Introduction
Cardiovascular disease is one of the major threats
to human life and health, and vascular senescence is an important
cause of its occurrence. Vascular senescence is also considered to
be an independent risk factor for cardiovascular diseases (1). Senescence, which is thought to be
irreversible, is considered to contribute to alteration in cell
function, morphology, and gene expression (2), and thus has an important role in
diseases, including type 2 diabetes, cancer, neurodegeneration, and
age-associated cardiovascular diseases, such as atherosclerosis
(3). It is thought that vascular
smooth muscle cells (VSMCs) have a key role in vascular aging and
contribute to the initiation and progression of atherosclerosis
(4,5). Since no physiological stimuli are
known currently to cause senescent cells to re-enter the cell
cycle, the treatment of senescence remains a challenge (6). Thus, an in-depth understanding of
the molecular mechanisms of senescence and of potential molecular
targets for drug design is an important research direction for the
treatment of senescence.
Along with age and cancer (5), autophagy is considered to be another
factor affecting senescence (7).
Studies have demonstrated that autophagy has a crucial role in the
regulation of cellular senescence, through degradation of
aggregate-prone proteins and damaged organelles (8). The autophagy process is associated
with many proteins and signaling pathways, such as the autophagy
proteins autophagy-related gene 6 (Atg6)/Beclin1, and the
AMP-activated protein kinase (AMPK) and mammalian target of
rapamycin (mTOR) pathways (9-11).
Studies have demonstrated that the inhibition of mTOR promotes
longevity and expression of autophagy biomarkers, and that the
complex formed by Atg6/Beclin1 and phosphoinositide 3-kinase (PI3K)
was responsible for autophagosome formation (7,9,12).
However, the relation of autophagy-related signaling with
senescence requires further study.
Rapamycin, an antibiotic that stimulates autophagy
by inhibition of mTOR signaling (13), is thought to also influence the
aging process (14). As
previously reported, rapamycin suppresses replicative senescence in
rodent embryonic cells (15), and
is involved in regulation of cell senescence by different
mechanisms (16). A previous
study revealed that rapamycin treatment in mice promotes healthy
longevity by targeting aging, leading to increased lifespan and
health span (14). Additionally,
it was reported that microRNA (miR)-30a, also known as an
aged-related miRNA (17,18), regulates rapamycin-induced
autophagy in cancer cells by targeting Beclin1 (19). Furthermore, rapamycin also partly
decreases the effect of miR-30a on osteosarcoma cell apoptosis, by
activating autophagy through regulating Beclin1 and
microtubule-associated protein 1 light chain 3 β (LC3B) (20). However, deeper insights between
rapamycin and miR-30a still lack in vascular senescence.
To date, no study has focused on whether rapamycin
could regulate vascular senescence by modulating miR-30a and
autophagy. The present study aimed to investigate the effects of
rapamycin on miR-30a, as well as on autophagy and senescence, in
VSMCs.
Materials and methods
Cell culture and treatment
VSMC isolation and cell culture have been previously
described (21). The present
study was approved by the Ethics Committee of the Department of
Laboratory Animal Science, Central South University (Changsha,
China) prior to the experiments. Briefly, VSMCs were isolated from
the thoracic aorta of SD rats. A total of 6 male Sprague-Dawley
rats aged 5-6 weeks and weighting 160-220 g were purchased from
Human SJA Laboratory Animal Co., Ltd. (Changsha, China). All
animals were housed in micro-isolator cages with free access to
food and water in a light-controlled room under a 12/12 h
light/dark cycle and controlled temperature (23-25°C). Aortic VSMCs
were then cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich, Merck KGaA, Darmstradt, Germany) supplemented with
10% Gibco fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 100 µg/ml
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C and 5%
CO2. VSMCs of passage 5 (young) and passage 15 (aging)
at 70-90% confluence were used. All cells were divided into four
groups: Young VSMCs, young VSMCs with rapamycin, aging VSMCs, and
aging VSMCs with rapamycin. For the groups treated with rapamycin,
VSMCs were treated with 20 nM rapamycin (Sigma-Aldrich; Merck KGaA)
for 12 h. The untreated cells were used as controls.
Cell transfection
The miR-30a mimics and negative control (NC) were
chemically synthesized by GenePharma Co., Ltd. (Shanghai, China).
The sequences of miR-30a mimics and miR-NC were
5′-UGUAAACAUCCUCGACUGGAAG-3′ and 5′-UUCUCCGAACGUGUCACGUTT-3′,
respectively. The aging cells were pre-transfected with miR-30a
mimics or miR-NC with a final concentration of 50 nM for 48 h,
prior to treatment with rapamycin for 12 h. Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect
oligonucleotides and constructs into cell lines, according to the
manufacturer's instructions. The infection efficiency was confirmed
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) or western blotting 48 h post-transfection.
Cell cycle analysis
For cell cycle analysis, cells were fixed with 70%
cold ethanol overnight at 4°C, washed with PBS, and then stained
with 5 mg/ml propidium iodide in the presence of RNase A (10
µg/ml) for 30 min. Cell cycle phase distribution was
analyzed by flow cytometry as reported elsewhere (22).
