Introduction
Biliary cancer (BC) is an aggressive neoplasm with
high mortality. Although the incidence of BC is relatively low, it
is increasing (1,2). Based on its anatomical origin, BC
can be categorized as intrahepatic or extrahepatic
cholangiocarcinoma (CCA), or gallbladder cancer (3). The treatment options for BC include
surgery, chemotherapy and radiation therapy (4). Surgery is the most curative therapy
for BC, but most patients present an advanced disease stage at
diagnosis and are not suitable for surgical resection (5). Furthermore, numerous patients
exhibit recurrent BC even following complete surgical resection
(4). Several drugs are now used
for adjuvant or systemic chemotherapy in patients with advanced or
recurrent BC (6); a regimen based
on the combination of gemcitabine and cisplatin is regarded as a
first-line treatment for patients with advanced BC. Studies have
shown a survival benefit for patients with BC after receiving
chemotherapy (7,8). However, owing to its heterogeneity
and aggressiveness, the overall 5-year survival rate remains low
(9). Therefore, there is an
urgent requirement to develop novel therapeutic medicines for BC
treatment.
Reactive oxygen species (ROS) are active
oxygen-containing molecules that have long been considered as
by-products of cellular metabolism (10,11). ROS can be produced by
mitochondrial respiration, the NADPH oxidase complex and other
biochemical reactions (6,10). Excessive ROS production may be
harmful to cells, resulting in oxidative damage to DNA, proteins
and lipids, as well as genetic instability (11). Elevated ROS levels are often
observed in cancer cells (12),
which may be due to the compromised ROS-scavenging ability of these
cells (13). Increased ROS levels
can drive abnormal proliferation of cancer cells. Simultaneously,
elevated ROS levels increase the sensitivity of these cancer cells
to cell death, making them more vulnerable to ROS induction
(14). Therefore, a strategy to
induce anticancer effects is increasing ROS levels to selectively
kill cancer cells without causing damage to normal cells (9,15).
Recently, ROS-inducing drugs have been investigated to target
various cancer cells, including colon cancer (16), prostate cancer (17), pancreatic cancer (18) and glioblastoma (19) cells. Due to the heterogeneity of
BC, drugs targeting the signaling pathways of BC cells are not
currently available. Increased ROS production has been reported in
BC cells (20). Thus, it is
important to develop drugs that can induce ROS levels to interfere
with the redox balance in BC cells.
Piperlongumine (PL) is a natural product isolated
from the long pepper, Piper longum L. PL induces high levels
of ROS production (21). It
possesses selective anticancer activity in colon, ovarian,
prostate, breast, pancreatic, head and neck, and renal cancers via
multiple signaling pathways, including the p38/JNK, MAPK-C/EBO
homologous protein and NF-κB signaling pathways (20,22-24), causing apoptosis or autophagy in
cancer cells. However, whether PL can induce autophagy in BC cells
is yet to be determined. In this study, the cytotoxic effects of PL
in BC cells were investigated. In addition, autophagy in PL-treated
cells was assessed, and the underlying signaling pathways induced
by PL treatment were explored.
Materials and methods
Cell lines and cell culture
The human biliary epithelial tumor cell line HuCCT-1
and the gallbladder carcinoma cell line OCUG-1 were obtained from
the Japanese Collection of Research Bioresources Cell Bank. HuCCT-1
and OCUG-1 cells were cultured in RPMI 1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) and Dulbecco's Modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.), respectively, both
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), and antibiotic and antimycotic solution
(penicillin, 10 U/ml; streptomycin sulfate, 10 μg/ml;
amphotericin B, 25 ng/ml). These cell lines were tested for the
absence of mycoplasma contamination. The cells were incubated in a
humidified atmosphere containing 5% CO2 at 37°C.
Reagents and antibodies
PL was purchased from Cayman Chemical Company.
Proteins were extracted with M-PER mammalian protein extraction
reagent buffer (Thermo Fisher Scientific, Inc.) for western
blotting. Protease inhibitor was purchased from EMD Millipore.
Primary antibodies for caspase 3 (polyclonal, rabbit anti-human;
1:1,500; cat. no. 9662S), PARP (monoclonal, rabbit anti-human;
1:1,000; cat no. 9532S), cdc25C (monoclonal, rabbit anti-human;
1:1,000; cat. no. 4688S), cdc2 (monoclonal, rabbit anti-human;
1:1,000; cat. no. 9116S), cyclin D1 (monoclonal, mouse anti-human;
1:2,000; cat. no. 2926S), cyclin-dependent kinase (CDK)2
(monoclonal, rabbit anti-human; 1:1,000; cat. no. 2546S), P21
(monoclonal, rabbit anti-human; 1:1,000; cat. no. 2947S), P27
(monoclonal, rabbit anti-human; 1:1,000; cat. no. 3688S),
phosphorylated (p)-Erk1/2 [monoclonal, rabbit anti-human
(Thr202/Tyr204); 1:1,000; cat. no. 4377S], and Erk1/2 (monoclonal,
rabbit anti-human; 1:1,000; cat. no. 4695S) were all purchased from
Cell Signaling Technology, Inc. Antibodies against cyclin B1
(polyclonal, rabbit anti-human; 1:1,000; cat. no. GTX100911),
cyclin A2 (polyclonal, rabbit anti-human; 1:1,000; cat. no.
