Introduction
The entire plant of Maclura tricuspidata
Carr. (Cudrania tricuspidata) contains diverse
phytochemicals, including xanthones and flavonoids, which exert
various bioactivities and have various medicinal and nutritional
applications in East Asia (1).
The extracts or components of M. tricuspidata exhibit a
broad spectrum of pharmacological activities, including
anti-inflammatory (2,3), antioxidant (4) and antitumor effects (5,6).
Flavonoids, which represent a large subgroup of the phenolic class
of specialized plant metabolites, have been confirmed to possess
these beneficial effects, and >100 flavonoids have been isolated
from M. tricuspidata (1),
which are further classified into flavones, flavanones and
isoflavones. Of these, certain flavonoids exhibit potential
anti-inflammatory activity. The outcomes of isoflavones isolated
from M. tricuspidata on inflammatory responses induced by
endotoxins [lipopolysaccharide (LPS)] have previously been reported
(2,3). However, whether flavonoids influence
cell activation induced in an environment rich in oxygenated
cholesterol molecules remains to be elucidated.
Oxygenated cholesterol molecules (oxysterols) are
metabolites of cholesterol containing one or more hydroxyl groups
(7). They possess numerous potent
and diverse biological activities, of which several are implicated
in the development of major chronic diseases, including
atherosclerosis (8).
27-Hydroxycholesterol (27OHChol) is the major oxysterol present in
advanced atherosclerotic lesions, followed by 7-oxygenated
cholesterol molecules. These oxysterols comprise 75-85% of the
oxysterols detected in plaques from different sites (7,9).
The potent inflammatory activity of oxysterol is a major force
driving the progression and complication of diseases. Among the
aforementioned oxysterols, 27OHChol activates endothelial cells and
monocyte/macrophage cells, which in turn enhance the expression of
inflammatory mediators, including adhesion molecules, chemokines,
pattern recognition receptors and matrix metal-loproteinases (MMPs)
(10-12). The secreted mediators then amplify
inflammation by recruiting and activating monocytic cells and T
cells (12-14). As the resultant chain of sequences
in the presence of 27OHChol sustains and expands the inflammatory
process by activating vascular cells, it is considered a promising
therapeutic target for atherosclerosis (12).
Macrophages, which serve a central role in all stage
of the inflammatory response, are versatile. Macrophages are
activated towards an M1 phenotype upon stimulation by the
Gram-negative product LPS and by inflammation-related cytokines
tumor necrosis factor (TNF)-α or interferon (IFN)-γ, alone or in
combination. The M1 phenotypes produce toxic effector molecules,
including reactive oxygen species and nitric oxide, and
inflammatory cytokines, including C-C motif chemokine ligand
(CCL)2, interleukin (IL)-1β, tumor necrosis factor (TNF)-α and
IL-6. They are involved in polarized Th1 responses as inducers and
effector cells, and mediate resistance against intracellular
pathogens (15,16). M2-like polarized macrophages are
induced in response to Th2-related cytokines IL-4 or IL-13 (M2a),
to the concomitant triggering of Fcγ receptors, Toll-like receptors
and immune complexes (M2b) and to anti-inflammatory molecules,
including TGF-β, IL-10 and glucocorticoids (M2c) (17). The M2 phenotypes secrete
anti-inflammatory mediators, IL-10 and TGF-β, have high levels of
scavenger, mannose and galactose type receptors, and are involved
in polarized Th2 responses, immune regulation, dampening of
inflammation and tissue remodeling (15-17). M1 and M2 phenotypes are present in
human atherosclerotic plaques with distinct spatial distribution.
The fibrous caps of lesions exhibit no significant differences
between subsets, whereas M2 macrophages outnumber the M1 phenotype
in vascular adventitial tissue, and M1 macrophages dominate the
rupture-prone shoulder regions over M2-type cells (18), suggesting that the balance between
M1/M2 polarization is of critical importance to the homeostasis of
vascular tissue and progression/regression of the vascular
disease.
The present study was performed to investigate the
effects of isoflavones isolated from M. tricuspidata on the
responses of monocyte/macrophage to 27OHChol. This is the first
study, to the best of our knowledge, to report that a certain type
of flavonoid inhibits the activation of monocytes/macrophages to
the immunostimulatory phenotype in a milieu rich in oxygenated
cholesterol molecules.
Materials and methods
Cell culture
The human THP-1 monocyte/macrophage cells were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA; cat. no. TIB-20) and cultured in RPMI medium
1640 supplemented with 10% fetal bovine serum (FBS) at 37°C in a
humidified atmosphere of 5% CO2. Penicillin (50 U/ml)
and streptomycin (50 μg/ml) were added to prevent bacterial
contamination. A Jurkat cell line stably expressing C-C motif
chemokine receptor 5 (CCR5), which was constructed by transfecting
Jurkat cells (clone E6-1; ATCC; cat. no. TIB-152) with a eukaryotic
expression vector containing the CCR5 gene, was provided by Dr Y.
Yun (Ewha Womans University, Seoul, Korea) (19). The CCR5-expressing Jurkat T cells
were cultured in RPMI medium 1640 supplemented with 10% FBS in the
presence of geneticin.
Reagents
The 4′-O-methylalpinumisoflavone (mAI) and
alpinumisoflavone were isolated from the fruits of M.
tricuspidata, as previously described (20). 27OHChol (cat. no. sc-358756) and
antibodies against CD14 (cat. no. sc-9150), p65 (cat. no. c-8008),
phosphorylated p65 (cat. no. sc-136548), β-actin (cat. no.
sc-47778), anti-rabbit IgG-HRP (cat. no. sc-2357) and anti-mouse
IgG-HRP (cat. no. sc-2005) were purchased from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). Anti-Akt (cat. no. 4691)
and anti-phosphorylated Akt (cat. no. 4060) antibodies were
obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA).
LPS prepared from Escherichia coli K12 (cat. no. tlrl-eklps)
was purchased from InvivoGen (San Diego, CA, USA).
Treatment of cells
THP-1 cells (2.5×105 cells/ml) were
serum-starved overnight by incubation in RPMI 1640 medium
supplemented with 0.1% bovine serum albumin (BSA; GenDEPOT, Katy,
TX, USA). After the serum-starvation, cells were treated with
27OHChol in the presence of the indicated concentrations of mAI or
alpinumisoflavone. In the LPS response experiment, serum-starved
THP-1 cells were cultured for 24 h with 27OHChol in the presence of
mAI, followed by stimulation for 9 h with LPS (100 ng/ml).
