Introduction
Liver cancer is the third leading cause of
cancer-associated mortality worldwide (1) and systemic chemotherapy is the main
regimen for patients with late-stage liver cancer (2). Various chemotherapeutic regimens are
currently administered as first-line therapy and drug resistance is
a major clinical barrier to a successful treatment and modern
chemotherapeutics, including combination chemotherapies, against
liver cancer are still needed (3).
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) is a member of the TNF family
that initiates apoptosis via interaction with death receptors
(4). This interaction promotes
death-inducing signaling complex formation and caspase-8
activation, which in turn induce apoptosis (5). Interestingly, TRAIL is confirmed as
a safe and efficient anticancer therapeutic agent that targets
cancer cells (6). However,
various cancer cells are resistant to TRAIL (7) and underlying pathways of
TRAIL-resistance are associated with death receptors downregulation
(8,9) and upregulation of decoy receptors
(10). Therefore, the use of
TRAIL sensitizers is a mechanism towards overcoming
TRAIL-resistance.
6-Shogaol (6-sho) is a bioactive component in ginger
that has been widely used in traditional Chinese medicine (11,12). Additionally, 6-sho has
pharmacologic properties, including anti-inflammatory, anticancer
and antioxidant activities (13,14). Previous studies revealed that
6-sho initiates apoptosis in leukemia cells and liver, lung and
colorectal cancer cells (15-19). Molecular pathways describing
anticancer properties of 6-sho frequently include the activation of
caspases.
Autophagy is a cellular catabolic degradation system
that promotes the autophagosomal-lysosomal deterioration of
cytosolic proteins and other cellular components (20). The first step in autophagy is the
induction of vesicle nucleation, followed by the formation of
autophagosome. The second step is a docking and fusion mechanism,
in which the autophagolysosome is constructed by the fusion of the
autophagosome and lysosomes and finally, the autophagolysosome is
degraded into metabolic fuel by acid-containing enzymes (21). Under cellular stress, cell death
can be induced by autophagy (22-24). In addition, autophagy inhibitors,
including chloroquine (CQ), have been used in combination with
various chemotherapeutic drugs and have been confirmed to sensitize
tumor cells to apoptosis (25).
The tumor-suppressor protein 53 (p53) serves a vital
role in the cellular response to DNA damage and in the protection
of the genome from mutations (26). Previous studies have established a
major role of p53 in the regulation of DNA repair, cell cycle
arrest, apoptosis, senescence and autophagy (27-29). Several studies have revealed
increased reactive oxygen species (ROS) production in cancer cells,
which can be induced by various drugs (30) and increased ROS levels are
responsible for cell death in various cancer cells (31).
The current study aimed to elucidate the function of
6-sho as a sensitizing agent for TRAIL-induced apoptosis in Huh7
liver cancer cells. It was revealed that a combined regimen of
6-shol and TRAIL had a superior outcome compared with single
treatment using 6-sho or TRAIL.
Materials and methods
Cell culture
Human liver cancer cells (Huh7, Hep3B and HepG2)
were obtained from the American Type Culture Collection (Manassas,
VA, USA) and maintained in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Cells were cultured at 37°C with 5%
CO2 in humidified incubator.
Reagents
6-sho was acquired from Cayman Chemical Company (Ann
Arbor, MI, USA). TRAIL (200 ng/ml) was acquired from AbFrontier
Co., Ltd. (Seoul, South Korea). CQ diphosphate and
N-acetyl-L-cysteine (NAC) were purchased from Sigma-Aldrich
(Merck KGaA). CQ was dissolved in water to give a 10 mM stock
solution and aqueous NAC (10 mM) was prepared and added to cells 1
h at 37°C prior to treatment with TRAIL and 6-sho or CQ.
Cell viability assay
Huh7, HepG2 and Hep3B cells were seeded at
1.0×104 cells/well in 12-well plates, pre-exposed to
6-sho (20 µM) for 18 h at 37°C and were then treated with
TRAIL (200 ng/ml) for 2 h at 37°C. Cell morphology was assessed in
Huh7 and Hep3B cells under an inverted microscope (magnification,
×100) and cell viability was evaluated using crystal violet
staining in Huh7 and HepG2 cells as previously described (32).
