Introduction
Irritable bowel syndrome (IBS) is a highly prevalent
functional bowel disorder, which is characterised by the presence
of abdominal pain or discomfort, an alteration in bowel habits, and
diarrhoea and constipation without any structural cause. The
aetiology of IBS predominantly includes genetics, motility,
visceral hypersensitivity, diet, infection and inflammation,
alteration of intestinal flora and psychosocial factors (1,2).
Neonatal maternal separation (NMS) is a form of early-life stress,
which has been consistently used in rats to develop experimental
visceral hypersensitivity (3,4).
Visceral hypersensitivity has been widely considered a biological
marker of IBS; however, the underlying mechanism remains
unclear.
L-Glutamate is the major excitatory neurotransmitter
in the central nervous system. The actions of glutamate are
modulated by ionotropic receptors and metabotropic glutamate
receptors (mGluRs) (5). Based on
sequence homology and signal transduction mechanisms, mGluRs have
been divided into groups I, II and III; mGluR4, mGluR6, mGluR7 and
mGluR8 are group III mGluRs (5,6).
These receptors can modulate the effect and release of glutamate in
the central nervous system (7).
In addition, increasing evidence has indicated that mGluRs are
expressed in the periphery, such as the gastrointestinal system.
The glutamatergic system has also been implicated in the
pathophysiology of depression and anxiety; antidepressants have
been reported to inhibit glutamate release, a phenomenon mirrored
by activation of group III mGluRs (6,8).
As a member of the group III mGluRs, mGluR7 is of particular
interest because it is an important target for reducing anxiety and
stress-associated behaviours (8,9).
Selective activation of G-protein-coupled mGluR7 elicits
anxiolytic-like effects in mice by modulating γ-aminobutyric
acid-ergic neurotransmission (10). Furthermore, activating mGluR7 by
AMN082 (a selective agonist of mGluR7) induces a reduction in
immobility in a forced swim test and tail suspension test (11,12). Notably, mood disorders exhibit
high comorbidity with gastrointestinal dysfunction (13), and a high frequency of IBS
symptoms is observed in patients with panic disorder, generalised
anxiety disorder and major depressive disorder (14). Therefore, dysregulation of mGluR7
activity may be considered a significant factor underlying
stress-induced IBS. AMN082 is a recently discovered selective
mGluR7 agonist, which induces an increase in faecal water content
in stress-induced defecation (6);
however, to the best of our knowledge, the contribution of mGluR7
to early-life stress-induced visceral hypersensitivity in IBS
remains unexplored. Therefore, the present study aimed to
investigate a possible functional role of mGluR7 in the colon by
assessing agonist-induced alterations in visceral
hypersensitivity.
Several 5-hydroxytryptamine receptors (5-HTRs) are
expressed in the gut, including 5-HT1AR,
5-HT2AR, 5-HT2BR, 5-HT3R,
5-HT4R and 5-HT7R. Alterations in the levels
of 5-HT have been observed in experimental models of colitis and in
patients with IBS; however, the results are varied (15,16). Nitric oxide (NO) is a gaseous
messenger that serves an essential role in the physiology and
pathophysiology of the gastrointestinal tract. NO is synthesised by
NO synthase (NOS), which is classified into neuronal NOS,
endothelial NOS and inducible NOS (iNOS). It has been reported that
activation of the NO pathway alleviates the symptoms of IBS;
Paragomi et al demonstrated that the NOS inhibitor L-NAME
reverses the antinociceptive effects of sodium hydrogen sulphide on
colorectal distension in a chemically induced model of IBS in rats
(17). These findings indicated
that NO may serve a protective role against IBS; however, the
expression levels of NO in the rectum and plasma in IBS are not
consistent (3,17). In addition, the development of IBS
is associated with low-grade inflammation, and nuclear factor
(NF)-κB is a critical transcription factor for the inflammatory
response (18).
Anxiety-depression status may elevate interleukin (IL)-1β and IL-10
levels in patients with IBS (19), which leads to the occurrence or
aggravation of IBS. The present study demonstrated that mGluR7 may
serve an important role in visceral hypersensitivity by modulating
the function of NOS or inflammatory factors, thereby attenuating
visceral hypersensitivity in IBS. The results indicated that there
was no difference in 5-HTRs in any of the groups; therefore,
alterations in NOS and inflammatory factors were discussed in
detail.
Materials and methods
Animals and NMS
All animal protocols were approved by the Animal
Care and Use Committee at the Tongji University School of Medicine
(Shanghai, China) and were conducted according to the National
Institutes of Health (NIH) Guidelines for the Care and Use of
Animals in Research (NIH Publication No. 85-23, revised 1996)
(20). Seven 15-day pregnant
Sprague-Dawley rats were obtained from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). The rats were maintained under
a 12-h light/dark cycle with free access to food and water at 22°C
and 50% relative humidity.
All Sprague-Dawley pups (weight 5-6 g) were divided
into three groups: Normal control (NC) group, neonatal maternal
separation (NMS) group and NMS + AMN082 group (n=6/group). The pups
were maintained under a 12-h light/dark cycle with free access to
food and water at 22°C and 50% relative humidity. Pups in the NMS
and NMS + AMN082 groups were separated from their dams and placed
into individual cages in an adjacent room for 3 h (09:00-12:00) on
postnatal day (P)2-14 (date of birth is designated P0). The pups
were subsequently returned to the cages of their dams for the
remaining time. The rats in the NC group were allowed to remain in
standard cages with their dams. Pups were weaned on P22; only male
rats were used in the present study, in order to avoid alterations
associated with hormonal cycles. Each cage housed five rats;
visceral hypersensitivity was measured after 8 weeks of feeding
after weaning.
Behavioural testing to measure visceral
hypersensitivity Abdominal withdrawal reflex (AWR)
Visceral hypersensitivity responses to colorectal
distension (CRD) were measured by recording AWR scores, as
described previously (3,10). Prior to testing, the rats were
fasted and subjected to water deprivation, in order to reduce
faeces. The rats were anaesthetized with isoflurane (1.5%) in a
sealed cage (21), and a 6-cm
deflated latex balloon was inserted into the distal colon up to 1
cm from the anal verge. After a 15-min recovery period,
measurements of the visceral pain threshold were recorded when
various pressure was applied to the abdominal walls by inflating
the balloon (20, 40, 60 and 80 mmHg). Three measurements for each
animal were taken at 5-min recovery intervals. A previously
reported standard was used to evaluate the AWR score: 0, no
response to given pressure; 1, slight head movement without
abdominal muscle contraction; 2, contraction of the abdominal
muscles; 3, lifting of the abdominal wall; and 4, body arching and
lifting of pelvic structures (22).
Electromyography (EMG)
EMG was also used to quantitatively measure visceral
hypersensitivity at week 8. The surgical procedures for EMG were
performed as previously described (23,24). Rats were deeply anaesthetized with
30-40 mg/kg (3-4 mg/ml) 3% sodium pentobarbital (i.p.), no signs of
toxicity were observed. A pair of EMG electrodes was surgically
implanted in the lower left abdominal area to expose the external
oblique abdominal musculature, and the electrode was tunnelled
subcutaneously, exteriorised and secured at the back of the neck
for EMG recording. Rats were allowed to recover for ≥5 days.
