Introduction
The cornea has the physiological capacity to absorb
the majority of ultraviolet radiation (UVR), and protects the inner
eye from ultraviolet (UV)-induced oxidative damaging effects
(1). Corneal injury can produce
photophobia, an aversive sensitivity to light, and even blindness
(2), and corneal injury remains a
major reason for consultations in ophthalmology clinics worldwide
(3). The common pathological
characteristics of corneal injury include inflammatory factor
activation, vascular endothelial cell or inflammatory cell
infiltration into lesions, corneal edema, corneal
neovascularization (CNV) and scar formation (4). Photokeratitis can be caused by UV,
and a model of photokeratitis can be established with 400
mJ/cm2 of UVB irradiation to mice corneas (5). Patients with UV-induced
photokeratitis exhibit promoted apoptosis of corneal cells due to
inflammatory response, induction of reactive oxygen species (ROS),
and damage to the cell membrane and deoxyribonucleic acid (DNA)
(6). Treatment, including the
topical administration of peroxiredoxin-6, has been reported to
promote recovery from UV-induced corneal injury after 14 weeks
(7). Chemical burns and exposure
to UVB lead to corneal injury, which can be aggravated by
inflammatory responses, oxidative stress and neovascularization,
resulting in blindness (8,9).
Exposure to UV lasers can result in pathology to the cornea, lens
or retina of the primate eye (10). Specifically, irradiation of the
eyes with UV causes serious enzymatic disturbances and inflammatory
reactions in the cornea (7).
Chronic exposure to UVR causes damage to the anterior pole of the
eye, such as pterygium or photokeratitis, which is accompanied by
several corneal disorders, including inflammatory infiltration and
CNV (11). In the last decade,
several aspects of the wound healing process in different regions
of the cornea have been elucidated, and therapeutic approaches have
emerged with novel markers identified and novel treatment options,
including gene and microRNA therapies that have been tested in
experimental systems (12,13).
For example, hepatocyte growth factor (HGF), keratinocyte growth
factor (KGF) and their receptors have been associated with wound
healing in the cornea (14).
HGF comprises an α-chain and a β-chain, which
contain four kringle domains and a serine protease-like structure,
respectively (15); these
structures induce a three-phase response, resulting in the
formation of branched tubular structures in epithelial cells
(16). In addition, KGF, a member
of the fibroblast growth factor family, is a human mitogen that is
specific for epithelial cells and functions as a stromal mediator
of epithelial cell proliferation (17). KGF is secreted by stromal cells in
a variety of tissues, including the cornea, skin, prostate and
mammary gland (18). These two
paracrine growth factors, namely, HGF and KGF, influence corneal
epithelial cell metabolism (19).
They have specific effects on corneal epithelial cell cycle
regulatory proteins; for example, HGF can rescue epithelial cells
from apoptosis and KGF promotes cell survival in corneal epithelial
cells (20). CNV is a major cause
of vision impairment and corneal blindness (21) and may result from a disrupted
balance between the upregulation of angiogenic factors and the
downregulation of antiangiogenic factors (13). Additionally, vascular endothelial
growth factor (VEGF) is a multifunctional cytokine that is
overexpressed in several trans-plantable and autochthonous tumors,
in healing wounds and in chronic inflammatory disorders, including
psoriasis and rheumatoid arthritis (22). It is well established that CNV is
tightly regulated by a dynamic, natural equilibrium between local
proangiogenic factors, including VEGF, and antiangiogenic molecules
(7). The present study was
designed to thoroughly examine the effects of HGF and KGF gene
silencing on VEGF and its receptors on UVR-induced CNV in rats.
As stated above, factors causing corneal injury
include infection, physical injury, chemistry and UVR. However, the
repair of corneal injury and the mechanism of neovascularization
remain to be elucidated. Furthermore, in addition to ozone
depletion and environmental disruption, UVR has increased, causing
an increase in the number of patients suffering from UVR-induced
corneal injury (23). Therefore,
the present study examines the effects of HGF/KGF on
neovascularization in the rat model of UVR-induced corneal
injury.
Materials and methods
Ethics statement
This study protocol was approved by the Animal
Ethics Committee of the Second Hospital, Shanxi Medical University
(Taiyuan, China). All animal experiments were performed in line
with the approval of the Guide for the Care and Use of Laboratory
Animal by International Committees. Additionally, the
implementation of humane endpoints was in compliance with the
guideline of assessment for humane endpoints in animal experiments
(RB/T 173-2018). The humane endpoints were met when the rats
presented with massive hemorrhage or other hemorrhage, blindness,
dyspnea or impending death, or when the rats failed to develop the
characteristics of the model during the experiments.
Study subjects
A total of 46 clean, 6-week-old male Wistar rats
weighing 180-220 g (provided by the Laboratory Animal Center of Sun
Yat-sen University of Medical Sciences, Guangzhou, China), weighing
180-220 g, were used in the present study. The rats were fed at
22-24°C, with a humidity of 50-60%, with an alternating 12-h
light:dark cycle, and with free access to water and food. The rats
were randomly classified into normal and model groups (n=23 in each
group).
UVR-induced corneal injury model
construction
The right eyes collected from the rats in the model
group were used for the experiments. The UVR-induced corneal injury
model was constructed using an UVR exposure method, as previously
described (7,24). In detail, the rats received an
intraperitoneal injection of 10 g/l pentobarbital sodium (40 mg/kg)
and were subjected to pupillary dilation using a compound
tropicamide eye drop after being anesthetized. The eyes were placed
under a UV lamp with a wavelength of 280 nm for 20 min for 4
consecutive days and a cumulative radiation dose of 9
kJ/m2 based on the UV light meter. After the 2nd, 4th,
and 6th day of model construction, corneal damage in the rats was
examined using a routine slit-lamp, and a slit-lamp microscope was
used to observe the corneal damage during the modeling. Prior to
the observation, the rats were anesthetized and were subjected to
pupillary dilation using the tropicamide eye drops in order to
record the corneal damage and the corneal neovascularization.
Images were captured and recorded, and the corneas were scored in
accordance with the Dickey grading criteria (Table I) (25).
 | Table IDickey grading criteria for edematous
corneal opacity. |
Table I
Dickey grading criteria for edematous
corneal opacity.
| Grading | Surface
characteristics | Points |
|---|
| 0 stage | Transparent
cornea | 0 |
| I stage | Mild corneal hazy
opacities | 1 |
| II stage | Corneal opacities
but anterior chamber clearly visible | 2 |
| III stage | More corneal
opacities and anterior chamber less visible | 3 |
| IV stage | Marked corneal
opacities and anterior chamber not visible | 4 |
Hematoxylin and eosin (H&E)
staining
Corneal damage in the rats was examined under the
slit-lamp microscope. On the 6th day following exposure to UVR, the
corneas from the normal rats and model rats were collected and
sectioned to observe the pathological changes. The rats weighted
220-260 g at the time of sacrifice. The rats in each group were
anesthetized through intraperitoneal injection of 2% pentobarbital
sodium (30 mg/kg) and then sacrificed by cervical dislocation. The
cornea tissues were immediately collected using ophthalmic
scissors. The tissues then were fixed in Bouin solution for 24 h
and were dehydrated, cleared, embedded with paraffin and cut into
4-µm sections. The sections were deparaffinized, dehydrated,
conventionally treated with H&E staining, dehydrated by
gradient alcohol, cleared with xylene, and mounted with neutral
balsam. Finally, the sections were observed and images were
captured under an optical microscope.