Viability assay
The viability of the THP-1 cells was determined via
the trypan blue dye exclusion method using a Vi-Cell XR cell
counter (Beckman Coulter, Inc., Indianapolis, IN, USA). The
viability of cells cultured in medium alone was considered 100%.
Data are expressed as the mean ± standard deviation (n=3 replicates
for each group).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Reagents for RT were purchased from Promega
Corporation (Madison, WI, USA) unless otherwise stated. Total RNAs
isolated using TRIzol reagent (Thermo Fisher Scientific, Inc.) were
reverse transcribed at 42°C for 1 h with 100 U Moloney Murine
Leukemia Virus reverse transcriptase (cat. no. M1701) in a 10
μl reaction volume, containing 50 mM Tris-HCl (pH 8.3 at
25°C), 55 mM KCl, 3 mM MgCl2, 10 mM DTT, 1 μg oligo (dT) 15
primers (cat. no. C110B), 0.125 mM each dNTP (cat. no. U1511), and
40 U RNase inhibitor (cat. no. N2111). Subsequent qPCR was
performed following the protocol described by Kim et al
(21). Each 20-μl reaction
consisted of 10 μl of SYBR-Green Master Mix, 2 μl of
forward and reverse primers (10 pM each) of genes to be analyzed,
and cDNA template. The thermal cycling conditions consisted of 95°C
for 10 min, followed by 45 cycles at 95°C for 10 sec, 50°C for 10
sec and 72°C for 10 sec. The relative expression of each gene was
calculated as the ratio to housekeeping gene (GAPDH) using the
2−ΔΔCq method, as previously reported (22). The primers used were as follows:
CCL2, 5′-CAGCCAGATGCAATCAATGCC-3′ (forward) and
5′-TGGAATCCTGAACCCACTTCT-3′ (reverse); CCL3,
5′-AGTTCTCTGCATCACTTGCTG-3′ (forward) and 5′-CGG
CTTCGCTTGGTTAGGAA-3′ (reverse); CCL4, 5′-CTGGGT CCAGGAGTACGTGT-3′
(forward) and 5′-GCGGAGAGG AGTCCTGAGTA-3′ (reverse); CD14,
5′-ACGCCAGAACCT TGTGAGC-3′ (forward) and 5′-GCATGGATCTCCACC
TCTACTG-3′ (reverse); MMP-9, 5′-GCACGACGTCTT CCAGTACC-3′ (forward)
and 5′-CAGGATGTCATAGG TCACGTAGC-3′ (reverse); IL-1β,
5′-TGAGCTCGCCAGTG AAATGA-3′ (forward) and 5′-AGATTCGTAGCTGGATG
CCG-3′ (reverse); TNF-α, 5′-CCCAGGGACCTCTCT CTAATC-3′ (forward) and
5′-ATGGGCTACAGGCTTGTC ACT-3′ (reverse); C-X-C motif chemokine
ligand (CXCL)10, 5′-TGTACGCTGTACCTGCATCA-3′ (forward) and 5′-GGA
CAAAATTGGCTTGCAGGA-3′ (reverse); CXCL11, 5′-AAG
CAGTGAAAGTGGCAGAT-3′ (forward) and 5′-TAAGCC TTGCTTGCTTCGAT-3′
(reverse); CD80, 5′-GCAGGGA ACATCACCATCCA-3′ (forward) and
5′-TCACGTGGAT AACACCTGAACA-3′ (reverse); CD86, 5′-GGACTAGCAC
AGACACACGGA-3′ (forward) and 5′-CTTCAGAGGAGCA GCACCAGA-3′
(reverse); CD163, 5′-AAAAAGCCACAAC AGGTCGC-3′ (forward) and
5′-CTTGAGGAAACTGCAA GCCG-3′ (reverse); CD206, 5′-TGAATTGTACTGGTCTG
TCCT-3′ (forward) and 5′-CTGTGGTGCTGTGCATTTA TCT-3′ (reverse);
liver X receptor (LXR)α, 5′-AAGCCCTGC ATGCCTACGT-3′ (forward) and
5′-TGCAGACGCAGTG CAAACA-3′ (reverse); GAPDH, 5′-GAAGGTGAAGGTCG
GAGT-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′ (reverse).
Enzyme-linked immunosorbent assay
(ELISA)
The protein levels of CCL2, CCL3, CCL4 and soluble
CD14 (sCD14) secreted from cells were measured by using
commercially available ELISA kits following the manufacturer's
protocol (R&D Systems, Inc., Minneapolis, MN, USA).
Migration assay
Cell migration was investigated using Transwell
permeable supports (Costar; Corning, Inc., Corning, NY, USA) as
previously described (21). The
top wells of the 5-μm-pore polycarbonate Transwell inserts
were loaded with THP-1 cells or Jurkat T cells expressing CCR5
(5×105 cells in 100 μl of 0.1% BSA) and then
inserted into a reservoir filled with supernatants containing
chemoattractants. Following incubation for 2 h in a cell culture
CO2 chamber, the number of cells migrated into the
reservoir were counted using the Vi-Cell XR cell counter (Beckman
Coulter, Inc.).
MMP-9 gelatinolytic activity
The activity of MMP-9 was assessed by gelatin
zymography following the protocol described by Kim et al
(21). Following the removal of
cell debris by centrifugation at 760 × g for 10 min at 4°C, the
culture medium was concentrated 30-fold using the Vivaspin 2
centricon. An equal volume (20 μl) of each sample was
electrophoresed on 8% polyacrylamide gels containing 0.15% gelatin,
and the MMPs were renatured by the removal of SDS though immersion
of the gel in Triton X-100 [2.5% (v/v) in Tris-HCl, pH 7.5] for 1 h
with gentle agitation. Following a rinse in water, the gels were
incubated in an activation buffer (50 mM Tris-HCl pH 7.7, 0.2 M
NaCl, 5 mM CaCl2, 0.02% Brij 35 and 0.01%
NaN3) overnight at 37°C to allow proteolysis of the
gelatin substrate. To visualize proteolytic bands, the gels were
stained in 0.2% Coomassie Brilliant blue R-250 followed by
distaining with methanol/acetic acid (20/10%) solution. The gel was
photographed by using ZoomBrowser EX 5.0 (Canon, Inc., Tokyo,
Japan).
Flow cytometric analysis
The THP-1 cells were harvested by centrifugation at
200 × g for 5 min at room temperature, which was followed by
incubation for 40 min with anti-CD14 antibody conjugated with
fluorescent dye (Santa Cruz Biotechnology, Inc.) diluted to 1:100
in FACS buffer (2 mM EDTA and 0.2% BSA in PBS) at 4°C. The cells
were washed twice with PBS and resuspended in 1% paraformaldehyde
in phosphate-buffered saline (PBS). The fluorescence was analyzed
using a flow cytometer.
