Introduction
Polysaccharides are a type of polymer composed of
≥10 mono- or di-oligosaccharide residues coupled together with
glycosidic bonds (1), and serve
an important role in pharmacology and physiology (2). Polysaccharides act as barriers
between the cell wall and the environment, they mediate
host-pathogen interactions and form biofilm structures. In recent
years, active polysaccharides have been investigated due to their
immunomodulatory, antitumor, antiviral, antioxidant and
hypoglycemic effects (3-5). Furthermore, active polysaccharides
can regulate the immune system, activate immune cells, and
complement and promote cytokine formation (6).
Lactarius deliciosus Gray is a rare edible
mushroom with high nutritional value that has attracted increasing
attention (7). As one of the main
effective components of L. deliciosus, its polysaccharides
exhibit a broad spectrum of application, including anti-tumor
activity, macrophage immunostimulant activity and B cell
immunostimulant activity (8-11).
Therefore, studies on the structure and biological activity of
L. deliciosus polysaccharide are of great significance for
the application and development of L. deliciosus treatment.
In previous studies, certain water-soluble polysaccharides have
been extracted and purified from the fruiting bodies of wild L.
deliciosus (8-10). These studies also revealed that
L. deliciosus polysaccharides exhibit immunoregulatory and
anti-tumor activities (8-10). Furthermore, the polysaccharides of
L. deliciosus from different habitats exhibit different
structures (11). For example,
L. deliciosus polysaccharide is composed of
(1→6)-α-L-mannose and (2→3)-α-D-xylose at a ratio of 3:1 (8), while L. deliciosus
polysaccharide consists of (1→6)-α-D-Gal, (1→2,6)-α-D-Gal,
→6)-α-D-Gal and →4)-β-D-Glu in a ratio of 1:2:1:1 (11).
In the present study, Maerkang L. deliciosus
Gray polysaccharide was isolated and purified using hot water
extraction technology and column chromatography, respectively
(12-14). Chemical methods, high performance
gel permeation chromatography (HPGPC), fourier transform infrared
spectroscopy (FT-IR), nuclear magnetic resonance spectrum (NMR) and
gas chromatography-mass spectrometry (GC-MS) were used to
characterize the polysaccharide. The immunomodulatory ability of
LDG-M was also investigated. To the best of our knowledge, the
results of the present study provide a scientific basis for further
study on the pharmacological action, structure-activity association
and application of LDG-M.
Materials and methods
Chemicals
The fresh fruiting bodies of Maerkang L.
deliciosus Gray were collected from Maerkang County, which lies
in the Sichuan Aba Tibetan and Qiang Autonomous Prefecture. After
vacuum freeze-drying, the bodies were crushed and stored at 4°C for
use in the Key Laboratory of Southwest China Wildlife Resources
Conservation, College of Life Sciences, China West Normal
University, China. The fruiting bodies of Maerkang L.
deliciosus Gray were identified by Professor Tang Zongxiang
(Sichuan Agricultural University, China). The ethanol was purchased
from Swancor Shanghai Fine Chemical Co., Ltd. (Shanghai, China).
Sodium chloride was purchased from Sichuan Kelun Pharmaceutical
Co., Ltd. (Chengdu, China). Trifluoroacetic acid (TFA), standard
monosaccharide and dextran of different molecular weight were
purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin,
China). DEAE-cellulose column, Sephacryl S-300 gel column and
Sephadex G-200 column were purchased from Beijing Solarbio Science
& Technology Co., Ltd. (Beijing, China). Cell counting kit
(CCK)-8 (cat. no. CK04) was purchased from Dojindo Molecular
Technologies, Inc. (Shanghai, China). PBS buffer, RPMI-1640 medium,
phenol red free, 0.5% Trypsin-EDTA and fetal bovine serum (FBS)
were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Mouse interleukin (IL)-6 (cat. no. M6000B) and tumor necrosis
factor (TNF)-α (cat. no. MTA00B) ELISA kits were purchased from
R&D Systems China Co., Ltd. (Shanghai, China). Neutral red and
DMSO were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). MTT was purchased from Amresco, LLC (Solon, OH, USA).
Cell cycle and apoptosis analysis kit (cat. no. C1052) was
purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China).
The inverted fluorescence microscope was purchased from Leica
Microsystems (DMI4000B, Germany). The intelligent high-efficiency
centrifuge was purchased from Beckman Coulter, Inc. (Brea, CA, USA;
Avanti JXN-26). The refrigerated centrifuge was purchased from
Eppendorf (Hanburg, Germany; cat. no. 5418R). All analytical
reagents were of analytical grade.
Extraction of the polysaccharide from
Maerkang L. deliciosus Gray
The fresh fruiting bodies of Maerkang L.
deliciosus Gray were washed with water, dried at 60°C and
pulverized. The 300 g powder accurately weighed was boiled in water
for 6 h and the ratio of powder and water was 1:3 (15,16). The supernatant was collected by
centrifugation (16,670 × g for 20 min at 4°C; Avanti JXN-26,
Beckman Coulter, Inc.) and the precipitate was boiled in water for
6 h (repeated three times). All supernatants were concentrated to
300 ml. Four volumes of absolute ethanol were added to precipitate
crude polysaccharides. Flocculent precipitation was collected and
dried. Crude polysaccharides were weighed and dissolved with
distilled water, which was performed as previously described
(17). After adding the
supernatant to the activated DEAE cellulose-52 column (2×60 cm), it
was allowed them to stand for 10 min to fully bind. Sodium chloride
solutions with different concentrations (0.1, 0.2, 0.3, 0.4, 0.5
mol/l) were prepared as the mobile elution phase. After adding the
mobile phase, the liquid was collected and concentrated.
Phenol-sulfuric acid method was used to determine the
polysaccharide as described previously (18). The eluate was purified on Sephadex
G-200, then concentrated and centrifuged (12,400 × g for 20 min at
4°C; 5418R; Eppendorf). Small molecules in the supernatant were
removed by dialysis (7 kDa) for 48 h. For lyophilization, Maerkang
L. deliciosus Gray poly-saccharide (named LDG-M) was
obtained for further analysis on its structure and
bioactivities.
