Introduction
Osteonecrosis of the femoral head (ONFH) may be
induced by large doses of glucocorticoids, one of its most common
causes (1). During the natural
course of ONFH, ~80% of femoral heads collapse within 1-3 years,
leading to the development of hip osteoarthritis, which severely
affects hip joint function and eventually leads to artificial joint
replacement (2). At present, the
long-term therapeutic effects of artificial joint replacement are
unsatisfactory; therefore, certain young patients may need to
undergo 2-3 replacements, which causes substantial pain and is an
economic burden for patients and their families (3,4).
However, specific and effective protective drugs against ONFH for
clinical use are currently lacking, as the mechanisms of
hormone-induced osteonecrosis remain to be fully elucidated
(5).
MicroRNAs (miRNA/miRs) are a class of
single-stranded non-coding RNAs of 20-24 nucleotides in length and
are produced from single-stranded RNA precursors with a hairpin
loop structure that are 70-80 nucleotides in length following
shearing (6). miRNAs induce the
degradation or inhibit the translation of their target mRNAs
through specific binding with their 3′-untranslated region
(3′-UTR), thus allowing for post-transcriptional regulation of
genes. Therefore, the effects of miRNAs are considered to be a
common means of regulating gene expression at the
post-transcriptional level in multicellular organisms (7). In particular, miRNAs serve important
regulatory roles during cell proliferation, apoptosis,
differentiation, physiological development and pathological
processes (8).
Transforming growth factor (TGF)-β superfamily
members serve important roles during bone growth, bone wound
healing, skeletal muscle repair and cellular immune responses. Of
note, they are considered to be important regulatory factors during
wound healing, may be involved in the entire process of bone
healing, and are therefore a focus of studies in this field
(9,10).
The Smad signalling pathway is among those that
serve key regulatory roles in osteogenic differentiation (8). Members of the Smad protein family
are involved in each step of the Smad pathway, among which Smad7
functions in the medial steps of the signaling pathway. Smads
belong to the universal transporter class and have important roles,
which comprise binding with receptors Smad1-3, -5, -7 and -8 of the
Smad protein family, assisting Smad proteins to translocate to the
nucleus, and regulating the expression of downstream target genes
(6).
The Smad7 protein is an inhibitory Smad that may
block the biological effects induced by combined actions of other
activated Smads, thus enabling Smad7 to antagonize bone
morphogenetic protein (BMP) and TGF-β signals conferred through the
Smad pathway (11). BMPs, which
also belong to the TGF-β superfamily, are the only local growth
factors with the ability to independently induce bone tissue
formation (12). BMPs also serve
a leading role in regulating bone tissue formation. It has been
indicated by a previous study that a low BMP concentration may
induce directional migration of mesenchymal cells, while a moderate
BMP concentration promotes the chondrogenic and osteogenic
differentiation of mesenchymal cells, and a high BMP concentration
induces mesenchymal cell proliferation (13). As a major member of the BMP
family, BMP-2 mainly serves a role in the recruitment and
differentiation of mesenchymal cells and osteoblasts (14). Zeng et al (15) demonstrated that the miR-23a
cluster miR-23a/-27a/-24-2 promoted osteocyte differentiation in
osteoblasts by regulating TGF-β signalling. The present study
assessed the effects of miR-27a in steroid-induced ONFH and
investigated its potential underlying mechanisms of action.
Materials and methods
Animal model
A total of 20 Sprague Dawley rats (male, weight,
200-220 g) were purchased from Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China), and housed at 22-23°C,
55-60% humidity and 12-h light/dark cycle. The rats were randomly
divided into the control (n=6) and ONFH model groups (n=6). The
ONFH model rats were administered 10 mg/kg dexamethasone sodium
phosphate by intramuscular injection as references (16).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from serum or cells
(1×106 cell/ml) using TRIzol total RNA isolation reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer’s protocol and purified with the
Column DNA Erasol kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, 1 µg total RNA was used to synthesize
first-strand complementary DNA using avian myeloblastosis virus
reverse transcriptase (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer’s protocol. The thermocycling
conditions were as follows: 35°C for 40 min; and 85°C for 30
sec.
The relative expression was analysed by TaqMan miRNA
probes (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer’s protocol and a CFX96 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
PCR was initiated by a 5-min hold at 95°C, followed by 40 cycles of
denaturation at 95°C for 20 sec, annealing/extension at 60°C for 30
sec and 72°C for 30 sec. The following primer sequences were used:
miRNA27a forward, 5′-ACA GGC TAG CGC CGC CTA AC-3′ and reverse,
5′-CCT TAA GGC CCA AGA TTA CG-3′; and U6 forward, 5′-TCG CTT CGG
CAG CAC ATA TAC-3′ and reverse, 5′-TAT GGA ACG CTT CAC GAA TTT
G-3′. The relative levels were normalized to the control using the
equation 2−ΔΔCq (17).