Trypan blue exclusion assay
Cell viability was evaluated by trypan blue
exclusion assay (Sigma-Aldrich; Merck KGaA) using a hemocytometer
in Huh7 and Hep3B cells. Following each treatment, cells were then
trypsinized and re-suspended in PBS. Trypan blue dye solution
(0.4%) was added to the cell suspension for 5 min at room
temperature. Unstained cells were viable and stained cells were
dead. The total cell number and the number of trypan blue-positive
cells were counted using a light microscope (magnification, ×100)
in a blinded manner. The percentage of surviving cells was
calculated using the formula. Number of stained cells/number of
total cells ×100. Each experiments was performed in triplicate.
Immunofluorescent staining
Huh7 cells were cultured on poly-L-lysine coated
coverslips (Sigma-Aldrich; Merck KGaA). Following differentiation
and treatments, cells were fixed with 4% paraformaldehyde for 15
min at room temperature and permeabilized with 0.1% Triton X-100
for 5 min at room temperature. Cells were then incubated for 60 min
at room temperature with blocking solution (5% FBS in Tris-buffered
saline) followed by overnight incubation at 4°C with anti-p62
(1:250; cat. no. PA5-20839; Invitrogen; Thermo Fisher Scientific,
Inc.) and anti-p53 (1:250; cat. no. 9286; Cell Signaling
Technology, Inc., Danvers, MA, USA) antibodies. Following washing
with PBS, cells were incubated with fluorescence-labeled secondary
antibodies (Alexa Fluor® 488-conjugated donkey
polyclonal anti-rabbit; 1:500; cat. no. A-21206; and Texas
Red-X-conjugated goat polyclonal anti-mouse; 1:500; cat. no.
T-6390; both Thermo Fisher Scientific, Inc.) for 2 h at room
temperature in the dark. In addition, DAPI (1:1,000; cat. no.
D9564; Sigma-Aldrich; Merck KGaA) was used to non-specifically
stain the nuclei and samples were incubated with 50 µl DAPI
for 10 min at room temperature. Immunostaining was visualized under
a fluorescence microscope (magnification, ×400).
ROS determination
ROS formation was determined using the cell
permeable fluorescent marker dihydroethidium (DHE). Briefly,
following treatment, Huh7 cells (1.0×104 cells) were
treated with 5 µM DHE (Invitrogen; Thermo Fisher Scientific,
Inc.) for 30 min at room temperature in the dark. Fluorescence was
observed using a fluorescence plate reader at excitation and
emission wavelengths of 518 and 605 nm, respectively.
Transmission electron microscopy (TEM)
analysis
Following fixation of Huh7 cells in 2%
glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA, USA)
and 2% paraformaldehyde (EMS, USA) in 0.05 M sodium cacodylate (pH
7.2; Electron Microscopy Sciences) for 2 h at 4°C, specimens were
fixed in 1% osmium tetroxide (Electron Microscopy Sciences) for 1 h
at 4°C, dehydrated with increasing ethanol (25, 50, 70, 90 and
100%) for 5 min each at 4°C and embedded in epoxy resin (Embed 812;
Electron Microscopy Sciences) for 48 h at 60°C according to the
manufacturers' instructions. Ultrathin sections (60 nm) were
prepared using an LKB-III ultratome (Leica Microsystems GmbH,
Wetzlar, Germany) and were stained with 0.5% uranyl acetate
(Electron Microscopy Sciences) for 20 min and 0.1% lead citrate
(Electron Microscopy Sciences) for 7 min at room temperature.
Images were recorded on a Hitachi H7650 electron microscope
(magnification, ×10,000; Hitachi, Ltd., Tokyo, Japan) installed at
the Center for University-Wide Research Facilities (CURF) at
Chonbuk National University.