Determining the molecular weight of
LDG-M
A total of 10 mg LDG-M was dissolved in 1 ml double
distilled water, sonicated for 5 min and then filtered
(0.22-µm pore). HPGPC was used to determine the molecular
weight of LDG-M as previously described (19-21). The measured data were subjected to
Empower Pro GPC software analysis (Agilent Empower Pro GPC Data
Analysis Software for Agilent ChemStation; version B.01.02; Agilent
Technologies Inc., Santa Clara, CA, USA) with a standard curve
prepared from dextran to obtain molecular weight.
FT-IR analysis
A total of 5 mg LDG-M and fully dry potassium
bromide (KBr) were ground and mixed (22). Subsequently, the mixture was
pressed and scanned in the Fourier transform infrared spectrometer
at a range of 4,000-400 cm−1 as described previously
(23).
NMR
For NMR analysis, 20 mg LDG-M was weighed and
dissolved in D2O (24). The Varian Unity INOVA 400/45
(Varian Medical Systems, Inc., CA, USA) was used to detect the
1H NMR spectra and 13C NMR spectra, and
tetramethylsilane was used as an internal standard (11).
Methylation analysis and GC-MS
Methyl iodide was used to prepare methylated
polysaccharide (25). The
methylated product was dried and dissolved in 2 M TFA and
hydrolyzed at 100°C for 6 h. Silane reagent was added and the
derivatized product was used to perform the GC-MS experiment. The
temperature program was set as follows: Initial temperature
maintained at 80°C for 3 min, then raised to 200°C at a rate of
10°C/min and maintained at 200°C for another 10 min.
Cell lines and reagents
The mouse L929, B and macrophage RAW264.7 cell
lines, purchased from the cell bank of the Typical Culture
Preservation Committee of the Chinese Academy of Science (Shanghai,
China), were cultured in RPMI-1640 medium with 10% FBS, 1%
penicillin (100 IU/ml) and streptomycin (100 mg/l) in an incubator
at 37°C with 5% CO 2.
Pharmacological evaluation for B cells
and RAW264.7 cells stimulation
The pharmacological evaluation of B and RAW264.7
cell stimulation was examined using a CCK-8 assay (26). Cells were cultured in RPMI-1640
medium. When cells were in the logarithmic growth period, the
medium was added to dilute cells. A density of 1×105
cells/ml were added to 96-well plates at 100 µl/well and
incubated in an incubator for 24 h at 37°C with 5% CO2
(11). Different concentrations
of LDG-M prepared using cell culture medium (0.625, 1.25, 2.5, 5,
10, 20 µg/ml) were added to the 96-well plates at 100
µl/well. Furthermore, 5 µg/ml lipopolysaccharide
(LPS) was used as positive control (27) and the cell culture medium without
LDG-M was used as blank control. After incubated at 37°C for 24 h,
5 µl of CCK-8 reagent was added to each well and the cells
were cultured for an additional 2 h. A microplate reader was used
to detect the optical density (OD) at 450 nm. The formula used to
calculate cell viability (%) was as follows: [(Ac-As)/(Ac-Ab)]
×100%, where Ac was the absorbance of the control group, Ab was the
absorbance of the blank group, and as was the absorbance of the
experimental groups.
Pharmacological evaluation for macrophage
phagocytic activity
RAW264.7 cells (1×105 cells/ml) were
seeded into 96-well plates at 100 µl/well and incubated for
24 h. Subsequently, 100 µl cell culture medium (blank
control), LPS (final concentration 5 µg/ml, positive
control) and LDG-M solutions (0.625, 1.25, 2.5, 5, 10, 20, 40
µg/ml) were added to the 96-well plates. After incubation at
37°C for 24 h, neutral red reagent (0.075 g/l) was added to the
96-well plates. After 30 min, the neutral red reagent was discarded
and the cells were washed with PBS thrice, followed by the addition
of 200 µl lysis buffer (ethanol:glacial acetic acid, 1:1).
The 96-well plates were placed in an incubator for 2 h. The OD was
measured at 540 nm.
Effect of LDG-M on the inhibitory
activity of RAW264.7 macrophages on L929 tumor cells in vitro
RAW264.7 cells (effector cells, 1×105
cells/ml) and L929 cells (target cells, 1×106 cells/ml)
in the logarithmic growth period were cultured on 96-well plates
and incubated for 24 h. Effector cells (RAW264.7 cells) and target
cells (L929 cells) were added to 96-well plates at a ratio of 1:2
with 100 µl/well. Simultaneously, separate effector and
target cell groups were set at 200 µl/well. When cells were
adherent, supernatants were discarded. The followings groups were
used: Blank control (cell culture medium), positive control (LPS 5
µg/ml) and experimental (LDG-M 5 µg/ml). Following an
incubation at 37°C for 24 h, supernatants were removed and MTT (5
mg/ml) was added at 10 µl/well. The plate was then cultured
in incubator at 37°C for 4 h. The supernatants were discarded, and
DMSO was added at 100 µl/well for 15 min. The absorbance
value was measured at 570 nm. The inhibitory activity (%) of L929
tumor cells in the presence of macrophage RAW264.7 cells in
vitro was calculated according to the following formula: [1-(OD
value of experimental group-OD value of individual effector
cell)/OD value of individual target cell] ×100%.
Morphological observation of cells
Following treatment with LDG-M as aforementioned,
cells were observed under a Leica inverted fluorescence microscope
directly without staining (magnification, ×100). Leica Application
Suite X software (Leica Microsystems, Inc.; version 3.0.0.15697)
was used for the assessment of cell morphology.
Evaluating cytokine levels
In order to understand the alterations in the level
of cytokines, TNF-α and IL-6 secreted by RAW264.7 cells, ELISA kits
were used according to the manufacturers' protocols.
Effects of LDG-M on the cell cycle of B
cells
Cell cycle and apoptosis analysis kit were used to
evaluate the effects of LDG-M on the cell cycle of B cells. The
following groups were used: Blank control, experimental (LDG-M, 2.5
and 5 µg/ml) and positive control (LPS, 5 µg/ml). A
total of 1 ml 70% ethanol was added to cell plates at 4°C for 2 h
in order to immobilize cells. Subsequently, 0.5 ml propidium iodide
staining solution was added to each sample in a 37°C water bath for
30 min. The percentages of cells at the
G0/G1, S and G2/M phases were analyzed using
a flow cytometer as previously described (28).
Statistical analysis
Data are presented as the mean ± standard deviation.
Significant differences between the experimental and control groups
were analyzed using one-way ANOVA followed by Student-Newman-Keuls
test with SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
(27). There were six replicates
in each group. P<0.05 was used to indicate a statistically
significant difference.
