Introduction
Atherosclerosis (AS) is a common disease threatening
human health. In recent years, the morbidity of AS is markedly
increased in China (1). A
previous study demonstrated that the inflammatory response serves a
key role in the formation process of AS (2). Mononuclear macrophage and
lymphocyte, multiple growth factors, cytokines and adhesion
molecules are closely correlated with the formation, development
and rupture of the atherosclerotic plaque (2). Typically, coronary AS plaque rupture
and the induced thrombosis are the important causes leading to
acute coronary syndrome and death (3). Furthermore, plaque structure and
components also serve an important role in maintaining its
stability (1).
MicroRNA (miRNA/miR) is a class of highly conserved
non-coding small RNA about 19-25 bp in length, which (4) can bind with target genes (mRNA)
through base complementation or partial base complementation.
Therefore, it can degrade mRNA or restrain its translation and
regulate gene expression at a post-transcription level. miRNA
distribution in the human body is tissue specific, which serves a
key role in cell proliferation, transformation, migration,
metabolism and apoptosis (5).
AS is a complicated multi-factor disease, but its
pathogenesis remains unclear (6).
It is characterized by destroyed vascular endothelial cell
integrity, smooth muscle cell and fibroblast hyperplasia, and lipid
atherosclerotic plaque formation under the large and medium-sized
endarterium (7). Research
indicates that the acute and chronic overloads of reactive oxygen
species (ROS) exist in the whole process of AS, which will induce
endothelial cell injury and apoptosis. Therefore, ROS serves a key
role in the pathogenesis of AS. ROS mainly affect the growth,
migration, proliferation and activation of vascular cells (7). Under pathological conditions, ROS
are involved in inflammation, endothelial dysfunction, cell
proliferation and activation. Recent studies suggest that,
diabetes, hypertension, hyperlipidemia, obesity and smoking will
induce excessive ROS production (7,8).
Furthermore, they will increase lipid peroxidation injury to cells,
therefore promoting AS formation and development (8). Transforming growth factor (TGF)-β is
a multi-functional cytokine, which can regulate cell growth,
proliferation, differentiation, migration and apoptosis (9). In addition, it may stimulate the
synthesis and secretion of multiple active substances, including
cytokines, and inflammatory mediators. Therefore, it participates
in the extracellular matrix (ECM) constitution and degradation, and
has dual effects of suppression and promotion (10).
NADPH oxidase (NOX) is demonstrated in research to
be a major enzyme involved in ROS production in blood vessels
(11). Evidence proves that NOX4
expression in endothelial cells is markedly increased compared with
other Nox members (12). Research
data also prove that NOX4 serves a key role in endothelial redox
reactions and has an important functional regulatory effect on
endothelial cell physiology (13). Lai et al (14) demonstrated that miRNA-30e mediated
cardioprotection of ACE2 in rats with doxorubicin-induced heart
failure. The present study was designed to investigate the function
of miRNA-30e in atherosclerosis and to explore potential
mechanisms.
Materials and methods
Atherosclerosis in vivo model
C57BL/6 (n=8; male; 5-6 weeks; 18-20 g) and
Apoe−/− (n=8; 5-6 weeks; 18-20 g) mice were obtained
from the Animal center of Xiamen University (Xiamen, China). All
mice were housed at 22-23°C, 55-60% humidity, 12 h light and dark
cycle, and had free access to water. C57BL/6 mice were the normal
control group and were fed normal diet for 12 weeks. The
Apoe−/− mice were the AS group and were fed the high-fat
diet for 12 weeks. Animal experiments were approved by the Animal
Care and Utilization Committee of Xiamen Cardiovascular Hospital
Xiamen University. Following 12 weeks, mice were injected with 50
mg/kg pentobarbital sodium and then sacrificed using decollation.
The aorta vessel tissue was fixed using 4% para-formaldehyde for 24
h at room temperature and made paraffin sections (5 µm) for
Oil Red O staining at room temperature for 15 min. Tissues were
observed using a confocal fluorescent Zeiss Axioplan 2 microscope
(magnification, ×100; Carl Zeiss MicroImaging; Carl Zeiss AG,
Oberkochen, Germany).
Oxidative stress and ROS levels
Malondialdehyde (MDA; cat. no. A003-1), superoxide
dismutase (SOD; cat. no. A001-1-1), glutathione (GSH; cat. no.
A006-2) and glutathione-peroxidase (GSH-PX; cat. no. A005) levels
were measured using ELISA kits from cells or blood vessel tissue
(all from Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). The ROS level was measured in cells using ELISA kits (cat.
no. E004; Nanjing Jiancheng Bioengineering Institute). In addition,
ROS level was analyzed using ROS probe and observed using a Zeiss
Axioplan 2 (Carl Zeiss MicroImaging; Carl Zeiss AG).
Quantitative polymerase chain reaction
(qPCR) and gene microarray hybridization
Total RNA was extracted from cells or blood vessel
tissue using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Reverse transcription (RT) was
performed with the TaqMan miRNA RT kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) at 37°C for 30 min and 84°C for 10 sec.
qPCR was executed using the SYBR-Green Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) cycling profile: 95°C
for 20 sec, 40 cycles of amplification (95°C for 30 sec, 60°C for
30 sec and 72°C for 30 sec) and melt curve analysis. The primer
sequences for RT-qPCR: miR-30e 5′-GGG CAG TCT TTG CTA CTG TAA AC-3′
and 5′-GCC GCT GTA AAC ATC CGA CT-3′; U6 5′-GCT TCG GCA GCA CAT ATA
CTA AAA T-3′ and 5′-CGC TTC ACG AAT TTG CGT GTC AT-3′. The relative
expression levels of microRNA-30e were calculated using the
2−ΔΔCq method (15).
