Introduction
Dental implants have become the optimal treatment
for dentition defects or missing teeth. Osseointegration is a key
factor associated with implants and involves three stages:
Peri-implant bone formation, reconstruction, and maturation. There
are a series of events involved in the formation of bone, including
proliferation, migration, and the recruitment of osteoblasts
surrounding the implant surface. Previous studies have demonstrated
that the migration, recruitment and adsorption efficiency of
osteoblasts on the implant surface directly affects the occurrence,
development and final effect of osseo-integration (1). The process of osseointegration is
affected by numerous factors in patients with systemic diseases,
including diabetes, osteoporosis and cancer, and the success rates
of implants in these patients are markedly reduced. A systematic
review reported that, in the first year of loading, there is an
increasing trend in the failure of dental implant teeth in patients
with diabetes (2). Therefore, how
to improve the osseointegration of implants in patients with
certain diseases remains a focus of clinical investigations.
Various traditional Chinese medicine materials have
been shown to promote osteoblast proliferation and migration and
bone formation using in vivo and in vitro experiments
(3). Astragali Radix is the root
of Astragalus membranaceus (Fisch.) Bge.var.
Mongholicus (Bge.) Hsiao or EE Astragalus membranaceus
(Fisch.) Bge. Astragali Radix has been shown to exhibit a
range of pharmacological effects, including anticancer,
anti-inflammatory, antioxidant and anti-osteoporotic properties
(4-10). Astragaloside IV (AST-IV,
3-O-b-D-xylopyr-anosyl-6-O-b-D-glucopyranosylcyl-cloastragenol) is
the principal effective compound isolated from Astragali Radix. A
previous study reported that pretreatment with astragaloside IV
significantly reverses the loss of neuronal cell viability and
prevents MPP+-guided SH-SY5Y cell death, and that
astragaloside IV has a neuroprotective effect (11). Astragaloside IV can enhance the
proliferation of bone marrow mesenchymal stem cells in
vitro, so as to the neural stem cells (12,13) and promote angiogenesis and the
reproduction of human umbilical vein endothelial cells (14). The combined application of
astragaloside and tanshinone IIA promotes the migration of
mesenchymal stem cells in cardiovascular disease therapy (15). Furthermore, a previous study
revealed the effects of astragaloside IV on osteoclasts,
demonstrating that astragaloside IV caused the inhibition of
osteoclastogenesis and attenuation of osteolysis (16). However, the regulatory action of
astragaloside IV on osteoblasts and the associated mechanisms
remain, at present, to be fully elucidated.
A previous study demonstrated that the hedgehog
signaling pathway regulates the differentiation of skeletal muscle
cells in the process of skeletal repair and regeneration (17). In addition, the hedgehog signaling
pathway is associated with bone formation, which can promote the
proliferation and differentiation of chondrocytes and osteoblasts
(18-21).
The hedgehog signaling pathway is conserved in
evolution, and is key in embryonic development and homeostasis
regulation, growth and cell migration in adult tissues (22). The hedgehog family contains three
protein ligands, including sonic hedgehog (Shh), India hedgehog
(Ihh) and desert hedgehog (Dhh). These signal via a mechanism
involving two transmembrane proteins, namely smoothened (Smo) and
patched (Ptc). When a hedgehog protein binds to Ptc, Smo is
rendered constitutively active and activates an intracellular
signaling cascade, leading to the upregulated transcription of the
downstream nuclear transcription factor GLI family zinc finger
(Gli)1 and Gli2 (23). Aberrant
activation of the hedgehog signaling may lead to oncogenesis in
various tissues, including basal cell carcinoma, medulloblastoma,
pancreatic, colon and gastric cancer, and glioblastoma (24-28). However, the role of the hedgehog
signaling pathway in the proliferation and migration of
astragaloside IV in osteoblasts remains to be fully elucidated.
In the present study, human MG-63 and U-2OS
osteoblast-like cells were treated with astragaloside IV, following
which cell functions and the activation of hedgehog signaling were
evaluated. Furthermore, the Smo inhibitor, cyclopamine, was used to
inhibit the hedgehog signaling pathway, following which the gene
expression levels of SHH and GLI1 and cell functions
were evaluated.
Materials and methods
Reagents
Fetal bovine serum (FBS), modified Eagle's medium
(MEM) and McCoy's 5A medium were obtained from HyClone
Laboratories, GE Healthcare Life Sciences (Logan, UT, USA).
Penicillin/streptomycin solution, phosphate-buffered saline (PBS),
0.05% Trypsin-EDTA and dimethyl sulfoxide (DMSO) were obtained from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Astragaloside IV and cyclopamine (purity >99%, HPLC) were
obtained from Sigma-Aldrich, Merck Millipore (Darmstadt, Germany).
