Introduction
Stevioside, a natural sweet-tasting glycoside, is
found in Stevia rebaudiana. Isosteviol, a derivate of
stevioside, has been demonstrated to exhibit a variety of
beneficial pharmacological effects (1-5).
Isosteviol sodium salt (STVNa), which is a beyerane diterpene, a
more soluble and injectable form of isosteviol, has recently been
synthesized via acid hydrolysis of stevioside, and it has been
determined that STVNa exhibits neuro- and cardio-protective
properties (1-5). It has also been indicated that STVNa
attenuates right ventricular hypertrophy and pulmonary artery
remodeling in an experimental model of transverse aortic
constriction and ameliorates diabetic cardiomyopathy (6,7).
However, whether STVNa exhibits an effect on the development of
left ventricular hypertrophy (LVH) is, to the best of our
knowledge, yet to be determined.
LVH is defined as the enlargement and thickening of
the left ventricle walls, which form the main contractile chamber
of the heart. LVH is the ultimate outcome in a variety of
pathological states, including hypertension, valvular disease,
myocardial infarction and cardiomyopathy (8). This condition is usually associated
with the activation of β-adrenergic signaling and the consequent
increase in oxidative stress, protein synthesis, proto-oncogene
expression and the stimulation of mitogen activated protein kinases
and phosphatidyl inositol-3 kinases (9). The development of pathological LVH
is initially beneficial as it allows the heart to maintain its
cardiac pump function despite abnormal pressure and/or volume load.
However, this ultimately leads to depression of the intrinsic
contractile state of the myocardium and subsequent heart failure
(8). Additional therapeutic
strategies, which prevent LVH and heart failure are urgently
required (10).
Isoprenaline (Iso), a non-selective β-adrenoceptor
agonist, is widely used to induce LVH in animal experimental models
of cardiac hypertrophy (11-13). This model successfully mimics
sustained adrenergic stimulation, which is a major mechanism in the
pathogenesis of maladaptive cardiac hypertrophy (14). In the current study, this
particular model was used to assess whether STVNa modifies the
development of myocardial hypertrophy and if it does, to determine
the underlying mechanism governing this.
Materials and methods
Materials
All chemicals (including caffeine) used in the
current study were purchased from Sigma-Aldrich; Merck KGaA, unless
otherwise stated. H2DCFDA, JC-1 and Fluo-4 ester were purchased
from Invitrogen; Thermo Fisher Scientific, Inc. mitoTEMPO was
purchased from Enzo Life Sciences, Inc. Medium-199 (M199) was
purchased from Thermo Fisher Scientific, Inc. PCR reagent kit,
primers and markers were purchased from Takara Biotechnology Co.,
Ltd. STVNa, which is the sodium salt of isosteviol and is a
beyerane diterpene, was obtained via acid hydrolysis of stevioside,
and was synthesized by the Chemical Synthesis Group of Institute of
Biomedical and Pharmaceutical Sciences, Guangdong University of
Technology (Guangzhou, China).
Rats and experimental protocol
Sixty male Sprague-Dawley rats (weight, 200-250 g;
age, 6 weeks) were obtained from the Experimental Animal Center of
Guangzhou University of Chinese Medicine (Guangzhou, China). All
animal experimental protocols complied with the Guide for the Care
and Use of Laboratory Animals, which was published by the National
Institutes of Health. The current study was approved by the
Institutional Animal Research Committee of South China University
of Technology (Guangzhou, China). Sprague-Dawley rats were housed
in a room maintained at 24°C and 50% humidity with a 12-h
light/dark cycle and provided with standard food and water ad
libitum. Rats were randomly divided into three groups (60 in
total): Control group, treated with vehicle (0.9% NaCl; control)
(n=20); Iso group, treated with isoprenaline (5 mg/kg; Iso) (n=20);
Iso + STVNa group, treated with isoprenaline (5 mg/kg) with
isosteviol sodium (4 mg/kg; Iso + STVNa) (n=20). Vehicle and
compounds were injected intraperitoneally daily for 7 days, as
previously described (15).
Heart weight index measurement
Rats were weighed (body weight, BW), anesthetized by
sodium pentobarbital [intra-peritoneal (IP), 50 mg/kg] and
heparinized (IP, 1,000 U/kg). Rats were sacrificed by overdose of
sodium pentobarbital (>150 mg/kg). The thoracic cavity was
subsequently opened and the heart was harvested in a clean glass
dish, washed with cold saline solution and weighed [heart weight,
(HW)]. The atrium was cut off and the ventricle was separated and
weighed [left ventricle weight (LW)]. The tibia length was also
measured (Tibia). The heart weight indexes are represented by
ratios of HW/BW, HW/Tibia and LW/Tibia.
Histological analysis
Rat hearts were fixed in 10% formalin at 25°C for 8
h. Transverse sections were embedded in paraffin and were cut into
5 mm sections. Hematoxylin and eosin (H&E; hematoxylin staining
for 5 min, eosin staining for 2 min, at 25°C) were used to assess
the cardiomyocyte cross-sectional area. Images were captured with a
light microscope and analyzed using ImageJ 1.48 (National
Institutes of Health). A total of >50 cells were counted in each
independent heart from each group.
Isolation of cardiomyocytes and cells
treatment
Ventricular myocytes were isolated from untreated,
wild-type male Sprague-Dawley rats (200-250 g) as described
previously (16), with some
modifications. Heparinized (IP; 1,000 U/kg) animals were
anesthetized using sodium pentobarbital (IP, 50 mg/kg). Excised
hearts were transferred to a Langendorf perfusion apparatus and
perfused with Ca2+-free Tyrode's solution (NaCl 137 mM;
KCl 5.4 mM; NaH2PO4 1.2 mM; MgCl2.