Senescence-associated
(SA)-β-Galactosidase (gal) staining
Measurement of cellular senescence was conducted by
SA-β-gal staining. Briefly, cells were fixed with 4% formaldehyde
for 15 min at room temperature, washed with PBS, and then incubated
at 37°C overnight in SA-β-gal staining solution (1 mg/ml of X-gal;
40 mmol/l citric acid/sodium phosphate buffer, pH 6.0; 5 mmol/l
potassium ferrocyanide; 5 mmol/l potassium ferricyanide; 150
mmol/lNaCl; and 2 mmol/l MgCl2). Next day, the slides
were washed twice in PBS, mounted in glycerol, and observed in five
optical fields per sample using a light microscope.
Immunofluorescence
Immunofluorescence was conducted to evaluate the
expression of LC3B. The cells were fixed with 4% formaldehyde for
15 min at room temperature, permeabilized with 0.1% Triton X-100 in
PBS for 15 min at room temperature and then incubated with
anti-LC3B rabbit antibodies (cat. no. ab48394, 1:200; Abcam,
Cambridge, MA, USA) overnight at 4°C. The cells were then incubated
with Alexa Fluor® 594-conjugated goat anti-rabbit IgG
antibodies (cat. no. ab150080; 1:1,000; Abcam) for 1 h at room
temperature. The nuclei of ells were then counterstained with DAPI
for 10 min at room temperature. A Leica TCS-SP laser scanning
confocal microscope was used to capture the photomicrographs in
five optical fields per sample.
Dual luciferase reporter assay
For the dual luciferase reporter assay, the wild
type (WT) or a mutant (MUT) 3′-untranslated region (UTR) sequence
of Beclin1 was amplified and subcloned into the pGL3-basic
luciferase vector (Promega Corporation, Madison, WI, USA). VSMCs
were co-transfected with either the WT or MUT 3′-UTR sequence of
Beclin1 (1 µg), together with 25 nM miR-30a mimics or
negative control. After 48 h of transfection, relative luciferase
activity was measured with the Bright-Glo Luciferase Assay System
(Promega Corporation).
RT-qPCR
The expression levels of miR-30a and Beclin1 were
determined by RT-qPCR. Briefly, total RNA was extracted from VSMCs
with TRIzol reagent (Tiangen Biotech, Beijing, China) according to
the manufacturer's protocol. The PrimeScriptOne-Step RT-PCR kit
(Takara Biotechnology Co., Ltd., Dalian, China) was used to convert
RNA into cDNA. PCR reactions were conducted using
SYBR®-Green PCR Master Mix (Takara Biotechnology Co.,
Ltd.) in an ABI7500 System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers sequences for Beclin1 were: Forward
5′-GTGCTCCTGTGGAATGGAAT-3′ and reverse 5′-TGCAACACAGTCCAGAAAAGC-3′.
The primers for miR-30a were purchased from Takara Biotechnology
Co., Ltd., and were: Forward
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTTCCA-3′ and
reverse 5′-GGCGTGTAAACATCCTCGAC-3. The primers sequences for GAPDH
were: Forward 5′-GGATTTGGTCGTATTGGG-3′ and reverse
5′-GGAAGATGGTGATGGGATT-3′. The primers sequences for U6 were:
Forward 5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse
5′-GGAACGCTTCACGAATTT G-3′. Relative fold changes in mRNA
expression were calculated using the 2−ΔΔCq formula
(23). GAPDH and U6 served as
internal controls for mRNA and miRNA respectively.
Western blot analysis
The protein levels of LC3, p62, Beclin1, mTOR,
phosphorylated (p-) mTOR, p53, p21, p16 and SA-β-gal were
determined by western blotting. Briefly, total protein was
extracted from VSMCs using a RIPA buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and was quantitated with protein
assay reagent from Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
Then equal amount of samples (30 µg) were loaded on 10%
SDS-PAGE, followed by transfer to PVDF membranes. After blocking
with 5% non-fat milk at room temperature for 1 h, the membranes
were then incubated with primary antibodies (all purchased from
Abcam) targeting LC3B (cat. no. ab48394; 2 µg/ml), p62 (cat.
no. ab56416; 5 µg/ml), Beclin1 (cat. no. ab62557; 2
µg/ml), p-Beclin1 (cat. no. ab183335; 1:250), mTOR (cat. no.
ab2732; 1:2,000), p-mTOR (S2448; cat. no. ab109268; 1:100),
p-ribosomal protein S6 kinase B1 (S6K1; cat. no. ab2571; 1:500),
p-eukaryotic translation initiation factor 4E binding protein 1
(4EBP1; cat. no. ab75767; 1:1,000), p53 (cat. no. ab131442;
1:1,000), p21 (cat. no. ab109520; 1:1,000), p16 (cat. no. ab51243;
1:10,000), SA-β-gal (cat. no. ab9361; 0.5 µg/ml) and GAPDH
(cat. no. ab8245; 1:500) at 4°C overnight. Subsequently, the
membranes were incubated with the corresponding horseradish
peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG;
cat. no. ab6940 and goat anti-mouse IgG; cat. no. ab97035; both
1:500) at 37°C for 45 min. The target bands were then developed
using Super Signal West Pico Chemiluminescent Substrate kit
(Pierce; Thermo Fisher Scientific, Inc.) and analyzed by Image-Pro
Plus software (version 6.0; Media Cybernetics, Inc., Rockville, MD,
USA). GAPDH served as an internal control.
