Introduction
Sepsis is a life-threatening organ dysfunction,
caused by an overwhelming immune response to infection (1). The kidneys are frequently affected
by sepsis, which results in the development of acute kidney injury
(AKI), inducing unfavorable health outcomes. Previous
epidemiological investigations have revealed that patients with AKI
(mild or short) tend to have a higher risk of developing chronic-
and end-stage kidney disease at later stages of their life
(2). Volume resuscitation,
antimicrobial therapy and renal replacement therapy remain the main
types of treatment for sepsis-induced AKI (3). Recent investigations have focused on
the supportive measures that aim to keep the patient alive.
ω3 fatty acids (FAs), eicosapentaenoic acid (EPA)
and docosahexaenoic acid (DHA), are the main component of fish oils
(FOs) (4). Previous studies have
shown that enteral nutrition based on FOs affects the induction of
sepsis, which is the leading cause of AKI (5,6).
Sepsis is a systemic inflammatory response and a severe
postoperative infection, which is usually initiated by bacteria and
toxins (7). Following the release
of bacterial toxins, the immune response is activated, and organ
disorders may develop (8). The
sepsis-induced kidney inflammatory response involves the increased
production of pro-inflammatory factors, which may contribute to
high mortality (9).
Concomitantly, the release of TNFα, IL-1β and IL-6 contributes to
the inflammatory factors (9).
During the development of sepsis, the levels of these
pro-inflammatory factors are significantly elevated at the early
stage of disease onset affecting the induction of
pathophysiological responses (10). ω-3 FAs have shown considerable
potential in the prevention of chronic kidney disease in humans
(11). The ω-3 fatty acids,
especially EPA and DHA, exert anti-inflammatory effects in humans
(12). Shih et al
(13) demonstrated that FOs could
suppress AKI and the inflammatory response in septic mice. The
pathophysiology of sepsis remains controversial. In addition to
inflammatory reactions, sepsis-induced AKI often involves the
induction of oxidative stress and apoptosis (14,15). Previously, the administration of
an ω3-FA-rich infusion during parenteral nutrition was shown to
alleviate sepsis in patients (16); however, the detailed mechanisms of
this process remain unknown.
Blood urea nitrogen (BUN) and serum creatine (SCr)
are classic biomarkers for renal injury and have been reported as
biomarkers for delayed kidney injury (17). Accompanied with increased SCr
levels, the glomerular filtration rate (GFR) is reduced (18). The secretion of SCr contributes to
10-40% of total SCr; thus, reductions in GFR may be linked.
Additionally, the concentrations of Scr are notably influenced by
variable factors, including age, gender, diet and drugs (19). Thus, the levels of BUN and Scr are
not sufficient for the diagnosis of early kidney injury. Kidney
injury molecule-1 (KIM-1) and neutrophil gelatinase-associated
lipocalin (NGAL) have been detected in early renal tubular injury
(20). The expression of NGAL in
both blood and urine have been reported to indicate the presence,
severity and development of renal disease, especially in chronic
renal disease (21). KIM-1 is a
transmembrane protein not found in normal kidney; the expression of
KIM-1 and NGAL has been linked to renal ischemia, which usually
leads to AKI (22).
In the present study, the cecal ligation and
puncture (CLP) model was established to induce sepsis. This model
mimics the condition noted in patients with bowel perforation and
polymi-crobial infection (23).
The effects of FOs on sepsis-induced inflammation, apoptosis and
oxidative stress were investigated.
Materials and methods
Animals
Male Sprague Dawley rats (n=32; 7-weeks-old,
Qinglongshan Experimental Animal Center, China) weighing 250-330 g
were employed. A total of 3 rats were housed per cage and
maintained in a 12-h light/dark cycle at 25°C. The experimental
protocol was approved by the commission for animal experimentation
of the People's Hospital of the Xishuangbanna Dai Nationality
Autonomous Prefecture.
Following acclimation for 1 week, the rats with an
initial body weight of 280±25 g were randomly assigned to one of
the following groups: Sham-operated (n=8) and CLP (n=24). The
former group was used as a control group and the latter as the
experimental sepsis group. The CLP model group was randomly
assigned to one of the following subgroups: CLP sepsis (n=8) used
for model animals, CLP treated with dexamethasone [1 mg/kg,
intraperitoneal (i.p.) daily; n=8] used for positive control and
CLP treated with FO-containing fat emulsion (2 ml/kg i.p. daily;
n=8). The rat model was established by CLP-induced sepsis and
intraperitoneally administered with dexamethasone or FOs (Table I) once a day. The treatment was
provided 3 days prior to CLP operation and was continued until
animal sacrifice. The serum samples were collected from the inner
canthal orbital vein 24 h following CLP operation. All rats were
sacrificed by using pentobarbital sodium (200 mg/kg; i.p.
injection) following 96 h of CLP operation. The collected renal
tissues were harvested and all renal tissues from each rat was half
embedded in paraffin and half stored in −80°C refrigerator. Some
animals did not survive the operation.
 | Table IContent of fish oil. |
Table I
Content of fish oil.
