Introduction
Particulate matter (PM) or particle pollutants are a
complex mixture of solid and liquid particles of various sizes and
composition and are a widespread airborne contaminant (1).
Prevalence of PM increases with urbanization and industrialization
(1). PM components that are harmful to human health include
polycyclic aromatic hydrocarbons (PAHs), organic compounds,
bacteria, metals and carbon particles (2-4). Numerous studies have
demonstrated that PM induces oxidative stress and inflammatory
reactions, and that PM exposure may cause various diseases
following penetration and accumulation in respiratory and
cardiovascular systems (5,6).
Skin serves as an important interface between the
body and the environment, with the epidermal barrier preventing
environmental stress and dehydration (3,7,8). Previously, several
studies have demonstrated that PM causes skin barrier dysfunction
and skin inflammatory diseases, including atopic dermatitis and
psoriasis (3,9-13). PM promotes the generation of reactive oxygen
species (ROS) and inflammation (2,13,14). Increased ROS levels
serve an important role in DNA damage, whilst elevated oxidative
stress may activate mitogen-activated protein kinases (MAPKs) and
the transcription factor activator protein-1 (AP-1), which lead to
the release of proinflammatory cytokines (7,15,16).
Afzelin (3-O-α-L-rhamnopyranoside; Fig. 1A) is a flavonoid isolated from
Thesium chinense Turcz, which is widely distributed
throughout Korea and China (17,18). Previous studies have suggested
that afzelin has anti-inflammatory, anticancer and antibacterial
properties (19-21), and possesses DNA-protective, antioxidant and
anti-inflammatory properties in UVB-irradiated human skin cells
(22,23).
However, effects of afzelin on PM-mediated responses
have not yet been elucidated. Therefore, the present study aimed to
investigate protective effects of afzelin in PM-exposed cells. It
was reported that afzelin reduced ROS generation or attenuated
proinflammatory responses.
Materials and methods
Materials
Standard reference material (SRM) 1649b, mainly
composed of PAHs and dioxin (9), was purchased from the National
Institute of Standards and Technology (Gaithersburg, MD, USA) and
dispersed in distilled water (240 μg/ml). Specific
antibodies for western blot analysis, including anti-phosphorylated
(p)-p38 MAPK (cat. no. 9211), anti-p38 MAPK (cat. no. 8690),
anti-p-c-Fos (cat. no. 5248), anti-c-Fos (cat. no. 4384),
anti-p-c-Jun (Ser63; cat. no. 2361), anti-p-c-Jun (Ser73; cat. no.
3270) and anti-c-Jun (cat. no. 9165) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Anti-GAPDH (cat. no.
sc-47724) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Secondary antibodies, goat anti-rabbit (cat. no.
31460) and goat anti-mouse IgG-horseradish peroxidase (cat. no.
31430) were purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Human interleukin (IL)-1α (cat. no. DY200-05), IL-1β
(cat. no. DY201-05) and IL-6 (cat. no. DY206-05) ELISA kits were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Afzelin (purity, ≥98%) was purchased from Wuhan ChemFaces
Biochemical Co., Ltd. (Wuhan, China).
Cell culture
HaCaT cells (American Tissue Cell Collection,
Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; Hyclone; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin at 37°C in a
humidified 5% CO2 atmosphere. At confluence, cells were
treated with 0.05% trypsin/0.53 mM EDTA for 5 min at 37°C. HaCaT
cells used in subsequent experiments were between passages 20 and
24.
Cell viability assay
Cell viability was assessed using a commercial
water-soluble tetrazolium salt (WST-1) assay kit (EZ-CYTOX;
DoGenBio, Seoul, South Korea) according to the manufacturer’s
protocol. Briefly, cells were seeded at 5×103 cells/well
in 96-well plates. Following 24 h, cells were treated with various
concentrations of PM (0, 5, 10, 25, 50 or 100
μg/cm2) and afzelin (0, 50, 100 or 200 μM)
for 24, 48 or 72 h. Following incubation, WST-1 reagent solution
(10 μl) was added to each well containing 100 μl of
serum-free DMEM. Plates were incubated at 37°C for 1 h and the
absorbance measured at 450 nm using a microplate spectrophotometer.