6H2O 1.2 mM; HEPES 20 mM; taurine 30 mM; glucose 20 mM;
pH 7.4) for 5 min. The perfusion solution was then switched to
Ca2+-free Tyrode's solution containing collagenase II
(0.4 mg/ml) and protease (Sigma-Aldrich; Merck KGaA; 0.1 mg/ml).
After 30 min, ventricles were cut into small pieces, incubated in a
37°C water bath and separated into individual cardiomyocytes via
slow pipetting. The cells were filtered through a 200 nm mesh and
settled in Tyrode's solution containing 1.2 mM Ca2+ and
bovine serum albumin (BSA; 0.1%). Cells were subsequently
re-suspended in M199 (Invitrogen; Thermo Fisher Scientific, Inc.)
containing 10% FBS supplemented with BSA (0.1%) and transferred to
laminin coated culture dishes. After a 1.5 h of incubation in a
CO2 incubator (5% CO2; 95% O2),
the medium was replaced with serum free M199 (pH 7.4) supplemented
with 0.1% BSA. To induce hypertrophy, cells were treated with 5 µM
Iso (Sigma-Aldrich; Merck KGaA) for 24 h. To investigate the effect
of STVNa on Iso-induced hypertrophy, Iso (5 µM)-treated cells were
co-treated with a variety of STVNa concentrations (1, 5, 10 and 20
µM). The most effective STVNa concentration (5 µM; Fig. S1) was used for subsequent
experimentation.
Measurement of ROS, mitochondrial
membrane potential and calcium
For ROS measurement, cardiomyocytes were loaded with
10 µM H2DCFDA in serum-free medium at 37°C for 20 min in the dark
and then resuspended in 1 mM Ca2+ Tyrode's solution to
wash out residues of the dye. DCFDA was excited at 480 nm and
measured at 525 nm. For mitochondrial membrane potential
measurement, cardiomyocytes were incubated with 5 µM JC-1 at 37°C
in the dark for 30 min. Cells were washed twice in 1 mM
Ca2+ Tyrode's solution. Red fluorescence was exited at
585 nm and measured at 590 nm. Green fluorescence was exited at 514
nm and measured at 529 nm. For calcium measurement, cardiomyocytes
were exposed to 1 µM Fluo-4 AM at 37°C for 40 min for loading and
then washed twice in 1 mM Ca2+ Tyrode's solution. Fluo-4
was excited at 488 nm and measured at >520 nm. For analysis,
intensity of fluorescence for targeted cells was directly read
using the Zeiss LSM 710 confocal software (ZEN version 2011, Carl
Zeiss Meditec AG).
Measurement of cell surface area
Phase contrast images that were captured using a
light Olympus IX83 microscope (Olympus Corporation) were used to
measure the surface area of different groups using Image Pro-Plus
6.0 (National Institutes of Health) software. A total of 120 cells
from six different animals were analyzed to determine the
morphological changes that were induced by Iso.
Measurement of mRNA levels
The effect of STVNa on the hypertrophic response of
cardiomyocytes to Iso stimulus was assessed by monitoring BNP mRNA
expression using reverse transcription (RT)-quantitative (q) PCR.
Total RNA was extracted from cells using RNAiso Plus (Takara
Biotechnology Co., Ltd.; Total RNA extraction reagent) according to
the manufacturer's protocol. The concentration was determined by
measuring the absorbance at 260 nm and RNA purity was determined by
measuring 260/280 ratio using a NanoDrop 2000c Spectrophotometer
(Thermo Fisher Scientific, Inc.). Total RNA (0.5 µg) was
used for RT with the PrimeScript II First Strand cDNA synthesis kit
(cat. no. 6210A; Takara Biotechnology Co., Ltd.), following the
manufacturer's protocol. qPCR was performed by using ChamQ SYBR PCR
kit Q311-01 (Vazyme Biotech Co., Ltd.). Relative quantification of
gene expression was normalized to GAPDH. The nucleotide sequences
of the primers used were: BNP forward, 5′-CTG TGA CGG GCT GAG
GTT-3′ and reverse, 5′-GCA AGT TTG TGC TGG AAG-3′; GAPDH forward,
5′-GCA AGT TCA ACG GCA CAG-3′, and reverse, 5′-CGC CAG TAG ACT CCA
CGA C-3′.
Analysis of cardiomyocyte contractile
function
Cell contraction was recorded in the frame-scanning
mode and time-series mode using a Zeiss LSM 710 confocal
microscope. Cells were stimulated to contract at 1 Hz and scanned
for 5 min (100 ms/Frame). The rate of contraction and shortening
were measured and analyzed using the Zeiss LSM Imaging processing
software (ZEN version 2011; Carl Zeiss Meditec AG).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean from three experimental repeats. Statistical analysis was
performed using a one-way analysis of variance followed by the
Tukey post-hoc test (SigmaPlot v14; Jandel Corporation). P<0.05
was considered to indicate a statistically significant difference.
For the analysis of HW/BW, cross-sectional area of H&E
staining, cell surface area of cardiomyocytes, BNP mRNA expression,
ROS fluorescence intensity and Ca2+ fluorescence
intensity, the raw mean value of the control group was used as a
reference value, and the raw value of the control group was divided
by this value. The raw mean value of the control group was set at 1
and the data of the other groups were presented as the fold-change
of the control.
