Introduction
The incidence of type 2 diabetes mellitus (T2DM) has
rapidly increased in the last decade. In China alone, the number of
patients with diabetes rose from 20,400,000 in 1980 to 102,900,000
in 2014 (1). Patients with
diabetes often develop diabetic vascular complications, which are
known to be associated with impaired vascular remodeling and lack
of endothelial cell reconstruction function (2). Hyperlipidemia as a common
comorbidity of diabetes has also been reported as an important risk
factor for diabetic vascular complications (3). Palmitic acid (PA), which is one of
the most common saturated free fatty acids (FFAs) found in human
serum, is closely linked to the occurrence of lipotoxicity in
diabetes (4). Guo et al
(5) showed that PA significantly
inhibited endothelial progenitor cell (EPC) proliferation and rate
of migration, and their ability to form tube structures.
EPCs are a subpopulation of bone marrow mononuclear
cells, which are capable of generating new blood vessels in regions
of ischemia or infarction (6,7).
EPCs were first reported by Asahara et al (8), in 1977. It has been reported that
EPCs are associated with diabetic vascular complications (9). Loomans et al (10), reported that EPCs are important
regulators of impaired wound healing and reduced collateral
formation in type 1 diabetes. Other studies have reported that EPCs
isolated from human T2DM exhibit impaired viability, adhesion,
colony formation and tube formation (11-13). Although there were several ways to
improve EPC function in diabetes (14,15), these are limited ways to improve
the functions of EPC exposed to PA, and the underlying mechanism of
this process remains to be fully elucidated.
MicroRNAs (miRNAs) are small (20-24 nucleotides),
non-coding, single-stranded RNAs that were first identified in
Caenorhabditis elegans in 1993 (16,17). It is widely accepted that specific
miRNAs jointly regulate different EPC functions, including
proliferation, migration, tube formation, differentiation and
adhesion, in addition to EPC apoptosis and autophagy (18). miR-130a has been found to be
important in diabetes, as levels of miR-130a are lower than normal
in the EPCs of diabetic patients; upregulation of the expression of
miR-130a not only improves cell migration and colony formation but
also reduces the apoptosis of EPCs in diabetic patients (19). The phosphatase and tensin homolog
(PTEN) protein can affect EPC division and survival by
dephosphory-lating phosphoinositide-3,4,5-triphosphate to
inactivate the phosphoinositide-3 kinase (PI3K)/AKT pathway
(20,21). A study by Song et al
(22), showed that the
3′-untranslated region (3′UTR) of PTEN mRNA has an miR-130a binding
site, which is involved in regulating the survival and viability of
coronary artery endothelial cells. Therefore, miR-130a may serve a
role in protecting EPCs from PA-induced damage via the PTEN and the
PI3K/AKT pathway.
Metformin is a biguanide derivative, which is widely
prescribed as an oral anti-diabetic drug for T2DM. Yu et al
(23), reported that metformin
can promote angiogenesis by activating the AMP-activated protein
kinase (AMPK)/endothelial nitric oxide synthase pathway, which
improves migration and tube formation in bone marrow-derived EPCs
when these functions are impaired by high glucose levels. It was
also reported that metformin attenuated diabetic retinopathy and
diabetic vascular disease through combining with miRNAs (24,25). However, little is known regarding
the interplay between metformin and miR-130a in affecting the
PA-induced impairment of EPC angiogenesis. The present study
investigated the interaction between miR130a and the PTEN/PI3K/AKT
pathway as a potential therapeutic target for metformin in
PA-induced impaired EPC functions.
Materials and methods
Animals experiments
Animal care and all experimental protocols involving
animals were approved by the Animal Care and Use Committee of
Wenzhou Medical University (Wenzhou, China). A total of 50 adult
male Sprague-Dawley (SD) rats (specific pathogen-free; aged 6-8
weeks (200-320 g); Wenzhou Medical University Laboratory Animal
Center, Wenzhou, China) were used in the study. All rats were
housed at room temperature (21-25°C) with 45-50% humidity and a 12
h light/dark cycle, with free access to soft food and tap
water.
Isolation and culture of bone
marrow-derived-EPCs
The EPCs were isolated from the bone marrow of SD
rats and cultured according to established methods with minor
modifications (26,27). Briefly, five rats per batch were
sacrificed by cervical vertebra separation following anesthesia by
intraperitoneal injection of 320 mg/kg chloralhydrate. Bones (femur
and tibia of hind legs) from the 6-8 week-old male SD rats were
repeatedly flushed with homogenate rinse solution (Tianjin Hao Yang
Biological Manufacture Co., Ltd., Tianjin, China). The washing
fluid was centrifuged for 10 min at room temperature (700 × g) and
the pellet was resuspended in 3 ml sample diluent containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), and then was combined carefully under 3 ml
histopaque-1083 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Following centrifugation at 400 × g for 30 min at room temperature,
the mixture had separated into three layers. The cloudy layer was
gently removed and washed with PBS. Following three washes, the
mononuclear cells (MNCs) were resuspended in endothelial growth
factor-supplemented media (EGM-2 bullet kit; Lonza Group, Ltd.,
Basel, Switzerland) with 10% fetal bovine serum and plated on
fibronectin-coated culture plates (Sigma-Aldrich; Merck KGaA). The
cells were cultured at 37°C with 5% CO2 in a humidified
atmosphere. The medium was replaced following the first 48 h and
every 3 days thereafter. Late EPCs appeared after 14 days in
culture, and the third or fourth passage was used for later
analysis.
Characterization of bone
marrow-derived-EPCs
To identify the bone marrow-derived-EPCs,
immunofluorescence staining and flow cytometry were performed to
analyze the expression of the specific cell markers FLK-1 and CD34.
For the immunofluorescence staining, the EPCs that grew to 90% were
washed with PBS three times prior to fixation with 4%
paraformaldehyde. The cells were then incubated with FLK-1 (cat.
no. sc-393163; Santa Cruz Biotechnology, Inc., Dallas, TX, USA;
1:400) and CD34 (cat. no. 14486-1-AP; ProteinTech Group, Inc.,
Chicago, IL, USA; 1:200) antibodies at 4°C overnight. Following
incubation with the corresponding secondary antibodies at room
temperature for 1 h (cat. no. BYE010; Boyun Biotech, Shanghai,
China; 1:200) and DAPI nuclei dye, the EPCs were observed and
images were captured under a fluorescence microscope (932706; Nikon
Corporation, Tokyo, Japan). For flow cytometry (28), the adherent cells were digested
with EDTA-free trypsin and resuspended in PBS at the density of
5×106 cells/ml. The MNCs were then stained with
FITC-conjugated mouse anti-vascular endothelial growth factor
receptor 2 (cat. no. ab184903; Abcam, Cambridge, UK; 1:200) and
PE-conjugated mouse anti-CD34 (1:200; cat. no. MA1-10205; Thermo
Fisher Scientific, Inc.) for 30 min at room temperature. The same
fluorescein-labeled isotype IgG was used as a negative control for
each antibody. The cells were analyzed using a flow cytometer (BD
Biosciences, San Jose, CA, USA) and data were analyzed with FlowJo
7.6.1 (TreeStar, Inc., Ashland, OR, USA).
Cell proliferation
A Cell Counting Kit-8 (CCK-8) assay was used to
examine cell viability. The EPCs (5×103 cells/well) were
plated on 96-well plates and cultured in EGM-2 medium supplemented
with 10% FBS to induce attachment overnight. The medium was
replaced with EGM-2 plus the specified additives: i) 0.5 mM PA; ii)
50 µM metformin, and iii) metformin + PA. Following
incubation at 37°C with 5% CO2 for 48 h, the medium was
aspirated and fresh DMEM without serum but containing CCK-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added
(10 µl/well). Following culturing for 2 h at 37°C, the
absorbance was measured with a microplate reader
(SpectraMax® Plus384 Absorbance Microplate
Reader; Molecular Devices, LLC, Sunnyvale, CA, USA) at a wavelength
of 450 nm. Each group had five replicates and was assessed in
triplicate.