GTX103042), cyclin E1 (polyclonal, rabbit anti-human; 1:1,000; cat.
no. GTX103045) and CDK4 (poly-clonal, rabbit anti-human; 1:1,000;
cat. no. GTX102993) were obtained from GeneTex, Inc. The antibody
against autophagy-related protein, -Microtubule-associated protein
1A/1B light chain 3B (LC-3) antibody (polyclonal, rabbit
anti-human; 1:1,000; cat. no. AP1802A) was obtained from Abgent,
Inc. β-actin or GAPDH expression was used as an internal control
for western blotting. The antibody against β-actin (monoclonal,
mouse anti-human; 1:7,500; cat. no. sc-47778) was purchased from
Santa Cruz Biotechnology, Inc. The antibody against GAPDH
(monoclonal, rabbit anti-human; 1:1,000; cat. no. 2118S) was
purchased from Cell Signaling Technology, Inc.
N-acetyl-L-cysteine (NAC) and
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA)
were purchased from Sigma-Aldrich (Merck KGaA). PD98059 (cat. no.
9900; Cell Signaling Technology, Inc.), an inhibitor of
mitogen-activated protein kinase (MAPK) kinase (MEK1) activation
and the MAPK cascade, was used in the experiment. For experiments
involving this inhibitor, HuCCT-1 cells were pretreated in the
presence or absence of PD98059 (20 or 30 μM) at 37°C for 1 h
in a humidified incubator, followed by PL treatment (5 or 7.5
μM for western blotting; 10 or 20 μM for ROS
analysis) for 24 h. Cells were then harvested for analyses.
ROS analysis
To determine the ROS level in BC cells,
1×106 cells were seeded in 6-well plates. The cells were
pretreated with NAC (3 mM) or DMSO in a humidified incubator at
37°C for 1 h. Then, the cells were treated with 10 μM
CM-H2DCFDA, incubated at 37°C in the dark for 30 min.
Cells were treated with various concentrations of PL (10 or 20
μM for HuCCT-1 cells; 30 or 60 μM for OCUG-1 cells)
in a humidified incubator at 37°C for 1 h. After washing the cells,
cells were trypsinized and the ROS level was determined by using
flow cytometry (FACSCanto II; BD Biosciences) and analyzed by BD
FACSDiva software (v6.1.3; BD Biosciences).
Cell viability analysis and colony
formation assay
Cell viability was determined by using a Cell
Counting Kit-8 (CCK-8) proliferation assay (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's protocols. The absorbance
values were read at 490 nm at 2 h following the addition of CCK-8
reagent. Briefly, 1×104 HuCCT-1 cells were seeded in
96-well plates, pretreated with NAC as aforementioned and then
treated with 2.5, 5 or 10 μM PL, and incubated for 24 and 48
h at 37°C in a humidified incubator, at which point 100 μl
CCK-8 reagent (mixed 1:20 in medium) was added to each well. The
values of vehicle-treated cells were used for normalization. Three
independent experiments were conducted. The assay protocol for
OCUG-1 cells was the same, except for the concentration of PL (5,
10 or 20 μM). For the colony formation assay,
1×103 cells were seeded in 6-well plates. At day 7 after
cell plating, the cells were washed and fixed with 10% formaldehyde
at 25°C for 10 min, and stained with 0.05% crystal violet
(Sigma-Aldrich; Merck KGaA) at 25°C for 15 min. The colonies were
scanned and analyzed with ImageJ software (FiJi-win32; National
Institutes of Health).
Cell cycle analysis
For determination of cell cycle profile,
1×105 cells were seeded in 6-well plates. After serum
starvation for 24 h, the cells were treated with PL (5 or 10
μM for HuCCT-1 cells; 15 or 30 μM for OCUG-1 cells)
and/or NAC as aforementioned and harvested at 24 and 48 h. The
cells were then fixed by using 100% methanol for 24 h at 4°C.
Propidium iodide (PI; 0.05 mg/ml) solution containing RNase was
added to the methanol-fixed cells. The cells were then incubated at
25°C in the dark for 30 min. The DNA content was analyzed by
performing flow cytometry to determine the percentage of cells in
each phase of the cell cycle using Modfit LT 3.3 cell cycle
analysis software (Verity Software House).
Detection of apoptosis by flow cytometric
analysis
The percentage of apoptotic cells was determined
after PL treatment. The cells were treated with PL for 24 and 48 h,
harvested and subjected to Annexin V and PI staining (5 μl
Annexin V-FITC and 5 μl PI in 500 μl binding buffer)
at 25°C for 5 min in the dark using an apoptosis detection kit
(BioVision, Inc.). Apoptotic cells were determined using flow
cytometry (FACSCanto II; BD Biosciences) and analyzed using BD
FACSDiva software (v6.1.3). The apoptotic ratio was calculated
based on the percentage early + late apoptotic cells.
Cell lysate preparation and western
blotting
Cells were lysed and extracted by using M-PER
mammalian protein extraction reagent (Thermo Fisher Scientific,
Inc.) containing protease inhibitor cocktail (Cell Signaling
Technology, Inc.). Bio-Rad Protein Assay reagent (Bio-Rad
Laboratories, Inc.) was used to determine the concentration of
extracted protein. Protein (40 μg/lane) was subjected to
12.5% SDS-PAGE and electrotransferred onto a polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc.). The membrane was
blocked by incubation with 5% skim milk (Sigma-Aldrich; Merck KGaA)
at 25°C for 1 h, followed by probing with specific primary
antibodies at 4°C for 16 h. The membrane was then incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000;
cat. no. 111-035-003) or goat anti-mouse IgG (1:5,000; cat. no.
115-035-003) secondary antibodies (Jackson ImmunoResearch
Laboratories, Inc.) for 2 h at 25°C. Protein expression was
detected using an enhanced chemiluminescence horseradish peroxidase
substrate detection kit (cat. no. WBKLS0500; EMD Millipore) and
quantified using a UVP BioSpectrum 800 Imaging System (UVP,
LLC).
