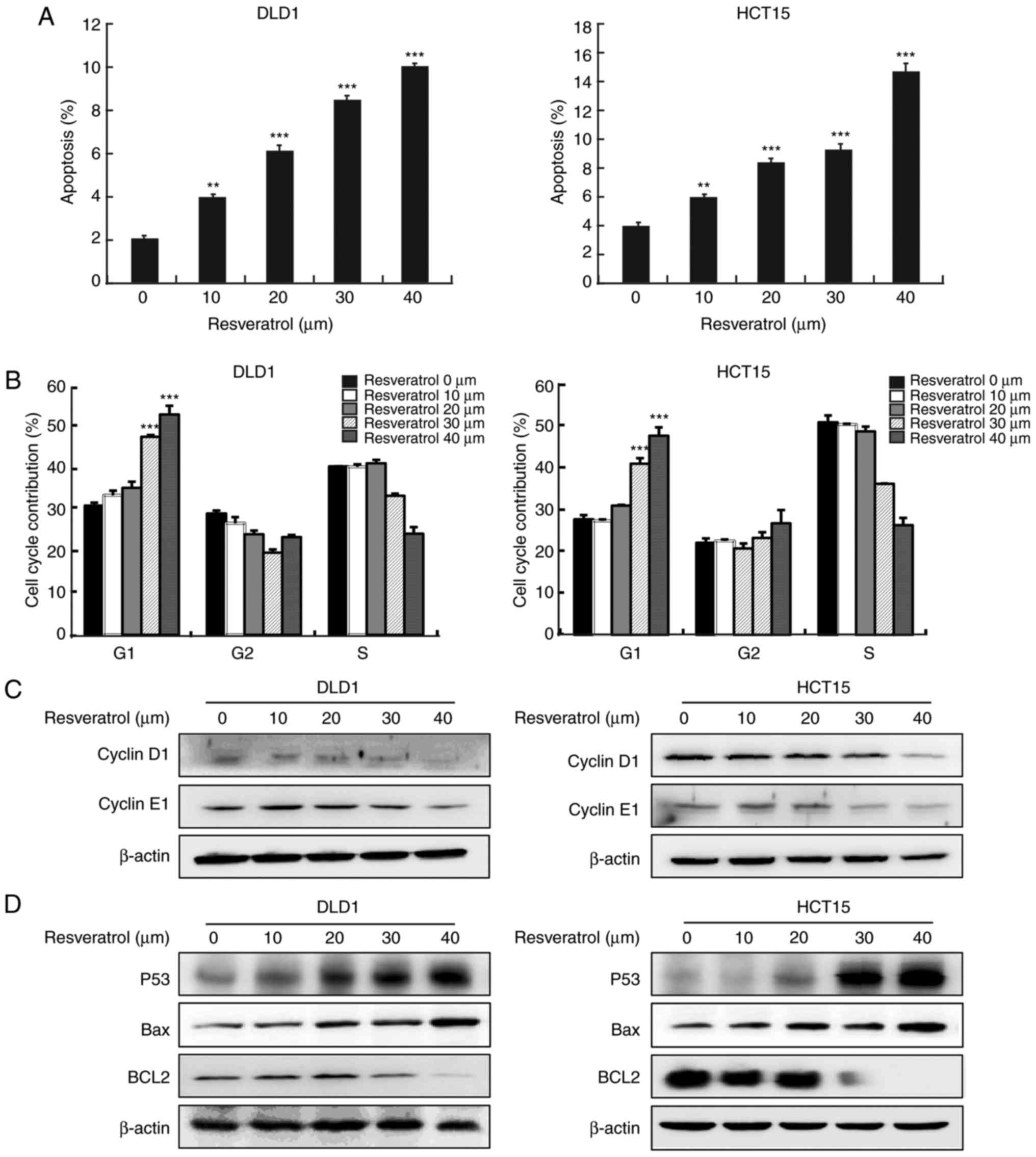
Resveratrol induces cell apoptosis and
cell cycle arrest at the G1 phase
Next, further analyses were conducted to determine
whether resveratrol could lead to the inhibition of cancer cell
growth by regulating cell cycle progression and apoptosis. The
results of Annexin V staining revealed that resveratrol treatment
resulted in a significant increase in the apoptosis rate in both
cell lines, DLD1 and HCT15 (Fig.
2A). Cell cycle analysis revealed that resveratrol
significantly induced cell cycle arrest at the G1 phase
(Fig. 2B). The effects of
resvera-trol were further verified by examining the expression
levels of cell apoptosis and cell cycle biomarkers, using western
blot analysis. The results revealed that resveratrol treatment
decreased cyclin D1 and cyclin E1 (Fig. 2C), increased p53, and decreased
Bcl2 protein expression levels in a dose-dependent manner (Fig. 2D).