Fasting and water deprivation, isoflurane anaesthesia and insertion
of the deflated latex balloon were conducted as in the AWR test.
Subsequently, the electrodes were connected to a BL-420F Data
Acquisition & Analysis system (Chengdu Techman Software Co.,
Ltd., Chengdu, China), and to record the EMG signal, various
pressure was applied to the abdominal walls by inflating the
balloon (20, 40, 60 and 80 mmHg). The procedure was repeated three
times and the EMG signals were recorded. Alterations in the EMG
signal response to CRD were determined by calculating the changes
in the area under the curve (AUC) of raw EMG amplitude responses to
CRD, based on the formula ΔAUC (AUC during CRD-AUC before CRD)
(3,21).
The selective mGluR7 agonist AMN082 (cat. no. 2385;
Tocris Bioscience, Bristol, UK) was dispersed in a suspension of
0.5% methylcellulose (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and was administered intraperitoneally (3 or 10 mg/kg) to
NMS rats in the NMS + AMN082 group 1 h prior to AWR and EMG
testing. The same volume of PBS (vehicle control) was administered
to rats in the NMS group. Doses were chosen based on previous
experiments indicating behavioural and physiological alterations in
response to this range (6,25).
Tissue preparation
Rats were anaesthetized by 3% pentobarbital sodium
(30 mg/kg i.p.) immediately following completion of the CRD study.
The distal colons were dissected before the rats were sacrificed by
decapitation. The colon samples were immediately frozen in liquid
nitrogen, and stored at −70°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the colon tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
RNA was reverse-transcribed to cDNA using a PrimeScript™ RT reagent
kit (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. mRNA transcripts were analysed by qPCR
using SYBR® Premix Ex Taq™ (Takara Bio, Inc.) on an
Applied Biosystems StepOne/StepOnePlus Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was
performed for 40 cycles, as follows: Initial denaturation at 95°C
for 10 min, denaturation at 95°C for 15 sec and annealing/extension
at 60°C for 1 min. The primers were designed and purchased from
Sangon Biotech Co., Ltd. (Shanghai, China), and the sequences are
shown in Table I. GAPDH was used
as a reference gene, and relative gene expression was determined
using the 2−ΔΔCq method (26).
 | Table ISequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| IL-10 |
5'-agtggagcaggtgaagaatga-3' |
5'-cacgtaggcttctatgcagttg-3' |
| TGF-β1 |
5'-tggagcctggacacacagta-3' |
5'-tagtagacgatgggcagtgg-3' |
| IFN-γ |
5'-tcatcgaatcgcacctgat-3' |
5'-ggatctgtgggttgttcacc-3' |
| iNOS |
5'-gagacaggaagtcggaagc-3' |
5'-gtgttgaaggcgtagctgaa-3' |
| eNOS |
5'-tgtagctgtgctggcataca-3' |
5'-ttgagttggctcatccatgt-3' |
| nNOS |
5'-tcattagcaatgaccgaagc-3' |
5'-aacattggaaagaccttggg-3' |
|
5-HT1AR |
5'-gatctcgctcacttggctca-3' |
5'-aaagcgccgaaagtggagta-3' |
|
5-HT2AR |
5'-aagccaccttgtgtgtgagt-3' |
5'-tgtggatggaccgttggaag-3' |
|
5-HT3R |
5'-atgactgctcagccatggga-3' |
5'-tggtggtggaagagggctat-3' |
|
5-HT4R |
5'-tggtttcggtgctggtgaatg-3' |
5'-tgatgctgtggtgagtaggaca-3' |
|
5-HT7R |
5'-atcacgagacccctcacgta-3' |
5'-tctgagcccatccgaagaga-3' |
| mGluR7 |
5'-tgtgagccctgtgatggata-3' |
5'-ccagtttgatgattgggatg-3' |
| GAPDH |
5'-agatccacaacggatacatt-3' |
5'-tccctcaagattgtcagcaa-3' |
Western blotting
Proteins were extracted from colon tissues using
radioimmunoprecipitation assay buffer supplemented with protease
inhibitors (Shanghai Shenggong Co., Ltd., Shanghai, China) and
protein concentration was measured using the Bicinchoninic Acid
Protein Assay kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). Subsequently, proteins (50 µg) were separated by 10%
SDS-PAGE and were transferred to a nitrocellulose membrane
(Whatman; GE Healthcare Life Sciences, Little Chalfont, UK). The
membrane was then blocked with 5% skimmed milk at room temperature
for 1 h and was incubated overnight at 4°C with anti-mGluR7
antibodies (1:1,000, rabbit anti-rat, cat. no. NB-91787; Novus
Biologicals, LLC, Littleton, CO, USA) or antibodies against total
NF-κB (p65, 1:1,000, rabbit anti-rat, cat. no. 9936S),
phosphorylated (p)-NF-κB (p65, 1:1,000, rabbit anti-rat, cat. no.
9936S) or β-actin (1:1,000, rabbit anti-rat, cat. no. 8457) (all
from Cell Signaling Technology, Inc., Danvers, MA, USA). The
membrane was then incubated with an IRDye®
800CW-conjugated secondary antibody (1:2,000, cat. no. A80-195P;
Rockland Immunochemicals, Inc., Limerick, PA, USA) for 1 h at room
temperature. Images were acquired using an Odyssey infrared imaging
system (LI-COR Biosciences, Lincoln, NE, USA). The blots were
semi-quantified by grey value analysis (ImageJ 1.50i; NIH,
Bethesda, MA, USA).
Immunohistochemistry
Colon tissues were fixed in 4% formalin overnight at
4°C; subsequently, paraffin-embedded colon tissues (length, 1 cm;
width, 0.5 cm) were mounted on slides, deparaffinised with xylene
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and
rehydrated with 1.5% H2O2 in PBS for 30 min
at room temperature. Tissues were incubated overnight at 4°C with
anti-mGluR7 antibody (1:500, rabbit anti-rat, cat. no. NB-91787;
Novus Biologicals LLC), or antibodies against cluster of
differentiation (CD)3 (1:100, rabbit anti-rat, cat. no. ab16669)
CD68 (1:200, mouse anti-rat, cat. no. ab31630) or myeloperoxidase
(MPO; 1:50, rabbit anti-rat, cat. no. ab9535) (Abcam, Cambridge,
UK). Secondary goat anti-rabbit antibodies or goat anti-mouse
antibodies (1:200, cat. nos. A0277 and A0286; Beyotime Institute of
Biotechnology, Shanghai, China) were then added to the tissues for
1 h at room temperature. After being incubated with
streptavidin-conjugated horseradish peroxidase (HRP) [Rabbit
Specific HRP/DAB (ABC) Detection IHC kit, cat. no. ab64261; Abcam]
for 10 min at room temperature, sections were stained with DAB for
1-10 min at room temperature (1:2; Gene Tech Biotechnology Co.,
Ltd., Shanghai, China); sections were then counterstained with
haematoxylin for 0.5-2 min at room temperature. The sections were
visualised under an Olympus BX41-32P02-FLB3 microscope (Olympus
Corporation, Tokyo, Japan). Images were captured using FluoView
software (FV1000, Olympus Corporation).
Statistical analysis
The experiments were repeated three times, and all
data are presented as the means ± standard error of the mean.