The chemical structure and molecular weight of astragaloside IV is
shown in Fig. 1. The
astragaloside was dissolved in DMSO, and the concentration of the
original solution was 25 µg/ml. The stock solution was
diluted according to the treatment group when used; the DMSO did
not exceed 0.5% (V/V).
 | Figure 1AST-IV enhances human osteoblast-like
cell growth and proliferation. (A) Structure of AST-IV (structural
formula, C41H68O14; molecular weight, 784.97). (B) MG-63 and (C)
U-2OS cells were treated with DMSO as controls or various
concentrations of AST-IV for 48 h. Cell viability was determined
with a Cell Counting Kit-8 assay. Following treatment of (D) MG-63
cells with AST-IV (1×10−2 µg/ml) and of (E) U-2OS
cells with AST-IV (1×10−3 µg/ml) or DMSO as a
control for 24, 48, 72 and 96 h, cell viability was determined.
*P<0.05, AST-IV group compared with control group and
#P<0.05, compared with the 24 h group, determined by
one-way analysis of variance. AST-IV, astragaloside IV; DMSO,
dimethyl sulfoxide. |
Cell culture
The MG-63 and U-2OS human osteosarcoma cell lines
were obtained from American Type Culture Collection (Manassas, VA,
USA). The MG-63 and U-2OS cells were cultured in MEM and McCoy's 5A
medium supplemented with 10% FBS, respectively. The two cell lines
were incubated at 37°C in a 5% CO2 humidified
atmosphere.
Cell Counting Kit-8 (CCK-8) assay
Cell growth was evaluated using a CCK-8 assay
(Dojindo Molecular Technologies, Inc., Shanghai, China). The cells
were plated in 96-well plates, 100 µl of medium was added to
each well, which contained 10,000 cells. The cells were cultured
overnight and were then treated with gradient dilutions of
astragaloside IV (1×10−5, 1×10−4,
1×10−3, 1×10−2, 1×10−1, 1, and 10
µg/ml concentrations). After 48 h, 100 µl CCK8
reagents were mixed into each well. Following an additional
incubation at 37°C for 4 h, the absorbance value of each well at
450 nm was measured using a VERS Amax microplate reader. The number
of living cells measured by the absorbance at the wavelength 450 nm
was measured with a monochromator microplate. Cell
viability=[(As-Ab)]/[(Ac-Ab)] ×100%; where As is the absorbance of
the astragaloside IV group, Ac is the absorbance of the control
group, and Ab is the absorbance of blank group.
Flow cytometry
The cells were seeded in 6-well plates with 2 ml of
complete growth medium/well, containing the appropriate number of
cells to yield 60% confluence 12 h following plating. After 12 h,
the complete growth medium was replaced with serum-free medium for
24 h. After 24 h, the cells were cultured in complete growth medium
and treated with astragaloside IV or astragaloside IV combined with
cyclopamine for 48 h. BrdU (1 mM/ml) was then added for 4 h at
37°C, and the cells were harvested. The cells were stained with
anti-BrdU and 7-AAD using the BD Pharmingen™ BrdU Flow kit (BD
Biosciences, San Diego, CA, USA) according to the manufacturer's
protocol. The cell cycle distribution was determined by flow
cytometry (FACSAria™ II, BD Biosciences).
Wound-healing assay
Cell migration was measured using a wound-healing
assay. The cells were seeded in 6-well plates and, when the cells
had formed a confluent monolayer, a p200 micro-pipette tip was used
to create a scratch in a straight line. Culture medium was used to
clean the cells once and they were incubated in 0.1% FBS culture
medium. Images of the cells at multiple points along the scratch
were captured every 24 h using an inverted microscope. Images were
captured at ×100 magnification.
Transwell cell migration assay
The cells were harvested following treatment with
astragaloside IV for 48 h. The cells were then suspended in
serum-free medium and placed in the upper chamber of the Transwell
array (8-µm pore size; Merck Millipore). Medium containing
20% FBS was added to the lower chamber, following incubation at
37°C for 48 h, a cotton swab was used to carefully wipe away the
cells in the upper chamber. The migrated cells that had adhered to
the membrane of the lower chamber, were fixed in 4% formaldehyde
solution for 10 min, and stained with 0.1% crystal violet for 15
min. The numbers of migrating cells were then counted with an
inverted microscope. The values were averaged and images were
captured.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The extraction of total cell RNA was performed using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
RNA integrity was analyzed on a 1.0% agarose gel and detection of
RNA quantity was performed using a NanoDrop 2000C Spectrophotometer
(Thermo Fisher Scientific, Inc.). Subsequently, 1 µg of
total RNA was reverse transcribed with a PrimeScript™ RT reagent
kit (Takara Biotechnology Co., Ltd., Dalian, China) for cDNA
synthesis and genomic DNA removal, according to the manufacturer's
protocol. The qPCR procedure was performed on the basis of the
instructions of the SYBR premix Ex Taq™ II kit (Tli RNaseH Plus;
Takara Biotechnology Co., Ltd.) and performed in triplicate using a
Takara real-time PCR system (Takara Biotechnology Co., Ltd.). The
thermocycling conditions were as follows: 30 sec at 95°C, followed
by 40 cycles at 95°C for 5 sec and 60°C for 30 sec. Gene-specific
primers were designed using the online primer design tool
Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). The
lengths of amplifications were between 100 and 250 bp. GAPDH served
as an internal control. The specificity of amplification was
evaluated by the analysis of the dissociation curve, and the
relative abundance of genes was determined using the
2−ΔΔCq method (29).