Statistical analysis
All experiments were performed at least three
independent times in triplicate. Data were presented as mean ±
standard deviation. Statistical analyses were performed with
Graphpad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Comparisons were conducted using one-way analysis of variance
followed by Tukey post hoc test for multiple comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Rapamycin inhibits senescence in VSMCs
and alleviates cell cycle arrest
SA-β-gal staining was conducted to examine the
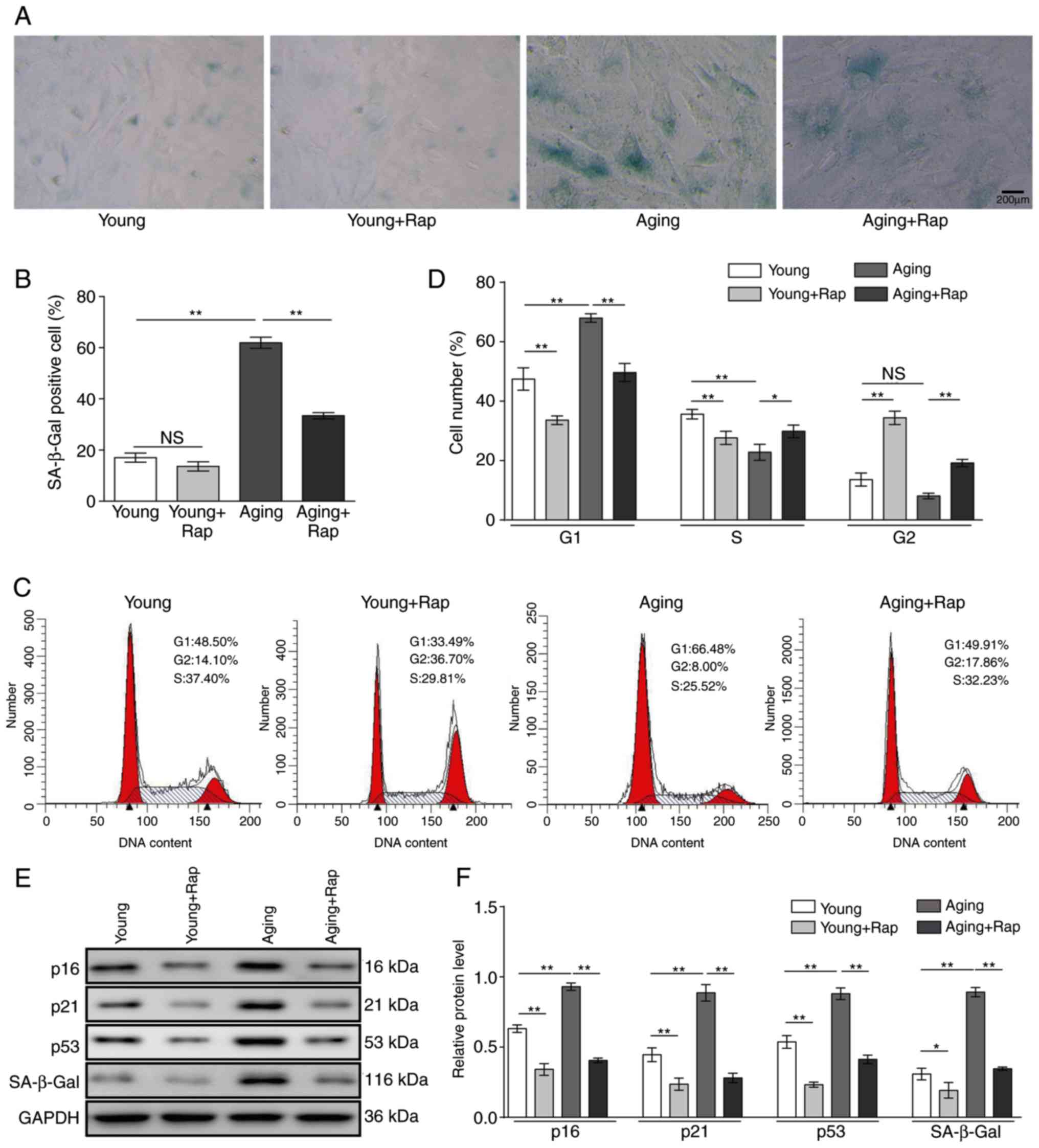
effect of rapamycin on senescence of VSMCs. As illustrated in
Fig. 1A and B, the ratio of
SA-β-gal-positive cells was significantly higher in aging cells
compared with young cells (P<0.01). However, following rapamycin
treatment, the ratio of SA-β-gal-positive cells significantly
decreased in aging cells (Fig.
1B), indicating that rapamycin could inhibit senescence in
VSMCs.
Flow cytometry analysis revealed that G1 arrest
occurred in aging cells and this cell cycle arrest was reduced when
cells were treated with rapamycin (Fig. 1C and D). Further analysis of p16,
p21, p53 and SA-β-gal expression levels by western blotting
demonstrated that rapamycin treatment reduced the increasing levels
of senescence-related proteins in aging cells (Fig. 1E and F). These results suggested
that rapamycin could inhibit senescence of VSMCs through inhibition
of cell cycle arrest and senescence-related proteins.