Western blot analysis
Western blotting was performed as described
previously (21). The cells were
lysed with lysis buffer (1% SDS, 1 mM NaVO3 and 10 mM Tris-HCl, pH
7.4) containing protease inhibitors, and the supernatants were
isolated following centrifugation (15,000 × g) for 5 min at 4°C.
Following determination of protein concentration using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.), an equal quantity of protein (20 μg) was separated by
10% SDS-PAGE and transferred onto polyvinylidene fluoride
membranes. Following blocking for 1 h in 5% skim milk in 0.1%
Tween-20/TBS at room temperature, the membranes were incubated with
primary antibodies diluted to 1:1,000 (v/v) in the blocking
solution overnight at 4°C. The membranes were then washed three
times with 0.1% Tween-20/TBS for 10 min each and incubated with
horseradish peroxidase-conjugated secondary antibodies diluted in
the blocking solution (1:8,000) for 1 h at room temperature.
Following three washes with washing buffer for 10 min each, the
bands were detected using ECL western blotting substrate.
Statistical analysis
Statistical analysis was performed via one-way
analysis of variance, followed by Dunnett's multiple comparison
test, using PRISM 5.0 (GraphPad Software Inc., San Diego, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
mAI exerts minimal effects on the
viability of monocytic cells
The present study determined the effects of
alpinumisoflavone and mAI, the two most abundant isoflavones
isolated from the fruits of M. tricuspidata, on the
viability of THP-1 cells (Fig.
1A). Cell viabilities were 92.4, 89.7 and 86.5% following
exposure for 48 h to 2.5, 5 and 10 μg/ml of mAI,
respectively. However, significant cytotoxicity was observed with
identical concentrations of alpinumisoflavone. When the monocytic
cells were treated with 1 μg/ml of mAI in the presence of
27OHChol at the concentration used of 2 μg/ml, viability was
not decreased (Fig. 1B).
Therefore, subsequent experiments were performed with mAI at
sub-cytotoxic concentrations.
mAI inhibits the expression of CCL2 and
monocytic cell migration
Whether mAI influences the activation of
monocytes/macrophages was investigated by measuring the expression
of CCL2 and monocytic cell migration. 27OHChol activated the THP-1
monocyte/macrophage cells as indicated by enhanced expression of
the CCL2 chemokine, however, the expression was inhibited by mAI in
a dose-dependent manner. The levels of CCL2 transcript increased
26.4-fold following stimulation with 27OHChol, whereas this
increase was suppressed to 19.3- 11.3- and 3.7-fold in the presence
of 0.01, 0.1 and 1 μg/ml of isoflavone, respectively
(Fig. 2A). However, the basal
expression level of CCL2 was not altered by treatment with mAI
(Fig. S1A). mAI affected the
secretion of CCL2 in a pattern similar to that observed with
transcription of the gene. The level of secreted CCL2 significantly
increased following stimulation with 27OHChol compared with that of
unstimulated THP-1 cells, however, the levels of secreted CCL2 were
dose-dependently reduced in the presence of mAI (Fig. 2B). Whether the decreased secretion
of CCL2 altered the migration of monocytic cells was also
investigated. An increase in monocytic cell migration was observed
in response to the supernatant isolated from cells stimulated with
27OHChol, however, the migration was significantly reduced in
response to the media isolated from cells treated with 0.1 and 1
μg/ml of the isoflavone (Fig.
2C). Collectively, these results suggested that mAI inhibited
the production of CCL2 induced by 27OHChol and thereby monocytic
cell migration.
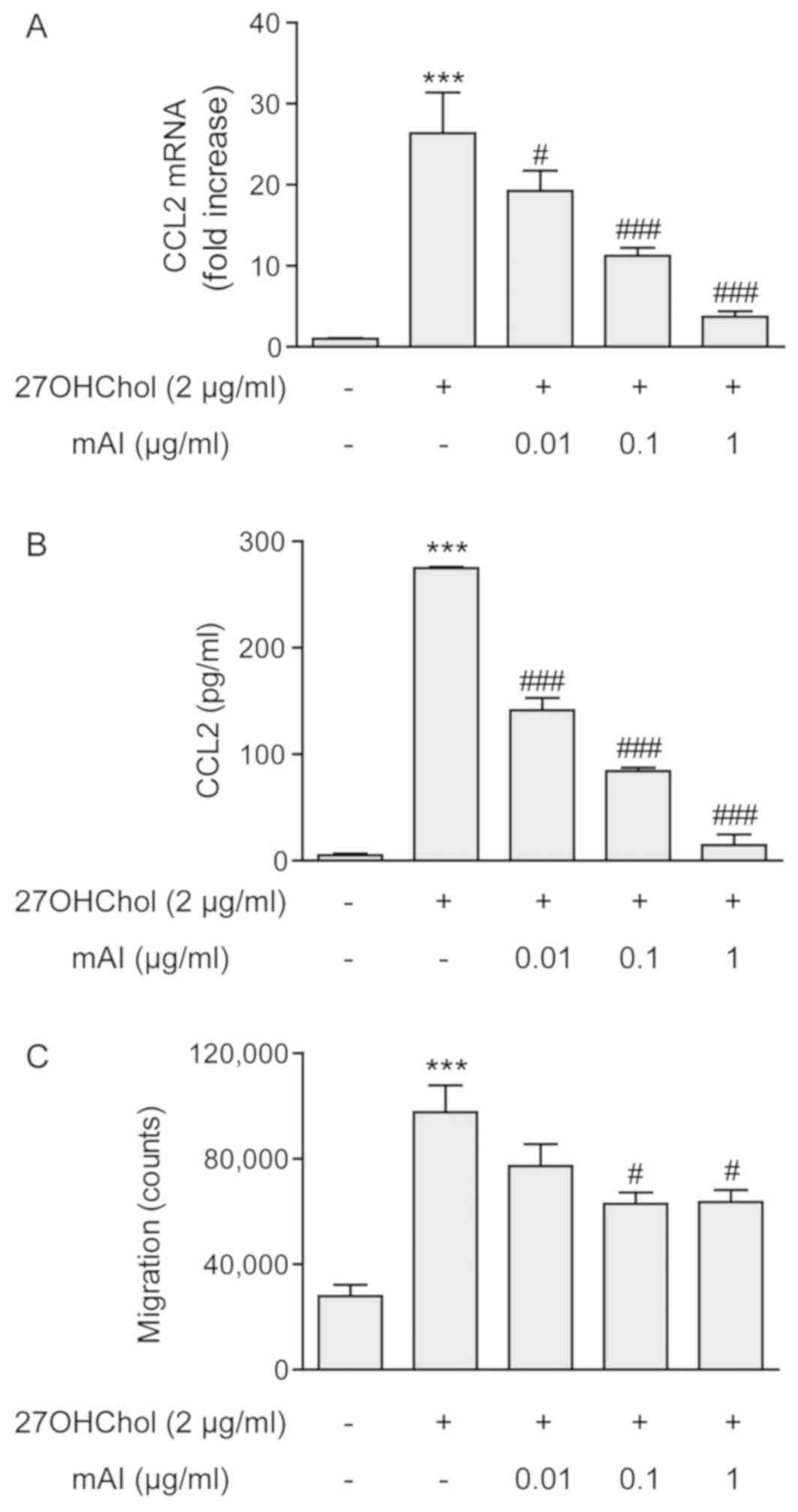 | Figure 2mAI decreases the expression of CCL2
and monocytic cell migration. THP-1 cells (2.5×105
cells/ml) were serum-starved by incubating overnight in RPMI medium
supplemented with 0.1% BSA (endotoxin-free) and cultured for 48 h
with 27OHChol in the presence of the indicated concentrations of
mAI. (A) CCL2 transcript levels were assessed by reverse
transcription-quantitative polymerase chain reaction analysis. The
y-axis values represent the increases in mRNA levels of CCL2
normalized to GAPDH levels, relative to that of the untreated THP-1
cells (control). ***P<0.001, vs. control;
###P<0.001, vs. 27OHChol; #P<0.05, vs.