Lentiviral vector construction
The primers and meaningless siRNA sequences
(Table II) of KGF-siRNA and
HGF-siRNA were designed according to the GeneBank gene sequence
using online design software (BLOCK-iT™ RNAi Designer; http://rnaidesigner.invitrogen.com/rnaiexpress/) from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The
primers and sequences were then connected with the plasmid
pcDNATM6.2-GM/EmGFP-siRNA (Invitrogen; Thermo Fisher Scientific,
Inc.), and these were added into competent Escherichia coli
TG1 bacteria (cat. no. ZY12, Shanghai Zeye Biotech Co., Ltd.,
Shanghai, China), and incubated at 37°C for 90 min and incubated at
37°C for 12 h. Sequencing and screening was conducted by Sangon
Biotech Co., Ltd. (Shanghai, China) to construct the lentiviral
vectors pcDNATM6.2-GM/EmGFP-negative-siRNA (non-targeting scramble
sequence), pcDNATM6.2-GM/EmGFP-KGF-siRNA (KGF-siRNA; siRNA
targeting KGF) and pcDNATM6.2-GM/EmGFP-HGF-siRNA (HGF-siRNA; siRNA
targeting HGF) (Biologic Wind Company, Shanghai, China). The blank
vector was pcDNATM6.2-GM/EmGFP-siRNA. The pcD-NATM6.2-
GM/EmGFP-HGF-siRNA and pcDNATM6.2-GM/EmGFP- KGF-siRNA vectors were
treated by double enzyme digestion with BamHII and
XhoI (Santa Cruz Biotechnology, Inc., Dallas TX, USA) to
obtain the inserted fragments and vector backbones, which were then
connected with T4 ligase, sequenced and identified to obtain the
lentiviral vector pcDNATM6.2-GM/EmGFP-KGF- HGF-siRNA (KGF- HGF-
siRNA).
 | Table IIsiRNA sequences for lentiviral vector
construction. |
Table II
siRNA sequences for lentiviral vector
construction.
| siRNA | Sequence
(5′-3′) |
|---|
| HGF-siRNA |
CAAGTGCAGTAACATATCTCCTGAA |
| KGF-siRNA |
CGGAACTCTTGTGTACCCAGCTGTT |
| NC-siRNA |
ACGTCTCGTGTATCCACCGTAAGCC |
Cell culture, transfection and
grouping
The rats from the normal and model groups were
sacrificed, and the corneal tissues were immediately extracted
using ophthalmic scissors. Briefly, the rat eyes were sterilized
using 75% ethyl alcohol and added to 1.5-ml centrifuge tubes
containing sterile phosphate-buffered saline (PBS) and 1% (v/v) 100
U/ml penicillin and streptomycin 100 µg/ml (Sangon Biotech
Co., Ltd.), followed by at least three washes. Excess sclera was
removed with the use of sterile ophthalmic scissors following
collection of the corneas along the corneal limbal rims. Under a
stereoscopic dissection microscope (Carl Zeiss GmbH, Oberkochen,
Germany), the corneal endothelia and stromata were cautiously
stripped off with sterile surgical forceps (thin-tipped). Finally,
the residual corneal stroma was again removed, gradually, following
which the straticulate epithelia were inoculated into 12-well
plates (26). The corneal
epithelial cells were separated and cultured in Roswell Park
Memorial Institute (RPMI)-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.), containing 10% fetal bovine serum (FBS; HyClone;
GE Healthcare Life Sciences, Logan, UT, USA), at 37°C with 5%
CO2. The cells were treated with 0.25% trypsin (Gibco;
Thermo Fisher Scientific, Inc.) and were triturated using the
RPMI-1640 medium containing 10% FBS to prepare the single cell
suspension, followed by conventional subculture. Cells at the
logarithmic growth phase were extracted for the subsequent
experiment.
The corneal epithelial cells from rats in the normal
and model groups were collected and assigned to a normal group
(non-UVR-exposed corneal epithelial cells from normal rats without
any treatment); blank group (corneal epithelial cells from model
rats transfected with pcDNATM6.2-GM/EmGFP-siRNA), negative control
(NC) group (corneal epithelial cells from the model rats
transfected with pcDNATM6.2-GM/EmGFP-negative-siRNA); si-HGF/KGF
group (HGF and KGF double gene silencing, corneal epithelial cells
from the model rats transfected with HGF-KGF-siRNA); si-HGF group
(HGF gene silencing, corneal epithelial cells from the model rats
transfected with HGF-siRNA) and si-KGF group (KGF gene silencing,
corneal epithelial cells from the model rats transfected with
KGF-siRNA). Only the UVR-exposed corneal tissues were infected with
lentivirus vectors. The healthy corneal tissues, together with
UVR-damaged corneal tissues infected with or without the lentivirus
NC vector, were set as controls to determine the biological
function of HGF/KGF in UVR-damaged corneal tissues. The
transfection procedures were as follows: Cells were subcultured on
the day prior to the transfection and were inoculated into 6-well
plates, with 1×106 cells in each well. When the cells
reached a confluence of 70-80%, they were transfected. The cells
were transfected using Lipofectamine 2000 reagent, according to the
manufacturer’s protocol (cat. no. 11668-019, Invitrogen; Thermo
Fisher Scientific, Inc.). A total of 250 µl Opti-MEM
serum-free medium (cat. no. 51985042, Gibco; Thermo Fisher
Scientific, Inc.) was used to dilute 100 ng of
pcDNATM6.2-GM/EmGFP-siRNA, pcDNATM6.2-GM/EmGFP-negative-siRNA,
HGF-KGF-siRNA, KGF-siRNA and HGF-siRNA lentiviral vectors (final
concentration of 50 nm), and they were gently mixed and incubated
at room temperate for 5 min. Another 250 µl Opti-MEM
serum-free medium was used to dilute the Lipofectamine 2000 (5
µl), and this was gently mixed and incubated at room
temperate for 5 min. The two abovementioned dilutions were mixed
and incubated at room temperate for 20 min, followed by their
addition into the cell culture wells. The transfected cells were
further incubated in a 5% CO2 incubator at 37°C. The
complete medium was replaced after 6-8 h, and the subsequent
experiments were conducted after 24-48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The corneas from rats in the normal and model groups
were placed in liquid nitrogen and were then ground into a uniform
powder. The total RNA was extracted using the TRIzol reagent kit
(cat. no. 15596-018, Invitrogen; Thermo Fisher Scientific, Inc.)
and the concentration and purity of the RNA were determined.
According to the instructions in the Primescript™ RT reagent kit
(cat. no. RRO37A, Takara Biotechnology Co., Ltd., Dalian, China),
the RNA was reverse transcribed into complementary DNA (cDNA), with
a total volume of 25 µl. The cDNA was diluted with 65
µl diethyl pyrocarbonate and was fully mixed. RT-qPCR
analysis was conducted using the cDNA according to the instructions
in the SYBR® Premix Ex Taq™ II kit (Takara Biotechnology
Co., Ltd.). The reaction system (50 µl) included 25
µl of SYBR Premix Ex Taq II (2X), 2 µl of PCR forward
primers, 2 µl of PCR reverse primers, l µl of ROX
Reference dye (50X), 4 µl of DNA template and 16 µl
of dH2O. The RT-qPCR procedure was performed using the
ABI PRISM® 7300 system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The reaction conditions were as follows:
Predenaturation at 95°C for 4 min; 40 cycles of denaturation at
94°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C
for 1 min; with extension at 72°C for 7 min after the final cycle.