Results
STVNa prevents the development of
Iso-induced cardiac hypertrophy
Experiment were carried out using rat heart tissues.
Treatment with Iso significantly increased HW/BW (1.00±0.02 vs.
1.53±0.05; P<0.05; n=6; Fig.
1), HW/tibia (0.23±0.01 g/cm vs. 0.33±0.01 g/cm; P<0.05;
n=6; Fig. 1) and LV/tibia
(0.15±0.01 g/cm vs. 0.23±0.01 g/cm; P<0.05; n=6; Fig. 1) ratios. This effect was inhibited
by STVNa (Fig. 1; HW/BW,
1.53±0.05 vs. 1.35±0.06; n=6; P=0.05; HW/tibia, 0.33±0.01 g/cm vs.
0.29±0.01 g/cm; n=6; P<0.05; LV/tibia, 0.23±0.01 g/cm vs.
0.20±0.01 g/cm; n=6; P=0.01). The histological analysis of
myocardial tissues demonstrated that cardiomyocyte cross sectional
areas were significantly increased in mice treated with Iso
(1.00±0.01 fold-change vs. 2.05±0.04 fold-change; n=6; P<0.05;
Fig. 2) and this increase was
partly inhibited by STVNa (1.56±0.02 fold-change; n=6; P>0.05
vs. control; Fig. 2). Similar
results were obtained subsequent to the examination of the effects
of Iso and STVNa treatments on cardiomyocytes size in vitro,
which were carried out using cardiomyocytes isolated from rats.
Although STVNa did not solely affect cardiomyocyte surface area
(control, 1.01±0.05 fold-change; STVNa, 1.04±0.03 fold-change, n=7
for each; P=0.639; Fig. 3) it
prevented an increase in this parameter that was induced by Iso
(Iso, 1.39±0.04 fold-change; Iso + STVNa, 1.15±0.03 fold-change;
n=7 for each; P<0.05; Fig.
3).
BNP mRNA is a well-established biomarker for cardiac
hypertrophy and heart failure (17). mRNA measurement was carried out
using cardiomyocytes isolated from untreated, wild-type rats. Iso
treatment significantly increased BNP mRNA levels in cardiomyocytes
(control, 1.00±0.10 fold-change; Iso, 2.25±0.25 fold-change; n=5