Statistical analyses were performed using SPSS 19.0 (IBM SPSS,
Armonk, NY, USA). Statistical significance of differences between
two groups was determined with Student's t-test, whereas multiple
groups were analysed by one-way analysis of variance followed by
the least significant difference test or Dunnett's T3 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Early NMS causes CRD-induced visceral
hypersensitivity in rats
Initially, pain threshold in response to CRD was
determined using the AWR test and EMG. Electromyogram activity in
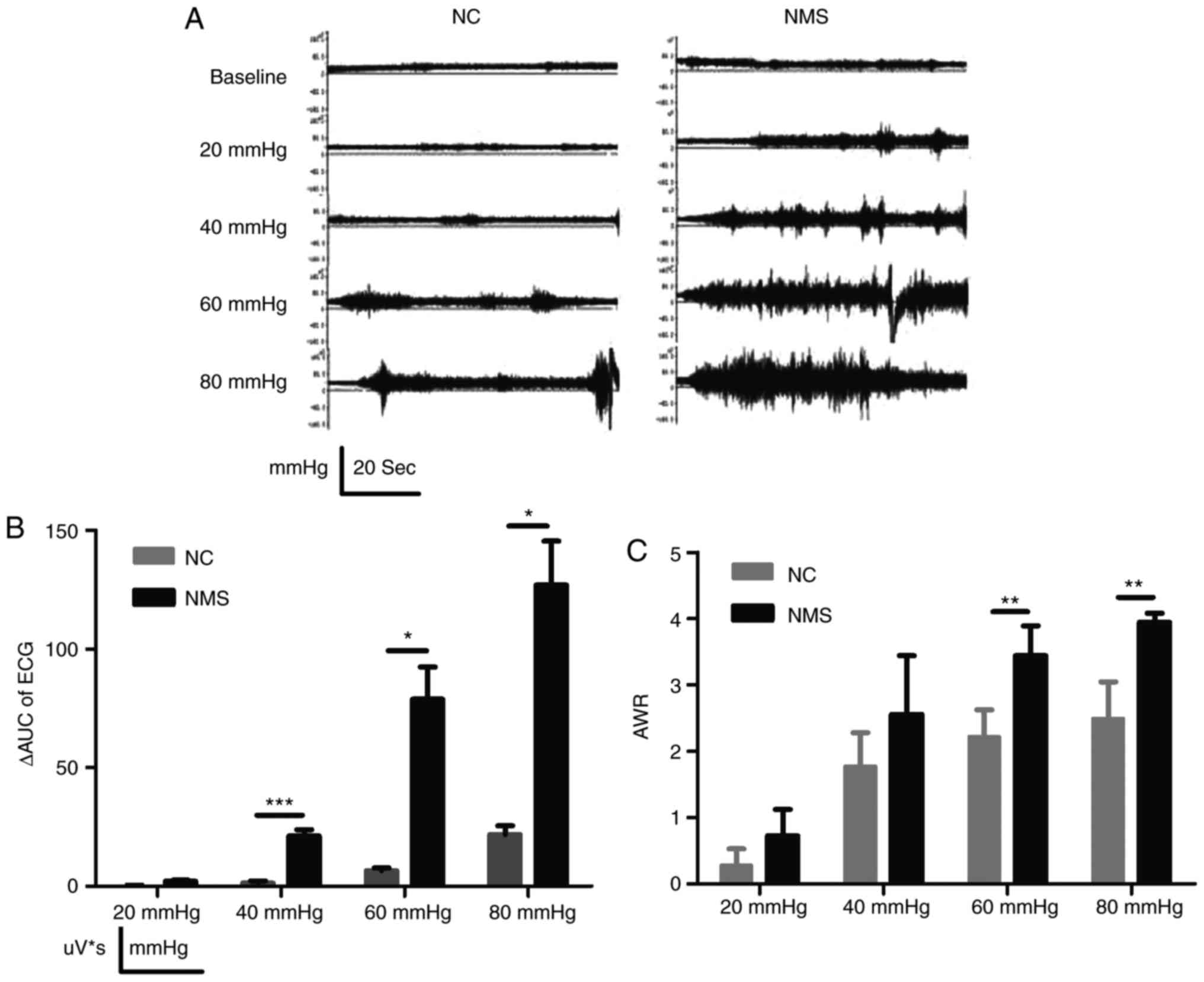
the NMS group was enhanced compared with in the NC group (Fig. 1A). To quantitatively measure EMG,
ΔAUC was used. Compared with in the NC group, the ΔAUC in the NMS
group was significantly increased at CRDs of 40, 60 and 80 mmHg
(P<0.001, P<0.05 and P<0.05, respectively; Fig. 1B). AWR in the NMS group was
significantly higher compared with in the NC group at CRDs of 60
and 80 mmHg (P<0.01 and P<0.01, respectively; Fig. 1C). These findings indicated that
NMS could simulate negative emotional events during early IBS, and
the model successfully induced visceral hypersensitivity.
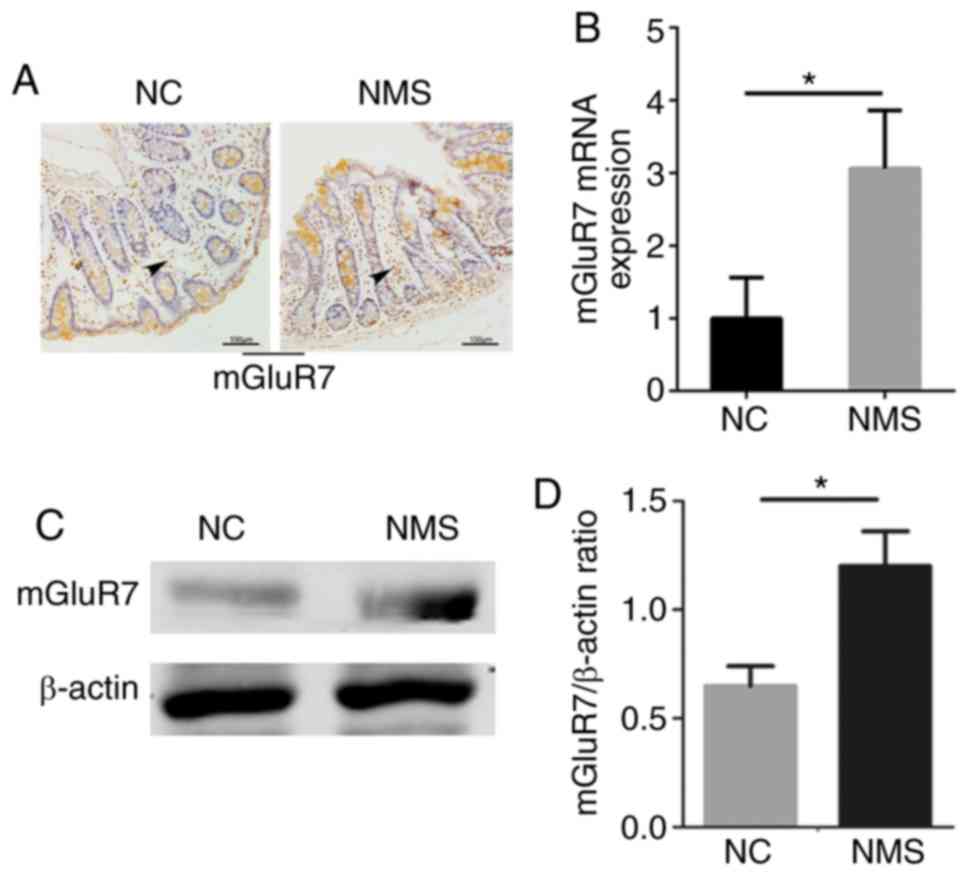
mGluR7 expression is significantly
increased in the colons of rats with visceral hypersensitivity
The expression levels of mGluR7 were increased in
the colon tissues of NMS rats compared with in the NC group, as
determined by immunohistochemistry. Notably, staining was primarily
observed in the mucosa, including a portion of cells at the surface
epithelium, as well as in the lamina propria, with particularly
strong staining in the bottom of the crypts (Fig. 2A). The mRNA and protein expression
levels of mGluR7 were increased in the colon tissues of NMS rats
compared with in the NC group (P<0.05; Fig. 2B and C). A significant difference
was detected between the two groups (P<0.05; Fig. 2D). These findings indicated that
mGluR7 expression was increased in the colons of rats with visceral
hypersensitivity; therefore, IBS episodes may be associated with
mGluR7 expression.