Cell viability was calculated using absorbance values and data were
normalized to the untreated control.
ROS assay
Intracellular ROS levels were measured by detecting
the fluorescence intensity of an oxidant-sensitive dye,
2′,7′-dichlorofluorescin diacetate (DCFH-DA). DCFH-DA diffuses into
cells and is deacetylated by cellular esterases to produce
non-fluorescent DCFH, which rapidly oxidizes to highly fluorescent
2′,7′-dichlorodihydrofluorescein (DCF) (24). Cells were seeded at
5×103 cells/well in black 96-well plates. Following 24
h, cells were incubated with afzelin (200 μM in ethanol) for
2 h prior to exposure to PM (25 μg/cm2) in
serum-free DMEM for 2 h. In another experiment, following the
initial 24 h culturing, cells were incubated with PM (25
μg/cm2) for 10 min prior to the addition of
afzelin (200 μM) and incubation for 2 h. Single treatment
controls were established by incubation with PM (25
μg/cm2) or afzelin (200 μM) for 2 h.
Medium was removed and cells were incubated with 20 μM
DCFH-DA for 30 min in the dark at 37°C. Fluorescence intensity was
detected at 485 nm excitation and 535 nm emission wavelengths.
Fluorescence intensity was recorded every 15 min for 2 h for the PM
only treatment group or at 2 h post PM treatment for all other
treatment groups. Relative intracellular ROS fluorescence intensity
was normalized by subsequent staining with PI (stock, 1 mg/ml;
dilution, 1:200) and measurement of PI (propidium iodide)
fluorescence. Intensity was calculated relative to the untreated
control.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from cells that were
pretreated with afzelin (200 μM) for 24 h followed by PM (25
μg/cm2) for 2 h using TRIzol reagent (Welgene,
Inc., Gyeongsan, South Korea) according to the manufacturer’s
protocol. cDNA was synthesized by RT using the RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) for 1 h
at 42°C. cDNA was amplified by qPCR with specific primers for
IL-1α, IL-1β, IL-6 and GAPDH and assays were performed using
PowerUp SYBR-Green Master mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Thermocycling conditions were as follows: 95°C
for 30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 30
sec and 72°C for 30 sec. Results were normalized to GAPDH and
quantified using the 2−ΔΔCq method (25). Primer
sequences were as follows: IL-1α forward, 5′-ATCAGTACC TCACGG CTG
CT-3′ and reverse, 5′-TGGGTATCTCAGGCATCTCC-3′; IL-1β forward,
5′-GGGCCTCAAGGAAAAGAATC-3′ and reverse, 5′-TTCTGCTTGAGAGGTGCTGA-3′;
IL-6 forward, 5′-GCCTTCGGTCCAGTTGCCTT-3′ and reverse,
5′-GCAGAATGAGATGAGTTGTC-3′; and GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′.
Western blot analysis
Cells (1.3×106/well) were cultured in
6-well plates. At confluence, cells were pretreated with afzelin
(200 μM) for 24 h and then incubated with PM (25
μg/cm2) for 1 h at 37°C. Cells were lysed using
1% Triton-X radioimmunoprecipitation assay buffer (50 mM NaCl, 1%
Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris-HCl
pH 8.0) containing a protease inhibitor cocktail (cOmplete™,
EDTA-free protease inhibitor; Roche Diagnostics, Indianapolis, IN,
USA). Cell debris was removed by centrifugation (10,000 × g, 10
min, 4°C) and the protein concentration was determined using the
bicinchoninic acid assay method. Equal amounts of protein (30
μg) were separated on 8% SDS-PAGE gels and proteins were
transferred to nitrocellulose membranes and blocked with 5% skimmed
milk for 1 h at 25°C. Subsequently, membranes were incubated with
primary antibodies (1:1,000) overnight at 4°C, followed by
horseradish peroxidase-conjugated secondary antibodies (1:2,000)
for 1 h at 25°C. Protein expression was detected using the
EzWestLumi plus system (ATTO Corporation, Tokyo, Japan). Protein
expression levels were quantified using Image J v1.8.0 (National
Institutes of Health, Bethesda, MD, USA) and normalized to
GAPDH.