for each; P<0.05; Fig. 4).
STVNa prevented this increase (1.61±0.25 fold-change; n=5;
P<0.05 vs. control; Fig. 4)
although it did not exhibit any effect on BNP mRNA levels when used
on its own (1.07±0.08 fold-change; n=5; P>0.05 vs. control;
Fig. 4).
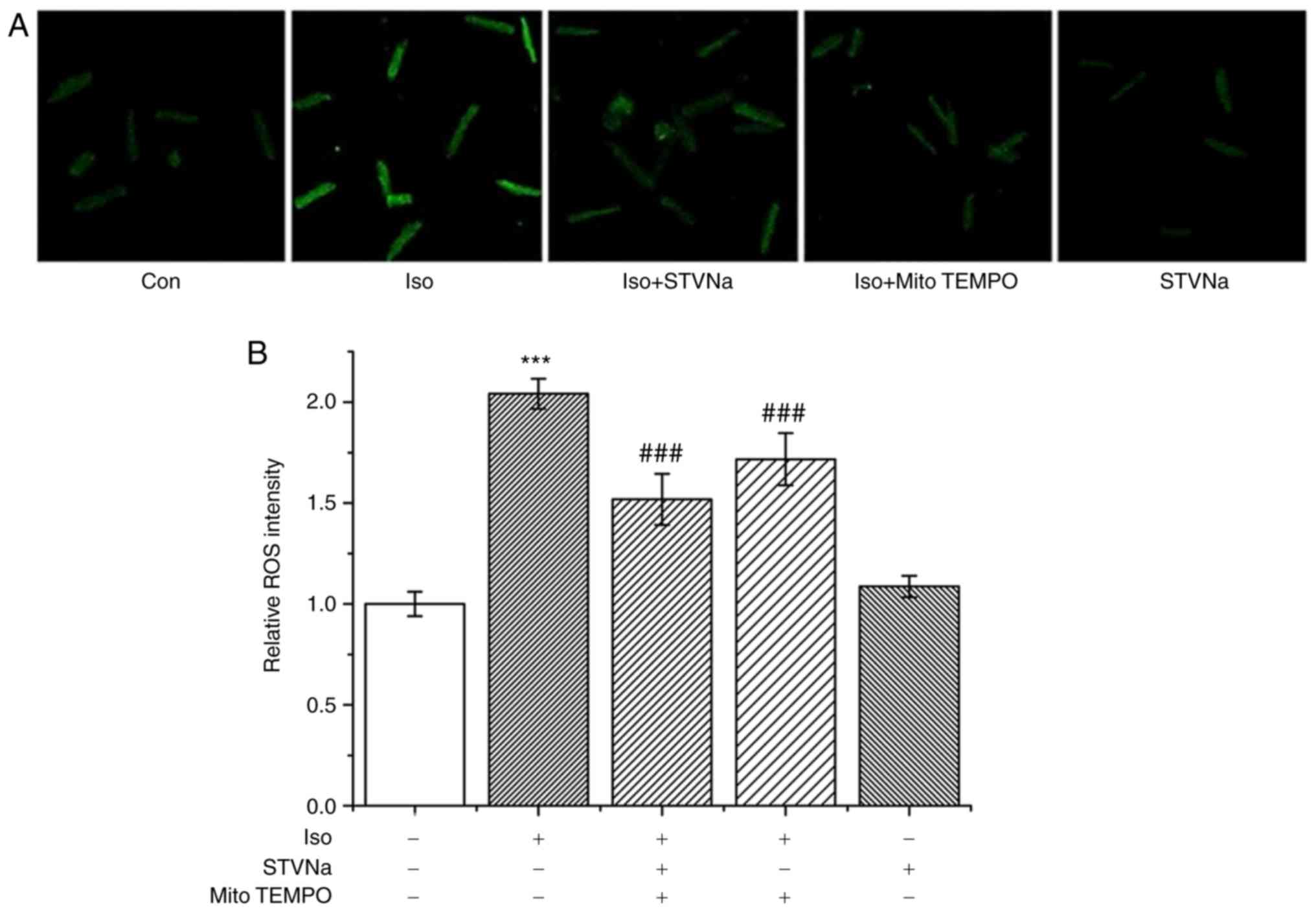
STVNa reduces ROS production in
cardiomyocytes treated with Iso
Iso treatment significantly increased ROS production
in cardiomyocytes as indicated by DCFH fluorescence (from 1.00±0.06
fold-change under control conditions to 2.04±0.07 fold-change when
treated with Iso; n=6 for each; P<0.001; Fig. 5). STVNa did not solely affect ROS
production (1.09±0.05 fold-change; n=6; P=0.524 vs. the control;
Fig. 5). However, STVNa prevented
the effect exhibited by Iso (1.52±0.13 fold-change; n=6; P=0.004
vs. Iso-treated group; Fig. 5) in
a similar manner to mitoTEMPO, a mitochondrial-targeted antioxidant
(1.72±0.13 fold-change; n=6; P=0.274 vs. Iso + STVNa-treated group;
Fig. 5). Experiments were carried
out using cardiomyocytes isolated from untreated, wild-type
rats.
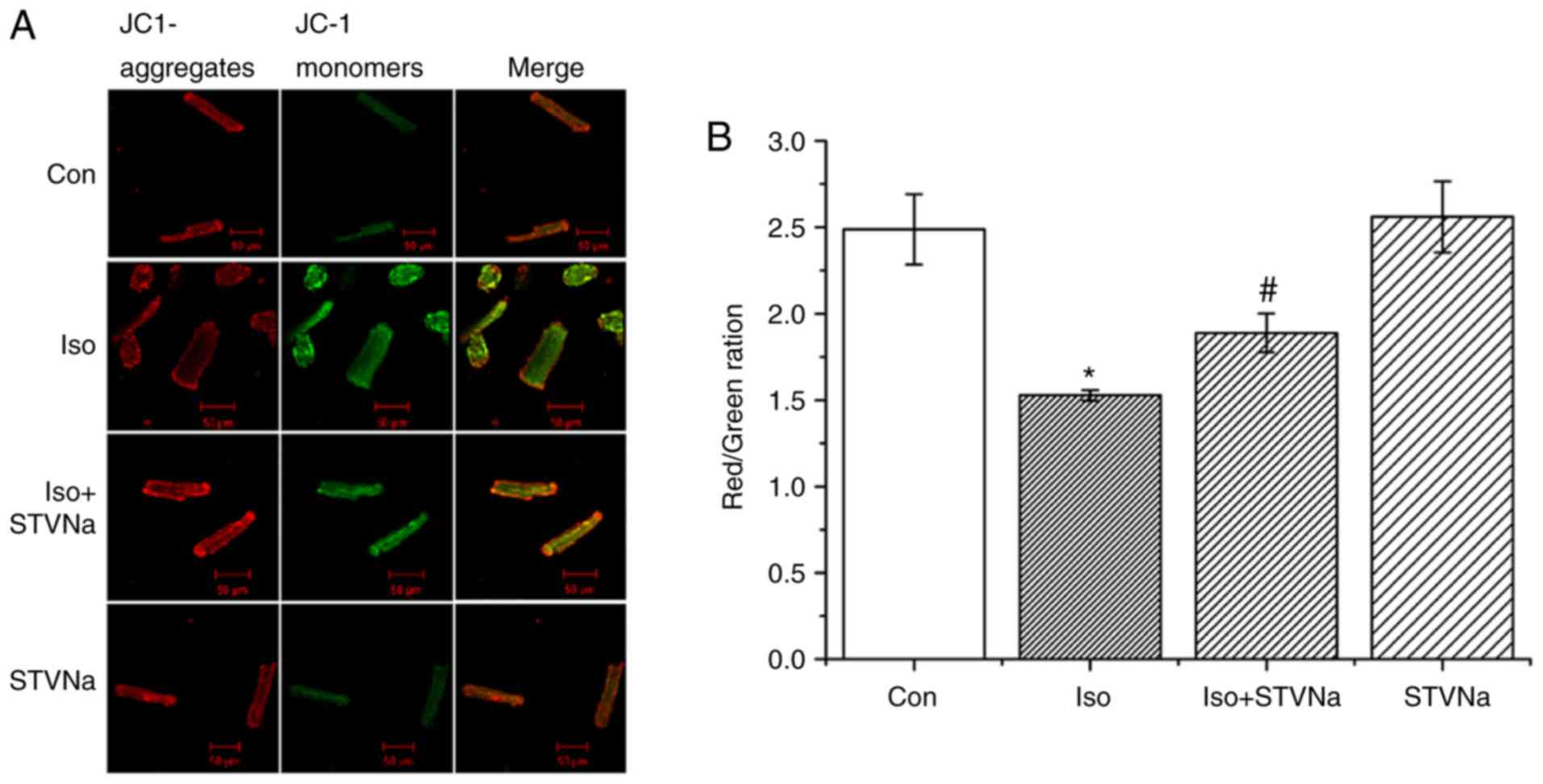
STVNa prevents mitochondrial membrane
depolarization induced by Iso treatment
Treatment with Iso led to mitochondrial membrane
depolarization in wild-type cardiomyocytes (isolated from untreated
wild-type rats) as indicated by the significant decrease that was
observed in channels ratio (from 2.49±0.20 under control conditions
to 1.53±0.03 following Iso treatment; n=6 for each; P<0.05;
Fig. 6). STVNa did not solely
affect mitochondrial membrane potential (2.56±0.20; n=6; P=0.746
vs. control; Fig. 6). However,
STVNa inhibited the effect of Iso treatment (1.89±0.11; n=6;
P=0.011 vs. Iso group; Fig.