Mitochondrial transmembrane potential
(MTP) analysis
Changes in MTP were assessed using the cationic
fluorescent marker, JC-1. Huh7 cells (2.0×104) were
maintained on cover slips in a 24-well plate, incubated with 10
µM JC-1 (Molecular Probes; Thermo Fisher Scientific, Inc.)
at 37°C for 30 min and washed with PBS. Cells were mounted with
DakoCytomation fluorescent mounting medium (cat. no. S3023; Dako;
Agilent Technologies GmbH, Waldbronn, Germany) and analyzed at 485
nm for excitation and at 530 nm for emission using a fluorescence
microscope (magnification, ×400).
Western blot assay
Immunoblotting was performed as described previously
(33). Briefly,
radioimmunoprecipitation assay buffer (Qiagen, Inc., Valencia, CA,
USA) was used to extract total proteins from Huh7 cells. The
supernatant was collected by centrifugation (13,282 × g; 4°C; 10
min). The protein concentration was determined using the Pierce BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). Proteins (30
µg) were separated on 10% SDS-PAGE gels and blotted onto
polyvinylidene fluoride membranes. Membranes were blocked with 5%
non-fat dried milk at 25°C for 1 h, followed by incubation with
primary antibodies overnight at 4°C. The β-actin antibody was from
Sigma-Aldrich (cat. no. A2228; 1:2,000, Merck KGaA, Darmstadt,
Germany), antibodies against microtubule-associated proteins 1A/1B
light chain 3B (LC3)-I/II (cat. no. 3868; 1:1,000), cleaved
caspase-3 (cas3; cat. no. 9661; 1:500) and p62 (cat. no. 5114;
1:1,000) were from Cell Signaling Technology, Inc., the p53 (cat.
no. sc-6243; 1:1,000) antibody was from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA) and the caspase-8 (cas8; cat. no. 551242;
1:1,000) antibody was from BD Biosciences (Franklin Lakes, NJ,
USA). Membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (cat. no. 4410; 1:2,000;
Cell Signaling Technology, Inc.) at 25°C for 1 h. The
immune-reactive protein bands were visualized using an enhanced
chemiluminescence detection system (GE Healthcare Life Sciences,
Chalfont, UK).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism (version 5.03; GraphPad Software, Inc., La Jolla, CA, USA).
All experiments were performed in triplicate and data are presented
as the mean ± standard error. Significant differences between
control and treated samples were analyzed using one-way analysis of
variance followed by Duncan's post-hoc test or Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
6-Sho sensitizes liver cancer cells to
TRAIL-induced apoptosis
Changes in cell morphology were determined using a
light microscope. Treatment with 6-sho or TRAIL alone only affected
cell death minimally and no morphological changes were observed in
TRAIL-treated cells compared to untreated cells (Fig. 1). However, combined treatment of
TRAIL and 6-sho at varying concentrations markedly increased cell
death compared with 6-sho or TRAIL alone (Fig. 1). Cell morphology (Fig. 1A and E) and trypan blue exclusion
assays (Fig. 1D and F) with Huh7
and Hep3B, and crystal violet assays (Fig. 1B and G) and mean density of
crystal violet assay data (Fig. 1C
and H) for Huh7 and Hep-G2 suggested that combined treatment
with TRAIL and 6-sho upregulated cell death compared with the
single treatments. The data indicated that 6-sho pretreatment
sensitized liver cancer cells to TRAIL-induced apoptosis.
Autophagy flux is induced by 6-sho in
Huh7 cells
LC3-II and p62 expressions increased following 6-sho
treatments in a dose-dependent manner (Fig. 2A). Immunofluorescent staining
further demonstrated that 6-sho increased p62 levels dependent on
the dose (Fig. 2B). TEM analysis
revealed increased autophagy and empty vacuoles in the untreated
control compared with 6-sho-treated cells (Fig. 2C). Combined treatment with TRAIL
and 6-sho increased the cleaved cas8 and cleaved cas3 levels
compared with the untreated or single treatments (Fig. 2D).
6-Sho enhances TRAIL-induced cell death
by attenuating autophagy flux
Cell morphology indicated increased cell death
through treatment with TRAIL and 6-sho (20 µM) or CQ (20
µM) (Fig. 3A). Combined
treatment regimen using CQ and TRAIL markedly increased cell death
(Fig. 3B) and significantly
decreased cell viability compared with the untreated and the single
treatment groups (Fig. 3C and D).