ELISA
Proinflammatory cytokine levels were analyzed using
ELISA. Cells (1.3×106/well) were cultured in 6-well
plates and were pretreated with afzelin (200 μM) for 24 h
followed by incubation with PM (25 μg/cm2) for 24
h. Subsequently, conditioned media from the cells were collected
and stored at −80°C. Cytokines released by the cells were
quantified using ELISA kits for human IL-1α, IL-1β and IL-6
according to the manufacturer’s protocol.
Statistical analysis
In vitro assays were performed ≥3 times. Data
are presented as the mean ± standard deviation. Data were analyzed
using one-way analysis of variance with Bonferroni post hoc tests.
Statistical analysis was performed using SPSS version 18.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of PM and afzelin on HaCaT
viability
To determine the cytotoxicity of PM and afzelin in
HaCaT cells, cells were treated with various concentrations of PM
(0, 10, 25, 50 or 100 μg/cm2) and afzelin (50,
100 or 200 μM) for 24, 48 or 72 h. Cell viability was
determined using the WST-1 assay. At 48 and 72 h, significant
decreases in viability were observed for PM at 50 and 100
μg/cm2 compared with the untreated control
(Fig. 1B). Therefore, subsequent
experiments were performed with 25 μg/cm2 PM. As
presented in Fig. 1C, afzelin not
significantly affected cell viability at ≤200 μM over 24, 48
or 72 h. The results indicated that afzelin at the tested
concentrations exhibited no cytotoxicity towards HaCaT cells.
Afzelin exerts intracellular ROS
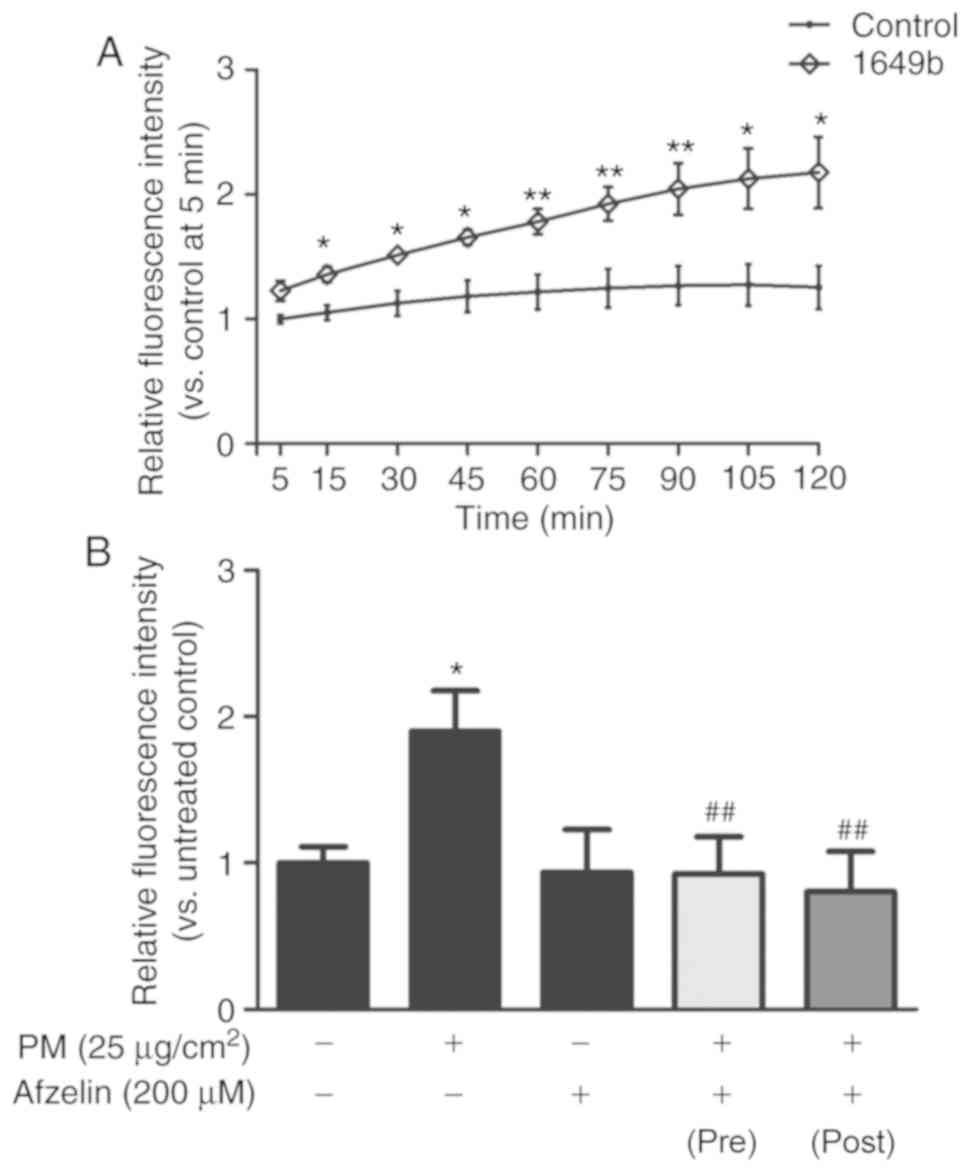
scavenging effects on PM-treated HaCaT cells
To confirm effects of PM on intracellular ROS
generation in HaCaT cells, cells were treated with PM (25
μg/cm2) for 2 h. Intracellular ROS levels were
detected by measuring the fluorescence intensity of the
oxidant-sensitive probe DCFH-DA over 120 min (Fig. 2A). PM treatment significantly
increased intracellular ROS levels in HaCaT cells in a
time-dependent manner compared with the untreated control. To
examine the intracellular ROS scavenging effect of afzelin, cells
were treated with afzelin (200 μM) for 2 h prior to or
following exposure to PM (25 μg/cm2). As
presented in Fig. 2B, afzelin
significantly reversed PM-induced ROS generation suggesting that
afzelin treatment may prevent and inhibit PM-induced intracellular
ROS generation in HaCaT cells.
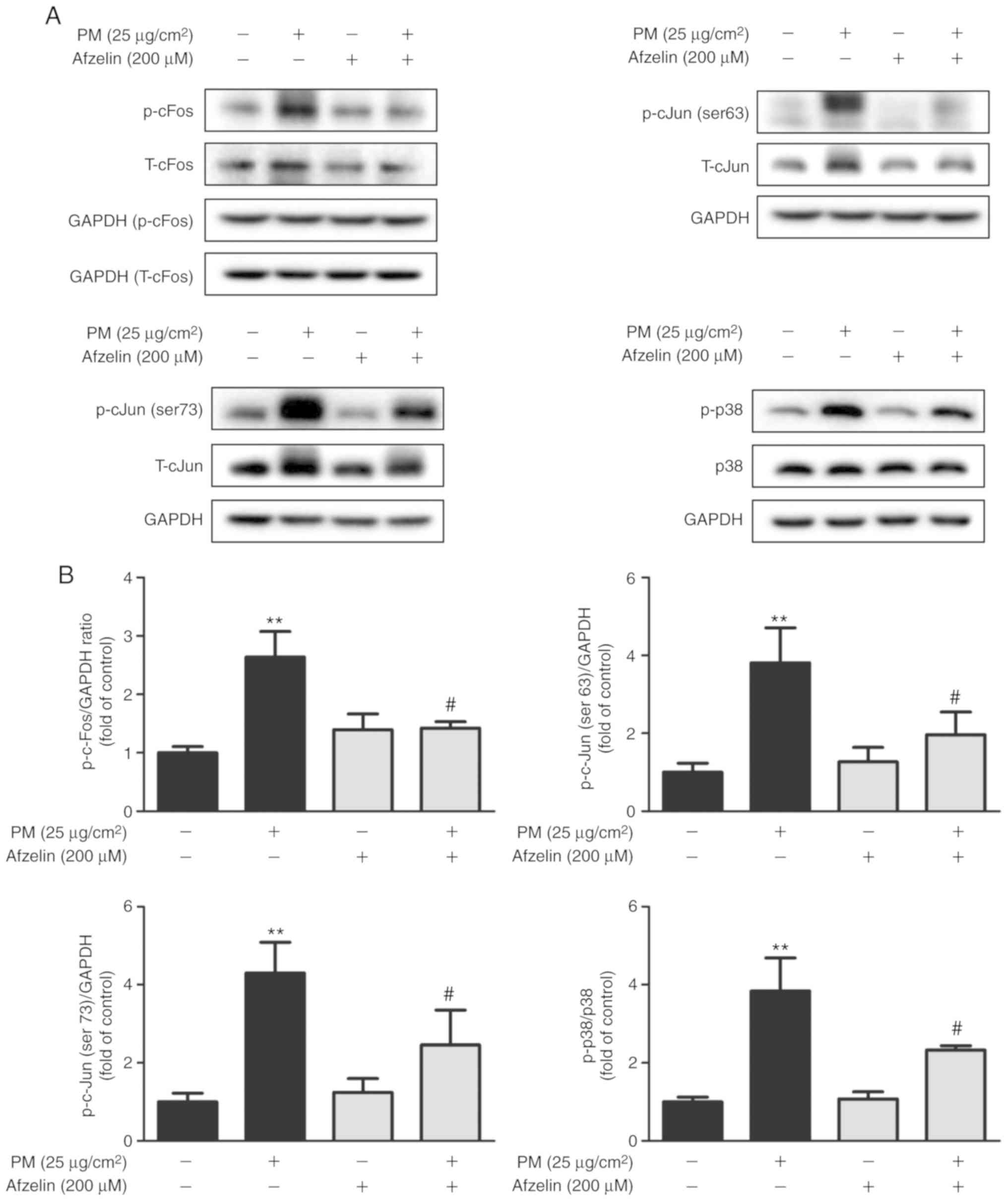
Afzelin affects AP-1 and p38 protein
expression in PM-treated HaCaT cells
Western blotting was used to assess effects of