Cell line, culture and transfection
MC3T3-E1 cells were purchased from the Cell Bank of
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and maintained in Dulbecco’s modified Eagle’s
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 µg/ml streptomycin at 37°C in a
humidified atmosphere containing 5% CO2. MC3T3-E1 cells
were transfected with miRNA-27a mimics, miRNA-27a inhibitor (5′-GCG
GAA CUU AGC CAC UGU GAA-3′ and antisense, 5′-CAG UAC UUU UGU GUA
GUA CAA-3′), TGF-β plasmid (5′-ACC CAT GCC TCC CTC TCG GA-3′ and
anti-sense, 5′-AGT GCA GCT AAG GCT CTG GCC-3′), Smad7 plasmid
(5′-TCA GCC TTT TGG AAT GTG TG-3′ and anti-sense, 5′-CCG CGT GCG
GAG GGG ACA GA-3′) and negative control mimic (Sangon Biotech Co.,
Ltd., Shanghai, China) with Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.).
Cell proliferation assay and alkaline
phosphatase (ALP) activity
The proliferation was assessed using an MTT assay. A
total of 150 µl MTT solution (5 mg/ml in PBS; Invitrogen;
Thermo Fisher Scientific, Inc.) was added to the cells
(1×103 cell/well) in a 96-well cell culture plate
following transfection at 48 h, followed by incubation at 37°C for
4 h. Following removal of the supernatant, 200 µl dimethyl
sulfoxide was added, followed by incubation at 37°C for 20 min. The
optical density (OD) was measured at 490 nm on a microplate reader
(Bio-Rad 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ALP activity was measured with an ALP activity assay
(A059-2; Nanjing Jiancheng Biology Engineering Institute) following
transfection for 48 h according to the manufacturer’s protocol. The
OD was measured at 405 nm on a microplate reader (Bio-Rad 550;
Bio-Rad Laboratories, Inc.).
Oil red O and ALP staining
For Oil red O staining, MC3T3-E1 cells
(1×105 cell/ml) were gently washed twice with PBS and
fixed with 15% neutral formalin for 1 h at room temperature.
Subsequently, the MC3T3-E1 cells were stained with oil red O
solution at 37°C for 30 min.
For ALP staining, the MC3T3-E1 cells
(1×105 cell/ml) were gently washed twice with PBS and
fixed with 4% neutral formalin for 5 min at room temperature.
MC3T3-E1 cells were gently washed twice with Tris-buffered
saline/Tween-20 (0.5% TBST) and stained with ALP (cat. no. D001-2;
Nanjing Jiancheng Biology Engineering Institute) at 37°C for 30 min
in the dark. Cell morphology and staining patterns were then
examined using a microscope.
Protein extraction and western blot
analysis
Cells (1×106 cell) were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) following transfection at 48 h and the protein
concentration in the supernatant was quantified with a
bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Total cell lysates were separated by 8-12% SDS-PAGE and transferred
to polyvinylidene difluoride membranes (EMD Millipore, Billerica,
MA, USA). Membranes were blocked in 5% non-fat milk in TBST for 1 h
at 37°C and hybridized with antibodies for B-cell
lymphoma-2-associated X protein (Bax; cat. no. sc-6236; 1:1,000;
Santa Cruz Biotechnology, Inc.), TGF-β (cat. no. sc-31609; 1:1,000;
Santa Cruz Biotechnology, Inc.), Smad7 (cat. no. sc-9183; 1:1,000;
Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no. sc-51631;
1:5,000; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
Subsequently, the membranes were incubated with anti-rabbit
immunoglobulin G secondary antibody (cat. no. sc-2004; 1:5,000;
Santa Cruz Biotechnology, Inc.) and bands were visualized with an
enhanced chemiluminescence system kit (EMD Millipore). Membranes
were analyzed using Image_Lab_3.0 (Bio-Rad Laboratories, Inc.).
Assessment of caspase activity
Total cell lysates prepared as in the western blot
protocol were used to measure caspase-3/9 activity with caspase-3
or caspase-9 activity kits (C1116 or C1158; Beyotime Institute of
Biotechnology). The OD was measured at 405 nm on a microplate
reader (Bio-Rad 550; Bio-Rad Laboratories, Inc.) according to the
manufacturer’s protocol.
Statistical analysis
Values are expressed as the mean ± standard
deviation using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Statistical analysis of differences between multiple groups was
performed using Student’s t-test or one-way analysis of variance
with Tukey’s post-hoc test. Experiments were repeated three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum miR-27a in ONFH rats
The levels of miR-27a in the serum of ONFH rats were
significantly decreased compared with those in normal controls
(Fig. 1A). HE staining
demonstrated that bone cell appeared mass deaths in ONFH group,
compared with control group (Fig.