Total RNA was hybridized using SurePrint G3 Mouse
Whole Genome Microarray (G4471A-021828; Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). Images were quantified
using Agilent Feature Extraction Software (version A.10.7.3.1;
Agilent Technologies, Inc.).
Cell culture, transfection and
grouping
Human umbilical vein endothelial cells (HUVECs) were
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc.) at 37°C in 5% CO2. In total,
100 ng small interfering (si)-Nox4 (cat. no. sc-41586; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), 100 ng microRNA-30e (5′-UGU
AAA CAU CCU UGA CUG GAA G-3′), 100 ng anti-microRNA-30e (5′-UCC AGU
CAA GGA UGU UUA CAU U-3′), 100 ng Snai1 plasmid (5′-CAC TAT GCC GCG
TCT TTC C-3′), 100 ng TGF-β plasmid (5′-ACC CAT GCC TCC CTC TCG
GC-3′) and 100 ng negative mimics (5′-CCC CCC CCC CCC CC-3′) were
transfected into HUVECs (1×105 cells/ml) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.).
After transfection at 37°C for 6 h, the medium was subsequently
replaced with DMEM containing 10% fetal bovine serum for 42 h and
treated with 500 µmol/l of H2O2 for 16
h.
Luciferase reporter assays
The 3’ untranslated region (UTR) of Snai1 was cloned
into pMIR-REPORT luciferase reporter plasmids (Promega Corporation,
Madison, WI, USA). Snai1 Plasmids and anti-microRNA-30e mimics were
co-transfected into HUVECs using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.). Following transfection at 37°C
for 48 h, cells were lysed using a dual luciferase reporter assay
kit (Promega Corporation) according to the manufacturer’s protocol.
The absolute values of firefly luminescence were normalized to
Renilla luciferase activity.
Western blotting
Total proteins from myocardial tissue were extracted
using radioimmunoprecipitation buffer lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) and protein contents
were measured using bicinchoninic acid assay. A total of 50
µg protein lysate of each sample was stacking on 4% SDS-PAGE
and transferred onto polyvinylidene difluoride membrane. The
membrane was blocked with 5% skim milk powder at room temperature
for 2 h and incubated with Snai1 (cat. no. sc-271977; 1:1,000),
TGF-β (cat. no. sc-52892; 1:1,000; both Santa Cruz Biotechnology,
Inc.), Samd2 (cat. no. ab122028; 1:1,000), Nox4 (cat. no. ab133303;
1:1,000; both Abcam, Cambridge, USA) and GAPDH (cat. no. sc-293335;
1:5,000; Santa Cruz Biotechnology, Inc.) at 4°C overnight. On the
next day, membrane was washed with Tris buffered saline 0.1% Tween
20 for three times and incubated with horseradish peroxidase
labeled secondary antibody (cat. no. sc-2004; 1:5,000; Abcam) at
room temperature for 1 h. The absorbance of each band was analyzed
using Image Lab 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
following enhanced chemiluminescence (cat. no. P0018AS; Beyotime
Institute of Biotechnology) staining.
Statistical analysis
All the data are expressed as mean ± standard error
of the mean (n=3). Statistical analysis was performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Student’s t-test or one-way
analysis of variance and Tukey’s post hoc test was used to analyze
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of miRNA-30e in an AS
model
The effects and mechanisms of miRNA-30e in were
first evaluated in an AS model. MDA levels were significantly
increased, while the levels of SOD, GSH and GSH-PX were
significantly decreased in mice with AS, compared with the normal
control group (P<0.01; Fig.
1A-D). As presented in Fig.
1E, the number of thrombi in mice of AS was increased compared
with the normal control group. The expression of miRNA-30e in the
AS model group was significantly reduced, compared with the normal
mice group (P<0.01; Fig.
1F-G). In conclusion, these results demonstrated that miRNA-30e
may be involved in AS.
 | Figure 1Expression of miRNA-30e in an
atherosclerosis model. (A) MDA, (B) SOD, (C) GSH and (D) GSH-PX,
(E) Oil Red O staining, (F) heat map and (G) quantitative
polymerase chain reaction for miRNA-622 expression. Scale bar, 400
µm. **P<0.01 vs. the control normal group.
Normal, control normal group; Atherosclerosis, Atherosclerosis
model group; miR/miRNA, microRNA; GSH-PX, glutathione-peroxidase;
MDA, malondiadehyde; SOD, superoxide. |
Downregulation of miRNA-30e increases
oxidative stress and ROS
To investigate the function of miRNA-30e
downregulation in AS, anti-miRNA-30e was used to reduce the
expression of miRNA-30e. As presented in Fig. 2A, anti-miRNA-30e significantly
inhibited the expression of miRNA-30e in an in vitro model,
compared with the negative group (P<0.01). Downregulation of
miRNA-30e significantly increased MDA levels, while the levels of
SOD, GSH and GSH-PX were significantly decreased, and significantly
promoted ROS levels in an in vitro model, compared with the
negative group (P<0.01; Fig.
2B-G).