afzelin on expression levels of the signaling molecules AP-1 and
p38 MAPK. Cells were treated with PM (25 μg/cm2)
for 1 h following pretreatment with afzelin (200 μM) for 24
h. Afzelin pretreatment significantly inhibited PM-induced
phosphorylation of p38 MAPK and AP-1 components c-Fos and c-Jun
(serine 63 and 73; Fig. 3).
Afzelin inhibits proinflammatory cytokine
expression in PM-treated HaCaT cells
To investigate if afzelin inhibited PM-induced
proinflammatory cytokine expression, HaCaT cells were pretreated
with afzelin (200 μM) for 24 h followed by PM (25
μg/cm2) for 2 or 24 h to determine mRNA or
protein levels, respectively. RT-qPCR and ELISA analysis were
performed to determine mRNA and protein levels, respectively, of
IL-1α, IL-1β and IL-6. PM treatment significantly increased mRNA
and protein levels of IL-1α, IL-1β and IL-6 in HaCaT cells compared
with the untreated cells (Fig.
4). As presented in Fig. 4A,
afzelin pretreatment significantly attenuated mRNA level increases
of IL-1α compared with the PM-treated cells, while no significant
changes were observed for IL-1β or IL-6. Furthermore, ELISA
revealed that afzelin pretreatment significantly suppressed the
PM-induced increase in IL-1α, but not IL-1β or IL-6 (Fig. 4B).