1B).
miR-27a regulates the proliferation and
osteogenic differentiation of MC3T3-E1 cells
miR-27a mimics were used to increase the miR-27a
expression in MC3T3-E1 cells. As demonstrated in Fig. 2A, transfection with miR-27a mimics
significantly increased miR-27a expression of the MC3T3-E1 cells
compared with that in the negative control transfection group.
Over-expression of miR-27a promoted osteogenic differentiation and
increased the proliferation of the MC3T3-E1 cells compared with
that in the negative control transfection group (Fig. 2B and C). The inhibitor of miR-27a
was used to decrease the levels of miR-27a in MC3T3-E1 cells.
Transfection with miR-27a inhibitor decreased the expression of
miR-27a, inhibited osteogenic differentiation and reduced cell
proliferation in the MC3T3-E1 cells compared with those in the
negative control transfection group (Fig. 3).
miR-27a mimic and inhibitor affect
caspase-3/9 activity and Bax protein expression in MC3T3-E1
cells
Subsequently, it was identified that miR-27a mimics
inhibited caspase-3/9 activity and Bax protein expression in
MC3T3-E1 cells (Fig. 4), while
the miR-27a inhibitor increased caspase-3/9 activity and Bax
protein expression in the MC3T3-E1 cells (Fig. 5), relative to the respective
control groups. These results indicate that miR-27a has an
anti-apoptotic function in osteoblasts.
miR-27a mimic and inhibitor affect ALP
activity, and BMP-2, runt-related transcription factor (Runx)2 and
osteonectin mRNA expression in MC3T3-E1 cells
As demonstrated in Fig. 3A, miR-27a mimics effectively
increased ALP activity, as well as BMP-2, Runx2 and osteonectin
mRNA expression (Fig. 6), while
miR-27a inhibitor suppressed ALP activity, as well as BMP-2, Runx2
and osteonectin mRNA expression in MC3T3-E1 cells, compared with
those in the respective control groups (Fig. 7). Therefore it was demonstrated
that miR-27a regulates osteogenic differentiation.
 | Figure 6miR-27a affects ALP activity, and Col
I, OCN and OPN mRNA expression in MC3T3-E1 cells. (A) ALP activity.
mRNA expression levels of (B) Col I, (C) OCN and (D) OPN in
MC3T3-E1 cells. ##P<0.01 vs. control group. Control,
negative control group; miR-27a, miR-27a mimics group. miR,
microRNA; ALP, alkaline phosphatase; OPN, osteopontin; OCN,
osteocalcin; Col I, collagen I. |
 | Figure 7Inhibition of miR-27a affects ALP
activity, and Col I, OCN and OPN mRNA expression in MC3T3-E1 cells.
(A) ALP activity. mRNA expression levels of (B) Col I, (C) OCN and
(D) OPN in MC3T3-E1 cells. ##P<0.01 vs. control
group. Groups: Control, negative control group; anti-miR-27a,
miR-27a inhibitor group. miR, microRNA; ALP, alkaline phosphatase;
OPN, osteopontin; OCN, osteocalcin; Col I, collagen I. |
miR-27a mimic and inhibitor affect TGF-β
and Smad7 protein expression in MC3T3-E1 cells
Western blot analysis was used to examine TGF-β and
Smad7 protein expression in MC3T3-E1 cells. It was observed that
miR-27a overexpression significantly promoted TGF-β and Smad7
protein expression (Fig. 8),
while the miR-27a inhibitor significantly suppressed TGF-β and
Smad7 protein expression in the MC3T3-E1 cells (Fig. 9), compared with those in the
respective control groups.
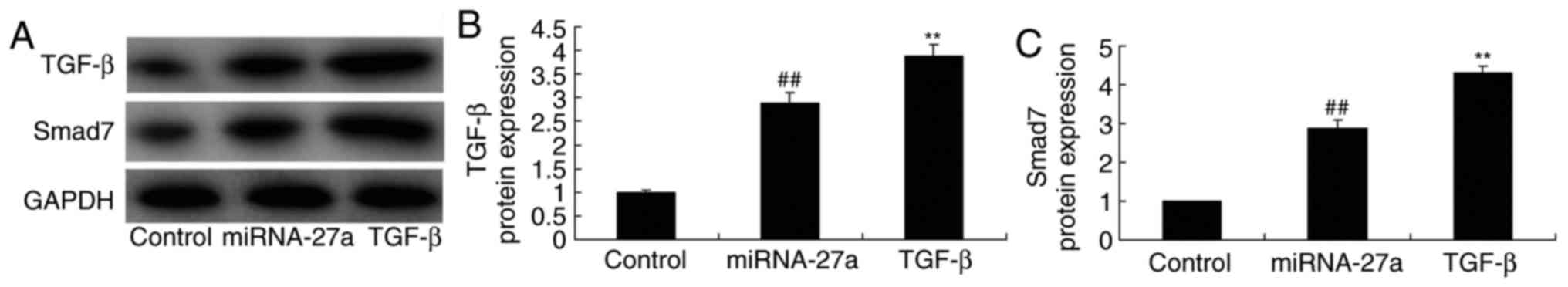
Ectopic overexpression of TGF-β enhances
miR-27a- mediated expression of TGF-β and Smad7 in MC3T3-E1
cells
MC3T3-E1 cells were transfected with miR-27a mimics
and simultaneously with TGF-β to evaluate the potential role of
TGF-β in the effect of miR-27a on osteogenic differentiation. As
demonstrated in Fig. 10,
TGF-β-expressing plasmid significantly increased TGF-β and Smad7
protein expression in MC3T3-E1 cells transfected with miR-27a
mimics, compared with that in cells transfected with miR-27a mimics
alone. In this study, the results demonstrated that TGF-β is an
important for the effects of miR-27a on osteogenic differentiation
of MC3T3-E1 cells.