6).
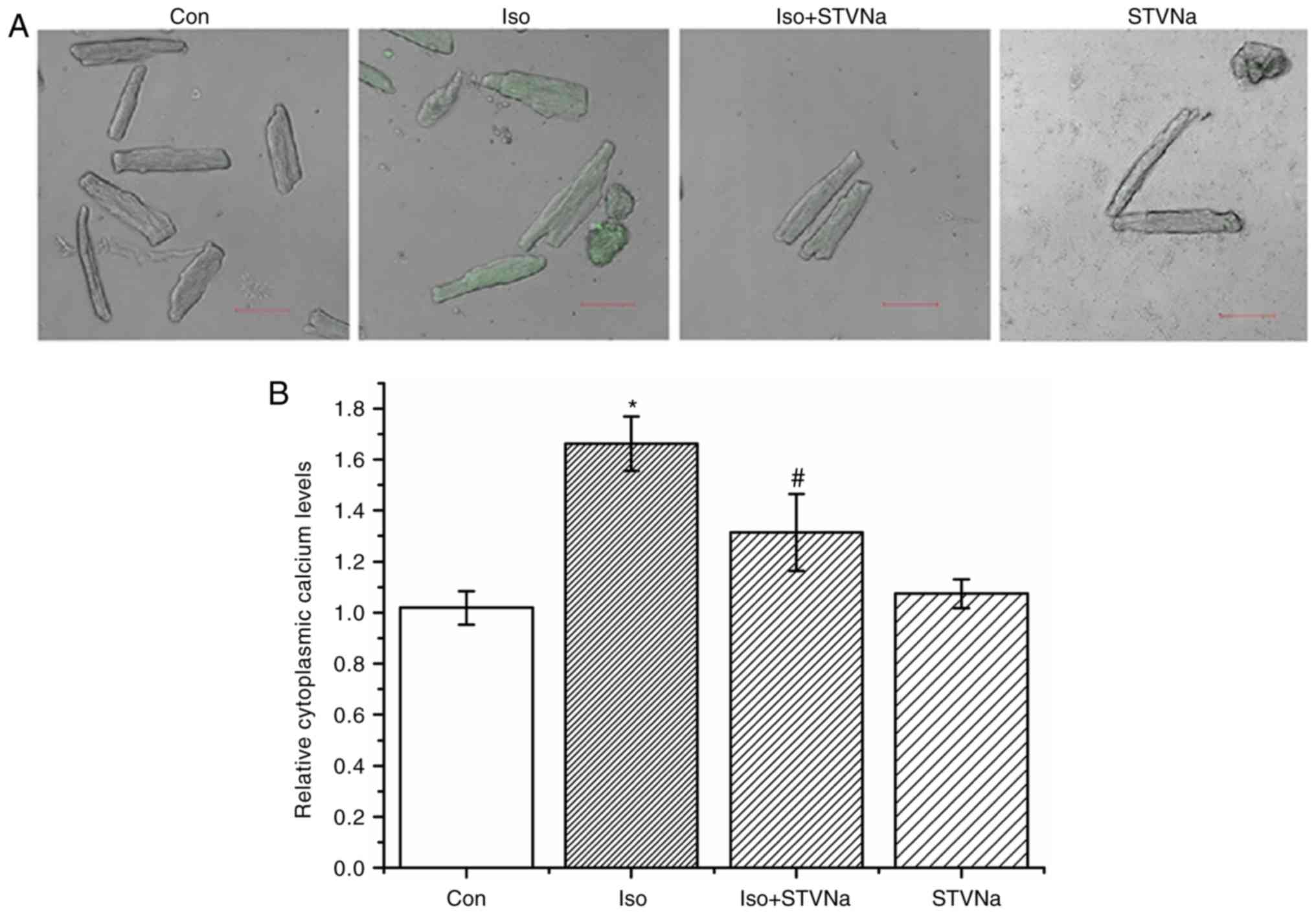
STVNa prevents Ca2+ loading
and impaired Ca2+ dynamics induced by Iso treatment
Cardiomyocytes were isolated from untreated
wild-type rats. Treatment with Iso induced intracellular
Ca2+ loading as reflected by the significant increase
observed in Fluo-4 fluorescence (from 1.02±0.07 fold-change under
control conditions to 1.66±0.11 fold-change when treated with Iso;
n=6 for each; P<0.05; Fig. 7).
STVNa did not solely affect intracellular Ca2+
(1.07±0.06 fold-change; n=6; P=0.707 vs. control; Fig. 7). However, STVNa inhibited the
effect of Iso treatment (1.31±0.15 fold-change; n=6; P=0.04 vs. Iso
group alone; Fig. 7).
