Introduction
Lower respiratory tract infections, including
pneumonia, are primary causes of patient mortality caused by
infection. The World Health Organization has estimated that
~3,500,000 mortalities worldwide result from this type of infection
annually (1). Community-acquired
pneumonia (CAP), a severe type of pneumonia, commonly needs
hospitalization. CAP is an important factor for adult mortality,
and even patients who successfully survive present a high mortality
rate in the following years (2,3).
In addition, pneumonia is estimated to account for ~15% of
mortality cases among adolescents worldwide (4). Although the occurrence of childhood
pneumonia has decreased, this decrease is not marked. In 2013,
~950,000 individuals younger than 5 years old succumbed to
pneumonia (5). Vaccines against
viruses, such as Haemophilus influenzae type b and
Streptococcus pneumoniae, have been introduced in several
countries; however, obtaining sufficient protection against these
viruses remains a big challenge in developing countries (5). Therefore, it is of great importance
to identify novel treatments for patients suffering from
pneumonia.
Lipopolysaccharide (LPS) is a strong stimulant for
the production of pro-inflammatory cytokines, including tumor
necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and type I
interferon, by Toll-like receptor (TLR)4 responses, resulting in
systemic inflammatory response syndrome (SIRS) (6). Toll-IL-1-resistance (TIR) domain,
which mediates the recruitment of myeloid differentiation factor 88
(MyD88), is a critical adaptor used by all TLRs and indispensable
for Toll signaling (7). The
recruitment of MyD88 to proximal TIR domains of activated TLRs
activates IL-1 receptor-associated kinase (IRAK) family members and
TNF receptor-associated factor 6 (TRAF6) (8). TRAF6 has been reported to
participate in the inflammation of lupus nephritis (9), regulating inflammatory cytokines of
bovine mammary epithelial cells (10) and ischemia/reperfusion injury
(11). TRAF6 may participate in
the progression of severe CAP (SCAP).
MicroRNAs (miRNAs) are a group of small, non-coding
RNAs that primarily regulate gene expression at the transcriptional
or post-transcriptional levels through targeting and binding to the
3′-untranslated region (3′UTR) of their target mRNAs (12). Therefore, miRNAs affect cellular
activities and disease processes (13). A large number of diseases,
including pulmonary diseases, may be attributed to the dysfunction
of miRNAs (14). Certain miRNAs
have been verified to be involved in the pathogenesis of pneumonia,
such as miR-155, miR-21 and miR-197, which have been reported to be
significantly increased in patients with lung cancer or pneumonia
(15).
In the present study, the aim was to investigate
whether specific miRNAs are able to target TRAF6 and regulate the
progression of SCAP in Ana-1 macrophages.
Materials and methods
Cell culture
The murine macrophage Ana-1 cell line was obtained
from the Institute of Biochemistry and Cell Biology (Chinese
Academy of Sciences, Shanghai, China). Cells were cultured in RPMI
1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% low endotoxin fetal calf serum (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA), 2 mM glutamine, 100
U/ml penicillin and 100 µg/ml streptomycin in an incubator
at 37°C in a humidified atmosphere with 5% CO2.
Additionally 293 cells were obtained from the American Type Culture
Collection (Manassas, MA, USA) and cultured in Dulbecco’s modified
Eagle medium Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin and
100 µg/ml streptomycin in an incubator at 37°C in a
humidified atmosphere with 5% CO2.
Assessment of cell viability
Ana-1 murine macrophages (100 µl) were seeded
into 96-well plates at the density of 5×104 cells/ml and
treated with different concentrations of LPS (0.01, 0.1 and 1
µg/ml) for the establishment of an SCAP in vitro
model (16). After 24 h of
incubation at 37°C in a humidified atmosphere with 5%
CO2, 10 µl (5 mg/ml) MTT solution (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was added to each well and
incubated for 4 h at 37°C in a humidified atmosphere with 5%
CO2. Subsequently, the formazan crystals in each well
were dissolved by the addition of DMSO, and the absorbance of each
well at 490 nm was read with a microplate reader. Wells with RPMI
1640 served as the blank, and cell culture medium (without LPS)
served as the control.
