Introduction
Lung development is the physiological basis of
breathing. Although, at present, the mortality rate of premature
infants has decreased, the incidence of lung developmental
diseases, such as bronchopulmonary dysplasia (BPD) and respiratory
distress syndrome (RDS) is increasing (1,2).
Lung development is a complex process that extends from the
embryonic period to the fetal period to birth, and involves cell
proliferation, differentiation and morphogenesis (3). The classic molecular pathways
include the Wnt, fibroblast growth factor (FGF) and bone
morphogenetic protein pathways, and the microRNAs (miRNAs/miRs)
involved in regulating lung development include miR-7, let-7,
miR-221, miR-30 and miR-93 (4,5).
A number of circular RNAs (circRNAs) have been
discovered in different kinds of cell lines and species (6). This small RNA is a novel class of
non-coding RNAs characterized by a covalently closed-loop structure
without a 5′-terminal cap or 3′-terminal poly tail, which is
generated through a special type of alternative splicing, termed
backsplicing (7). Generally,
there are three types of circRNA, including exonic, intronic and
retained-intronic circRNA (8).
Accumulating evidence has demonstrated that circRNAs can act as
both an miRNA sponge and RNA blinding protein sponge, which
regulates alternative splicing and gene transcription (8-10).
This suggested that the sponge activity of circRNA affects the
pathway regulation by targeting miRNAs. Furthermore, previous
studies have shown that circRNAs have a spatiotemporal specificity
during species development. It was reported that circRNAs are
differentially expressed during nervous system development in
drosophila (11), porcine embryos
(12) and human fetuses (13). However, the role of circRNAs in
lung development remains unknown.
The 5 stages of lung development consist of the
embryonic (first 13 days), pseudoglandular (13-18 days),
cana-licular (18-20 days), saccular (20 days-full term) and
alveolar (following birth) phases (14). During the canalicular phase,
lamellar bodies begin to emerge, marking a significant point in
lung maturation. During the saccular phase, alveolar ducts and air
sacs form, an event that is critical for postnatal gas exchange
(14). During the alveolar phase
there is complete alveoli formation (14,15). Therefore, in the present study, 3
representative time points [embryonic day (E) 19, E21 and postnatal
(P) day 3] were selected for circRNA high-throughput sequencing. In
addition, 4 time points (E16, E19, E21 and P3) were selected for
reverse transcription-PCR (RT-PCR) verification to provide further
insight for rat lung development. To the best of our knowledge,
circRNA high-throughput sequencing was used for the first time to
perform differential expression profiling of circRNAs during rat
lung development.
Materials and methods
Rat fetal lung tissues
A total of 12 10-week-old healthy pregnant female
Sprague-Dawley rats (270-360 g) obtained from The Animal Center of
Nanjing Medical University, were raised in a specific-pathogen-free
animal facility at 21-25°C, with a humidity 45-65%, a 12 h
light/dark cycle, and ordinary feed and drinking water at The
Animal Center of Nanjing Medical University. The duration of the
gestation period for the rat strain used in the present study was
23 days. The animal use protocol has been reviewed and approved by
The Nanjing Medical University Animal Ethical and Welfare Committee
(approval no. IACUC-1809020). All pregnant SD rats were sacrificed
by cervical dislocation after anesthesia with 2% chloral hydrate
(400 mg/kg), and then all the rat fetuses at E16, E19 and E21 were
sacrificed with direct decapitation, while the rat fetuses at P3
were sacrificed with direct cervical dislocation. The whole fetal
lungs were isolated from the rat fetuses on E16, E19, E21 and P3.
These 4 time points were named as the E16 group, E19 group, E21
group and P3 group. There were 3 pregnant rats in each group
following a random contrast rule. The lung tissues were washed with
normal saline after isolation. In total, 1 sample of fetal lungs
was randomly selected for morphological observation in each group,
E19, E21 and P3. In the E19, E21 and P3 groups, 3 samples of fetal
lungs in each group were mixed to produce 1 sample to be used in
high-throughput sequencing. In the E16, E19, E21 and P3 groups, 10
samples of fetal lungs were used for RT-PCR.
Hematoxylin and eosin staining
The lung tissues were fixed with 4% paraformaldehyde
for 24 h at 4°C, embedded in paraffin, and cut into longitudinal
sections of 4 µm. Then, staining and mounting were
performed, according to the method of hematoxylin and eosin
staining (16). The lung tissues
were observed under a light microscope (DM2500; Leica Microsystems
GmbH; magnifications, ×20 and 40).
Library construction and sequencing
Total RNA was isolated from tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. RNA purity was assessed
using ND-1000 Nanodrop (Thermo Fisher Scientific, Inc.) requiring
A260/280 ≥1.8, A260/A230 ≥2.0. RNA integrity (RIN) was evaluated
using Agilent 2200 TapeStation (Agilent Technologies, Inc.)
requiring RIN ≥7.0. Briefly, ribosomal RNAs were removed from total
RNA using the Epicentre Ribo-Zero rRNA Removal kit (Illumina,
Inc.). Then, RNA was treated with RNase R (Epicentre; Illumina,
Inc.) and fragmented to ~200 bp. Subsequently, the purified RNA
fragments were subjected to first strand and second strand cDNA
synthesis. The RT temperature protocol consisted of the first
strand cDNA synthesis (10 min at 25°C, 15 min at 42°C, 15 min at
70°C and hold at 4°C) and the second strand cDNA synthesis
(incubate in a thermal cycler for 1 h at 16°C, with heated lid set
at 40°C), following adaptor ligation and enrichment with a
low-cycle using the NEBNext® Ultra™ RNA Library Prep kit
for Illumina (NEB; Illumina, Inc.) according to the manufacturer's
protocol. The purified library products were evaluated using the
Agilent 2200 TapeStation and Qubit® 2.0 (Thermo Fisher
Scientific, Inc.) and then sequenced on HiSeq 3000 with 2×150 bp
mode.
Pre-processing of sequencing
reads/quality control
Raw reads were treated with Trimmomatic tools
(V0.36) to remove adapters (17).
Following reads quality control: Scan the read with a 4-base wide
sliding window, cutting when the average quality per base drops
below 15, drop reads which are less than 35% of initiation read
length. Then, the reads quality was inspected using FastQC software
(Version no. 0.11.8; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/)
then output statistical result.
Identification and quantification of
circRNAs
A total of two algorithms, CIRI2 (v2.0.6; https://sourceforge.net/projects/ciri/files/CIRI2/)
and CIRCexplorer2 [V2; (18)],
were used to detect circRNAs. Reads were mapped to human reference
genome GRCh37/hg19 (http://genome.ucsc.edu/) by Burrows-Wheeler-Aligner
(BWA)-MEM [BWA-0.6; (19)] or
Tophat (Bowtie 2; http://ccb.jhu.edu/software/tophat/tutorial.shtml),
respectively. CIRI2 detects the paired chiastic clipping signals
from the mapping information of reads by local alignment with
BWA-MEM and combines with systematic filtering steps to remove
potential false positives. CIRCexplorer2 uses TopHat and
TopHat-Fusion alignment output to detect circRNAs. If a circRNA can
be detected by both methods, it will be considered as an identified
cirRNA. Back-spliced junction reads identified in CIRI2 were
combined and scaled to Reads Per Million mapped reads (bwamem
mapping) to quantify every circRNA. Some previous studies used fold
change >2, P<0.05 (20,21) and a previous study used fold
change >1.5, P<0.05 (22)
to screen the differential expression of circRNAs between two
samples. In the present study, the criteria were defined as fold
change ≥1.5 and P<0.05 to explore as many differentially
expressed circRNAs as possible among three groups.