EPC migration assay
A cell migration assay was conducted in a 24-well
Millicell (Corning Incorporated, Corning, NY, USA) containing a
microporous (8.0-mm) membrane. Briefly, the EPCs (3×104
cells/well) were resuspended in EBM-2 medium (EGM-2 bullet kit;
Lonza Group, Ltd.) and added to the upper chambers. The lower
chambers (24-well plates) were mixed with EGM-2 medium in the
presence of metformin (50 µM, Sigma-Aldrich; Merck KGaA), PA
(0.5 mM, Sigma-Aldrich; Merck KGaA) or both. Following incubation
for 24 h, the non-migrated cells were removed with cotton-tipped
swabs. Those cells attached to the lower chambers were fixed with
10% paraformaldehyde for 30 min. Following DAPI staining for 30
min, the average cell number was counted manually in random fields
(magnification, ×100) with an inverted microscope (Olympus CKX41;
Olympus Corporation, Tokyo, Japan) in each well.
In vitro tube formation assay
The in vitro angiogenic activity of the EPCs
was assessed using a Matrigel tube experiment, as described
previously (24,29). In brief, Matrigel (cat. no.
356234; BD Biosciences) was thawed at 4°C overnight, and 150
µl Matrigel per well was added to a 48-well plate and
incubated at 37°C for 30 min to polymerize. The transfected EPCs
(3×104 cells/well) were cultured in the presence or
absence of metformin, PA or their combination for 12 h. Tube-like
structures were observed under an inverted microscope (Olympus
CKX41; Olympus Corporation). The numbers of tube-like structures in
the images were measured using ImageJ software (National Institutes
of Health, Bethesda, MD, USA). At least six random areas per well
were analyzed, and the experiments were repeated three times using
EPCs from three independent isolations.
miR-130a transfection
For the overexpression or inhibition of miR-130a,
the EPCs were transfected with miR-130a mimics (50 nmol/l, 5′-CAG
UGC AAU GUU AAA AGG GCA U-3′) or inhibitor (200 nmol/l, 5′-AUG CCC
UUU UAA CAU UGC A CU G-3′) and the corresponding negative controls
(Guangzhou RiboBio Co, Ltd., Guangzhou, China) for 6 h using
LipoFiter™ transfection reagent according to the manufacturer's
protocol (Hanbio Biotechnology Shanghai Co., Ltd., Shanghai,
China). Following 6 h of transfection, the medium was replaced with
10% FBS EGM-2 consisting of either PA or metformin for further
analysis.
Prediction of miR-130a targets
The target of miR-130a was predicted by using
TargetScan 7.1 (http://www.targetscan.org). Combining previous
literature on miR-130a and angio-genesis (22,30), PTEN was selected for further
analysis as a potential target of this miRNA.
Treatment with metformin
The EPCs were cultured in EGM-2 with 10% fetal
bovine serum containing either PA (0.5 mM, Sigma-Aldrich; Merck
KGaA) or metformin (50 μM, Sigma-Aldrich; Merck KGaA) for 24
h. For the miR-130a overexpression experiment, the medium was
replaced with PA or metformin 6 h following transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the harvested cells
using TRIzol reagent (Thermo Fisher Scientific, Inc.). To quantify
the mRNA levels of PTEN, RNA was quantified and reverse transcribed
into complementary DNA using specific stem-loop reverse
transcription primers. The mRNA first strand synthesis was
performed using a HiScript II Q RT SuperMix for qPCR (Vazyme
Biotech, Co., Ltd., Piscataway, NJ, USA), and qPCR was operated
using a ChamQ™ Universal SYBR qPCR Master mix (Vazyme Biotech, Co.,
Ltd.) on a Bio-Rad CFX96™ R-T PCR machine (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The Vazyme cycling conditions were as
follows: 95°C for 30 sec, followed by 39 cycles at 95°C for 10 sec
and 60°C for 30 sec. A melting curve every 0.5°C between 65 and
95°C for 15 min was obtained. GAPDH was used as a loading control.
To analyze the expression of miRNAs, cDNA was synthesized using the
miRcute Plus miRNA First-Strand cDNA Synthesis kit (Tiangen Biotech
Co., Ltd., Beijing, China) and RT-qPCR was performed using an
miRcute Plus miRNA qPCR Detection kit (cat. no. FP411; Tiangen
Biotech, Co., Ltd.). The Tiangen cycling conditions were as
follows: 95°C for 15 min, followed by 44 cycles at 94°C for 20 sec
and 60°C for 34 sec. A melting curve every 0.5°C between 65 and
95°C for 15 min was obtained. U6 was used as an internal control.
The PCR primers used in the present study were synthesized by
Sangon Biotech, Co., Ltd., (Shanghai, China) and the sequences are
listed in Table I. The PTEN
forward, 5′-ATG TTC AGT GGC GGA ACT TG-3′ primer and the reverse
primers for miR-130a, miR-132, miR-34a, miR-221 and U6 were
synthesized by Tiangen Biotech Co., Ltd., and were include in the
kit applied for the synthesis of cDNA. The results were subjected
to melting curve analysis and the relative gene expression levels
of miRNAs were determined. The levels of PTEN genes were analyzed
using the 2−ΔΔCq method (31).
 | Table ISequences of polymerase chain
reaction primers. |
Table I
Sequences of polymerase chain
reaction primers.
| Primer | Sequence |
|---|
| miR-130a-F |
5′-GCGCGCAGTGCAATGTTAAAAGGGCAT-3′ |
| miR-132-F |
5′-ACGCGTAACAGTCTACAGCCATGGTCG-3′ |
| miR-34a-F |
5′-ACGCTGGCAGTGTCTTAGCTGGTTGT-3′ |
| miR-221-F |
5′-CGCGAGCTACATTGTCTGCTGGGTTTC-3′ |
| U6-F |
5′-AGAGAAGATTAGCATGGCCCCTG-3′ |
| PTEN-F |
5′-ATGTTCAGTGGCGGAACTTG-3′ |
| PTEN-R |
5′-GTCACCACACACAGGCAATG-3′ |
| GAPDH-F |
5′-GACATGCCGCCTGGAGAAAC-3′ |
| GAPDH-R |
5′-AGCCCAGGATGCCCTTTAGT-3′ |
Western blotting
The protein expression levels of PTEN, AKT and
phosphorylated (P-)AKT were detected by western blot analysis. The
EPCs were washed with pre-cooled PBS, lysed in RIPA solution, and
centrifuged at 12,000 g for 30 min (4°C). Protein concentration
were measured using the BCA method (Beyotime Institute of
Biotechnology, Beijing, China). The proteins (30 μg/lane)
were separated on a 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred onto a polyvinylidene difluoride
membrane. Following blocking of the nonspecific binding sites with
5% non-fat milk in Tris-buffered saline-Tween 20 (TBS-T), the
membranes were incubated overnight with primary antibody against
PTEN (1:1,000; cat. no. 9188; Cell Signaling Technology, Inc.,
Danvers, MA, USA), AKT (1:1,000; cat. no. 9272; Cell Signaling
Technology, Inc.), and P-AKT (1:1,000; cat. no. AF0016; Affinity
Biosciences, Cell Signal Transduction, Cincinnati, OH, USA) at 4°C
overnight. The membranes were washed with TBS-T and incubated with
goat anti-rabbit IgG, peroxidase-conjugated secondary antibodies
(1:5,000; Biosharp Life Sciences, Hefei, China) at room temperature
for 1 h. The signals were visualized using chemiluminescence
detection reagents (Pierce ECL Plus; Thermo Fisher Scientific,
Inc.). The relative band intensities were determined by the Image
Lab software 4.1 (Bio-Rad Laboratories, Inc.). GAPDH was used as an
internal control. The results of the analysis were are expressed as
a relative ratio of the target protein to the internal reference.
All experiments were repeated three times.
Statistical analysis
All data were analyzed with the statistical software
GraphPad Prism v.7.0 (GraphPad Software, Inc., San Diego, CA, USA).
The statistical analysis was performed using one-way analysis of
variance among groups. All continuous data are expressed as the
mean ± standard deviation, and P<0.05 was considered to indicate
a statistically significant difference.