Results
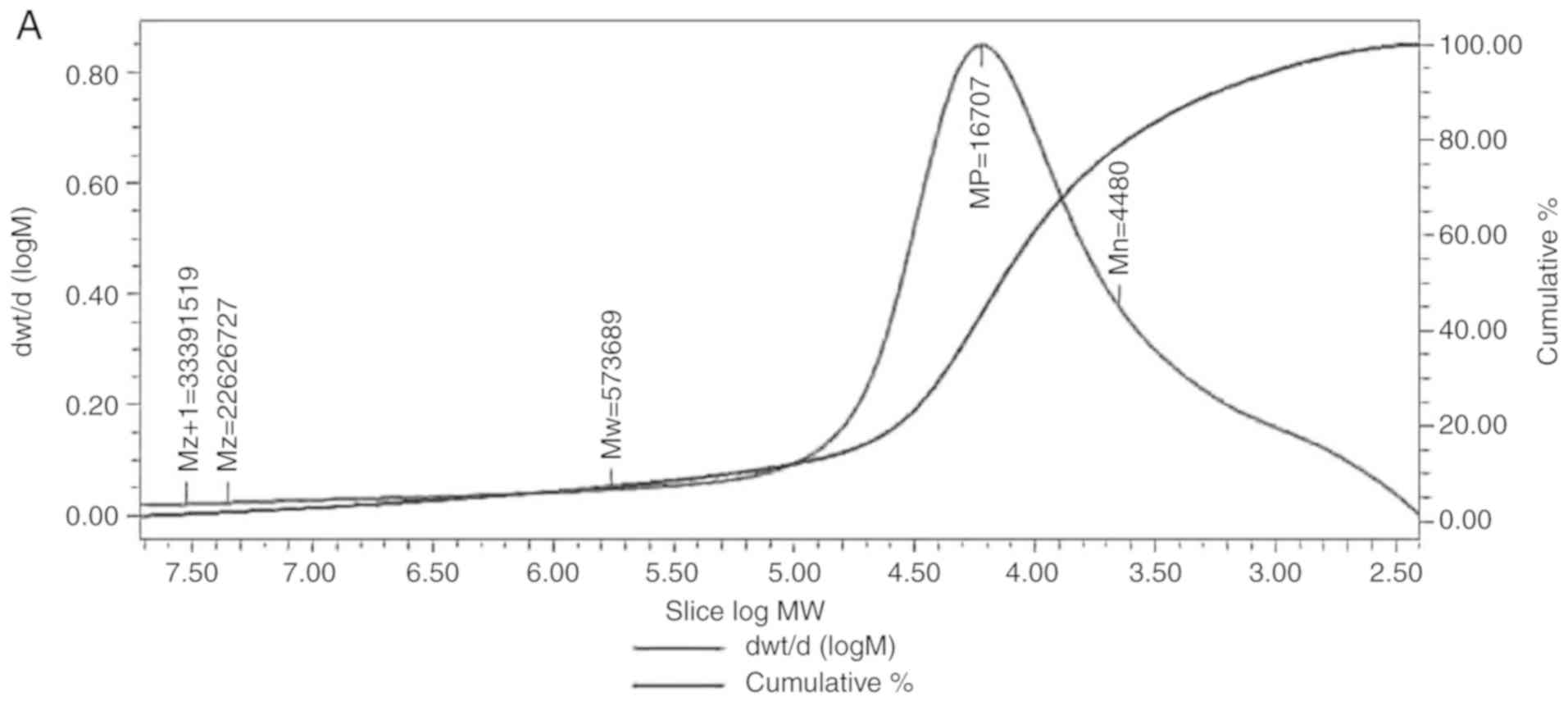
Determining the molecular weight of
LDG-M
HPGPC was used to evaluate the relative molecular
weight of the polysaccharide, using the Z+1-average molecular
weight (Mz+1), Z-average molecular weight (Mz), weight-average
molecular weight (Mw), peak molecular weight (Mp) and
number-average molecular weight (Mn). The peaks of LDG-M on HPGPC
were broadly symmetrical. The Mz+1 of LDG-M was 33391519 Da, Mz was
2626727 Da, Mw was 573689 Da, Mp was 16707 Da, and Mn was 4480 Da
(Fig. 1A).
FT-IR analysis
The working principle of infrared spectroscopy is
due to different vibration levels. According to the results for
LDG-M, no absorption peak at wavelengths of 280 and 260 nm were
present, indicating no protein and nucleic acid in the LDG-M
sample. The FT-IR spectra of LDG-M displayed typical polysaccharide
absorption peaks in the range of 4,000-400 cm−1
(Fig. 1B). A broad absorption
peak at 3,443.46 cm−1 was designated as a OH stretching
vibration peak, 2,920.91 cm−1 was designated as a CH
stretching vibration peak, 1,639.88 cm−1 was designated
as a CO stretching vibration peak, 1,401.54 cm−1 was
designated as a bending vibration peak of CH2, CH and
OH, 1,093.48 cm−1 was designated as a CO stretching
vibration peak and 6,18.19 cm−1 was designated as a CH
rocking vibration peak (29,30).
Analysis of the NMR results
The proton spectrum of LDG-M is shown in Fig. 1C. In the 1H NMR (400
Hz) spectrum, δ 5.03, δ 4.92 and δ 4.92 ppm indicated that LDG-M
had three anomeric protons, suggesting that LDG-M consisted of at
least three monosaccharides. The overlapping proton signal at δ
4.92 ppm was assigned to β-pyranose unit, whereas another signal at
δ 5.03 ppm was attributed to α-pyranose forms (21). The proton signal for water was δ
4.70 ppm. The signals in δ 3.49-4.14 ppm were the proton signal of
H2-H6 of glucose and H2-H5 of lyxose.