TGF-β contributes to the function of
miR-27a in osteogenic differentiation of MC3T3-E1 cells
Next, the role of TGF-β in the function of miR-27a
in osteogenic differentiation was investigated. Ectopic
overexpression of TGF-β significantly increased the effect of
miR-27a mimics on the proliferation and osteogenic differentiation
of MC3T3-E1 cells when compared with that of cells transfected with
miR-27a mimics alone (Fig.
11).
TGF-β enhances the effects of miR-27a on
caspase-3/9 activity and Bax protein expression in MC3T3-E1
cells
Ectopic overexpression of TGF-β increased the
inhibitory effect of miR-27a on caspase-3/9 activity and Bax
protein expression in MC3T3-E1 cells when compared with those in
cells transfected with miR-27a mimics alone (Fig. 12).
TGF-β enhances the effects of miR-27a on
ALP activity, as well as BMP-2, Runx2 and osteonectin mRNA
expression in MC3T3-E1 cells
Ectopic overexpression of TGF-β promoted the effects
of miR-27a on ALP activity, as well as on BMP-2, Runx2 and
osteonectin mRNA expression in MC3T3-E1 cells when compared with
those in cells transfected with miR-27a only, suggesting that
miR-27a/TGF-β expression to activate osteogenic differentiation of
MC3T3-E1 cells (Fig. 13).
 | Figure 13TGF-β enhances the effects of miR-27a
on ALP activity, and Col I, OCN and OPN mRNA expression in MC3T3-E1
cells. (A) ALP activity. mRNA expression levels of (B) Col I, (C)
OCN and (D) OPN in MC3T3-E1 cells. ##P<0.01 vs.
control group, **P<0.01 vs. miR-27a mimics group.
Groups: Control, negative control group; miR-27a, miR-27a mimics
group; TGF-β, miR-27a mimics + TGF-β group. TGF, transforming
growth factor; miR, microRNA; ALP, alkaline phosphatase; OPN,
osteopontin; OCN, osteocalcin; Col I, collagen I. |
Ectopic overexpression of Smad7 enhances
miR-27a-mediated expression of Smad7 in MC3T3-E1 cells
To determine the potential role of Smad7 in the
function of miR-27a on the osteogenic differentiation of MC3T3-E1
cells, transfection with Smad7 mimics was performed. As
demonstrated in Fig. 14,
Smad7-expressing plasmid enhanced Smad7 protein expression in
MC3T3-E1 cells following miR-27a transfection, compared with that
in cells transfected with miR-27a alone. These results demonstrated
that miR-27a regulates Smad7 to activate osteogenic differentiation
of MC3T3-E1.
Smad7 enhances the stimulatory effect of
miR-27a on the osteogenic differentiation of MC3T3-E1 cells
via
Vector-mediated upregulation of Smad7 promoted the
effect of miR-27a mimics on the proliferation and osteogenic
differentiation of MC3T3-E1 cells compared with that in the group
transfected with miR-27a mimics alone (Fig. 15).
Smad7 enhances the anti-apoptotic effects
of miR-27a on MC3T3-E1 cells
Vector-medicated upregulation of Smad7 also enhanced
the inhibitory effect of miR-27a on caspase-3/9 activity and Bax
protein expression in MC3T3-E1 cells compared with that in cells
transfected with miR-27a alone (Fig.
16).