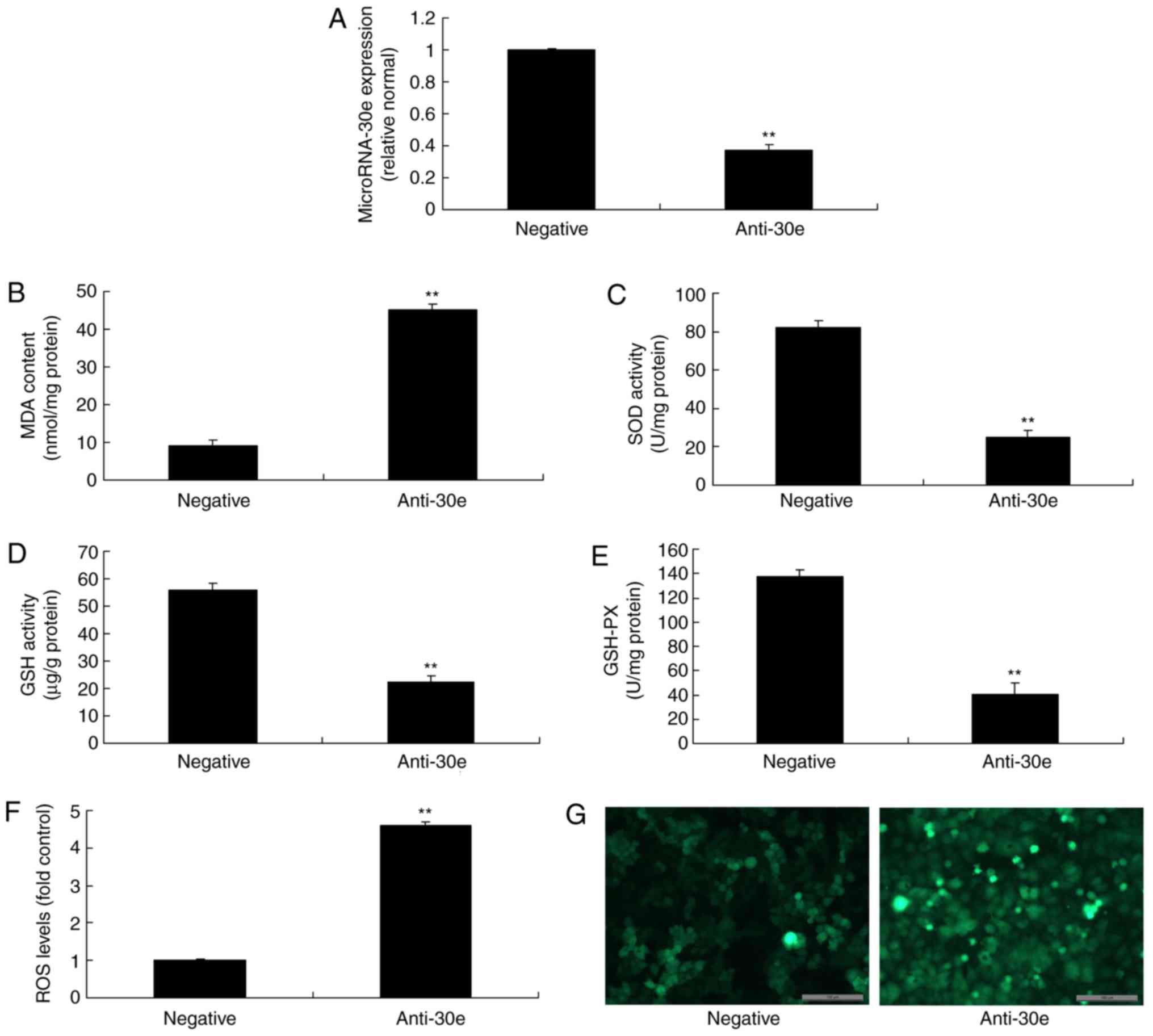 | Figure 2Downregulation of miRNA-30e increases
oxidative stress and ROS. (A) miRNA-30e expression, (B) MDA, (C)
SOD, (D) GSH and (E) GSH-PX, (F) ROS levels and (G) green
fluorescent protein staining of cells following anti-miRNA30e
transfection. Scale bar, 100 µm. **P<0.01 vs.
the negative normal group. miR/miRNA, microRNA; GSH-PX,
glutathione-peroxidase; MDA, malondiadehyde; SOD, superoxide; ROS,
reactive oxygen species; Negative, negative control group;
anti-30e, downregulation of microRNA-30e group. |
Over-expression of miRNA-30e reduces
oxidative stress and ROS
Then an miRNA-30e mimics was used to increase the
expression of miRNA-30e in an in vitro model, compared with
the negative group (Fig. 3A).
Overexpression of miRNA-30e reduced MDA levels, increased the
levels of SOD, GSH and GSH-PX, and decreased ROS levels in an in
vitro model, compared with the negative group (Fig. 3B-G). Therefore, it was concluded
that miRNA-30e regulated ROS and oxidative stress in AS.