The β-actin gene was used as the internal reference, and all
primers (Table III) were
designed and synthesized by Wuhan Bojie Biological Engineering
Company (Wuhan, China). The relative expression of the target genes
was calculated using the 2−∆∆Cq method. The formula was
as follows: ΔΔCq=ΔCq experimental group-ΔCq control group and
ΔCq=Cq target gene-Cq β-actin. Cq represents the number of
amplification cycles when the real-time fluorescence intensity
reached a set threshold value, and amplification was performed in
logarithmically growing cells at the same time (27). Tissue samples from 10 rats were
collected from each group, and the experiment was repeated three
times. The above method was also used for the cell experiments.
 | Table IIIPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table III
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) |
|---|
| VEGF | F:
TGCACCCACGACAGAAGGGGA |
| R:
TCACCGCCTTGGCTTGTCACAT |
| Flk-1 | F:
TAGCGGGATGAAATCTTTGG |
| R:
GACTGTGCATGTCAGCGTCT |
| HGF | F:
CTGCTCTATAATGCGCAAATGG |
| R:
TGGACTCATGTCATTGCAAGCT |
| KGF | F:
GCACTACACTAATGCAC |
| R:
AAAGAAATCTCCCTGCTGG |
| β-actin | F:
CACCCGCGAGTACAACCTTC |
| R:
CCCATACCCACCATCACACC |
Western blot analysis
The corneas from rats in the normal and model groups
were placed in liquid nitrogen, were ground into a uniform powder,
added to lysis buffer (cat. no. C0481, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and centrifuged (30,237 × g) at 4°C for 15 min;
the supernatant obtained was saved for later use. The bicinchoninic
acid assay was conducted for protein quantitation. The 10% sodium
dodecyl sulfate (SDS) separation gel and spacer gel were prepared,
and the total protein (40 µg) and SDS sample buffer were
mixed and denatured following boiling at 100°C for 5 min. Following
ice-bath treatment and centrifugation at 179 × g for 5 min at room
temperature, each lane was loaded with the samples for
electrophoresis separation. The proteins in the gel were
transferred onto a nitrocellulose membrane, which was blocked with
5% skim milk at 4°C overnight. The membrane was then incubated
overnight at 4°C with primary antibodies against KGF (1:1,000, cat.
no. ab131162), HGF (1:5,000, cat. no. ab178395), VEGF (1:1,000,
cat. no. ab32152), kinase insert domain receptor (Flk-1; 1:1,000,
cat. no. ab11939) and β-actin (1:1,000, cat. no. ab8277). All
antibodies were from Abcam (Cambridge, MA, USA). Subsequently, the
membrane was rinsed with Tris-buffered saline with Tween-20 (TBST)
three times (5 min each) at room temperature. The membrane was then
incubated with a secondary antibody horseradish peroxidase-labeled
IgG (1:500) at 37°C for 1 h. The membrane was rinsed in PBS three
times (5 min each). The reaction membrane was completely immersed
in an electrochemiluminescence solution, developed in a dark room
and images were captured. β-actin was considered the internal
reference. The ratio of the target band to the internal reference
band was used to determine the relative protein level. Tissue
samples from 10 rats were collected from each group. The experiment
was repeated three times. The above method was also used for the
cell experiments, which were performed following 48 h of
transfection.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) assay
Following transfection for 48 h, 100 µl of
the cell suspension in each group was inoculated into 96-well
plates (2×103 cells per well) and was incubated with 5%
CO2 at 37°C. The four-time points were set as 0, 24, 48
and 72 h. At 4 h prior to culture ending, 20 µl of MTT (5
mg/ml) solution was added in each well. After 4 h, the supernatant
was discarded. Dimethyl sulfoxide (100 µl in each well) was
dropped into the wells, and were shaken for 5 min. The optical
density (OD) value of each well was determined using a micro-plate
reader (Multiskan FC, Thermo Fisher Scientific, Inc.) at a
wavelength of 590 nm. A total of 10 parallel wells were set in each
group, and the mean value was obtained. The experiment was repeated
three times and was performed by the same individual. Cell
viability curves were drawn with the time point as the abscissa and
the OD value as the ordinate.
Flow cytometry
Following transfection of the cells in each group
for 48 h, the supernatant was discarded, and the cells were washed
once with a PBS balanced salt solution. The cells were treated with
0.25% trypsin solution. The digestion solution was discarded when
the cells were observed to shrink and became round under the
microscope. Medium containing serum was used to terminate the
digestion. The cells were separated from the well following
trituration, and made into a mixed cell suspension followed by
centrifugation at 179 × g for 5 min at room temperature; the
supernatant was discarded. The cells were rinsed with PBS twice,
fixed in precooled 70% ethanol for 30 min, and were then
centrifuged at 179 × g for 5 min at room temperature and collected.
Following this, the cells were washed with PBS, stained with 1%
propidium iodide (PI) containing RNase for 30 min and washed twice
with PBS to wash away the PI. The volume of the cells was adjusted
to 1 ml using a PBS balanced salt solution. A BD-Aria FACSCalibur
flow cytometer (FACSCalibur; Beckman Coulter, Inc., Brea, CA, USA)
was used to detect the cell cycle (28). Tissue samples from 10 rats in each
group were detected, and the experiment was repeated three
times.
Following transfection for 48 h, the cells were
treated with ethylenediaminetetraacetic acid-free trypsin and were
collected in a flow tube. Following centrifugation, the supernatant
was discarded. The cells were rinsed in cold PBS three times, and
the supernatant was discarded following centrifugation at 179 × g
for 5 min at room temperature. According to the instructions in the
Annexin V-fluorescein isothiocyanate (FITC)/PI double staining cell
apoptosis assay kit (cat. no. C1065, Beyotime Institute of
Biotechnology, Shanghai, China), Annexin V-FITC, PI and HEPES
buffer (1:2:50) were formulated to make the Annexin V-FITC/PI
double staining solution. The cells were resuspended (100 µl
staining solution for 1×106 cells), shaken, mixed and
incubated at room temperate for 15 min. Subsequently, the cells
were added to HEPES buffer (1 ml) and were oscillated to mix. A
flow cytometer was used to record the apoptosis conditions at a
wavelength at 488 nm. Band pass filters at 525 and 620 nm were used
to detect the FITC and PI fluorescence, respectively. Tissue
samples from 10 rats were collected in each group, and the
experiment was repeated three times. The healthy living cells in
the lower left quadrant of the scatter plots are expressed as
(FITC−/PI−). The mechanically damaged cells
in the upper left quadrant are expressed as
(FITC−/PI+). The early apoptotic cells in the
lower right quadrant are expressed as
(FITC+/PI−). The late apoptotic and necrotic
cells in the upper right quadrant are expressed as
(FITC+/PI+). Apoptotic rate = percentage of
early apoptotic cells + percentage of late apoptotic cells.