Bioinformatics analysis
Gene Ontology (GO; http://www.geneontology.org/) and Kyoto Encyclopedia
of Genes and Genomes (KEGG; release 88.0; http://www.genome.jp/) were used to analyze the parent
genes to predict circRNA functions. Each differentially expressed
circRNAs-targeted miRNAs was predicted with miRanda (August 2010
Release; http://www.microrna.org/microrna/home.do), RNAhybrid
(RNAhybrid.2.1; http://bibiserv.techfak.uni-bielefeld.de/download/tools/rnahybrid.html)
and TargetScan (Release 7.2; http://www.targetscan.org) software. A total of four
software packages [TargetScan (Release 7.2; http://www.targetscan.org), miRDB (5.0; http://www.mirdb.org/), miRTarBase (Release 7.0;
http://mirtarbase.mbc.nctu.edu.tw/php/index.php) and
miRWalk (miRWalk.2.0; http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/index.html)]
were used to predict the target genes of miRNAs in a circRNA-miRNA
network. Then, these target genes were subjected to GO and KEGG
analysis.
RT-PCR
Total RNA was isolated from 10 samples (fetal lungs)
in each group using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The RNA quantity control and
concentration were detected using a NanoDrop2000 Spectrophotometer
(Thermo Fisher Scientific, Inc.). Subsequently, 1 µg total
RNA was isolated as aforementioned and was converted into cDNA
using the HiScript® II Q Select RT SuperMix for qPCR
(R232-01; Vazyme) according to the manufacturer's protocol [4
µl 5X HiScript II Select qRT SuperMix, 1 µl Random
hexamers (50 ng/µl) and 1 µg RNA]. The RT reaction
was conducted at 37°C for 15 min and 85°C for 2 min. Next, the
RT-PCR reaction was performed using AceQ® qPCR (Q131-01;
Vazyme). For RT-PCR, 1 µl cDNA was added to 9 µl
master mix, including 5 µl SYBR® Green Master Mix
(Low Rox Premixed; Q131-01; Vazyme), 0.2 µl reverse and
forward primers, and 3.6 µl diethypyro-carbonate water.
Then, the PCR was performed with an ABI 7500 thermal cycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
reaction conditions included an initial step at 95°C for 5 min, and
40 cycles at 90°C for 15 sec and at 60°C for 15 sec, 72°C for 1 min
and final extension at 72°C for 10 min. The primer sequences used
are listed in Table I. PCR was
performed in each plate with GAPDH as an endogenous control. All
primer sequences were designed and synthesized by Guangzhou RiboBio
Co., Ltd. The relative level of each circRNA expression was
calculated using the 2−∆∆Cq method (23).
 | Table IPrimers for reverse
transcription-PCR. |
Table I
Primers for reverse
transcription-PCR.
| Gene name | Primers | Temperature,
°C |
|---|
|
rno_circ:chr7:24777879-24784993 | F:
5′-TTCCAACGCTGAGGACGCT-3′
R: 5′-CGTCTTCAATGTCATAGCCGCT-3′ | 60 |
|
rno_circ:chr14:14620910-14624933 | F:
5′-GTCGGGTATTGTGCTGCTTG-3′
R: 5′-GGTTAAAGTGGGTCTCTGGACA-3′ | 60 |
|
rno_circ:chr3:1988750-1998592 | F:
5′-GACAATGCGGGTGCCAATA-3′
R: 5′-GCGTCCAAGTGGTTGTTCTCT-3′ | 60 |
| GAPDH | F:
5′-GAACGGGAAGCTCACTGG-3′
R: 5′-GCCTGCTTCACCACCTTCT-3′ | 60 |
Statistical analysis
All data are presented as the mean ± SD. All
experiments were repeated independently at least three times. The
data were analyzed using SPSS 17.0 (SPSS, Inc.) and GraphPad Prism
5.0 (GraphPad Software, Inc.) statistical packages. One-way ANOVA
followed by Newman-Keuls was applied to analyze statistical
significance among four groups. P<0.05 was considered to
indicated a statistically significant difference.
Results
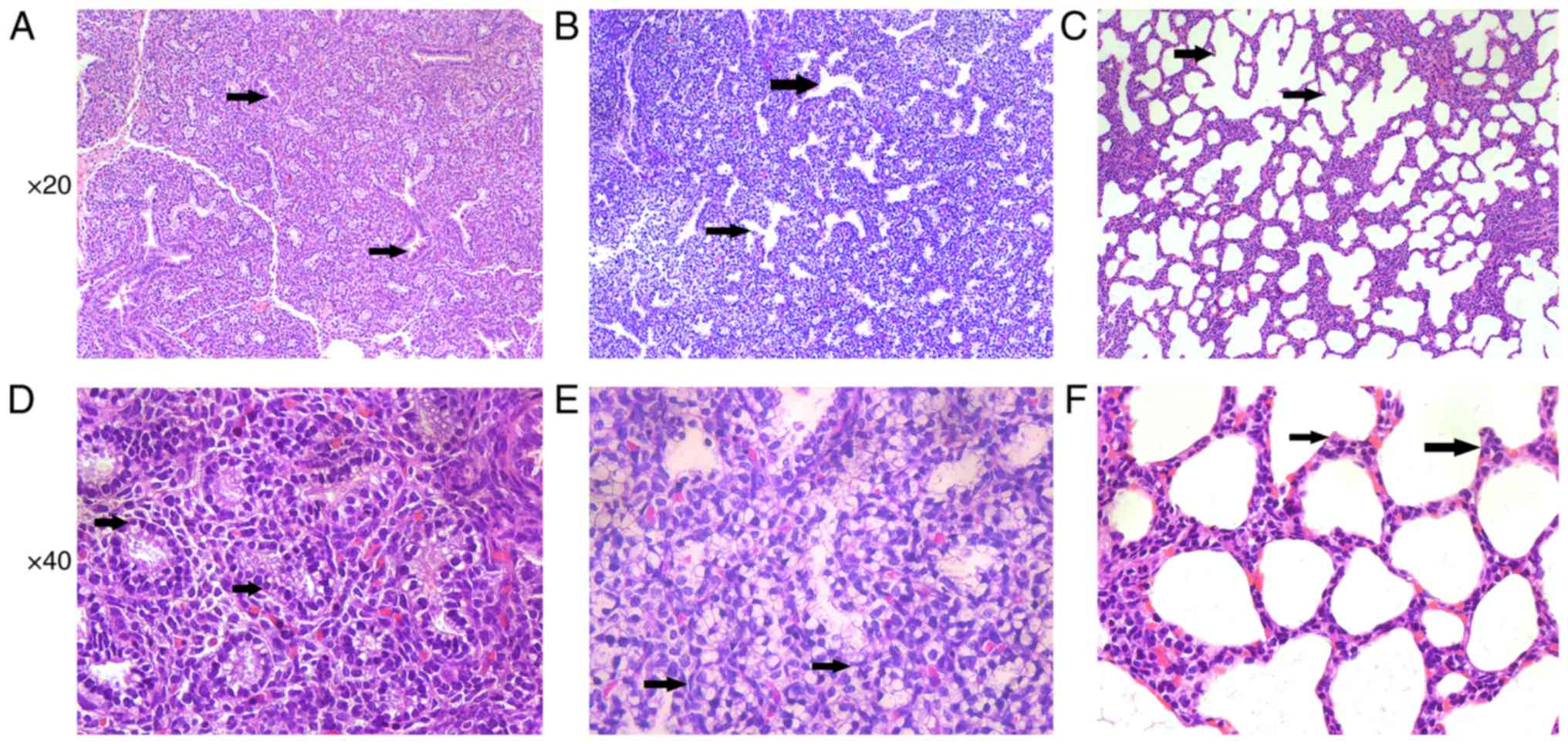
Morphological results of rat lung tissue
at E19, E21 and P3
In the E19 group, the respiratory bronchiolitis and
the alveolar sacs began to take shape. The interstitial tissue
became thinner than before (Fig.
1A). The airway tubes were lined with cuboidal epithelium
(Fig. 1D). In the E21 group, the
airspaces expanded rapidly and the connective tissue between the
airspaces diminished (Fig. 1B),
and the epithelium became flat (Fig.