The findings suggested that 6-sho sensitized cells to TRAIL-induced
cell death by attenuating autophagy flux.
6-Sho enhances the TRAIL-induced
apoptotic pathway by attenuating autophagy flux
LC3-II and p62 expression levels were markedly
increased in Huh7 cells treated with 6-sho or CQ compared with
untreated cells. This confirmed that 6-sho inhibited autophagy flux
and observed effects were enhanced with CQ treatment (Fig. 4A). Immunofluorescent staining
demonstrated enhanced p62 protein levels in the 6-sho and CQ
treated cells compared to the untreated cells (Fig. 4B). Combined treatment regimen with
TRAIL and CQ or 6-sho increased cleaved cas3 and cleaved cas8
protein levels (Fig. 4C).
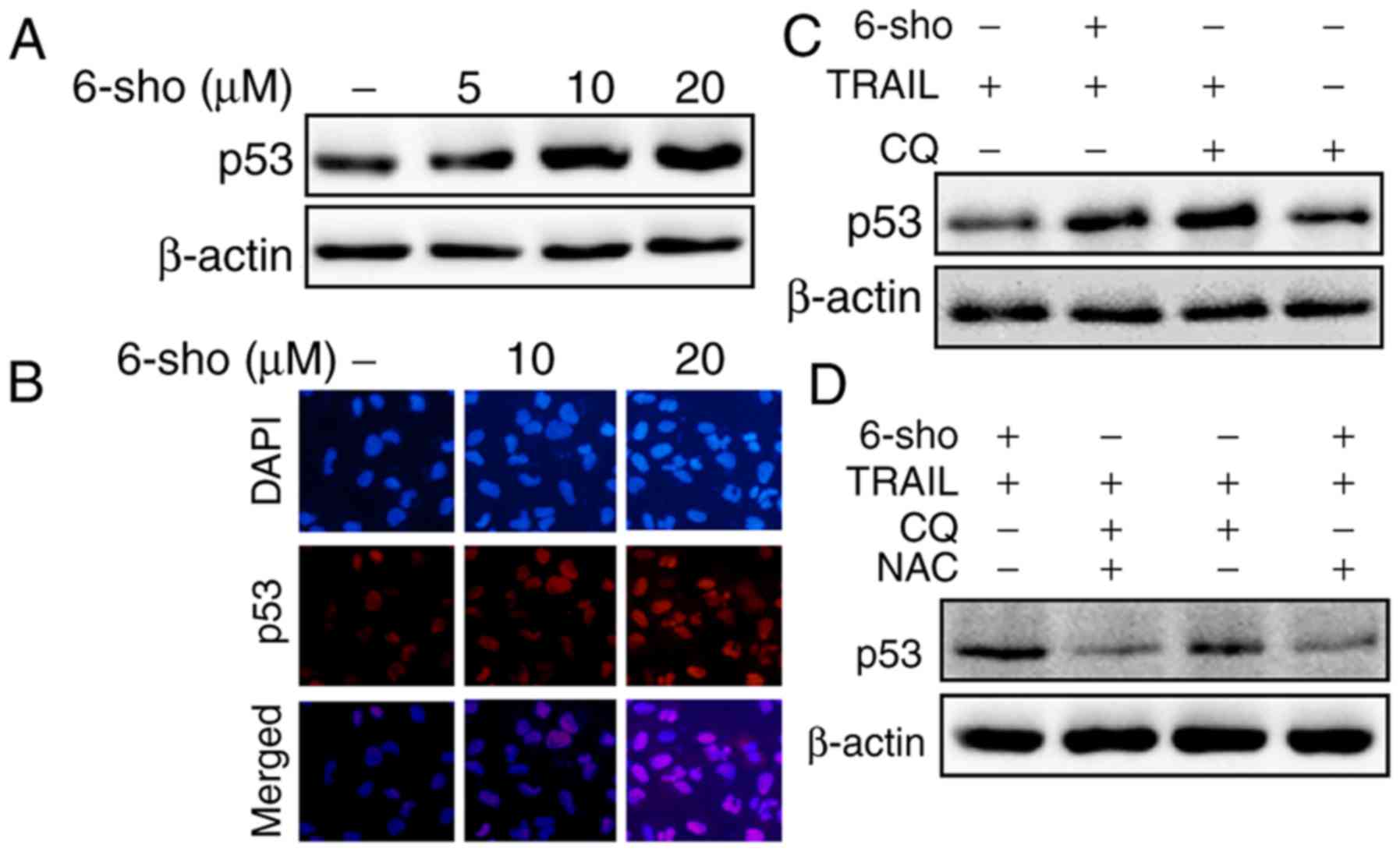
6-Sho enhances TRAIL-mediated p53
expression
6-Sho treatment increased p53 expression in Huh7
cells in a dose-dependent manner (Fig. 5A); immunofluorescent staining
further confirmed these findings (Fig. 5B). p53 levels were increased in
cells treated with TRAIL combined with 6-sho or CQ compared with
the TRAIL or CQ single treatment (Fig. 5C). The increase in p53 expression
was attenuated when the cells were preincubated with
N-acetyl-L-cysteine (NAC) for 1 h prior to treatment with
TRAIL and 6-sho or CQ (Fig.
5D).
Attenuation of autophagy stimulates ROS
production and changes in the MTP
In Fig. 6A, it was
demonstrated that ROS levels were significantly increased in Huh7
cells treated with TRAIL combined with 6-sho or CQ compared to the
TRAIL single treatment (P<0.01) and this increase was reversed
in cells pretreated with NAC prior to TRAIL and 6-sho or CQ
treatment. Green fluorescence, as observed in cells treated with
TRAIL combined with 6-sho or CQ, indicated lower MTP values and
pretreatment with NAC restored MTP values (Fig. 6B). Yellow-orange fluorescence
indicated correct potential of the intracellular mitochondrial
membrane (80-100 mV) from the polarized mitochondria. The findings