Immunofluorescence assay
HuCCT-1 cells cultured on coverslips were fixed with
ice-cold acetone and methanol (2:1) at -20°C for 30 min and were
then blocked with 5% normal horse serum (Gibco; Thermo Fisher
Scientific, Inc.) in phosphate-buffered saline (PBS) for 30 min at
25°C. The cells were then washed with PBS and incubated for 16 h at
4°C with LC-3 antibody (1:100 in 5% normal horse serum). After
washing with PBS, the cells were incubated with goat ant-rabbit IgG
secondary antibody conjugated with Alexa Fluor® 488
(1:7,500; cat. no. A-11008; Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at 25°C. Next, the cells were washed again with PBS
and stained with 1 μg/ml DAPI in PBS at 25°C for 5 min. The
coverslips were mounted with anti-fading solution (Sigma-Aldrich;
Merck KGaA) and examined under fluorescence microscopy
(magnification, 200; Olympus 1X81; Olympus Corporation).
Statistical analysis
Statistical significance was determined using the
GraphPad Prism 5.0 software (GraphPad Software, Inc.). The data
were analyzed using two-way analysis of variance followed by
Bonferroni post hoc test for multiple comparisons. All in
vitro experiments were repeated three times and the results are
presented as means ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
PL exhibits growth inhibitory activity
against BC cells, which is attenuated by a ROS inhibitor
To determine the inhibitory effects of PL on BC cell
proliferation, HuCCT-1 and OCUG-1 cells were treated with different
concentrations of PL for 24 and 48 h. Cell survival rates were
analyzed using a CCK-8 assay. PL treatment produced a significant
cytotoxic effect on both HuCCT-1 (Fig. 1A) and OCUG-1 (Fig. 1B) cells. HuCCT-1 exhibited greater
sensitivity to PL treatment, with IC50 values of 24.8
and 4.2 μM at 24 and 48 h, respectively. The IC50
of PL for OCUG-1 cells at 24 and 48 h was 22.2 and 13.8 μM,
respectively. Cell proliferation was then evaluated using a
clonogenic assay. As shown in Fig.
1C and D, PL treatment decreased the colony number in a
concentration-dependent manner, and HuCCT-1 cells exhibited a more
pronounced response to PL treatment than OCUG-1 cells.
It was then determined whether the cytotoxicity of
PL to HuCCT-1 and OCUG-1 cells was due to an imbalance in redox
status. ROS levels were measured via flow cytometric analysis in
PL-treated cells in the presence or absence of the ROS scavenger
NAC. The data from cytometric analysis demonstrated that PL
treatment increased ROS levels in a concentration-dependent manner
(Fig. S1). Furthermore, the
reduction in ROS levels was associated with the rescue of cell
death in NAC-treated cells (Fig.
1). The reversal of cell death by NAC showed a significant
difference, most notably in high-concentration PL-treated cells and
those treated with PL for a long duration. Collectively, these data
suggested that PL exhibits substantial cytotoxicity by interfering
with the redox balance in HuCCT-1 and OCUG-1 cells.
PL interferes with cell cycle progression
in HuCCT-1 and OCUG-1 cells
To determine the mechanism underlying the
antiproliferative effects of PL, the cell cycle distribution
profiles of HuCCT-1 and OCUG-1 cells following PL treatment were
determined via flow cytometric analysis. As shown in Fig. 2A, HuCCT-1 cells showed G2/M phase
arrest after PL treatment. The G2/M cell distribution was increased
from 20.8±2.8 and 14.3±1.1% in the vehicle control group to
45.4±6.8 and 30.3±7.0% in 5 μM PL-treated HuCCT-1 cells
after treatment for 24 and 48 h, respectively. In contrast, OCUG-1
cells exhibited G0/G1 phase arrest after PL treatment (Fig. 2B). The G0/G1 cell distribution
increased from 46.8±1.5 and 63.3±0.4% in the vehicle control group
to 87.5±0.62 and 75.1±1.5% in 15 μM PL-treated OCUG-1 cells
after treatment for 24 and 48 h, respectively. Similar cell cycle
arrest was observed in 10 and 30 μM PL-treated HuCCT-1 and
OCUG-1 cells. Pretreatment with NAC partially reversed the cell
cycle arrest induced by PL in both HuCCT-1 and OCUG-1 cells.
Additionally, an increase in the sub-G1 cell population was
observed with higher PL concentrations and longer exposure time (48
h). Pretreatment with NAC decreased the sub-G1 cell population
induced by PL in both HuCCT-1 and OCUG-1 cells. These data
suggested that cell cycle arrest was associated with the
antiproliferative effects induced by PL treatment in HuCCT-1 and
OCUG-1 cells.
To further verify the induction of cell cycle arrest
by PL, the expression of cell cycle-associated checkpoint proteins
was analyzed via western blotting. Cdc25C is a key protein in
regulating cell cycle entry into mitosis (25). Cyclin B1-cdc2 complex activation
controls cell cycle mitotic progression (26). Cyclin A2 is involved in G2/M
transition (22). As shown in
Fig. 3A, treatment with PL
reduced the protein levels of cdc25C, cyclin B1, cdc2 and cyclin A2
in HuCCT-1 cells. Similarly, the decrease in the expression of
these G2/M-regulating proteins induced by PL was attenuated by NAC
pretreatment (Fig. 3A).
The proteins p21 and p27 are cyclin-dependent kinase
inhibitors that bind to and inhibit the activity of CDK2, CDK1 and
CDK4/6 complexes; both p21 and p27 are thus regarded as regulators
of cell cycle progression to G1 phase (27). The results of western blotting
showed that PL treatment increased the expression of p21 and p27
proteins, and decreased the expression of cyclin E1/D2 and CDK2/4
in OCUG-1 cells (Fig. 3B). This
finding indicated that PL treatment induces G0/G1 cell cycle arrest
in OCUG-1 cells. Similar to that in HuCCT-1 cells, G0/G1 cell cycle
arrest induced by PL in OCUG-1 cells can be reversed by NAC
treatment (Fig. 3B).