1E). In the P3 group, the terminal alveoli formed rapidly
(Fig. 1C) and the secondary
septation appeared, which divides alveolar ducts into terminal
alveoli. The double capillary network turned into a single
capillary system (Fig. 1F).
circRNA expression profile during lung
development
The present study examined differentially expressed
circRNAs during rat lung development by high-throughput sequencing
in the E19, E21 and P3 groups. With CIRI2 and CIRC explorer
packages, 9,734, 8,429 and 9,169 overlapped circRNAs were predicted
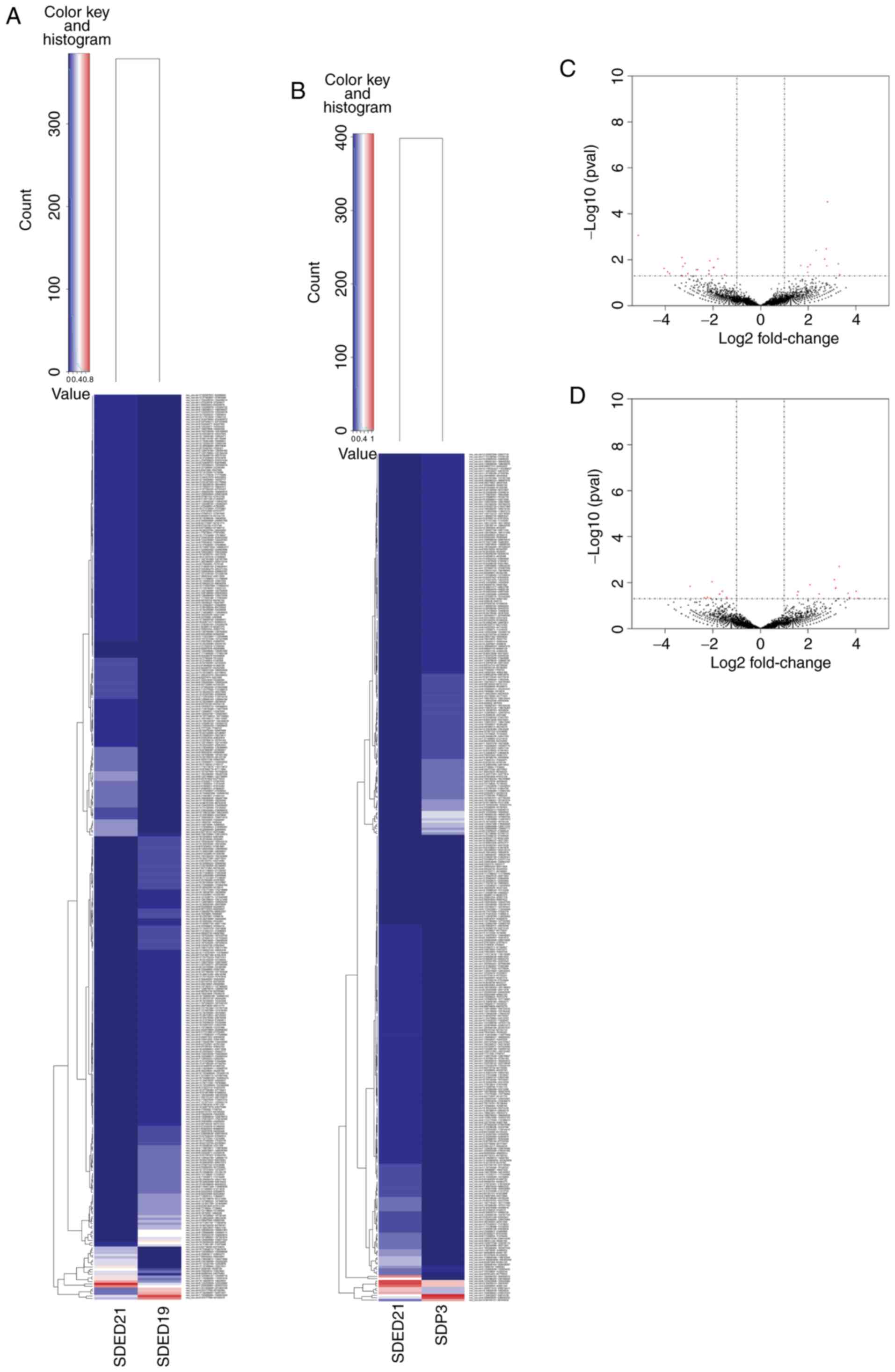
in the E19, E21 and P3 groups, respectively. As illustrated in
Fig. 2A and C, 375 of these
exhibited differentially expressed circRNAs between E19 and E21
groups (fold change ≥1.5; P<0.05; Table SI), among which 200 were
upregulated and the remaining 175 were downregulated in group E21.
Meanwhile, as shown in Fig. 2B and
D, 358 of these exhibited differentially expressed circRNAs
between E21 and P3 (fold change ≥1.5; P<0.05; Table SI), among which 164 were
upregulated and 194 were downregulated in group P3.
From all these differentially expressed circRNAs
(Table SI), a specific criterion
was set for screening circRNAs, which continuously regulate lung
development. The circRNAs must meet the criteria that the
fold-changes of E19 vs. E21 ≥1.5 and E21 vs. P3 ≥1.5. Then, 1
consistently upregulated and 1 consistently downregulated circRNAs
were screened (Table II). In
addition, 4 circRNAs presented first upregulation and then
downregulation (Table III). In
contrast, 1 circRNA was first downregulated and then upregulated
(Table III).
 | Table IISpecific fold-changes of the
consistently up- and downregulated circular RNAs in three groups
(E19, E21 and P3). |
Table II
Specific fold-changes of the
consistently up- and downregulated circular RNAs in three groups
(E19, E21 and P3).
| Name | E19 vs. E21
| E21 vs. P3
| Gene symbol |
|---|
| log2FoldChange | Regulation | log2FoldChange | Regulation |
|---|
|
rno_circ:chr7:24777879-24784993 |
−2.11547721741994 | Down |
−1.54432051622381 | Down | Polr3b |
|
rno_circ:chr19:24859866-24860320 |
2.92599941855622 | Up |
2.05324202127698 | Up | Adgre5 |
 | Table IIISpecific fold-changes of first
increased and then decreased and first decreased and then increased
circular RNAs. |
Table III
Specific fold-changes of first
increased and then decreased and first decreased and then increased
circular RNAs.
| Name | E19 vs. E21
| E21 vs. P3
| Gene symbol |
|---|
| P-value | log2FoldChange | Regulation | P-value | log2FoldChange | Regulation |
|---|
|
rno_circ:chr14:14620910-14624933 | 0.003912883 | 2.3310112 | Up | 0.032264398 | −1.722379793 | Down | Fras1 |
|
rno_circ:chr1:255636891-255637533 | 0.018024097 | 1.685443894 | Up | 0.009200469 | −2.028644787 | Down | Btaf1 |
|
rno_circ:chr3:1988750-1998592 | 0.000002489 | Positive
infinite | Up | 0.0485656156 | −2.096861539 | Down | Ehmt1 |
|
rno_circ:chr4:65761402-65770025 | 0.000015442 | Positive
infinite | Up | 0.014539291 | −2.955108543 | Down | Atp6v0a4 |
|
rno_circ:chr10:16724062-16730140 | 0.027029049 | −2.64385619 | Down | 0.001996808 | 3.313660479 | Up | Crebrf |
RT-PCR of the significantly
differentially expressed circRNAs
When PCR verification was performed, the specified
cycle threshold value of rno_circ:chr19:24859866-24860320,
rno_circ:chr4:65761402-65770025, rno_circ:chr1:255636891-255637533
and rno_circ:chr10:16724062-16730140 could not be detected
successfully at different time points (data not shown). Therefore,
3 out of the 7 significantly differentially expressed circRNAs were
confirmed successfully by RT-PCR in groups E16, E19, E21 and P3.