suggested that 6-sho-induced autophagy flux attenuation enhanced
TRAIL-induced apoptosis via ROS production and MTP reduction.
Discussion
TRAIL is a member of the TNF superfamily, first
discovered in the 1990s (34,35) and is regarded as a selective
antitumor drug inducing apoptosis in tumor cells with minimal
effects on healthy cells (36).
TRAIL has gained increasing attention due to its therapeutic role
as a tumor cell-specific apoptosis inducer (37). 6-Sho has multiple anticancer
effects, including attenuation of proliferation and invasion, and
initiation of cell death (38).
Autophagy is a highly conserved catabolic pathway exhibiting
decreased levels of proteins and organelles that enhance survival
and it occurs in physiological and pathological situations
(39). Activation of several
autophagy-associated genes, including LC3,
phosphatidylinositol-4,5-bisphosphate 3-kinase, p62 and beclin
(40-42), induces autophagosome formation.
p53 induces cell death by promoting apoptosis moderators (43). ROS is associated with cell death,
and promotes apoptosis and autophagy through various signaling
mechanisms (44-46).
Studies have demonstrated that liver cancer cells
are resistant to TRAIL-induced apoptosis (47,48). In the present study, Huh7 cells
were used in all experiments, while HepG2 and Hep3B we cells were
used in cell viability assay. This study was demonstrated that a
combined regimen of 6-sho and TRAIL caused a marked induction of
cell death in liver cancer cells compared with the single
treatments. The main focus of the current study was on autophagy
and caspase signaling, including caspase-8 and caspase-3. Combined
treatment of 6-sho and TRAIL upregulated cleaved cas3 and cleaved
cas8 compared with single treatments mediating autophagy. Further
caspases, including caspase-9, may serve pivotal roles in
6-sho-mediated TRAIL activity and further investigations are
required regarding mitochondrial pathways, including cytochrome c,
MTP and the activation of caspase-9.