Selective mGluR7 agonist AMN082 (10
mg/kg) attenuates CRD-induced visceral hypersensitivity in NMS
rats
The effects of AMN082 on alterations in mGluR7
expression and visceral hypersensitivity were detected. Following
treatment with AMN082, the protein expression levels of mGluR7 were
increased (Fig. 3A). The
electromyogram activity in the NMS + AMN082 (10 mg/kg) group was
attenuated compared with in the NMS group (Fig. 3B). Compared with in the NMS group,
the ΔAUC in the NMS + AMN082 (10 mg/kg) group was significantly
decreased at CRDs of 40, 60 and 80 mmHg (P<0.01, P<0.01 and
P<0.05, respectively; Fig.
3C). No significant alteration was observed between the NMS and
NMS + AMN082 (3 mg/kg) groups. Furthermore, the AWR in the NMS +
AMN082 (10 mg/kg) group was significantly reduced compared with in
the NMS group at CRDs of 60 and 80 mmHg (P<0.01 and P<0.05,
respectively; Fig. 3D). At a dose
of 3 mg/kg, the AWR results in the NMS + AMN082 group were not
significantly different compared with in the NMS group. These
findings suggested that 10 mg/kg AMN082 significantly activated
mGluR7 and attenuated CRD-induced visceral hypersensitivity in NMS
rats.
AMN082 (10 mg/kg) suppresses the
expression levels of pro-inflammatory cytokines and increases the
expression levels of anti-inflammatory cytokines in colon tissues
of NMS rats
Since 10 mg/kg AMN082 significantly attenuated
CRD-induced visceral hypersensitivity in NMS rats, the present
study explored the underlying mechanisms. The mRNA expression
levels of the anti-inflammatory cytokines IL-10 and TGF-β1, the
pro-inflammatory cytokine IFN-γ, NOS and 5-HTRs were detected in
colon tissues by RT-qPCR (Fig.
4). IL-10 was decreased in the NMS group compared with in the
NC group (P<0.01), whereas IFN-γ was increased (P<0.05).
Notably, treatment with 10 mg/kg AMN082 significantly increased the
release of IL-10 (P<0.01) and TGF-β1 (P<0.01), and suppressed
the release of IFN-γ (P<0.05). NOS and 5-HTRs exhibited almost
no change in expression; however, iNOS was significantly decreased
in the NMS group compared with in the NC group (P<0.05). Since
the release of inflammatory cytokines is an indicator of the
inflammatory response, these results indicated that AMN082 may
inhibit inflammation in the colons of NMS rats.
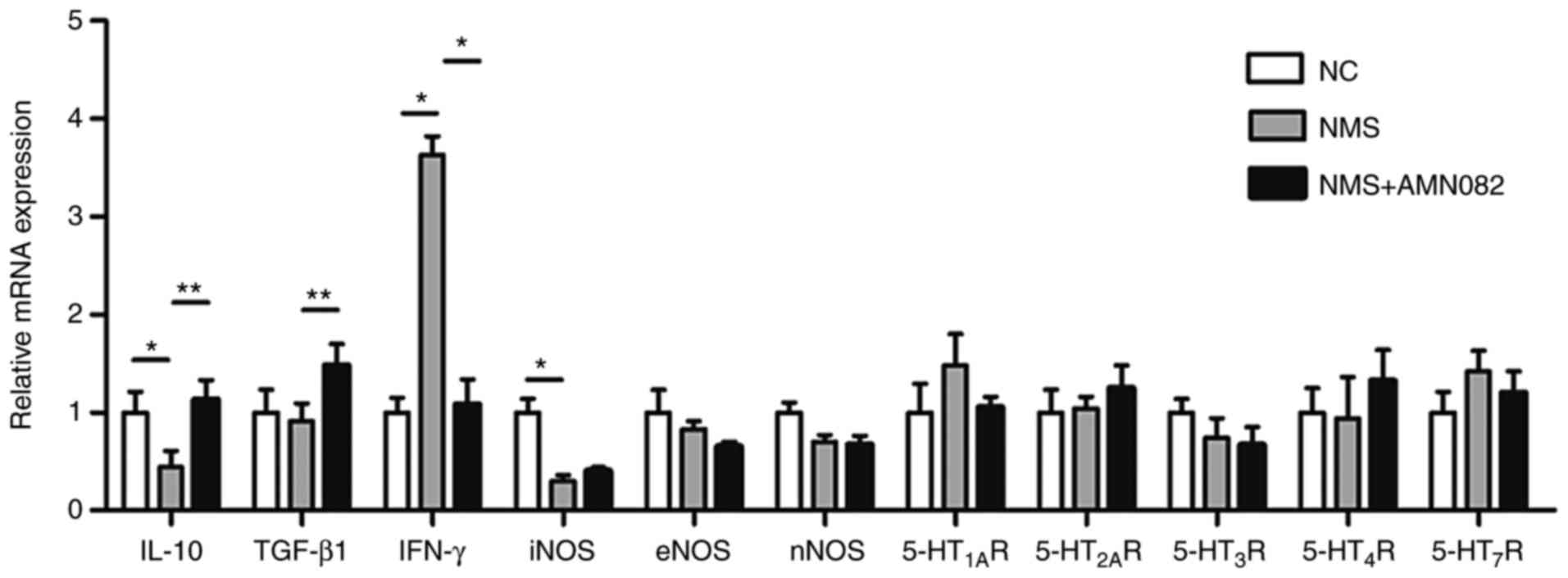 | Figure 4Effects of AMN082 (10 mg/kg) on the
mRNA expression levels of 5-HT receptors, NOS and inflammatory
cytokines. IL-10 and TGF-β1 data were analysed by one-way ANOVA and
least significant difference test; iNOS and IFN-γ data were
analysed by one-way ANOVA and Dunnett's T3 test. Data are expressed
as the means ± standard error of the mean (number of rats=6).