The primer sequences were as follows: SHH forward, 5'-GTG GCC GAG
AAG ACC CTA-3' and reverse, 5'-CAA AGC GTT CAA CTT GTC CTT A-3';
GLI1 forward, 5'-CGG GCA CCA TCC ATT TCT AC-3' and reverse, 5'-GGC
ACA GTC AGT CTG CTT TCC T-3'; and GAPDH forward, 5'-GCA CCG TCA AGG
CTG AGA AC-3' and reverse, 5'-TGG TGA AGA CGC CAG TGG A-3'.
Western blot analysis
Total protein was isolated with RIPA lysis buffer
(Pulilai Gene Technology Co., Ltd., Beijing, China). The cells were
lysed in RIPA buffer on ice for 30 min, and supernatant was then
collected through centrifugation at 12,000 × g for 5 min at 4°C.
Protein concentrations were determined with a BCA protein assay kit
(Pulilai Gene Technology Co., Ltd.). Total proteins (40 µg
protein/lane) were separated by 12% SDS-PAGE and transferred onto a
PVDF membrane. The membrane was blocked with 5% (W/V) non-fat milk
at room temperature for 1 h, incubated with primary antibodies
against Shh (1:1,000; cat. no. 2207), Gli1 (1:1,000; cat. no. 3538)
and β-actin (1:1,000; cat. no. 4970) at 4°C overnight, and then
incubated with anti-rabbit IgG horseradish peroxidase-conjugated
secondary antibody (1:2,000; cat. no. 7074) (all from Cell
Signaling Technology, Inc., Danvers, MA, USA) at room temperature
for 1 h. The bands were visualized with an ECL reagent (Thermo
Fisher Scientific, Inc.). β-actin was used as the loading
control.
Statistical analysis
All results are expressed as the mean ± standard
deviation of three independent experiments performed in triplicate.
Statistical analyses were performed using the SPSS 17.0 software
package (SPSS, Inc., Chicago, IL, USA) and one-way analysis of
variance with Dunnett's test was used to compare the means between
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Astragaloside IV promotes the
proliferation of human osteoblast-like cells
To investigate the effects of astragaloside IV on
osteogenesis in human osteoblast-like cells, cell growth and
proliferation were analyzed. The structure of astragaloside IV was
drawn using Chemdraw 14.0 (Fig.
1A). A CCK-8 assay was performed to detect cell growth. The
MG-63 and U-2OS cells were treated with increasing concentrations
of astragaloside IV (1×10−5, 1×10−4,
1×10−3, 1×10−2, 1×10−1, 1, and 10
µg/ml) for 48 h. The results showed that astragaloside IV at
the indicated concentrations (MG-63, 1×10−2
µg/ml; U-2OS, 1×10−3 µg/ml) significantly
increased the growth of the human osteoblast-like cells (P<0.05,
Fig. 1B and C). As a further
demonstration of the association between time and the concentration
of astragaloside IV following 24, 48, 72 and 96 h of treatment, the
MG-63 and U-2OS cells treated with astragaloside IV for 48 h
(MG-63, 1×10−2 µg/ml; U-2OS, 1×10−3
µg/ml) had significantly decreased cell proliferation rate
was observed (P<0.05, Fig. 1D and
E). Taken together, the results indicated that astragaloside IV
increased the growth and proliferation of the human osteoblast-like
cells.
Astragaloside IV stimulates the migration
of human osteoblast-like cells
The migratory capacity of osteoblasts is key in
osteogenesis. To evaluate the functions of astragaloside IV in
MG-63 and U-2OS cell migration efficiency, a wound-healing assay
and Transwell cell migration assay were performed. Astragaloside IV
was observed to markedly induce the wound-healing of MG-63 and
U-2OS cells following pre-treatment for 48 h (P<0.05, Fig. 2A and B). Furthermore, the results
of the Transwell cell migration analysis demonstrated that the cell
migration efficiencies of the MG-63 and U-2OS cells were enhanced
following astragaloside IV treatment for 24 or 48 h, and the
differences were significant when compared with the control group
(P<0.05, Fig. 2C and D). These
results indicated that astragaloside IV is critical for stimulating
the migration of human osteoblast-like cells.