Previously, 6-sho was reported to initiate cell
cycle arrest and autophagy in A549 cells (49). The present findings confirmed that
LC3-II and p62 expression increased following 6-sho treatments. It
was further observed that a combined regimen of TRAIL and 6-sho or
CQ attenuated cell viability and increased cell death compared with
single treatment regiments. Previously, it has been reported that
p53 is a mediator for CQ-induced apoptotic initiation in cancer
cells (50). The present findings
confirmed that TRAIL combined with 6-sho or CQ increased p53
expression compared with single treatment groups. Previous evidence
revealed that CQ augments dysfunctional mitochondria and ROS
production in prostate cancer cells (51). The present findings suggested that
treatment of 6-sho enhanced TRAIL-mediated apoptosis via ROS
production and MTP reduction.
In conclusion, treatment with 6-sho and TRAIL
induced apoptosis via p53 and ROS, suggesting that the
administration of TRAIL in combination with 6-sho is a suitable
therapeutic treatment of TRAIL-resistant Huh7 liver cells.
Funding
The present study was supported by the National
Research Foundation of Korea (grant no. 2016R1A2B2009293).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
UMDN and S-YP designed the study. UMDN performed the
experiments. UMDN and S-YP analyzed the data and prepared the
manuscript. All authors reviewed the results and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Dhanasekaran R, Limaye A and Cabrera R:
Hepatocellular carcinoma: Current trends in worldwide epidemiology,
risk factors, diagnosis, and therapeutics. Hepat Med. 4:19–37.
2012.PubMed/NCBI
|
|
2
|
Chi HC, Chen SL, Cheng YH, Lin TK, Tsai
CY, Tsai MM, Lin YH, Huang YH and Lin KH: Chemotherapy resistance
and metastasis-promoting effects of thyroid hormone in
hepatocarcinoma cells are mediated by suppression of FoxO1 and Bim
pathway. Cell Death Dis. 7:e23242016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei F, Jiang X, Gao HY and Gao SH:
Liquiritin induces apoptosis and autophagy in cisplatin
(DDP)-resistant gastric cancer cells in vitro and xenograft nude
mic in vivo. Int J Oncol. 51:1383–1394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Helmy SA, El-Mesery M, El-Karef A, Eissa
LA and El Gayar AM: Chloroquine upregulates TRAIL/TRAILR2
expression and potentiates doxorubicin anti-tumor activity in
thioacetamide-induced hepatocellular carcinoma model. Chem Biol
Interact. 279:84–94. 2018. View Article : Google Scholar
|
|
5
|
Falschlehner C, Emmerich CH, Gerlach B and
Walczak H: TRAIL signalling: Decisions between life and death. Int
J Biochem Cell Biol. 39:1462–1475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trivedi R and Mishra DP: Trailing TRAIL
resistance: Novel targets for TRAIL sensitization in cancer cells.
Front Oncol. 5:692015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar
|
|
8
|
Zhang Y and Zhang B: TRAIL resistance of
breast cancer cells is associated with constitutive endocytosis of
death receptors 4 and 5. Mol Cancer Res. 6:1861–1871. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozoren N, Fisher MJ, Kim K, Liu CX, Genin
A, Shifman Y, Dicker DT, Spinner NB, Lisitsyn NA and El-Deiry WS:
Homozygous deletion of the death receptor DR4 gene in a
naso-pharyngeal cancer cell line is associated with TRAIL
resistance. Int J Oncol. 16:917–925. 2000.
|
|
10
|
Sanlioglu AD, Dirice E, Aydin C, Erin N,
Koksoy S and Sanlioglu S: Surface TRAIL decoy receptor-4 expression
is correlated with TRAIL resistance in MCF7 breast cancer cells.
BMC Cancer. 5:542005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali BH, Blunden G, Tanira MO and Nemmar A:
Some phytochemical, pharmacological and toxicological properties of
ginger (Zingiber officinale Roscoe): A review of recent research.