In the 13C NMR spectra of LDG-M (Fig. 1D), the signals of δ 102.69, δ
102.67 and δ 100.79 ppm were anomeric carbon peaks, which indicated
that LDG-M had α and β anomeric configurations (11). It was in accord with the results
of FT-IR and 1H NMR. In virtue of inexistence of signals
among δ 160-180 ppm, accordingly, no carboxyl group was present in
LDG-M. Due to the absence of a chemical shift at δ 106-109 ppm, no
furan ring was present in LDG-M. According to the literature
(31), the chemical shift at δ
102.69 ppm could belong to C1 of residue A (1,4,6-linked β-D-Glcp),
the chemical shift at δ 102.67 ppm could belong to C1 of residue B
(1,6-linked β-D-Glcp) and the chemical shift at δ 100.79 ppm could
belong to C1 of residue C (1-linked α-D-Lyxp), respectively. The
strong signals among δ 80-δ 69 ppm may be assigned to the carbon
signal of C2-C6 of glucose and C2-C5 of lyxose. Signals of every
carbon atom are listed in Table
I.
 | Table I13C NMR chemical shift
data (δ, ppm) for LDG-M. |
Table I
13C NMR chemical shift
data (δ, ppm) for LDG-M.
| Sugar residues | Chemical shift, δ
(ppm)
|
|---|
| C1 | C2 | C3 | C4 | C5 | C6 |
|---|
| β-D-Glcp (A) | 102.69 | 71.29 | 70.88 | 72.06 | 73.00 | 69.35 |
| β-D-Glcp (B) | 102.67 | 71.71 | 79.50 | 71.27 | 72.50 | 71.11 |
| α-D-Lyxp (C) | 100.79 | 71.78 | 72.19 | 71.15 | 72.78 | - |
The proton chemical shift at δ 5.03, δ 4.92, δ 4.92
ppm and a cross peak at 5.03/3.89, 4.92/3.92, 4.92/3.79 ppm were
readily obtained from H-H correlated spectroscopy (Fig. 2A), which implied that the chemical
shift of H2 were δ 3.89, δ 3.92 and δ 3.79 ppm, respectively.
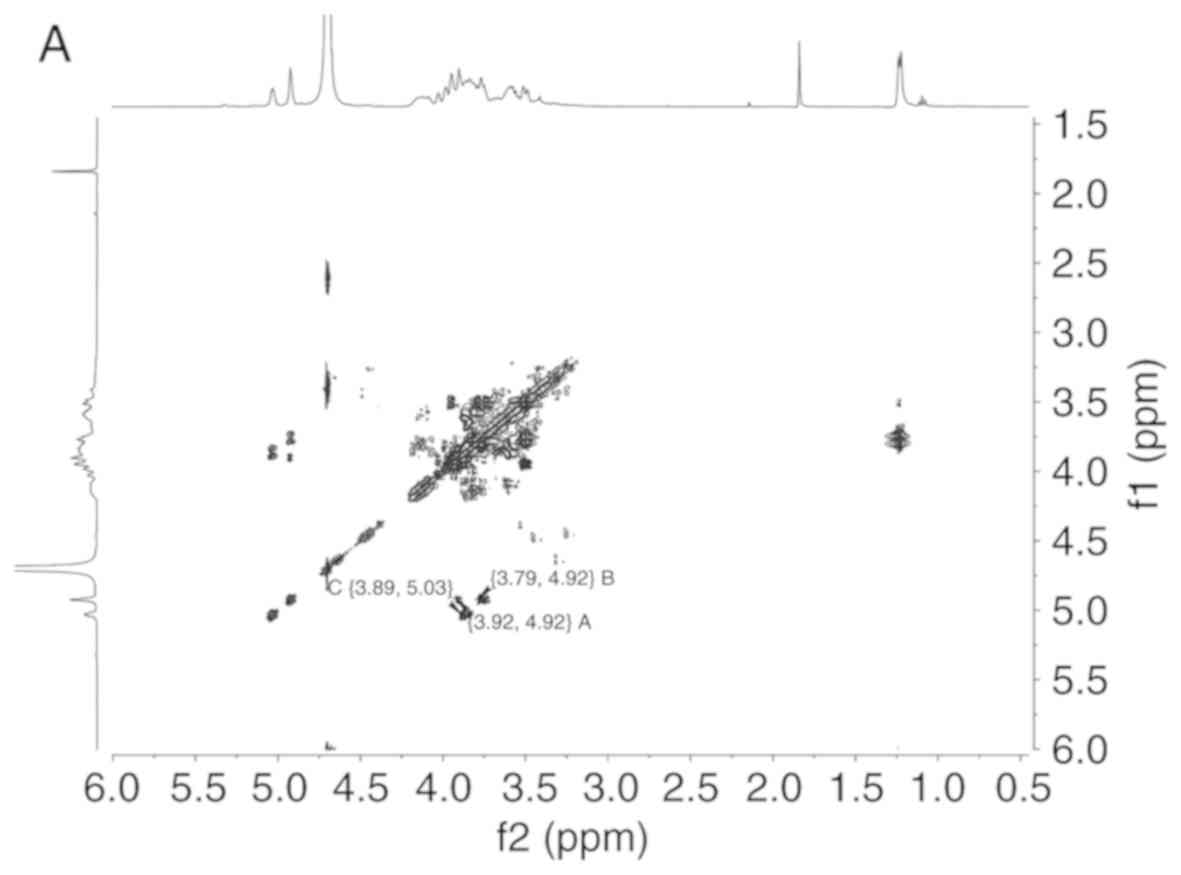 | Figure 2LDG-M 2D NMR spectrum, GC-MS fragment
ion peaks and predicted chemical structure of LDG-M. (A) H-H COSY
spectrum of LDG-M. (B) HMBC spectrum of LDG-M. (C) The fragment ion
peaks of 2,3,4-tri-O-Me-1,6-bis-O-trimethylsilyl-Glc. LDG-M 2D NMR
spectrum, GC-MS fragment ion peaks and predicted chemical structure
of LDG-M. (D) The fragment ion peaks of
2,3-di-O-Me-1,4,6-tris-O-trimethylsilyl-Glc. (E) The fragment ion
peaks of 2,3,4-tri-O-Me-1-O-trimethylsilyl-Lyx. (F) Predicted
chemical structure of LDG-M; the asterisk symbol (*) represents the
connection of the same polysaccharide unit. COSY, correlated
spectroscopy; HMBC, heteronuclear multiple bond correlation; GC-MS,
gas chromatography-mass spectrometry. |
Long-range 1H-13C
heteronuclear multiple bond correlation (HMBC) spectrum was used to
analyze the association between monosaccharide residues of LDG-M
(Fig. 2B). A H1/C3 association
was identified in the 1H-13C HMBC spectrum,
such as δ 4.92/δ 70.88 ppm corresponding to residue A, δ 4.92/δ
79.50 ppm corresponding to residue B and δ 5.03/δ 72.19 ppm
corresponding to residue C, respectively, which was consistent with
the GC-MS analysis.