Rapamycin inhibits miR-30a expression and
promotes autophagy in VSMCs
To examine the effect of rapamycin on the expression
of miR-30a and autophagy of VSMCs, the expression of miR-30a and
Beclin1 was determined by RT-qPCR. The results demonstrated that in
aging cells miR-30a was significantly upregulated while Beclin1 was
significantly downregulated compared with young cells (P<0.01;
Fig. 2A and B), indicating that
both miR-30a and Beclin1 might be associated with senescence in
VSMCs. However, when treated with rapamycin, miR-30a was
signifi-cantly downregulated in both young and aging cells
(P<0.05; Fig. 2A).
Additionally, the expression of Beclin1 was significantly
upregulated in both young and aging cells following rapamycin
treatment (P<0.05; Fig. 2B).
These results clearly demonstrated that rapamycin inhibited miR-30a
expression while it induced Beclin1 expression.
 | Figure 2Rapamycin inhibits miR-30a expression
and promotes autophagy in VSMCs. (A) Relative expression levels of
miR-30a and (B) Beclin1 were determined by reverse
transcription-quantitative polymerase chain reaction in young and
aging VSMCs treated with 20 nM rapamycin for 12 h. (C)
Representative blots of LC3, Beclin1, p-Beclin1, p62, mTOR, p-mTOR,
p-S6K1 and p-4EBP1 protein expression levels in young and aging
VSMCs treated with 20 nM rapamycin for 12 h. (D) Quantitative
analysis for LC3-II/LC3-I ratio, Beclin1, p62, mTOR and p-mTOR
protein expression. (E) Immunofluorescence analysis for LC3B in
young and aging VSMCs treated with 20 nM rapamycin for 12 h.
Results are presented as mean ± standard deviation (n=3).
*P<0.05 and **P<0.01, with comparisons
indicated by lines. VSMCs, vascular smooth muscle cells; LC3,
microtubule-associated protein 1 light chain 3 β; p-,
phosphorylated; mTOR, mammalian target of rapamycin; S6K1,
ribosomal protein S6 kinase B1; 4EBP1, eukaryotic translation
initiation factor 4E binding protein 1; Rap, rapamycin. |
To further investigate the effect of rapamycin on
autophagy in VSMCs, the expression of LC3, Beclin1, p62, mTOR and
p-mTOR was determined by western blotting. As illustrated in
Fig. 2C and D, the ratio of
LC3-II/LC3-I and the expression of Beclin1 and p-Beclin1 were
significantly downregulated, while the expression of p62, mTOR,
p-mTOR, p-S6K1 and p-4EBP1 was significantly upregulated in aging
cells compared with young cells (P<0.05). These findings
indicate that autophagy-related proteins and mTOR signaling were
associated with cell aging. Similarly, when treated with rapamycin,
the ratio of LC3-II/LC3-I and the expression of both Beclin1 and
p-Beclin1 were all significantly upregulated, while the expression
of p62, mTOR, p-mTOR, p-S6K1 and p-4EBP1 significantly
down-regulated (Fig. 2C and D).
Immunofluorescence analysis of LC3B protein expression demonstrated
the same results (Fig. 2E). In
both young and aging cells, when treated with rapamycin, the
expression of LC3B markedly increased compared with the untreated
cells (Fig. 2E), suggesting that
rapamycin might promote autophagy of VSMCs to influence the cell
aging process.
miR-30a directly downregulates
Beclin1
To investigate the relationship between miR-30a and
Beclin1, aging cells were treated with rapamycin or miR-30a mimics
and the expression of miR-30a and Beclin1 was determined. The
results demonstrated that expression of miR-30a was significantly
upregulated following transfection with miR-30a mimics and
significantly downregulated following rapamycin treatment
(P<0.01; Fig. 3A). This
indicated that the transfection of miR-30a mimics was successful.
When treated with miR-30a mimics, expression of Beclin1
significantly decreased in aging cells, and the rapamycin-induced
upregulation of Beclin1 was significantly reversed at both the mRNA
and protein levels (P<0.01; Fig.
3B-D), indicating that miR-30a could downregulate the
expression of Beclin1.
To further examine the function of miR-30a on
regulating Beclin1, a dual luciferase reporter assay was conducted.
Bioinformatics analysis by software target scan 5.1 (http://www.targetscan.org) revealed that there is a
potential miR-30a binding site on the 3′-UTR of Beclin1 in rats
(Fig. 3E). Furthermore, the
sequence of miR-30a was identical and the homology of Beclin1 was
high in both rat and human, so the binding sites of miR-30a and
Beclin1 were the same in rat and human (Fig. 3E). The results from the luciferase
assay demonstrated that the relative luciferase activity in
WT-Beclin1 was significantly downregulated by miR-30a mimics, while
no significant change was observed with MUT-Beclin1 (Fig. 3F), further confirming that miR-30a
directly bound to the Beclin1 3′-UTR.