To examine any potential changes in Ca2+
dynamics, the transient Ca2+ was assessed in
cardiomyocytes. The amplitude of calcium transient (F/F0)
significantly decreased (from 2.64±0.25 under control conditions to
1.67±0.07 when treated with Iso; n=9 for each; P=0.013; Fig. 8) and the time of Ca2+
uptake was significantly extended (T50 values were 0.29±0.02 sec
under control conditions and 0.38±0.02 sec when treated with Iso;
n=9 for each; P=0.006; Fig. 8).
Co-treatment with STVNa prevented the effects of Iso treatment
(F/F0, 2.67±0.30; n=8; P=0.860 vs. control; T50, 0.28±0.02 S; n=9;
P=0.711 vs. control; Fig. 8).
STVNa prevents the sarcoplasmic reticulum
(SR) Ca2+ depletion that is induced by Iso
Caffeine (10 mM) was used to measure the quantities
of Ca2+ stored in SR. Iso significantly decreased the
quantity of Ca2+ in SR (from 12.65±0.42 under control
conditions to 8.03±0.55 when treated with Iso; n=6 for each;
P<0.05; Fig. 9). STVNa
inhibited this effect (10.30±0.43; n=6; P=0.004 vs. the Iso group;
Fig. 9).
STVNa prevents the Iso-induced impairment
of cardiomyocytes contractile function
Treatment with Iso decreased shortening and rate of
contraction of wild-type cardiomyoctyes (shortening, from
6.76±0.67% under control conditions to 5.13±0.30% when treated with
Iso; n=8 for each; P=0.067; rate of contraction, from
0.28±0.03%.S-1 under control conditions to
0.17±0.02%.S-1 when treated with Iso; n=8 for each;
P=0.009; Fig. 10). STVNa
inhibited both effects exhibited by Iso treatment (shortening,
7.81±0.49%; n=8; P=0.004 vs. Iso; rate of contraction,
0.30±0.02%.S-1; n=8; P=0.003 vs. Iso; Fig. 10).
Discussion
A chronic increase in sympathetic activation occurs
during hypertension, obesity, sleep apnea and mental stress, and
this can promote the development of cardiac hypertrophy and heart
failure through the sustained stimulation of β-adrenergic receptors
(18). The results of the current
study demonstrated that sustained stimulation with Iso induces
cardiac hypertrophy, which is in agreement with the
well-established features of the experimental model used (11). The current study revealed that the
induction of cardiac hypertrophy by Iso was associated with i)
increased ROS, ii) mitochondrial membrane depolarization, iii)
intracellular Ca2+ loading, iv) impaired Ca2+
transients and v) impaired cardiac contractility.
A central mechanism that is associated with the
development of cardiac hypertrophy is an increase in ROS and the
subsequent oxidative stress (19). The activation of β-adrenoreceptors
has been specifically linked with ROS generation and cardiac
hypertrophy (20,21). Oxidative stress has been indicated
to activate extracellular signal regulated kinase 1/2 and stimulate
protein synthesis in ventricular remodeling (13,22). It has also been suggested that
compounds attenuating oxidative stress may also attenuate cardiac
hypertrophy (23). In the present
study, it was demonstrated that STVNa did not solely affect ROS
levels, but prevented an Iso-induced increase in ROS, making this
compound a potential therapeutic candidate for use in the
prevention of cardiac hypertrophy.
In addition to ATP synthesis, the electron transport
chain of mitochondria is a significant source of ROS (24), which, in turn, can damage
mitochondria and affect the activity and function of mitochondrial
ion channels. Any alterations in mitochondrial ion channel function
and mitochondrial homeostasis is reflected in the mitochondrial
membrane potential. Mitochondrial membrane depolarization is a
well-established indicator of disturbed mitochondrial homeostasis
(25). The results of the current
study indicated that Iso-treatment induced mitochondrial membrane
depolarization, which is supported by previous studies (26-28) that have used this experimental
model. STVNa did not solely affect mitochondrial membrane potential
but prevented the mitochondrial membrane depolarization that was
induced by Iso. These results suggested that STVNa prevented
increases in ROS and consequently, mitochondrial damage.
Intracellular Ca2+ homeostasis is crucial
for cardiac contractile function (29). Intracellular Ca2+
levels have been demonstrated to reflect the overall metabolic
condition of cardiomyocytes (30,31). In the present study,
Iso-pretreatment was revealed to increase intracellular
Ca2+ and impair intracellular Ca2+ transients
and cardiomyocytes contractility. These effects exhibited by Iso
were expected, due to the results of multiple studies that
indicated that sustained stimulation with β-agonists increased
intracellular Ca2+ levels and impaired contractility
(32-34). The sustained increase in
Ca2+ activated the protein phosphatase calcineurin and
its target, the NFAT family of transcription factors, which are
critical mediators of pathological hypertrophy (35). Links between mitochondrial
impairment, intracellular Ca2+ loading, impaired
contractility and cardiac hypertrophy resulting in heart failure
are well established (36). STVNa
was revealed to prevent all negative events associated with
sustained activation of β-adrenoceptors, including cardiac
hypertrophy, ROS production, mitochondrial membrane depolarization,
impaired Ca2+ homeostasis and cardiomyocytes
contractility.