Results
Characterization of bone marrow-derived
EPCs
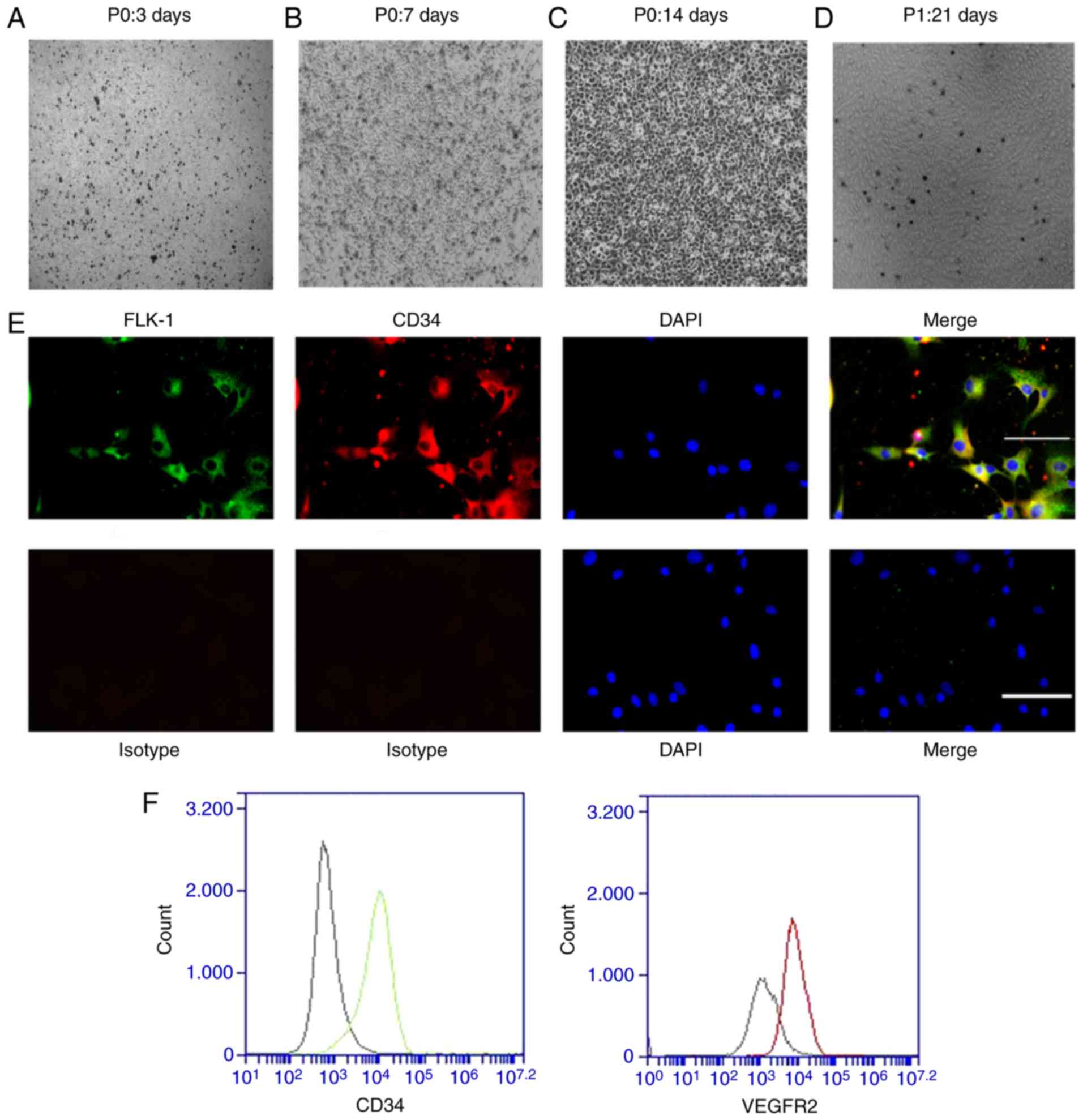
The EPCs derived from rat bone marrow were isolated
by density gradient centrifugation and cultured in
fibronectin-coated six-well plates. Following 3 days of culture in
endothelial cell growth medium EGM-2, most cells were adherent to
the well surfaces (Fig. 1A);
non-adherent cells were removed by replacing the medium. On day 7
of culture, the EPCs were in spindle-like cell colonies, which
indicated the formation of early EPCs (Fig. 1B). Following another 7 days (on
the 14th day of culture), the cell colonies gradually became
larger, and the cells exhibited a cobblestone-like morphology
indicating their progression into late EPCs (Fig. 1C). The first generation of late
EPCs (Fig. 1D) were used for
phenotypic identification and subsequent experiments. To
demonstrate that these primary cells were EPCs, immunofluorescence
assays and flow cytometry were performed using the first generation
of EPCs. FLK-1-positive cells were stained green and CD34-positive
cells were stained red. Most EPCs were positive for both FLK-1 and
CD34, as shown by their yellow coloration in Fig. 1E (Merge). The flow cytometry
confirmed that most of the cells were positive for CD34 and FLK-1
(Fig. 1F). This result is
consistent with that of previous studies (8,32).
Metformin protects EPCs from PA-induced
dysfunction
To examine the function of EPCs, the cells were
cultured in EGM-2 with or without PA. Transwell and Matrigel assays
were used to measure the migration and angiogenic potential of the
EPCs exposed to PA. As shown in Fig.
2A, the migration potential of the EPCs exposed to PA was
inhibited, as the number of cells migrating to the lower chambers
was significantly lower in the PA-treated EPCs than in the control
cells (Fig. 2A and B). The
PA-treated EPCs also formed fewer numbers of tubes than the
non-treated control EPCs (Fig. 2C and
D), indicating that PA reduced the angiogenic potential of
EPCs. Cell proliferation was also analyzed using CCK-8. The EPCs
exposed to PA exhibited significantly lower proliferation than the
control EPCs that were not exposed to PA (Fig. 2E). However, treatment of metformin
eliminated the effects of PA on the EPCs (migrating ability,
Fig. 2A and B; tube formation,
Fig. 2C and D; proliferation,
Fig. 2E). These data showed that
metformin not only improved EPC proliferation (Fig. 2E), but also migration and
angiogenesis (Fig. 2A-D), which
indicated that metformin may prevent EPCs from PA-induced
impairment.
 | Figure 2Metformin alleviates PA-induced
injury to the proliferation and functions of bone marrow-derived
EPCs. Bone marrow-derived EPCs were exposed to PA (0.5 mM) with or
without metformin (50 μM) for 24 h. (A) EPC migration was
determined by a Transwell assay (magnification, ×200) and (B)
quantified. (C) EPC tube formation was examined using a Matrigel
tube experiment (magnification, ×100) and (D) quantified. (E) Cell
viability was investigated using a Cell Counting Kit-8 assay. Three
independent experiments were performed for each experiment.
**P<0.01, vs. control; ##P<0.01, vs.
PA. Data shown in the graphs represent the mean ± standard
deviation. EPCs, endothelial progenitor cells; PA, palmitic acid;
C, cells treated without PA or metformin; M, cells treated with
metformin; P, cells exposed to PA. M+P, cells treated with
metformin and PA. |
Metformin attenuates the PA-induced
reduction in expression levels of miR-130a and PTEN in EPCs
Based on an extensive literature review, four miRNAs
(miR-132, miR-34a, miR-221 and miR-130a) were identified as being
associated with angiogenesis (24,33,34), the levels of which may be affected
by EPC exposure to PA and/or metformin. In brief, the EPCs were
maintained in PA (0.5 mM) for 24 h, following which the expression
levels of the four miRNAs were measured using RT-qPCR analysis. As
shown in Fig. 3A, the levels of
miR-130a were significantly lower in the PA-treated EPCs than in
the EPCs of the control group. It was also found that the
PA-treated EPCs exhibited significantly higher mRNA and protein
levels of PTEN than the control EPCs (Fig. 3B-D). However, the levels of
p-AKT/AKT were significantly decreased following treatment with PA
compared with the control (Fig.