Assessment of TNF-α and IL-1β
secretion
Ana-1 macrophages were plated into 24-well plates at
a density of 5×105 cells/well, stimulated with LPS (0.1
µg/ml) and incubated overnight at 37°C in a humidified
atmosphere with 5% CO2. On the following day, the
expression levels of TNF-α and IL-1β in the culture supernatants of
Ana-1 macrophages were evaluated by commercially available
enzyme-linked immunosorbent assay (ELISA) kits for mouse TNF-α
(cat. no. EK0527) and IL-1β (cat. no. EK0394; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) according to the
manufacturer’s protocols.
Detection of mRNA levels by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
In total, 1×106 Ana-1 macrophages were
plated into a 6-well culture plate and incubated for 24 h at 37°C
in a humidified atmosphere with 5% CO2. RNA were
isolated by TRIzol reagent (Thermo Fisher Scientific, Inc.) and
then the amount of RNA was measured by NanoDrop Thermo Fisher
Scientific, Inc. prior to reverse transcribed into cDNA by the
RevertAid First Strand cDNA Synthesis kit Thermo Fisher Scientific,
Inc. , according to the protocols provided by the manufacturer, the
protocol was as follows: 25°C for 5 min followed by at 42°C for 60
min. Terminate by heating at 70°C for 5 min. PCR was then conducted
with a TaKaRa PrimeScript™ One Step RT-PCR kit PCR kit purchased
from Takara Biotechnology Co., Ltd. (Dalian, China). mRNA was
amplified from the cDNA templates for 35 cycles with the following
primers: TNF-α forward, 5′-AAA TTC GAG TGA CAA GCC TGT AG-3′, and
reverse, 5′-GAG AAC CTG GGA GTA GAC AAG GT-3′; IL-1β forward,
5′-CAA GTG TCT GAA GCA GCT ATG G-3′, and reverse, 5′-GAG ATT TGA
AGC TGG ATG CTC T-3′; and GAPDH forward, 5′-GAG GAC CAG GTT GTC TCC
TG-3′, and reverse, 5′-GGA TGG AAT TGT GAG GGA GA-3′. The thermo
cycling conditions were: denaturation at 95°C (5 min), 40 cycles of
amplification and quantification at 5°C (25 sec) and 62°C (40 sec),
and the melting curve at 60°C for 1 min. The amounts of TNF-α and
IL-1β were determined by and normalized to the amount of GAPDH
cDNA, serving as the internal control. The 2−ΔΔCq method
was used for the quantification of mRNA levels (17).
Detection of miRNAs by RT-qPCR
For the detection of miRNA levels, total RNA was
isolated from the cells using the mirVana kit (Thermo Fisher
Scientific, Inc.). The amount of RNA was measured by NanoDrop
(Thermo Fisher Scientific, Inc.). RNA 10 ng) was converted into
cDNA by TaqMan® MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Subsequently,
qPCR reactions were conducted by specific primers
TaqMan® MicroRNA Assay) and TaqMan® Universal
PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
on a Bio-Rad PCR system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The PCR conditions for miRNA detection were conducted
according to the standard protocol, as follows: 50°C pre-incubation
for 2 min, 95°C incubation for 10 min, followed by 40 cycles of
95°C for 15 sec, and 60°C for 1 min. Primers were: miR124-3p,
5′-TGC GGT AAG GCA CGC GGG AAT-3′. U6 5′-CTC GCT TCG GCA GCA CA-3′.
U6 small nuclear RNA served as an endogenous control (17). The 2−ΔΔCq method was
used for the quantification of miRNA levels.
Western blot analysis
A total of 1×106 Ana-1 macrophages/well
were plated into a 6-well culture plate and incubated for 24 h at
37°C in a humidified atmosphere with 5% CO2. The Ana-1
macrophages were then lysed by a dissociation solution RIPA (Roche
Diagnostics, Basel, Switzerland) with phosphatase, protease
inhibitors and phenylmethane sulfonyl fluoride. The proteins
extracted from the macrophages were measured by BCA kit (Beyotime
Institue of Biotechnology, Haimen, China), then electrophoresed on
SDS-polyacrylamide gels (10%) and transferred to nitrocellulose
membranes. Next, the membranes were incubated with 5% blocking
buffer at room temperature for 1 h, and then incubated with primary
antibodies against phosphorylated (p)-p38 mitogen-activated protein
kinase (MAPK; mAb 4511; 1:1,000), TRAF6 (mAb 8028; 1:1,000) and
nuclear factor (NF)-κB (mAb 8242; 1:1,000; all purchased from Cell
Signaling Technology, Inc., Boston, MA, USA) at 4°C overnight. On
the following day, the membranes were incubated with a horseradish
peroxidase peroxidase-conjugated secondary antibody (cat. no.