Resveratrol binds with AKT1 and AKT2
kinases
To elucidate the underlying mechanism of
resveratrol’s effects, potential targets of resveratrol were
screened by Schrödinger software (release 2017) (31). The results predicted that
resveratrol may bind with AKT1 and AKT2. To elucidate the potential
binding site, an in silico docking study was conducted using
the induced fit docking module in the Schrödinger software. The
results indicated that resveratrol interacts with the ATP-binding
pockets of AKT1 (Fig. 3A-a and b)
and AKT2 (Fig. 3A-c and d). For
the binding of resveratrol with AKT1, the carbonyl oxygen of
resveratrol interacts with the residues Glu228, Ala230 and Glu234
of AKT1 individually through hydrogen bonds (Fig. 3A-b). For the binding of
resve-ratrol with AKT2, the carbonyl oxygen of resveratrol
interacts with the residues Glu230, Ala232, Glu279 and Asp293 of
AKT2 individually through hydrogen bonds (Fig. 3A-d). Thus, AKT1/2 activity might
be dependent upon the presence of these residues. These
computational results also indicated that resveratrol may elicit
ATP-competitive inhibitory effects on the AKT1/2 kinase activity.
To confirm the docking model, an in vitropull-down assay was
performed. The results demonstrated that Sepharose beads conjugated
to resveratrol bound with AKT1 and AKT2 in both the DLD1 and the
HCT15 cell lysates, but the Sepharose beads alone did not bind with
AKT1 and AKT2 (Fig. 3B).
Additionally, the expression of p-STAT3 (Tyr705), which is a
downstream target, was suppressed by treatment with resveratrol in
a dose-dependent manner (Fig.
3C). The total protein levels of STAT3 were not affected by
treatment with resvera-trol (Fig.
3C). These results illustrate that the AKT signaling pathway is
involved in the inhibitory effect of resveratrol in colon cancer
cells.
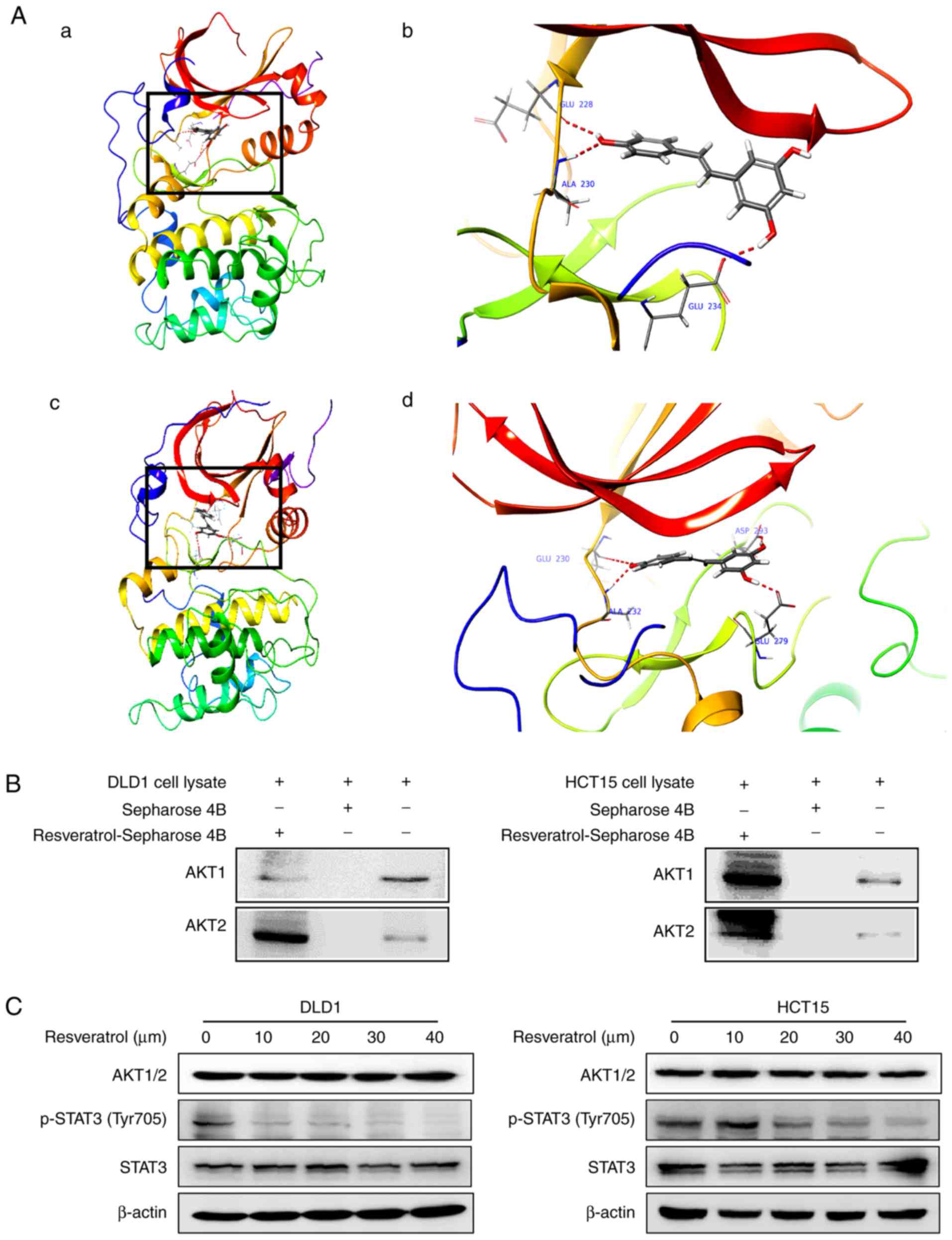 | Figure 3Resveratrol binds to AKT1 and AKT2.