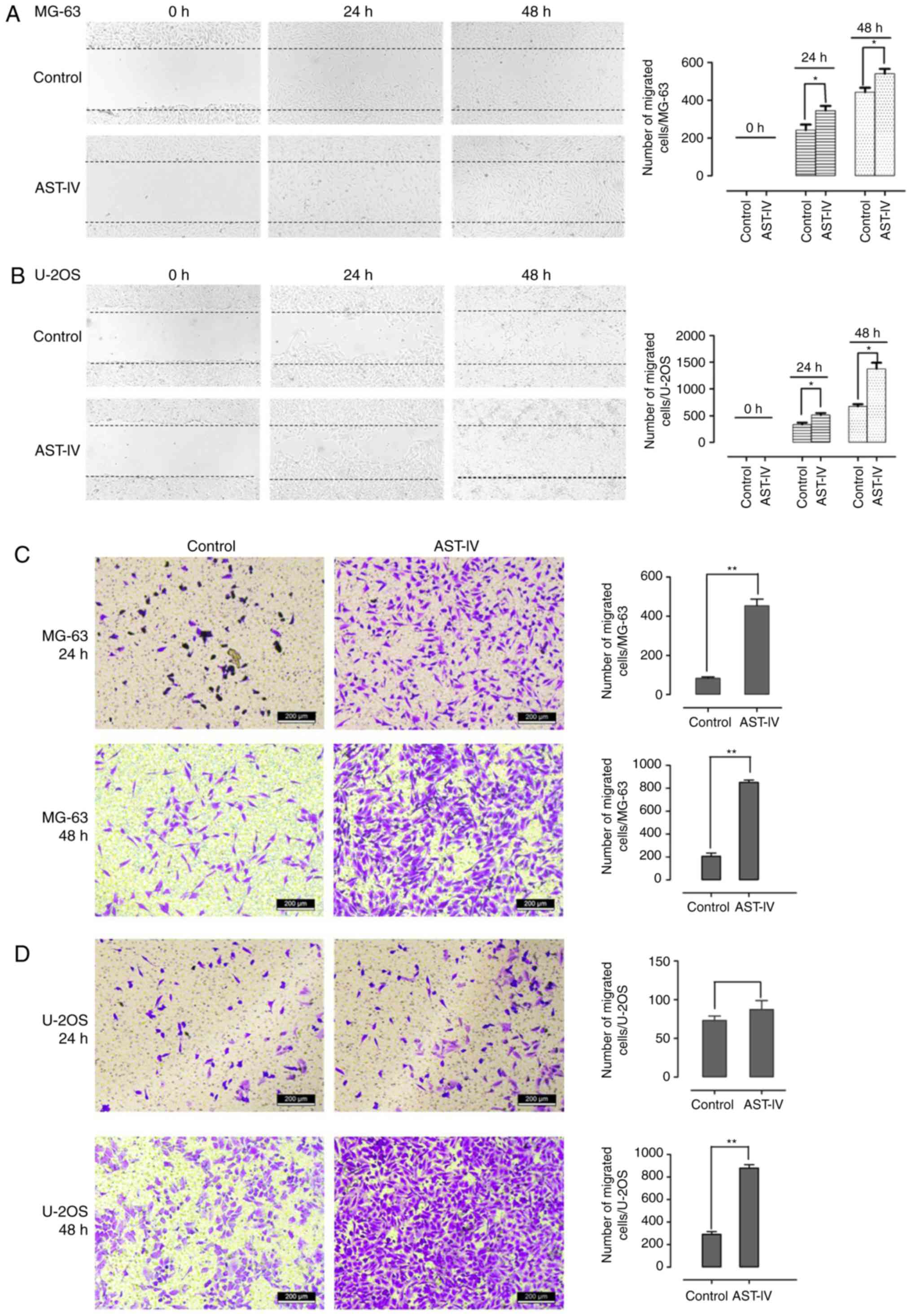 | Figure 2AST-IV increases the migration of
MG-63 and U-2OS cells in vitro. When the cells had been
treated with dimethyl sulfoxide as a control or AST-IV (MG-63,
1×10−2 µg/ml; U-2OS, 1×10−3
µg/ml) for 48 h, a wound-healing assay for the (A) MG-63 and
(B) U-2OS cells, and a Transwell cell migration assay for the (C)
MG-63 and (D) U-2OS cells, were performed to evaluate the effects
of AST-IV on the metastasis of the cell lines, shown by
representative images. The number of migrated cells per high-power
field was determined, as shown in the graphs. Data are presented as
the mean + standard deviation (n=3), *P<0.05 and
**P<0.01, determined by one-way analysis of variance. AST-IV,
astragaloside IV. |
Astragaloside IV enhances the hedgehog
signaling pathway
Several studies have suggested that the Shh-induced
hedgehog pathway is crucial for regulating the proliferation and
migration of tumor cells and promoting the proliferation,
differentiation and maturation of osteoblasts. The present study
investigated whether astragaloside IV affects the hedgehog
signaling pathway. To investigate the role of the hedgehog
signaling pathway in astragaloside IV-enhanced cell proliferation
and migration, the human osteoblast-like cells were treated with
cyclopamine, which is an inhibitor of Smo, to inhibit the hedgehog
pathway (30). The MG-63 and
U-2OS cells were treated with increasing concentrations of
cyclopa-mine (2.5, 5.0, 7.5, and 10 µmol/l) for 48 h. The
cell viability analysis using the CCK-8 assay demonstrated that
cyclopa-mine significantly suppressed the growth of the MG-63 and
U-2OS cells in a dose-dependent manner (Fig. 3A and B).