Analysis of GC-MS
The GC-MS results on LDG-M methylation are presented
in Table II. According to the
GC-MS results, it may be suggested that the fragment ion peaks of
LDG-M were consistent with the data of the D-configuration
monosaccharide fragment ion peaks. Therefore, the D-configuration
was the configuration of glucose residues that could be determined
(Fig. 2C-E). In the present
study, it may be suggested that the β-D-glucosepyranose residues
were 2,3,4-tri-O-methyl-1,6-bis-O-trimethysilyl-substitued and
2,3-di-O-methyl-1,4,6-tris-O-trimethysilyl-substituted and the
α-D-lyxopyranose residues were
2,3,4-tri-O-methyl-1-O-tri-methysilyl-substitued (Fig. 2C-E). According to the LDG-M
methylation results, the amount of (1→6)-linked-β-D-glu
cosepyranose and (1→4,6)-linked-β-D-glucosepyranose was the
highest, which constituted the main chain of the structure. In
addition, the side chain was comprised of the α-D-lyxopyranose
residues. LDG-M had repeating units of a backbone of
(1→6)-β-D-glucosepyranose and (1→4,6)-β-D-glucosepyranose, and a
branch of a (1→4)-α-D-lyxopyranose residue.
 | Table IIGC-MS results of methylation analysis
of LDG-M. |
Table II
GC-MS results of methylation analysis
of LDG-M.
| Methylated
sugar | Linkage | m/z |
|---|
|
1,6-bis-O-trimethylsilyl-Glc | 1,6- | 59 73 88 101 117
133 159 205 229 265 287 319 351 |
|
1,4,6-tris-O-trimethylsilyl-Glc | 1,4,6- | 59 73 88 103 117
133 147 159 191 205 232 259 287 303 319 345 377 409 |
|
1-O-trimethylsilyl-Lyx | 1- | 73 88 133 174
217 |
On the basis of the aforementioned experimental
data, the conceivable structure of LDG-M was confirmed, composing
of a backbone of 1,6-linked-β-D-glucose and
1,4,6-linked-β-D-glucose and one (1→4)-linked-α- D-lyxopyranose
residue as a branch (Fig.
2F).
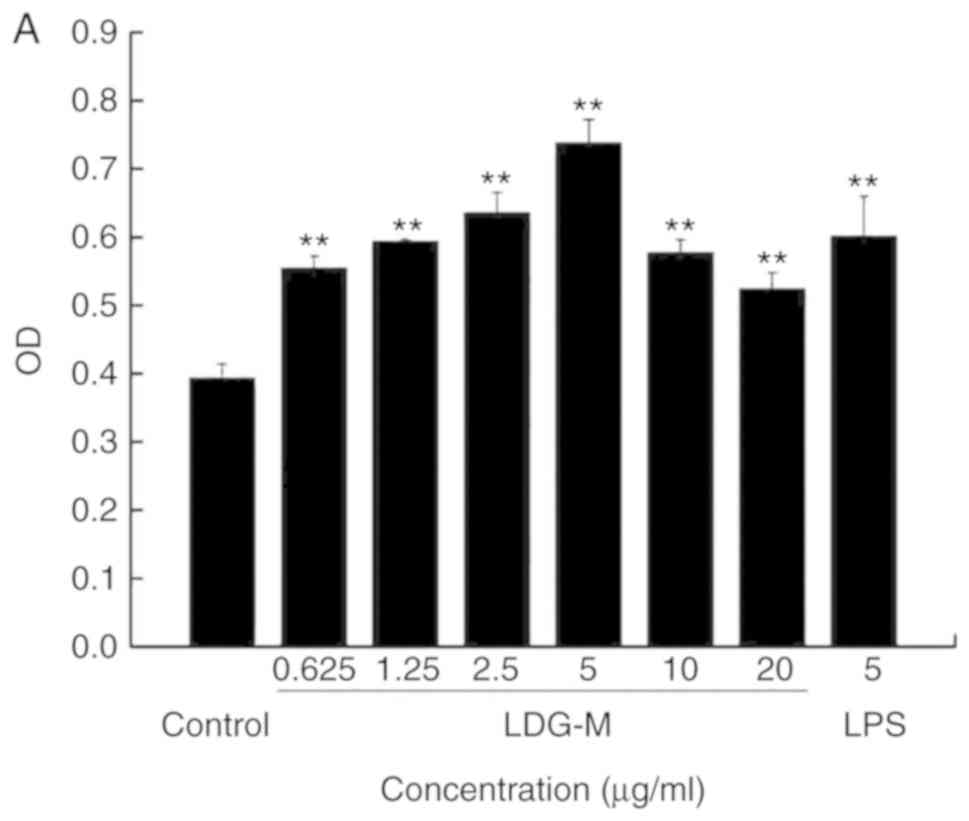
Effects of LDG-M on the proliferation of
RAW264.7 cells in vitro
The results demonstrated that the LDG-M group
(0.625, 1.25, 2.5, 5, 10, 20 µg/ml) significantly promoted
the proliferation of RAW264.7 cells compared with the blank control
group (P<0.01), as shown in Fig.
3A. The proliferation efficiency reached the maximum with an
increase of 87.8% at 5 µg/ml compared with the blank control
group.
Effect of LDG-M on the phagocytic
function of RAW264.7 cells
Neutral red is a fluorescent reagent with a large
molecule and strong absorption peak at 540 nm. As a macromolecular
substance, neutral red enters macrophages only through endocytosis.
Therefore, the phagocytosis of macrophages can be measured using
neutral red (32). Compared with
the blank control group, LDG-M significantly promoted the
phagocytosis of RAW264.7 cells (Fig.
3B). At a concentration of 2.5 µg/ml LDG-M, the
phagocytic activity reached the maximum, which was increased by
61.4% compared with that of the blank control group.
Effect of LDG-M on RAW264.7 cells
secreting cytokines
Compared with the blank control group, LDG-M
significantly promoted the secretion of TNF-α from RAW264.7 cells
(Fig. 3C). When the concentration
of LDG-M was 2.5 µg/ml, the secretion of TNF-α reached the
maximum, which was 14.4 times higher compared with that of the
blank control group.
Compared with the blank control group, LDG-M
significantly promoted the secretion of IL-6 from RAW264.7 cells
(Fig. 3D). When the concentration
of LDG-M was 5 µg/ml, the secretion of IL-6 reached the
maximum, which was 24.5 times higher compared with that of the
blank control group.