(A) Computational docking model of the binding between resveratrol
and AKT1/2. (A-a) The binding sites of AKT1 are Ala230, Glu228 and
Glu234 and (b) an enlarged view of the binding. (A-c) The binding
sites of AKT2 are Ala232, Glu279, Glu230 and Asp293 and (d) an
enlarged view of the binding. (B) Ex vivo pull-down assay
between resveratrol and AKT1 or AKT2 with DLD1 and HCT15 cell
lysates. (C) Representative images from western blot analysis of
the expression of p-STAT3, total STAT3 and AKT1/2, following
treatment with vehicle or resveratrol (10, 20, 30 or 40 μM).
AKT, AKT serine/threonine kinase; STAT3, signal transducer and
activator of transcription 3; p-, phosphorylated. |
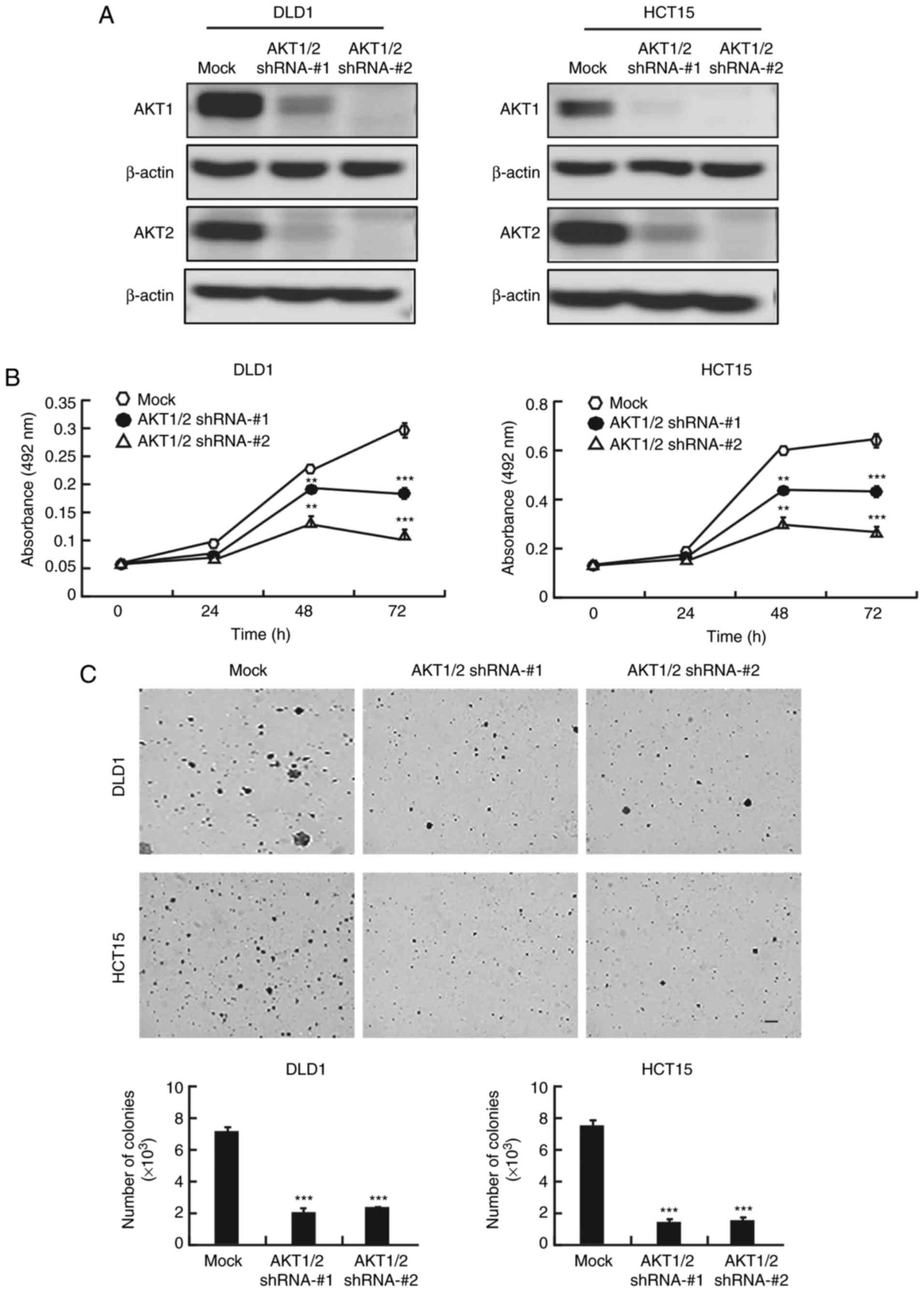
AKT1/2 knockdown inhibits colon cancer
cell proliferation and colony formation
Because of the crucial role of AKT in colon cancer,
the present study examined whether knocking down AKT expression
would produce an antitumor effect in colon cancer cells. After AKT1
and AKT2 were knocked down with short hairpin RNA (shRNA) in DLD1
and HCT15 colon cancer cells, the expression of AKT1 and AKT2 was
markedly decreased (Fig. 4A).