Rapamycin alleviates senescence and cell
cycle arrest in VSMCs by inhibiting miR-30a
At last, the present study investigated the effects
of miR-30a on senescence and the cell cycle in VSMCs. Similar to
the aforementioned results, the ratio of SA-β-gal positive cells
was significantly upregulated when cells were transfected with
miR-30a mimics (P<0.01; Fig. 4A
and B). In addition, the rapamycin inhibitory effect on
senescence of VSMCs was significantly reversed by overexpression of
miR-30a (P<0.01; Fig. 4A and
B). Notably, transfection reagent Lipofectamine 2000 alone had
no significant effect on VSMC senescence (Fig. 4A and B).
 | Figure 4Rapamycin alleviates senescence and
cell cycle arrest in VSMCs by inhibiting miR-30a. VSMCs were
transfected with miR-30a mimics or negative control for 48 h, and
then treated with 20 nM rapamycin for 12 h. (A) Senescence was
determined by SA-β-gal staining. Representative images are shown.
Scale bar, 300 µm. (B) SA-β-gal-positive cell rates in the
different groups. (C) Representative plots and (D) quantification
of flow cytometry analysis for cell cycle phase distribution. (E)
Protein expression levels of p16, p21, p53 and SA-β-gal were
determined by western blotting. Representative blots are shown. (F)
Quantitative analysis of indicated proteins. Results are presented
as mean ± standard deviation (n=3). *P<0.05 and
**P<0.01, with comparisons indicated by lines. VSMCs,
vascular smooth muscle cells; SA-β-gal,
senescence-associated-β-Galactosidase; NC, negative control; Rap,
rapamycin; ns, not significant. |
Flow cytometry analysis revealed that G1 arrest was
significantly promoted in aging cells following transfection with
miR-30a mimics (Fig. 4C and D).
Western blotting results demonstrated that overexpression of
miR-30a could significantly increase the levels of the
senescence-related proteins p16, p21, p53 and SA-β-gal (P<0.01;
Fig. 4E and F). Furthermore, the
rapamycin inhibition effects on both cell cycle arrest and
senescence-related protein expression were reversed upon
transfection with miR-30a mimics (P<0.05; Fig. 4E and F). These results suggested
that miR-30a could promote senescence and cell cycle arrest in
VSMCs, and that rapamycin might inhibit senescence of VSMCs through
inhibition of miR-30a.
Rapamycin promotes autophagy in VSMCs via
inhibiting miR-30a
The effect of miR-30a on autophagy of VSMCs was then
investigated by determining the protein expression of
autophagy-related proteins LC3, Beclin1, p62, mTOR, p-mTOR, p-S6K1
and p-4EBP1. As illustrated in Fig.
5A and B, the LC3-II/LC3-I ratio and Beclin1 expression levels
were significantly downregulated, while the expression levels of
p62, mTOR, p-mTOR, p-S6K1 and p-4EBP1 were significantly
upregulated in aging cells following transfection with miR-30a
mimics, suggesting that miR-30a could inhibit autophagy of aging
cells. Additionally, miR-30a mimics could significantly reverse the
alteration of all autophagy-related proteins induced by rapamycin
(Fig. 5A and B). Similar results
were also observed by immunofluorescence analysis of LC3B
expression. In both control cells and cells treated with rapamycin,
when transfected with miR-30a mimics, the expression of LC3B
markedly decreased (Fig. 5C). The
present results fully demonstrated that miR-30a could inhibit
autophagy of VSMCs and that rapamycin could induce autophagy of
VSMCs through inhibition of miR-30a.
 | Figure 5Rapamycin promotes autophagy in VSMCs
by inhibiting miR-30a. VSMCs were transfected with miR-30a mimics
or negative control for 48 h, and then treated with 20 nM rapamycin
for 12 h. (A) Protein expression levels of LC3, Beclin1, p62, mTOR,
p-mTOR, p-S6K1 and p-4EBP1 were determined by western blotting.
Representative blots are shown. (B) Quantitative analysis of
indicated proteins. (C) Immunofluorescence analysis for LC3B.
Results are presented as mean ± standard deviation (n=3).
*P<0.05 and **P<0.01, with comparisons
indicated by lines. VSMCs, vascular smooth muscle cells; LC3,
microtubule-associated protein 1 light chain 3 β; mTOR, mammalian
target of rapamycin; p-, phosphorylated; S6K1, ribosomal protein S6
kinase B1; 4EBP1, eukaryotic translation initiation factor 4E
binding protein 1; NC, negative control; Rap, rapamycin. |
Discussion
In the present study, rapamycin treatment was
demonstrated for the first time to upregulate Beclin1 and to
activate autophagy by downregulating miR-30a, which further
alleviated the senescence of VSMCs. The present study provided
novel insights into the inhibitory effects of rapamycin on
senescence of VSMCs, and may serve as a basis for a potential
clinical application of rapamycin in treatment of
senescence-related diseases.
Rapamycin could influence the senescence processes.
As reported, rapamycin inhibits senescence of mouse hematopoietic
stem cells (24), and modulates
cell senescence and inflammation by different mechanisms (16). A previous study focused on the
effect of rapamycin in aged mice and reported that rapamycin could
enhance the resistance of aged mice to pneumococcal pneumonia
through reduced cellular senescence (25). All the above results are
consistent with the present findings that rapamycin could inhibit
senescence. Additionally, the current study demonstrated that
rapamycin could inhibit cell cycle arrest. A study on the
relationship between rapamycin and normal aging demonstrated that
rapamycin could block cell aging by inhibiting cell cycle arrest
(26), which is consistent with
the current findings. The p53, p21 and p16 proteins are generally
considered to be associated with cell cycle arrest and the
activation of p53/p21 signaling is thought to contribute to cell
cycle arrest (27,28). In the present study, rapamycin was
demonstrated to inhibit the expression of cell cycle-related
proteins p53, p21 and p16, suggesting that rapamycin inhibited cell
cycle arrest via repressing the p53/p21 signaling pathway. These
results confirmed that rapamycin could alleviate senescence of
VSMCs as well as inhibit cell cycle arrest.