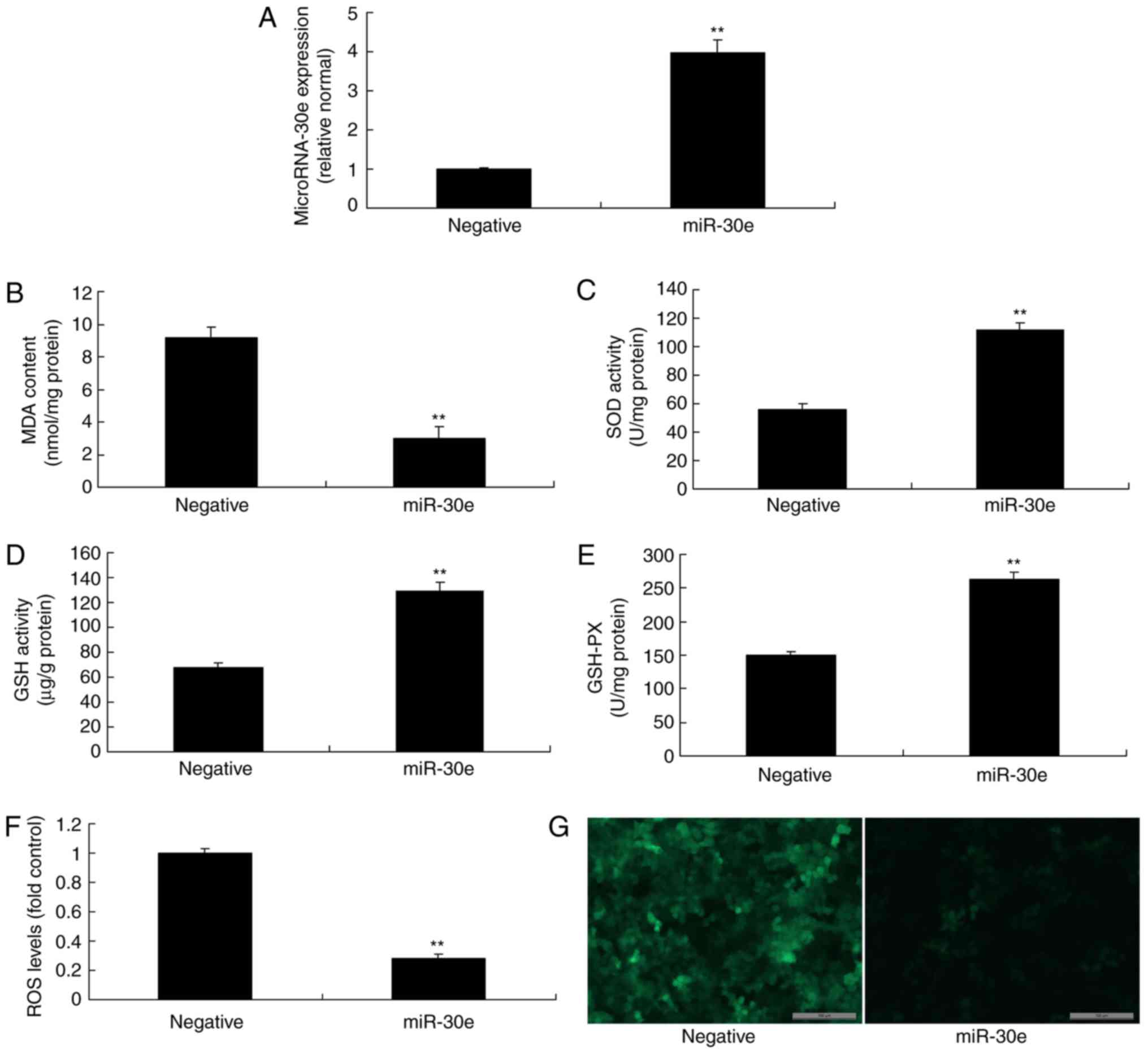 | Figure 3Overexpression of miRNA-30e reduces
oxidative stress and ROS. (A) miRNA-30e expression, (B) MDA, (C)
SOD, (D) GSH and (E) GSH-PX, (F) ROS levelsand (G) green
fluorescent protein staining of cells following anti-miRNA30e
transfection. Scale bar, 100 µm. **P<0.01 vs.
the negative normal group. miR/miRNA, microRNA; GSH-PX,
glutathione-peroxidase; MDA, malondiadehyde; SOD, superoxide; ROS,
reactive oxygen species; Negative, negative control group; miR-30e,
over-expression of microRNA-30e group. |
miRNA-30e regulates TGF-β-mediated
Nox4-dependent oxidative stress by Snai1
Furthermore, the mechanism of miRNA-30e on ROS and
oxidative stress was analyzed in AS. The results of the Gene chip
demonstrated that downregulation of miRNA-30e induced the
expression of Snai1 in vitro, compared with the negative
group (Fig. 4A). Snai1 was a
putative target of miRNA-30e, confirmed by Luciferase reporter