Statistical analysis
All data from the experiments were analyzed using
SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). The count
data (success rate of model establishment) are expressed as the
percentage. The measurement data are presented as the mean ±
standard deviation. Comparisons between two groups were measured
using a paired t-test and unpaired Student’s t-test. Comparisons
among multiple groups were conducted by one-way analysis of
variance, and Tukey’s post hoc test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Successful establishment of the rat model
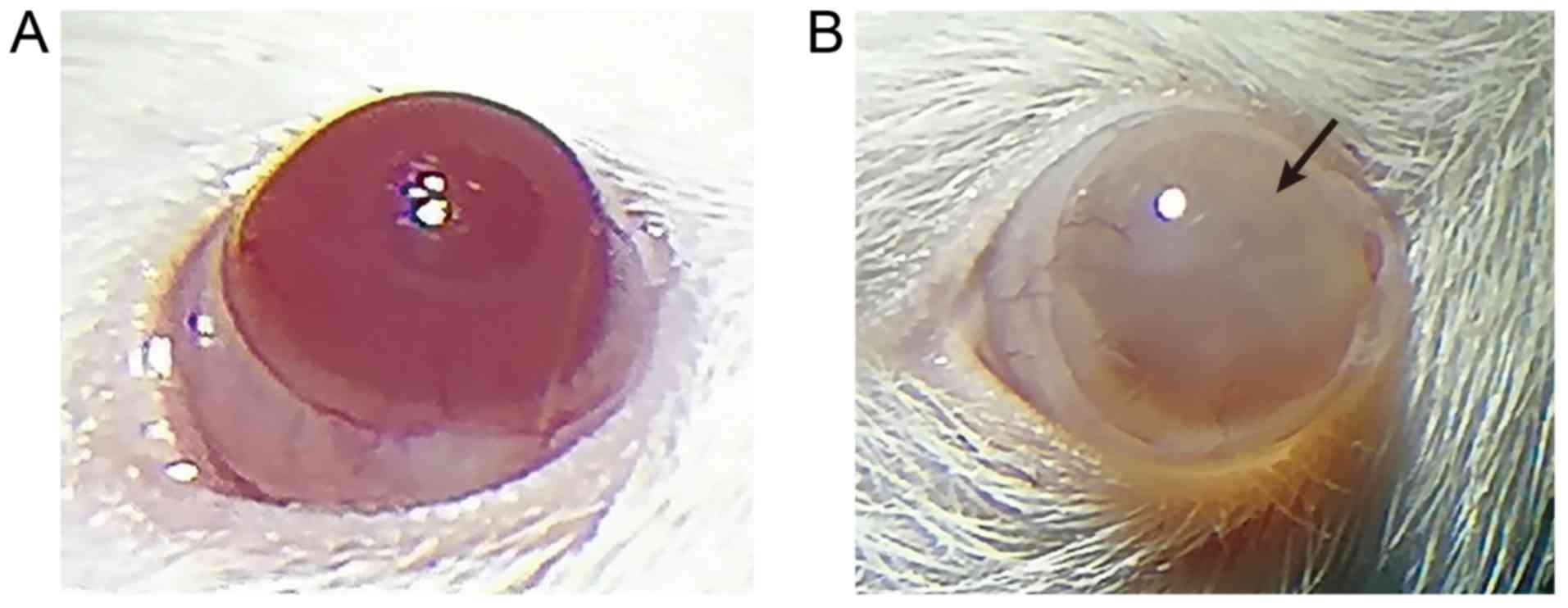
of UVR-induced corneal injury
A model of corneal injury induced by UVR was
established. Following UVR exposure, the injuries during modeling
of the rats were observed under a slit-lamp microscope on the 2nd,
4th and 6th days. Based on the state of injury, the rats in the
model group were scored as grade IV using the Dickey edematous
corneal opacity grading standard, suggesting successful modeling,
and the success rate was 100%. The results from the corneas
observed under the slit-lamp microscope (Fig. 1A and B, Table IV) showed that the normal cornea
was completely transparent and without vessels, and the surrounding
vessels were mainly on the edge of the cornea and formed the vessel
net. Neovascularization was not apparent in the model group
(Fig. 1B) as serious corneal
opacity was observed (degree IV pathology), and the anterior
chamber was not observed. Cataract induced by chronic UVR may be a
major factor accounting for this phenomenon (29). On the 6th day after UVR exposure,
serious corneal opacities were visible in the model rats and the
opacity reached degree IV pathology, which demonstrated successful
modeling (23,30). Therefore, the model of rats with
corneal injury induced by UVR was successfully established.
 | Table IVCorneal opacity scores in the normal
rats and model rats of ultraviolet radiation-induced corneal
injury. |
Table IV
Corneal opacity scores in the normal
rats and model rats of ultraviolet radiation-induced corneal
injury.
| Group | Corneal
opacity |
|---|
| Normal | 0 |
| Model | 3.62±0.31a |
Pathological changes in the corneal
tissues of model rats
H&E staining can be used to determine whether UV
induces corneal epithelial cell proliferation, during which corneal
cell thickness is reduced (5,6,31).
In the present study, H&E staining was used to observe
pathological changes in the corneal tissues. The results showed
that the normal corneal epithelium exhibited between five and six
layers of regularly arranged epithelia, which had a clear boundary
with the Bowman’s layer. The Bowman’s layer was uniform in
thickness, and the stromal layer showed an orderly arranged
collagenous fiber bundle. In the model group, the rats had severe
corneal epithelial cell hyperplasia and edema (Fig. 2A). In addition, the
immunohistochemistry (Fig. 2B)
used to examine the expression of platelet endothelial cell
adhesion molecule-1 (CD31; an angiogenesis marker) suggested that,
compared with the normal group, the model group had upregulated
expression of CD31. These data showed abnormal morphology of the
corneal tissues and CNV in the model rats.
mRNA and protein levels of KGF and HGF
are high in the corneal epithelial tissues of model rats
Subsequently, the mRNA and protein levels of KGF,
HGF, VEGF and Flk-1 were measured by RT-qPCR and western blot
analyses. As shown in Fig. 3A-C,
compared with the normal group, the mRNA and protein levels of the
aforementioned genes in the model group were significantly
increased (P<0.05), which revealed that the model rats had high
expression levels of KGF and HGF.
 | Figure 3Elevated mRNA and protein levels of
KGF, HGF, VEGF and Flk-1 are observed in model rats. (A) Relative
mRNA levels of KGF, HGF, VEGF and Flk-1 in the corneal tissues. (B)
Relative protein levels of KGF, HGF, VEGF and Flk-1 in the corneal
tissues. (C) Gray values of the relative protein bands; *P<0.05,
vs. normal group. The mRNA and protein level measurement data are
expressed using the mean ± standard deviation; and the data were
normalized to the level in the normal group. Data were analyzed
using a one-way analysis of variance, and the experiment was
repeated three times independently. KGF, keratinocyte growth
factor; HGF, hepatocyte growth factor; VEGF, vascular endothelial
growth factor. |
Successful transfer of vectors and
sequences into the corneal epithelial cells of the model rats
A fluorescence microscope was then used to examine
the expression of the vectors and sequences. Following cell
transfection, the cells in the normal group showed no green
fluorescence. By contrast, significant green fluorescence was
present in the blank, NC, si-KGF, si-HGF and si-HGF/KGF groups
(expression rate reaching 80%; Fig.
4A and B). This demonstrated that the lentiviral vectors
pcDNATM6.2-GM/EmGFP-KGF-HGF-siRNA, pcDNATM6.2-GM/EmGFP-KGF-siRNA,
pcDNATM6.2-GM/EmHGF-siRNA, pcDNATM6.2-GM/EmGFP-siRNA and
pcDNATM6.2-GM/EmGFP-negative-siRNA were all introduced into the
corneal epithelial cells and were effectively expressed in the
cells.
mRNA and protein levels of KGF, HGF, VEGF
and Flk-1 are decreased by si-HGF/KGF
To investigate the mRNA and protein levels of KGF,
HGF, VEGF and Flk-1 in the transfected cells, RT-qPCR and western
blot analyses were conducted. The findings (Fig. 5A-C) revealed that, compared with
the normal group, the mRNA and protein levels of KGF, HGF, VEGF and
Flk-1 were significantly increased in the blank, NC, si-KGF, si-HGF
and si-HGF/KGF groups (all P<0.05). Compared with the blank
group, the mRNA and protein levels of VEGF and Flk-1 were notably
downregulated in the si-KGF, si-HGF and si-HGF/KGF groups
(P<0.05). The si-KGF and si-HGF groups showed lower mRNA and
protein levels of HGF and KGF than the blank group (P<0.05). The
si-HGF/KGF group also exhibited significantly decreased mRNA and
protein levels of KGF and HGF (P<0.05). No significant
differences were observed between the NC and blank groups
(P>0.05). The si-HGF/KGF group exhibited lower mRNA and protein
levels of VEGF and Flk-1 than the si-KGF group and si-HGF group,
with a significant difference (P<0.05). These results suggested
that the gene silencing of both HGF and KGF decreased the
expression of KGF, HGF, VEGF and Flk-1.