Food Chem Toxicol. 46:409–420. 2008. View Article : Google Scholar
|
|
12
|
Haniadka R, Rajeev AG, Palatty PL, Arora R
and Baliga MS: Zingiber officinale (ginger) as an anti-emetic in
cancer chemotherapy: A review. J Altern Complement Med. 18:440–444.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li F, Nitteranon V, Tang X, Liang J, Zhang
G, Parkin KL and Hu Q: In vitro antioxidant and anti-inflammatory
activities of 1-dehydro-[6]-gingerdione, 6-shogaol,
6-dehydroshogaol and hexahydrocurcumin. Food Chem. 135:332–337.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dugasani S, Pichika MR, Nadarajah VD,
Balijepalli MK, Tandra S and Korlakunta JN: Comparative antioxidant
and anti-inflammatory effects of [6]-gingerol, [8]-gingerol,
[10]-gingerol and [6]-shogaol. J Ethnopharmacol. 127:515–520. 2010.
View Article : Google Scholar
|
|
15
|
Chen CY, Liu TZ, Liu YW, Tseng WC, Liu RH,
Lu FJ, Lin YS, Kuo SH and Chen CH: 6-shogaol (alkanone from ginger)
induces apoptotic cell death of human hepatoma p53 mutant Mahlavu
subline via an oxidative stress-mediated caspase-dependent
mechanism. J Agric Food Chem. 55:948–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu R, Zhou P, Peng YB, Xu X, Ma J, Liu Q,
Zhang L, Wen XD, Qi LW, Gao N and Li P: 6-Shogaol induces apoptosis
in human hepatocellular carcinoma cells and exhibits anti-tumor
activity in vivo through endoplasmic reticulum stress. PLoS One.
7:e396642012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H,
Sang S and Ho CT: 6-Shogaol induces apoptosis in human colorectal
carcinoma cells via ROS production, caspase activation, and GADD
153 expression. Mol Nutr Food Res. 52:527–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Q, Peng YB, Zhou P, Qi LW, Zhang M,
Gao N, Liu EH and Li P: 6-Shogaol induces apoptosis in human
leukemia cells through a process involving caspase-mediated
cleavage of eIF2α. Mol Cancer. 12:1352013. View Article : Google Scholar
|
|
19
|
Kim MO, Lee MH, Oi N, Kim SH, Bae KB,
Huang Z, Kim DJ, Reddy K, Lee SY, Park SJ, et al: [6]-shogaol
inhibits growth and induces apoptosis of non-small cell lung cancer
cells by directly regulating Akt1/2. Carcinogenesis. 35:683–691.
2014. View Article : Google Scholar
|
|
20
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bergmann A: Autophagy and cell death: No
longer at odds. Cell. 131:1032–1034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Codogno P, Mehrpour M and Proikas-Cezanne
T: Canonical and non-canonical autophagy: Variations on a common
theme of self-eating. Nat Rev Mol Cell Biol. 13:7–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura T, Takabatake Y, Takahashi A and
Isaka Y: Chloroquine in cancer therapy: A double-edged sword of
autophagy. Cancer Res. 73:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiu HW, Su YC and Hong JR: Betanodavirus
B2 protein triggers apoptosis and necroptosis in lung cancer cells
that suppresses autophagy. Oncotarget. 8:94129–94141.
2017.PubMed/NCBI
|
|
27
|
Kruse JP and Gu W: Modes of p53
regulation. Cell. 137:609–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toledo F and Wahl GM: Regulating the p53
pathway: In vitro hypotheses, in vivo veritas. Nat Rev Cancer.
6:909–923. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di J, Huang H, Qu D, Tang J, Cao W, Lu Z,
Cheng Q, Yang J, Bai J, Zhang Y and Zheng J: Rap2B promotes
proliferation, migration, and invasion of human breast cancer
through calcium-related ERK1/2 signaling pathway. Sci Rep.
5:123632015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao J, Zhang L, Li J, Wu T, Wang M, Xu G,
Zhang F, Liu L, Yang J and Sun S: A novel pyrazolone-based
derivative induces apoptosis in human esophageal cells via reactive
oxygen species (ROS) generation and caspase-dependent
mitochondria-mediated pathway. Chem Biol Interact. 231:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Menzies FM, Fleming A and Rubinsztein DC:
Compromised autophagy and neurodegenerative diseases. Nat Rev
Neurosci. 16:345–357. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nazim UM, Moon JH, Lee JH, Lee YJ, Seol
JW, Eo SK, Lee JH and Park SY: Activation of autophagy flux by
metformin down-regulates cellular FLICE-like inhibitory protein and
enhances TRAIL- induced apoptosis. Oncotarget. 7:23468–23481. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nazim UM, Moon JH, Lee YJ, Seol JW and
Park SY: PPARγ activation by troglitazone enhances human lung
cancer cells to TRAIL-induced apoptosis via autophagy flux.