| Main
components | Contents (g/100
ml) |
|---|
| Fish oil | 10 |
| EPA | 1.25-2.82 |
| DHA | 1.44-3.09 |
| Myristic acid | 0.1-0.6 |
| Palmitic acid | 0.25-1 |
| Palmitoleic
acid | 0.3-0.9 |
| Stearic acid | 0.05-0.2 |
| Oleic acid | 0.6-1.3 |
| Linoleic acid | 0.1-0.7 |
| ALA | ≤0.2 |
| SDA | 0.05-0.4 |
| Eicosanoic
acid | 0.05-0.3 |
| ARA | 0.1-0.4 |
| Docosanoic
acid | ≤0.15 |
| docosapentaenoic
acid | 0.15-0.45 |
| Vitamin E | 0.015-0.0296 |
Sepsis renal injury was induced by CLP as previously
described (24). Briefly, rats
were fast with access to water for 12 h before the experiments. All
rats were anesthetized with isoflurane inhalation. The hypogastric
region was shaved and disinfected with alcohol, cutting ~2 cm long
in middle of abdomen. The cecum was subsequently exposed and
ligated just below the ileocecal valve with a 3-0 silk and
punctured with an 18-gauge needle. A droplet of feces was then
squeezed from the puncture hole. The bowel was placed back into the
abdominal cavity and the incision was sutured with 4-0 silk. The
sham-operated rats underwent CLP surgery except cecal ligation and
perforation. All rats were given 0.9% saline solution immediately
after surgery for fluid resuscitation.
Survival rate assessment
In each group, the survival rate of the rats (n=8)
was evaluated over a period of 4 days following operation.
Following CLP surgery, the rats were observed every 12 h for a
total of 96 h. The mortality percentage was recorded. All animals
that did not survive the operation during the 12-24 h period was
recorded. The human endpoint in this assay was determined according
to previous investigation (25).
All animals were monitored three times daily for humane endpoints
and were euthanized when the humane endpoints were reached. Humane
endpoints included loss of 20% weight from surgery, or the clinical
score reached 5. The scores were denoted based on hunched posture
(0-2), ruffled coat (0-2), diarrhea (0-2), dehydrated eyes (0-2),
decreased body temperature (0-1) and reluctance to move (0-2).
Hematoxylin and eosin staining (H&E)
staining
The induction of sepsis was performed via CLP and
the rats were sacrificed following 96 h of treatment. Renal and
small intestinal tissues were fixed in 4% formaldehyde at room
temperature for >24 h for histopathological analysis.
Subsequently, the fixed tissues were embedded in paraffin. Sections
(5 μm) were sliced. After deparaffinization and rehydration,
the sections were stained with hematoxylin (Sigma-Aldrich; Merck
KGaA) for 5 min and then stained with eosin (Sigma-Aldrich) for 3
min at room temperature. The samples were subsequently visualized
by optical microscopy (Olympus Corporation) at x100
magnification.
Assessment of serum and renal biochemical
parameters
Blood biochemical analysis was performed according
to previous investigations. The blood samples were extracted 24 h
following sham or CLP operation and centrifuged at 1400 x g to
prepare serum at 4°C for 10 min. SCr levels were evaluated by a
creatinine enzymatic assay kit (MAK080-1KT, Sigma-Aldrich, Merck
KGaA) and measured at 570 nm. BUN was detected with an enzymatic
assay kit (MAK006-1KT, Sigma-Aldrich, Merck KGaA) and measured at
570 nm. The expression levels of NGAL (ab119602, Abcam) and
KIM-1(ab119597, Abcam) were measured using corresponding kits and
measured at 450 nm. All measurements were conducted with a
multiscan spectrum spectrophotometer (Thermo Fisher Scientific
Inc.)
Evaluation of inflammatory factors ELISA
assays
The serum samples were extracted 24 h following sham
or CLP operation. The expression levels of tumor necrosis factor
(TNF)-α (ER006-96), interleukin (IL)-1β (ER008-96), and IL-6
(ER003-96) in the serum were determined by ELISA kits (Shanghai
ExCell Biology, Inc.) following the manufacturer's protocol. The
samples were centrifuged at 1,500 x g for 15 min at 4°C.
Subsequently, the supernatant was collected, and the measurements
were conducted at 450 nm using a multiscan spectrum
spectrophotometer (Thermo Fisher Scientific Inc.).
Immunofluorescence staining
The expression levels of TNF-α, IL-1β, and IL-6 were
detected in renal and intestinal tissues by immunofluorescence.
Sections (5 μm) of renal and intestinal tissues were
obtained. The samples were incubated with blocking solution (5%
bovine serum albumin in PBS) and permeabilized (0.1% Triton X-100
in PBS). Primary antibodies against TNF-α (1:100, ab6671, Abcam),
IL-1β (1:100, ab9722, Abcam), and IL-6 (1:200, YB-0782R, Yubo
Biological Technology Ltd.) were used for sequential double
immunofluorescence staining for 2 h at room temperature in the
dark. The goat anti-rabbit IgG H&L (Alexa Fluor®
488) secondary antibody (1:500, ab1500, Abcam) were conjugated with
fluorescein isothiocyanate. The sections were mounted in an
anti-fading agent (DAPI; Invitrogen; Thermo Fisher Scientific,
Inc.) for 5 min at room temperature, and subsequently imaged and
analyzed with a fluorescence microscope (Olympus Corporation) at
x100 magnification.
Assessment of antioxidant activity and
oxidative stress markers in renal tissues
The levels of oxidative stress biomarkers were
determined according to a previous investigation (26). The renal tissues were homogenized
in 50 mmol/l phosphate buffer, and subsequently centrifuged at
12,000 x g for 20 min at 4°C. The supernatant was collected from
the samples for the detection of the markers of oxidative stress.