27OHChol.(B) Culture media were isolated, and the protein levels of
CCL2 in the media were measured by enzyme-linked immunosorbent
assay. ***P<0.001, vs. control;
###P<0.001, vs. 27OHChol. (C) Monocytic cells were
exposed to the conditioned media isolated above, and migration of
monocytic cells was measured using a chemotaxis assay.
***P<0.001, vs. control; #P<0.05, vs.
27OHChol. Data in all graphs are expressed as the mean ± standard
deviation (n=3 replicates for each group). CCL2, C-C motif
chemokine ligand 2; mAI, 4′-O-methylalpinumisoflavone;
27OHChol, 27-hydroxycholesterol. |
mAI downregulates the expression of
CD14
The present study also investigated whether mAI has
any effects on the expression of CD14 pattern recognition receptor.
Stimulation of the THP-1 cells with 27OHChol resulted in an
increased percentage of cells expressing membrane CD14 (mCD14),
from 11.2 to 27.9%, compared with that in untreated cells. However,
the increase was suppressed to 25.9, 18.0 and 8.4%, following
treatment with 0.01, 0.1 and 1 μg/ml of mAI, respectively,
as determined by flow cytometry (Fig.
3A). Whether mAI affected the cellular level of CD14 was
determined by western blotting. The protein level of CD14 in the
THP-1 cells increased following stimulation with 27OHChol, however,
treatment with mAI resulted in decreased levels of cellular CD14
(Fig. 3B). The effects of mAI on
sCD14 release were also determined. The THP-1 cells released a
basal level of sCD14 (129.9±7.6 pg/ml) into the media, and this was
significantly increased to 315.3±15.6 pg/ml following stimulation
with 27OHChol (Fig. 3C). However,
the release of sCD14 was significantly reduced in a dose-dependent
manner by treatment with varying concentrations of the isoflavone.
The level of sCD14 released from cells was reduced to the basal
level following treatment with 1 μg/ml of mAI.
As mAI inhibited the expression of CD14 at the
protein level, its effects on the transcription of the gene were
investigated. The levels of CD14 transcript increased 6.2-fold
following stimulation with 27OHChol, compared with control cells
cultured in medium, however, this was reduced to 3.9- and 3.5-fold
in the presence of 0.1 and 1 μg/ml of mAI, respectively
(Fig. 3D). However, the basal
expression of CD14 was not influenced by treatment with mAI
(Fig. S1B). Collectively, these
results indicated that mAI suppressed the expression of CD14
induced by 27OHChol at the transcriptional and protein levels.