Oncotarget. 8:26819–26831. 2017.PubMed/NCBI
|
|
34
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Falschlehner C, Ganten TM, Koschny R,
Schaefer U and Walczak H: TRAIL and other TRAIL receptor agonists
as novel cancer therapeutics. Adv Exp Med Biol. 647:195–206. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Green KL, Brown C, Roeder GE, Southgate TD
and Gaston K: A cancer cell-specific inducer of apoptosis. Hum Gene
Ther. 18:547–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han MA, Woo SM, Min KJ, Kim S, Park JW,
Kim DE, Kim SH, Choi YH and Kwon TK: 6-Shogaol enhances renal
carcinoma Caki cells to TRAIL-induced apoptosis through reactive
oxygen species-mediated cytochrome c release and down-regulation of
c-FLIP(L) expression. Chem Biol Interact. 228:69–78. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferraro E and Cecconi F: Autophagic and
apoptotic response to stress signals in mammalian cells. Arch
Biochem Biophys. 462:210–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie Z and Klionsky DJ: Autophagosome
formation: Core machinery and adaptations. Nat Cell Biol.
9:1102–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohsumi Y and Mizushima N: Two
ubiquitin-like conjugation systems essential for autophagy. Semin
Cell Dev Biol. 15:231–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jia L, Macey MG, Yin Y, Newland AC and
Kelsey SM: Subcellular distribution and redistribution of Bcl-2
family proteins in human leukemia cells undergoing apoptosis.
Blood. 93:2353–2359. 1999.PubMed/NCBI
|
|
43
|
Chen L, Xiong YQ, Xu J, Wang JP, Meng ZL
and Hong YQ: Juglanin inhibits lung cancer by regulation of
apoptosis, ROS and autophagy induction. Oncotarget. 8:93878–93898.
2017.PubMed/NCBI
|
|
44
|
Park SY and Kim Y: Surfactin inhibits
immunostimulatory function of macrophages through blocking
NK-kappaB, MAPK and Akt pathway. Int Immunopharmacol. 9:886–893.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang TC, Lai CC, Shiu SL, Chuang PH, Tzou
BC, Lin YY, Tsai FJ and Lin CW: Japanese encephalitis virus
down-regulates thioredoxin and induces ROS-mediated ASK1-ERK/p38
MAPK activation in human promonocyte cells. Microbes Infect.
12:643–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: How can ROS activate MAPK pathways. J Signal
Transduct. 2011:7926392011. View Article : Google Scholar
|
|
47
|
Yamanaka T, Shiraki K, Sugimoto K, Ito T,
Fujikawa K, Ito M, Takase K, Moriyama M, Nakano T and Suzuki A:
Chemotherapeutic agents augment TRAIL-induced apoptosis in human
hepatocellular carcinoma cell lines. Hepatology. 32:482–490. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shankar S and Srivastava RK: Enhancement
of therapeutic potential of TRAIL by cancer chemotherapy and
irradiation: Mechanisms and clinical implications. Drug Resist
Updat. 7:139–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hung JY, Hsu YL, Li CT, Ko YC, Ni WC,
Huang MS and Kuo PL: 6-Shogaol, an active constituent of dietary
ginger, induces autophagy by inhibiting the AKT/mTOR pathway in
human non-small cell lung cancer A549 cells. J Agric Food Chem.
57:9809–9816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Saleem A, Dvorzhinski D, Santanam U,
Mathew R, Bray K, Stein M, White E and DiPaola RS: Effect of dual
inhibition of apoptosis and autophagy in prostate cancer. Prostate.
72:1374–1381. 2012. View Article : Google Scholar : PubMed/NCBI
|