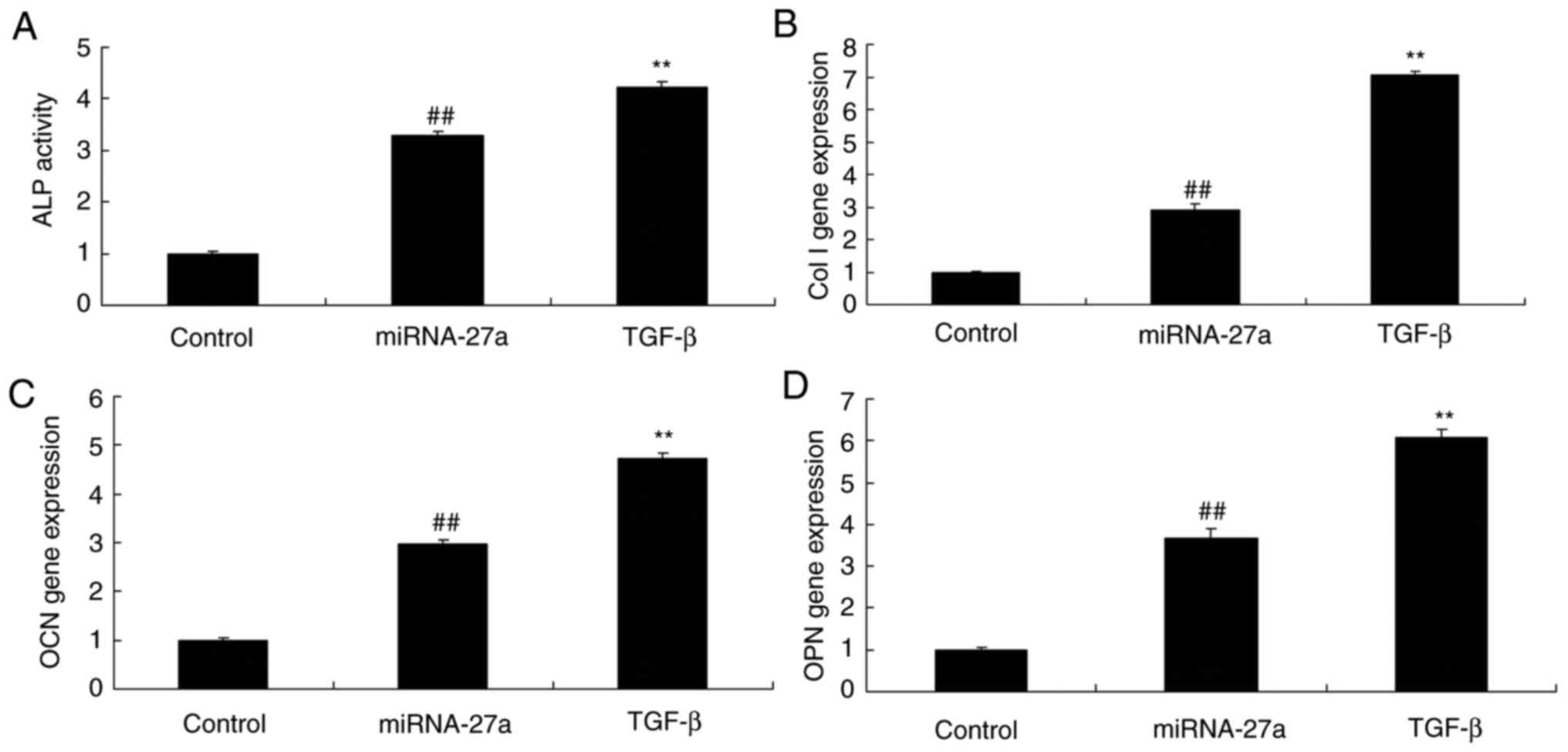
Smad7 amplifies the effects of miR-27a on
ALP activity, as well as BMP-2, Runx2 and osteonectin mRNA
expression in MC3T3-E1 cells
To evaluate the potential role of Smad7 in the
effect of miR-27a on the osteogenic differentiation of MC3T3-E1
cells, ALP activity and BMP-2, Runx2 and osteonectin mRNA
expression were measured following transfection with Smad7 and/or
miR-27a mimics. As demonstrated in Fig. 17, upregulation of Smad7 increased
the effects of miR-27a on ALP activity and BMP-2, Runx2 and
osteonectin mRNA expression in the MC3T3-E1 cells, compared with
that in the group transfected with miR-27a mimics alone.
 | Figure 17Smad7 enhances the effects of miR-27a
on ALP activity, and Col I, OCN and OPN mRNA expression in MC3T3-E1
cells. (A) ALP activity. mRNA expression levels of (B) Col I, (C)
OCN and (D) OPN in MC3T3-E1 cells. ##P<0.01 vs.
control group, **P<0.01 vs. miR-27a mimics group.