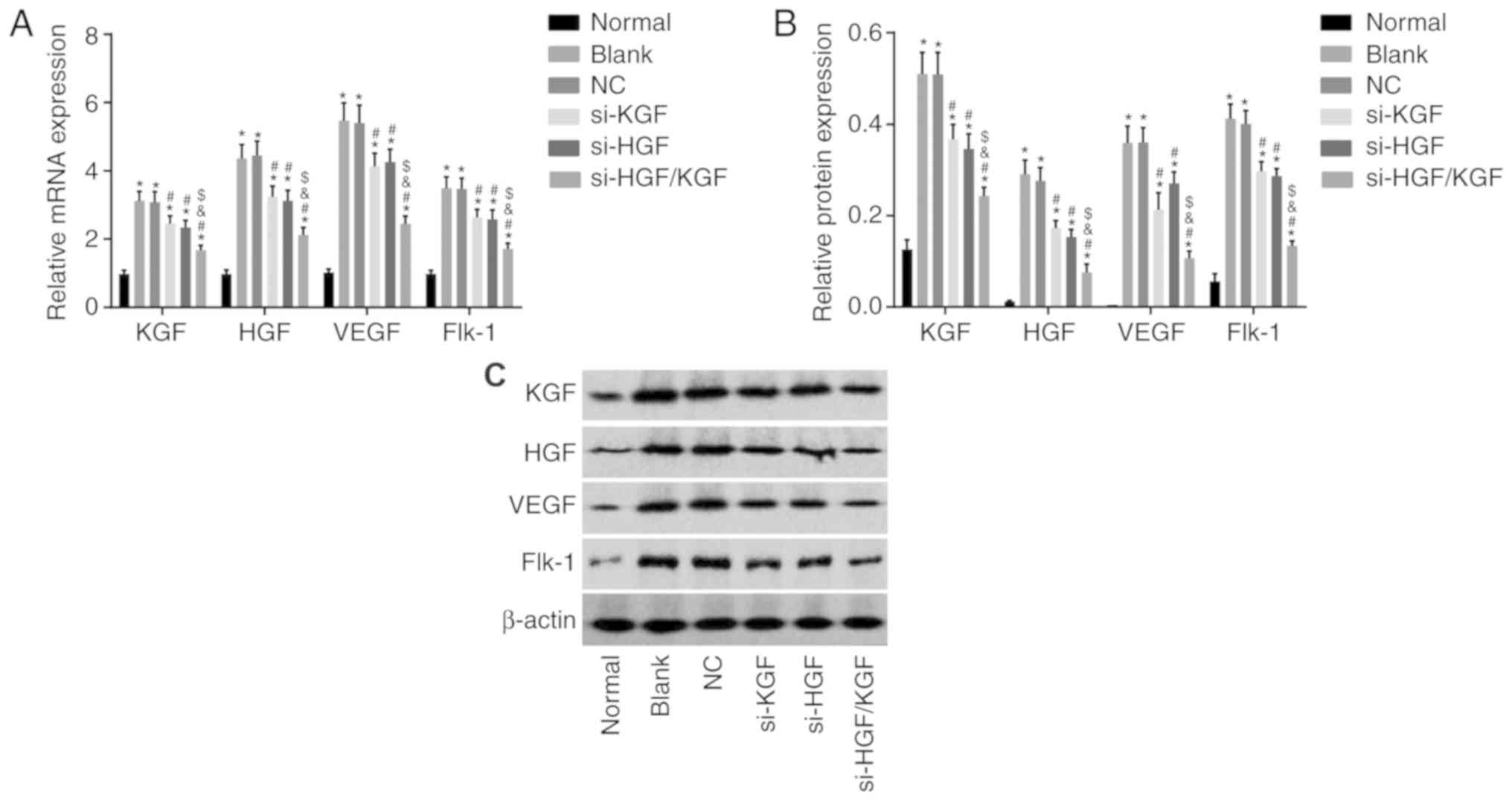 | Figure 5HGF and KGF silencing reduces the
levels of KGF, HGF, VEGF and Flk-1 in the corneal epithelial cells
of model rats. The mRNA and protein expression levels of KGF, HGF,
VEGF and Flk-1 were determined following 48 h of transduction. (A)
Relative mRNA levels of KGF, HGF, VEGF and Flk-1 in each group
following 48 h of transduction, as determined by reverse
transcription-quantitative polymerase chain reaction analysis. (B)
Relative protein levels of KGF, HGF, VEGF and Flk-1 in each group
following 48 h of transduction. (C) Gray values of the relative
protein bands, as determined by western blot analysis;
*P<0.05, vs. normal group; #P<0.05, vs.
blank group; &P<0.05, vs. si-HGF group;
$P<0.05, vs. si-KGF group. The mRNA and protein
levels are measurement data expressed as the mean ± standard
deviation, and data were normalized to the level in the normal
group. Data were analyzed using one-way analysis of variance and
Tukey’s post hoc test. The experiment was repeated three times
independently (n=3). NC, negative control; KGF, fibroblast growth
factor; HGF, hepatocyte growth factor; VEGF, vascular endothelial
growth factor; Flk-1, kinase insert domain receptor; si, small
interfering RNA. |
KGF and HGF gene silencing decreases the
proliferation of corneal epithelial cells in the model rats
An MTT assay was conducted to examine cell
proliferation in each group, and the results (Fig. 6) demonstrated that the
proliferation of the corneal epithelial cells from each group
increased as time proceeded (P<0.05). Compared with the normal
group, the other groups showed increased cell proliferation (all
P<0.05). Compared with the blank group, the si-KGF, si-HGF and
si-HGF/KGF groups exhibited inhibited cell proliferation (all
P<0.05). No significant differences were observed between the
blank and NC groups. Additionally, no significant differences were
observed between the si-KGF and si-HGF groups (all P>0.05).
Compared with the si-KGF and si-HGF groups, the si-HGF/KGF group
exhibited decreased cell proliferation (all P>0.05). The above
results demonstrated that the knockdown of KGF and HGF may
downregulate the proliferation ability of corneal epithelial cells
in the model rats.
 | Figure 6Viabilities of the corneal epithelial
cells are reduced by KGF and HGF gene silencing following 48 h of
transduction, as assessed by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
assay. *P<0.05, vs. normal group;
#P<0.05, vs. blank group; &P<0.05,
vs. si-HGF group; $P<0.05, vs. si-KGF group. The
experiment was repeated three times. The measurement data are
expressed as the mean ± standard deviation; and data at different
time points were analyzed using repeated analysis of variance, and
examined using Tukey’s test; OD, optical density; NC, negative
control; KGF, fibroblast growth factor; HGF, hepatocyte growth
factor; Flk-1, kinase insert domain receptor; si, small interfering
RNA. |
KGF and HGF gene silencing promotes
apoptosis of corneal epithelial cells of model rats
To characterize the role of KGF and HGF gene
silencing in corneal epithelial cells, with regard to cell cycle
and apoptosis, PI staining and Annexin V-FITC/PI double staining
were conducted. As shown in Fig. 7A
and B, compared with the normal group, the other five groups
exhibited decreased ratios of corneal epithelial cells in the G1
phase and increased ratios in the S phase (all P<0.05). Compared
with the blank group, the si-KGF, si-HGF and si-HGF/KGF groups
exhibited increased cell ratios in the G1 phase and decreased
ratios in the S phase (all P<0.05). No significant differences
were observed in the ratio of the cell distribution in the G1 and S
phases between the blank and NC groups (P>0.05). There were also
no notable differences in the ratios of the cell distribution in
the G1 and S phases between the si-KGF and si-HGF groups
(P>0.05). Compared with the si-KGF and si-HGF groups, the
si-HGF/KGF group exhibited a significantly increased ratio of
corneal epithelial cells in the G1 phase and decreased ratio in the
S phase (all P<0.05).