A recent study has demonstrated that STVNa
sensitizes ATP-sensitive K+ (KATP) channels,
in the mitochondria and sarcolemma, to KATP channel
openers (37). The results of
this aforementioned study, which revealed that STVNa did not affect
mitochondrial membrane potential, is in agreement with the
consensus that STVNa does not solely activate KATP
channels (37), but rather makes
channels more sensitive to endogenous channel openers. It has also
been previously established that lactate, a product of anaerobic
metabolism in the heart, activates KATP channels
irrespective of high intracellular ATP levels (38,39). The activation of mitochondrial and
sarcolemmal KATP channels has been demonstrated to
regulate intracellular Ca2+ homeostasis (30,31). Therefore, the regulation of
Ca2+ homeostasis by STVNa corresponds with its ability
to sensitize KATP channels to KATP channel
openers.
In conclusion, the current study demonstrated that
STVNa prevents the development of cardiac hypertrophy, which is
induced by Iso by preventing ROS generation, protecting
mitochondrial function and regulating intracellular Ca2+
homeostasis. These results suggest that STVNa should be a potential
therapeutic strategy against cardiac hypertrophy and heart failure
in the future.
In the current study, the therapeutic effect of
STVNa and the underlying mechanism by matching in vitro and
in vivo experiments was defined. However, ex vivo
experiments were not performed, which would provide another layer
of tests for the present hypothesis. This could be viewed as a
limitation of the present study although the in vitro and
in vivo experiments match each other very well and strongly
support the conclusions.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by a grant from the
'Major Science and Technology Projects', Bureau of Science,
Technology & Information, Guangzhou City, 2013 (Category
reference number 164; grant. no. 201300000051) and the National
Natural Science Foundation of China (grant. no. 31300940). AJ was
supported by the University of Nicosia Medical School.
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and WT designed the experiments. YC, HB, HS, FF,
ZF and NL performed the experiments and YC, HS and AJ analyzed the
data. AJ and YC wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal experimental protocols complied with the
Guide for the Care and Use of Laboratory Animals, published by the
United States National Institutes of Health. The current study was
approved by the Institutional Animal Research Committee of South
China University of Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu H, Sun Xo, Tian F, Zhang H, Liu Q and
Tan W: Neuroprotective effects of isosteviol sodium injection on
acute focal cerebral ischemia in rats. Oxid Med Cell Longev.
2016.1379162:2016.
|
|
2
|
Zhang H, Sun X, Xie Y, Zan J and Tan W:
Isosteviol sodium protects against permanent cerebral ischemia
injury in mice via inhibition of NF-κB-mediated inflammatory and
apoptotic responses. J Stroke Cerebrovasc Dis. 26:2603–2614. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zan J, Zhang H, Lu MY, Beng HM, Zhong KL,
Sun XO and Tan W: Isosteviol sodium injection improves outcomes by
modulating TLRs/NF-κB-dependent inflammatory responses following
experimental traumatic brain injury in rats. Neuroreport.
29:794–803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhong KL, Lu MY, Liu F, Mei Y, Zhang XJ,
Zhang H, Zan J, Sun XO and Tan W: Isosteviol sodium protects neural
cells against hypoxia-induced apoptosis through inhibiting MAPK and
NF-κB pathways. J Stroke Cerebrovasc Dis. 28:175–184. 2019.
View Article : Google Scholar
|
|
5
|
Sun X, Yang Y, Xie Y, Shi X, Huang L and
Tan W: Protective role of STVNa in myocardial ischemia reperfusion
injury by inhibiting mitochondrial fission. Oncotarget.
9:1898–1905. 2017.
|
|
6
|
Liu Q, Hu H, Hu T, Han T, Wang A, Huang L,
Tan Q and Tan W: STVNa attenuates right ventricle hypertrophy and
pulmonary artery remodeling in rats induced by transverse aortic
constriction. Biomed Pharmacother. 101:371–378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang SG, Liu XY, Ye JM, Hu TT, Yang YY,
Han T and Tan W: Isosteviol ameliorates diabetic cardiomyopathy in
rats by inhibiting ERK and NF-κB signaling pathways. J Endocrinol.
238:47–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dini FL, Galeotti GG, Terlizzese G,
Fabiani I, Pugliese NR and Rovai I: Left ventricular mass and
thickness: Why does it matter? . Heart Fail Clin. 15:159–166. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertero E and Maack C: Metabolic
remodelling in heart failure Nat Rev Cardiol. 15:457–470. 2018.
|
|
10
|
Haselhuhn LR, Brotman DJ and Wittstein IS:
Heart failure guidelines: What you need to know about the 2017
focused update. Cleve Clin J Med. 86:123–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tse J, Powell JR, Baste CA, Priest RE and
Kuo JF: Isoproterenol-induced cardiac hypertrophy: Modifications in
characteristics of beta-adrenergic receptor, adenylate cyclase, and
ventricular contraction. Endocrinology. 105:246–255. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morisco C, Zebrowski DC, Vatner DE, Vatner
SF and Sadoshim J: Beta-adrenergic cardiac hypertrophy is mediated
primarily by the beta(1)-subtype in the rat heart. J Mol Cell
Cardiol. 33:561–573. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osadchii OE: Cardiac hypertrophy induced
by sustained beta-adrenoreceptor activation: Pathophysiological
aspects. Heart Fail Rev. 12:66–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nichtova Z, Novotova M, Kralova E and
Stankovicova T: Morphological and functional characteristics of
models of experimental myocardial injury induced by isoproterenol.
Gen Physiol Biophys. 31:141–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krenek P, Kmecova J, Kucerova D, Bajuszova
Z, Musil P, Gazova A, Ochodnicky P, Klimas J and Kyselovic J:
Isoproterenol-induced heart failure in the rat is associated with
nitric oxide-dependent functional alterations of cardiac function.