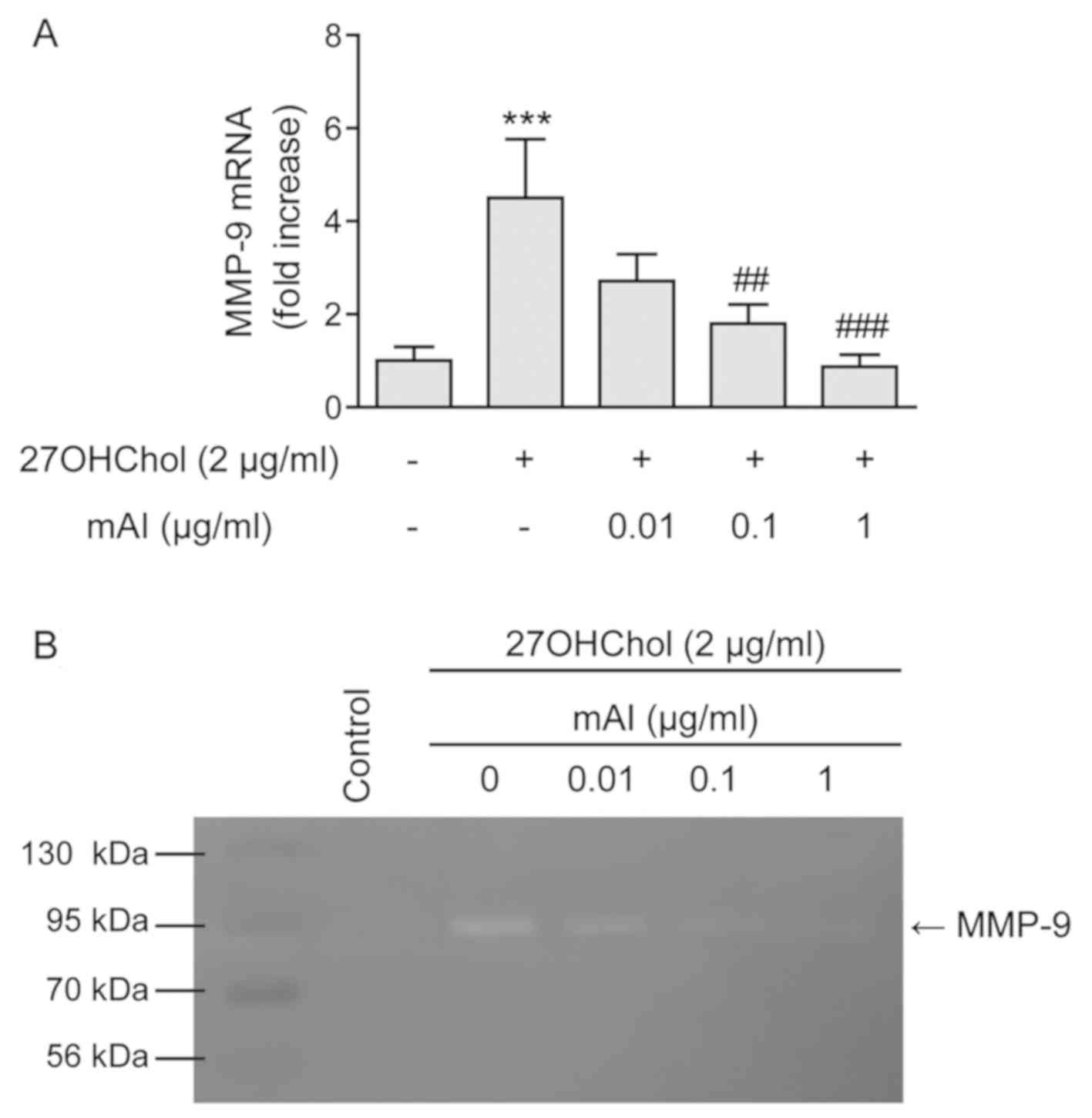
mAI impairs the secretion of active
MMP-9
Activated monocyte/macrophage cells secrete active
MMP-9 (22). Therefore, the
present study investigated whether mAI influenced the expression of
MMP-9. The stimulation of THP-1 cells with 27OHChol resulted in a
4.5-fold increase in the transcript levels of MMP-9, however, the
level decreased to 2.7-, 1.8- and 0.9-fold in the presence of 0.01,
0.1 and 1 μg/ml of the isoflavone, respectively (Fig. 4A). However, treatment with mAI
alone did not cause a change in the basal level of the MMP-9
transcript (Fig. S1B). The
activity of secreted MMP-9 was also analyzed by gelatin zymography
(Fig. 4B). The stimulation of
THP-1 cells with 27OHChol resulted in increased MMP-9 activity in
the supernatant. However, the gelatinolytic activity elevated by
27OHChol was attenuated by treatment with mAI. These results
indicated that mAI inhibited the secretion of active MMP-9.
mAI suppresses the expression of M1
markers
M1 phenotype macrophages enhance the secretion of
inflammatory and immunostimulatory mediators, whereas M2-type
macrophages release immunoregulatory cytokines (18). Therefore, the present study
investigated whether mAI affected macrophage polarization by
examining the expression of M1 markers. The transcript levels of M1
markers CCL2, IL-1β, TNF-α, CXCL10, CXCL11, CD80 and CD86 increased
following stimulation with 27OHChol, and the levels of all seven
markers were significantly reduced by treatment with mAI (Fig. 5A). The effects of mAI on the M2
marker were also investigated. 27OHChol increased the transcript
levels of three M2 markers of CD163, CD206 and LXR-α. The IL-10
transcript was not detected by RT-qPCR analysis (data not shown).
The levels of CD163 were significantly reduced by treatment with
mAI, whereas the isoflavone did not significantly affect the
expression of CD206 or LXR-α (Fig.
5B). Overall, these results suggested that mAI mainly regulates
the expression of M1 markers.
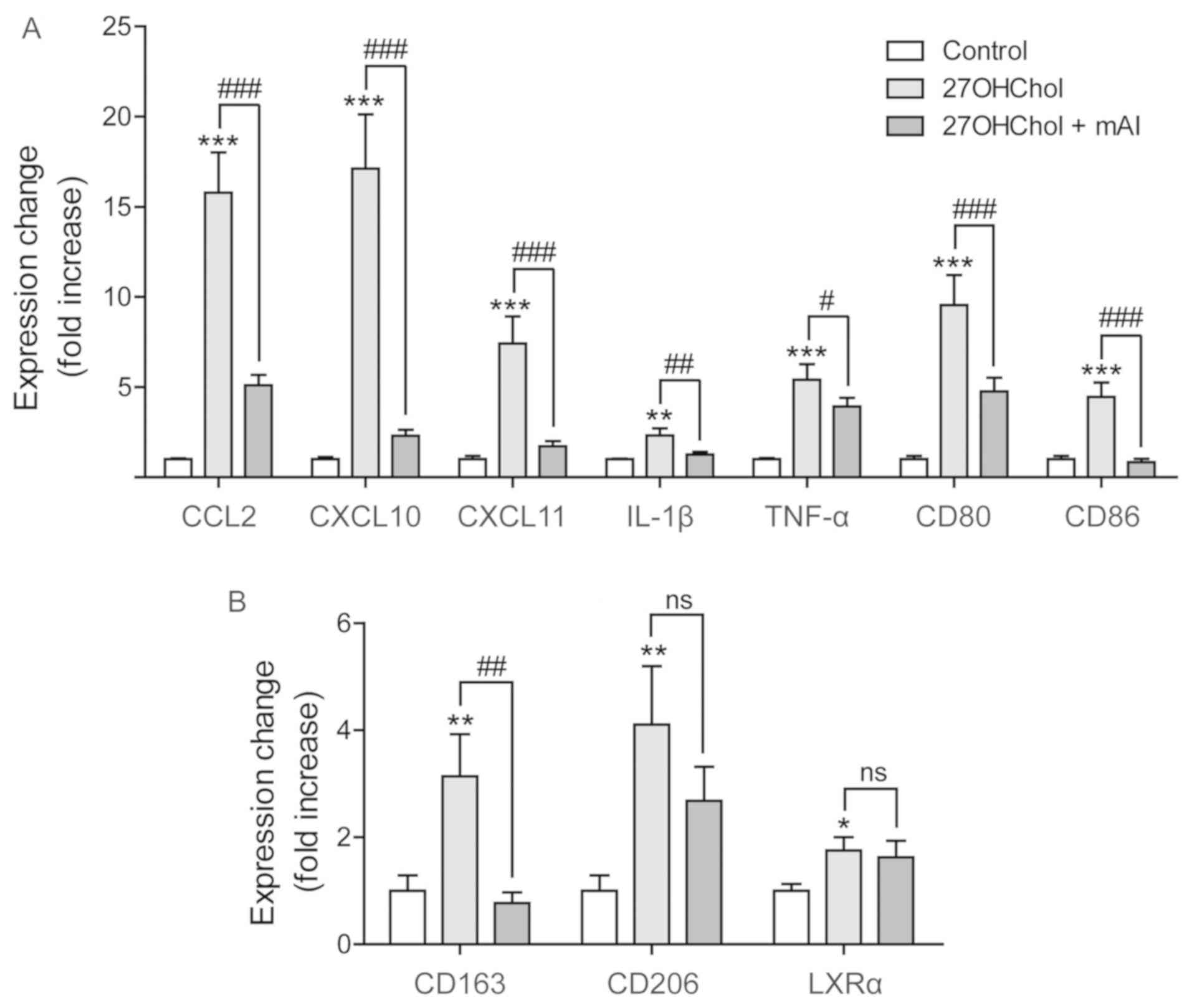 | Figure 5Effects of mAI on M1/M2 polarization
markers. Serum-starved THP-1 cells (2.5×105 cells/ml)
were cultured for 48 h with 27OHChol (2 μg/ml) in the
presence of mAI (1 μg/ml). The transcript levels of markers
for (A) M1 and (B) M2 polarization were assessed by reverse
transcription-quantitative polymerase chain reaction analysis. Data
are expressed as the mean ± standard deviation (n=3 replicates for
each group). ***P<0.001, vs. control;