*P<0.05, **P<0.01. 5-HT,
5-hydroxytryptamine; 5-HT1AR, 5-HT 1A receptor;
5-HT2AR, 5-HT 2A receptor; 5-HT3R, 5-HT 3
receptor; 5-HT4R, 5-HT 4 receptor; 5-HT7R,
5-HT 7 receptor; ANOVA, analysis of variance; eNOS, endothelial
nitric oxide synthase; IFN-γ, interferon-γ; IL-10, interleukin-10;
iNOS, inducible nitric oxide synthase; NC, normal control; NMS,
neonatal maternal separation; nNOS, neuronal nitric oxide synthase;
TGF-β1, transforming growth factor-β1. |
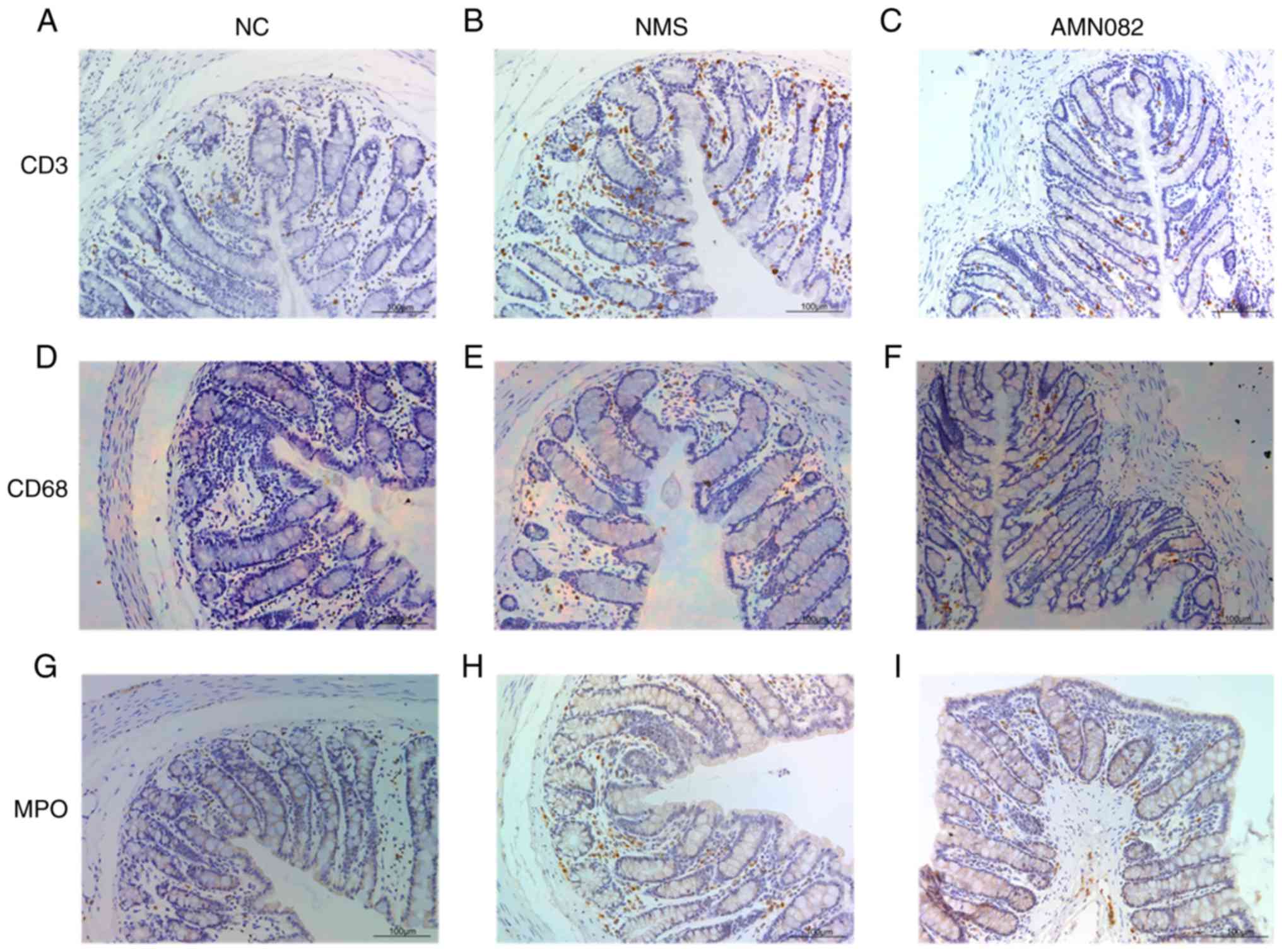
AMN082 (10 mg/kg) reduces the expression
levels of MPO and the number of CD3+ cells in the
intestinal mucosa of NMS rats
MPO enzymatic activity is an index of neutrophil
infiltration in colon tissue; and CD3+ is a specific
membrane surface molecule on T lymphocytes, which is often used as
a marker of T lymphocytes; CD68 is a marker of macrophages or
monocytes, Both T cells and macrophages participate in regulating
the intestinal immune system (27,28); therefore, MPO, CD3 and CD68 were
detected in the present study (Fig.
5). NMS rats exhibited increased MPO expression levels, and CD3
and CD68 infiltration in colon tissue compared with the NC group,
and staining was primarily observed in the mucosa (Fig. 5A, B, D, E, G and H). Notably, CD68
staining was the least obvious. In addition, the NMS + AMN082 (10
mg/kg) group exhibited markedly decreased CD3+ T cell
infiltration compared with the NMS group (Fig. 5B and C). Similarly, administration
of 10 mg/kg AMN082 markedly reduced MPO expression compared with
the NMS group (Fig. 5H and I);
there was no difference in CD68 expression in the NMS + AMN082 (10
mg/kg) group compared with the NMS group (Fig. 5E and F).
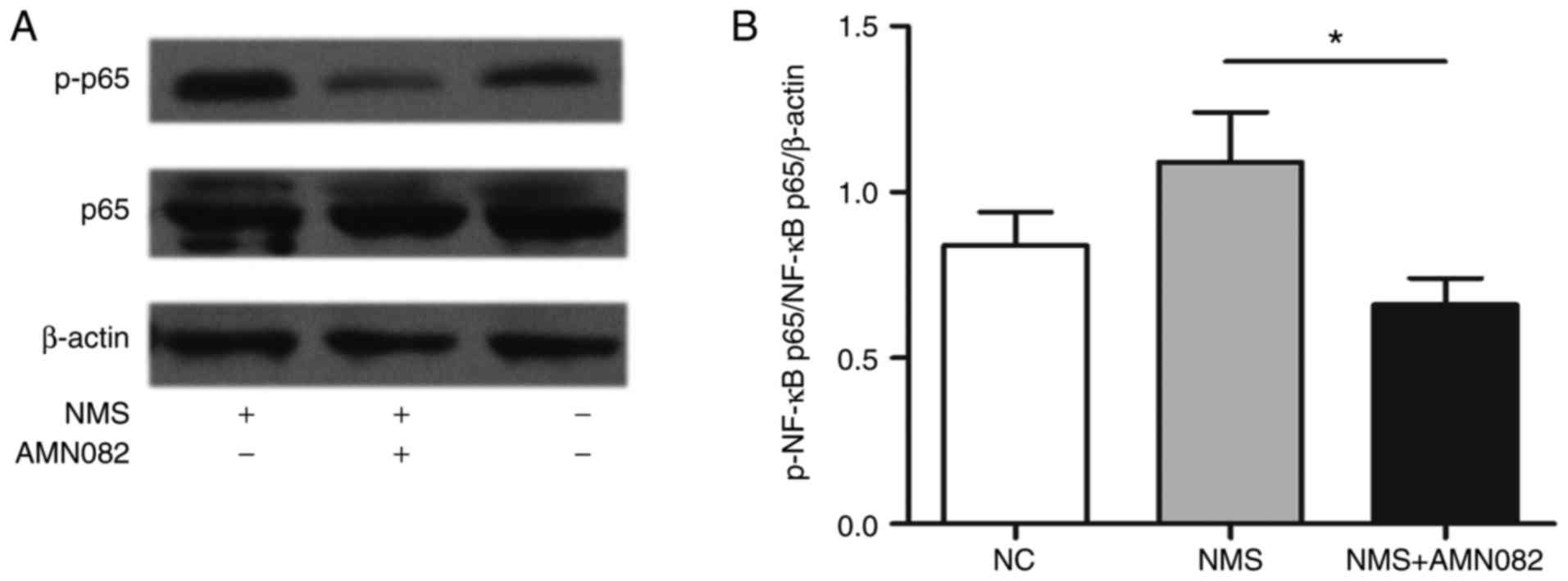
AMN082 (10 mg/kg) inhibits activation of
NF-κB in the colon of NMS rats
NF-κB is a critical transcription factor in the
inflammatory response. This protein functions as a pro-inflammatory
factor that participates in the pathophysiology of intestinal
inflammatory diseases (29).