 | Figure 3AST-IV regulates the mRNA and protein
levels of hedgehog signaling pathway components in MG-63 and U-2OS
cells. (A) MG-63 and (B) U-2OS cells were treated with CP (2.5,
5.0, 7.5, and 10.0 µg/ml) for 48 h, and cell growth was
assessed using a Cell Counting Kit-8 array. Data are presented as
the mean + standard deviation of three independent experiments. (C)
Components detected in the hedgehog signaling pathway. Expression
levels of GLI1 and SHH genes were detected in MG-63
and U-2OS cells following treatment with dimethyl sulfoxide as a
control or AST-IV (MG-63, 1×10−2 µg/ml; U-2OS,
1×10−3 µg/ml) or AST-IV (MG-63, 1×10−2
µg/ml; U-2OS. 1×10−3 µg/ml) combined with
CP (2.5 µmol/l) for 48 h. mRNA levels in the (D) MG-63 and
(E) U-2OS cells were analyzed by reverse transcription-quantitative
polymerase chain reaction analysis and normalized to GAPDH. Protein
expression levels in (F) MG-63 and (G) U-2OS were analyzed by
western blot analysis. Actin was used as an internal control.
*P<0.05 and **P<0.01. Experiments were
performed in triplicate, determined by one-way analysis of
variance. AST-IV, astragaloside IV; CP, cyclopamine; GLI1, GLI
family zinc finger 1; SHH, sonic hedgehog. |
Ptch1 and Ptch2 are 12-pass transmembrane proteins
that function as Hh receptors. When the hedgehog signal Shh binds
to Ptch (Ptch1 and Ptch2), suppressor of fused (Sufu), as a
suppressor of fused kinase, positively regulates hedgehog signaling
and the active nuclear transcription factor Gli1 (Fig. 3C).
The present study aimed to detect the mechanism of
action of astragaloside IV combined with cyclopamine on components
of the hedgehog signaling pathway. The results of the RT-qPCR and
western blot analyses results demonstrated that the mRNA and
protein levels of SHH and GLI1 were significantly upregulated in
MG-63 and U-2OS cells treated with astragaloside IV for 48 h
(Fig. 3D and E). The mRNA and
protein expression levels of GLI1 and SHH were marginally elevated
in cells treated with astragaloside IV combined with cyclopamine,
however the increase was markedly reduced, compared with that in
MG-63 and U-2OS cells treated with astragaloside IV (Fig. 3F and G).
The results of the analysis of gene expression
levels of SHH and GLI1 in MG-63 and U-2OS cells
following treatment with astragaloside IV suggested that
astragaloside IV promoted activation of the hedgehog signaling
pathway.
Hedgehog signaling pathway inhibitor
eliminates astraga- loside IV-induced proliferation and migration
of the osteoblast-like cells
The present study then analyzed the effect of
astragaloside IV combined with cyclopamine on cell proliferation in
human osteoblast-like cells. In the MG-63 cells, the two drugs
acted together to inhibit cell proliferation, and the percentage of
cells in the S phase was reduced. In the U-2OS cells, there was no
effect on cell proliferation compared with the control (Fig. 4A and B). These results indicated
that the effect of astragaloside IV on osteoblast-like cell
proliferation was reduced by cyclopamine.
Furthermore, the Transwell cell migration assay was
used to assess the impact of cyclopamine on cell migration
efficiency. The results demonstrated that the cell migration
ability of MG-63 and U-2OS cells was promoted following treatment
with astragaloside IV combined with cyclopamine for 48 h
(P<0.05, Fig. 4C and D);
however, the cell migration efficiency was lower than that
resulting from the treatment of osteoblast-like cells with
astragaloside IV alone. Taken together, these findings suggested
that astragaloside IV-promoted cell proliferation and migration,
and that these effects were significantly inhibited by hedgehog
signaling pathway inhibition.
Discussion
In the process of bone remodeling, bone is
continuously broken down and reformed, which occurs through the
balance between osteoblasts and osteoclasts (31). Osseointegration comprises a
cascade of complex mechanisms. First, the drilling of an implant
cavity is a traumatic insult to bone and leads to distinct phases
of wound healing (32,33). Secondly, new bone is generated
from the borders of the drill hole (distance osteogenesis) or from
osteogenic cells on the surface of the implant (contact
osteogenesis). In distance osteogenesis, osteo-blasts migrate to
the surface of the implant cavity, whereas in contact osteogenesis,
osteogenic cells migrate directly onto the implant surface and
generate new bone (34).