**P<0.01, vs. control; *P<0.05, vs.
control; ###P<0.001, vs. 27OHChol;
##P<0.01, vs. 27OHChol; #P<0.05, vs.
27OHChol; ns, not significant; mAI,
4′-O-methylalpinumisoflavone; 27OHChol,
27-hydroxycholesterol; CCL2, C-C motif chemokine ligand 2; CXCL,
C-X-C motif chemokine ligand; IL-1β, interleukin-1β; TNF-α, tumor
necrosis factor-α; LXRα, liver X receptor α. |
mAI inhibits the production of CCR5
ligands and influences the migration of CCR5-expressing cells
Monocytic cells activated by 27OHChol secrete
chemokines in addition to CCL2, which enhance the migration of
CCR5-positive cells (13).
Therefore, the present study investigated the effects of mAI on the
expression of CCR5 ligands (CCL3 and CCL4) involved in the
migration of the cell. The transcript levels of CCL3 and CCL4 were
significantly elevated following stimulation with 27OHChol, and
this elevation was significantly suppressed in a dose-dependent
manner in the presence of 0.1 and 1 μg/ml mAI (Fig. 6A). However, mAI did not alter the
basal expression of CCL3 or CCL4 (Fig. S1A). mAI affected the secretion of
CCL3 and CCL4 in a similar pattern to that observed for their
transcription. Stimulation with 27OHChol resulted in enhanced
secretion of CCL3 and CCL4, and their secretion was significantly
suppressed by treatment with mAI (Fig. 6B). A migration assay was performed
using CCR5-expressing Jurkat T cells. The supernatant isolated from
cells exposed to 27OHChol significantly enhanced the migration of
the CCR5-expressing cells (Fig.
6C). However, the migration was significantly decreased when
the cells were exposed to supernatant isolated following treatment
with 1 μg/ml mAI. Overall, these results suggested that mAI
inhibited the production of CCR5 ligands induced by 27OHChol and
affected the migration of CCR5-expressing cells.
 | Figure 6mAI inhibits the expression of CCL3
and CCL4 and reduces migration of CCR5-positive T cells.
Serum-starved THP-1 cells (2.5×105 cells/ml) were
cultured for 48 h with 27OHChol (2 μg/ml) in the presence of
the indicated concentrations of mAI. (A) Levels of CCL3 and CCL4
transcripts were assessed by reverse transcription-quantitative
polymerase chain reaction analysis. ***P<0.001, vs.
control; ###P<0.001, vs. 27OHChol;
##P<0.01, vs. 27OHChol. (B) Secretion of CCL3 and
CCL4 proteins into the media was measured by enzyme-linked
immunosorbent assay following isolation of culture media.
***P<0.001, vs. control; ###P<0.001,
vs. 27OHChol. (C) CCR5-expressing Jurkat T cells were exposed to
the conditioned media, and migration of the cells was measured by
chemotaxis assay. *P<0.05, vs. control;
#P<0.05, vs. 27OHChol. In all graphs, data are
expressed as the mean ± standard deviation (n=3 replicates for each
group). mAI, 4′-O-methylalpinumisoflavone; 27OHChol,
27-hydroxycholesterol; CCR5, C-C motif chemokine receptor 5; CCL,
C-C motif chemokine ligand. |
mAI impairs the phosphorylation of the
nuclear factor (NF)-κB p65 subunit, but not of Akt
The transcription factor NF-κB serves a central role
in the inducible expression of inflammatory genes (23). Therefore, whether mAI had any
effects on the expression or phosphorylation of the NF-κB p65
subunit was investigated (Fig.
7). Increased phosphorylation of p65 was observed following
stimulation with 27OHChol, which was almost completely inhibited by
treatment with 1 μg/ml mAI. However, this flavonoid did not
influence the level of cellular p65 protein. The effects of mAI on
Akt were also investigated. The phosphorylation of Akt was elevated
upon stimulation with 27OHChol, but no alterations were observed in
phosphorylation or cellular protein levels of Akt following mAI
treatment. These results indicated that mAI regulated the
phosphorylation of the NF-κB p65 subunit.
Discussion
The present study demonstrated inhibition of the
27OHChol-induced expression of multiple chemokines, including CCL2,
CCL3 and CCL4, by treatment with mAI. CCL2 recruits monocytes to
the sites of inflammation, thereby amplifying inflammation
(24). CCL3 and CCL4 interact
with CCR5 and enhance the migration of Th1 lymphocytes, as this
receptor is characteristic of Th1 cells (25). Th1 cells produce IFN-γ, IL-2 and
TNF-α, and evoke cell-mediated immunity and phagocyte-dependent
inflammation (26). The decreased
levels of chemokines result in the reduced migration of the
corresponding aforementioned cell types. mAI also inhibits the
secretion of active MMP-9, which is required for the migration of
macrophages during inflammatory responses (27). The results of the present study
suggest that these inhibitory effects are not due to cytotoxicity,
but are specific; treatment with mAI and 27OHChol did not decrease
cell viability, and treatment with mAI alone had no effects on the
basal expression of the multiple genes, including CCL2, CCL3, CCL4
and MMP-9. The secretion of high levels of chemokines and active
MMP-9 indicated the activation of monocyte/macrophage cells.