Groups: Control, negative control group; miR-27a, miR-27a mimics
group; Smad7, miR-27a mimics + Smad7 group. miR, microRNA; ALP,
alkaline phosphatase; OPN, osteopontin; OCN, osteocalcin; Col I,
collagen I. |
Discussion
With the extensive application of corticosteroids in
clinical practice, it has gradually become apparent that they are
key inducing factors of osteonecrosis. Corticosteroid-induced ONFH
is the most common type of ONFH in Chinese patients (3) and numerous research groups have
focused on investigating and exploring its pathogenesis for a
number of years. However, specific and effective therapeutic
methods for the treatment of hormone-induced ONFH are still lacking
in the clinic at present, due to an incomplete understanding of its
precise pathogenesis (18). In
the present study, the serum levels of miR-27a were decreased in a
rat model of ONFH when compared with those in normal controls.
miRNAs are a class of small non-coding RNAs that
collectively regulate thousands of genes; it is estimated that
miRNAs regulate the expression of 30% of human genes, generally by
blocking the expression or promoting the degradation of their
target mRNAs through binding with their 3′-UTR (8). Although the roles of miRNAs have
been investigated for numerous diseases, few studies have reported
on their regulatory role in the differentiation of stem cells and
osteoblasts, or their differential expression profiles in bone
diseases including osteoporosis and ONFH (19). The present study identified that
miR-27a expression promoted osteogenic differentiation, and reduced
caspase-3/9 activity and Bax protein expression in MC3T3-E1 cells,
while the miR-27a inhibitor inhibited osteogenic differentiation
and increased caspase-3/9 activity and Bax protein expression in
MC3T3-E1 cells, compared with that in the respective control
groups. In contrast to these results, Wang et al (20) reported that miR-27a promoted the
apoptosis of cochlear sensory epithelium and may thus have opposing
functions in the survival of different cell types.
TGF-β is an important cytokine involved in the
function and metabolism of bone cells, and according to a previous
study, it not only exerts a mitogenic effect on bone cells, but
also reduces ossein loss, increases the bone deposition rate and
promotes osteoblast differentiation (13). Suppression of programmed cell
death is also among the mechanisms by which TGF-β prolongs cell
survival (21). TGF-β has also
been reported to promote metaplasia of the periosteal and
aponeurotic layers on the greater trochanter surface of the femoral
head of articular cartilage (10). The present study demonstrated that
miR-27a significantly promoted TGF-β and Smad7 protein expression,
while the miR-27a inhibitor significantly suppressed TGF-β and
Smad7 protein expression in MC3T3-E1 cells when compared with that
in the control groups. Similarly, Zeng et al (15) demonstrated that the miR-23a
cluster miR-23a/-27a/-24-2 promoted osteocyte differentiation in
osteoblasts by regulating TGF-β signaling.
The Smad pathway is responsible for transducing BMP
and TGF-β signals during osteogenic and chondrogenic
differentiation (13). BMPs and
TGF-β belong to the TGF-β superfamily, with BMPs, as the largest
family in the TGF-β superfamily, classified as acid glycoproteins
that are extensively distributed over the extracellular matrix
(21). In particular, BMP-2 is an
important extracellular signaling molecule that promotes osteogenic
differentiation and bone formation (22). Regulation via the BMP-2/Smad/Runx2
pathway leads to increases and decreases in bone mass during the
growth, metabolism and development of bone tissues, in addition to
bone formation and reconstruction, the osteogenic differentiation
of stem cells, the maturation of osteoblasts and the secretion and
mineralization of extracellular matrix (23). BMP-2 regulates the transcription
of genes involved in osteogenesis by activating the Smad pathway,
which thus enhances its osteogenic effects (24). Type I and II serine/threonine
kinase receptors are receptors of the TGF-β receptor family; type I
receptors are also known as activin-receptor-like kinases. The two
receptor types may be activated by TGF-β signaling to form
tetramers, in which type II receptors phosphorylate type I
receptors. In the present study, upregulation of Smad7 protein
expression enhanced the effects of miR-27a overexpression on
osteoblastic differentiation, cell proliferation, ALP activity,
osteonectin mRNA expression and Smad7 protein expression in
MC3T3-E1 cells. Wang et al (25) demonstrated that miR-27a
ameliorates chronic kidney disease-induced muscle atrophy. Smad7
enhanced osteogenic differentiation via a different/additional
mechanism, and an experiment with Smad7 knockdown to inhibit the
effect of miR-27a on osteogenic differentiation would have provided
more information. The present study did not investigate the direct
or indirect binding of miR-27a and TGF or Smad7 by a luciferase
assay, which is one of its limitations, however the regulation is
probably an indirect one and that the steps in between are likely
to be those reported by Chae et al (26).
The accumulation of activated Smad compounds in the
nucleus serves a crucial role in the transmission of TGF-β signals
from transmembrane receptors to the cell nucleus (27). It is currently thought that the
distribution of Smads is in a dynamic balanced state between the
cell nucleus and cytoplasm, which means that Smads undergo constant
trafficking between the cytoplasm and nucleus in the presence or
absence of signal stimulation to reach a certain equilibrium
(13). Part of the mechanism
regulating the nuclear-cytoplasmic trafficking of Smad has
previously been documented (27).