It has been reported that rapamycin promotes
autophagy in multiple cells or diseases, such as neural tissue
damage, melanoma cells and stem cells (16,29). However, the effects of rapamycin
on autophagy and related mechanisms in the process of vascular
senescence remain unclear. As reported, the serine/threonine kinase
unc-51 like autophagy activating kinase 1 (ULK1), an upstream
component of autophagy initiation, is phosphorylated and inhibited
by mTOR (11). Furthermore, ULK1
induces autophagy by phosphorylating Beclin1 (30) and the guanine nucleotide exchange
factor DENN domain containing 3 (DENND3) (31). A previous study demonstrated that
rapamycin inhibits ULK phosphorylation and induces autophagy
(32). This might imply that
rapamycin may promote ULK-mediated Beclin1 and DENND3
phosphorylation by inhibiting the effect of mTOR on ULK expression,
thereby resulting in autophagy. In the present study, it was
demonstrated that in both young and aging VSMCs, rapamycin induced
autophagy by increasing the LC3-II/LC3-I ratio and the expression
of Beclin1 and p-Beclin1, as well as inhibiting expression of
miR-30a and mTOR. Althought rapamycin is a well-known mTOR
inhibitor, the effect of rapamycin on mTOR expression levels is
different in different cell lines. It has been reported that
rapamycin has no effect on the expression of mTOR in the human
cells Hela, HEK293T and DU145 (33), however other studies have
demonstrated that rapamycin decreases mTOR levels in the human
U87-MG cell line (34) and mouse
bone marrow mesenchymal stem cells (35). This difference may be due to the
different cell lines, and requires further confirmation studies. In
the present study, rapamycin was demonstrated to decrease mTOR
levels in VSMCs. Additionally, miR-30a mimics have been reported to
blunt activation of rapamycin-induced autophagy induced in cancer
cells (19). Another study
demonstrated that rapamycin could partly decrease the expression of
miR-30a (20). Since miR-30a has
been proven to be a potent suppressor of autophagy (36,37), it can be speculated that the
promotion effect of rapamycin on autophagy might be partly due to
the downregulation of miR-30a.
miR-30a is considered an aging-related miRNA
(17). Studies have also showed
that miR-30a induces cell cycle arrest. Overexpression of miR-30a
increases cell apoptosis and induces cell cycle arrest in non-small
cell lung cancer (38). miR-30a
has also been demonstrated to promote cell cycle arrest at the G1
phase (39). The present study
revealed that miR-30a induced G1 cycle arrest and promoted cellular
senescence in aging VSMCs, while rapamycin significantly reversed
the miR-30a-mediated cycle arrest and senescence effects through
inhibition of p53/p21 signaling. These results suggested that
rapamycin alleviated cycle arrest of VSMCs via downregulating
miR-30a, which then further inhibited senescence of VSMCs.
Beclin1 is a factor closely related with autophagy,
and it is significantly upregulated when autophagy occurs. Studies
have already proven that Beclin1 is a target of miR-30a (19). miR-30a has also been demonstrated
to suppress Beclin1-mediated autophagy and further sensitized tumor
cells to cis-platinum (40). A
previous study reported that downregulation of miR-30a could
release cerebral chemic injury through enhancing Beclin1-mediated
autophagy (41). The present
study further demonstrated that overexpression of miR-30a could
inhibit the autophagy of VSMCs by regulating expression of Beclin1,
p62, p-mTOR and mTOR, by directly binding the 3′-UTR of Beclin1.
These results strongly indicate that rapamycin might induce
autophagy by inducing expression of Beclin1 through inhibition of
miR-30a in a vascular model. Although a previous study has
suggested the relationship among miR-30a, Beclin1 and rapamycin in
cancer cells (19), this had not
been elucidated in vascular cells and may be different depending on
cell type. To the best of our knowledge, the effects of rapamycin
and miR-30a on senescence have not been studied before in a
vascular model. The present study demonstrated for the first time
that rapamycin-induced miR-30a downregulation could alleviate VSMC
senescence by regulating autophagy.
In conclusion, the current study firstly
demonstrated that rapamycin inhibited the senescence of VSMCs by
downregulating miR-30a, which resulted in upregulation of Beclin1
and activation of autophagy. These results clearly demonstrated
that miR-30a might be a novel target for the induction of autophagy
by rapamycin, and rapamycin might be a potential treatment method
for senescence-related diseases.
Funding
This study was supported by the Youth Science
Project of National Natural Science Foundation of China (grant no.