Therefore, the results of the present study suggest that mAI is
likely to inhibit activation of monocytic cells and thereby
regulate inflammation and Th1 responses in an environment rich in
27OHChol.
CD14, a pattern recognition receptor, recognizes and
binds to pathogen-associated molecular patterns, including LPS, and
enhances responses to them (28).
As mAI downregulated the expression of mCD14 and sCD14, whether the
isoflavone altered responses to LPS was investigated. The
transcript levels of the CCL2 gene increased following stimulation
with 27OHChol or LPS alone (Fig.
8A). The addition of LPS to 27OHChol-stimulated cells resulted
in a significant elevation of the levels of CCL2. However, the
enhanced transcription of CCL2 was significantly attenuated by
treatment with mAI. The isoflavone also influenced the secretion of
CCL2 in a pattern similar to that of transcription of the gene
(Fig. 8B). These results suggest
that mAI prevents the overproduction of CCL2 induced by LPS, which
is in agreement with a previous study that reported the inhibition
of LPS-induced expression of inflammatory molecules in murine
microglial cells, following treatment with this isoflavone
(3). Collectively, these findings
suggest that mAI downregulates the expression of CD14, thereby
weakening responses to LPS.
 | Figure 8Effects of mAI on enhanced expression
of CCL2 induced by 27OHChol + LPS. Serum-starved THP-1 cells
(2.5×105 cells/ml) were cultured for 24 h with 27OHChol
(2 μg/ml) in the presence of the indicated concentrations of
mAI, followed by stimulation for 9 h with or without LPS (100
ng/ml) from Escherichia coli K12. (A) Levels of the CCL2
transcript were assessed by reverse transcription-quantitative
polymerase chain reaction analysis. ***P<0.001, vs.
control; +++P<0.001, vs. 27OHChol;
###P<0.001, vs. 27OHChol + LPS. (B) Culture media
were isolated, and the level of CCL2 protein secreted into the
media was measured by enzyme-linked immuno-sorbent assay.
**P<0.01, vs. control; ++P<0.01, vs.
27OHChol; ###P<0.001, vs. 27OHChol + LPS;
##P<0.01, vs. 27OHChol + LPS. In both graphs, data
are expressed as the mean ± standard deviation (n=3 replicates for
each group). mAI, 4′-O-methylalpinumisoflavone; 27OHChol,
27-hydroxycholesterol; LPS, lipopolysaccharide; CCL2, C-C motif
chemokine ligand 2. |
The present study attempted to determine the
molecular mechanisms underlying the inhibitory effects of mAI.
NF-κB, of which the most common and well-characterized form is the
p65/p50 heterodimer, induces the expression of genes involved in
inflammation following phosphorylation and translocation into the
nucleus (29). Akt induces the
expression of chemokines and pattern recognition receptors in
response to 27OHChol (13,14,30).
Therefore, the effects of mAI on Akt and the p65 subunit, which is
responsible for the high transcription activating potential of
NF-κB, were investigated. mAI inhibited the phosphorylation of p65,
whereas the phosphorylation of Akt was unchanged, indicating
specific inhibition of the phosphorylation of p65. In addition, the
decreased levels of the phosphorylated p65 subunit coincided with
decreased inflammatory mediators following treatment with mAI,
which is consistent with a previous study that reported the
inhibited phosphorylation of p65 and p50 subunits following LPS
stimulation in the presence of this isoflavone (3). Collectively, these findings suggest
that mAI is likely to exert its effects via the post-translational
modification of NF-κB.
Macrophages polarize to different functional
phenotypes, M1 and M2, in response to microenvironmental stimuli.
The present study demonstrated that mAI primarily suppresses the
expression of M1 markers, suggesting the regulation of polarization
to the M1 macrophage phenotype. The M1-type macrophages produce
high levels of pro-inflammatory cytokines and promote Th1
responses, whereas the M2-type macrophages promote tissue and
functional recovery (15). The
inhibitory effects suggest that the isoflavone can suppress Th1
responses and subsequent inflammatory responses, as demonstrated by
reduced chemokine expression and immune cell migration. Taken
together, these findings suggest that mAI regulates inflammation by
inhibiting the expression of chemokines and affecting the
polarization to M1-type macrophages. The negative effects of mAI on
macrophage M1 polarization may also be linked to attenuated
responses to LPS (2,3).
The present study demonstrated that mAI impairs the
inflammatory and immunostimulatory effects of 27OHChol on
monocytes/macrophages at the cellular and molecular levels. As
27OHChol is the most abundant cholesterol oxidation product in
atherosclerotic lesions (9), the
results of the present study suggest that mAI can reduce
inflammation in the lesions (Fig.
9). Further investigation is required to evaluate the
beneficial effects of mAI using animal models.
 | Figure 9Beneficial effects of mAI in a milieu
rich in 27OHChol. 27OHChol induced the expression of adhesion
molecules on endothelial cells, enhanced the migration of monocytic
cells and Th1 cells, and activates monocytic cells to produce
pro-inflammatory molecules. mAI inhibits the activation of
monocytes/ macrophages induced by 27OHChol, and decreases the
production of MMP-9 and chemokines, including CCL2, CCL3 and CCL4.
The decreased concentration of chemokines results in impaired
migration of the inflammatory cells to the sites of inflammation.
mAI also attenuates responses to lipopolysaccharide via the
downregulation of CD14. The anti-inflammatory effects may slow
progression and reduce complications of diseases in which 27OHChol
is involved in the pathogenesis. mAI,
4′-O-methylalpinumisoflavone; 27OHChol, 27-hydroxycholester;
MMP-9, matrix metalloproteinase-9; CCL, C-C motif chemokine ligand;
mCD14, membrane CD14; sCD14, soluble CD14; ICAM-1, intercellular
adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1;
CCR-2, C-C chemokine receptor type 2. |
Supplementary Materials
Abbreviations:
|
mAI
|
4′-O-methylalpinumisoflavone
|
|
27OHChol
|
27-hydroxycholesterol
|
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
NRF-2017R1A2B4002800).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KK, DL, and SKE designed the study and analyzed the
data; JL, BYK, YS, and DHG performed the experiments and analyzed
the data; JL, BYK, and KK wrote the manuscript. All authors
approved the final version of the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Y. Yun at Ewha
Womans University (Seoul, Korea) for providing the Jurkat cell line
that stably expresses CCR5.