Intestinal inflammation may provide an initial stimulus for a
persistent state of visceral hypersensitivity (30). To investigate the mechanism
underlying the anti-inflammatory activity of AMN082, the effects of
AMN082 on activation of the NF-κB pathway in rats were
investigated. Alterations in the expression levels of p-NF-κB p65
in the colons of NMS rats were evaluated by western blotting
(Fig. 6A); p-NF-κB p65 was
upregulated in NMS rats compared with in the NC group. Conversely,
AMN082 treatment significantly reduced p-NF-κB expression compared
with in the NMS group (Fig. 6B;
P<0.05).
Discussion
The present study demonstrated that early NMS
induced visceral hypersensitivity in rats and increased the
expression of mGluR7 in the colon. However, selective activation of
mGluR7 via AMN082 (10 mg/kg) attenuated CRD-induced visceral
hypersensitivity. In addition, AMN082 modulated the immune response
in NMS rats by regulating the balance between pro-inflammatory
factors and anti-inflammatory factors, and suppressing low-grade
inflammation via the reduction of NF-κB activation.
A strong body of evidence supports the role of
glutamate as a primary neurotransmitter in the vagal circuitry,
which is involved in key gastrointestinal functions (31). In addition, mGluRs appear to be
relevant not only for the modulation of gastrointestinal vagovagal
reflexes, but also for the process of digestion as a whole
(8). mGluR4 has been detected in
normal human colon epithelium (32), and an accelerating effect on
guinea pig colon motility and longitudinal muscle contractions has
been observed upon application of mGluR8 agonists on isolated
tissue (33). Furthermore, mGluR7
mRNA and protein are expressed in the mouse colon mucosa, and
treatment with the selective mGluR7 agonist AMN082 induces an
increase in faecal water content in stress-induced defecation
(6). Previous evidence has
indicated that overexpression of the N-methyl-D-aspartate receptor
(an ionotropic receptor) serves an important role in the formation
of post-inflammatory visceral hypersensitivity (34). Therefore, it was hypothesised that
mGluR7 may be relevant to visceral hypersensitivity. The majority
of studies regarding IBS and AMN082 (mGlu7 receptor agonist) have
used animal models; AMN082 is active in the central nervous system
via direct central injection, and is also active outside of the
central nervous system via oral or intraperitoneal injection
(6,35,36). The present study demonstrated that
mGluR7 expression was enhanced in NMS rats, as determined by
assessing immunohistochemistry, mRNA levels and protein levels.
Therefore, it was suggested that mGluR7 may have an important role
in the visceral hypersensitivity of IBS; these results were similar
to those of Julio-Pieper et al (6). A high frequency of IBS symptoms has
been detected in patients with panic disorder, generalised anxiety
disorder and major depressive disorder, and another study reported
that mGluR7-knockout animals display an anxious phenotype (8). The present study confirmed that
activating mGluR7 attenuated CRD-induced visceral hypersensitivity.
This conclusion was consistent with previous literature reports,
and represented a breakthrough in understanding the association
between mGluR7 and the pathogenesis of IBS.
It has been reported that the abnormal perception of
visceral stimuli, or visceral hypersensitivity, is an important
underlying mechanism of IBS, and intestinal inflammation may
provide an initial stimulus for a persistent state of visceral
hypersensitivity (31). Patients
with post-infectious IBS exhibit no signs of overt inflammation but
exhibit persistent minor increases in epithelial T lymphocytes and
mast cells (37), thus suggesting
that long-term inflammatory alterations may be responsible for
colonic hypersensitivity. Furthermore, it has been indicated that a
T helper (Th)1/Th2 immune imbalance is closely associated with
patients with IBS with diarrhoea (D-IBS); IFN-γ is a Th1-mediated
cytokine that promotes the inflammatory response, whereas IL-10 is
a Th2-mediated cytokine that inhibits the inflammatory response
(38). In IL-10-deficient mice,
the majority of animals suffer from chronic enterocolitis, thus
suggesting that IL-10 is an essential immunoregulator in the
intestinal tract (39). TGF-β1 is
one of the most common members of the TGF-β family, which possesses
an anti-inflammatory effect. Furthermore, NF-κB is an important
transcription factor that is mainly involved in inflammatory and
immune responses. NF-κB-dependent inflammatory mediators, including
IL-6, are associated with the maternal separation model of IBS, and
the downstream activation of extracellular signal-regulated kinase
(ERK), Janus kinase (JAK)-signal transducer and activator of
transcription (STAT) and NF-κB signalling cascades (40). Furthermore, stimulating the
production of pro-inflammatory cytokines, including IL-1β and
tumour necrosis factor (TNF)-α, contributes to visceral
hypersensitivity through the Toll-like receptor 4 (TLR4)/myeloid
differentiation primary response 88/NF-κB signalling pathway in the
spinal cord in a neonatal colonic irritation rat model (41). In addition, NO is involved in
inhibition of the transmission and perception of visceral
hypersensitivity (42). A
previous study revealed that ioglitazone (hypoglycemic drug)
reduces visceral hypersensitivity, increases nociceptive
thresholds, NO production and iNOS activity in D-IBS rats (17). In the present study, iNOS
expression was decreased in the NMS group, which could further
reduce the synthesis of NO, resulting in enhanced intestinal
motility and increased visceral hypersensitivity. Similar to the
aforementioned findings, a decrease in iNOS expression may be
associated with the occurrence of visceral hypersensitivity in
IBS.
In the present study, decreased IFN-γ, and increased
TGF-β1 and IL-10 levels were observed in the intestinal mucosa of
AMN082-treated rats. Furthermore, the expression levels of CD3 and
MPO were decreased in the intestinal mucosa of AMN082-treated rats.