81501212) and the Youth Project of Hunan Provincial Natural Science
Foundation Project (grant no. 2018JJ3768).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
PT and YL designed the experiments. PT, HW, JZ, XM
and XC performed the experiments. PT, YJW, YW and JZ analyzed the
data. PT and YL wrote and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The protocols involving animals were approved by the
Ethics Committee of the Department of Laboratory Animal Science,
Central South University (Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sueta D, Koibuchi N, Yu H, Toyama K,
Uekawa K, Katayama T, Ma MJ, Nakagawa T, Waki H, Maeda M, et al:
Blood pressure variability, impaired autonomic function and
vascular senescence in aged spontaneously hypertensive rats are
ameliorated by angiotensin blockade. Atherosclerosis. 236:101–107.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Uryga AK, Reinhold J, Figg N,
Baker L, Finigan A, Gray K, Kumar S, Clarke M and Bennett M:
Vascular smooth muscle cell senescence promotes atherosclerosis and
features of plaque vulnerability. Circulation. 132:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mistry Y, Poolman T, Williams B and
Herbert KE: A role for mitochondrial oxidants in stress-induced
premature senescence of human vascular smooth muscle cells. Redox
Biol. 1:411–417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campisi J: Aging, cellular senescence, and
cancer. Annu Rev Physiol. 75:685–705. 2013. View Article : Google Scholar
|
|
6
|
Birch J, Barnes PJ and Passos JF:
Mitochondria, telomeres and cell senescence: Implications for lung
ageing and disease. Pharmacol Ther. 183:34–49. 2018. View Article : Google Scholar
|
|
7
|
Gewirtz DA: Autophagy and senescence: A
partnership in search of definition. Autophagy. 9:808–812. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gewirtz DA: Autophagy and senescence in
cancer therapy. J Cell Physiol. 229:6–9. 2014.
|
|
9
|
Tan P, Wang YJ, Li S, Wang Y, He JY, Chen
YY, Deng HQ, Huang W, Zhan JK and Liu YS: The PI3K/Akt/mTOR pathway
regulates the replicative senescence of human VSMCs. Mol Cell
Biochem. 422:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong M, Leung L and Nighot PK: Role of
autophagy related protein ATG6/Beclin 1 in intestinal tight
junction barrier. Gastroenterology. 152(Suppl 1): S1192017.
View Article : Google Scholar
|
|
11
|
Kim J and Guan KL: Regulation of the
autophagy initiating kinase ULK1 by nutrients: Roles of mTORC1 and
AMPK. Cell Cycle. 10:1337–1338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pattingre S, Espert L, Biardpiechaczyk M
and Codogno P: Regulation of macroautophagy by mTOR and Beclin1
complexes. Biochimie. 90:313–323. 2008. View Article : Google Scholar
|
|
13
|
Zhan JK, Wang YJ, Wang Y, Wang S, Tan P,
Huang W and Liu YS: The mammalian target of rapamycin signalling
pathway is involved in osteoblastic differentiation of vascular
smooth muscle cells. Can J Cardiol. 30:568–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilkinson JE, Burmeister L, Brooks SV,
Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong
R, Wood LK, et al: Rapamycin slows aging in mice. Aging Cell.
11:675–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pospelova TV, Leontieva OV, Bykova TV,
Zubova SG, Pospelov VA and Blagosklonny MV: Suppression of
replicative senescence by rapamycin in rodent embryonic cells. Cell
Cycle. 11:2402–2407. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rong W, Zhen Y, Sunchu B, Caples K, Zhao
S, Dang I and Perez VI: Rapamycin modulates cell senescence and
inflammation by different mechanisms. Exp Gerontol. 94:126–127.
2017. View Article : Google Scholar
|
|
17
|
Muther C, Jobeili L, Garion M, Heraud S,
Thepot A, Damour O and Lamartine J: An expression screen for
aged-dependent microRNAs identifies miR-30a as a key regulator of
aging features in human epidermis. Aging (Albany NY). 9:2376–2396.
2017. View Article : Google Scholar :
|
|
18
|
Lin X, Zhan JK, Wang YJ, Tan P, Chen YY,
Deng HQ and Liu YS: Function, role, and clinical application of
MicroRNAs in vascular aging. Biomed Res Int. 2016:60213942016.
View Article : Google Scholar
|
|
19
|
Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X,
Liu CG and Yang JM: Regulation of autophagy by a beclin 1-targeted
microRNA, miR-30a, in cancer cells. Autophagy. 5:816–823. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu R, Liu S, Chen H and Lao L:
MicroRNA-30a downregulation contributes to chemoresistance of
osteosarcoma cells through activating Beclin-1-mediated autophagy.
Oncology Reports. 35:1757–1763. 2016. View Article : Google Scholar
|
|
21
|
Lee KY, Kim JR and Choi HC:
Genistein-induced LKB1-AMPK activation inhibits senescence of VSMC
through autophagy induction. Vascul Pharmacol. 81:75–82. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Qian X, Sun X, Lin C, Jing Y, Yao
Y, Ma Z, Kuai M, Lu Y, Kong X, et al: Liuwei Dihuang, a traditional
Chinese medicinal formula, inhibits proliferation and migration of
vascular smooth muscle cells via modulation of estrogen receptors.
Int J Mol Med. 42:31–40. 2018.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Luo Y, Li L, Zou P, Wang J, Shao L, Zhou D
and Liu L: Rapamycin enhances long-term hematopoietic
reconstitution of ex vivo expanded mouse hematopoietic stem cells
by inhibiting senescence. Transplantation. 97:20–29. 2014.