AKT1/2 knockdown markedly decreased the growth of both cell lines
at 48 and 72 h (Fig. 4B). In
addition,
AKT1/2 knockdown exhibited a significant inhibitory
effect on anchorage-independent colony growth by decreasing the
number and size of colonies compared to the number and size of the
colonies in the mock group (Fig.
4C).
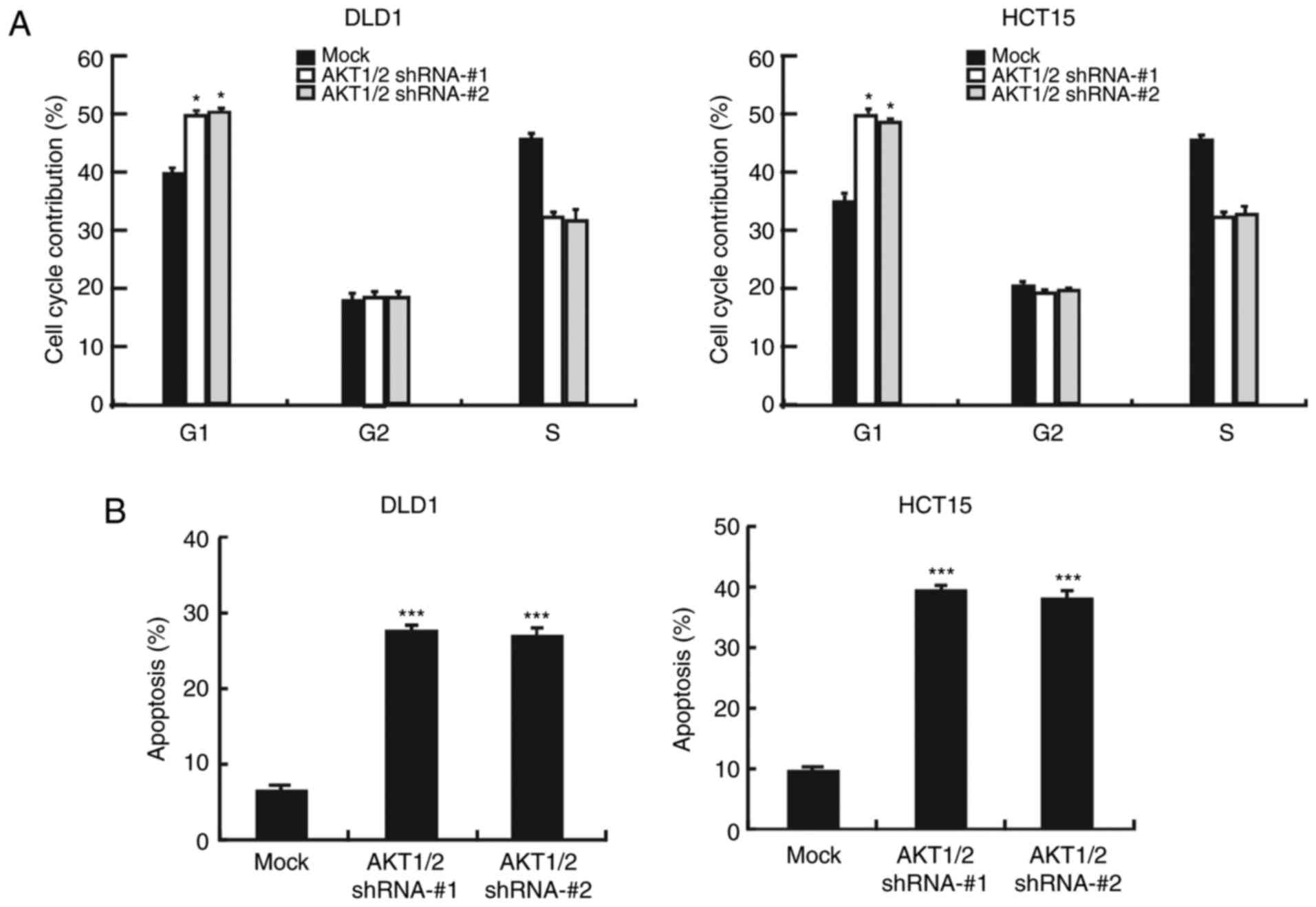
Next, it was examined whether silencing AKT1/2 could
affect cancer cell apoptosis or cell cycle progression. Upon AKT1/2
knockdown, the number of cells in the G1 phase of the
cell cycle increased, indicating that the cells suffered cell cycle
arrest at the G1 phase (Fig. 5A). Furthermore, flow cytometry
analysis revealed that the number of apoptotic cells increased
following AKT1/2 silencing (Fig.