PL induces apoptosis and autophagy in
HuCCT-1 and OCUG-1 cells
Whether PL can induce apoptotic cell death in BC
cells was further analyzed. HuCCT-1 and OCUG-1 cells were treated
with different concentrations of PL for 48 h. Apoptotic cells were
determined via flow cytometry using Annexin V/PI double staining.
As presented in Fig. 4A and B, PL
treatment resulted in increased Annexin-V staining in a
concentration-dependent manner, which indicates apoptotic cell
death. The apoptotic ratio in HuCCT-1 cells was 10.6±3.3% at 5
μM and 26.0±4.9% at 10 μM PL vs. 4.7±1.4% in the
vehicle control cells. The apoptotic ratio in OCUG-1 cells was
30.9±6.3% at 15 μM and 55.1±4.5% at 30 μM PL vs.
28.3±3.4% in the vehicle control cells. When the cells were
pretreated with NAC, the apoptotic ratio decreased from 26.0±4.9 to
6.1±2.1% and 55.1±4.5 to 20.8±2.3% in HuCCT-1 and OCUG-1 cells,
respectively. Western blotting was then performed to determine
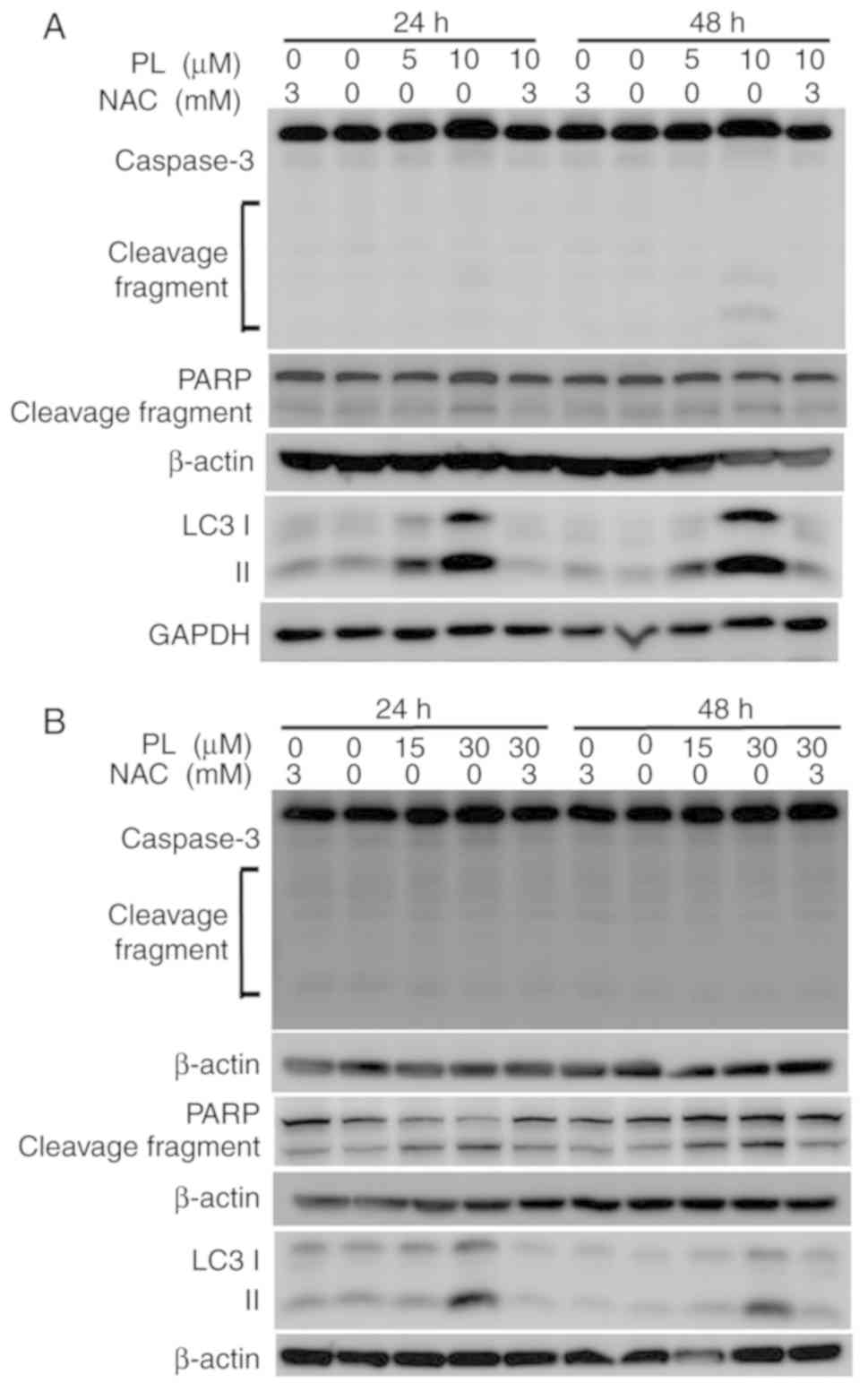
whether caspases were involved in PL-induced apoptosis. However,
only a faint increase in caspase 3 cleavage was observed in HuCCT-1
cells (Fig. 5A), with no clear
increase in OCUG-1 cells after PL treatment, as determined via
western blot analysis (Fig. 5B).
Similar to the results of caspase 3 cleavage, no clear increase in
PARP cleavage was detected after PL treatment (Fig. 5A and B).
During autophagy, cytoplasmic LC3-I is conjugated to
phosphatidyl ethanolamine to form LC3-II (28). As shown in Fig. 5A and B, LC3-II expression
increased after PL treatment in HuCCT-1 and OCUG-1 cells. A
reduction in LC3-II expression was observed when the cells were
pretreated with NAC prior to PL application.