Effect of LDG-M on the inhibitory
activity of RAW264.7 macrophages on L929 tumor cells in vitro
The results demonstrated that the inhibitory
activity of RAW264.7 cells on L929 cells reached 49.36% at an LDG-M
concentration of 5 µg/ml, while it was 32.94% in the blank
control group (Fig. 3E and F).
Cell morphology observation revealed that RAW264.7 cells grew well
and L929 cells grew weakly under the stimulation of LDG-M compared
with the blank control group. This was consistent with the above
statistical data, which confirmed that LDG-M could enhance the
inhibitory activity of RAW264.7 cells on L929 cells.
Effect of LDG-M on B cells activation and
B cell cycle in vitro
B lymphocytes that can secrete antibodies were
derived from bone marrow pluripotent stem cells and was used as a
model of humoral immunity. Compared with the blank control group,
the LDG-M and LPS groups significantly promoted the proliferation
of B cells (Fig. 4A). At a
concentration of 2.5 µg/ml LDG-M, the proliferation
efficiency reached the maximum at 39.9%. Using the Leica
Microsystems inverted fluorescence microscope to observe the cell
morphology and the alterations to cell numbers (Fig. 4B), it is evident that compared
with the blank control group, the number of B cells in the LDG-M
group significantly increased and were grouped together more.
 | Figure 4Effect of LDG-M on B cells. (A)
Effects of LDG-M on the proliferation of B cells in vitro.
(B) Cell morphological observations on the proliferation of B cells
(magnification, ×100). a, Blank control group; b-g, Experimental
group (LDG-M 0.625, 1.25, 2.5, 5, 10, 20 µg/ml,
respectively); h, Positive control group (LPS 5 µg/ml). (C)
Effect of LDG-M on B cell cycle in vitro. a, Blank control
group; b-c, Experimental group (LDG-M 2.5, 5 µg/ml,
respectively); d, Positive control group (LPS 5 µg/ml). (D)
Statistical analysis of LDG-M on B cell cycle in vitro.
**P<0.01, *P<0.05. LDG-M, Maerkang
L. deliciosus Gray polysaccharide; LPS,
lipopolysaccharide. |
Cell cycle is a process involving cell division and
involves cell interphases and the cell division phase. Cells are
dormant when they are in the G0 phase. Cell interphase is divided
into the G1, S and G2 phases. The G1 phase involves the synthesis
of RNA and ribosomes. The S phase includes the synthesis of DNA and
histones, and in general, once the cells enter the S phase, cell
division continues until the G1 phase of the next cycle. The G2
phase involves the mitosis preparation period when protein
synthesis is completed, and the M phase is the cell division
period. The results for cell cycle analysis are shown in Fig. 4C-D. LDG-M was demonstrated to
promote cell cycle progression in B lymphocytes, inducing cell
division. In Fig. 4D, compared
with the blank control group, when the concentration of LDG-M was
2.5 µg/ml, the number of B lymphocytes in the
G0/G1 and G2/M phases decreased significantly
from 66 to 58.2%, and from 17 to 11.6%, respectively, while the
number of cells in S phase increased significantly from 17.6 to
30.8%. The percentage of cells in the G0/G1
phase was the lowest at 2.5 µg/ml. However, when the
concentration of LDG-M was 5 µg/ml, the number of B
lymphocytes in the G0/G1 phase did not change
significantly. The B cell cycle assay showed that when the
concentration of LDG-M was 2.5 µg/ml, the number of B cells
in the S phase was the highest, but whether it is associated with
the proliferation of B cells remains to be investigated.
Discussion
Maerkang L. deliciosus Gray is considered a
healthy food in Asia, and demonstrates various pharmacological
activities, including anticancer, antimicrobial (33), antihyperlipidemic, antifatigue,
antioxidation (34) and
immunological activities. Polysaccharides are involved in various
biological roles, such as regulating immune function, identifying
cell and cell interactions and transporting intercellular
substances (35). Additionally,
polysaccharides have been used for the diagnose and treatment of
cancer (36-38), with a prominent feature in
enhancing host immune function (39,40). Via the stimulation of immune
cells, such as B lymphocytes, T lymphocytes, dendritic cells,
macrophages and natural killer cells, polysaccharides exhibit
anti-tumor effects (41).
Polysaccharides in the preparation of drugs are also used as an
alternative adjuvant (42).
In the present study, a novel polysaccharide was
isolated from the fruiting body of Maerkang L. deliciosus
Gray with a molecular weight of 573,689 Da, named LDG-M. The
composition of LDG-M primarily includes β-D-glucose and α-D-lyxose
with a ratio of 2:1. LDG-M is composed of a backbone of
1,6-linked-β-D-glucose and 1,4,6-linked-β-D-glucose, and branches
consisting of one (1→4)-linked-α-D-lyxopyranose residue.
Furthermore, the immunoregulatory activities results showed that
LDG-M could promote the proliferation and phagocytosis of
macrophages, and induce cytokine (TNF-α and IL-6) release. It could
also significantly promote the proliferative activity of B cells
and promote B cells to enter S phase. According to macrophage
proliferation, phagocytosis and cytokine secretion assays in the
literature and previous reports (27,31), LPS (5 µg/ml) was chosen as
the positive control. The combination of macrophages and L929 cells
was used to assess the inhibitory effect of macrophages stimulated
by the polysaccharide on tumor cells, where LPS was also used as
the positive control group. LPS may slightly activate the
proliferation of L929 cells; however, the experimental results
showed that LDG-M significantly enhanced the inhibitory activity of
RAW264.7 cells on L929 cells, which further indicated that LDG-M
exhibited immunological activities.
Although the concentration of LPS used (5
µg/ml) was not a suitable physiological concentration and no
intergroup analysis was performed, the current study was primarily
performed to evaluate the effect of the polysaccharide on the
proliferation of immune cells, including macrophages and B cells.
Furthermore, this study aimed to investigate whether the
stimulatory effect was dose-dependent in vitro rather than
to identify an optimal concentration between the groups in
vivo. It is also worth noting that the in vitro active
concentration of the polysaccharide is different from that in
vivo. Consequently, the active concentration in the current
study is not suitable for use in vivo. Therefore, whether
LDG-M has the corresponding biological activity in vivo and
whether it is dose-dependent remains to be further studied. In
short, this study introduced LDG-M as a valuable source with unique
immunoregulatory properties.