References
|
1
|
Xin LT, Yue SJ, Fan YC, Wu JS, Yan D, Guan
HS and Wang CY: Cudrania tricuspidata: An updated review on
ethnomedicine, phytochemistry and pharmacology. RSC Advances.
7:31807–31832. 2017. View Article : Google Scholar
|
|
2
|
Jeong GS, Lee DS and Kim YC:
Cudratricusxanthone A from Cudrania tricuspidata suppresses
pro-inflammatory mediators through expression of anti-inflammatory
heme oxygenase-1 in RAW264.7 macrophages. Int Immunopharmacol.
9:241–246. 2009. View Article : Google Scholar
|
|
3
|
Lim JY, Hwang BY, Hwang KW and Park SY:
Methylalpinu- misoflavone inhibits lipopolysaccharide-induced
inflammation in microglial cells by the NF-kappaB and MAPK
signaling pathway. Phytother Res. 26:1948–1956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho EJ, Yokozawa T, Rhyu DY, Kim SC,
Shibahara N and Park JC: Study on the inhibitory effects of Korean
medicinal plants and their main compounds on the
1,1-diphenyl-2-picrylhy-drazyl radical. Phytomedicine. 10:544–551.
2003. View Article : Google Scholar
|
|
5
|
Lee IK, Kim CJ, Song KS, Kim HM, Koshino
H, Uramoto M and Yoo ID: Cytotoxic benzyl dihydroflavonols from
Cudrania tricuspidata. Phytochemistry. 41:213–216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou YS, Hou AJ and Zhu GF: Isoprenylated
xanthones and flavonoids from Cudrania tricuspidata. Chem
Biodivers. 2:131–138. 2005. View Article : Google Scholar
|
|
7
|
Brown AJ and Jessup W: Oxysterols and
atherosclerosis. Atherosclerosis. 142:1–28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gargiulo S, Gamba P, Testa G, Leonarduzzi
G and Poli G: The role of oxysterols in vascular ageing. J Physiol.
594:2095–2113. 2016. View
Article : Google Scholar :
|
|
9
|
Garcia-Cruset S, Carpenter KL, Guardiola
F, Stein BK and Mitchinson MJ: Oxysterol profiles of normal human
arteries, fatty streaks and advanced lesions. Free Radic Res.
35:31–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SM, Kim BY, Eo SK, Kim CD and Kim K:
27-Hydroxy- cholesterol up-regulates CD14 and predisposes monocytic
cells to superproduction of CCL2 in response to lipopolysaccharide.
Biochim Biophys Acta. 1852:442–450. 2015. View Article : Google Scholar
|
|
11
|
Morello F, Saglio E, Noghero A, Schiavone
D, Williams TA, Verhovez A, Bussolino F, Veglio F and Mulatero P:
LXR-activating oxysterols induce the expression of inflammatory
markers in endothelial cells through LXR-independent mechanisms.
Atherosclerosis. 207:38–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Umetani M, Ghosh P, Ishikawa T, Umetani J,
Ahmed M, Mineo C and Shaul PW: The cholesterol metabolite
27-hydroxycholesterol promotes atherosclerosis via proinflammatory
processes mediated by estrogen receptor alpha. Cell Metab.
20:172–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SM, Kim BY, Lee SA, Eo SK, Yun Y, Kim
CD and Kim K: 27-Hydroxycholesterol and 7alpha-hydroxycholesterol
trigger a sequence of events leading to migration of
CCR5-expressing Th1 lymphocytes. Toxicol Appl Pharmacol.
274:462–470. 2014. View Article : Google Scholar
|
|
14
|
Kim SM, Lee SA, Kim BY, Bae SS, Eo SK and
Kim K: 27-Hydroxycholesterol induces recruitment of monocytic cells
by enhancing CCL2 production. Biochem Biophys Res Commun.
442:159–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment.
F1000Prime Rep. 6:132014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Italiani P and Boraschi D: From monocytes
to M1/M2 macrophages: Phenotypical vs. functional differentiation.
Front Immunol. 5:5142014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stöger JL, Gijbels MJ, van der Velden S,
Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E and de
Winther MP: Distribution of macrophage polarization markers in
human atherosclerosis. Atherosclerosis. 225:461–468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park D, Park I, Lee D, Choi YB, Lee H and
Yun Y: The adaptor protein Lad associates with the G protein beta
subunit and mediates chemokine-dependent T-cell migration. Blood.
109:5122–5128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hiep NT, Kwon J, Kim DW, Hwang BY, Lee HJ,
Mar W and Lee D: Isoflavones with neuroprotective activities from
fruits of Cudrania tricuspidata. Phytochemistry. 111:141–148. 2015.
View Article : Google Scholar
|
|
21
|
Kim BY, Son Y, Lee J, Choi J, Kim CD, Bae
SS, Eo SK and Kim K: Dexamethasone inhibits activation of
monocytes/macrophages in a milieu rich in 27-oxygenated
cholesterol. PLoS One. 12:e01896432017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manicone AM and McGuire JK: Matrix
metalloproteinases as modulators of inflammation. Semin Cell Dev
Biol. 19:34–41. 2008. View Article : Google Scholar
|
|
23
|
Bhatt D and Ghosh S: Regulation of the
NF-κB-mediated transcription of inflammatory genes. Front Immunol.
5:712014. View Article : Google Scholar
|
|
24
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loetscher P, Uguccioni M, Bordoli L,
Baggiolini M, Moser B, Chizzolini C and Dayer JM: CCR5 is
characteristic of Th1 lymphocytes. Nature. 391:344–345. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romagnani S: T-cell subsets (Th1 versus
Th2). Ann Allergy Asthma Immunol. 85:9–18; quiz 18-21. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanania R, Sun HS, Xu K, Pustylnik S,
Jeganathan S and Harrison RE: Classically activated macrophages use
stable microtubules for matrix metalloproteinase-9 (MMP-9)
secretion. J Biol Chem. 287:8468–8483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kirkland TN and Viriyakosol S:
Structure-function analysis of soluble and membrane-bound CD14.
Prog Clin Biol Res. 397:79–87. 1998.PubMed/NCBI
|
|
29
|
Christian F, Smith EL and Carmody RJ: The
Regulation of NF-κB Subunits by Phosphorylation. Cells. 5:1–19.
2016. View Article : Google Scholar
|
|
30
|
Heo W, Kim SM, Eo SK, Rhim BY and Kim K:
FSL-1, a Toll-like receptor 2/6 agonist, induces expression of
interleukin-1 alpha in the presence of 27-hydroxycholesterol.
Korean J Physiol Pharmacol. 18:475–480. 2014. View Article : Google Scholar
|