activity which was significantly increased in an in vitro
model, compared with the negative group (P<0.01; Fig. 4B and C). Downregulation of
miRNA-30e significantly induced the expression of the Snai1 protein
in an in vitro model, compared with the negative group
(P<0.01; Fig. 4D).
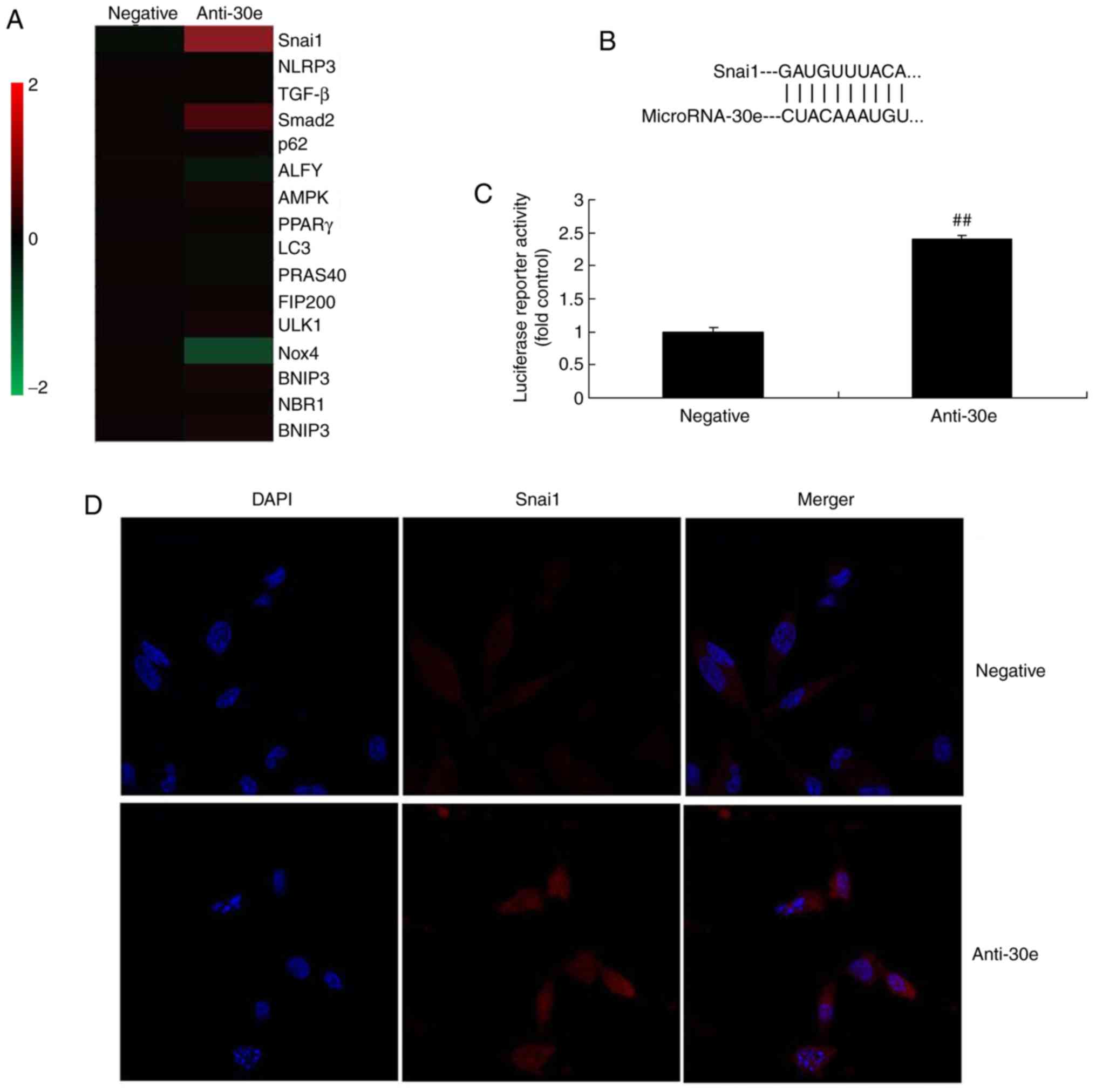 | Figure 4miRNA-30e regulates TGF-β-mediated
NADPH oxidase 4-dependent oxidative stress by Snai1. (A) Heat map
for signaling pathway, (B) Snai1 was a putative target of miRNA-30e
and (C) luciferase reporter activity, (D) Snai1 protein expression.
(E) Snai1, (F) TGF-β, (G) Smad2 and (H) Nox4 protein expression by
statistical analysis, and (I) western blotting analysis.
##P<0.01 vs. the negative normal group. miR/miRNA,
microRNA; TGF, transforming growth factor; Nox4, NAPDH oxidase 4;
Smad, mothers against decapentaplegic homolog; negative, negative
control group; anti-30e, downregulation of microRNA-30e group. |
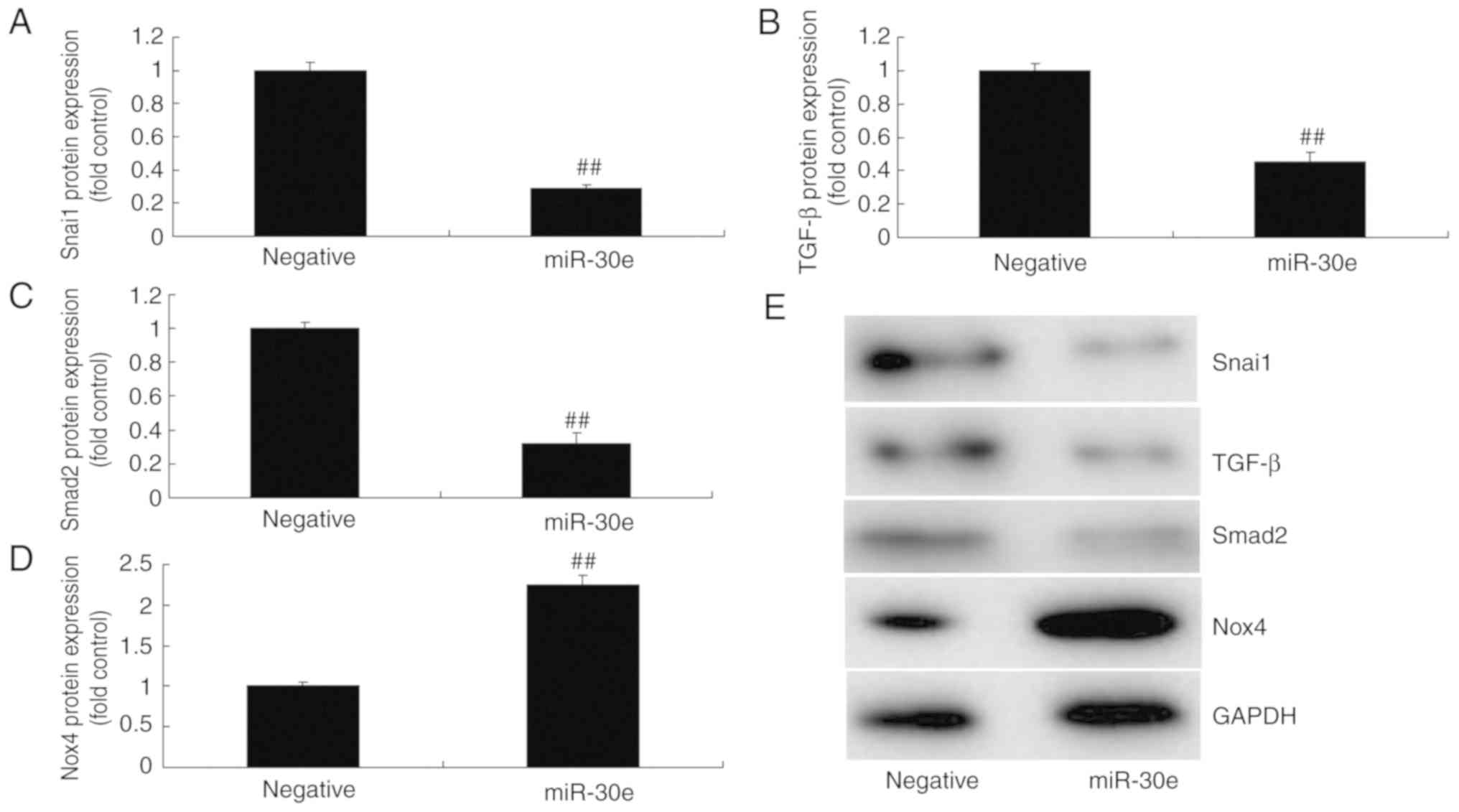
As presented in Fig.