Among them, 2 circRNAs first presented upregulation and then
downregulation (rno_circ:chr14:14620910-14624933 and
rno_circ:chr3:1988750-1998592; Table III). Another differentially
expressed circRNA is rno_circ:chr7:24777879-24784993 (Table II), which showed consistent
downregulation.
Analysis by RT-PCR revealed that these three
circRNAs exhibited changes in their expression levels, which were
consistent with the results of circRNA high-throughput sequencing.
The relative expressions of these 3 circRNAs are shown in Fig. 3. It is worth noting that
rno_circ:chr7:24777879-24784993 showed a continuously downregulated
tendency at the four continuous time points (E16, E19, E21 and P3;
Fig. 3C). Meanwhile, the relative
expression level of rno_circ:chr3:1988750-1998592 in the E16 group
was significantly lower than E19 (Fig. 3B). Similarly, the relative
expression level of rno_circ:chr14:14620910-14624933 in the E16
group was significantly lower than E21 as well (Fig. 3A).
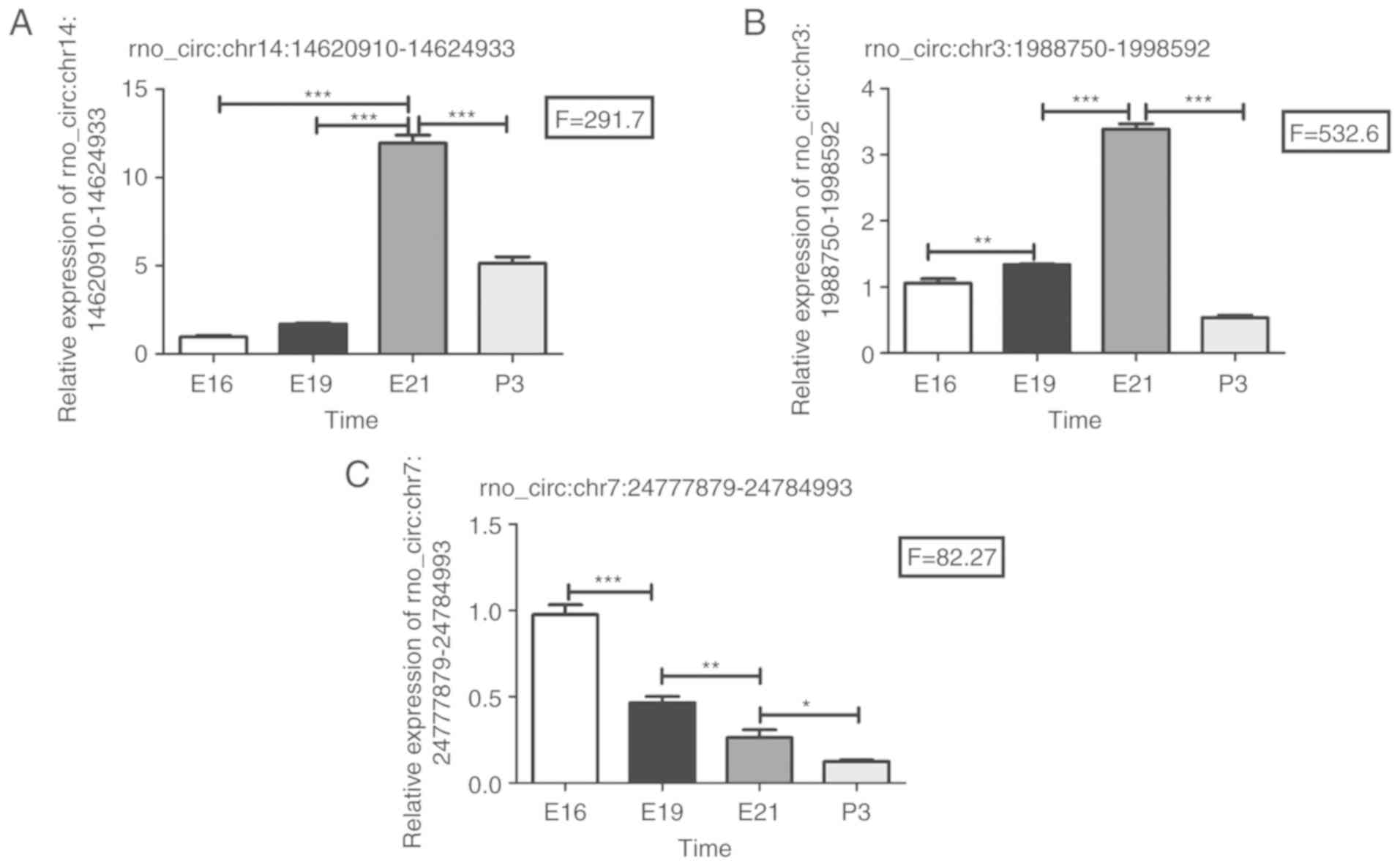 | Figure 3Validation of circular RNA
high-throughput sequencings results by reverse transcription-PCR.
(A) Relative expression of rno_circ:chr14:14620910-14624933 in four
groups (E16, E19, E21 and P3) showed a tendency of first
upregulation and then downregulation. (B) Relative expression of
rno_circ:chr3:1988750-1998592 in four groups (E16, E19, E21 and P3)
showed a tendency of first upregulation and then downregulation.
(C) Relative expression of rno_circ:chr7:24777879-24784993 in four
groups (E16, E19, E21 and P3) showed consistent downregulation.
Triplicate assays were performed from each RNA sample. Data are
normalized using GAPDH as an endogenous control for RNA input.
Error bars represent the mean ± SD. All exhibited statistical
significance of reverse transcription-PCR was tested by one-way
ANOVA followed by Newman-Keuls. *P<0.05,
**P<0.001, ***P<0.0005. E, embryonic
day; P, post-natal day. |
Bioinformatic analysis of differentially
expressed circRNAs
The functions of circRNAs may be associated with
their parent genes, as circRNAs share the same parent genes with
mRNA and compete with them by linear splicing (24,25). Therefore, parent genes of
differentially expressed circRNAs were subjected to KEGG and GO
analysis based on their mRNAs to explore the potential functions of
circRNAs. GO and KEGG analysis suggested that these differentially
expressed circRNAs are involved in biological process, cellular
component, molecular function and several biological pathways, such
as the cGMP-PKG, Hippo, Wnt, regulating pluripotency of stem cells,
TGF-β and PI3K-Akt signaling pathways. Notably, many of these
signaling pathways were associated with lung development closely.
The top 30 KEGG pathways of differentially expressed circRNAs
between E19 and E21 are shown in Fig.
4A. Similarly, the top 30 KEGG pathways which were identified
for the parent genes of the upregulated and downregulated circRNAs
between P3 and E21 are illustrated in Fig. 4B. In addition, the role of these
differentially expressed circRNAs were further investigated by GO
analysis. Fig. 4C and D showed
the top 10 enriched GO terms in molecular function, cellular
component and biological process of differentially expressed
circRNAs among three groups.