 | Figure 7KGF and HGF gene silencing induces
cell cycle arrest in corneal epithelial cells. (A) Cell cycle
distribution in each group following 48 h of transduction, as
assessed by PI staining. (B) Percentages of cells in
G0/G1, S and G2/M phase in each group
following 48 h of transduction; *P<0.05, vs. normal
group; #P<0.05, vs. blank group;
&P<0.05, vs. si-HGF group; $P<0.05,
vs. si-KGF group; The experiment was repeated three times
independently. The measurement data are expressed as the mean ±
standard deviation and were analyzed using one-way analysis of
variance and Tukey’s post hoc test. NC, negative control; KGF,
fibroblast growth factor; HGF, hepatocyte growth factor; si, small
interfering RNA; PI, propidium iodide. |
As shown in Fig. 8A
and B, Annexin V-FITC/PI double staining demonstrated that the
apoptotic rate of the corneal epithelial cells was significantly
reduced compared with that of the normal group (P<0.05). The
cell apoptotic rate was notably increased in the si-KGF, si-HGF and
si-HGF/KGF groups compared with that in the blank group (all
P<0.05). No significant difference in apoptotic rate was
identified between the blank and NC groups (P>0.05). There were
also no notable differences in the apoptotic rate between the
si-KGF and si-HGF groups (P>0.05). Compared with the si-KGF and
si-HGF groups, the apoptotic rate was significantly increased in
the si-HGF/KGF group (all P<0.05). All the above results
demonstrated that KGF and HGF gene silencing promoted the apoptosis
of corneal epithelial cells in the model rats.
 | Figure 8KGF and HGF gene silencing promotes
apoptosis of corneal epithelial cells. (A) Flow chart of cell
apoptosis in each group following 48 h of transduction, as detected
by Annexin V-FITC/PI double staining. (B) Apoptotic cell rates in
each group. *P<0.05, vs. normal group;
#P<0.05, vs. blank group; &P<0.05,
vs. si-HGF group; $P<0.05, vs. si-KGF group; The
experiment was repeated three times. The measurement data are
expressed as the mean ± standard deviation and were analyzed using
a one-way analysis of variance and Tukey’s post hoc test. NC,
negative control; KGF, fibroblast growth factor; HGF, hepatocyte
growth factor; Flk-1, kinase insert domain receptor; si, small
interfering RNA; FITC/PI, fluorescein isothiocyanate/propidium
iodide. |
Discussion
UVR is identified as the most common cause of
radiation injury to the eye (32). UVR evokes photokeratitis, which is
accompanied by increased corneal hydration and changes in corneal
transparency, leading to increased light absorption (33). Despite their harmfulness, certain
microRNAs and genes are found to have potent protective effects on
UV-induced corneal damage (12,13). Shi et al stated that
peroxiredoxin 6 (PRDX6) protein can be used to treat UV-induced
corneal injury (7), and the
present study suggested that silencing of HGF and KGF inhibits the
expression of VEGF and its receptor, therefore, assisting in
recovery from UV-induced corneal injury. The treatment provided by
Shi et al comprised increasing PRDX6 at the protein level
and the strategy used in the present study comprised siRNA-mediated
gene silencing, both of which are innovative. In the present study,
it was found that HGF and KGF gene silencing possessed the ability
to attenuate UVR-induced corneal injury and CNV by suppressing the
expression of VEGF and its receptors.
The findings obtained in the present study showed
that KGF and HGF were expressed at high levels in the rats with
UVR-induced corneal injury and CNV, and the knockdown of KGF and
HGF inhibited the proliferation and promoted the apoptosis of
corneal epithelial cells. KGF is a well-established mitogen for
keratinocytes, which promotes early differentiation and inhibits
terminal differentiation of cultured keratinocytes (34). HGF acts by binding to a specific
receptor, c-Met, and is reported to promote keratinocyte
proliferation and stimulate keratinocyte metalloproteinase
production in response to skin injury, migration and proliferation
(35). An intact corneal
epithelium is essential for maintaining vision and protecting
against infection (36). Healing
of epithelial wounds in a healthy cornea occurs relatively fast
(20). HGF, KGF and their
receptors are associated with homeostasis and injury healing in the
cornea (14). In addition, it was
previously reported that HGF and KGF are expressed in the cornea in
response to injury (20). HGF and
KGF exhibit a lasting influence on corneal epithelial cell survival
and cell growth, and on the regulation of levels of proteins that
control apoptosis and cell cycle, which include p53, poly
(adenosine diphosphate-ribose) polymerase, retinoblastoma protein,
cyclins, CDKs, and cell cycle inhibitor p27kip (17). Both HGF and KGF are important in
corneal injury; HGF and KGF promote the proliferation and migration
of corneal epithelial cells (37,38).
In the present study, the knockdown of both the HGF
and KGF genes prevented corneal epithelial cell proliferation and
promoted apoptosis. Podskochy and Fagerholm demonstrated that
UV-damage can decrease the number of apoptotic cells, thus,
inhibiting cell apoptosis based on a single dose of UVR (30). However, in the present study, cell
proliferation and apoptosis were observed following repeated UVR
exposure, which has been reported to increase the resistance of
corneal stroma cells to apoptosis; therefore, corneal stroma cells
exhibit apoptosis resistance. Acute UVB exposure leads to
photokeratitis and triggers the apoptosis of corneal cells
(6). Prolonged eye exposure to UV
induces photokeratitis, which affects all tissues of the cornea
(39) and induces the expression
of various inflammatory agents, including nuclear factor-κ-light
chain enhancer of activated B cells (NF-κB) and prostaglandin E2
(40). Photokeratitis is
generally associated with lost or injured corneal epithelial cells
due to natural and artificial UVR (33,41). UV exposure affects all tissues of
the cornea, and causes apoptosis of corneal cells through direct
cell membrane damage, DNA damage and ROS induction as a result of
an inflammatory reaction (39).
Under UVR, corneal damage is mediated through increased levels of
lipid peroxidation products (malondialdehyde and 4-hydroxynonenal),
inflammatory mediators (NF-κB and cyclooxygenase-2), cell death
factors (Fas receptor) and matrix metalloproteinases (MMP-9 and
MMP-2) (42). Consistently, the
present study found that excessive exposure to UVR in the cornea
may induce photokeratitis, damage to the epithelium, corneal
opacities, edema and CNV.
Corneal transparency and vascularity are important
for maintaining proper optical performance of the cornea (43). CNV involves the development of new
vascular structures in areas that were previously avascular
(44). CNV can be induced by
infection, inflammation, degeneration or delayed wound-healing
disorders in the ocular surfaces (45). VEGF, a potent angiogenic
stimulator, promotes proliferation, migration, proteolytic activity
and capillary tube formation by endothelial cells (46). During CNV, VEGF is expressed at
high levels in the vascular endothelial cells of the limbal vessels
and in newly formed vessels in the stroma, but its expression is
weak in keratocytes (47).
Correspondingly, VEGF is significantly increased in the
vascularized cornea compared with the normal cornea (47). It was previously reported that
VEGF is involved in the process of UVR-induced CNV (7). The expression of VEGF is elevated by
HGF and KGF, indicating their involvement in the regulation of
angiogenesis (48,49). In the present study, the protein
level of VEGF was significantly reduced by the knockdown of both
HGF and KGF.