View Article : Google Scholar
|
|
25
|
Hinojosa CA, Mgbemena V, Van Roekel S,
Austad SN, Miller RA, Bose S and Orihuela CJ: Enteric-delivered
rapamycin enhances resistance of aged mice to pneumococcal
pneumonia through reduced cellular senescence. Exp Gerontol.
47:958–965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blagosklonny MV: Progeria, rapamycin and
normal aging: Recent breakthrough. Aging (Albany NY). 3:685–691.
2011. View Article : Google Scholar
|
|
27
|
Qi LW, Zhang Z, Zhang CF, Anderson S, Liu
Q, Yuan CS and Wang CZ: Anti-colon cancer effects of 6-Shogaol
through G2/M cell cycle arrest by p53/p21-cdc2/cdc25A crosstalk. Am
J Chin Med. 43:743–756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan D, Jiang C, Hua X, Wang T and Chai Y:
Cell cycle arrest and apoptosis induced by aspidin PB through the
p53/p21 and mitochondria-dependent pathways in human osteosarcoma
cells. Anticancer Drugs. 26:931–941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sotthibundhu A, McDonagh K, von Kriegsheim
A, Garcia-Munoz A, Klawiter A, Thompson K, Chauhan KD, Krawczyk J,
Mcinerney V, Dockery P, et al: Rapamycin regulates autophagy and
cell adhesion in induced pluripotent stem cells. Stem Cell Res
Ther. 7:1662016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Russell RC, Tian Y, Yuan H, Park HW, Chang
YY, Kim J, Kim H, Neufeld TP, Dillin A and Guan KL: ULK1 induces
autophagy by phosphorylating Beclin-1 and activating VPS34 lipid
kinase. Nat Cell Biol. 15:741–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu J, Fotouhi M and McPherson PS:
Phosphorylation of the exchange factor DENND3 by ULK in response to
starvation activates Rab12 and induces autophagy. EMBO Rep.
16:709–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiao YA, Kolwicz SC, Basisty N, Gagnidze
A, Zhang J, Gu H, Djukovic D, Beyer RP, Raftery D, MacCoss MJ, et
al: Rapamycin transiently induces mitochondrial remodeling to
reprogram energy metabolism in old hearts. Aging (Albany NY).
8:314–327. 2016. View Article : Google Scholar
|
|
33
|
Sarbassov DD, Ali SM, Kim DH, Guertin DA,
Latek RR, Erdjument-Bromage H, Tempst P and Sabatini DM: Rictor, a
novel binding partner of mTOR, defines a rapamycin-insensitive and
raptor-independent pathway that regulates the cytoskeleton. Curr
Biol. 14:1296–1302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang M, Ke Y, Sun X, Yu L, Yang Z, Zhang
Y, Du M, Wang J, Liu X and Huang SJ: Mammalian target of rapamycin
signaling is involved in the vasculogenic mimicry of glioma via
hypoxia-inducible factor-1α. Oncol Rep. 32:1973–1980. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gu Z, Tan W, Ji J, Feng G, Meng Y, Da Z,
Guo G, Xia Y, Zhu X, Shi G and Cheng C: Rapamycin reverses the
senescent phenotype and improves immunoregulation of mesenchymal
stem cells from MRL/lpr mice and systemic lupus erythematosus
patients through inhibition of the mTOR signaling pathway. Aging
(Albany NY). 8:1102–1114. 2016. View Article : Google Scholar
|
|
36
|
Fu XT, Shi YH, Zhou J, Peng YF, Liu WR,
Shi GM, Gao Q, Wang XY, Song K, Fan J and Ding ZB: MicroRNA-30a
suppresses autophagy-mediated anoikis resistance and metastasis in
hepatocellular carcinoma. Cancer Lett. 412:108–117. 2018.
View Article : Google Scholar
|
|
37
|
Zhang L, Cheng R and Huang Y: MiR-30a
inhibits BECN1-mediated autophagy in diabetic cataract. Oncotarget.
8:77360–77368. 2017.PubMed/NCBI
|
|
38
|
Geng GJ, Yang YT, Jiang J, Yu XY and Fa
XE: MicroRNA-30a suppresses non-small-cell lung cancer by targeting
Myb-related protein B. Exp Ther Med. 15:1633–1639. 2018.PubMed/NCBI
|
|
39
|
Wang X, Xu X, Wei W, Yu Z, Wen L, He K and
Fan H: MicroRNA-30a-5p promotes replication of porcine circovirus
type 2 through enhancing autophagy by targeting 14-3-3. Arch Virol.
162:2643–2654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen
X, Chen X, Zhang CY, Zhang Q and Zen K: MicroRNA-30a sensitizes
tumor cells to cis-platinum via suppressing beclin 1-mediated
Autophagy. J Biol Chem. 287:4148–4156. 2012. View Article : Google Scholar :
|
|
41
|
Wang P, Liang J, Li Y and Li J, Yang X,
Zhang X, Han S, Li S and Li J: Down-regulation of miRNA-30a
alleviates cerebral isch-emic injury through enhancing beclin
1-mediated autophagy. Neurochem Res. 39:1279–1291. 2014. View Article : Google Scholar : PubMed/NCBI
|