4E-I, downregulation of miRNA-30e induced the protein
expression of Snai1, TGF-β and Smad2 and suppressed Nox4 protein
expression in an in vitro model, compared with the negative
group. In contrast, over-expression of miRNA-30e significantly
suppressed the protein expression of Snai1, TGF-β and Smad2
(P<0.01) and significantly induced that of Nox4 in an in
vitro model, compared with the negative group (P<0.01;
Fig. 5). These results
demonstrated that miRNA-30e regulated Snai1/TGF-β/Nox4 protein
expression to affect ROS/oxida-tive stress in AS.
The activation of Snai1 attenuates the
effects of miRNA-30e on oxidative stress in vitro
Therefore, to further evaluate the role of Snai1 in
the effects of miRNA-30e on oxida-tive stress in an in vitro
model, a Snai1 plasmid was used to significantly increase the
protein expression of Snai1, TGF-β and Smad2 (P<0.01) and
significantly suppressed that of Nox4 in an in vitro model
by miRNA-30e, compared with the miRNA-30e group (P<0.01;
Fig. 6A-E). The activation of
Snai1 significantly attenuated the effects of miRNA-30e on the
inhibition of MDA and ROS levels, and activation of SOD, GSH and
GSH-PX levels in an in vitro model, compared with the
miRNA-30e group (P<0.01; Fig.
6F-K). Snai1 is a target spot for the effects of miRNA-30e on
oxida-tive stress in AS.
 | Figure 6Activation of Snai1 reduces the
effects of miRNA-30e on the oxidative stress in vitro model.
(A) Snai1, (B) TGF-β, (C) Smad2 and (D) Nox4 protein expression by
statistical analysis, and (E) western blotting analysis, (F) MDA,
(G) SOD, (H) GSH and (I) GSH-PX, (J) ROS levels and (K) green
fluorescent protein transfection. Scale bar, 100 µm.
**P<0.01 vs. the negative normal group,
##P<0.01 vs. over-expression of miRNA-30e group.
miR/miRNA, microRNA; TGF, transforming growth factor; Nox4, NAPDH
oxidase 4; Smad, mothers against decapentaplegic homolog; Negative,
negative control group; miR-30e, over-expression of miRNA-30e
group; Snai1, over-expression of miRNA-30e and Snai1 plasmid
group. |
The activation of TGF-β attenuates the
effects of miRNA-30e on oxidative stress in an in vitro model
Next, the function of TGF-β in the effects of
miRNA-30e on oxidative stress in an in vitro model was
investigated further. To this end, a TGF-β plasmid significantly
induced the protein expression of TGF-β, Smad2 and significantly
suppressed Nox4 in an in vitro model following miRNA-30e,
compared with the miRNA-30e group (P<0.01; Fig. 7A-D). Additionally, the activation
of TGF-β attenuated the effects of miRNA-30e on the inhibition of
MDA and ROS levels, and activation of SOD, GSH and GSH-PX levels in
an in vitro model, compared with the microRNA-30e group
(Fig. 7E-K). These results
support that TGF-β is an important player for the effects of
miRNA-30e on oxidative stress in AS.
 | Figure 7Activation of TGF-β reduces the
effects of miRNA-30e on the oxidative stress in vitro model.
(A) TGF-β, (B) Smad2 and (C) Nox4 protein expression by statistical
analysis and (D) western blotting analysis, (E) MDA, (F) SOD, (G)
GSH and (H) GSH-PX (I) ROS levels and (J) green fluorescent
protein. **P<0.01 vs. the negative normal group,
##P<0.01 vs. the over-expression of microRNA-30e
group. miR/miRNA, microRNA; TGF, transforming growth factor; Nox4,
NAPDH oxidase 4; Smad, mothers against decapentaplegic homolog;
Negative, negative control group; miR-30e, over-expression of
microRNA-30e group; TGF-β, over-expression of microRNA-30e and
TGF-β plasmid group. |
The inhibition of Nox4 attenuates the
effects of miRNA-30e on oxidative stress in an in vitro model
Finally, si-Nox4 could significantly suppress the
protein expression of Nox4 in an in vitro model following
miRNA-30e transfection, compared with the miRNA-30e group
(P<0.01; Fig. 8A). Then, the
inhibition of Nox4 significantly attenuated the effects of
miRNA-30e on the inhibition of MDA and ROS levels, and activation
of SOD, GSH and GSH-PX levels in an in vitro model, compared
with the miRNA-30e group without Nox4 inhibition (P<0.01;
Fig. 8B-G). In conclusion, the
inhibition of Nox4 attenuated the effects of miRNA-30e on oxidative
stress in vitro.