5B). The expression levels of cyclin D1 and cyclin E2, markers
of the G1 phase, were markedly decreased following
AKT1/2 silencing (Fig. 5C). In
addition, Bax protein levels were increased, but Bcl2 protein
levels were decreased in AKT1/2 knockdown cells, in both the DLD1
and HCT15 cell lines (Fig. 5D).
Finally, STAT3 phosphorylation was also decreased in the AKT1/2
knockdown cells (Fig. 5E). These
findings suggest that the AKT/STAT3 signaling pathway has an
important role in colon cancer cells.
Discussion
Colorectal cancer (CRC) is the most common
gastrointestinal tract cancer worldwide. Approximately 50% of those
diagnosed will succumb to colorectal cancer, making it the second
leading cause of cancer-related deaths in both sexes. Despite
decades of research and some promising discoveries, the mainstay of
colorectal cancer treatment remains based on cytotoxic chemotherapy
agents, such as irinotecan or oxaliplatin combined with
fluoropyrimidine and leucovorin (FOLFIRI or FOLFOX6 regimens). Both
the FOLFIRI and FOLFOX6 regimens have shown modest outcomes when
used as first-line therapies. For colon cancer therapy,
5-fluo-rouracil (5-FU) and leucovorin have been the only options.
The addition of irinotecan or oxaliplatin increases overall
survival (OS) to 18 months. The present study demonstrated that
resveratrol effectively inhibited the proliferation and colony
formation of human colon cancer cells DLD1 and HCT15, and also
induced cell apoptosis and G1 phase cell cycle arrest.
These findings suggested that resveratrol might be a promising
cancer prevention agent or therapeutic agent. As a natural
compound, resveratrol may have fewer side effects and lower
toxicity to normal colon epithelial cells. Indeed, the present
results demonstrated that resveratrol treatment had no effect in
the viability of the normal HCEC cell line.
Multiple critical protein-encoding genes and
pathways are believed to be responsible for tumorigenesis.
Colorectal tumors contain a median of 76 mutations, and 15 of these
affect candidate cancer genes. Increased understanding of the
genetic and genomic changes in colorectal cancer has helped to
direct therapies and predict responses. Genetic and epigenetic
errors in signal transduction pathways result in malignant
transformations and have thus emerged as key candidates for
targeted molecular therapies. Over the past 10 years, the number of
targeted agents used in various malignancies has increased
dramatically. Currently, there are seven FDA-approved targeted
agents for colorectal cancer, with many more in development and in
clinical trials. The addition of targeted therapies over the past
10 years has improved OS in colorectal cancer by between 20 and 24
months. Resveratrol is a naturally occurring phytochemical that is
produced by plants. Resveratrol has been demonstrated to have
diverse biological properties, including anti-inflammatory,
antioxidant, antiviral, neuroprotective, antifungal and anticancer
properties (32,33). Resveratrol has been reported to
inhibit cancer cell growth via the AKT signaling pathway (34). The PI3K/AKT pathway is an
important endogenous protective mechanism that can prevent cell
death and cell damage and is also associated with cancer
progression and involved in drug resistance (35). AKT activation may lead to cell
survival and can also inhibit apoptosis via the phosphorylation of
BCL2 associated agonist of cell death (Bad) and caspase-9. STAT3 is
an important transcription factor, and resveratrol can inhibit
colon cancer cell proliferation via the STAT3 signaling pathway
(36). Therefore, inhibition of
this signaling pathway may be significant for cancer therapy. The
computational docking results in the present study revealed that
resveratrol interacted with the ATP-binding pockets of AKT1 and
AKT2. In vitropull-down assays confirmed that Sepharose
beads conjugated to resveratrol bound AKT1 and AKT2 in colon cancer
cell lysates. In addition, silencing AKT1/2 inhibited cell growth,
increased apoptosis and induced G1 phase arrest. Several
phase I clinical trials have investigated the potential of
resveratrol for the treatment of patients with colon cancer
(https://clinicaltrials.gov), performed
by Nguyen et al (37),
Patel et al (38) and
Howells et al (39). The
small sample size and the possible confounding effect of
medications limited the conclusions reached; no definitive
conclusions were obtained from any single trial. However, a set of
well-designed and performed trials may provide more information,
and this is a long lasting process.