Discussion
PM exposure has various adverse effects on human
skin; the association between PM exposure and skin was elucidated
by epidemiological studies connecting PM with skin inflammation,
skin cancer and skin diseases, including atopic dermatitis, eczema
and psoriasis (1,2,10,26). The current study used SRM 1649b as a
standard PM, which is mainly composed of PAHs. PAHs possess the
ability to damage the plasma membrane, alter cell physiology and
induce cell death (9,27).
Afzelin is a flavonoid compound isolated from the
Thesium chinense Turcz, which has been used in traditional
Korean and Chinese medicine to treat inflammatory diseases (17,18)
and has been reported to have anti-inflammatory, anticancer and
antibacterial properties (19-21). Recent reports demonstrated the
anti-inflammatory effects of afzelin in UVB-irradiated skin cells
(22,23). However, the effects of afzelin on PM exposure have not
yet been clarified. Therefore, the present study investigated
whether afzelin affected inflammatory responses of HaCaT cells
exposed to PM.
Afzelin had no significant cytotoxicity at ≤200
μM and cells in subsequent experiments were treated with 200
μM afzelin. Various environmental pollutants can directly or
indirectly produce ROS, and increased ROS levels serve a role in
the pathogenesis of human skin disorders and reduce skin function
(2,7). It was revealed that intracellular ROS generation was
elevated in HaCaT cells treated with 25 μg/cm2
PM. PM enhances oxidative stress by contributing to mitochondrial
injury (28,29). Furthermore, increased oxidative stress activates
the MAPKs and AP-1 signaling pathways (7,15,16). Findings of the
current study suggested that afzelin significantly alleviated
intracellular ROS generation in PM-treated HaCaT cells. Many
antioxidants exert activity by functioning as hydrogen or electron
donors (30). It was suggested that the hydroxyl groups of afzelin
exert a protective effect against oxidative stress, indicating that
afzelin may protect skin keratinocytes from PM-induced oxidative
stress and regulate MAPKs and AP-1 levels via oxidative stress
management. It was investigated whether afzelin inhibited
PM-induced p38 MAPK and AP-1 phosphorylation, which is associated
with the regulation of proinflammatory cytokine release (7,15,16).
The current study demonstrated that afzelin pretreatment
significantly inhibited the phosphorylation of p38 MAPK and AP-1
components in PM-treated HaCaT cells, indicating an
anti-inflammatory effect of afzelin that may be mediated by p38
MAPK and AP-1 signaling. There are many processes between
transcription and translation in PM-induced inflammatory signaling
and the protein and mRNA analysis time in the current study was
determined by the highest expression of each target. Therefore,
PM-induced phosphorylation levels of p38 and AP-1 were analyzed in
HaCaT cells at different times.
As several studies have reported that air pollutants
enhance the secretion of proinflammatory cytokines (2,31-33), the
current study investigated whether afzelin suppressed the release
of proinflammatory cytokines, including IL-1α, IL-1β and IL-6, in
PM-treated HaCaT cells. Afzelin pretreatment attenuated mRNA and
protein secretion levels of IL-1α in PM-treated HaCaT cells.
Production of IL-1α by keratinocytes causes skin diseases,
including contact and atopic dermatitis (32,33). IL-1β and IL-6
levels were not significantly affected by afzelin pretreatment of
PM-treated HaCaT cells. Further studies are required to elucidate
the anti-inflammatory activities of afzelin in animal models with
PM-induced atopic dermatitis.
In conclusion, the results of the present study
indicated that afzelin exerts anti-inflammatory effects in HaCaT
cells exposed to PM, potentially by inhibiting mRNA and protein
expression of IL-1α. Anti-inflammatory properties of afzelin may be
associated with downregulating the p38 MAPK and AP-1 signaling
pathways. Afzelin demonstrated antioxidant activities by
alleviating intracellular ROS generation in PM-treated HaCaT cells.