The concentration of malondialdehyde (MDA) was detected with an MDA
assay kit (Beijing Solarbio Life Sciences). The activity level of
super-oxide dismutase (SOD) and glutathione peroxidase (GSH-Px)
were evaluated by commercial kits (Jiancheng Bioengineering
Institute). All detection was conducted according to manufacturer's
protocols.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL) staining
The TUNEL assay was performed as described
previously (27). The
paraffin-embedded renal tissues were sliced into 5 μm
sections at room temperature. After deparaffinization, the samples
were rehydrated with gradient concentration (90, 80, 70%) of
ethanol and immersed in 4% formaldehyde in PBS for 9 min.
Subsequently, the sections were treated with proteinase K (20
μg/ml) and incubated in a nucleotide mixture containing
fluorescein-12-dUTP and TdT for 1 h at 37°C. The cell nuclei were
stained with 4,6-diamidino-2-phenylindole for 5 min at room
temperature, and the sections were imaged with a fluorescence
microscope (Olympus Corporation).
Western blotting
Frozen renal tissues were thawed and total protein
extraction was performed by RIPA Lysis and Extraction Buffer
(Thermo Fisher Scientific). The proteins were analyzed by
immunoblotting using SDS-PAGE (10%) and transferred to
polyvinylidene fluoride membranes. The blots were probed with
antibodies against the following proteins: Bcl-2-associated X
protein (Bax; 1:1,000, cat. no. 2772), Bcl-2, cleaved-caspase3
(1:1,000, cat. no. 9664), caspase 3 (1:1,000, cat. no. 9662),
phosphorylated (p)-JAK2 (1:1,000, cat. no. 3776), JAK2 (1:1,000,
cat. no. 3230), p-STAT3 (1:2,000, cat. no. 9145), STAT3 (1:1,000,
cat. no. 12640), p-p38MAPK (1:1,000, cat. no. 4511), p-IκBα
(1:1,000, cat. no. 5209), IκBα (1:1,000, cat. no. 4812), p-p65
(1:1,000, cat. no. 3031), p65 (1:1,000, cat. no. 8242), and GAPDH
(1:1,000, cat. no. 5174; all Cell Signaling Technology, Inc.).
Incubation with the primary antibodies was performed overnight at
4°C. The following morning, the membranes were washed and incubated
with horseradish peroxidase-conjugated secondary anti-rabbit IgG
(1:2,000, cat. no. 7074, Cell Signaling Technology, Inc.) for 2 h.
The expression levels of each protein were determined using
enhanced chemiluminescence reagents (ChemiDoc™XRS, Bio-Rad
Laboratories, Inc.). The images were collected and quantified using
Quantity One Software v4.6.6 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Experiments were performed in triplicate, and data
are presented as mean ± standard deviation. GraphPad Prism 6
(GraphPad Software, Inc.) software was used to analyze the results.
Kaplan-Meier analysis was performed to derive survival curves and
survival differences was analyzed by log-rank test. Multiple
comparisons were assessed by analysis of variance followed by a
Bonferroni post-hoc test. P<0.05 was considered statistically
significant.
Results
Effects of FOs in sepsis on the survival
of septic rats
To investigate the effects of FOs on the survival
rate of septic rats, the survival rate between the CLP group and
FOs-treated group was compared. A total of 8 rats did not survive
48 h of sepsis induction in the CLP group. The survival rate was
reduced to 25% at 48 h and reached 0% at 72 h. Following treatment
of the animal with dexamethasone, the survival rate was increased
to 69% within 48 h. The survival rate was estimated to 71% at 48 h
following FOs intervention. The average survival rate of the FOs
and dexamethasone groups was significantly higher than that of the
CLP group (Fig. 1A).
Effects of FOs on renal injury induced by
CLP
H&E staining was performed for histopathological
evaluation (Fig. 1B).
Histopathological alterations are direct indications of renal
injury. In contrast to the control group, the model group presented
glomerular atrophy, dilation of the renal capsule cavity,
destruction of tubular structures, and local focal epithelial cell
necrosis. However, pretreatment with FOs or dexamethasone
alleviated glomerular atrophy and epithelial necrosis.
Effects of FOs on CLP-induced renal
dysfunction
Renal injury was assessed in rats subjected to CLP
for 24 h. The levels of SCr, and BUN, NGAL and KIM-1 in the serum
were significantly elevated in the model group at 24 h post-CLP
compared with the control group. However, treatment with
dexamethasone and FOs significantly diminished the levels of SCr,
BUN, NGAL and KIM-1 compared with those of the model group
(Fig. 2).
Effects of FOs on CLP-induced
inflammatory cytokines expression in the serum
The expression levels of TNFα, IL-1β and IL-6 were
determined by ELISA and by immunofluorescence assays in serum
samples from CLP-rats. The results indicated that the expression
levels were significantly increased in the model group compared
with the control group, whereas administration of FOs or
dexamethasone significantly suppressed the induction of TNFα, IL-1β
and IL-6 expression in the CLP group (Fig. 3A). Immunofluorescence analysis of
the renal tissue samples verified the aforementioned results
(Fig. 3B). The data indicated
that FOs could reduce the induction of inflammation in the CLP
group.
Effects of FOs on CLP-induced oxidative
stress and apoptosis
The induction of inflammation is usually accompanied
with the induction of oxidative stress and apoptosis (28). The effects of FOs on CLP-induced
renal oxidation injury were presented in Fig. 4A. The results indicated that CLP
induced a significant increase in the MDA levels compared with the
control group. In addition, the activity of the antioxidant enzymes
SOD and GSH-Px was significantly decreased in the CLP model
compared with the control group. The MDA levels of the CLP rats
that received treatment with dexamethasone and FOs were reduced by
60.4 and 57.7% compared with those of the CLP model group.