In summary, the present study revealed that
resveratrol inhibited colon cancer cell proliferation and colony
formation, and induced cell apoptosis and G1 phase
arrest. The mechanism of the anticancer effects of resveratrol was
demonstrated to occur, at least partially, via inhibiting the
AKT/STAT3 signaling pathway (Fig.
6). Taken together, the present results suggest that
resveratrol may be a promising chemopreventive or therapeutic drug
for colon cancer. Although resveratrol has a clear inhibitory
effect on colon cancer cell proliferation and growth in
vitro and ex vivo, these effects have not been confirmed
yet in animal models and humans. Further research and clinical
trials are warranted to fully elucidate the effects of resveratrol
on human cancer.
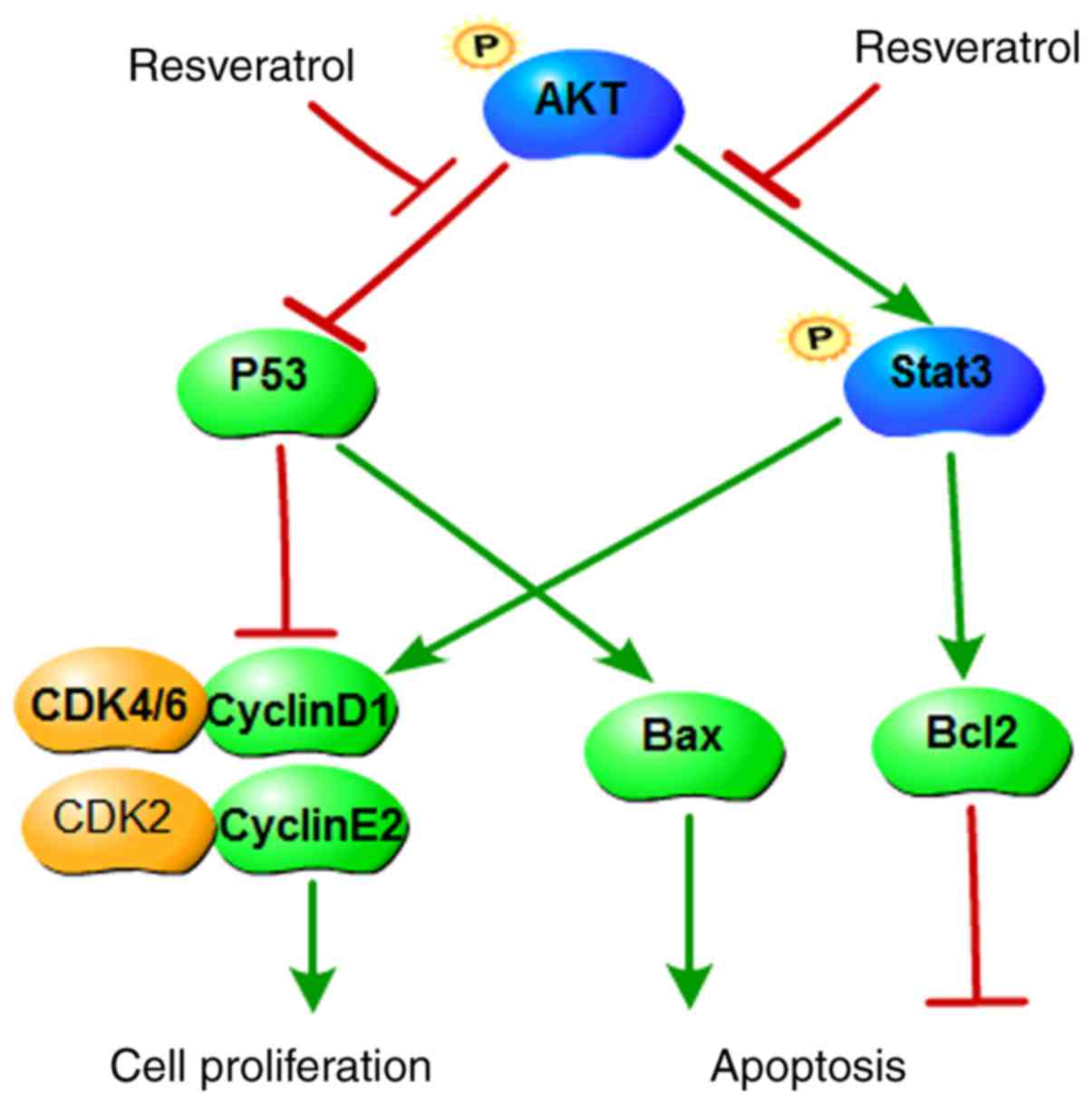 | Figure 6A proposed scheme illustrating the
roles of AKT in colon cancer and its regulation by resveratrol. AKT
upregulates Bcl2 and cyclin D1, promotes STAT3 phosphorylation, and
downregulates p53 and Bax. Subsequently, AKT promotes cell cycle
progression, prevents apoptosis and increases cell proliferation.