3E). To investigate how metformin affected the expression
levels of miR-130a, PTEN and p-AKT in EPCs exposed to PA, the EPCs
were cultured in EGM-2-containing PA (0.5 mM) and metformin (50
μM) for 24 h. The EPCs exposed to both metformin and PA had
significantly higher levels of miR-130a and p-AKT than the EPCs
exposed to PA alone (Fig. 3A and
E). By contrast, the mRNA and protein expression levels of PTEN
were lower in the EPCs treated with metformin and PA compared with
those in the EPCs exposed to PA only (Fig. 3B-D). These data implied that
metformin may attenuate the PA-induced reduction of miR-130a/p-AKT
and increase of PTEN in EPCs.
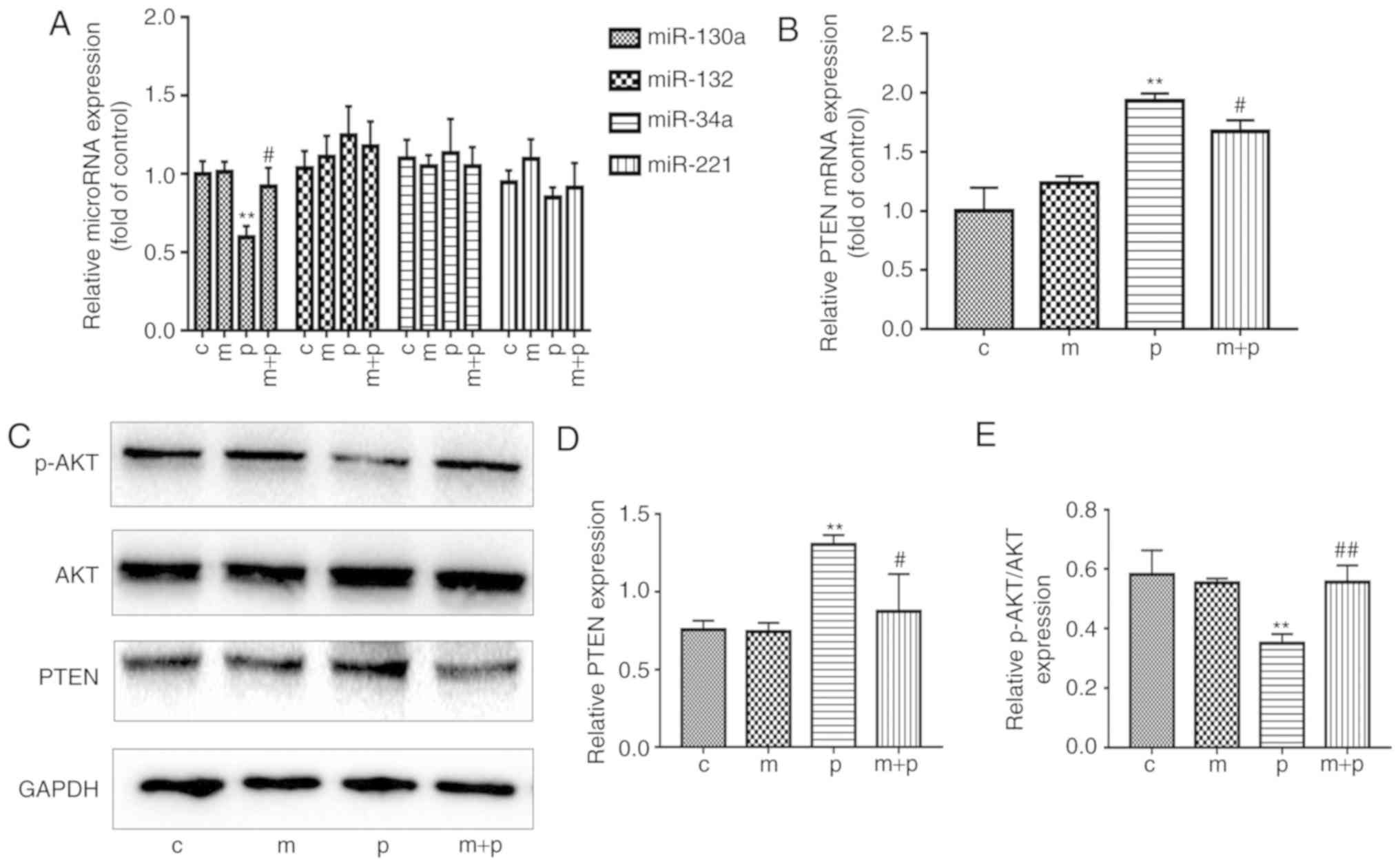 | Figure 3Metformin reverses the PA-induced
reduction in expression of miR-130a and PTEN in EPCs. EPCs were
cultured with metformin or 0.5 mM PA or both for 24 h. (A) Relative
expression levels of miR-130a, miR-132, miR-34a and miR-221 in EPCs
were determined by RT-qPCR analysis. (B) Relative mRNA expression
of PTEN was detected by RT-qPCR analysis. (C) Representative gel
images and histogram values of (D) PTEN and (E) AKT and p-AKT.
Three independent repeats were performed for each experiment.
**P<0.01, vs. control; #P<0.05 and
##P<0.01, vs. PA. The data shown in graphs represent
the mean ± standard deviation. miR, microRNA; EPCs, endothelial
progenitor cells; PA, palmitic acid; C, cells treated without PA or
metformin; M, cells treated with metformin; P, cells exposed to PA.
M+P, cells treated with metformin and PA; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; PTEN,
phosphatase and tensin homolog; p-AKT, phosphorylated AKT. |
miR-130a regulates the expression of PTEN
in EPCs
Analyses using TargetScan softwareindicated that
PTEN is a potential target of miR-130a regulation. The association
between miR-130a and PTEN was confirmed in a previous study, which
showed that miR-130a specifically inhibited the expression of PTEN
via a 3′UTR region in the PTEN gene (22). To confirm whether miR-130a
regulates the expression levels of PTEN in EPCs, the present study
investigated the mRNA and protein expression levels of PTEN in EPCs
transfected with miR-130a mimics or miR-130a inhibitor. EPCs
overexpressing miR-130a had significantly lower mRNA and protein
levels of PTEN than those transfected with the corresponding
negative control (NC) (Fig.
4A-C). By contrast, EPCs transfected with miR-130a inhibitors
shown an opposite trend (Fig.
4E-G). These data indicated that miR-130a negatively regulated
the expression of PTEN in EPCs.