PL induces autophagy via ROS-activated
Erk signaling pathway
An increase in LC3-II suggests that PL treatment
induces autophagy in HuCCT-1 and OCUG-1 cells. Therefore, the
pathways involved in this activation were investigated. As shown in
Fig. 6A, Erk phosphorylation
increased after PL treatment, indicating that PL activated the Erk
pathway in HuCCT-1 cells. However, Erk phosphorylation was not
observed in OCUG-1 cells at 48 h after PL treatment (Fig. 6B). These results indicated that PL
activated the Erk pathway in HuCCT-1 cells, but only transiently in
OCUG-1 cells. Pretreatment with NAC decreased p-Erk levels in
HuCCT-1 cells only, without affecting total Erk expression,
suggesting that activation of the Erk signaling pathway occurs via
ROS induction in PL-treated HuCCT-1 cells. Erk phosphorylation
occurred concomitantly with an increase in LC3-II expression, and
the reduction in p-Erk levels with NAC pretreatment was concomitant
with the reduction in LC3-II (Fig.
6A). Conversely, NAC pretreatment did notably reduce Erk
phosphorylation in OCUG-1 cells (Fig.
6B). During autophagy, LC3-II aggregates and is recruited to
the autophagosomal membrane (28). Aggregated LC3-II, termed LC3
puncta, can be detected using immunofluorescence staining. LC-3
puncta were analyzed in PL-treated cells using an LC3-specific
antibody. As shown in Fig. 6C, an
clear increase in LC-3 puncta was detected after PL treatment, and
a decrease with Erk inhibition in pretreated HuCCT-1 cells. These
data suggested that PL treatment induces autophagy via ROS-mediated
Erk signaling. To further determine whether ROS accumulation was
upstream of Erk activation in PL-treated HuCCT-1 cells, an MEK
inhibitor, PD98059, was used to analyze ROS levels in PL-treated
HuCCT-1 cells. The results of flow cytometry showed that
pretreatment with the MEK inhibitor did not affect ROS levels in
PL-treated cells (Fig. S2).
Then, HuCCT-1 cells were pretreated with PD98059 followed by PL
treatment, and apoptosis was analyzed using flow cytometry. The
apoptotic cell ratio increased from 13.2±3.2% in PL-treated cells
to 16.5±3.0% in cells pretreated with PD98059 followed by PL
application (Fig. 7A). It was
hypothesized that if autophagy was blocked by Erk inhibition in
PL-treated HuCCT-1 cells, the cells would undergo apoptosis.
However, there was no significant difference in the apoptotic rate
of HuCCT-1 cells with or without pretreatment with PD98059
(Fig. 7A). The expression of
apoptosis-associated proteins was detected by western blotting.
PD98059 decreased the LC3-II level in PL-treated HuCCT-1 cells, but
increased the cleaved forms of caspase 3 and PARP (Fig. 7B). The relative level of LC3-II
for each treatment was quantified using a UVP image system. A
significant reduction in LC3-II was observed following
pre-treatment with a high concentration of PD98059 (30 μM)
before application of 7.5 μM PL to HuCCT-1 cells (Fig. 7C).
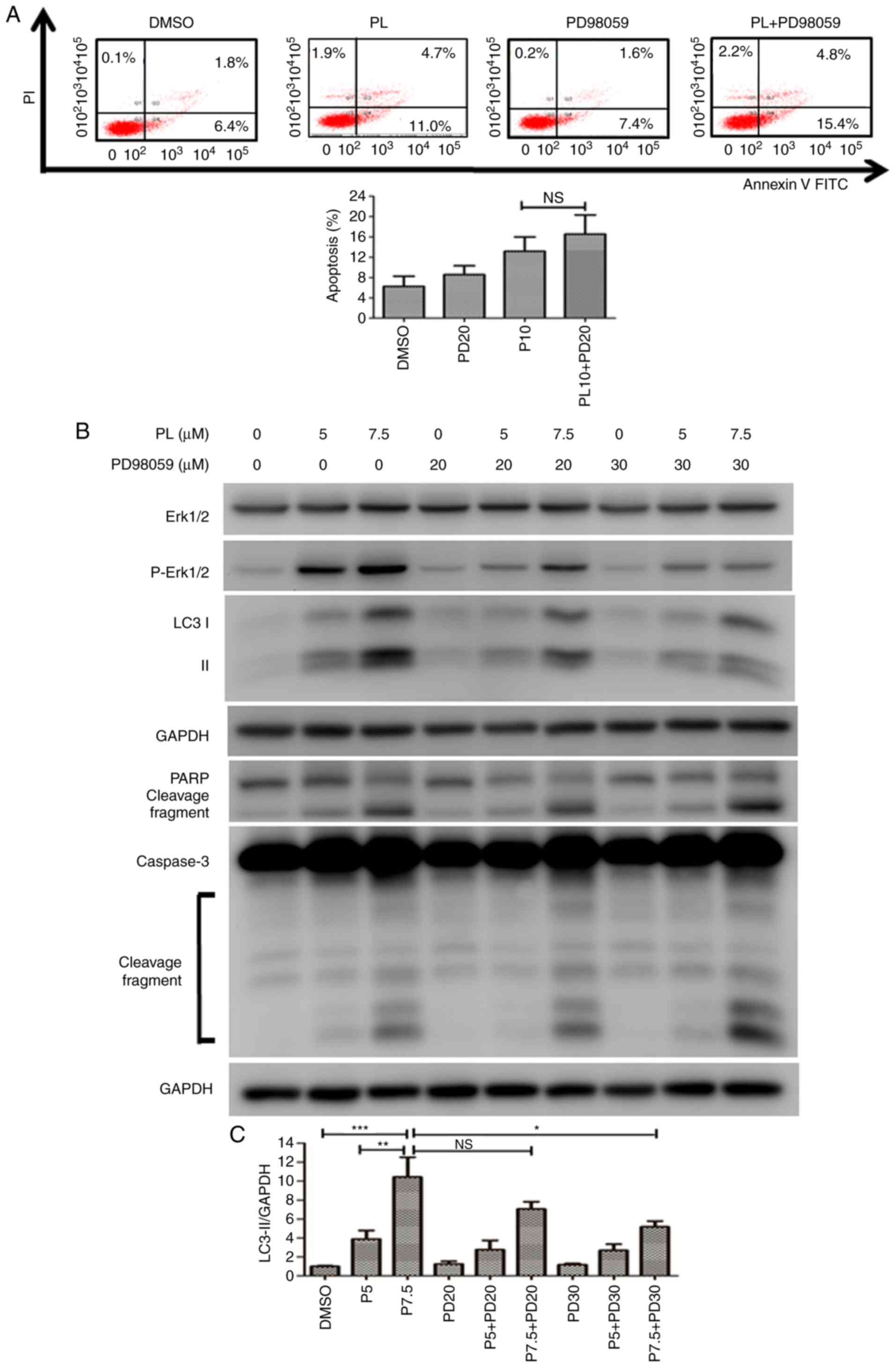 | Figure 7Inhibition of the Erk signaling
pathway increases apoptosis in PL-treated HuCCT-1 cells. (A)
HuCCT-1 cells were pretreated in the presence or absence of the MEK
inhibitor PD98059 (20 μM) followed by treated with 10
μM PL for 24 h. Apoptotic cells were analyzed by flow
cytometry. The x- and y-axes represent the fluorescence intensity
of Annexin V-FITC and PI fluorescence in HuCCT-1 cells,
respectively. (B) HuCCT-1 cells were treated with 5 or 7.5
μM PL in the presence or absence of various concentrations