 | Figure 8Inhibition of Nox4 reduces the effects
of miRNA-30e on the oxidative stress in vitro model. Nox4
protein expression by (A) statistical analysis and (B) western
blotting analysis, (C) MDA, (D) SOD, (E) GSH and (F) GSH-PX, (G and
H) ROS levels. Negative, negative control group; miR-30e,
over-expression of microRNA-30e group; si-Nox4, over-expression of
microRNA-30e and si-Nox4 group. **P<0.01 vs. the
negative normal group, ##P<0.01 vs. the
over-expression of miRNA-30e group. miR/miRNA, microRNA; TGF,
transforming growth factor; Nox4, NAPDH oxidase 4; Smad, mothers
against decapentaplegic homolog. |
Discussion
Angiogenesis is a vital influencing factor during AS
plaque development. Local angiogenesis is correlated with plaque
stability (16). The increasing
angiogenesis in the plaque will accumulate lipid and various
inflammatory cells within the plaque. Finally, it will result in
matrix degradation, fibrous cap thinning, plaque rupture and
therefore induce a severe cardiovascular event (17). miRNA research suggests that miRNA
serves a key role in the fields of tumor disease and AS. Recently,
multiple AS-associated miRNAs have been identified in the
cardiovascular system, which may provide a novel clue for the early
diagnosis and treatment selection for AS (18). The results of the present study
demonstrated that the expression of miRNA-30e in the AS model group
was reduced, compared with the normal mice group. Lai et al
(14) demonstrated that miRNA-30e
mediated cardioprotection of ACE2 in rats with doxorubicin-induced
heart failure.
OS is present in the whole process of AS, from fat
lesion formation to plaque rupture. Furthermore, it mediates the
functional alterations and injury of vascular endothelial cells,
smooth muscle cells and mononuclear macrophages (19). Therefore, it is of great
significance to illustrate the molecular mechanism of cellular OS
in AS genesis and development. This is important to delay AS
progression and prevent the incidence of acute cardiovascular and
cerebrovascular events (19). In
this study, it was demonstrated that the downregulation of
miRNA-30e increased MDA levels and reduced SOD, GSH and GSH-PX
levels in vitro model. Jin et al (20) reported that miR-30e-UCP2 pathway
regulates alcoholic hepatitis progress through suppression of
oxidative stress.
Current research suggests that ROS may induce AS
genesis and development through the following mechanisms (21). ROS can react with the multivalent
unsaturated fatty acid in the biomembrane and therefore directly
induce lipid peroxidation in the biomembrane. As a result, a large
amount of lipid peroxides and aldehydes will be produced, therefore
increasing the membrane permeability and causing tissue injury. ROS
can promote the degeneration of intracellular proteins and enzymes,
and cause protein function loss and enzyme inactivation. In
addition, it can destroy nucleic acids and chromosomes, resulting
in DNA strand breaking, chromosome mutation or rupture (21). Furthermore, ROS can affect the
redox status and ion channel of cells, causing protein nitration or
inactivation. Therefore, it will restrict mitochondrial
respiration, promote endothelial cell degeneration, necrosis and
apoptosis, and destroy the vascular endothelial integrity. In the
present study, the downregulation of miRNA-30e promoted ROS levels
in the in vitro model. These results demonstrated that the
downregulation of miRNA-30e promoted oxidative stress and ROS
levels in vitro model of AS.
TGF-β is a multi-functional protein associated with
the development and trauma repair of tissues. TGF-β participates in
immune function regulation and tumor genesis in the body. There are
3 subtypes in the human body, which share similar biological
characteristics. Among them, TGF-β1 is present in the greatest
proportion and has the highest activity (21). It mainly exerts its regulatory
role through autocrine and paracrine routes involving the cells of
the vascular wall including smooth muscle cells and vascular
endothelial cells. These results suggest that down-regulation of
miRNA-30e induced Snai1, TGF-β and Smad2 protein expression and
suppressed Nox4 protein expression in an in vitro model.
Zhang et al (22)
demonstrated that miR-30e attenuates isoproterenol-induced cardiac
fibrosis via Snai1/TGF-β.
OS-induced ROS accumulation serves a vital role in
AS genesis and development. Nox is a major enzyme in ROS production
in blood vessels, which takes part in AS genesis and development
(11). Studies report that
exogenous angiotensin (Ang)II can increase the Nox-origin ROS
production in smooth muscle cells, which will therefore damage
their structure and induce vascular remodeling (12). Exogenous AngII will upregulate the
peroxidasin homolog protein and mRNA expression in smooth muscle
cells, accompanying the increase in intracellular
H2O2 (23).
Therefore, miRNA-30e regulates Snai1/TGF-β/Nox4 to reduce oxidative
stress in an in vitro model of AS.
In conclusion, it was demonstrated that the
expression of microRNA-30e was reduced in an AS model and
miRNA-30e/Snai1/TGF-β/Nox4 signaling pathway to reduce oxidative
stress and negative feedback regulates ROS production in AS model
(Fig. 9). The results of the
present study provide novel insights for miRNA in defending against
oxida-tive stress, which miRNA-30e may represent a novel target for
AS.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
YC designed the experiments. MZ and WZ performed the
experiments. YC and MZ analyzed the data. MZ wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Animal Care
and Utilization Committee of Xiamen Cardiovascular Hospital Xiamen
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Wang SK, Green LA, Drucker NA,
Motaganahalli RL, Fajardo A and Murphy MP: Rationale and design of
the clinical and histologic analysis of mesenchymal stromal cells
in AmPutations (CHAMP) trial investigating the therapeutic
mechanism of mesenchymal stromal cells in the treatment of critical
limb ischemia. J Vasc Surg. 68:176–181.e1. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delgado-Lista J, Perez-Martinez P,
Garcia-Rios A, Alcala-Diaz JF, Perez-Caballero AI, Gomez-Delgado F,
Fuentes F, Quintana-Navarro G, Lopez-Segura F, Ortiz-Morales AM, et
al: CORonary Diet Intervention with Olive oil and cardiovascular
PREVention study (the CORDIOPREV study): Rationale, methods, and
baseline characteristics: A clinical trial comparing the efficacy
of a Mediterranean diet rich in olive oil versus a low-fat diet on
cardiovascular disease in coronary patients. Am Heart J. 177:42–50.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nicholls SJ, Puri R, Wolski K, Ballantyne
CM, Barter PJ, Brewer HB, Kastelein JJ, Hu B, Uno K, Kataoka Y, et
al: Effect of the BET protein inhibitor, RVX-208, on progression of
Coronary Atherosclerosis: Results of the Phase 2b, Randomized,
Double-Blind, Multicenter, ASSURE Trial. Am J Cardiovasc Drugs.