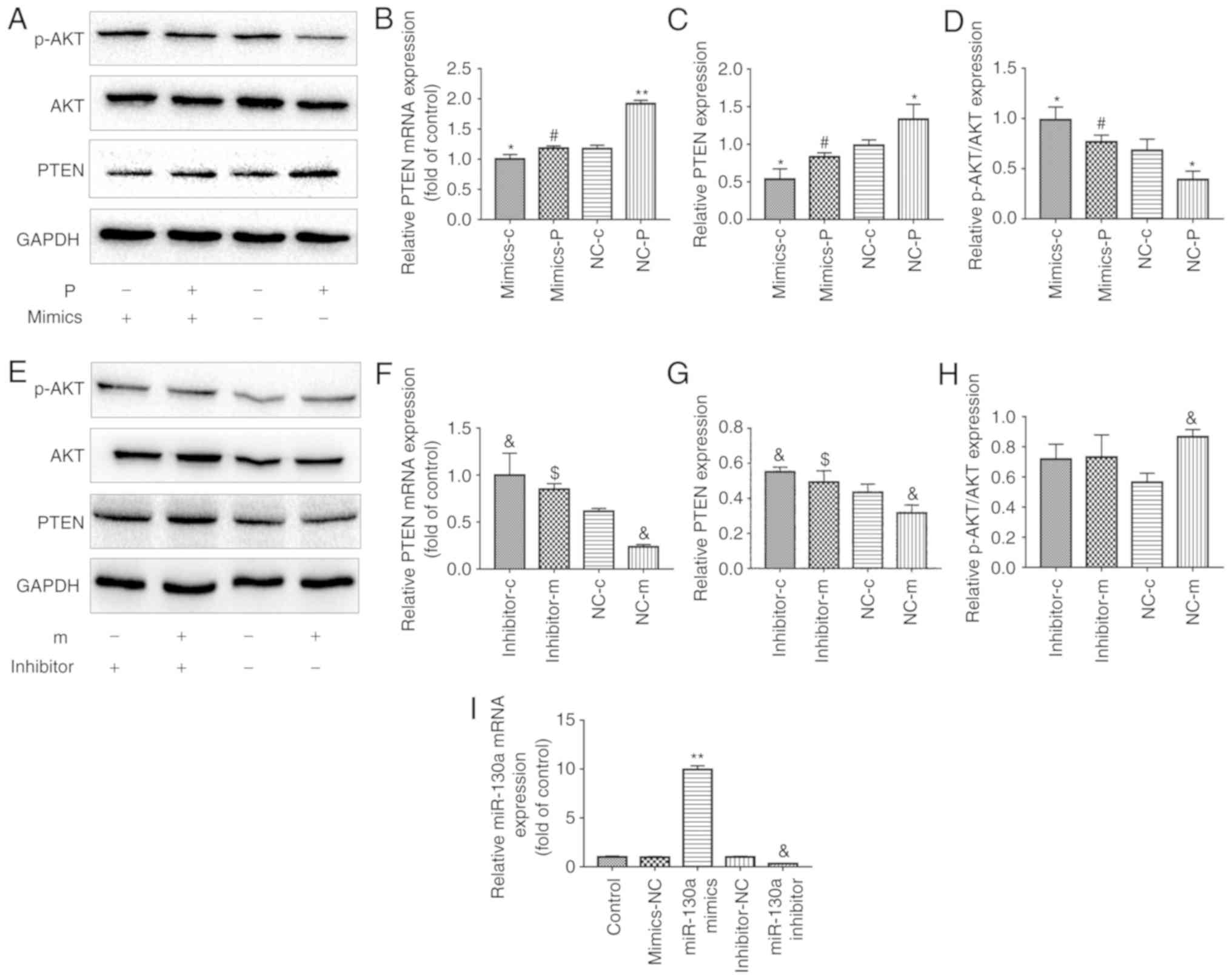 | Figure 4Metformin protection of EPC
angiogenesis and proliferation from PA is associated with the
regulation of miR130a, PTEN and the PI3K/AKT pathway. EPCs were
transfected with either a miR-130a mimics or a miR-130a inhibitor
and the corresponding NC. After 6 h, cells were exposed to media
containing either PA or metformin for another 24 h. In the
mimic-treated group, (A) PTEN, AKT and P-AKT proteins were examined
by western blotting, and (B) mRNA expression of PTEN was detected
by RT-qPCR analysis. Protein levels of (C) PTEN and (D) P-AKT/AKT
were quantified. In the inhibitor-treated group, (E) PTEN, AKT and
P-AKT proteins were examined by western blotting, and (F) mRNA
expression of PTEN was detected by RT-qPCR analysis. Protein levels
of (G) PTEN and (H) P-AKT/AKT were quantified.
*P<0.05 and **P<0.01, vs. NC-c;
#P<0.05, vs. NC-p; &P<0.05, vs.
NC-c; $P<0.05, vs. NC-m). (I) Relative expression of
miR-130a was determined by RT-qPCR analysis.
**P<0.01, vs. mimics-NC; &P<0.05,
vs. inhibitor-NC. Three independent experiments were performed for
each experiment. The data shown in the graphs represent the mean ±
standard deviation. miR, microRNA; EPCs, endothelial progenitor
cells; PA, palmitic acid; C, cells treated without PA or metformin;
M, cells treated with metformin; P, cells exposed to PA;
mimics/inhibitor, EPCs transfected with miR-130a mimics/inhibitor;
NC, EPCs transfected with the corresponding scrambled control;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; PTEN, phosphatase and tensin homolog; p-AKT,
phosphorylated AKT. |
miR-130a/PTEN and PI3K/AKT pathways are
involved in the protective effects of metformin on EPCs
As mentioned above, metformin improved the functions
of EPCs when exposed to PA. However, whether the miR-130a/PTEN and
AKT pathways were involved remained unclear. The EPCs exposed to PA
or metformin were treated with miR-130a mimics or inhibitors. The
increased expression of miR-130a was correlated with the activated
PI3K/AKT pathway, as the p-AKT ratio was higher (Fig. 4D and I). Regarding the expression
of PTEN, opposite results were observed at both the mRNA and
protein levels (Fig. 4A-C). As
shown in Fig. 5A-E, the impaired
tube formation, migration and cell viability of the EPCs induced by
PA was attenuated in the EPCs transfected with miR-130a mimics. To
further investigate whether the endothelial-protective action of
metformin was miR-130a-dependent, the effects of metformin in EPCs
were examined following transfection with an miR-130a inhibitor. As
expected, the inhibition of miR-130a in EPCs resulted in a reverse
effect of metformin in the expression of PTEN (Fig. 4E-G) and miR-130a (Fig. 4I). However, no significant effects
on PI3K/AKT were observed in EPCs transfected with miR-130a
inhibitor (Fig. 4E and H).
Similarly, the effects of metformin on angiogenesis and cell
viability significantly decreased in EPCs transfected with the
miR-130a inhibitor (Fig. 6A-E).
In summary, the ameliorative effect of metformin on PA-induced
changes in EPC proliferation, cell migration and tube formation may
be associated with an increase in the expression levels of miR-130a
and a decrease in the levels of PTEN, which may be associated with
activation of the PI3K/AKT signaling pathway.
 | Figure 5miR-130a is involved in the
PA-induced impaired proliferation and functions of bone
marrow-derived EPCs. EPCs were transfected with either scrambled
control or miR-130a mimics. After 6 h, cells were cultured with and
without PA. (A) Images (magnification, ×200) and (B) quantification
of EPC migration ability when incubated with or without PA. (C)
Images (magnification, ×100) and (D) quantification of EPC tube
formation when incubated with and without PA. (E) Cell viability
following treatment with and without PA. Three independent
experiments were performed for each experiment.
*P<0.05 and **P<0.01, vs. NC-con;
#P<0.05, vs. NC-PA. The data shown in the graphs
represent the mean ± standard deviation. miR, microRNA; EPCs,
endothelial progenitor cells; PA, palmitic acid; mimics, EPCs
transfected with miR-130a mimics; NC, EPCs transfected with
scrambled control miR-130a; con, control. |
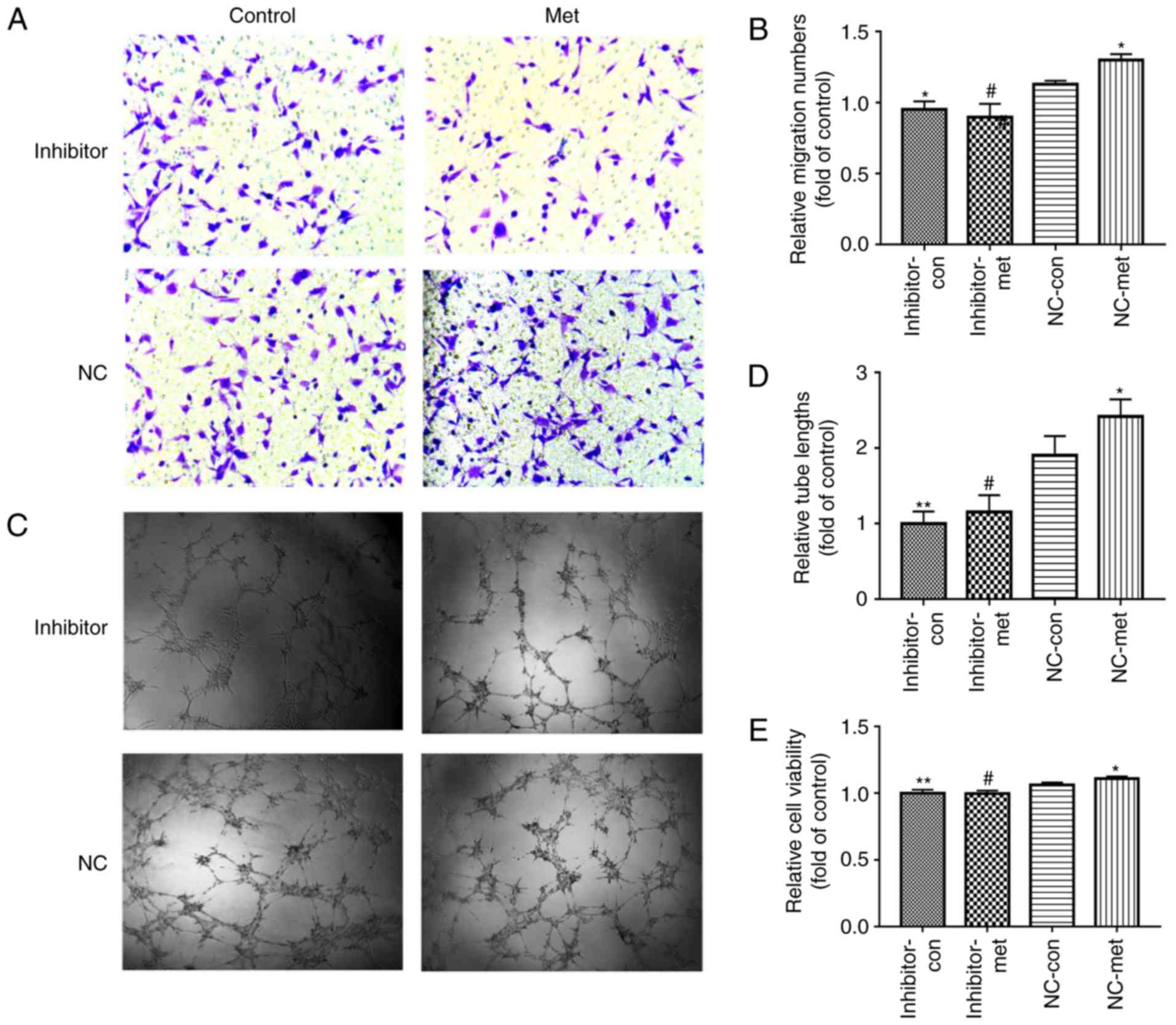 | Figure 6Downregulation of miR-130a reverses
the metformin-induced EPC proliferation and angiogenesis
improvement when exposed to PA. EPCs were treated with miR-130a
inhibitor or NC. After 6 h, the medium was replaced with medium or
without metformin. (A) EPC migration was determined using a
Transwell assay (magnification, ×200). (B) Relative numbers of
cells migrated. (C) Tube formation was examined by a Matrigel tube
experiment (magnification, ×100). (D) Relative tube lengths. (E)
Cell viability was investigated using a Cell Counting Kit-8 assay.