BM2006; 1:1,000; Wuhan Boster Biological Technology, Ltd.). The
membranes were visualized using a superenhanced chemiluminescence
detection system (Beyotime Institute of Biotechnology).
Densitometric analysis was then performed by Quantity One 4.62
(Bio-Rad Laboratories, Inc.) to determine the protein levels by
normalizing the band density to internal control antibody GAPDH
(cat. no. sc-32233; 1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA).
TargetScan
Using the online tool TargetScan version 7.1
(http://www.targetscan.org/vert_71/),
the interaction between miR-124-3p and TRAF6 was predicted.
miR-124-3p was predicted to target the position 42-48 of TRAF6
3′UTR with the context ++ score percentile as high as 95.
Construction and transfection of
plasmids
The wild-type (WT) 3′UTR plasmids of TRAF6 were
cloned by primers (XhoI site forward,
5′-CGCTCGAGttgtgttcaaaaactaggaaccata-3′, and NotI site
reverse, 5′-GCG CGG CCG Ctg gga aca ggg cag gtc aga-3′) and
inserted into the 3′UTR of the Renilla luciferase gene of
the psiCHECK2 vector (Promega Corporation, Madison, MI, USA). The
mutant type (Mut) 3′UTR plasmids of TRAF6 were produced by
site-directed mutagenesis. The putative promoters of miR-124-3p on
genome loci were cloned by primers (KpnI site forward,
5′-CGG GTA CCG GTG CAG GGG TTC GAA ACT G-3′, and BglII site
reverse, 5′-GCA GAT CTA ATC GGG GAG CCA GAG TTC C-3′) and inserted
into upstream of lucif-erase gene in the pGL3-Basic vector (Promega
Corporation), resulting in the pGL3-124 vector. Subsequently, 293
cells were transfected with these plasmids using Lipofectamine
2000™ (Thermo Fisher Scientific, Inc.) at 0.5-1.5 µg/ml.
Dual-luciferase reporter assay
Validation of miR-124-3p binding to the 3′UTR of
TRAF6 was conducted by a dual-luciferase reporter assay. Briefly,
miR-124-3p mimics or miR-NC mimics were co-transfected with TRAF6
WT or Mut 3′UTR plasmids into the 293 cells for 48 h. Luciferase
assay was then performed by the Modulus™ microplate micromode
reader (Turner Biosystems; Promega Corporation) with a
Dual-Luciferase Reporter Assay system (Promega Corporation). The
relative luciferase activity was evaluated by calculating the ratio
of Renilla over Firefly luciferase activity.
Statistical analysis
Quantitative data are presented as the mean ±
standard error of the mean. Statistical analysis was performed by
SPSS version 13.0 software (version 13.0; SPSS, Inc., Chicago, IL,
USA). Student’s t-test was performed for comparisons between two
subgroups. One-way analysis of variance followed by Bonferroni’s
post hoc test was performed for analysis of experiments with more
than two subgroups. Graphs were obtained by GraphPad Prism software
(version 5.04; GraphPad Software, Inc., San Diego, CA, USA).
Experiments were repeated at least three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of LPS on the viability of Ana-1
macrophages
In order to determine a non-cytotoxic concentration
of LPS for establishing the SCAP model in vitro, Ana-1
murine macrophages were treated with different concentrations of
LPS (0.01, 0.1 and 1 µg/ml). After 24 h of incubation, an
MTT assay was used for the detection of the effects of LPS on the
viability of Ana-1 macrophages. The results of the MTT assay
indicated that, compared with cells in the control group, there was
no significant difference in the viability of cells treated with
0.01 or 0.1 µg/ml LPS (P>0.05). However, the viability of
cells that were treated with 1 µg/ml LPS was significantly
lower compared with that in the control group (P<0.05; Fig. 1). Therefore, the LPS concentration
of 0.1 µg/ml was used in subsequent experiments.