These results indicated that activating mGluR7 may result in an
imbalance of Th1/Th2 immunity. Upregulated IL-10 reduces
antigen-specific human T-cell proliferation by diminishing the
antigen-presenting capacity of monocytes (43), which then inhibits the secretion
of TNF-α and results in the inhibition of NF-κB pathway activation.
A previous report suggested that during acute infection of IBS,
TGF-β1 is increased in the muscle layer (44); therefore, in AMN082-treated NMS
rats, upregulated TGF-β1 may inhibit the maturation of dendritic
cells and Th1 activity, and enhance Th2 activity. Conversely,
downregulated IFN-γ can reduce the activation of macrophages and
inhibit Th1 activity, also resulting in the inhibition of NF-κB
pathway activation. The decreased expression of NF-κB in
AMN082-treated NMS rats is consistent with the aforementioned
conclusions. When NF-κB is activated various downstream
inflammatory cytokines are produced. These findings indicated that
activating mGluR7 may reduce NF-κB-associated amplification of the
inflammatory response, thereby protecting the intestinal mucosal
barrier and reducing intestinal permeability. Subsequently, the
number of antigens that pass through the intestine and immune cell
interactions are reduced. When the initial inflammatory stimulus is
weakened, the persistent state of visceral hypersensitivity may be
relieved.
The present study did not assess the mGluR7
signalling pathway in vitro; therefore, further studies may be
conducted using a cell model and mGluR7 small interfering RNA. To
examine the effects of mGluR7 more detail, it would be beneficial
to determine the effects of mGluR7 gene knockdown or antagonism in
rats. In addition, the association between mGluR7 and other
characteristics of IBS, such as diarrhoea and constipation, should
be evaluated; in these future studies, stool characteristics,
including stool particles and faecal water content, may be
detected. Other IBS-associated inflammatory signalling pathways,
including ERK, JAK-STAT, TLR4 and NF-κB signalling cascades
(40,41), may also have a relationship with
altered mGluR7 in rats with visceral hypersensitivity; further
studies are required to evaluate this.
Stressful life events and experimental stress
exacerbate symptoms and visceral hypersensitivity in patients with
functional gastrointestinal disorders, such as IBS (13). In conclusion, the present study
supported the idea of a complex interaction between the
gastrointestinal tract and the brain, namely, the brain-gut axis.
The present data demonstrated that mGluR7 may have an important
role in attenuating visceral hypersensitivity in IBS, in addition
to regulating the central components of chronic stress, and
regulating fluid and electrolyte transport in the intestine. The
mechanisms underlying these effects may be associated with
inhibition of NF-κB activation and reduced inflammation.
Funding
No funding was received.
Availability of data and material
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LMS and YBL were involved in the conception and
design, and conducted most of the study. JHX and QYW assisted with
the immunohistochemistry, western blotting and RT-qPCR experiments.
LMS was involved in the acquisition, analysis and interpretation of
data, and drafted the article. FL and JD made contributions to the
conception and design of the study, and analysed and interpreted
the data; they were also involved in revising the manuscript
critically for important intellectual content and gave final
approval of the version to be published. All authors participated
sufficiently in the work to take public responsibility for
appropriate portions of the content and agreed to be accountable
for all aspects of the work. The final manuscript was read and
approved by all authors.
Ethics approval and consent to
participate
All animal experiments were performed following
approval by the Animal Care and Use Committee of Tongji University
School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Zhao K, Yu L, Wang X, He Y and Lu B:
Clostridium butyricum regulates visceral hypersensitivity of
irritable bowel syndrome by inhibiting colonic mucous low grade
inflammation through its action on NLRP6. Acta Biochim Biophys Sin
(Shanghai). 50:216–223. 2018. View Article : Google Scholar
|
|
2
|
Rodiño-Janeiro BK, Vicario M,
Alonso-Cotoner C, Pascua- García R and Santos J: A review of
microbiota and irritable bowel syndrome: Future in therapies. Adv
Ther. 35:289–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tjong YW, Ip SP, Lao L, Wu J, Fong HH,
Sung JJ, Berman B and Che CT: Role of neuronal nitric oxide
synthase in colonic distension-induced hyperalgesia in distal colon
of neonatal maternal separated male rats. Neurogastroenterol Motil.
23:e666-e2782011. View Article : Google Scholar
|
|
4
|
Coutinho SV, Plotsky PM, Sablad M, Miller
JC, Zhou H, Bayati AI, McRoberts JA and Mayer EA: Neonatal maternal
separation alters stress-induced responses to viscerosomatic
nociceptive stimuli in rat. Am J Physiol Gastrointest Liver
Physiol. 282:G307–G316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernández-Montoya J, Avendaño C and
Negredo P: The glutamatergic system in primary somatosensory
neurons and its involvement in sensory input-dependent plasticity.
Int J Mol Sci. 19:E692017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Julio-Pieper M, Hyland NP, Bravo JA, Dinan
TG and Cryan JF: A novel role for the metabotropic glutamate
receptor-7: Modulation of faecal water content and colonic
electrolyte transport in the mouse. Br J Pharmacol. 160:367–375.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Golubeva AV, Moloney RD, O'Connor RM,
Dinan TG and Cryan JF: Metabotropic glutamate receptors in central
nervous system diseases. Curr Drug Targets. 17:538–616. 2016.
View Article : Google Scholar
|
|
8
|
Julio-Pieper M, O'Connor RM, Dinan TG and
Cryan JF: Regulation of the brain-gut axis by group III
metabotropic glutamate receptors. Eur J Pharmacol. 698:19–30. 2013.
View Article : Google Scholar
|
|
9
|
Kłak K, Pałucha A, Brański P, Sowa M and
Pilc A: Combined administration of PHCCC, a positive allosteric
modulator of mGlu4 receptors and ACPT-I, mGlu III receptor agonist
evokes antidepressant-like effects in rats. Amino Acids.
32:169–172. 2007. View Article : Google Scholar
|
|
10
|
Stachowicz K, Brañski P, Kłak K, van der
Putten H, Cryan JF, Flor PJ and Andrzej P: Selective activation of
metabotropic G-protein-coupled glutamate 7 receptor elicits
anxiolytic-like effects in mice by modulating GABAergic
neurotransmission. Behav Pharmacol. 19:597–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Chaer ED, Kawasaki M and Pasricha PJ: A
new model of chronic visceral hypersensitivity in adult rats
induced by colon irritation during postnatal development.
Gastroenterology. 119:1276–1285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palucha A, Klak K, Branski P, van der
Putten H, Flor PJ and Pilc A: Activation of the mGlu7 receptor
elicits antidepressant-like effects in mice. Psychopharmacology
(Berl). 194:555–562. 2007. View Article : Google Scholar
|
|
13
|
Shah E, Rezaie A, Riddle M and Pimentel M:
Psychological disorders in gastrointestinal disease: Epiphenomenon,
cause or consequence. Ann Gastroenterol. 27:224–230. 2014.
|
|
14
|
Gros DF, Antony MM, McCabe RE and Swinson
RP: Frequency and severity of the symptoms of irritable bowel
syndrome across the anxiety disorders and depression. J Anxiety
Disord. 23:290–296. 2009. View Article : Google Scholar
|
|
15
|
Yan C, Xin-Guang L, Hua-Hong W, Jun-Xia L
and Yi-Xuan L: Effect of the 5HT4 receptor and serotonin
transporter on visceral hypersensitivity in rats. Braz J Med Biol
Res. 45:948–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoffman JM, Tyler K, MacEachern SJ,
Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL,
Galligan JJ, et al: Activation of colonic mucosal 5-HT(4) receptors
accelerates propulsive motility and inhibits visceral
hypersensitivity. Gastroenterology. 142:844–854. e8442012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paragomi P, Rahimian R, Kazemi MH,
Gharedaghi MH, Khalifeh-Soltani A, Azary S, Javidan AN, Moradi K,
Sakuma S and Dehpour AR: Antinociceptive and antidiarrheal effects
of pioglitazone in a rat model of diarrhoea-predominant irritable
bowel syndrome: Role of nitric oxide. Clin Exp Pharmacol Physiol.