The nuclear-cytoplasmic trafficking of Smad allows cells to sense
changes in TGF-β signaling in a continuous manner, which enables
rapid cellular responses to changes in signalling (13). Smad7, along with Smad6, is
classified as an inhibitory Smad, although it differs from Smad6,
which specifically inhibits BMP signalling (21), as Smad7 inhibits BMP and TGF-β
signals to exert marked negative regulatory effects (21). Furthermore, overexpression of
Smad7 has been demonstrated to inhibit BMP-induced osteogenesis
(21). The results of the present
study demonstrated that vector-mediated upregulation of TGF-β
enhanced the effects of miR-27a mimics on osteoblastic
differentiation, cell proliferation, ALP activity, as well as the
expression of BMP-2, Runx2 and osteonectin mRNA and Smad7 protein
in MC3T3-E1 cells. TGF-β-expressing plasmid increased TGF-β and
Smad7 protein expression in MC3T3-E1 cells transfected with miR-27a
mimics, miR-27a induced TGF-β expression to activate osteogenic
differentiation of MC3T3-E1 cells. Smad7-expressing plasmid
enhanced Smad7 protein expression in MC3T3-E1 cells following
miR-27a transfection. Chae et al (26) demonstrated that miR-27a induced
the TGF-β signaling pathway by targeting Smad2 and -4 in lung
cancer. The present study only analyzed the extent to which the
expression of miR-27a regulated TGF-β/Smad7 signaling in
osteoblasts, which is a limitation of the present study. An
analysis of the expression profile of downstream genes following
transfection with miR-27a mimics in MC3T3-E1 cells should be
pursued in a future study. In addition, these experiments with
additional overexpression of TGF-β may not provide sufficient
mechanistic evidence. It may have been more appropriate to inhibit
TGF-β to then demonstrate that the effect of miR-27a is
abrogated
In conclusion, the present study demonstrated that
the effects of miR-27a on TGF-β/Smad7 signaling in osteoblasts may
be a potential mechanism by which miR-27a regulates steroid-induced
ONFH. miR-27a was indicated to have a role in regulating osteoblast
differentiation and cell proliferation, although its exact role in
the pathogenesis of ONFH remains to be elucidated.
Funding
No funding received.
Availability of data and materials
The analysed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YB designed the experiment; YL, SJ, KS, HZ and SM
performed the experiment; YB and YL analysed the data; YB wrote the
manuscript.
Ethics approval and consent to
participate
Full ethical approval was granted by the Medical
Ethics Committee of Cangzhou City Central Hospital (Cangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Zaki MH, Lamkanfi M and Kanneganti TD: The
Nlrp3 inflammasome: Contributions to intestinal homeostasis. Trends
Immunol. 32:171–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lv Q, Wang K, Qiao S, Yang L, Xin Y, Dai Y
and Wei Z: Norisoboldine, a natural AhR agonist, promotes Treg
differentiation and attenuates colitis via targeting glycolysis and
subsequent NAD+/SIRT1/SUV39H1/H3K9me3 signaling pathway.
Cell Death Dis. 9:2582018. View Article : Google Scholar
|
|
3
|
Sands BE, Joshi S, Haddad J, Freudenberg
JM, Oommen DE, Hoffmann E, McCallum SW and Jacobson E: Assessing
colonic exposure, safety, and clinical activity of SRT2104, a novel
oral SIRT1 activator, in patients with mild to moderate ulcerative
colitis. Inflamm Bowel Dis. 22:607–614. 2016. View Article : Google Scholar :
|
|
4
|
Chen A, Chen Z, Xia Y, Lu D, Yang X, Sun
A, Zou Y, Qian J and Ge J: Liraglutide attenuates NLRP3
inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS
pathway in H9c2 cells. Biochem Biophys Res Commun. 499:267–272.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang HX, Wang ZT, Lu XX, Wang YG, Zhong J
and Liu J: NLRP3 gene is associated with ulcerative colitis (UC),
but not Crohn’s disease (CD), in Chinese Han population. Inflamm
Res. 63:979–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Jiang L, Che F, Lu Y, Xie Z and
Wang H: Arctigenin attenuates ischemic stroke via SIRT1-dependent
inhibition of NLRP3 inflammasome. Biochem Biophys Res Commun.
493:821–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baba Y, Shigemi Z, Hara N, Moriguchi M,
Ikeda M, Watanabe T and Fujimuro M: Arctigenin induces the
apoptosis of primary effusion lymphoma cells under conditions of
glucose deprivation. Int J Oncol. 52:505–517. 2018.