It has been shown that corneal injury can lead to
the inflammatory response and damage to sensory nerves (50,51). However, the present study only
focused on the effects of HGF/KGF in VEGF and its receptors in
corneal epithelial cells, rather than the biological function of
corneal nerves. KGF and HGF can influence the proliferation and
migration of epithelial cells by activating the p38/extracellular
signal-regulated kinase 1/2 signaling pathway (37,52). Therefore, silencing of KGF or HGF
alone can suppress cell proliferation and migration, and silencing
of both leads to the same effects. In the present study, HGF
silencing reduced the expression of KGF, and KGF silencing reduced
the expression of HGF. Interleukin (IL)-1β and IL-1α have been
reported to upregulate the expression of HGF and KGF (53), based on which it was hypothesized
that the silencing of HGF or KGF alone not only inhibits corneal
epithelial cell proliferation, but also downregulates the
expression of cell-secreted IL-1β and IL-1α, thus indirectly
influencing the promotive effects of IL-1β and IL-1α on KGF/HGF. As
a result, HGF gene silencing may lead to decreased KGF, and KGF
gene silencing may lead to decreased HGF. Although the present
study found that HGF may interact with KGF in UVR-induced CNV, the
interaction between HGF and KGF remains to be fully elucidated.
Confirmation with more reliable data is required in subsequent
investigations. In addition, the specific channel of small
molecules and siRNA entering corneal cells, and whether they
causing injury to other organs with involvement of the circulatory
system in humans remains to be elucidated. Therefore, rat model
established in the present study is not applicable to humans, and
requires attention in future investigations. Despite this, the
findings of the present study support a theoretical basis for a
novel target in corneal wound healing following UVR exposure.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Shanxi
Scholarship Council of China (grant no. 2016-062); the Fund Program
for the Scientific Activities of Selected Returned Overseas
Professionals in Shanxi Province (grant no. 2017-144) and the Fund
Program for Doctor of Shanxi Medical University (grant no.
BS201712).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
Conception and design: MH, TH, YW, YHW, WSQ, LZD and
CQZ; analysis and interpretation: MH, TH, YW, YHW, WSQ, LZD and
CQZ; data collection: MH, TH, YW, YHW, WSQ, LZD and CQZ; manuscript
preparation: MH, TH and YW; statistical analysis: LZD and CQZ;
critical revision of the manuscript: MH, TH, YW, YHW, WSQ, LZD and
CQZ; final approval of the manuscript: MH, TH, YW, YHW, WSQ, LZD
and CQZ. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study protocol was approved by the Animal
Ethics Committee of the Second Hospital, Shanxi Medical University.
All animal experiments were performed in line with the approval of
the Guide for the Care and Use of Laboratory Animal by
International Committees.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsai CF, Lu FJ and Hsu YW: Protective
effects of Dunaliella salina - a carotenoids-rich alga-against
ultraviolet B-induced corneal oxidative damage in mice. Mol Vis.
18:1540–1547. 2012.
|
|
2
|
Green PG, Alvarez P and Levine JD: Topical
tetrodotoxin attenuates photophobia induced by corneal injury in
the rat. J Pain. 16:881–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Couture C, Zaniolo K, Carrier P, Lake J,
Patenaude J, Germain L and Guérin SL: The tissue-engineered human
cornea as a model to study expression of matrix metalloproteinases
during corneal wound healing. Biomaterials. 78:86–101. 2016.
View Article : Google Scholar
|
|
4
|
Lu Y, Feng J, Yang L, Tang H, Jin J and Xu
X: Anti-inflammatory effects of a synthetic peptide derived from
pigment epithelium-derived factor on
H2O2-induced corneal injury in vitro. Chin
Med J (Engl). 127:1438–1444. 2014.
|
|
5
|
Lennikov A, Kitaichi N, Fukase R, Murata
M, Noda K, Ando R, Ohguchi T, Kawakita T, Ohno S and Ishida S:
Amelioration of ultraviolet-induced photokeratitis in mice treated
with astaxanthin eye drops. Mol Vis. 18:455–464. 2012.PubMed/NCBI
|
|
6
|
Lennikov A, Kitaichi N, Kase S, Noda K,
Horie Y, Nakai A, Ohno S and Ishida S: Induction of heat shock
protein 70 ameliorates ultraviolet-induced photokeratitis in mice.
Int J Mol Sci. 14:2175–2189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi H, Yu HJ, Wang HY, Wang WT, Jin SH,
Zhu P, Li SJ, Rong CT and Li JY: Topical administration of
peroxiredoxin-6 on the cornea suppresses inflammation and
neovascularization induced by ultraviolet radiation. Invest
Ophthalmol Vis Sci. 53:8016–8028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu XJ, Liu X, Chen YY, Zhao Y, Xu M, Han
XJ, Liu QP, Yi JL and Li JM: Involvement of NADPH oxidases in
alkali burn-induced corneal injury. Int J Mol Med. 38:75–82. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen MH, Tsai CF, Hsu YW and Lu FJ:
Epigallocatechin gallate eye drops protect against ultraviolet
B-induced corneal oxidative damage in mice. Mol Vis. 20:153–162.
2014.PubMed/NCBI
|
|
10
|
Zuclich JA: Ultraviolet-induced
photochemical damage in ocular tissues. Health Phys. 56:671–682.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Golu A, Gheorghişor I, Bălăşoiu AT, Baltă
F, Osiac E, Mogoantă L and Bold A: The effect of ultraviolet
radiation on the cornea-experimental study. Rom J Morphol Embryol.
54:1115–1120. 2013.
|
|
12
|
Seo M, Choi JS, Rho CR, Joo CK and Lee SK:
MicroRNA miR-466 inhibits Lymphangiogenesis by targeting
prospero-related homeobox 1 in the alkali burn corneal injury
model. J Biomed Sci. 22:32015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu PH, Yeh LK, Lin HC, Jung SM, Ma DH,
Wang IJ, Wu HH, Shiu TF and Chen J: Deletion of the FHL2 gene
attenuating neovascularization after corneal injury. Invest
Ophthalmol Vis Sci. 49:5314–5318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilson SE, Chen L, Mohan RR, Liang Q and
Liu J: Expression of HGF, KGF, EGF and receptor messenger RNAs
following corneal epithelial wounding. Exp Eye Res. 68:377–397.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumoto K, Funakoshi H, Takahashi H and
Sakai K: HGF-Met pathway in regeneration and drug discovery.
Biomedicines. 2:275–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boccaccio C, Andò M, Tamagnone L, Bardelli
A, Michieli P, Battistini C and Comoglio PM: Induction of
epithelial tubules by growth factor HGF depends on the STAT
pathway. Nature. 391:285–288. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Finch PW, Rubin JS, Miki T, Ron D and
Aaronson SA: Human KGF is FGF-related with properties of a
paracrine effector of epithelial cell growth. Science. 245:752–755.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng CC, Wang DY, Kao MH and Chen JK: The
growth-promoting effect of KGF on limbal epithelial cells is
mediated by upregulation of DeltaNp63alpha through the p38 pathway.
J Cell Sci. 122:4473–4480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McBain VA, Forrester JV and McCaig CD:
HGF, MAPK, and a small physiological electric field interact during
corneal epithelial cell migration. Invest Ophthalmol Vis Sci.
44:540–547. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chandrasekher G, Pothula S, Maharaj G and
Bazan HE: Differential effects of hepatocyte growth factor and
keratinocyte growth factor on corneal epithelial cell cycle protein
expression, cell survival, and growth. Mol Vis. 20:24–37.
2014.PubMed/NCBI
|
|
21
|
Li X, Zhou Q, Hanus J, Anderson C, Zhang
H, Dellinger M, Brekken R and Wang S: Inhibition of multiple
pathogenic pathways by histone deacetylase inhibitor SAHA in a
corneal alkali-burn injury model. Mol Pharm. 10:307–318. 2013.
View Article : Google Scholar :
|
|
22
|
Dvorak HF, Nagy JA, Feng D, Brown LF and
Dvorak AM: Vascular permeability factor/vascular endothelial growth
factor and the significance of microvascular hyperpermeability in
angiogenesis. Curr Top Microbiol Immunol. 237:97–132.