Resveratrol inhibits the function of AKT and its downstream
targets, therefore inducing cell cycle arrest and apoptosis, as
well as inhibiting cell proliferation. AKT, AKT serine/threonine
kinase; Bcl2, BCL2 apoptosis regulator; STAT3, signal transducer
and activator of transcription 3; p53, tumor protein p53; Bax, BCL2
associated X; CDK, cyclin-dependent kinase. |
Acknowledgments
Not applicable.
Funding
This study was supported by the Medical Science
Research projects of Henan Province (grant no. 201702248), the
Science and Technology Research Projects of Henan Province (grant
no. 182102310376) and the Science and Technology Research Projects
of Henan Province (grant no. 182102310125).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
DL, SL and ZG designed the study. GW and GJ
performed the kinase and pull down assays. KY, ZZ, LB and YG
analyzed the data. NL, WD, BC, YL and XC performed all other
experiments. DL and GJ wrote the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
3
|
Juan ME, Vinardell MP and Planas JM: The
daily oral administration of high doses of trans-resveratrol to
rats for 28 days is not harmful. J Nutr. 132:257–260. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol:
Mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aluyen JK, Ton QN, Tran T, Yang AE,
Gottlieb HB and Bellanger RA: Resveratrol: Potential as anticancer
agent. J Dietary. (Suppl 9): S45–S56. 2012. View Article : Google Scholar
|
|
6
|
Bai Y, Yang H, Zhang G, Hu L, Lei Y, Qin
Y, Yang Y, Wang Q, Li R and Mao Q: Inhibitory effects of
resveratrol on the adhesion, migration and invasion of human
bladder cancer cells. Mol Med Rep. 15:885–889. 2017. View Article : Google Scholar
|
|
7
|
Rossi EL, Khatib SA, Doerstling SS, Bowers
LW, Pruski M, Ford NA, Glickman RD, Niu M, Yang P, Cui Z, et al:
Resveratrol inhibits obesity-associated adipose tissue dysfunction
and tumor growth in a mouse model of postmenopausal claudin-low
breast cancer. Mol Carcinog. 57:393–407. 2018. View Article : Google Scholar :
|
|
8
|
Jiang Q, Yang M, Qu Z, Zhou J and Zhang Q:
Resveratrol enhances anticancer effects of paclitaxel in HepG2
human liver cancer cells. BMC Complement Altern Med. 17:4772017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
10
|
Zhou HB, Yan Y, Sun YN and Zhu JR:
Resveratrol induces apoptosis in human esophageal carcinoma cells.
World J Gastroenterol. 9:408–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng X, Jia B, Song X, Kong QY, Wu ML,
Qiu ZW, Li H and Liu J: Preventive potential of resveratrol in
carcinogen-induced rat thyroid tumorigenesis. Nutrients.
10:E2792018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Z, Chen K, Cheng L, Yan B, Qian W,
Cao J, Li J, Wu E, Ma Q and Yang W: Resveratrol and cancer
treatment: Updates. Ann NY Acad Sci. 1403:59–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng
ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, et al: The PTEN/PI3K/Akt and
Wnt/β-catenin signaling pathways are involved in the inhibitory
effect of resveratrol on human colon cancer cell proliferation. Int
J Oncol. 45:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saiko P, Szakmary A, Jaeger W and Szekeres
T: Resveratrol and its analogs: Defense against cancer, coronary
disease and neuro-degenerative maladies or just a fad? Mutat Res.
658:68–94. 2008. View Article : Google Scholar
|
|
15
|
Mahyar-Roemer M, Katsen A, Mestres P and
Roemer K: Resveratrol induces colon tumor cell apoptosis
independently of p53 and precede by epithelial differentiation,
mitochondrial proliferation and membrane potential collapse. Int J
Cancer. 94:615–622. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malhotra A, Bath S and Elbarbry F: An
organ system approach to explore the antioxidative,
anti-inflammatory, and cytoprotective actions of resveratrol. Oxid
Med Cell Longev. 2015:8039712015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tessitore L, Davit A, Sarotto I and
Caderni G: Resveratrol depresses the growth of colorectal aberrant
crypt foci by affecting bax and p21(CIP) expression.