|
|
8
|
Cheng X, Wang H, Yang J, Cheng Y, Wang D,
Yang F, Li Y, Zhou D, Wang Y, Xue Z, et al: Arctigenin protects
against liver injury from acute hepatitis by suppressing immune
cells in mice. Biomed Pharmacother. 102:464–471. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Y, Lou Z and Lee SH: Arctigenin
represses TGF-β-induced epithelial mesenchymal transition in human
lung cancer cells. Biochem Biophys Res Commun. 493:934–939. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daci A, Neziri B, Krasniqi S, Cavolli R,
Alaj R, Norata GD and Beretta G: Arctigenin improves vascular tone
and decreases inflammation in human saphenous vein. Eur J
Pharmacol. 810:51–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cox SL, Orgeret F, Gesta M, Rodde C,
Heizer I, Weimerskirch H and Guinet C: Processing of acceleration
and dive data on-board satellite relay tags to investigate diving
and foraging behaviour in free-ranging marine predators. Methods
Ecol Evol. 9:64–77. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Tang HL, Chen Y, Fan Q, Shao YT, Jia
M, Wang JC and Yang CM: Malondialdehyde and SOD-induced changes of
gastric tissues in acute gastric mucosal injury under positive
acceleration. Genet Mol Res. 14:4361–4368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Sarosiek I, Bashashati M, Alvarez A, Hall
M, Shankar N, Gomez Y, McCallum RW and Sarosiek J: Lubiprostone
accelerates intestinal transit and alleviates small intestinal
bacterial overgrowth in patients with chronic constipation. Am J
Med Sci. 352:231–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng HC, Bae Y, Dawson BC, Chen Y, Bertin
T, Munivez E, Campeau PM, Tao J, Chen R and Lee BH: MicroRNA
miR-23a cluster promotes osteocyte differentiation by regulating
TGF-beta signalling in osteoblasts. Nat Commun. 8:150002017.
View Article : Google Scholar
|
|
16
|
Wang X, Qian W, Wu Z, Bian Y and Weng X:
Preliminary screening of differentially expressed circulating
microRNAs in patients with steroidinduced osteonecrosis of the
femoral head. Mol Med Rep. 10:3118–3124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neudecker V, Brodsky KS, Clambey ET,
Schmidt EP, Packard TA, Davenport B, Standiford TJ, Weng T,
Fletcher AA, Barthel L, et al: Neutrophil transfer of miR-223 to
lung epithelial cells dampens acute lung injury in mice. Sci Transl
Med. 9:eaah53602017. View Article : Google Scholar
|
|
18
|
Guo W, Hu S, Elgehama A, Shao F, Ren R,
Liu W, Zhang W, Wang X, Tan R, Xu Q, et al: Fumigaclavine C
ameliorates dextran sulfate sodium-induced murine experimental
colitis via NLRP3 inflammasome inhibition. J Pharmacol Sci.
129:101–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Yang X, He Y, Wang W, Zhang J, Zhang
W, Jing T, Wang B and Lin R: Negative regulation of NLRP3
inflammasome by SIRT1 in vascular endothelial cells. Immunobiology.
222:552–561. 2017. View Article : Google Scholar
|
|
20
|
Wang Y, Lin C, He Y, Li A, Ni W, Sun S, Gu
X, Li J and Li H: Mir-27a promotes apoptosis of cochlear sensory
epithelium in Cx26 knockout mice. Front Biosci (Landmark Ed).
21:364–373. 2016. View
Article : Google Scholar
|
|
21
|
Li R, Liu J, Li Q, Chen G and Yu X:
miR-29a suppresses growth and metastasis in papillary thyroid
carcinoma by targeting AKT3. Tumour Biol. 37:3987–3996. 2016.
View Article : Google Scholar
|
|
22
|
Tian H, Liu C, Zou X, Wu W, Zhang C and
Yuan D: MiRNA-194 regulates palmitic acid-induced toll-like
receptor 4 inflammatory responses in THP-1 cells. Nutrients.
7:3483–3496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui Q, Hou Y, Wang Y, Li X, Liu Y, Ma X,
Wang Z, Wang W, Tao J, Wang Q, et al: Biodistribution of
arctigenin-loaded nanoparticles designed for multimodal imaging. J
Nanobiotechnology. 15:272017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao C, Li Y, Huan L, Zhang Y, Zhao F, Wang
Q, Liang L, Ding J, Liu L, Chen T, et al: NF-κB signaling relieves
negative regulation by miR-194 in hepatocellular carcinoma by
suppressing the transcription factor HNF-1α. Sci Signal.
8:ra752015. View Article : Google Scholar
|
|
25
|
Wang B, Zhang C, Zhang A, Cai H, Price SR
and Wang XH: MicroRNA-23a and MicroRNA-27a mimic exercise by
ameliorating CKD-induced muscle atrophy. J Am Soc Nephrol.
28:2631–2640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chae DK, Ban E, Yoo YS, Kim EE, Baik JH
and Song EJ: MIR-27a regulates the TGF-β signaling pathway by
targeting SMAD2 and SMAD4 in lung cancer. Mol Carcinog.
56:1992–1998. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nie H, Song C, Wang D, Cui S, Ren T, Cao
Z, Liu Q, Chen Z, Chen X and Zhou Y: MicroRNA-194 inhibition
improves dietary-induced non-alcoholic fatty liver disease in mice
through targeting on FXR. Biochim Biophys Acta Mol Basis Dis.
1863.3087–3094. 2017.
|