Eur J Heart Fail. 11:140–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Claycomb WC and Palazzo MC: Culture of the
terminally differentiated adult cardiac muscle cell: A light and
scanning electron microscope study. Dev Biol. 80:466–482. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sergeeva IA and Christoffels VM:
Regulation of expression of atrial and brain natriuretic peptide,
biomarkers for heart development and disease. Biochem Biophys Acta.
1832.2403–2412. 2013.
|
|
18
|
Shin E, Ko KS, Rhee BD, Han J and Kim N:
Different effects of prolonged β-adrenergic stimulation on heart
and cerebral artery. Integr Med Res. 3:204–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang GX, Kimura S, Nishiyama A, Shokoji
T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A and Abe Y:
Cardiac oxidative stress in acute and chronic isoproterenol-infused
rats. Cardiovasc Res. 65:230–238. 2005. View Article : Google Scholar
|
|
20
|
Srivastava S, Chandrasekar B, Gu Y, Luo J,
Hamid T, Hill BG and Prabhu SD: Downregulation of cuzn-superoxide
dismutase contributes to beta-adrenergic receptor-mediated
oxidative stress in the heart. Cardiovasc Res. 74:445–455. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takimoto E and Kass DA: Role of oxidative
stress in cardiac hypertrophy and remodeling. Hypertension.
49:241–248. 2007. View Article : Google Scholar
|
|
22
|
Vidal M, Wieland T, Lohse MJ and Lorenz K:
β-Adrenergic receptor stimulation causes cardiac hypertrophy via a
Gβγ/Erk-dependent pathway. Cardiovasc Res. 96:255–264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cha HN, Choi JH, Kim YW, Kim JY, Ahn MW
and Park SY: Metformin inhibits isoproterenol-induced cardiac
hypertrophy in mice. Korean J Physiol Pharmacol. 14:377–384. 2010.
View Article : Google Scholar
|
|
24
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar
|
|
25
|
Jovanović A: Cardioprotective signaling:
Past, present and future. Eur J Pharmacol. 833:314–319. 2018.
View Article : Google Scholar
|
|
26
|
Zhou B, Wu LJ, Li LH, Tashiro S, Onodera
S, Uchiumi F and Ikejima T: Silibinin protects against
isoproterenol-induced rat cardiac myocyte injury through
mitochondrial pathway after up-regulation of sirt1. J Pharmacol
Sci. 102:387–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Xu J, Long Z, Wang C, Wang L, Sun
P, Li P and Wang T: Hydrogen (H2) inhibits
isoproterenol-induced cardiac hypertrophy via antioxidative
pathways. Front Pharmacol. 7:3922016.
|
|
28
|
Remondino A, Kwon SH, Communal C, Pimentel
DR, Sawyer DB, Singh K and Colucci WS: Beta-Adrenergic
receptor-stimulated apoptosis in cardiac myocytes is mediated by
reactive oxygen species/c-Jun NH2-terminal kinase-dependent
activation of the mitochondrial pathway. Circ Res. 92:136–138.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eisner DA, Caldwell JL, Kistamás K and
Trafford AW: Calcium and excitation-contraction coupling in the
heart. Circ Res. 121:181–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du Q, Jovanović S, Clelland A, Sukhodub A,
Budas G, Phelan K, Murray-Tait V, Malone L and Jovanović A:
Overexpression of SUR2A generates a cardiac phenotype resistant to
ischaemia. FASEB J. 20:1131–1141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sukhodub A, Sudhir R, Du Q, Jovanović S,
Reyes S and Jovanović A: Nicotinamide-rich diet improves physical
endurance by up-regulating SUR2A in the heart. J Cell Mol Med.
15:1703–1712. 2011. View Article : Google Scholar
|
|
32
|
de Lucia C, Eguchi A and Koch WJ: New
insights in cardiac β-adrenergic signaling during heart failure and
aging. Front Pharmacol. 9:9042018. View Article : Google Scholar
|
|
33
|
Rengo G, Lymperopoulos A and Koch WJ:
Future g protein-coupled receptor targets for treatment of heart
failure. Curr Treat Options Cardiovasc Med. 11:328–338. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bristow MR, Ginsburg R, Umans V, Fowler M,
Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, et
al: Beta 1- and beta 2-adrenergic-receptor subpopulations in
nonfailing and failing human ventricular myocardium: Coupling of
both receptor subtypes to muscle contraction and selective beta
1-receptor downregulation in heart failure. Circ Res. 59:297–309.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dorn GW II and Force T: Protein kinase
cascades in the regulation of cardiac hypertrophy. J Clin Invest.
115:527–537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bertero E and Maack C: Calcium signaling
and reactive oxygen species in mitochondria. Circ Res.
122:1460–1478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan Z, Wen T, Chen Y, Huang L, Lin W, Yin
C and Tan W: Isosteviol sensitizes sarcKATP channels towards
pinacidil and potentiates mitochondrial uncoupling of diazoxide in
Guinea Pig ventricular myocytes. Oxid Med Cell Longev.
2016.6362812:2016.
|
|
38
|
Jovanović S, Du Q, Sukhodub A and
Jovanović A: M-LDH physically associated with sarcolemmal K
channels mediates cytoprotection in heart embryonic H9C2 cells. Int
J ATP Biochem Cell Biol. 41:2295–2301. 2009. View Article : Google Scholar
|
|
39
|
Jovanović S, Du Q, Sukhodub A and
Jovanović A: Dual mechanism of cytoprotection afforded by M-LDH in
embryonic heart H9C2 cells. Biochim Biophys Acta. 1793:1379–1386.
2009. View Article : Google Scholar
|