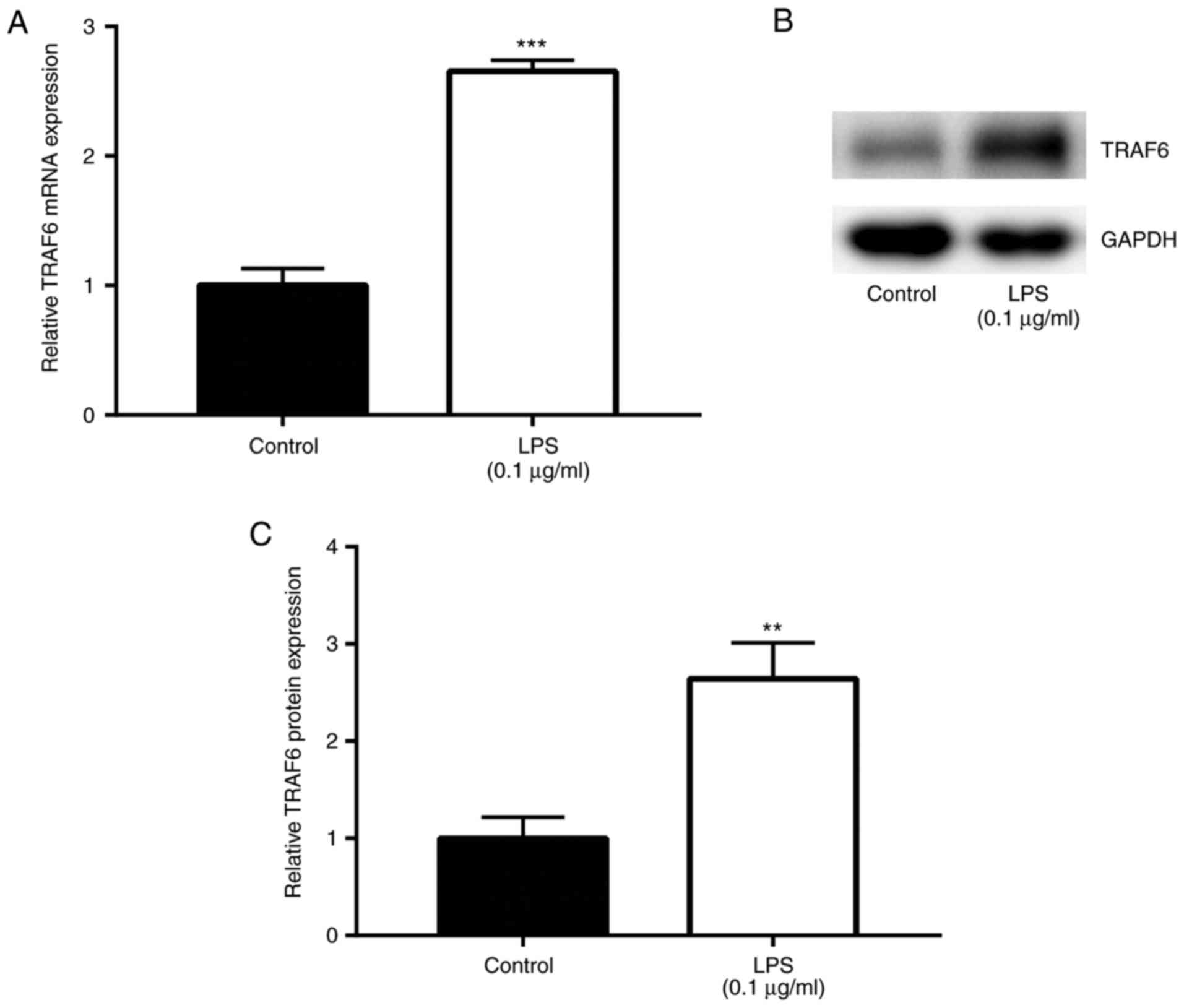
LPS induces elevation of TRAF6 in
SCAP
Following stimulation of Ana-1 macrophages with LPS
(0.1 µg/ml) and incubation at 37°C for 24 h to establish an
SCAP model (16), the effects of
LPS on TRAF6 expression were explored by RT-qPCR and western blot
analyses. The results of RT-qPCR revealed that TRAF6 mRNA level was
significantly upregulated by LPS induction (0.1 µg/ml) when
compared with that in the control group (P<0.01; Fig. 2A). In addition, the western blot
analysis results demonstrated that, compared with the control
group, TRAF6 protein level was markedly higher following exposure
to LPS (0.1 µg/ml; P<0.01; Fig. 2B and C). These data suggested the
promoting role of TRAF6 in the in vitro model of SCAP.