Carcinogenesis. 21:1619–1622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheaib B, Auguste A and Leary A: The
PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities
and challenges. Chin J Cancer. 34:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colakoglu T, Yildirim S, Kayaselcuk F,
Nursal TZ, Ezer A, Noyan T, Karakayali H and Haberal M:
Clinicopathological significance of PTEN loss and the
phosphoinositide 3-kinase/Akt pathway in sporadic colorectal
neoplasms: Is PTEN loss predictor of local recurrence? Am J Surg.
195:719–725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar
|
|
22
|
Fumarola C, Bonelli MA, Petronini PG and
Alfieri RR: Targeting PI3K/AKT/mTOR pathway in non small cell lung
cancer. Biochem Pharmacol. 90:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal E, Chaudhuri A, Leiphrakpam PD,
Haferbier KL, Brattain MG and Chowdhury S: Akt inhibitor MK-2206
promotes anti-tumor activity and cell death by modulation of AIF
and Ezrin in colorectal cancer. BMC Cancer. 14:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Z, Wang Z, Liu X and Wang D: New
development of inhibitors targeting the PI3K/AKT/mTOR pathway in
personalized treatment of non-small-cell lung cancer. Anticancer
Drugs. 26:1–14. 2015. View Article : Google Scholar
|
|
25
|
Pal I and Mandal M: PI3K and Akt as
molecular targets for cancer therapy: Current clinical outcomes.
Acta Pharmacol Sin. 33:1441–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh TC, Lin CY, Bennett DJ, Wu E and Wu
JM: Biochemical and cellular evidence demonstrating AKT-1 as a
binding partner for resveratrol targeting protein NQO2. PLoS One.
9:e1010702014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neradugomma NK, Subramaniam D, Tawfik OW,
Goffin V, Kumar TR, Jensen RA and Anant S: Prolactin signaling
enhances colon cancer stemness by modulating Notch signaling in a
Jak2-STAT3/ERK manner. Carcinogenesis. 35:795–806. 2014. View Article : Google Scholar :
|
|
28
|
Kang FB, Wang L, Jia HC, Li D, Li HJ,
Zhang YG and Sun DX: B7-H3 promotes aggression and invasion of
hepatocellular carcinoma by targeting epithelial-to-mesenchymal
transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int.
15:452015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Turkson J: STAT proteins as novel targets
for cancer drug discovery. Expert Opin Ther Targets. 8:409–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roig AI, Eskiocak U, Hight SK, Kim SB,
Delgado O, Souza RF, Spechler SJ, Wright WE and Shay JW:
Immortalized epithelial cells derived from human colon biopsies
express stem cell markers and differentiate in vitro.
Gastroenterology. 138:1012–1021. e1011–e1015. 2010. View Article : Google Scholar
|
|
31
|
Schrödinger Suite 2017. Schrödinger LLC;
New York, NY: 2017
|
|
32
|
Vang O: Resveratrol: Challenges in
analyzing its biological effects. Ann NY Acad Sci. 1348:161–170.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carter LG, D’Orazio JA and Pearson KJ:
Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat
Cancer. 21:R209–R225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SY, Hyun MY, Go KC, Zouboulis CC and
Kim BJ: Resveratrol exerts growth inhibitory effects on human SZ95
sebocytes through the inactivation of the PI3-K/Akt pathway. Int J
Mol Med. 35:1042–1050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang F, Gao JY, Chen H, Du ZH, Zhang XQ
and Gao W: Targeted inhibition of the phosphoinositide 3-kinase
impairs cell proliferation, survival, and invasion in colon cancer.
Onco Targets Ther. 10:4413–4422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Zhu W, Li J, Liu M and Wei M:
Resveratrol suppresses the STAT3 signaling pathway and inhibits
proliferation of high glucose-exposed HepG2 cells partly through
SIRT1. Oncol Rep. 30:2820–2828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nguyen AV, Martinez M, Stamos MJ, Moyer
MP, Planutis K, Hope C and Holcombe RF: Results of a phase I pilot
clinical trial examining the effect of plant-derived resveratrol
and grape powder on Wnt pathway target gene expression in colonic
mucosa and colon cancer. Cancer Manag Res. 1:25–37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patel KR, Brown VA, Jones DJ, Britton RG,
Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA,
et al: Clinical pharmacology of resveratrol and its metabolites in
colorectal cancer patients. Cancer Res. 70:7392–7399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Howells LM, Berry DP, Elliott PJ, Jacobson
EW, Hoffmann E, Hegarty B, Brown K, Steward WP and Gescher AJ:
Phase I randomized, double-blind pilot study of micronized
resveratrol (SRT501) in patients with hepatic metastases-safety,
pharmaco-kinetics, and pharmacodynamics. Cancer Prev Res.
4:1419–1425. 2011. View Article : Google Scholar
|