of the MEK inhibitor PD98059 for 24 h. Expression levels of Erk,
PARP, LC3 and caspase 3 proteins were analyzed via western
blotting. (C) Quantification of LC3-II expression relative to the
DMSO control group is presented. Data are presented as the mean ±
SD of at least three independent experiments.
*P<0.05, **P<0.01,
***P<0.001; n.s., not significant. LC3,
microtubule-associated protein 1A/1B light chain 3B; MEK,
mitogen-activated protein kinase kinase; p, phosphorylated; PARP,
poly(ADP-ribose) polymerase; PI, propidium iodide; PL,
piperlongumine. |
Discussion
BC represents a heterogeneous malignancy with poor
prognosis. Therefore, there is an urgent requirement to develop new
drugs for BC treatment. The aim of the present study was to
determine the inhibitory effects of PL on BC cell growth. It was
found that PL exhibited cytotoxicity in HuCCT-1 and OCUG-1 cells by
interfering with redox balance via ROS production. It was also
determined that PL induced cell cycle arrest, apoptosis and
autophagy via ROS-activated Erk signaling in HuCCT-1 cells, and
transiently in OCUG-1 cells. The present findings indicated that PL
is a potential drug for BC treatment.
Cancer cells are more vulnerable to ROS-mediated
effects owing to elevated ROS levels (15). PL inhibits the antioxidant enzyme
glutathione S-transferase P, leading to elevated ROS production and
subsequently resulting in cancer cell death (29,30). In the present study, it was
revealed that PL treatment at lower doses induced ROS production in
HuCCT-1 cells, indicating that HuCCT-1 cells were sensitive to PL
treatment. The IC50 calculated using a CCK-8 assay
supported this hypothesis. HuCCT-1 cells exhibited more sensitive
responses than OCUG-1 cells to PL treatment, with IC50
values of 4.2 and 13.8 μM at 48 h, respectively. Recently,
PL has been shown to exhibit cytotoxicity in other CCA cells
(KKU-055, KKU-100, KKU-139, KKU-213 and KKU-214) (31). PL showed similar IC50
values in these cells, ranging between 4 and 20 μM,
suggesting that PL is a potent anticancer drug for treating
CCA.
High ROS levels in cells result in cell cycle arrest
and subsequent cell death (32).
It was demonstrated that PL interfered with cell cycle progression
in both HuCCT-1 and OCUG-1 cells. It is proposed that PL may induce
G2/M cell cycle arrest in HuCCT-1 cells. Phosphorylation and
dephosphorylation of cdc2 at tyrosine 15 by Wee1 and cdc25,
respectively, cooperatively control the cdc2/cyclin B complex and
regulate cell cycle entry into mitosis (33). Therefore, phosphorylation of cdc2
at tyrosine 15 is an effective marker of G2/M cycle arrest;
however, only the protein expression levels of cdc25C, cyclin B1,
cdc2 and cyclin A2 were analyzed in this study.
The expression levels of antioxidant genes may
determine differential responses after PL treatment (31). Thongsom et al (31) determined the mRNA expression
levels of five antioxidant genes, including PARK1, TXN,
NQO1, HO-1 and SOD2, in CCA cells. Among the
proteins encoded by these genes, heme oxygenase-1 (HO-1) expression
may be a major factor contributing to the antioxidant capacity of
CCA cells. Consistent with this finding, HO-1 expression
contributed to the differential responses of breast cancer cells to
PL treatment (34). HO-1
expression is also inversely associated with survival in patients
with CCA (35). The higher
antioxidant capacity of OCUG-1 cells may be due to upregulated
expression of antioxidant genes compared with HuCCT-1 cells;
however, this requires further investigation.