Subsequently, the current study aimed to identify the miRNAs that
target the 3′UTR of TRAF6 and investigate the effect on TRAF6
expression.
TRAF6 is targeted by miR-124-3p in Ana-1
macrophages
Using the online tool TargetScan version 7.1
(http://www.targetscan.org/vert_71/),
miR-124-3p was predicted to target the position 42-48 of TRAF6
3′UTR with the context ++ score percentile as high as 95 (Fig. 3A). Next, using a dual-luciferase
reporter assay, significantly decreased luciferase activity was
detected in the miR-124-3p mimic group as compared with that in the
miR-NC mimic group in cells transfected with TRAF6 WT P<0.01;
Fig. 3B . However, there was no
significant difference in luciferase activity between the
miR-124-3p mimic and miR-NC mimic groups in cells transfected with
TRAF6 Mut (P>0.05; Fig. 3B),
indicating that miR-124-3p targeted TRAF6 in the established in
vitro model of SCAP.
LPS induces reduction of miR-124-3p
expression in SCAP cell model
Following stimulation of Ana-1 macrophages with LPS
(0.1 µg/ml and incubation at 37°C for 24 h, the effects of
LPS on miR-124-3p expression were explored. The results of RT-qPCR
demonstrated that, compared with the control group, there was a
significantly lower miR-124-3p expression subsequent to LPS (0.1
µg/ml) stimulation in Ana-1 macrophages (P<0.01; Fig. 4A). This suggested the suppressing
role of miR-124-3p in the SCAP cell model. Furthermore, the effects
of miR-124-3p mimic and miR-NC mimic on the expression of
miR-124-3p were assessed in LPS-treated Ana-1 macrophages. It was
observed that, compared with cells in the miR-NC mimic group, the
administration of miR-124-3p mimic significantly upregulated the
expression of miR-124-3p (P<0.01; Fig. 4B).
miR-124-3p inhibits the expression of
TRAF6 in the SCAP cell model
Next, the effects of miR-124-3p mimic and miR-NC
mimic on the expression of TRAF6 in LPS-treated Ana-1 macrophages
were assessed. The results revealed that, compared with cells in
the miR-NC mimic group, administration of miR-124-3p mimic
significantly downregulated the mRNA level of TRAF6 (P<0.001;
Fig. 5A). In addition, the
effects of miR-124-3p mimic on TRAF6 protein level were detected by
western blot assay, and the protein level exhibited the similar
change pattern as mRNA level (P<0.01; Fig. 5B and C). Taken together, the
results indicated that miR-124-3p inhibited the expression of TRAF6
in the SCAP cell model, suggesting the significant roles of
miR-124-3p and TRAF6 in SCAP.
miR-124-3p attenuates SCAP
To further investigate the effects of miR-124-3p in
SCAP, RT-qPCR and ELISA were performed to determine the changes in
the expression levels of inflammatory cytokines. Ana-1 cells were
randomly divided into four groups, including the control, LPS, LPS
+ miR-NC mimic and LPS + miR-124-3p mimic groups. Initially,
RT-qPCR was used to determine the mRNA levels of TNF-α and IL-1β
produced by Ana-1 cells. It was observed that, compared with cells
in the control group, there were significantly higher mRNA levels
of TNF-α (4-fold) and IL-1β (5-fold) in the LPS group (P<0.01),
while pre-treatment with miR-124-3p mimic significantly reduced the
LPS-induced upregulation of TNF-α (2.5-fold) and IL-1β (3-fold)
mRNA levels (P<0.01; Fig. 6).
By contrast, miR-NC mimic exhibited no significant effect on the
LPS-induced changes in the cytokine levels (P>0.05).
ELISA was subsequently performed for the detection
of the protein levels of TNF-α and IL-1β released from Ana-1 cells.
The results indicated that, compared with cells in the control
group, significantly higher protein levels of TNF-α (0.8±0.1
µg/ml) and IL-1β (2.5±0.2 µg/ml) were observed in the
LPS-induced group (P<0.01), whereas pre-treatment with
miR-124-3p mimic significantly reduced the LPS-induced upregulation
of the protein levels of TNF-α (0.4±0.2 µg/ml) and IL-1β
(1.9±0.3 µg/ml; P<0.01; Table I). miR-NC mimic, however, had no
significant influence on these LPS-induced changes (P>0.05).