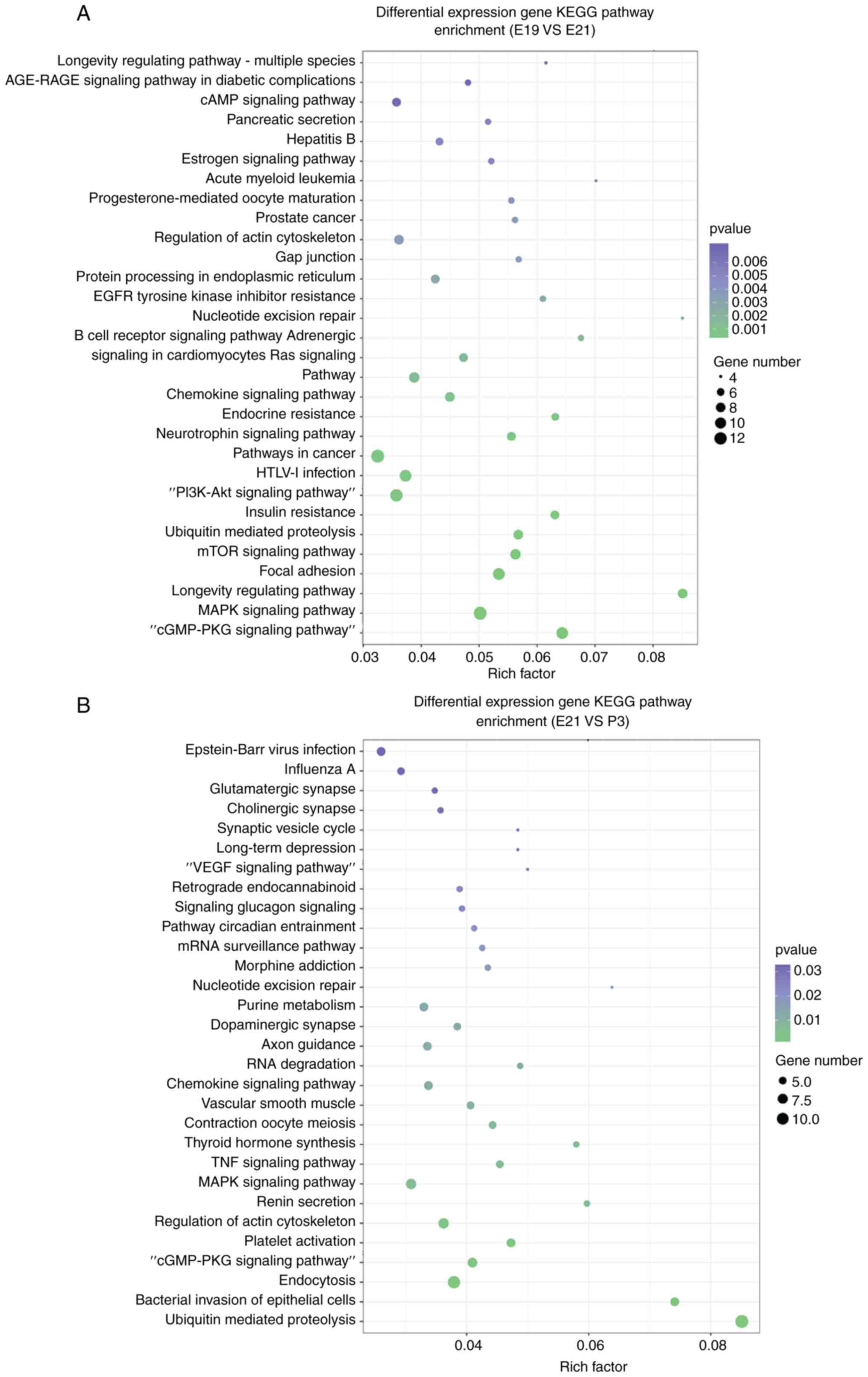 | Figure 4KEGG pathway analysis and GO analysis
of the parent genes of circRNAs. circRNAs with fold change ≥1.5 and
P<0.05 were selected from the differentially expressed circRNAs
in three groups, and the parent genes of these circRNAs were
identified using bioinformatics tools. (A) Top 30 KEGG pathways of
differentially expressed circRNAs in E19 compared with E21 using
KEGG analysis, according to the number of enriched genes. (B) Top
30 KEGG pathways of differentially expressed circRNAs in E21
compared with P3 using KEGG analysis, according to the number of
enriched genes. (C) Top 10 enriched GO terms in the molecular
function, cellular component and biological process categories of
differentially expressed circRNAs in E19 compared with E21. (D) Top
10 enriched GO terms in the molecular function, cellular component
and biological process categories of differentially expressed
circRNAs in E21 compared with P3. KEGG, Kyoto Encyclopedia of Genes
and Genomes; GO, Gene Ontology; circRNAs, circular RNAs; E,
embryonic day; P, post-natal day. |
circRNA-miRNA network
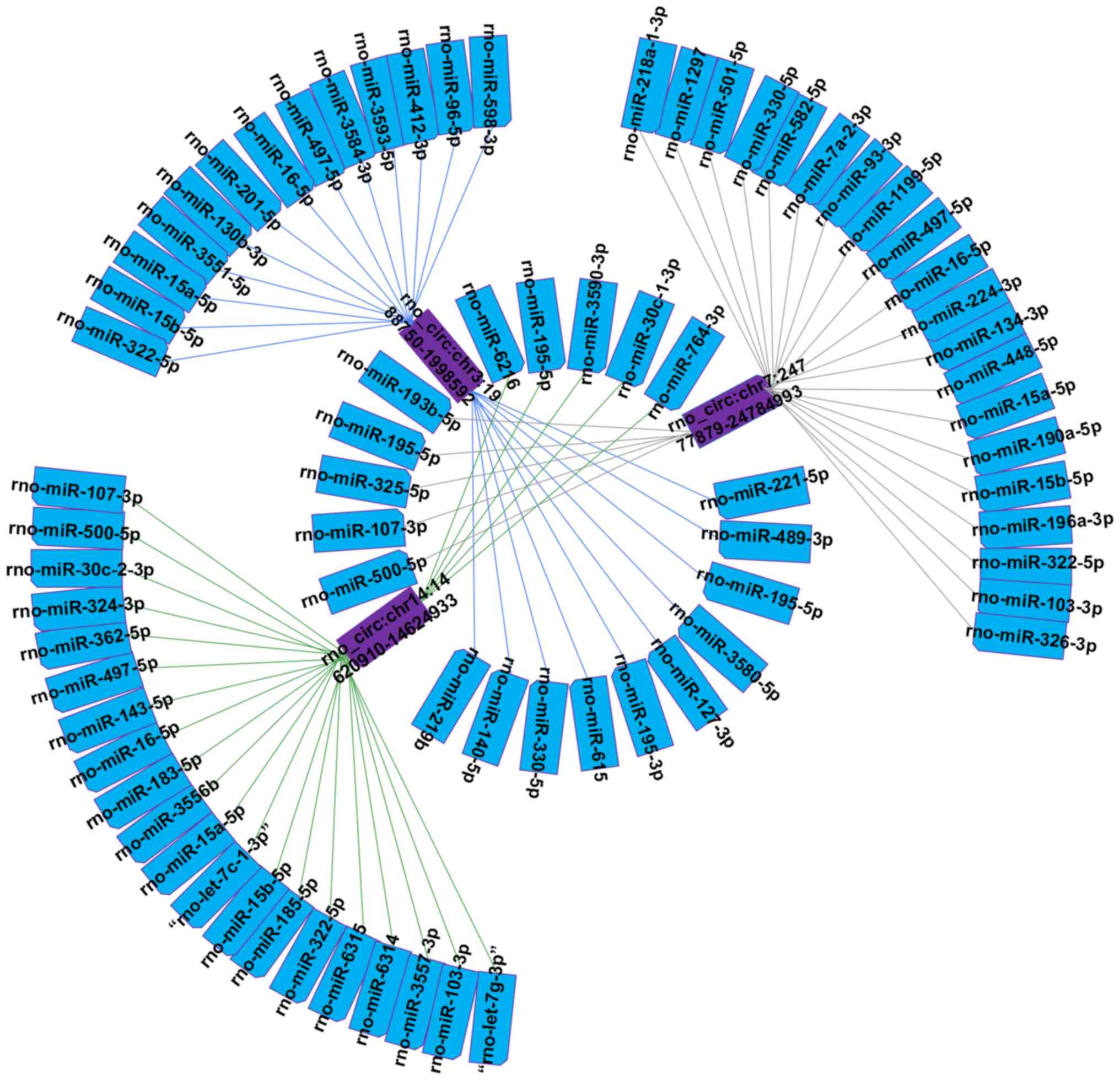
The 3 circRNAs verified by RT-PCR were selected to
construct a representative circRNA-miRNA network (Fig. 5). The downregulated
rno_circ:chr7:24777879-24784993 was predicted to increase the
expression level of 25 miRNAs. Rno_circ:chr14:14620910-14624933 and
rno_circ:chr3:1988750-1998592 were predicted to downregulate 25 and
23 miRNAs, respectively. In particular, it was identified that
rno_circ:chr14:14620910-14624933 can act as a let-7 family sponge.