Three independent experiments were performed for each experiment.
*P<0.05 and **P<0.01, vs. NC-con;
#P<0.05, vs. NC-met. The data shown in the graphs
represent the mean ± standard deviation. miR, microRNA; EPCs,
endothelial progenitor cells; PA, inhibitor, EPCs transfected with
miR-130a inhibitor; NC, EPCs transfected with miR-130a negative
control; con, control; met, metformin. |
Discussion
EPCs are known to be involved in vascularization and
angiogenesis during embryonic development and in adults (35). It has been reported that diabetes
can reduce the viability and angiogenic potential of EPCs, which is
a likely reason why patients with diabetes are at a high risk of
developing cardiovascular problems, chronic renal failure and
peripheral vascular complications (36-38). Several studies have shown that
metformin can increase the angiogenic potential of EPCs in diabetes
(23,35). However, whether metformin can
protect the EPCs from PA-induced dysfunctions, and whether the
potential mechanism involves miRNAs remain to be elucidated. In the
present study, it was found that metformin improved the
proliferation, migration and tube formation capacities of
PA-exposed EPCs; high levels of PA were used to mimic metabolic
disorders in vitro. The results demonstrated that metformin
exerted its ameliorative effects via the upregulation of miR-130a
and downregulation of PTEN in PA-exposed EPCs.
Diabetes is recognized as a metabolic syndrome
characterized by the dysregulation of glucose, fat and protein
metabolism. Song et al (39), showed that the senescence and
impaired angiogenic functions of EPCs exposed to high glucose and
FFAs, as observed in diabetes, were partly mediated by the sirtuin
1/P53/P21 signaling pathway. Another previous study indicated that
PA partially suppressed the expression and activities of cathepsin
L and S, leading to reduced endothelial cell proliferation and
invasion, with a concomitant increase in apoptosis (2). Furthermore, the PI3K/AKT/forkhead
box O1 pathway was found to be involved in PA-induced reduction in
endothelial cell angiogenesis (40). The present study showed that PA
significantly decreased the proliferation, migration and tube
formation of EPCs, and these effects of PA on EPCs were attenuated
by treatment with metformin. The results of present study are
consistent with a report by Han et al (41), which showed that metformin not
only improved wound closure in db/db mice but also increased the
numbers and angiogenic potential of EPCs. However, Li et al
(42) also reported that
metformin inhibits endothelial progenitor cell migration by
decreasing matrix metalloproteinase-2 and -9, via the
AMPK/mammalian target of rapamycin/autophagy pathway, which differs
from the findings of the present study. The different effects of
metformin on EPC migration among these studies may be due to the
different concentration of metformin used; the concentration of
metformin used by Li et al (42) was 10 mM, which was substantially
higher than that in the present study (50 μM). High
concentrations of metformin have been found to inhibit tumor growth
and proliferation, and is considered to be a promising drug for
treating tumors (43,44), although a low concentration of
metformin exerted effects on accelerating wound healing in diabetic
mice and improved the functions of EPCs (23). Metformin is a widely used
first-line hypoglycemic agent that affects angiogenesis in numerous
medical conditions, including esophageal squamous cell cancers,
retinal vascular endothelial cell cancers and hepatocellular
carcinomas (45-47). Until now, the mechanism by which
metformin protects EPCs from PA has remained unclear.
Several studies have found that endothelial
progenitor-specific miRNAs can regulate the proliferative,
migration and tube formation capacities of EPCs (48,49). miR-130a, is present in numerous
cells and is known to have important effects on multiple functions.
Duan et al (50), showed
that miR-130a can function as an oncogene to promote the
proliferation and migration of gastric cells. Liang et al
(51), showed that miR-130a can
also protect glomerular cells from lipopolysaccharide-induced
injury by activating the PI3K/AKT pathway and ameliorating
inflammation. In EPCs, the expression of miR-130a was found to
decrease in diabetes, which was associated with high levels of
glucose and lipids (19). In the
present study, the expression of miR-130a in PA-exposed EPCs was
significantly lower than that in control EPCs, which was similar to
the results reported by Ye et al (19), in which the expression levels
miR-130a were lower than normal in the EPCs of diabetic patients.
The present study also found that treating PA-exposed EPCs with
metformin reversed this effect. The overexpression of miR-130a
using miR-130a mimics also improved the proliferation, migration
and tube formation capacities of EPCs exposed to PA. However,
metformin did not effectively reverse the PA-induced dysfunction in
EPCs transfected with miR-130a inhibitor. Taken together, the
results indicated that miR-130a may be involved in the effects
exerted by metformin on PA-exposed EPCs.
The present study demonstrated that the
downregulation of miR-130a in PA-exposed EPCs led to an increased
expression of PTEN, which is considered to be a major regulator of
angiogenesis in endothelial cells (30). A study by Song et al
(22), showed that PTEN is a
target of miR-130a. PTEN is also involved in the angiogenic effects
of human umbilical cord exosomes and the extracellular vesicles of
lung cancer on endothelial cells (52,53). Furthermore, it was also reported
that the expression of PTEN was significantly higher in skeletal
muscle cells with glucose-induced insulin resistance than that in
normal skeletal muscle cells, and metformin significantly reduced
insulin resistance by decreasing the expression levels of PTEN in
these cells (54). In human
keratinocyte cell lines, metformin was found to inhibit cell growth
in a dose-dependent manner by inhibiting the miR-21/PTEN/AKT
pathway (55). The present study
showed that the increased expression levels of PTEN in PA-treated
EPCs were reversed by treatment with metformin or by the
overexpression of miR-130a induced by miR-130a mimics. By contrast,
down-regulating the expression of miR-130a with an miR-130a
inhibitor reversed the decreased expression of PTEN induced by
metformin. These results indicated that the mechanism by which
metformin affected PA-exposed EPCs may be mediated by miR-130a and
PTEN. However, Gao et al (56) demonstrated that miR-130a acted as
an oncogene targeting tissue factor pathway inhibitor 2 and reduced
cell growth and angiogenesis through FAK/PI3K/Rac1/mouse double
minute 2 signaling in hemangiomas, which appears contrary to the
results of the present study. Others reported that miR-130a
promoted gastric cancer migration, invasion and proliferation by
targeting the tumor suppressor gene Runt-related transcription
factor 3 (57). Therefore
miR-130a regulates the function of cells through several different
pathways, depending on the type of tumor or cell. Therefore,
further investigation of miR-130a is warranted. It is well accepted
that PTEN suppresses the PI3K/AKT pathway by negatively regulating
the activity of AKT, which is involved in regulating the
proliferation, migration and angiogenesis of EPCs (58-61). The present study found that the
overexpression of miR-130a activated the PI3K/AKT pathway, whereas
EPCs with inhibited expression of miR-130a exhibited no significant
changes in this pathway. miR-130a and PTEN may have principal roles
in the protective effects of metformin in ameliorating PA-induced
EPC dysfunction. The present study may serve as a foundation for
preclinical studies using combinations of metformin with miR-130a
inhibitor or mimics as therapeutic strategies to promote EPC
angiogenesis in diabetes-associated vascular complications.