Moreover, the activity levels of SOD and GSH-Px in animals treated
with dexamethasone were increased by 135.8 and 134.5%, whereas FOs
treatment resulted in increases of 104.0 and 104.3%,
respectively.
 | Figure 4Effects of FOs on oxidative stress
and apoptosis. (A) Comparison of MDA, SOD and GSH-Px levels among
the four groups. (B) Apoptosis was determined by TUNEL analysis
during four groups at x100 magnification. (C) The expression of
apoptotic proteins (Bax, Bcl-2, Caspase3) in four groups.
***P<0.001 vs. Control. #P<0.05,
##P<0.01, ###P<0.001 vs. Model. Bax,
Bcl-2-associated X protein; FOs, fish oils; GSH-Px, glutathione
peroxidase; SOD, superoxide dismutase; TUNEL, Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling. |
TUNEL staining
The induction of apoptosis in the present animal
model was stimulated by CLP treatment (Fig. 4B). The percentage of
TUNEL-positive cells was increased in the CLP model group compared
with the control group. Treatment of the rats with dexamethasone
and FOs markedly decreased the number of apoptotic cells. Moreover,
the expression levels of the apoptosis-related proteins were
detected (Fig. 4C). Bcl-2
expression levels were significantly reduced, whereas the
expression levels of Bax and cleaved-caspase 3 were significantly
increased in the CLP model compared with those of the control
(Fig. 4C). The ratio of Bax/Bcl-2
was significantly increased in the model group, while dexamethasone
and FOs treatment significantly reduced the ratio (Fig. 4C). This indicated the effects of
FOs on cell apoptosis. Therefore, administration of dexamethasone
and FOs caused a notable decline in the induction of apoptosis by
CLP.
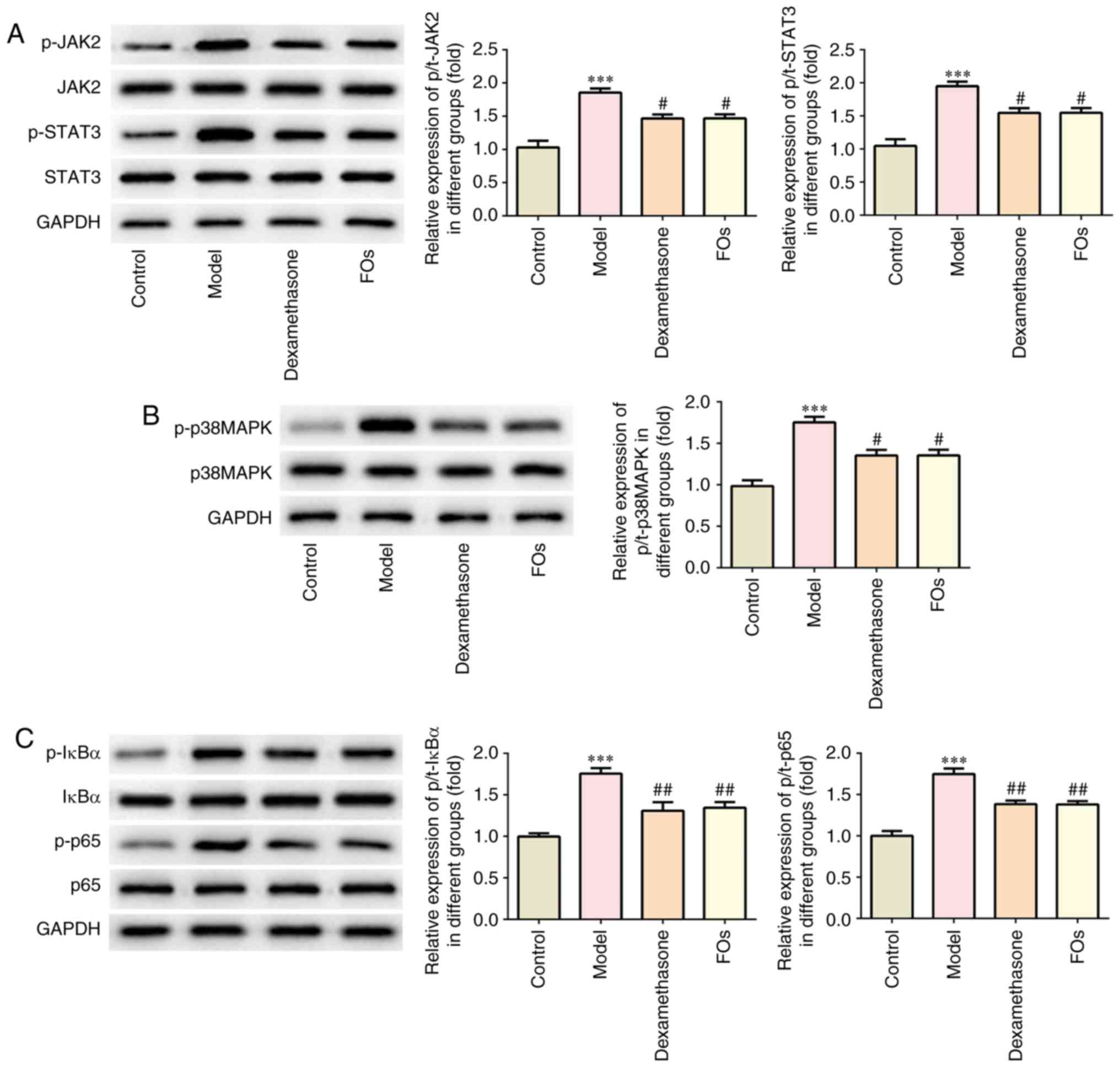
FOs negatively regulates the activation
of JAK/STAT3, p38-MAPK, and nuclear factor-κB (NF-kB) signaling
pathways
FOs were determined to affect the activation of
JAK2/STAT3, p38-MAPK and NF-κB proteins in the renal tissues of CLP
rats. Our results indicated that the phosphorylation levels of
JAK2, STAT3, p38MAPK, IκBα and p65 were significantly reduced in
renal tissues of CLP rats treated with FOs compared with the model
group (Fig. 5).