Acknowledgments
Thanks to Professor Tang Zongxiang of Sichuan
Agricultural University for the identification of the wild Maerkang
Lactarius deliciosus Gray.
Funding
This project was supported by the Science and
Technology Support Project of Sichuan Province (grant nos.
2018JY0087 and 2018NZ0055), the Cultivate Major Projects of Sichuan
Province (grant no. 16CZ0018), Nanchong Science and Technology
Bureau of Sichuan Province (grant no. 16YFZJ0043), the Talent
Program of China West Normal University (grant nos. 17YC328,
17YC136 and 17YC329), the National Training Project of China West
Normal University (grant no. 17c039) and the Innovative Team
Project of China West Normal University (grant no. CXTD2017-3).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YH conceived and designed the experiments of the
current study. SS and LF performed the experiments and acquired
data. XD and YH drafted the manuscript and revised it critically.
All authors discussed the results and implications, and commented
on the manuscript at all stages. All authors gave final approval of
the current version to be published. Furthermore, all authors agree
to be accountable for all aspects of the work in ensuring that
questions associated with the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Wang M, Yang XB, Zhao JW, Lu CJ and Zhu W:
Structural characterization and macrophage immunomodulatory
activity of a novel polysaccharide from Smilax glabra Roxb.
Carbohydr Polym. 156:390–402. 2017. View Article : Google Scholar
|
|
2
|
Yu Y, Shen MY, Song QQ and Xie J:
Biological activities and pharmaceutical applications of
polysaccharide from natural resources: A review. Carbohydr Polym.
183:91–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho CW, Han CJ, Rhee YK, Lee YC, Shin KS,
Shin JS, Lee KT and Hong HD: Cheonggukjang polysaccharides enhance
immune activities and prevent cyclophosphamide-induced
immunosuppression. Int J Biol Macromol. 72:519–525. 2015.
View Article : Google Scholar
|
|
4
|
Li N, Li L, Fang JC, Wong JH, Ng TB, Jiang
Y, Wang CR, Zhang NY, Wen TY, Qu LY, et al: Isolation and
identification of a novel polysaccharide-peptide complex with
antioxidant, anti-proliferative and hypoglycemic activities from
the abalone mushroom. Biosci Rep. 32:221–228. 2012. View Article : Google Scholar
|
|
5
|
Li LJ, Li MY, Li YT, Feng JJ, Hao FQ and
Lun Z: Adjuvant activity of Sargassum pallidum polysaccharides
against combined newcastle disease, infectious bronchitis and avian
influenza inactivated vaccines. Mar Drugs. 10:2648–2660. 2012.
View Article : Google Scholar :
|
|
6
|
Huang M, Wang FQ, Zhou XH, Yang H and Wang
Y: Hypoglycemic and hypolipidemic properties of polysaccharides
from Enterobacter cloacae Z0206 in KKAy mice. Carbohydr Polym.
117:91–98. 2015. View Article : Google Scholar
|
|
7
|
Chen XD, Wu QX, Zhao J, Su T, Lu YM, Zhang
WN, Wang Y and Chen Y: Immunomodulatory effect of a polysaccharide
fraction on RAW 264.7 macrophages extracted from the wild Lactarius
deliciosus. Int J Biol Macromol. 128:732–739. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding X, Hou YL and Hou WR: Structure
feature and antitumor activity of a novel polysaccharide isolated
from Lactarius deliciosus Gray. Carbohydr Polym. 89:397–402. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou Y, Ding X, Hou W, Song B, Wang T, Wang
F and Zhong J: Immunostimulant activity of a novel polysaccharide
isolated from Lactarius deliciosus (L. ex Fr) Gray. Indian J Pharm
Sci. 75:393–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou YL, Wang M, Zhao DQ, Liu L, Ding X and
Hou W: Effect on macrophage proliferation of a novel polysaccharide
from Lactarius deliciosus (L. ex Fr) Gray. Oncol Lett.
17:2507–2515. 2019.PubMed/NCBI
|
|
11
|
Hou YL, Liu L, Ding X, Zhao D and Hou W:
Structure elucidation, proliferation effect on macrophage and its
mechanism of a new heteropolysaccharide from Lactarius deliciosus
Gray. Carbohydr Polym. 152:648–657. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Y, Ding X, Wang M, Hou Y, Hou W and
Yue C: Structure and antioxidant activity of a novel polysaccharide
derived from Amanita caesarea. Mol Med Rep. 14:3947–3954. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding X, Hou Y, Zhu Y, Wang P, Fu L, Zhu H,
Zhang N, Qin H, Qu W, Wang F and Hou W: Structure elucidation,
anticancer and antioxidant activities of a novel polysaccharide
from Gomphus clavatus Gray. Oncol Rep. 33:3162–3170. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding X, Hou Y, Hou W, Zhu Y, Fu L and Zhu
H: Structure elucidation and anti-tumor activities of water-soluble
oligosaccharides from Lactarius deliciosus (L. ex Fr) Gray.
Pharmacogn Mag. 11:716–723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding X, Feng S, Cao M, Li MT, Tang J, Guo
CX, Zhang J, Sun Q, Yang ZR and Zhao J: Structure characterization
of polysaccharide isolated from the fruiting bodies of Tricholoma
matsutake. Carbohydr Polym. 81:942–947. 2010. View Article : Google Scholar
|
|
16
|
Hou Y, Ding X, Hou W, Zhong J, Zhu H, Ma
B, Xu T and Li J: Anti-microorganism, anti-tumor, and immune
activities of a novel polysaccharide isolated from Tricholoma
matsutake. Pharmacogn Mag. 9:244–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang M, Jiang C, Ma L, Zhang Z, Cao L, Liu
J and Zeng X: Preparation, preliminary characterization and
immunostimulatory activity of polysaccharide fractions from the
peduncles of Hovenia dulcis. Food Chem. 138:41–47. 2013. View Article : Google Scholar
|
|
18
|
Zhou X, Wang H, Wang B, Fu L, Yuan M, Liu
J, Zhou L and Ding C: Characterization and antioxidant activities
of polysaccharides from the leaves of Lilium lancifolium Thunb. Int
J Biol Macromol. 92:148–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao W, Luo Z, Liu D, Ning Z, Yang J and
Ren J: Structure characterization of a novel polysaccharide from
Dictyophora indusiata and its macrophage immunomodulatory
activities. J Agric Food Chem. 63:535–544. 2015. View Article : Google Scholar
|
|
20
|
Jing Y, Huang L, Lv W, Tong H, Song L, Hu
X and Yu R: Structural characterization of a novel polysaccharide
from pulp tissues of Litchi chinensis and its immunomodulatory
activity. J Agric Food Chem. 62:902–911. 2014. View Article : Google Scholar
|
|
21
|
Lee JS, Synytsya A, Kim HB, Choi DJ, Lee
S, Lee J, Kim WJ, Jang S and Park YI: Purification,
characterization and immunomodulating activity of a pectic
polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba
L.). Int Immunopharmacol. 17:858–866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamnev AA, Tugarova AV, Dyatlova YA,
Tarantilis PA, Grigoryeva OP, Fainleib AM and De Luca S:
Methodological effects in Fourier transform infrared (FTIR)
spectroscopy: Implications for structural analyses of
biomacromolecular samples. Spectrochim Acta A Mol Biomol Spectrosc.