Therefore, the proliferative ability and migratory behavior of
osteoblasts are key in osseointegration (35).
The optimization of the implant surface assists in
promoting osseointegration by promoting the migration, adhesion,
proliferation and differentiation of osteoblasts. Sandblasting,
etching, and hydrophilicity have been successfully used on the
implant surface. To further improve the speed and quality of
osseointegration, novel methods of surface modification, including
discrete crystal deposition, laser ablation, surface coating and
surface treatment with proteins, drugs or growth factors, are being
investigated (36). The present
study demonstrated that astragaloside IV can promote the
proliferation and migration of human osteoblast-like cells.
The effects of various doses of astragaloside IV
(1×10−5-10 µg/ml) for 48 h on human osteosarcoma
cells in vitro were examined in the present study. The
results indicated that astragaloside IV promoted MG-63 cell and
U-2OS cell proliferation and migration, respectively, at relatively
low concentrations (MG-63 cells, 1×10−2 µg/ml;
U-2OS cells, 1×10−3 µg/ml). Several previous
studies have reported that a high concentration of astragaloside IV
(10-100 µg/ml) can inhibit tumor cell growth, migration and
invasion in lung cancer (37),
breast cancer (8), hepatoma
(38), and glioma (39). In addition, the content of
astragaloside IV in Astragali Radix is ~0.04%, and the clinical
application dosage of Astragali Radix is ~20 g per day. Therefore,
there is ~8 µg of astragaloside IV in 20 g of Astragali
Radix. The results of the in vitro experiments in the
present study indicated that astragaloside IV promoted MG-63 cell
and U-2OS cell proliferation and migration, respectively, at
relatively low concentrations (MG-63 cells, 1×10−2
µg/ml; U-2OS cells, 1×10−3 µg/ml). This
result indicated that the dosage of astragaloside IV required in
promoting the proliferation and migration of osteoblast-like cells
is lower than the clinical application dosage of Astragali
Radix.
Previous studies have reported that numerous
cytokines are involved in the proliferation and migration of bone
cells, including bone morphogenetic protein, insulin like growth
factor, and wingless and int ligands. Furthermore, they have
demonstrated that high mobility group box 1 protein (HMGB1)
significantly promotes the migration of osteoblasts in vitro
and the Toll-like receptor (TLR)2/TLR4-dependent nuclear factor-κB
pathway is involved in HMGB1-induced osteoblast migration (40-43). In the present study the hedgehog
signaling pathway was found to be involved in the process of
astragaloside IV-enhanced cell proliferation and migration in MG-63
and U-2OS cells.
Shh is a 45-kDa signal protein that regulates the
proliferation, differentiation and morphology of numerous cell
types. Several studies have reported that the hedgehog signaling
pathway is important in the proliferation and differentiation of
osteoblasts, and is involved in fracture healing and bone repair
(44,45). Gli1 and Gli2 proteins are the main
transcription factors in hedgehog signaling. Shh can activate Gli1
and Gli2, and high protein expression levels of Gli1 and Gli2
indicate that the hedgehog signaling pathway is activated. The
activation of Gli1 and Gli2 can directly promote the expression of
a set of genes, including oncogenes and genes involved in cell
cycle, for example, Cyclin D, Cyclin E and Myc.
In the present study, the expression of key proteins
in the hedgehog signaling pathway in human osteoblast-like cells
were detected following treatment with astragaloside IV. The
results demonstrated that astragaloside IV caused a marked increase
in the mRNA and protein levels of GLI1 and SHH, culminating in the
observation that astragaloside IV activated hedgehog signaling.
To further investigate whether astragaloside IV
potentiated the osteogenesis of human osteoblast cells via the
hedgehog signaling pathway, the cells were treated with
cyclopamine. Cyclopamine is an inhibitor of the hedgehog signaling
pathway, is naturally produced and belongs to the group of
steroidal jerveratrum alkaloids. The results indicated that the
effect of astragaloside IV on cell proliferation and migration was
markedly reduced by cyclopamine. Following treatment with
astragaloside IV combined with cyclopamine in MG-63 and U-2OS
cells, the increase in the expression of genes involved in hedgehog
signaling was not statistically significant.
In conclusion, the findings of the present study
suggested that activation of the hedgehog signaling pathway by
astragaloside IV significantly enhanced human osteoblast-like cell
proliferation and migration, and that astragaloside IV may serve as
a growth factor to promote osseointegration. To the best of our
knowledge, the present study is the first to demonstrate the effect
of astragaloside IV on osteoblasts and that the hedgehog signaling
pathway was the direct target of astragaloside IV. These results
identify a therapeutic target for the promotion of bone formation
in implants and a rationale for the development of astragaloside IV
for use in clinical therapy.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371115) and the
Fundamental Research Funds for the Central Public Welfare research
Institutes (grant no. ZZ11-071).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and YH designed the study. JL and YC produced the
outline and reviewed the manuscript. YC and LHG analyzed all the
results. LHG performed the majority of the experiments and drafted
the manuscript. RTZ performed the statistical analysis and figure
editing. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Li JY, Zheng LW, Ma L, Kwong DL, Cheung LK
and Pow EH: Effect of fluoride-modified titanium surface on early
adhesion of irradiated osteoblasts. Biomed Res Int. 19752:2015.
|
|
2
|
Annibali S, Pranno N, Cristalli MP, La
Monaca G and Polimeni A: Survival analysis of implant in patients
with diabetes mellitus: A systematic review. Implant Dent.