Funding
This study was supported by the Natural Science
Foundation Project of Zhejiang Province (grant no.
LY16H070006).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XMG and XQW performed the research and drafted the
manuscript. MJL, HLL, SYF, LYW and XQY were involved in performing
experiments and performed the statistical analysis. WYL was
contributed to the discussion of the experimental process and
ideas. FXS conceived the study, and was involved in its design and
coordination. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Independent
Ethics Committee of Wenzhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
NCD Risk Factor Collaboration (NCD-RisC):
Worldwide trends in diabetes since 1980: A pooled analysis of 751
population-based studies with 4.4 million participants. Lancet.
387:1513–1530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Shan Y, Li Y, Luo X and Shi H:
Palmitate impairs angiogenesis via suppression of cathepsin
activity. Mol Med Rep. 15:3644–3650. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farmer JA: Diabetic dyslipidemia and
atherosclerosis: Evidence from clinical trials. Curr Diab Rep.
8:71–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Listenberger LL, Han X, Lewis SE, Cases S,
Farese RV Jr, Ory DS and Schaffer JE: Triglyceride accumulation
protects against fatty acid-induced lipotoxicity. Proc Natl Acad
Sci USA. 100:3077–3082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo WX, Yang QD, Liu YH, Xie XY, Wang M
and Niu RC: Palmitic and linoleic acids impair endothelial
progenitor cells by inhibition of Akt/eNOS pathway. Arch Med Res.
39:434–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Devanesan AJ, Laughlan KA, Girn HRS and
Homer-Vanniasinkam S: Endothelial progenitor cells as a therapeutic
option in peripheral arterial disease. Eur J Vasc Endovasc Surg.
38:475–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculo-genesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Egan CG, Lavery R, Caporali F, Fondelli C,
Laghi-Pasini F, Dotta F and Sorrentino V: Generalised reduction of
putative endothelial progenitors and CXCR4-positive peripheral
blood cells in type 2 diabetes. Diabetologia. 51:1296–1305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loomans CJ, de Koning EJ, Staal FJ,
Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B,
Rabelink TJ and van Zonneveld AJ: Endothelial progenitor cell
dysfunction: A novel concept in the pathogenesis of vascular
complications of type 1 diabetes. Diabetes. 53:195–199. 2004.
View Article : Google Scholar
|
|
11
|
Tepper OM, Galiano RD, Capla JM, Kalka C,
Gagne PJ, Jacobowitz GR, Levine JP and Gurtner GC: Human
endothelial progenitor cells from type II diabetics exhibit
impaired proliferation, adhesion, and incorporation into vascular
structures. Circulation. 106:2781–2786. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barthelmes D, Irhimeh MR, Gillies MC,
Karimipour M, Zhou M, Zhu L and Shen WY: Diabetes impairs
mobilization of mouse bone marrow-derived Lin(-)/VEGF-R2(+)
progenitor cells. Blood Cells Mol Dis. 51:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trombetta A, Togliatto G, Rosso A,
Dentelli P, Olgasi C, Cotogni P and Brizzi MF: Increase of palmitic
acid concentration impairs endothelial progenitor cell and bone
marrow-derived progenitor cell bioavailability: Role of the
STAT5/PPARgamma transcriptional complex. Diabetes. 62:1245–1257.
2013. View Article : Google Scholar :
|
|
14
|
Badr G, Hozzein WN, Badr BM, Al Ghamdi A,
Saad Eldien HM and Garraud O: Bee venom accelerates wound healing
in diabetic mice by suppressing activating transcription factor-3
(ATF-3) and inducible nitric oxide synthase (iNOS)-mediated
oxidative stress and recruiting bone marrow-derived endothelial
progenitor cells. J Cell Physiol. 231:2159–2171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abplanalp WT, Conklin DJ, Cantor JM,
Ginsberg MH, Wysoczynski M, Bhatnagar A and O'Toole TE: Enhanced
integrin α4β1-mediated adhesion contributes to a mobilization
defect of endothelial progenitor cells in diabetes. Diabetes.
65:3505–3515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qu K, Wang Z, Lin XL, Zhang K, He XL and
Zhang H: MicroRNAs: Key regulators of endothelial progenitor cell
functions. Clin Chim Acta. 448:65–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye M, Li D, Yang J, Xie J, Yu F, Ma Y, Zhu
X, Zhao J and Lv Z: MicroRNA-130a targets MAP3K12 to modulate
diabetic endothelial progenitor cell function. Cell Physiol
Biochem. 36:712–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song MS, Carracedo A, Salmena L, Song SJ,
Egia A, Malumbres M and Pandolfi PP: Nuclear PTEN regulates the
APC-CDH1 tumor-suppressive complex in a phosphatase-independent
manner. Cell. 144:187–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiojima I and Walsh K: Role of Akt
signaling in vascular homeostasis and angiogenesis. Circ Res.
90:1243–1250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song CL, Liu B, Shi YF, Liu N, Yan YY,
Zhang JC, Xue X, Wang JP, Zhao Z, Liu JG, et al: MicroRNA-130a
alleviates human coronary artery endothelial cell injury and
inflammatory responses by targeting PTEN via activating
PI3K/Akt/eNOS signaling pathway. Oncotarget. 7:71922–71936. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu JW, Deng YP, Han X, Ren GF, Cai J and
Jiang GJ: Metformin improves the angiogenic functions of
endothelial progenitor cells via activating AMPK/eNOS pathway in
diabetic mice. Cardiovasc Diabetol. 15:882016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arunachalam G, Lakshmanan AP, Samuel SM,
Triggle CR and Ding H: Molecular interplay between microRNA-34a and
Sirtuin1 in hyperglycemia-mediated impaired angiogenesis in
endothelial cells: Effects of metformin. J Pharmacol Exp Ther.
356:314–323. 2016. View Article : Google Scholar
|
|
25
|
Zhang Y, Chen F and Wang L: Metformin
inhibits development of diabetic retinopathy through
microRNA-497a-5p. Am J Transl Res. 9:5558–5566. 2017.
|
|
26
|
Dai X, Tan Y, Cai S, Xiong X, Wang L, Ye
Q, Yan X, Ma K and Cai L: The role of CXCR7 on the adhesion,
proliferation and angiogenesis of endothelial progenitor cells. J
Cell Mol Med. 15:1299–1309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brandl A, Yuan Q, Boos AM, Beier JP,
Arkudas A, Kneser U, Horch RE and Bleiziffer O: A novel early
precursor cell population from rat bone marrow promotes
angiogenesis in vitro. BMC Cell Biol. 15:122014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dai X, Yan X, Zeng J, Chen J, Wang Y, Chen
J, Li Y, Barati MT, Wintergerst KA, Pan K, et al: Elevating CXCR7
improves angiogenic function of EPCs via Akt/GSK-3beta/Fyn-mediated
Nrf2 activation in diabetic limb ischemia. Circ Res. 120:e7–e23.