To explore the respective potential functions of the three
circRNAs, the target genes of these miRNAs were predicted. In
addition, KEGG and GO analysis of miRNAs were conducted to gain
insight into each of the three candidate circRNAs. KEGG analysis
suggested that the 3 circRNAs involved in some signaling pathways,
like Wnt, Hippo, PI3K-Akt, NF-κB and vascular endothelial growth
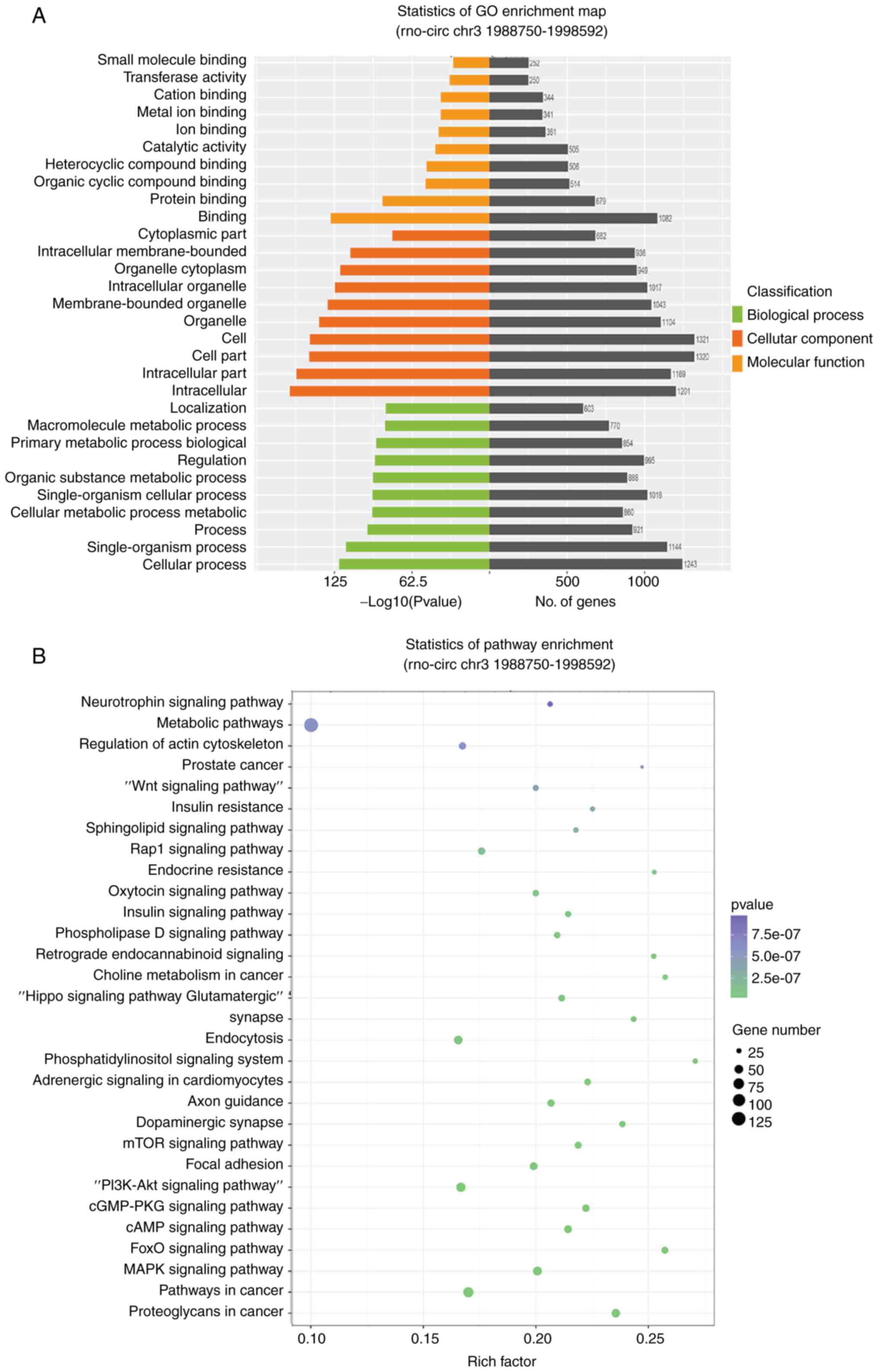
factor (VEGF) signaling pathways. Fig. 6A, C and E showed that the top 10
enriched GO terms of these miRNAs are similar to the analysis of
differentially expressed circRNAs in the three categories.
Additionally, the top 30 KEGG pathways for the three miRNAs are
demonstrated in Fig. 6B, D and
F.
Discussion
In the present study, differentially expressed
circRNAs were identified in fetal lung development for the first
time, to the best of our knowledge. Of these differentially
expressed circRNAs, rno_circ:chr7:24777879-24784993 was
consistently downregulated among the three groups. Although when
PCR verification was performed, the specified cycle threshold value
of 4 circRNAs could not be detected successfully at different time
points. It was hypothesized that there are two possible reasons for
this result. One possibility is that the designed primer might be
defective, and the other possibility might be that the content of
some circRNA was too low to be detected at specific time points.
Following the study by Li et al (26), which examined the function of
circRNAs on the regulation of their parental genes during
transcription, several previous studies reported that the splicing
efficiency of certain genes was negatively correlated with the
level of circRNA, suggesting that there are competitive splicing
mechanisms between circular transcripts and linear processing
(25-27). These findings suggested that
specific circRNAs could regulate their parent genes in both a
negative and a positive manner at different levels (25-27). In the present study, a significant
amount of KEGG and GO terms associated with pulmonary angiogenesis
[NF-κB and VEGF signaling pathways (28,29)] and vascular development [Hippo and
TGF-β signaling pathways (30-32)] were identified. Lung maturation
requires the involvement of various signaling pathways, including
the Hippo, Wnt, TGF-β and PI3K-Akt signaling pathways (30-37).
For example, the Hippo signaling pathway plays a
critical role in lung development (30). Evidence has demonstrated that the
Hippo pathway effector Yap is a key regulator of the formation and
branching of epithelial tubes, and mammalian STE20-like protein
kinase 1/2 during the differentiation of lung epithelial cells
(30,31). At the same time, the TGF-β
signaling pathway interacts with the Hippo pathway to regulate
epithelial-mesenchymal interactions, distal airway formation and
alveolarization (32). In
addition, the Wnt signaling pathway, essential during lung
development, was also identified. Mice with wnt2 knockout were
characterized by decreased cell proliferation and dilated
endothelial vasculature (33).
Additionally, mice with loss of wnt7 succumbed to respiratory
failure due to hypoplastic lungs and abnormal vascular development
(34). The Wnt signaling pathway
has also been shown to cooperate with the Hippo and PI3K-Akt
signaling pathways to regulate epithelial-mesenchymal interactions
that are the basis for fetal lung morphogenesis (35-37). In conclusion, the signaling
pathways identified in the present study play a vital role in lung
development. However, how these circRNAs influence the downstream
signaling pathways remains unknown.
It is widely accepted that circRNAs can act as miRNA
sponges; however, the potential functions of circRNAs are unclear.
As miRNAs regulate a large set of biological processes, the circRNA
sponge activity also affects these processes (10). In addition, several previous
studies have reported that circRNAs could affect the expression of
miRNAs and regulate the signaling pathways in several diseases,
including lung cancer (38-40). Therefore, the miRNAs of these
differentially expressed circRNAs were predicted. Among them were
some specific miRNAs such as cystic fibrosis- [miR-126 (41)], asthma- [miR-221 (42,43)], BPD- [miR-29 (44) and miR-152 (45)] and RDS- linked miRNAs [miR-26a
(46)]. It was hypothesized that
these differentially expressed circRNAs were closely associated
with lung development and diseases. In the circRNA-miRNA network of
three candidate circRNAs, it was observed that
rno_circ:chr7:24777879-24784993 was the sponge of miR-93. miR-93
was differentially expressed in developing mouse embryos, and was
found to promote lung development by regulating VEGF expression and
stem cell differentiation (47).