25:663–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang ND, Han T, Huang BK, Rahman K, Jiang
YP, Xu HT, Qin LP, Xin HL, Zhang QY and Li YM: Traditional Chinese
medicine formulas for the treatment of osteoporosis: Implication
for antiosteoporotic drug discovery. J Ethnopharmacol. 189:61–80.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang WD, Zhang C, Liu RH, Li HL, Zhang
JT, Mao C, Moran S and Chen CL: Preclinical pharmacokinetics and
tissue distribution of a natural cardioprotective agent
astragaloside IV in rats and dogs. Life Sci. 79:808–815. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo Y, Qin Z, Hong Z, Zhang X, Ding D, Fu
JH, Zhang WD and Chen J: Astragaloside IV protects against ischemic
brain injury in a murine model of transient focal ischemia.
Neurosci Lett. 363:218–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang WJ, Hufnagl P, Binder BR and Wojta
J: Antiinflammatory activity of astragaloside IV is mediated by
inhibition of NF-kappaB activation and adhesion molecule
expression. Thromb Haemost. 90:904–914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Tang D, Zang W, Yin G, Dai J, Sun
YU, Yang Z, Hoffman RM and Guo X: Synergistic inhibitory effect of
traditional Chinese medicine astragaloside IV and curcumin on tumor
growth and angiogenesis in an orthotopic nudemouse model of human
hepatocellular carcinoma. Anticancer Res. 37:465–473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang K, Lu Q, Li Q, Ji Y, Chen W and Xue
X: Astragaloside IV inhibits breast cancer cell invasion by
suppressing Vav3 mediated Rac1/MAPK signaling. Int Immunopharmacol.
42:195–202. 2017. View Article : Google Scholar
|
|
9
|
Xia B, Xu B, Sun Y, Xiao L, Pan J, Jin H
and Tong P: The effects of Liuwei Dihuang on canonical
Wnt/β-catenin signaling pathway in osteoporosis. J Ethnopharmacol.
153:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang SC, Kim HJ and Kim MH: Effects of
Astragalus membranaceus with supplemental calcium on bone mineral
density and bone metabolism in calcium-deficient ovariectomized
rats. Biol Trace Elem Res. 151:68–74. 2013. View Article : Google Scholar
|
|
11
|
Zhang ZG, Wu L, Wang JL, Yang JD, Zhang J,
Zhang J, Li LH, Xia Y, Yao LB, Qin HZ and Gao GD: Astragaloside IV
prevents MPP+-induced SH-SY5Y cell death via the inhibition of
Bax-mediated pathways and ROS production. Mol Cell Biochem.
364:209–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Yu L, She T, Gan Y, Liu F, Hu Z,
Chen Y, Li S and Xia H: Astragaloside IV attenuates Toll-like
receptor 4 expression via NF-κB pathway under high glucose
condition in mesenchymal stem cells. Eur J Pharmacol. 696:203–209.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haiyan H, Rensong Y, Guoqin J, Xueli Z,
Huaying X and Yanwu X: Effect of Astragaloside IV on neural stem
cell transplantation in Alzheimer's disease rat models. Evid Based
Complemen. Alternat Med. 2016:31069802016.
|
|
14
|
Wang S, Chen J, Fu Y and Chen X: Promotion
of Astragaloside IV for EA-hy926 cell proliferation and angiogenic
activity via ERK1/2 pathway. J Nanosci Nanotechnol. 15:4239–4244.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie J, Wang H, Song T, Wang Z, Li F, Ma J,
Chen J, Nan Y, Yi H and Wang W: Tanshinone IIA and astragaloside IV
promote the migration of mesenchymal stem cells by up-regulation of
CXCR4. Protoplasma. 250:521–530. 2013. View Article : Google Scholar
|
|
16
|
Li M, Wang W, Geng L, Qin Y, Dong W, Zhang
X, Qin A and Zhang M: Inhibition of RANKL-induced
osteoclastogenesis through the suppression of the ERK signaling
pathway by astragaloside IV and attenuation of
titanium-particle-induced osteolysis. Int J Mol Med. 36:1335–1344.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alman BA: The role of hedgehog signaling
in skeletal health and disease. Nat Rev Rheumatol. 11:552–560.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jemtland R, Divieti P, Lee K and Segre GV:
Hedgehog promotes primary osteoblast differentiation and increases
PTHrP mRNA expression and iPTHrP secretion. Bone. 32:611–620. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Andre P, Ye L and Yang YZ: The
Hedgehog signalling pathway in bone formation. Int J Oral Sci.