PL was reported to induce apoptosis in CCA cell
lines (KKU-055, KKU-100, KKU-139, KKU-213 and KKU-214) (31). In the present study, it was found
that PL also induced autophagy in HuCCT-1 and OCUG-1 cells, as
indicated by an increase in LC3-II in a concentration-dependent
manner. The balance between ROS and autophagy affects cellular
homeostasis and survival (36,37). Drugs that induce ROS production
may promote cancer cell death by activating autophagy. PL has been
reported to induce autophagy in kidney, prostate and breast cancer
(23), and leukemia cells
(38). The increase in LC3-II was
reversed by the application of NAC prior to PL treatment,
suggesting that ROS were involved in autophagy induction.
Numerous signaling pathways can be involved in
autophagy induction. Previously, it was reported that PL exhibits
anticancer activity by affecting PI3K/AKT, p38/JNK, MEK/Erk and
NF-κB signaling pathways in cancer cells (24,38-40). In the present study, only the Erk
pathway was analyzed; it was revealed that the Erk pathway was
involved in the anticancer activity of PL in HuCCT-1 cells. Upon
inhibition of the Erk signaling pathway, LC3-II expression and
puncta levels decreased. Apoptosis was promoted after inhibition of
Erk signaling in HuCCT-1 cells. Various molecules have been
reported to be involved in the crosstalk between autophagy and
apoptosis (41). In the present
study, it was not determined as to whether an interaction between
autophagy and apoptosis played a role in the effects of PL. Further
studies are required to examine the relationship between autophagy
and apoptosis following PL treatment in HuCCT-1 cells.
BCs can be divided into intrahepatic and
extrahepatic CCA, and gallbladder cancers (3). HuCCT-1 cells were established from
malignant cells originating from the intrahepatic bile duct tree,
whereas OCUG-1 cells were derived from metastatic peritoneal
effusion of gallbladder carcinoma. In the present study, HuCCT-1
and OCUG-1 cells were used to evaluate the cytotoxic effects of PL
on BCs. The response of OCUG-1 cells after PL treatment varied from
that of HuCCT-1 cells in terms of cell cycle distribution and
signaling pathway activation; this may result from their different
origins.
In conclusion, it was demonstrated that PL exhibits
anticancer activity in HuCCT-1 and OCUG-1 cells. PL treatment
interfered with redox balance, and induced apoptosis and autophagy.
This is the first report, to our knowledge, showing that PL induced
autophagy in BC cells. It was further demonstrated that the Erk
signaling pathway was involved in PL-induced autophagy in HuCCT-1
cells. However, this is a preliminary report of the treatment of BC
with PL. Further studies, including in vivo animal
experiments, should be conducted to determine the efficacy of PL as
a treatment of BC.
Supplementary Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ditmanson
Medical Foundation Chiayi Christian Hospital (grant no.
R107-008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SYC designed the research and collected the data.
HYH performed the experiments. HPL performed experiments and helped
with the literature search. CYF designed the study and drafted the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banales JM, Cardinale V, Carpino G,
Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes
SJ, Fouassier L, et al: Expert consensus document:
Cholangiocarcinoma: Current knowledge and future perspectives
consensus statement from the European network for the study of
cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol.
13:261–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghouri YA, Mian I and Blechacz B: Cancer
review: Cholangiocarcinoma. J Carcinog. 14:12015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahn DH and Bekaii-Saab T: Biliary cancer:
Intrahepatic cholangiocarcinoma vs. extrahepatic cholangiocarcinoma
vs. gallbladder cancers: Classification and therapeutic
implications. J Gastrointest Oncol. 8:293–301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blechacz B: Cholangiocarcinoma: Current
knowledge and new developments. Gut Liver. 11:13–26. 2017.
View Article : Google Scholar :
|
|
5
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar
|
|
6
|
Chun YS and Javle M: Systemic and adjuvant
therapies for intrahepatic cholangiocarcinoma. Cancer Control.
24:10732748177292412017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee J, Park SH, Chang HM, Kim JS, Choi HJ,
Lee MA, Jang JS, Jeung HC, Kang JH, Lee HW, et al: Gemcitabine and
oxaliplatin with or without erlotinib in advanced biliary-tract
cancer: A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 13:181–188. 2012. View Article : Google Scholar
|
|
8
|
Goff LW, Cardin DB, Whisenant JG, Du L,
Koyama T, Dahlman KB, Salaria SN, Young RT, Ciombor KK, Gilbert J,
et al: A phase I trial investigating pulsatile erlotinib in
combination with gemcitabine and oxaliplatin in advanced biliary
tract cancers. Invest New Drugs. 35:95–104. 2017. View Article : Google Scholar :
|
|
9
|
Uenishi T, Yamamoto T, Takemura S and Kubo
S: Surgical treatment for intrahepatic cholangiocarcinoma. Clin J
Gastroenterol. 7:87–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sabharwal SS and Schumacker PT:
Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles'
heel? . Nat Rev Cancer. 14:709–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017.8416763:2017.
|
|
12
|
Cohen Z, Maimon Y, Samuels N and Berger R:
Role of reactive oxygen species in the anticancer activity of
botanicals: Comparing sensitivity profiles. Oncol Lett.
13:2642–2648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? . Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar
|
|
15
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin S, Li Y, Zamyatnin AA Jr, Werner J and
Bazhin AV: Reactive oxygen species and colorectal cancer. J Cell
Physiol. 233:5119–5132. 2018. View Article : Google Scholar
|
|
17
|
Huang H, Xie H, Pan Y, Zheng K, Xia Y and
Chen W: Plumbagin triggers ER stress-mediated apoptosis in prostate
cancer cells via induction of ROS. Cell Physiol Biochem.
45:267–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Li J, Zong L, Chen X, Chen K,
Jiang Z, Nan L, Li X, Li W, Shan T, et al: Reactive oxygen species
and targeted therapy for pancreatic cancer. Oxid Med Cell Longev.