Histology
H&E staining of intestinal tissues derived from
the CLP model group revealed renal injury as demonstrated by tissue
destruction and inflammation. This was reversed by dexamethasone
and FOs treatment (Fig. 6A).
Immunofluorescence revealed that FO treatment suppressed the
production of the pro-inflammatory factors, IL-1β, IL-6 and TNFα in
the small intestine of CLP rats (Fig.
6B).
Discussion
In the present study, the survival rates of rats
subjected to CLP-induced sepsis were prolonged following treatment
with FOs and dexamethasone. A recent study has shown that in a
similar in vivo model, the administration of FOs alone led
to ~70% survival at 48 h following CLP induction (29). Dexamethasone alleviated the
induction of inflammation caused by sepsis and reduced the extent
of lipid peroxidation (30). In
the present study, dexamethasone was used as a positive control.
The current investigation evaluated the therapeutic effects of FOs
as a pharmaconutrient in the absence of standard administration of
food and water. The levels of SCr, BUN, KIM-1 and NGAL were
measured to evaluate kidney injury as described previously
(20).
Previous investigations have shown that ω3 FAs can
reduce inflammation, fibrosis and oxidative stress, which are
associated with renal function abnormalities (31-33). The protective effects of FOs on
AKI and their associated mechanism of action were examined. In the
CLP model, kidney injury was evident with distinct changes in the
levels of biochemical markers histopathological markers of renal
function and injury. The results indicated that treatment of the
GLP rats with FOs alleviated the changes in the expression of the
histopathological markers, increased the animal survival duration,
and decreased the levels of SCr, BUN, KIM-1 and NGAL. NGAL is a
reliable marker for GFR function (34,35). The reduced NGAL levels after
pretreatment of FOs suggests that FOs may alleviate renal injury by
improving GFR. The down-regulation of SCr and BUN further confirmed
this assumption. AKI patients with the existence of KIM-1
expression usually have poor prognosis (36). FOs reduce KIM-1 expression in
CLP-induced rats, suggesting the potential effects of FOs on
prognosis of sepsis-induced renal injury; however, further
investigation is required.
Excessive inflammation is a critical step during
septic shock (37). In sepsis,
multiple mediators of inflammation participate in organ injury
(37). The proinflammatory
cytokines IL-1β, IL-6 and TNFα are produced by damaged renal tubule
cells or extrarenal cells, serving as potential critical
contributors to renal injury (38). The current study confirmed that
the anti-inflammatory effects of FOs were mediated by regulation of
pro-inflammatory cytokine secretions as reported previously
(39). In addition, the data
demonstrated that CLP-induced septic intestinal injury could be
reduced by FOs treatment. H&E staining of intestinal tissues
derived from the CLP model group confirmed the induction of renal
injury as demonstrated by tissue destruction and inflammation. This
was reversed by dexamethasone and FOs treatment. In addition,
immunofluorescence staining indicated that FOs inhibited the
CLP-induced production of the pro-inflammatory factors, IL-1β, IL-6
and TNFα in the small intestine. These results supported the
aforementioned findings regarding the effects of external FOs on
sepsis-induced organ dysfunction. The inhibitory effects of FOs on
inflammation may suppress the progression of AKI.
Oxidative stress is a critical step required for the
pathogenesis of AKI (40).
Oxidative stress, as well as inflammation, can promote the severity
of sepsis (41). Excessive
oxidative stress is one of the main reason inflammation is induced
(41). Early in 1994, FOs have
been found to promote antioxidant enzyme expression in murine lupus
nephritis (39). Free radicals
increased the levels of lipid peroxidation and led to the
generation of reactive aldehyde metabolites, such as MDA (42). The antioxidant defense system
includes the enzymes (SOD and GSH-Px) that exert protective effects
on lipid peroxidation (43). In
the present study, FOs caused a significant inhibition in the
levels of MDA, and promoted the activity of SOD and GSH-Px in the
tissues of septic rats. This suggested that they could suppress
CLP-induced oxidative stress by increasing the activity levels of
the antioxidant enzymes. Therefore, FOs may attenuate renal injury
in the rat CLP model by inhibiting oxidative stress.
Furthermore, the present study reported the
suppressive effects of FOs on the induction of apoptosis caused by
CLP. The data showed that increased induction of apoptosis was
noted in renal tissues from the CLP group, while pretreatment of
the rats with FOs could effectively alleviate renal injury by
reducing the levels of apoptosis. The expression levels of specific
apoptotic proteins were determined. The Bcl-2 family of proteins
exerts key regulatory functions with regard to apoptosis induction.