The present findings suggested that afzelin may have the potential
of preventing air pollution-induced inflammatory skin diseases.
Funding
This research was supported by the Basic Science
Research Foundation of Korea funded by the Ministry of Science, ICT
& Future Planning (grant no. NRF-2017R1C1B1007523).
Availability of data and materials
The datasets analyzed in the study are available
from the corresponding author on reasonable request.
Authors’ contributions
JHK, MK and KYP designed the study. JHK and MK
performed the experiments. JHK, MK, JMK, MKL, SJS and KYP analyzed
and interpreted the data. JHK, MK, MKL, SJS and KYP prepared the
manuscript. All authors read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Kim KE, Cho D and Park HJ: Air pollution
and skin diseases: Adverse effects of airborne particulate matter
on various skin diseases. Life Sci. 152:126–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mancebo SE and Wang SQ: Recognizing the
impact of ambient air pollution on skin health. J Eur Acad Dermatol
Venereol. 29:2326–2332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Magnani ND, Muresan XM, Belmonte G,
Cervellati F, Sticozzi C, Pecorelli A, Miracco C, Marchini T,
Evelson P and Valacchi G: Skin damage mechanisms related to
airborne particulate matter exposure. Toxicol Sci. 149:227–236.
2016. View Article : Google Scholar
|
|
4
|
Kampa M and Castanas E: Human health
effects of air pollution. Environ Pollut. 151:362–367. 2008.
View Article : Google Scholar
|
|
5
|
Du Y, Xu X, Chu M, Guo Y and Wang J: Air
particulate matter and cardiovascular disease: The epidemiological,
biomedical and clinical evidence. J Thorac Dis. 8:E8–E19.
2016.PubMed/NCBI
|
|
6
|
Guarnieri M and Balmes JR: Outdoor air
pollution and asthma. Lancet. 383:1581–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bickers DR and Athar M: Oxidative stress
in the pathogenesis of skin disease. J Invest Dermatol.
126:2565–2575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Segre JA: Epidermal barrier formation and
recovery in skin disorders. J Clin Invest. 116:1150–1158. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan TL, Wang PW, Aljuffali IA, Huang CT,
Lee CW and Fang JY: The impact of urban particulate pollution on
skin barrier function and the subsequent drug absorption. J
Dermatol Sci. 78:51–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong
HK and Ahn K: Symptoms of atopic dermatitis are influenced by
outdoor air pollution. J Allergy Clin Immunol. 132:495–498.e1.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vierkotter A, Schikowski T, Ranft U,
Sugiri D, Matsui M, Krämer U and Krutmann J: Airborne particle
exposure and extrinsic skin aging. J Invest Dermatol.
130:2719–2726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin SP, Li Z, Choi EK, Lee S, Kim YK, Seo
EY, Chung JH and Cho S: Urban particulate matter in air pollution
penetrates into the barrier-disrupted skin and produces
ROS-dependent cutaneous inflammatory response in vivo. J Dermatol
Sci. S0923–1811. 30202–30210. 2018.PubMed/NCBI
|
|
13
|
Lee CW, Lin ZC, Hu SC, Chiang YC, Hsu LF,
Lin YC, Lee IT, Tsai MH and Fang JY: Urban particulate matter
down-regulates filaggrin via COX2 expression/PGE2 production
leading to skin barrier dysfunction. Sci Rep. 6:279952016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krutmann J, Liu W, Li L, Pan X, Crawford
M, Sore G and Seite S: Pollution and skin: From epidemiological and
mechanistic studies to clinical implications. J Dermatol Sci.
76:163–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CW, Lin ZC, Hsu LF, Fang JY, Chiang
YC, Tsai MH, Lee MH, Li SY, Hu SC, Lee IT and Yen FL: Eupafolin
ameliorates COX-2 expression and PGE2 production in particulate
pollutants-exposed human keratinocytes through ROS/MAPKs pathways.