Taken together, these results suggested that miR-124-3p attenuated
SCAP, which is evidenced by the reduction of LPS-induced cytokine
release from Ana-1 cells following the administration of miR-124-3p
mimics. However, the molecules that are responsible for the
aforementioned changes need to be further investigated.
 | Table IEffect of LPS treatment and
miR-124-3p overexpres-sion on TNF-α and IL-1β concentration
(µg/ml) in Ana-1 cells. |
Table I
Effect of LPS treatment and
miR-124-3p overexpres-sion on TNF-α and IL-1β concentration
(µg/ml) in Ana-1 cells.
| Group | TNF-α | IL-1β |
|---|
| Control | 0.32±0.08 | 1.12±0.24 |
| LPS (0.1
µg/ml) | 0.88±0.12a | 2.55±0.29a |
| LPS (0.1
µg/ml) + miR-NC mimic | 0.84±0.13b | 2.46±0.27b |
| LPS (0.1
µg/ml) + miR-124-3p mimic | 0.43±0.09b | 1.92±0.13b |
miR-124-3p attenuates SCAP by inhibiting
LPS-induced p38 MAPK phosphorylation and NF-κB activation
Studies have indicated that NF-κB activation and
MAPK phosphorylation, particularly p38 MAPK, are prerequisites for
the production of inflammatory cytokines in stimulated macrophages
18,1 . To investigate the effects of miR-124-3p in LPS-induced
NF-κB activation and p38 MAPK phosphorylation, Ana-1 cells were
randomly divided into four groups, including the control, LPS, LPS
+ miR-NC mimic and LPS + miR-124-3p mimic groups. The effects of
miR-124-3p on LPS-induced p38 MAPK phosphorylation and NF-κB
activation were then examined in Ana-1 macrophages of the SCAP
model in vitro. As presented in Fig. 7A and B, compared with cells in the
control group, treatment of Ana-1 macrophages with LPS
significantly increased p-p38 MAPK protein expression and NF-κB
activity (P<0.01), which were markedly attenuated upon
pretreatment with miR-124-3p mimic (P<0.01). miR-NC mimic
exhibited no significant effect on the LPS-induced changes
(P>0.05). These results suggested that miR-124-3p suppressed
SCAP by inhibiting LPS-induced activation of p38 MAPK and the NF-κB
signaling pathway in macrophages.
Discussion
TRAF6 has been reported to be involved in the
process of inflammation during lupus nephritis , regulation of
inflammatory cytokines of bovine mammary epithelial cells (10) and ischemia/reperfusion injury
(11). In addition, recent
studies have reported that miRNAs serve important roles in
regulating genes that are correlated with the immune system
(20), including macrophages,
microglia, dendritic cells and T cells (21). For instance, miR-146 negatively
regulated MyD88-NF-κB following bacterial infection by targeting
IRAK-1 and TRAF6 in THP-1 macrophage cells (22), while miRNA-200a-3p functioned in
severe pneumonia by targeting SOCS6 (23). Furthermore, miR-146a-5p is a
negative regulator of TRAF6, negatively limiting the immune
response (24). Recently, miR-124
was also reported to regulate TRAF6 in osteosarcoma (25) and microglial immunosuppression
(26). To the best of our
knowledge, the present study revealed for the first time that
miR-124-3p targeted the 3′UTR of TRAF6 and negatively regulated
TRAF6 in an in vitro SCAP model in Ana-1 cells, indicating
the significant roles of miR-124-3p and TRAF6 in SCAP. However, the
molecular mechanisms by which LPS mediates the activation of immune
cells are not completely understood. Therefore, the present study
subsequently investigated the molecules that may respond to LPS
induction and may be regulated by miR-124-3p.
It has previously been reported that LPS treatment
resulted in an evident elevated expression of proinflammatory
cytokines, such as TNF-α in mice (27). As the major effector cells of the
immune-associated response, activated macrophages produce a wide
spectrum of inflammatory cytokines, including TNF-α and IL-1β, to
augment the inflammatory response 28 . TNF-α is considered as an
early cytokine, which is associated with the early stage of
inflammatory response and serves a crucial role in the
establishment of inflammatory response 2 , while IL-1β is
considered as a late cytokine, which is associated with the late
stage of inflammatory response and serves a crucial role in the
enhancement of inflammatory response (30). Consequently, TNF-α and IL-1β were
investigated in the present SCAP in vitro model.