2016.1616781:2016.
|
|
19
|
Sharma V, Joseph C, Ghosh S, Agarwal A,
Mishra MK and Sen E: Kaempferol induces apoptosis in glioblastoma
cells through oxidative stress. Mol Cancer Ther. 6:2544–2553. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Lv H, Yang W, Li T, Fang T, Lv G,
Han Q, Dong L, Jiang T, Jiang B, et al: SVCT-2 determines the
sensitivity to ascorbate-induced cell death in cholangiocarcinoma
cell lines and patient derived xenografts. Cancer Lett. 398:1–11.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Möhler H, Pfirrmann RW and Frei K:
Redox-directed cancer therapeutics: Taurolidine and Piperlongumine
as broadly effective antineoplastic agents (Review). Int J Oncol.
45:1329–1336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong D and Ferrell JE Jr: The roles of
cyclin A2, B1, and B2 in early and late mitotic events. Mol Biol
Cell. 21:3149–3161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makhov P, Golovine K, Teper E, Kutikov A,
Mehrazin R, Corcoran A, Tulin A, Uzzo RG and Kolenko VM:
Piperlongumine promotes autophagy via inhibition of Akt/mTOR
signalling and mediates cancer cell death. Br J Cancer.
110:899–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng J, Son DJ, Gu SM, Woo JR, Ham YW,
Lee HP, Kim WJ, Jung JK and Hong JT: Piperlongumine inhibits lung
tumor growth via inhibition of nuclear factor kappa B signaling
pathway. Sci Rep. 6:263572016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sur S and Agrawal DK: Phosphatases and
kinases regulating CDC25 activity in the cell cycle: Clinical
implications of CDC25 overexpression and potential treatment
strategies. Mol Cell Biochem. 416:33–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi HJ, Fukui M and Zhu BT: Role of
cyclin B1/Cdc2 up-regulation in the development of mitotic
prometaphase arrest in human breast cancer cells treated with
nocodazole. PLoS One. 6:pp. e243122011, View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Georgakilas AG, Martin OA and Bonner WM:
p21: A two-faced genome guardian. Trends Mol Med. 23:310–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanida I, Ueno T and Kominami E: LC3 and
autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harshbarger W, Gondi S, Ficarro SB, Hunter
J, Udayakumar D, Gurbani D, Singer WD, Liu Y, Li L, Marto JA and
Westover KD: Structural and biochemical analyses reveal the
mechanism of glutathione S-Transferase Pi 1 inhibition by the
anti-cancer compound piperlongumine. J Biol Chem. 292:112–120.
2017. View Article : Google Scholar :
|
|
30
|
Piska K, Gunia-Krzyżak A, Koczurkiewicz P,
Wójcik-Pszczoła K and Pękala E: Piperlongumine (piplartine) as a
lead compound for anticancer agents-Synthesis and properties of
analogues: A mini-review. Eur J Med Chem. 156:13–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thongsom S, Suginta W, Lee KJ, Choe H and
Talabnin C: Piperlongumine induces G2/M phase arrest and apoptosis
in cholangiocarcinoma cells through the ROS-JNK-ERK signaling
pathway. Apoptosis. 22:1473–1484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Verbon EH, Post JA and Boonstra J: The
influence of reactive oxygen species on cell cycle progression in
mammalian cells. Gene. 511:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perry JA and Kornbluth S: Cdc25 and wee1:
Analogous opposites? . Cell Div. 2:122007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HN, Jin HO, Park JA, Kim JH, Kim JY,
Kim B, Kim W, Hong SE, Lee YH, Chang YH, et al: Heme oxygenase-1
determines the differential response of breast cancer and normal
cells to piperlongumine. Mol Cells. 38:327–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kongpetch S, Puapairoj A, Ong CK,
Senggunprai L, Prawan A, Kukongviriyapan U, Chan-On W, Siew EY,
Khuntikeo N, The BT and Kukongviriyapan V: Haem oxygenase 1
expression is associated with prognosis in cholangiocarcinoma
patients and with drug sensitivity in xenografted mice. Cell
Prolif. 49:90–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen YF, Liu H, Luo XJ, Zhao Z, Zou ZY, Li
J, Lin XJ and Liang Y: The roles of reactive oxygen species (ROS)
and autophagy in the survival and death of leukemia cells. Crit Rev
Oncol Hematol. 112:21–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Azad MB, Chen Y and Gibson SB: Regulation
of autophagy by reactive oxygen species (ROS): Implications for
cancer progression and treatment. Antioxid Redox Signal.
11:777–790. 2009. View Article : Google Scholar
|
|
38
|
Wang H, Wang Y, Gao H, Wang B, Dou L and
Li Y: Piperlongumine induces apoptosis and autophagy in leukemic
cells through targeting the PI3K/Akt/mTOR and p38 signaling
pathways. Oncol Lett. 15:1423–1428. 2018.PubMed/NCBI
|
|
39
|
Chen SY, Liu GH, Chao WY, Shi CS, Lin CY,
Lim YP, Lu CH, Lai PY, Chen HR and Lee YR: Piperlongumine
suppresses proliferation of human oral squamous cell carcinoma
through cell cycle arrest, apoptosis and senescence. Int J Mol Sci.
17:pp. pii E6162016
|
|
40
|
Yamaguchi Y, Kasukabe T and Kumakura S:
Piperlongumine rapidly induces the death of human pancreatic cancer
cells mainly through the induction of ferroptosis. Int J Oncol.
52:1011–1022. 2018.PubMed/NCBI
|
|
41
|
Su M, Mei Y and Sinha S: Role of the
crosstalk between autophagy and apoptosis in cancer. J Oncol.
2013.102735:2013.
|