2017. View Article : Google Scholar
|
|
29
|
Yan X, Cai S, Xiong X, Sun W, Dai X, Chen
S, Ye Q, Song Z, Jiang Q and Xu Z: Chemokine receptor CXCR7
mediates human endothelial progenitor cells survival, angiogenesis,
but not proliferation. J Cell Biochem. 113:1437–1446. 2012.
View Article : Google Scholar
|
|
30
|
Unseld M, Chilla A, Pausz C, Mawas R,
Breuss J, Zielinski C, Schabbauer G and Prager GW: PTEN expression
in endothelial cells is down-regulated by uPAR to promote
angiogenesis. Thromb Haemost. 114:379–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Yu S, Li Z, Zhang W, Du Z, Liu K, Yang D
and Gong S: Isolation and characterization of endothelial
colony-forming cells from mononuclear cells of rat bone marrow. Exp
Cell Res. 370:116–126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Mao H, Chen JY, Wen S, Li D, Ye M
and Lv Z: Increased expression of microRNA-221 inhibits PAK1 in
endothelial progenitor cells and impairs its function via
c-Raf/MEK/ERK pathway. Biochem Biophys Res Commun. 431:404–408.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Che F, Du H, Zhang W, Cheng Z and Tong Y:
MicroRNA-132 modifies angiogenesis in patients with ischemic
cerebrovascular disease by suppressing the NFkappaB and VEGF
pathway. Mol Med Rep. 17:2724–2730. 2018.
|
|
35
|
Ambasta RK, Kohli H and Kumar P: Multiple
therapeutic effect of endothelial progenitor cell regulated by
drugs in diabetes and diabetes related disorder. J Transl Med.
15:1852017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fadini GP, Miorin M, Facco M, Bonamico S,
Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A,
Agostini C and Avogaro A: Circulating endothelial progenitor cells
are reduced in peripheral vascular complications of type 2 diabetes
mellitus. J Am Coll Cardiol. 45:1449–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh
W, Sung J, Jeon ES, Oh HY and Kim DK: Decreased number and impaired
angiogenic function of endothelial progenitor cells in patients
with chronic renal failure. Arterioscler Thromb Vasc Biol.
24:1246–1252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song X, Yang B, Qiu F, Jia M and Fu G:
High glucose and free fatty acids induce endothelial progenitor
cell senescence via PGC-1alpha/SIRT1 signaling pathway. Cell Biol
Int. 41:1146–1159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ke J, Wei R, Yu F, Zhang J and Hong T:
Liraglutide restores angiogenesis in palmitate-impaired human
endothelial cells through PI3K/Akt-Foxo1-GTPCH1 pathway. Peptides.
86:95–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Han X, Tao Y, Deng Y, Yu J, Sun Y and
Jiang G: Metformin accelerates wound healing in type 2 diabetic
db/db mice. Mol Med Rep. 16:8691–8698. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li WD, Li NP, Song DD, Rong JJ, Qian AM
and Li XQ: Metformin inhibits endothelial progenitor cell migration
by decreasing matrix metalloproteinases, MMP-2 and MMP-9, via the
AMPK/mTOR/autophagy pathway. Int J Mol Med. 39:1262–1268. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ashinuma H, Takiguchi Y, Kitazono S,
Kitazono-Saitoh M, Kitamura A, Chiba T, Tada Y, Kurosu K, Sakaida
E, Sekine I, et al: Antiproliferative action of metformin in human
lung cancer cell lines. Oncol Rep. 28:8–14. 2012.PubMed/NCBI
|
|
44
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The anti-diabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han J, Li Y, Liu X, Zhou T, Sun H, Edwards
P, Gao H, Yu FS and Qiao X: Metformin suppresses retinal
angiogenesis and inflammation in vitro and in vivo. PLoS One.
13:e01930312018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao
ZQ, Yu LG and Guo XL: Metformin incombination with curcumin
inhibits the growth, metastasis, and angiogenesis of hepatocellular
carcinoma in vitro and in vivo. Mol Carcinog. 57:44–56. 2018.
View Article : Google Scholar
|
|
47
|
Yang Y, Jin G, Liu H, Liu K, Zhao J, Chen
X, Wang D, Bai R, Li X, Jang Y, et al: Metformin inhibits
esophageal squamous cell carcinoma-induced angiogenesis by
suppressing JAK/STAT3 signaling pathway. Oncotarget. 8:74673–74687.
2017.PubMed/NCBI
|
|
48
|
Wang N, Chen C, Yang D, Liao Q, Luo H,
Wang X, Zhou F, Yang X, Yang J, Zeng C and Wang WE: Mesenchymal
stem cells-derived extracellular vesicles, via miR-210, improve
infarcted cardiac function by promotion of angiogenesis. Biochim
Biophys Acta Mol Basis Dis. 1863:2085–2092. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liang L, Zhao L, Zan Y, Zhu Q, Ren J and
Zhao X: MiR-93-5p enhances growth and angiogenesis capacity of
HUVECs by down-regulating EPLIN. Oncotarget. 8:107033–107043. 2017.
View Article : Google Scholar :
|
|
50
|
Duan J, Zhang H, Qu Y, Deng T, Huang D,
Liu R, Zhang L, Bai M, Zhou L, Ying G and Ba Y: Onco-miR-130
promotes cell proliferation and migration by targeting TGFbetaR2 in
gastric cancer. Oncotarget. 7:44522–44533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liang H, Yang K, Xin M, Liu X, Zhao L, Liu
B and Wang J: MiR-130a protects against lipopolysaccharide-induced
glomerular cell injury by upregulation of klotho. Pharmazie.
72:468–474. 2017.
|
|
52
|
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo
J, Li HM, Zhang WS, Chen CY and Xie H: Exosomes from human
umbilical cord blood accelerate cutaneous wound healing through
miR-21-3p-mediated promotion of angiogenesis and fibroblast
function. Theranostics. 8:169–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zheng Y, Liu L, Chen C, Ming P, Huang Q,
Li C, Cao D, Xu X and Ge W: The extracellular vesicles secreted by
lung cancer cells in radiation therapy promote endothelial cell
angiogenesis by transferring miR-23a. PeerJ. 5:e36272017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang DF, Yang HJ, Gu JQ, Cao YL, Meng X,
Wang XL, Lin YC and Gao M: Suppression of phosphatase and tensin
homolog protects insulin-resistant cells from apoptosis. Mol Med
Rep. 12:2695–2700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Deng Y and Ma W: Metformin inhibits hacat
cell viability via the miR-21/PTEN/Akt signaling pathway. Mol Med
Rep. 17:4062–4066. 2018.
|
|
56
|
Gao F, Wang FG, Liu RR, Xue F, Zhang J, Xu
GQ, Bi JH, Meng Z and Huo R: Epigenetic silencing of miR-130a
ameliorates hemangioma by targeting tissue factor pathway inhibitor
2 through FAK/PI3K/Rac1/mdm2 signaling. Int J Oncol. 50:1821–1831.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang H, Yu WW, Wang LL and Peng Y:
MiR-130a acts as a potential diagnostic biomarker and promotes
gastric cancer migration, invasion and proliferation by targeting
RUNX3. Oncol Rep. 34:1153–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Oudit GY, Sun H, Kerfant BG, Crackower MA,
Penninger JM and Backx PH: The role of phosphoinositide-3 kinase
and PTEN in cardiovascular physiology and disease. J Mol Cell
Cardiol. 37:449–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cen M, Hu P, Cai Z, Fang T, Zhang J and Lu
M: TIEG1 defi-ciency confers enhanced myocardial protection in the
infarcted heart by mediating the Pten/Akt signalling pathway. Int J
Mol Med. 39:569–578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nip H, Dar AA, Saini S, Colden M, Varahram
S, Chowdhary H, Yamamura S, Mitsui Y, Tanaka Y, Kato T, et al:
Oncogenic microRNA-4534 regulates PTEN pathway in prostate cancer.
Oncotarget. 7:68371–68384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu L, Li Z, Dong X, Xue X, Liu Y, Xu S,
Zhang J, Han J, Yang Y and Wang H: Polydatin protects diabetic
heart against ischemia-reperfusion injury via Notch1/Hes1-mediated
activation of Pten/Akt signaling. Oxid Med Cell Longev.
2018:27506952018. View Article : Google Scholar : PubMed/NCBI
|