193:558–564. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong H, Mao D, Zhai M, Zhang Z, Sun G and
Jiang G: Macrophage activation induced by the polysaccharides
isolated from the roots of Sanguisorba officinalis. Pharm Biol.
53:1511–1515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Leeuwen SS, Kuipers BJH, Dijkhuizen L
and Kamerling JP: Development of a (1)H NMR
structural-reporter-group concept for the analysis of prebiotic
galacto-oligosaccharides of the [β-d-Galp-(1→x)]n-d-Glcp type.
Carbohyd Res. 400:54–58. 2014. View Article : Google Scholar
|
|
25
|
Maity P, Nandi AK, Manna DK, Pattanayak M,
Sen IK, Bhanja SK, Samanta S, Panda BC, Paloi S, Acharya K and
Islam SS: Structural characterization and antioxidant activity of a
glucan from Meripilus giganteus. Carbohydr Polym. 157:1237–1245.
2017. View Article : Google Scholar
|
|
26
|
Liu L, Jia J, Zeng G, Zhao Y, Qi X, He C,
Guo W, Fan D, Han G and Li Z: Studies on immunoregulatory and
anti-tumor activities of a polysaccharide from Salvia miltiorrhiza
Bunge. Carbohydr Polym. 92:479–483. 2013. View Article : Google Scholar
|
|
27
|
Zhu H, Ding X, Hou Y, Li Y and Wang M:
Structure elucidation and bioactivities of a new polysaccharide
from Xiaojin Boletus speciosus Frost. Int J Biol Macromol.
126:697–716. 2019. View Article : Google Scholar
|
|
28
|
Haneef J, Parvathy M, Thankayyan RSK,
Sithul H and Sreeharshan S: Bax translocation mediated
mitochondrial apoptosis and caspase dependent photosensitizing
effect of ficus religiosa on cancer cells. PLoS One. 7:e400552012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Huang J, Wang W, Li Q, Chen Y,
Feng W, Zheng D, Zhao T, Mao G, Yang L and Wu X: Extraction,
purification, characterization and antioxidant activities of
polysaccharides from Cistanche tubulosa. Int J Biol Macromol.
93:448–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Wen XY, Zhang XQ, Pu HM, Kan J and
Jin CH: Extraction, characterization and in vitro antioxidant
activity of polysaccharides from black soybean. Int J Biol
Macromol. 72:1182–1190. 2015. View Article : Google Scholar
|
|
31
|
Liu L, Ding X, Hou Y, Song B, Zhao D and
Hou W: Structural characterization and immunological activity of a
novel hetero-polysaccharide from Lactikporus supharells (Fr) murr.
Latin Am J Pharm. 36:2386–2396. 2017.
|
|
32
|
Gomez Perez M, Fourcade L, Mateescu MA and
Paquin J: Neutral Red versus MTT assay of cell viability in the
presence of copper compounds. Anal Biochem. 535:43–46. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dulger B, Yilmaz F and Gucin F:
Antimicrobial activity of some Lactarius species. Pharm Biol.
40:304–306. 2002. View Article : Google Scholar
|
|
34
|
Fernandes Â, Antonio AL, Barreira J,
Botelho L, Oliveira MB, Martins A and Ferreira I: Effects of gamma
irradiation on the chemical composition and antioxidant activity of
Lactarius deliciosus L. wild edible mushroom. Food Bioproc Technol.
6:2895–2903. 2013. View Article : Google Scholar
|
|
35
|
Dwek RA: Glycobiology: Toward
understanding the function of sugars. Chem Rev. 96:683–720. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan S, Zhang J, Nie W, Zhou W, Jin L, Chen
X and Lu J: Antitumor effects of polysaccharide from Sargassum
fusiforme against human hepatocellular carcinoma HepG2 cells. Food
Chem Toxicol. 102:53–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zong A, Cao H and Wang F: Anticancer
polysaccharides from natural resources: A review of recent
research. Carbohydr Polym. 90:1395–1410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie JH, Liu X, Shen MY, Nie SP, Zhang H,
Li C, Gong DM and Xie MY: Purification, physicochemical
characterisation and anticancer activity of a polysaccharide from
Cyclocarya paliurus leaves. Food Chem. 136:1453–1460. 2013.
View Article : Google Scholar
|
|
39
|
Li S, Gao A, Dong S, Chen Y, Sun S, Lei Z
and Zhang Z: Purification, antitumor and immunomodulatory activity
of polysaccharides from soybean residue fermented with Morchella
esculenta. Int J Biol Macromol. 96:26–34. 2017. View Article : Google Scholar
|
|
40
|
Li QM, Wang JF, Zha XQ, Pan LH, Zhang HL
and Luo JP: Structural characterization and immunomodulatory
activity of a new polysaccharide from jellyfish. Carbohydr Polym.
159:188–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Li S, Wang X, Zhang L and Cheung
PCK: Advances in lentinan: Isolation, structure, chain conformation
and bioactivities. Food Hydrocoll. 25:196–206. 2011. View Article : Google Scholar
|
|
42
|
Bisen PS, Baghel RK, Sanodiya BS, Thakur
GS and Prasad GB: Lentinus edodes: A macrofungus with
pharmacological activities. Curr Med Chem. 17:2419–2430. 2010.
View Article : Google Scholar : PubMed/NCBI
|