1999.PubMed/NCBI
|
|
23
|
Das P, Pereira JA, Chaklader M, Law A,
Bagchi K, Bhaduri G, Chaudhuri S and Law S: Phenotypic alteration
of limbal niche-associated limbal epithelial stem cell deficiency
by ultraviolet-B exposure-induced phototoxicity in mice. Biochem
Cell Biol. 91:165–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujihara T, Nagano T, Endo K, Nakamura M
and Nakata K: Lactoferrin protects against UV-B irradiation-induced
corneal epithelial damage in rats. Cornea. 19:207–211. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dickey JB, Cassidy EM and Bouchard CS:
Periocular FK-506 delays allograft rejection in rat penetrating
keratoplasty. Cornea. 12:204–207. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du S, Han B, Li K, Zhang X, Sha X and Gao
L: Lycium barbarum polysaccharides protect rat corneal epithelial
cells against ultraviolet B-induced apoptosis by attenuating the
mitochondrial pathway and inhibiting JNK phosphorylation. Biomed
Res Int. 2017:58068322017. View Article : Google Scholar :
|
|
27
|
Schefe JH, Lehmann KE, Buschmann IR, Unger
T and Funke-Kaiser H: Quantitative real-time RT-PCR data analysis:
Current concepts and the novel ‘gene expression’s CT difference’
formula. J Mol Med (Berl). 84:901–910. 2006. View Article : Google Scholar
|
|
28
|
Wood JC: Principles of gating. Curr Protoc
Cytom Chapter. 1(Unit 1): 82001.
|
|
29
|
Jose JG and Pitts DG: Wavelength
dependency of cataracts in albino mice following chronic exposure.
Exp Eye Res. 41:545–563. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Podskochy A and Fagerholm P: Repeated UVR
exposures cause keratocyte resistance to apoptosis and hyaluronan
accumulation in the rabbit cornea. Acta Ophthalmol Scand.
79:603–608. 2001. View Article : Google Scholar
|
|
31
|
Ibrahim OM, Kojima T, Wakamatsu TH, Dogru
M, Matsumoto Y, Ogawa Y, Ogawa J, Negishi K, Shimazaki J, Sakamoto
Y, et al: Corneal and retinal effects of ultraviolet-B exposure in
a soft contact lens mouse model. Invest Ophthalmol Vis Sci.
53:2403–2413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pauloin T, Dutot M, Joly F, Warnet JM and
Rat P: High molecular weight hyaluronan decreases UVB-induced
apoptosis and inflammation in human epithelial corneal cells. Mol
Vis. 15:577–583. 2009.PubMed/NCBI
|
|
33
|
Cejka C, Ardan T, Sirc J, Michálek J,
Beneš J, Brůnová B and Rosina J: Hydration and transparency of the
rabbit cornea irradiated with UVB-doses of 0.25 J/cm(2) and 0.5
J/cm(2) compared with equivalent UVB radiation exposure reaching
the human cornea from sunlight. Curr Eye Res. 36:607–613. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marchese C, Rubin J, Ron D, Faggioni A,
Torrisi MR, Messina A, Frati L and Aaronson SA: Human keratinocyte
growth factor activity on proliferation and differentiation of
human keratinocytes: Differentiation response distinguishes KGF
from EGF family. J Cell Physiol. 144:326–332. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weidner KM, Behrens J, Vandekerckhove J
and Birchmeier W: Scatter factor: Molecular characteristics and
effect on the invasiveness of epithelial cells. J Cell Biol.
111:2097–2108. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Azfar MF, Khan MF and Alzeer AH:
Protocolized eye care prevents corneal complications in ventilated
patients in a medical intensive care unit. Saudi J Anaesth.
7:33–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sharma GD, He J and Bazan HE: p38 and
ERK1/2 coordinate cellular migration and proliferation in
epithelial wound healing: Evidence of cross-talk activation between
MAP kinase cascades. J Biol Chem. 278:21989–21997. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen TC and Chang SW: Effect of mitomycin
C on IL-1R expression, IL-1-related hepatocyte growth factor
secretion and corneal epithelial cell migration. Invest Ophthalmol
Vis Sci. 51:1389–1396. 2010. View Article : Google Scholar
|
|
39
|
Cullen AP, Chou BR, Hall MG and Jany SE:
Ultraviolet-B damages corneal endothelium. Am J Optom Physiol Opt.
61:473–478. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schein OD: Phototoxicity and the cornea. J
Natl Med Assoc. 84:579–583. 1992.PubMed/NCBI
|
|
41
|
Mangan MS, Arici C, Atalay E, Tanyildiz B
and Orucoglu F: Four cases of pediatric photokeratitis present to
the emergency department after watching the same theater show. Turk
J Ophthalmol. 45:226–228. 2015. View Article : Google Scholar
|
|
42
|
Liou JC, Teng MC, Tsai YS, Lin EC and Chen
BY: UV-blocking spectacle lens protects against UV-induced decline
of visual performance. Mol Vis. 21:846–856. 2015.PubMed/NCBI
|
|
43
|
Ambati BK, Nozaki M, Singh N, Takeda A,
Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb
MT, et al: Corneal avascularity is due to soluble VEGF receptor-1.
Nature. 443:993–997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Azar DT: Corneal angiogenic privilege:
Angiogenic and anti-angiogenic factors in corneal avascularity,
vasculogenesis, and wound healing (an American Ophthalmological
Society thesis). Trans Am Ophthalmol Soc. 104:264–302. 2006.
|
|
45
|
Zhang SX and Ma JX: Ocular
neovascularization: Implication of endogenous angiogenic inhibitors
and potential therapy. Prog Retin Eye Res. 26:1–37. 2007.
View Article : Google Scholar
|
|
46
|
Dvorak HF, Brown LF, Detmar M and Dvorak
AM: Vascular permeability factor/vascular endothelial growth
factor, micro-vascular hyperpermeability, and angiogenesis. Am J
Pathol. 146:1029–1039. 1995.PubMed/NCBI
|
|
47
|
Philipp W, Speicher L and Humpel C:
Expression of vascular endothelial growth factor and its receptors
in inflamed and vascularized human corneas. Invest Ophthalmol vis
Sci. 41:2514–2522. 2000.PubMed/NCBI
|
|
48
|
Matsumura A, Kubota T, Taiyoh H, Fujiwara
H, Okamoto K, Ichikawa D, Shiozaki A, Komatsu S, Nakanishi M, Kuriu
Y, et al: HGF regulates VEGF expression via the c-Met receptor
downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine
cells. Int J Oncol. 42:535–542. 2013. View Article : Google Scholar
|
|
49
|
Narita K, Fujii T, Ishiwata T, Yamamoto T,
Kawamoto Y, Kawahara K, Nakazawa N and Naito Z: Keratinocyte growth
factor induces vascular endothelial growth factor-A expression in
colorectal cancer cells. Int J Oncol. 34:355–360. 2009.PubMed/NCBI
|
|
50
|
Pal-Ghosh S, Tadvalkar G and Stepp MA:
Alterations in corneal sensory nerves during homeostasis, aging,
and after injury in mice lacking the heparan sulfate proteoglycan
syndecan-1. Invest Ophthalmol Vis Sci. 58:4959–4975. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pan Z, Fukuoka S, Karagianni N, Guaiquil
VH and Rosenblatt MI: Vascular endothelial growth factor promotes
anatomical and functional recovery of injured peripheral nerves in
the avascular cornea. FASEB J. 27:2756–2767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chandrasekher G, Kakazu AH and Bazan HE:
HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in
corneal epithelial cells: Its relevance in wound healing. Exp Eye
Res. 73:191–202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Weng J, Mohan RR, Li Q and Wilson SE: IL-1
upregulates keratinocyte growth factor and hepatocyte growth factor
mRNA and protein production by cultured stromal fibroblast cells:
Interleukin-1 beta expression in the cornea. Cornea. 16:465–471.
1997. View Article : Google Scholar : PubMed/NCBI
|