NF-κB serves an important role in the expression of
LPS-induced proinflammatory cytokines 2 . Furthermore, LPS
functions by activating NF-κB and p38 MAPK signaling pathways in
mouse macrophages (31). A
previous study revealed that treatment of monocytes with LPS led to
a significant increase in TNF-α and IL-1β levels (22). Consistent with these previous
findings, the present study demonstrated LPS treatment resulted in
increased levels of inflammatory cytokines TNF-α and IL-1β, as well
as enhanced NF-κB activity and phosphorylation of p38 MAPK. These
LPS-induced increases were attenuated by miR-124-3p overexpression,
suggesting the protective role of this miRNA in SCAP by attenuating
inflammation.
In conclusion, the present study demonstrated that
LPS increased levels of TNF-α, IL-1β, enhanced NF-κB activity and
p-p38, which were attenuated by miR-124-3p overexpression,
suggesting that miR-124-3p may serve as a therapeutic target for
SCAP.
Funding
No funding was received.
Availability of data and materials
The materials and data are available on specific
request.
Authors’ contributions
WG conducted all the experiments in the present
study, while HY conceived the project and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Acknowledgments
The authors would like to thank The Second Hospital
of Shandong University for their support in the present study.
References
|
1
|
Organization WH: The top 10 causes of
death. July. 2013, Available at: http://www.Whoint/mediacentre/factsheets/fs310/enurisimpleWhoint/mediacentre/factsheets/fs310/en
Accessed July 2014.
|
|
2
|
Wunderink RG and Waterer GW: Clinical
practice. Community-acquired monocytespneumonia. N Engl J Med.
370:543–551. 2014. View Article : Google Scholar
|
|
3
|
Said MA, Johnson HL, Nonyane BA,
Deloria-Knoll M, O’Brien KL; AGEDD Adult Pneumococcal Burden Study
Team; Andreo F, Beovic B, Blanco S, Boersma WG, et al: Estimating
the burden of pneumococcal pneumonia among adults: A systematic
review and meta-analysis of diagnostic techniques. PLoS One.
8:e602732013. View Article : Google Scholar :
|
|
4
|
Rakha MA, Abdelmoneim AN, Farhoud S,
Pièche S, Cousens S, Daelmans B and Bahl R: Does implementation of
the IMCI strategy have an impact on child mortality? A
retrospective analysis of routine data from Egypt. BMJ Open.
3:e0018522013. View Article : Google Scholar
|
|
5
|
Floyd J, Wu L, Hay Burgess D, Izadnegahdar
R, Mukanga D and Ghani AC: Evaluating the impact of pulse oximetry
on childhood pneumonia mortality in resource-poor settings. Nature.
528:S53–S59. 2015. View Article : Google Scholar
|
|
6
|
Beutler B and Rietschel ET: Innate immune
sensing and its roots: The story of endotoxin. Nat Rev Immunol.
3:169–176. 2003. View
Article : Google Scholar
|
|
7
|
Janssens S and Beyaert R: A universal role
for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci.
27:474–482. 2002. View Article : Google Scholar
|
|
8
|
Li S, Strelow A, Fontana EJ and Wesche H:
IRAK-4: A novel member of the IRAK family with the properties of an
IRAK-kinase. Proc Natl Acad Sci USA. 99:5567–5572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng CZ, Shu YB, Luo YL and Luo J: The
role of miR-146a in modulating TRAF6-induced inflammation during
lupus nephritis. Eur Rev Med Pharmacol Sci. 21:1041–1048. 2017.
|
|
10
|
Wang XP, Luoreng ZM, Zan LS, Li F and Li
N: Bovine miR-146a regulates inflammatory cytokines of bovine
mammary epithelial cells via targeting the TRAF6 gene. J Dairy Sci.
100:7648–7658. 2017. View Article : Google Scholar
|
|
11
|
He X, Zheng Y, Liu S, Shi S, Liu Y, He Y,
Zhang C and Zhou X: MiR-146a protects small intestine against
ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB
pathway. J Cell Physiol. 233:2476–2488. 2018. View Article : Google Scholar
|
|
12
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar
|
|
13
|
Wong KY, Huang X and Chim CS: DNA
methylation of microRNA genes in multiple myeloma. Carcinogenesis.