Similarly, miR-221, closely linked to
rno_circ:chr3:1988750-1998592, was reported to target two Hox genes
known to have important functions in embryonic lung branching
morphogenesis and epithelial cell fate (43). In particular,
rno_circ:chr14:14620910-14624933 can act as a let-7 family sponge.
let-7 and its family members, which were highly conserved across
species in functions and sequence, were originally discovered in
the nematode Caenorhabditis elegans, and regulate cell
proliferation and differentiation (48,49). Numerous previous studies have
reported that let-7 was expressed in lung tissue and directly
regulated RAS expression (49-53). The RAS/mitogen-activated protein
kinase signaling pathway is one of the major downstream targets of
the FGF signaling pathway (54).
The FGF signaling pathway plays a key role in lung development,
including lung bud formation, pulmonary branching morphogenesis and
lung epithelial cell proliferation (55-57). Therefore, it was hypothesized that
these circRNAs were associated with lung development.
To further explore the functions of 3 candidate
circRNAs (rno_circ:chr7:24777879-24784993,
rno_circ:chr14:14620910-14624933 and
rno_circ:chr3:1988750-1998592), circRNA-miRNA network analysis was
performed, in addition to GO and KEGG analysis of the
miRNA-targeting genes. The Wnt, Hippo, TGF-β and PI3K-Akt signaling
pathways were all identified in the KEGG analysis. In addition to
these pathways, the VEGF and NF-κB signaling pathways were also
included, although some pathways were not included in the top 30
KEGG terms.
In the clinical setting, with the development of
neonatology, the survival rate of premature infants is increasing.
However, the incidence of lung development-related diseases, such
as BPD, RDS and cystic fibrosis, have been gradually increasing as
well, severely influencing the prognosis of preterm infants
(58). The lungs of premature
infants with BPD and RDS are characterized by a reduced alveolar
number, thickened septa, malformed pulmonary circulation and lack
of pulmonary surfactant, which, in combination with different risk
factors, ultimately gives rise to lung injury and lung-related
diseases (1,2). Therefore, exploring the
physiopathological mechanisms of lung development is crucial. The
present study identified certain circRNAs possibly associated with
lung development. However, it is necessary for future studies to
carry out homologous database analysis in humans and rats to
validate these predicted miRNAs, target genes and signaling
pathways. Future studies may aim to demonstrate the importance of
selected circRNAs in regulating lung development using knocked out
rat models. Finally, more experimental and clinical data are
required to demonstrate that the selected circRNAs may play a
unique, beneficial and essential role in lung development. A
challenge will be to further delineate the mechanisms of circRNAs,
to further progress the development of therapeutic strategies to
selectively block or enhance these mechanisms to effectively
prevent or treat lung development-related diseases. We hope to
clarify the potential physiopathological mechanism of human lung
development, and then these certain circRNAs could become the
therapeutic target in the future.
In conclusion, the present study is the first study,
to the best of our knowledge, to profile differentially expressed
circRNAs at 3 key time points during rat lung development. In
total, 7 consistently differentially expressed circRNAs were
identified. The circRNA-miRNA interactions were also predicted and
circRNA-miRNA networks were constructed for 3 candidate circRNAs
(rno_circ:chr14:14620910-14624933, rno_circ:ch r3:1988750 -1998592
a nd rno_circ:chr7:24777879-24784993). These results supported that
these novel circRNAs participate in lung development. These
findings may also help clarify the physiopathological mechanisms of
normal rat lung development, and may further provide a
physiopathological basis for lung development-related diseases.
Supplementary Data
Acknowledgments
The authors would like to thank Dr Zhi-Dan Bao
affiliated with The Department of Neonates, Children's Hospital of
Nanjing Medical University for her revision of the manuscript.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81601321) and
Jiangsu Science and Education Talents Program (grant no.
QNRC2016092).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, XYZ and XGZ conceived and designed the
experiments. YS, JP and ZS performed the experiments. YS, JP, ZS,
XC and RC analyzed the data. YS, JP, YY and YXZ drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal use protocol has been reviewed and
approved by The Nanjing Medical University Animal Ethical and
Welfare Committee (approval no. IACUC-1809020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Strueby L and Thebaud B: Advances in
bronchopulmonary dysplasia. Expert Rev Respir Med. 8:327–338. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Condò V, Cipriani S, Colnaghi M, Bellù R,
Zanini R, Bulfoni C, Parazzini F and Mosca F: Neonatal respiratory
distress syndrome: Are risk factors the same in preterm and term
infants? J Matern Fetal Neonatal Med. 30:1267–1272. 2017.
View Article : Google Scholar
|
|
3
|
Johar D, Siragam V, Mahood TH and Keijzer
R: New insights into lung development and diseases: The role of
microRNAs. Biochem Cell Biol. 93:139–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herriges M and Morrisey EE: Lung
development: Orchestrating the generation and regeneration of a
complex organ. Development. 141:502–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ameis D, Khoshgoo N, Iwasiow BM, Snarr P
and Keijzer R: MicroRNAs in lung development and disease. Paediatr
Respir Rev. 22:38–43. 2017.PubMed/NCBI
|
|
6
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebbesen KK, Hansen TB and Kjems J:
Insights into circular RNA biology. RNA Biol. 14:1035–1045. 2017.
View Article : Google Scholar :
|
|
8
|
Zhang ZC, Guo XL and Li X: The novel roles
of circular RNAs in metabolic organs. Genes Dis. 5:16–23. 2017.
View Article : Google Scholar
|
|
9
|
Wilusz JE: A 360° view of circular RNAs:
From biogenesis to functions. Wiley Interdiscip Rev RNA. 9. pp.
e14782018, View Article : Google Scholar
|
|
10
|
Kulcheski FR, Christoff AP and Margis R:
Circular RNAs are miRNA sponges and can be used as a new class of
biomarker. J Biotechnol. 238:42–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Westholm JO, Miura P, Olson S, Shenker S,
Joseph B, Sanfilippo P, Celniker SE, Graveley BR and Lai EC:
Genome-wide analysis of drosophila circular RNAs reveals their
structural and sequence properties and age-dependent neural
accumulation. Cell Rep. 9:1966–1980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Venø MT, Hansen TB, Venø ST, Clausen BH,
Grebing M, Finsen B, Holm IE and Kjems J: Spatio-temporal
regulation of circular RNA expression during porcine embryonic
brain development. Genome Biol. 16:2452015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szabo L, Morey R, Palpant NJ, Wang PL,
Afari N, Jiang C, Parast MM, Murry CE, Laurent LC and Salzman J:
Statistically based splicing detection reveals neural enrichment
and tissue-specific induction of circular RNA during human fetal
development. Genome Biol. 16:1262015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mullassery D and Smith NP: Lung
development. Semin Pediatr Surg. 24:152–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Kai G, Pu XD, Qing K, Guo XR and
Zhou XY: Expression profile of microRNAs in fetal lung development
of sprague-dawley rats. Int J Mol Med. 29:393–402. 2012.
|
|
16
|
Anderson J: An introduction to routine and
special staining. 2012
|
|
17
|
Bolger AM, Lohse M and Usadel B:
Trimmomatic: A flexible trimmer for illumina sequence data.
Bioinformatics. 30:2114–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao Y, Wang J and Zhao F: CIRI: An
efficient and unbiased algorithm for de novo circular RNA
identification. Genome Boil. 16:42015. View Article : Google Scholar
|
|
19
|
Li H and Durbin R: Fast and accurate short
read alignment with burrows-wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dang Y, Ouyang X, Zhang F, Wang K, Lin Y,
Sun B, Wang Y, Wang L and Huang Q: Circular RNAs expression
profiles in human gastric cancer. Sci Rep. 7:90602017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Li J, Sun H, Kong Z, Yan G, Wang X,
Wang Y, Wen X, Liu Y, Zheng XH, et al: High-throughput data of
circular RNA profiles in human temporal cortex tissue reveals novel
insights into temporal lobe epilepsy. Cell Physiol Biochem.