The Bax to Bcl-2 ratio has been used as a marker for the activation
of caspase 3 (44,45). The present study indicated that
the expression levels of cleaved caspase-3 and Bax were increased,
whereas the expression levels of Bcl-2 were decreased in the CLP
model group. Moreover, pretreatment of the rats with FOs could
reverse the changes noted in the expression levels of these
proteins. The results indicated that FOs exhibited a strong
protective effect against CLP induced apoptosis.
It has been reported that FOs inhibit the NF-κB
signaling pathway in sepsis-induced liver injury (46). The activation of p38 has been
shown to increase the expression levels of the inflammatory
cytokines, such as TNF-α, IL-1β and IL-6 in microglial cells
(47). Furthermore, TNF-α serves
a critical role in the endothelial dysfunction of patients with
sepsis via activating the p38-MAPK and the NF-κB pathways (48). The suppression of the
TNFα/p38-MAPK/caspase-3 signaling pathway is also considered a key
mechanism in the prevention of sepsis-induced myocardial injury in
rats (49). In the present study,
the results indicated that FOs inhibited p38-MAPK and NF-κB
signaling activation by CLP. In addition, the JAK/STAT signaling
pathway is involved in immune and inflammatory responses, by
regulating the expression of several target genes (50,51). JAK is an upstream regulator of
STAT activation, which has been rarely investigated in
sepsis-induced organ dysfunction. In the current study, the
activation of JAK2/STAT3 signaling pathway was inhibited by FOs.
This was noted in the renal tissues from CLP rats. The results
indicated that FOs could inhibit the CLP-induced activation of the
NF-κB, p38-MAPK, and JAK2/STAT3 signaling pathways. Whether the
effects of FOs on renal function and induction of oxidative stress,
apoptosis and inflammation were mediated by these signaling
pathways remains unclear. Thus, further investigation is
required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to data
confidentiality in our hospital but are available from the
corresponding author on reasonable request.
Authors' contributions
ZL drafted the manuscript, data analysis, and made
substantial contributions to the design of the present study and
resolved the problems during investigation. JJ prepared
experimental samples and detection. XS prepared the manuscript, and
was involved in western blotting and ELISA. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiment protocol was approved by the
Commission for Animal Experimentation of the people's hospital of
Xishuangbanna Dai Nationality Autonomous Prefecture.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greenberg JH, Coca S and Parikh CR:
Long-term risk of chronic kidney disease and mortality in children
after acute kidney injury: A systematic review. BMC Nephrol.
15:1842014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honore PM, Jacobs R, Hendrickx I, Bagshaw
SM, Joannes-Boyau O, Boer W, De Waele E, Van Gorp V and Spapen HD:
Prevention and treatment of sepsis-induced acute kidney injury: An
update. Ann Intensive Care. 5:512015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Redivo DDB, Jesus CHA, Sotomaior BB,
Gasparin AT and Cunha JM: Acute antinociceptive effect of fish oil
or its major compounds, eicosapentaenoic and docosahexaenoic acids
on diabetic neuropathic pain depends on opioid system activation.
Behav Brain Res. 372:1119922019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao S, Ren J, Sun L, Gu G, Yuan Y and Li
J: Fish oil-supplemented parenteral nutrition prolongs survival
while beneficially altering phospholipids' fatty acid composition
and modulating immune function in rat sepsis. Shock. 36:184–190.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ostermann M and Chang RW: Acute kidney
injury in the intensive care unit according to RIFLE. Crit Care
Med. 35:1837–1843; quiz 1852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tancevski I, Nairz M, Duwensee K, Auer K,
Schroll A, Heim C, Feistritzer C, Hoefer J, Gerner RR, Moschen A,
et al: Fibrates ameliorate the course of bacterial sepsis by
promoting neutrophil recruitment via CXCR2. EMBO Mol Med.
6:810–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Napier BA, Andres-Terre M, Massis LM,
Hryckowian AJ, Higginbottom SK, Cumnock K, Casey KM, Haileselassie
B, Lugo KA, Schneider DS, et al: Western diet regulates immune
status and the response to LPS-driven sepsis independent of
diet-associated microbiome. Proc Natl Acad Sci USA. 116:3688–3694.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao YZ, Tu YY, Chen X, Wang BL, Zhong YX
and Liu MH: Protective effect of Ulinastatin against murine models
of sepsis: Inhibition of TNF-α and IL-6 and augmentation of IL-10
and IL-13. Exp Toxicol Pathol. 64:543–547. 2012. View Article : Google Scholar
|
|
10
|
Hotchkiss RS, Monneret G and Payen D:
Sepsis-induced immunosuppression: From cellular dysfunctions to
immunotherapy. Nat Rev Immunol. 13:862–874. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saglimbene VM, Wong G, van Zwieten A,
Palmer SC, Ruospo M, Natale P, Campbell K, Teixeira-Pinto A, Craig
JC and Strippoli GFM: Effects of omega-3 polyunsaturated fatty acid
intake in patients with chronic kidney disease: Systematic review
and meta-analysis of randomized controlled trials. Clin Nutr. Mar
14–2019, Epub ahead of print.
|
|
12
|
Zhou Q, Zhang Z, Wang P, Zhang B, Chen C,
Zhang C and Su Y: EPA+DHA, but not ALA, improved lipids and
inflammation status in hypercholesterolemic adults: A randomized,
double-blind, placebo-controlled trial. Mol Nutr Food Res. 63:pp.
e18011572019, View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shih JM, Shih YM, Pai MH, Hou YC, Yeh CL
and Yeh SL: Fish oil-based fat emulsion reduces acute kidney injury
and inflammatory response in antibiotic-treated polymicrobial
septic mice. Nutrients. 8:1652016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quoilin C, Mouithys-Mickalad A, Lecart S,
Fontaine-Aupart MP and Hoebeke M: Evidence of oxidative stress and
mitochondrial respiratory chain dysfunction in an in vitro model of
sepsis-induced kidney injury. Biochim Biophys Acta. 1837.1790–1800.