33:1629–1638. 2012. View Article : Google Scholar
|
|
14
|
Christopher AF, Kaur RP, Kaur G, Kaur A,
Gupta V and Bansal P: MicroRNA therapeutics: Discovering novel
targets and developing specific therapy. Perspect Clin Res.
7:68–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abd-El-Fattah AA, Sadik NA, Shaker OG and
Aboulftouh ML: Differential microRNAs expression in serum of
patients with lung cancer, pulmonary tuberculosis, and pneumonia.
Cell Biochem Biophys. 67:875–884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Luo G, Yuan J, Wang Y, Yang X,
Wang X, Li G, Liu Z and Zhong N: Vitamin C mitigates oxidative
stress and tumor necrosis factor-alpha in severe community-acquired
pneumonia and LPS-induced macrophages. Mediators Inflamm.
2014:4267402014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Li Y, Reddy MA, Miao F, Shanmugam N, Yee
JK and Hawkins D: Role of the histone H3 lysine 4
methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent
inflammatory genes. J Biol Chem. 283:26771–26781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dean JL, Brook M, Clark AR and Saklatvala
J: p38 mitogen-activated protein kinase regulates cyclooxygenase-2
mRNA stability and transcription in lipopolysaccharidetreated human
monocytes. J Biol Chem. 274:264–269. 1999. View Article : Google Scholar
|
|
20
|
Nahid MA, Satoh M and Chan EK: MicroRNA in
TLR signaling and endotoxin tolerance. Cell Mol Immunol. 8:388–403.
2011. View Article : Google Scholar
|
|
21
|
Quinn SR and O’Neill LA: A trio of
microRNAs that control Toll-like receptor signalling. Int Immunol.
23:421–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar
|
|
23
|
Hoffmann J, Machado D, Terrier O, Pouzol
S, Messaoudi M, Basualdo W, Espínola EE, Guillen RM, Rosa-Calatrava
M, Picot V, et al: Viral and bacterial co-infection in severe
pneumonia triggers innate immune responses and specifically
enhances IP-10: A translational study. Sci Rep. 6:385322016.
View Article : Google Scholar :
|
|
24
|
Griss K, Bertrams W, Sittka-Stark A,
Seidel K, Stielow C, Hippenstiel S, Suttorp N, Eberhardt M, Wilhelm
J, Vera J and Schmeck B: MicroRNAs constitute a negative feedback
loop in streptococcus pneumoniae-induced macrophage activation. J
Infect Dis. 214:288–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng Q, Zhang W, Xu X, Li J, Mu H, Liu X,
Qin L, Zhu X and Zheng M: The effects of TRAF6 on proliferation,
apoptosis and invasion in osteosarcoma are regulated by miR-124.
Int J Mol Med. 41:2968–2976. 2018.PubMed/NCBI
|
|
26
|
Qiu S, Feng Y, LeSage G, Zhang Y, Stuart
C, He L, Li Y, Caudle Y, Peng Y and Yin D: Chronic morphine-induced
microRNA-124 promotes microglial immunosuppresison by modulating
P65 and TRAF6. J Immunol. 194:1021–1030. 2015. View Article : Google Scholar
|
|
27
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar
|
|
28
|
Dinarello CA: A clinical perspective of
IL-1β as the gatekeeper of inflammation. Eur J Immunol.
41:1203–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi AK, Yoon JG, Hong SC, Redford TW and
Krieg AM: Lipopolysaccharide and CpG DNA synergize for tumor
necrosis factor-alpha production through activation of NF-kappa B.
Int Immunol. 13:1391–1404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaushansky K, Broudy VC, Harlan JM and
Adamson JW: Tumor necrosis factor-alpha and tumor necrosis factor-β
(lympho-toxin) stimulate the production of granulocyte-macrophage
colony-stimulating factor, macrophage colony-stimulating factor,
and IL-1 in vivo. J Immunol. 141:3410–3415. 1988.PubMed/NCBI
|
|
31
|
An H, Xu H, Yu Y, Zhang M, Qi R, Yan X,
Liu S, Wang W, Guo Z, Qin Z and Cao X: Up-regulation of TLR9 gene
expression by LPS in mouse macrophages via activation of NF-kappa
B, ERK and p38 MAPK signal pathways. Immunol Lett. 81:165–169.
2002. View Article : Google Scholar
|