J Ethnopharmacol. 189:300–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCubrey JA, LaHair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parveen Z, Deng Y, Saeed MK, Dai R, Ahamad
W and Yu YH: Antiinflammatory and analgesic activities of Thesium
chinense Turcz extracts and its major flavonoids, kaempferol and
kaemp-ferol-3-O-glucoside. Yakugaku Zasshi. 127:1275–1279. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee IK, Kim KH, Choi SU, Lee JH and Lee
KR: Phytochemical constituents of Thesium chinense TURCZ and their
cytotoxic activities in vitro. Natural Product Sciences.
15:246–249. 2009.
|
|
19
|
Kim SK, Kim HJ, Choi SE, Park KH, Choi HK
and Lee MW: Anti-oxidative and inhibitory activities on nitric
oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids
from seeds of prunus tomentosa thunberg. Arch Pharm Res.
31:424–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diantini A, Subarnas A, Lestari K, Halimah
E, Susilawati Y, Supriyatna, Julaeha E, Achmad TH, Suradji EW,
Yamazaki C, et al: Kaempferol-3-O-rhamnoside isolated from the
leaves of Schima wallichii korth inhibits MCF-7 breast cancer cell
proliferation through activation of the caspase cascade pathway.
Oncol Lett. 3:1069–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SY, So YJ, Shin MS, Cho JY and Lee J:
Antibacterial effects of afzelin isolated from Cornus macrophylla
on Pseudomonas aeruginosa, a leading cause of illness in
immunocompromised individuals. Molecules. 19:3173–3180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin SW, Jung E, Kim S, Kim JH, Kim EG,
Lee J and Park D: Antagonizing effects and mechanisms of afzelin
against UVB-induced cell damage. PLoS One. 8:e619712013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung E, Kim JH, Kim MO, Jang S, Kang M, Oh
SW, Nho YH, Kang SH, Kim MH, Park SH and Lee J: Afzelin positively
regulates melanogenesis through the p38 MAPK pathway. Chem Biol
Interact. 254:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eruslanov E and Kusmartsev S:
Identification of ROS using oxidized DCFDA and flow-cytometry.
Methods Mol Biol. 594:57–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Drakaki E, Dessinioti C and Antoniou CV:
Air pollution and the skin. Front Environ Sci. 2:112014. View Article : Google Scholar
|
|
27
|
Tekpli X, Holme JA, Sergent O and
Lagadic-Gossmann D: Importance of plasma membrane dynamics in
chemical-induced carcinogenesis. Recent Pat Anticancer Drug Discov.
6:347–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li N, Xia T and Nel AE: The role of
oxidative stress in ambient particulate matter-induced lung
diseases and its implications in the toxicity of engineered
nanoparticles. Free Radic Biol Med. 44:1689–1699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Huang J, Wang L, Chen C, Yang D,
Jin M, Bai C and Song Y: Urban particulate matter triggers lung
inflammation via the ROS-MAPK-NF-κB signaling pathway. J Thorac
Dis. 9:4398–4412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lobo V, Patil A, Phatak A and Chandra N:
Free radicals, antioxidants and functional foods: Impact on human
health. Pharmacogn Rev. 4:118–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ushio H, Nohara K and Fujimaki H: Effect
of environmental pollutants on the production of pro-inflammatory
cytokines by normal human dermal keratinocytes. Toxicol Lett.
105:17–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi H, Shin DW, Kim W, Doh SJ, Lee SH and
Noh M: Asian dust storm particles induce a broad toxicological
transcriptional program in human epidermal keratinocytes. Toxicol
Lett. 200:92–99. 2011. View Article : Google Scholar
|
|
33
|
Li Q, Kang Z, Jiang S, Zhao J, Yan S, Xu F
and Xu J: Effects of ambient fine particles PM2.5 on
human HaCaT cells. Int J Environ Res Public Health. 14:E722017.
View Article : Google Scholar
|