16:55–65. 2016. View Article : Google Scholar
|
|
4
|
Wei Y, Zhu M and Schober A: Macrophage
MicroRNAs as therapeutic targets for atherosclerosis, metabolic
syndrome, and cancer. Int J Mol Sci. 19:pii: E17562018. View Article : Google Scholar
|
|
5
|
Zhang X, Shi H, Wang Y, Hu J, Sun Z and Xu
S: Down-regulation of hsa-miR-148b inhibits vascular smooth muscle
cells proliferation and migration by directly targeting HSP90 in
atherosclerosis. Am J Transl Res. 9:629–637. 2017.PubMed/NCBI
|
|
6
|
Stanek A, Cholewka A, Wielkoszynski T,
Romuk E and Sieroń A: Whole-body cryotherapy decreases the levels
of inflammatory, oxidative stress, and atherosclerosis plaque
markers in male patients with active-phase ankylosing spondylitis
in the absence of classical cardiovascular risk factors. Mediators
Inflamm. 2018.8592532:2018.
|
|
7
|
Ahotupa M: Oxidized lipoprotein lipids and
atherosclerosis. Free Radic Res. 51:439–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Wang W, Wang N, Tall AR and Tabas
I: Mitochondrial oxidative stress promotes atherosclerosis and
neutrophil extracellular traps in aged mice. Arterioscler Thromb
Vasc Biol. 37:e99–e107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hassan MO, Duarte R, Dix-Peek T, Dickens
C, Naidoo S, Vachiat A, Grinter S, Manga P and Naicker S:
Transforming growth factor-β protects against inflammation-related
atherosclerosis in South African CKD Patients. Int J Nephrol.
2018.8702372:2018.
|
|
10
|
Terada K, Yamada H, Kikai M, Wakana N,
Yamamoto K, Wada N, Motoyama S, Saburi M, Sugimoto T, Irie D, et
al: Transplantation of periaortic adipose tissue inhibits
atherosclerosis in apoE−/− mice by evoking
TGF-β1-mediated anti-inflammatory response in transplanted graft.
Biochem Biophys Res Commun. 501:145–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu H, Wang Z, Sun Z, Ni Y and Zheng L:
GATA4 protects against hyperglycemiainduced endothelial dysfunction
by regulating NOX4 transcription. Mol Med Rep. 17:1485–1492.
2018.
|
|
12
|
Hu P, Wu X, Khandelwal AR, Yu W, Xu Z,
Chen L, Yang J, Weisbrod RM, Lee KSS, Seta F, et al: Endothelial
Nox4-based NADPH oxidase regulates atherosclerosis via soluble
epoxide hydrolase. Biochim Biophys Acta Mol Basis Dis.
1863.1382–1391. 2017.
|
|
13
|
Shen D, Tian L, Shen T, Sun H and Liu P:
Alpha-lipoic acid protects human aortic endothelial cells against
H2O2-induced injury and inhibits atherosclerosis in ovariectomized
low density lipoprotein receptor knock-out mice. Cell Physiol
Biochem. 47:2261–2277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai L, Chen J, Wang N, Zhu G, Duan X and
Ling F: MiRNA-30e mediated cardioprotection of ACE2 in rats with
Doxorubicin-induced heart failure through inhibiting
cardio-myocytes autophagy. Life Sci. 169:69–75. 2017. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Misialek JR, Bekwelem W, Chen LY, Loehr
LR, Agarwal SK, Soliman EZ, Norby FL and Alonso A: Association of
white blood cell count and differential with the incidence of
atrial fibrillation: The atherosclerosis risk in communities (ARIC)
Study. PLoS One. 10:e01362192015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karunakaran D and Rayner KJ: Macrophage
miRNAs in atherosclerosis. Biochim Biophys Acta. 1861.2087–2093.
2016.
|
|
18
|
Ma S, Tian XY, Zhang Y, Mu C, Shen H,
Bismuth J, Pownall HJ, Huang Y and Wong WT: E-selectin-targeting
delivery of microRNAs by microparticles ameliorates endothelial
inflammation and atherosclerosis. Sci Rep. 6:229102016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng X, Su W, Tao X, Sun M, Ying R, Wei W
and Wang B: Oxidation prevents HMGB1 inhibition on PDGF-induced
differentiation of multipotent vascular stem cells to smooth muscle
cells: A possible mechanism linking oxidative stress to
atherosclerosis. Biomed Res Int. 2018.4019814:2018.
|
|
20
|
Jin X, Yu MS, Huang Y, Xiang Z and Chen
YP: MiR-30e-UCP2 pathway regulates alcoholic hepatitis progress by
influencing ATP and hydrogen peroxide expression. Oncotarget.
8:64294–64302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gopoju R, Panangipalli S and Kotamraju S:
Metformin treatment prevents SREBP2-mediated cholesterol uptake and
improves lipid homeostasis during oxidative stress-induced
atherosclerosis. Free Radic Biol Med. 118:85–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Chang H, Zhang H and Zhang L:
MiR-30e attenuates isoproterenol-induced cardiac fibrosis through
suppressing Snai1/TGF-β Signaling. J Cardiovasc Pharmacol.
70:362–368. 2017.PubMed/NCBI
|
|
23
|
Zhao W, Feng H, Guo S, Han Y and Chen X:
Danshenol A inhibits TNF-α-induced expression of intercellular
adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells.
Sci Rep. 7:129532017. View Article : Google Scholar
|