7:73–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura T, Aikawa T, Iwamoto-Enomoto M,
Iwamoto M, Higuchi Y, Pacifici M, Kinto N, Yamaguchi A, Noji S,
Kurisu K, et al: Induction of osteogenic differentiation by
hedgehog proteins. Biochem Biophys Res Commun. 237:465–469. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enomoto-Iwamoto M, Nakamura T, Aikawa T,
Higuchi Y, Yuasa T, Yamaguchi A, Nohno T, Noji S, Matsuya T, Kurisu
K, et al: Hedgehog proteins stimulate chondrogenic cell
differentiation and cartilage formation. J Bone Miner Res.
15:1659–1668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: Paradigms and principles. Genes
Dev. 15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mas C and Ruiz i Altaba A: Small molecule
modulation of HH-GLI signaling: Current leads, trials and
tribulations. Biochem Pharmacol. 80:712–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukaya M, Isohata N, Ohta H, Aoyagi K,
Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H, Sakamoto
H, et al: Hedgehog signal activation in gastric pit cell and in
diffuse-type gastric cancer. Gastroenterology. 131:14–29. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bar EE, Chaudhry A, Lin A, Fan X, Schreck
K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, et al:
Cyclopamine mediated Hedgehog pathway inhibition depletes stem-like
cancer cells in glioblastoma. Stem Cells. 25:2524–2533. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Briscoe J and Thérond PP: The mechanisms
of Hedgehog signalling and its roles in development and disease.
Nat Rev Mol Cell Biol. 14:416–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Chen JK, Taipale J, Young KE, Maiti T and
Beachy PA: Small molecule modulation of Smoothened activity. Proc
Natl Acad Sci USA. 99:14071–14076. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karsenty G: The complexities of skeletal
biology. Nature. 423:316–318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Von Wilmowsky C, Moest T, Nkenke E,
Stelzle F and Schlegel KA: Implants in bone: Part I. A current
overview about tissue response, surface modification sand future
perspectives. Oral Maxillofac Surg. 18:243–257. 2014. View Article : Google Scholar
|
|
33
|
Terheyden H, Lang NP, Bierbaum S and
Stadlinger B: Osseointegration-communication of cells. Clin Oral
Implants Res. 23:1127–1135. 2012. View Article : Google Scholar
|
|
34
|
Zhou R, Wei D, Cao J, Feng W, Cheng S, Du
Q, Li B, Wang Y, Jia D and Zhou Y: Synergistic effects of surface
chemistry and topologic structure from modified microarc oxidation
coatings on Ti implants for improving osseointegration. ACS Appl
Mater Interfaces. 7:8932–8941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andrukhov O, Huber R, Shi B, Berner S,
Rausch-Fan X, Moritz A, Spencer ND and Schedle A: Proliferation,
behavior, and differentiation of osteoblasts on surfaces of
different micro-roughness. Dent Mater. 32:1374–1384. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smeets R, Stadlinger B, Schwarz F,
Beck-Broichsitter B, Jung O, Precht C, Kloss F, Gröbe A, Heiland M
and Ebker T: Impact of dental implant surface modifications on
osseointegration. Biomed Res Int. 2016:62856202016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH,
Wang YC, Ye BG, Cao MQ, Gao DM and Tang ZY: Astragaloside IV
inhibits metastasis in hepatoma cells through the suppression of
epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin
pathway. Oncol Rep. 37:1725–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sims NA and Gooi JH: Bone remodeling:
Multiple cellular interactions required for coupling of bone
formation and resorption. Semin Cell Dev Biol. 19:444–451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Javed A, Bae JS, Afzal F, Gutierrez S,
Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS and
Lian JB: Structural coupling of Smad and Runx2 for execution of the
BMP2 osteogenic signal. J Biol Chem. 283:8412–8422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li MJ, Li F, Xu J, Liu YD, Hu T and Chen
JT: rhHMGB1 drives osteoblast migration in a TLR2/TLR4- and
NF-κB-dependent manner. Biosci Rep. 36:e003002016. View Article : Google Scholar
|
|
44
|
Horikiri Y, Shimo T, Kurio N, Okui T,
Matsumoto K, Iwamoto M and Sasaki A: Sonic hedgehog regulates
osteoblast function by focal adhesion kinase signaling in the
process of fracture healing. PLoS One. 8:e767852013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kimura H, Ng JM and Curran T: Transient
inhibition of the Hedgehog pathway in young mice causes permanent
defects in bone structure. Cancer Cell. 13:249–260. 2008.
View Article : Google Scholar : PubMed/NCBI
|


