2014.
|
|
15
|
Abd-Ellatif RN, Hegab II, Atef MM, Sadek
MT and Hafez YM: Diacerein protects against glycerol-induced acute
kidney injury: Modulating oxidative stress, inflammation, apoptosis
and necroptosis. Chem Biol Interact. 306:47–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heller AR, Rössler S, Litz RJ, Stehr SN,
Heller SC, Koch R and Koch T: Omega-3 fatty acids improve the
diagnosis-related clinical outcome. Crit Care Med. 34:972–979.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leelahavanichkul A, Souza AC, Street JM,
Hsu V, Tsuji T, Doi K, Li L, Hu X, Zhou H, Kumar P, et al:
Comparison of serum creati-nine and serum cystatin C as biomarkers
to detect sepsis-induced acute kidney injury and to predict
mortality in CD-1 mice. Am J Physiol Renal Physiol. 307:F939–F948.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nair S, O'Brien SV, Hayden K, Pandya B,
Lisboa PJ, Hardy KJ and Wilding JP: Effect of a cooked meat meal on
serum creati-nine and estimated glomerular filtration rate in
diabetes-related kidney disease. Diabetes Care. 37:483–487. 2014.
View Article : Google Scholar
|
|
19
|
Blantz RC: Pathophysiology of pre-renal
azotemia. Kidney Int. 53:512–523. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo QH, Chen ML, Chen ZL, Huang C, Cheng
AC, Fang J, Tang L and Geng Y: Evaluation of KIM-1 and NGAL as
early indicators for assessment of gentamycin-induced
nephrotoxicity in vivo and in vitro. Kidney Blood Press Res.
41:911–918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rysz J, Gluba-Brzózka A, Franczyk B,
Jabłonowski Z, Ciałkowska-Rysz A, et al: Novel biomarkers in the
diagnosis of chronic kidney disease and the prediction of its
outcome. Int J Mol Sci. 18:pp. pii E17022017, View Article : Google Scholar
|
|
22
|
Yuan SM: Acute kidney injury after cardiac
surgery: Risk factors and novel biomarkers. Braz J Cardiovasc Surg.
34:352–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doi K, Leelahavanichkul A, Yuen PS and
Star RA: Animal models of sepsis and sepsis-induced kidney injury.
J Clin Invest. 119:2868–2878. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stevens NE, Chapman MJ, Fraser CK, Kuchel
TR, Hayball JD and Diener KR: Therapeutic targeting of HMGB1 during
experimental sepsis modulates the inflammatory cytokine profile to
one associated with improved clinical outcomes. Sci Rep.
7:58502017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng L, Li L, Lu S, Li K, Su Z, Wang Y,
Fan X, Li X and Zhao G: The protective effect of dexmedetomidine on
LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB
and PI3K/Akt/mTOR pathways. Mol Immunol. 94:7–17. 2018. View Article : Google Scholar
|
|
27
|
Jia Y, Li Z, Feng Y, Cui R, Dong Y, Zhang
X, Xiang X, Qu K, Liu C and Zhang J: Methane-rich saline
ameliorates sepsis-induced acute kidney injury through
anti-inflammation, antioxidative, and antiapoptosis effects by
regulating endoplasmic reticulum stress. Oxid Med Cell Longev.
2018.4756846:2018.
|
|
28
|
Hussain T, Tan B, Yin Y, Blachier F,
Tossou MC and Rahu N: Oxidative stress and inflammation: What
polyphenols can do for us? . Oxid Med Cell Longev.
2016.7432797:2016.
|
|
29
|
Tian T, Zhao Y, Huang Q and Li J: N-3
polyunsaturated fatty acids improve inflammation via inhibiting
sphingosine kinase 1 in a rat model of parenteral nutrition and
CLP-induced sepsis. Lipids. 51:271–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shalmani AA, Ghahremani MH, Jeivad F,
Shadboorestan A, Hassanzadeh G, Beh-Pajooh A, Ganbari-Erdi M,
Kasirzadeh S, Mojtahedzadeh M and Sabzevari O: Monomethyl fumarate
alleviates sepsis-induced hepatic dysfunction by regulating
TLR-4/NF-κB signalling pathway. Life Sci. 215:152–158. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peake JM, Gobe GC, Fassett RG and Coombes
JS: The effects of dietary fish oil on inflammation, fibrosis and
oxidative stress associated with obstructive renal injury in rats.
Mol Nutr Food Res. 55:400–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
An WS, Kim HJ, Cho KH and Vaziri ND:
Omega-3 fatty acid supplementation attenuates oxidative stress,
inflammation, and tubulointerstitial fibrosis in the remnant
kidney. Am J Physiol Renal Physiol. 297:F895–F903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jangale NM, Devarshi PP, Bansode SB,
Kulkarni MJ and Harsulkar AM: Dietary flaxseed oil and fish oil
ameliorates renal oxidative stress, protein glycation, and
inflammation in streptozotocin-nicotinamide-induced diabetic rats.