45:677–691. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Zhu MC, Kalionis B, Wu JZ, Wang
LL, Ge HY, Chen CC, Tang XD, Song YL, He H and Xia SJ:
Characteristics of circular RNA expression in lung tissues from
mice with hypoxiainduced pulmonary hypertension. Int J Mol Med.
42:1353–1366. 2018.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Huang Li Z, Bao C, Chen C, Lin L, Wang M,
Zhong X, Yu G, Hu B, Dai WL, et al: Corrigendum: Exon-intron
circular RNAs regulate transcription in the nucleus. Nat Struct Mol
Biol. 24:1942017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with Pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Li Z, Bao C, Chen C, Lin L, Wang M,
Zhong X, Yu G, Hu B, Dai WL, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Boil.
22:256–264. 2015. View Article : Google Scholar
|
|
27
|
Hsiao KY, Sun HS and Tsai SJ: Circular
RNA-New member of noncoding RNA with novel functions. Exp Boil Med
(Maywood). 242:1136–1141. 2017. View Article : Google Scholar
|
|
28
|
Alvira CM: Nuclear factor-kappa-B
signaling in lung development and disease: One pathway, numerous
functions. Birth Defects Res A Clin Mol Teratol. 100:202–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Woik N and Kroll J: Regulation of lung
development and regeneration by the vascular system. Cell Mol Life
Sci. 72:2709–2718. 2018. View Article : Google Scholar
|
|
30
|
Mahoney JE, Mori M, Szymaniak AD, Varelas
X and Cardoso WV: The hippo pathway effector Yap controls
patterning and differentiation of airway epithelial progenitors.
Dev Cell. 30:137–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin C, Yao E and Chuang PT: A conserved
MST1/2-YAP axis mediates Hippo signaling during lung growth. Dev
Biol. 403:101–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saito A and Nagase T: Hippo and TGF-β
interplay in the lung field. Am J Physiol Lung Cell Mol Physiol.
309:L756–L767. 2015.PubMed/NCBI
|
|
33
|
Goss AM, Tian Y, Tsukiyama T, Cohen ED,
Zhou D, Lu MM, Yamaguchi TP and Morrisey EE: Wnt2/2b and
beta-catenin signaling are necessary and sufficient to specify lung
progenitors in the foregut. Dev Cell. 17:290–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shu W, Jiang YQ, Lu MM and Morrisey EE:
Wnt7b regulates mesenchymal proliferation and vascular development
in the lung. Development. 129:4831–4842. 2002.PubMed/NCBI
|
|
35
|
Zhang M, Shi J, Huang Y and Lai L:
Expression of canonical WNT/beta-CATENIN signaling components in
the developing human lung. BMC Dev Biol. 12:212012. View Article : Google Scholar
|
|
36
|
Moura RS, Carvalho-Correia E, daMota P and
Correia-Pinto J: Canonical Wnt signaling activity in early stages
of chick lung development. PLoS One. 9:e1123882014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Volckaert T and De Langhe SP: Wnt and FGF
mediated epithelial-mesenchymal crosstalk during lung development.
Dev Dyn. 244:342–366. 2015. View Article : Google Scholar
|
|
38
|
Yao Y, Hua Q and Zhou Y: CircRNA
has_circ_0006427 suppresses the progression of lung adenocarcinoma
by regulating miR-6783-3p/DKK1 axis and inactivating
Wnt/beta-catenin signaling pathway. Biochem Biophys Res Commun.
508:37–45. 2019. View Article : Google Scholar
|
|
39
|
Chen D, Ma W, Ke Z and Xie F: CircRNA
has_circ-100395 regulates miR-1228/TCF21 pathway to inhibit lung
cancer progression. Cell Cycle. 17:2080–2090. 2018. View Article : Google Scholar
|
|
40
|
Han J, Zhao G, Ma X, Dong Q, Zhang H, Wang
Y and Cui J: CircRNA circ-BANP-mediated miR-503/LARP1 signaling
contributes to lung cancer progression. Biochem Biophys Res Commun.
503:2429–2435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oglesby IK, Bray IM, Chotirmall SH,
Stallings RL, O'Neill SJ, McElvaney NG and Greene CM: miR-126 is
downregulated in cystic fibrosis airway epithelial cells and
regulates TOM1 expression. J Immunol. 184:1702–1709. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao D, Zhuang N, Ding Y, Kang Y and Shi
L: MiR-221 activates the NF-κB pathway by targeting A20. Biochem
Biophys Res Commun. 472:11–18. 2016. View Article : Google Scholar
|
|
43
|
Mujahid S, Nielsen HC and Volpe MV:
MiR-221 and miR-130a regulate lung airway and vascular development.
PLoS One. 8:e559112013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dong J, Carey WA, Abel S, Collura C, Jiang
G, Tomaszek S, Sutor S, Roden AC, Asmann YW, Prakash YS and Wigle
DA: MicroRNA-mRNA interactions in a murine model of
hyperoxia-induced bronchopulmonary dysplasia. BMC Genomics.
13:2042012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu YT, Chen WJ, Hsieh WS, Tsao PN, Yu SL,
Lai CY, Lee WC and Jeng SF: MicroRNA expression aberration
associated with bronchopulmonary dysplasia in preterm infants: A
preliminary study. Respir Care. 58:1527–1535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang XQ, Zhang P, Yang Y, Qiu J, Kan Q,
Liang HL, Zhou XY and Zhou XG: Regulation of pulmonary surfactant
synthesis in fetal rat type II alveolar epithelial cells by
microRNA-26a. Pediatr Pulmonol. 49:863–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Long J, Wang Y, Wang W, Chang BH and
Danesh FR: Identification of microRNA-93 as a novel regulator of
vascular endothelial growth factor in hyperglycemic conditions. J
Boil Chem. 285:23457–23465. 2010. View Article : Google Scholar
|
|
48
|
Roush S and Slack FJ: The let-7 family of
microRNAs. Trends Cell Biol. 18:505–516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Büssing I, Slack FJ and Grosshans H: let-7
microRNAs in development, stem cells and cancer. Trends Mol Med.
14:400–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mondol V and Pasquinelli AE: Let's make it
happen: The role of let-7 microRNA in development. Curr Top Dev
Biol. 99:1–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Johnson CD, Esquela-Kerscher A, Stefani G,
Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J,
Shingara J, et al: The let-7 microRNA represses cell proliferation
pathways in human cells. Cancer Res. 67:7713–7722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS Is regulated by the let-7 MicroRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shinya M, Koshida S, Sawada A, Kuroiwa A
and Takeda H: Fgf signalling through MAPK cascade is required for
development of the subpallial telencephalon in zebrafish embryos.
Development. 128:4153–4164. 2001.PubMed/NCBI
|
|
55
|
Min H, Danilenko DM, Scully SA, Bolon B,
Ring BD, Tarpley JE, DeRose M and Simonet WS: Fgf-10 is required
for both limb and lung development and exhibits striking functional
similarity to Drosophila branchless. Genes Dev. 12:3156–3161. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cardoso WV and Lü J: Regulation of early
lung morphogenesis: Questions, facts and controversies.
Development. 133:1611–1624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Park WY, Miranda B, Lebeche D, Hashimoto G
and Cardoso WV: FGF-10 is a chemotactic factor for distal
epithelial buds during lung development. Dev Biol. 201:125–134.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Colin AA, McEvoy C and Castile RG:
Respiratory morbidity and lung function in preterm infants of 32 to
36 weeks' gestational age. Pediatrics. 126:115–128. 2010.
View Article : Google Scholar : PubMed/NCBI
|