41:118–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J: Correlation between
anxiety-depression status and cytokines in diarrhea-predominant
irritable bowel syndrome. Exp Ther Med. 6:93–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. The National Academies Press;
Washington, DC: 1996
|
|
21
|
van den Wijngaard RM, Stanisor OI, van
Diest SA, Welting O, Wouters MM, Cailotto C, de Jonge WJ and
Boeckxstaens GE: Susceptibility to stress induced visceral
hypersensitivity in maternally separated rats is transferred across
generations. Neurogastroenterol Motil. 25:e780-e7902013. View Article : Google Scholar
|
|
22
|
Li L, Xie R, Hu S, Wang Y, Yu T, Xiao Y,
Jiang X, Gu J, Hu CY and Xu GY: Upregulation of cystathionine
beta-synthetase expression by nuclear factor-kappa B activation
contributes to visceral hypersensitivity in adult rats with
neonatal maternal deprivation. Mol Pain. 8:892012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumoto K, Takagi K, Kato A, Ishibashi
T, Mori Y, Tashima K, Mitsumoto A, Kato S and Horie S: Role of
transient receptor potential melastatin 2 (TRPM2) channels in
visceral nociception and hypersensitivity. Exp Neurol. 285:41–50.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van den Wijngaard RM, Stanisor OI, van
Diest SA, Welting O, Wouters MM, de Jonge WJ and Boeckxstaens GE:
Peripheral α-helical CRF (9-41) does not reverse stress-induced
mast cell dependent visceral hypersensitivity in maternally
separated rats. Neurogastroenterol Motil. 24:274–282. e1112012.
View Article : Google Scholar
|
|
25
|
Li X, Xi ZX and Markou A: Metabotropic
glutamate 7 (mGlu7) receptor: A target for medication development
for the treatment of cocaine dependence. Neuropharmacology.
66:12–23. 2013. View Article : Google Scholar :
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Xiao J, Shao L, Shen J, Jiang W, Feng Y,
Zheng P and Liu F: Effects of ketanserin on experimental colitis in
mice and macrophage function. Int J Mol Med. 37:659–668. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao J, Lu Z, Sheng J, Song Y, Jiang W,
Liu F and Zheng P: 5-fluorouracil attenuates dextran sodium
sulfate-induced acute colitis in mice. Mol Med Rep. 13:2821–2828.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karrasch T and Jobin C: NF-kappaB and the
intestine: Friend or foe. Inflamm Bowel Dis. 14:114–124. 2008.
View Article : Google Scholar
|
|
30
|
Wouters MM: Histamine antagonism and
postinflammatory visceral hypersensitivity. Gut. 63:1836–1837.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hornby PJ: Receptors and transmission in
the brain-gut axis. II. Excitatory amino acid receptors in the
brain-gut axis. Am J Physiol Gastrointest Liver Physiol.
280:G1055–G1060. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang HJ, Yoo BC, Lim SB, Jeong SY, Kim WH
and Park JG: Metabotropic glutamate receptor 4 expression in
colorectal carcinoma and its prognostic significance. Clin Cancer
Res. 11:3288–3295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tong Q and Kirchgessner AL: Localization
and function of metabotropic glutamate receptor 8 in the enteric
nervous system. Am J Physiol Gastrointest Liver Physiol.
285:G992–G1003. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Z, Gao J and Wei L: Changes of
expressions of NR2A and NR2B in anterior cingulate cortex of
post-inflammatory visceral hypersensitive rats. Chin J
Gastroenteml. 15:330–334. 2010.In Chinese.
|
|
35
|
Pałucha-Poniewiera A, Szewczyk B and Pilc
A: Activation of the mTOR signaling pathway in the
antidepressant-like activity of the mGlu5 antagonist MTEP and the
mGlu7 agonist AMN082 in the FST in rats. Neuropharmacology.
82:59–68. 2014. View Article : Google Scholar
|
|
36
|
Wang WY, Wang H, Luo Y, Jia LJ, Zhao JN,
Zhang HH, Ma ZW, Xue QS and Yu BW: The effects of metabotropic
glutamate receptor 7 allosteric agonist N,
N'-dibenzhydrylethane-1,2- diamine dihydrochloride on developmental
sevoflurane neurotoxicity: Role of extracellular signal-regulated
kinase 1 and 2 mitogen-activated protein kinase signaling pathway.
Neuroscience. 205:167–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohman L and Simrén M: Pathogenesis of IBS:
Role of inflammation, immunity and neuroimmune interactions. Nat
Rev Gastroenterol Hepatol. 7:163–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li YQ, Zhang HN, Zuo XL, Yuan HP, Lu XF
and Li MJ: Study on the shifting of Thl/Th2 balance of large
intestinal mueosa in patients with irritable bowel syndrome. Chin J
of Dig. 24:728–731. 2004.In Chinese.
|
|
39
|
Kühn R, Löhler J, Rennick D, Rajewsky K
and Müller W: Interleukin-10-deficient mice develop chronic
enterocolitis. Cell. 75:263–274. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O'Malley D, Liston M, Hyland NP, Dinan TG
and Cryan JF: Colonic soluble mediators from the maternal
separation model of irritable bowel syndrome activate submucosal
neurons via an interleukin-6-dependent mechanism. Am J Physiol
Gastrointest Liver Physiol. 300:G241–G252. 2011. View Article : Google Scholar
|
|
41
|
Chen ZY, Zhang XW, Yu L, Hua R, Zhao XP,
Qin X and Zhang YM: Spinal toll-like receptor 4-mediated signalling
pathway contributes to visceral hypersensitivity induced by
neonatal colonic irritation in rats. Eur J Pain. 19:176–186. 2015.
View Article : Google Scholar
|
|
42
|
Rodella L, Rezzani R, Agostini C and
Bianchi R: Induction of NADPH-diaphorase activity in the rat
periaqueductal gray matter after nociceptive visceral stimulation.
Brain Res. 793:333–336. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Waal Malefyt R, Haanen J, Spits H,
Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H
and de Vries JE: Interleukin 10 (IL-10) and viral IL-10 strongly
reduce antigen-specific human T cell proliferation by diminishing
the antigen-presenting capacity of monocytes via downregulation of
class II major histocompatibility complex expression. J Exp Med.
174:915–924. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Akiho H, Deng Y, Blennerhassett P,
Kanbayashi H and Collins SM: Mechanisms underlying the maintenance
of muscle hypercontractility in a model of postinfective gut
dysfunction. Gastroenterology. 129:131–141. 2005. View Article : Google Scholar : PubMed/NCBI
|