J Physiol Biochem. 72:327–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bolignano D, Donato V, Coppolino G, Campo
S, Buemi A, Lacquaniti A and Buemi M: Neutrophil
gelatinase-associated lipocalin (NGAL) as a marker of kidney
damage. Am J Kidney Dis. 52:595–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McIlroy DR, Wagener G and Lee HT:
Neutrophil gelatinase-associated lipocalin and acute kidney injury
after cardiac surgery: The effect of baseline renal function on
diagnostic performance. Clin J Am Soc Nephrol. 5:211–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tu Y, Wang H, Sun R, Ni Y, Ma L, Xv F, Hu
X, Jiang L, Wu A, Chen X, et al: Urinary netrin-1 and KIM-1 as
early biomarkers for septic acute kidney injury. Ren Fail.
36:1559–1563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
You B, Zhang YL, Luo GX, Dang YM, Jiang B,
Huang GT, Liu XZ, Yang ZC, Chen Y, Chen J, et al: Early application
of continuous high-volume haemofiltration can reduce sepsis and
improve the prognosis of patients with severe burns. Crit Care.
22:1732018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SW, Chen SW, Kim M, Brown KM, Kolls
JK, D'Agati VD and Lee HT: Cytokines induce small intestine and
liver injury after renal ischemia or nephrectomy. Lab Invest.
91:63–84. 2011. View Article : Google Scholar
|
|
39
|
Chandrasekar B and Fernandes G: Decreased
pro-inflammatory cytokines and increased antioxidant enzyme gene
expression by omega-3 lipids in murine lupus nephritis. Biochem
Biophys Res Commun. 200:893–898. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pavlakou P, Liakopoulos V, Eleftheriadis
T, Mitsis M and Dounousi E: Oxidative stress and acute kidney
injury in critical illness: Pathophysiologic
mechanisms-biomarkers-interventions, and future perspectives. Oxid
Med Cell Longev. 2017.6193694:2017.
|
|
41
|
Galley HF: Oxidative stress and
mitochondrial dysfunction in sepsis. Br J Anaesth. 107:57–64. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Levent G, Ali A, Ahmet A, Polat EC, Aytaç
C, Ayşe E and Ahmet S: Oxidative stress and antioxidant defense in
patients with chronic hepatitis C patients before and after
pegylated interferon alfa-2b plus ribavirin therapy. J Transl Med.
4:252006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Segura-Aguilar J, Cortes-Vizcaino V,
Llombart-Bosch A, Ernster L, Monsalve E and Romero FJ: The levels
of quinone reductases, superoxide dismutase and glutathione-related
enzymatic activities in diethylstilbestrol-induced carcinogenesis
in the kidney of male Syrian golden hamsters. Carcinogenesis.
11:1727–1732. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McClintock DS, Santore MT, Lee VY,
Brunelle J, Budinger GR, Zong WX, Thompson CB, Hay N and Chandel
NS: Bcl-2 family members and functional electron transport chain
regulate oxygen deprivation-induced cell death. Mol Cell Biol.
22:94–104. 2002. View Article : Google Scholar
|
|
45
|
Dang J, Jia R, Tu Y, Xiao S and Ding G:
Erythropoietin prevents reactive oxygen species generation and
renal tubular cell apop-tosis at high glucose level. Biomed
Pharmacother. 64:681–685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li CC, Yang HT, Hou YC, Chiu YS and Chiu
WC: Dietary fish oil reduces systemic inflammation and ameliorates
sepsis-induced liver injury by up-regulating the peroxisome
proliferator-activated receptor gamma-mediated pathway in septic
mice. J Nutr Biochem. 25:19–25. 2014. View Article : Google Scholar
|
|
47
|
Taves S, Berta T, Liu DL, Gan S, Chen G,
Kim YH, Van de Ven T, Laufer S and Ji RR: Spinal inhibition of p38
MAP kinase reduces inflammatory and neuropathic pain in male but
not female mice: Sex-dependent microglial signaling in the spinal
cord. Brain Behav Immun. 55:70–81. 2016. View Article : Google Scholar
|
|
48
|
Liang Y, Li X, Zhang X, Li Z, Wang L, Sun
Y, Liu Z and Ma X: Elevated levels of plasma TNF-α are associated
with microvas-cular endothelial dysfunction in patients with sepsis
through activating the NF-κB and p38 mitogen-activated protein
kinase in endothelial cells. Shock. 41:275–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang M, Wang X, Bai B, Zhang R, Li Y and
Wang Y: Oxymatrine protects against sepsis-induced myocardial
injury via inhibition of the TNF-α/p38-MAPK/caspase-3 signaling
pathway. Mol Med Rep. 14:551–559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pena G, Cai B, Liu J, van der Zanden EP,
Deitch EA, de Jonge WJ and Ulloa L: Unphosphorylated STAT3
modulates alpha 7 nico-tinic receptor signaling and cytokine
production in sepsis. Eur J Immunol. 40:2580–2589. 2010. View Article : Google Scholar
|
|
51
|
Yuan FH, Chen YL, Zhao Y, Liu ZM, Nan CC,
Zheng BL, Liu XY and Chen XY: microRNA-30a inhibits the liver cell
proliferation and promotes cell apoptosis through the JAK/STAT
signaling pathway by targeting SOCS-1 in rats with sepsis. J Cell
Physiol. 234:17839–17853. 2019.PubMed/NCBI
|