Introduction
The biological activities of mesenchymal stem cells
(MSCs), such as anti-inflammatory activities, render them a
promising tool for cell-based regenerative medicine (1). Among adult stem cells,
adipose-derived MSCs (AdMSCs) can be readily obtained in large
quantities using a simple procedure, and rapidly expanded in
vitro (2). AdMSCs, capable of
differentiating into osteoblasts, adipocytes and chondrocytes via
the activation of several key transcription factors, have been
shown to be effective in the treatment of atrophy, fibrosis,
retraction, and ulcers, as well as in wound healing. In addition,
the immunomodulatory function of AdMSCs has been effective in
treating hematological and immunological diseases (2,3).
Several studies to date have revealed the considerable potential of
AdMSCs in regenerative medicine (4,5).
Melatonin (N-acetyl-5-methoxytryptamine), mainly
synthesized from tryptophan and produced by the pineal gland, is
involved in diverse physiological activities, including sleep cycle
regulation, cell proliferation control, and antioxidant and
anti-inflammatory activities (6).
In general, melatonin, which regulates physiological and
endocrinological functions, is used for treating sleep disorders,
such as insomnia (7). Recently,
melatonin was reported to exhibit both proliferation-promoting and
anti-inflammatory properties, not only by enhancing the
self-renewing potential of stem cells, but also by reducing the
expression of pro-inflammatory factors (8,9).
Several studies have demonstrated that the melatonin receptors type
(MT) 1 and 2 can be detected in adipose tissue, and daily melatonin
treatment inhibits adipose accumulation (10,11). In addition, melatonin has been
demonstrated to suppress the adipocyte differentiation of murine
pre-adipocytes (12). In this
regard, melatonin exhibits the potential to prevent obesity, as
well as obesity-related metabolic diseases, although the mechanisms
underlying melatonin-related reduction in adiposity via
adipogenesis remain unknown (13).
Previous studies have shown that the MT1 and MT2
melatonin receptors are expressed in human MSCs (14). Melatonin is known to improve the
functionality of MSCs via its cognate receptors (15). Melatonin pretreatment enhances the
therapeutic potential of MSCs by maintaining their
self-renewability during in vitro expansion (16). Melatonin also influences MSC
survival in in vivo animal studies (17). Another study reported that
melatonin prevented the replicative senescence of AdMSCs through a
sirtuin 1-dependent pathway (18). Successful in vitro
expansion of MSCs is an essential procedure for clinical
application, because of cellular aging due to long-term passaging
of cells. Nevertheless, studies regarding whether a small molecule
such as melatonin can improve the proliferation, differentiation
and immunomodulatory ability of AdMSCs are still lacking.
Furthermore, the mechanisms underlying the biological effects of
melatonin in AdMSCs have not been elucidated.
The maintenance of stemness in MSCs is regulated by
a complex network, including intracellular and extracellular
signaling (19). Diverse
strategies have been applied to improve the expansion and stemness
of MSCs by providing a favorable microenvironment (20,21). The present study aimed to
establish such a strategy, using the small molecule melatonin to
improve AdMSC cell therapy. Such small cell-permeable molecules can
provide robust and reproducible results, and affect signaling
pathways (22). Additionally, the
present study investigated whether melatonin treatment could
improve the proliferative activity and anti-inflammatory effects of
MSCs, and whether it could regulate the tri-lineage differentiation
potential of MSCs compared with normal culture conditions.
Furthermore, the melatonin effects on the proliferative and
immunomodulatory properties of AdMSCs via the melatonin receptors
were confirmed using the melatonin antagonist, luzindole.
Materials and methods
Isolation of AdMSCs
Adipose tissues were obtained from 3 different
normal healthy donors (female; age, 42, 53 and 55 years, collected
at CHA Hospital on September-December 2018) after providing written
informed consent. This study was approved by the Institutional
Review Board of CHA General Hospital, Seoul, Korea. AdMSCs were
isolated using freshly prepared 0.1% collagenase type I solution
(Invitrogen; Thermo Fisher Scientific, Inc.). After incubation at
37°C for 1 h, an equal amount of growth medium [DMEM with 10% FBS
and 1% penicillin/streptomycin (all Thermo Fisher Scientific, Inc)]
was added to the mixture to arrest enzyme activity. After
centrifugation at 1,200 × g for 5 min, the upper layer of the
supernatant was removed, and the lower part of the mixture was
filtered through a 70 µm cell strainer. After centrifugation
at 1,200 × g for 5 min, the supernatant was removed and the pellet
was resuspended with growth medium by pipetting. After
centrifugation at 1,200 × g or 5 min, the supernatant was removed,
and the cells were cultured in DMEM, supplemented with 10% FBS and
1% penicillin/streptomycin. The cells were cultured at 37°C under
5% CO2, and the media were supplemented with 5 ng/ml
basic fibroblast growth factor (Invitrogen; Thermo Fisher
Scientific, Inc.) every 3 or 4 days. The cultivated cells exhibited
the typical spindle shape of AdMSCs. The cells were positive for
expression of the surface antigens CD73, CD90 and CD105 (data not
shown). Cells were passaged with trypsin-EDTA (Invitrogen; Thermo
Fisher Scientific, Inc.) when they reached 80-90% confluence. The
cultured AdMSCs were frozen until the cells were used at passages
5-10. Melatonin (Sigma-Aldrich; Merck KGaA) and luzindole
(Sigma-Aldrich; Merck KGaA) were dissolved in DMSO, and the cells
were photographed after melatonin treatment under an inverted light
phase-contrast microscope (IX-71; Olympus Corporation). Experiments
were performed three times from three different donors for the
present study.
Cell proliferation assay
AdMSCs were plated at a density of 1×103
cells/well in 96-well plates to determine the proliferation rate of
AdMSCs. Each well of the culture plate was treated with varying
concentrations (0.5-50 µM) of melatonin for 3 days, along
with 5 µM of luzindole. In all experiments, when melatonin
was added to the well, it was marked as 'melatonin' or MT, and
untreated control was labeled as 'control' or 'CON.' In addition,
when luzindole was added along with melatonin to the cells, these
were labeled as LU + MT. Proliferation activity was determined
using the EZ-Cytox kit (DaeilLab Services) based on the WST
reagent. The results were analyzed according to the manufacturer's
instructions. Data were expressed as the mean ± SD of three
independent experiments.
Reverse transcription (RT-) PCR
Total RNA was extracted using a RiboEx solution
(GeneAll Biotechnology Co., Ltd.). Standard reverse transcription
was performed using a Maxime™ RT Premix (Intron Biotechnology,
Inc.). To amplify target genes, 25 ng cDNA was used along with the
PCR primers (Bioneer Corporation) using the conditions listed in
Table I. The amplification
program consisted of 24-35 cycles of the following conditions: 94°C
for 30 sec; annealing at 62°C for 30 sec, and extension at 72°C for
30 sec, followed by a final amplification step for 10 min at 72°C.
The PCR products were visualized by agarose gel electrophoresis.
GAPDH was used as an internal reference control. One representative
example of three independent experiments is shown.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene symbol | Primer sequence
(5′-3′) | Annealing
temperature (°C) |
|---|
| MT1 | F:
TGTCGATATTTAACAACGGGTGG
R: CGATGCCGGTGATGTTGAA | 58 |
| MT2 | F:
GCATGGCCTACCACCGAATC
R: AATAGATGCGTGGGTCGTACT | 58 |
| CD73 | F:
TATTGCACTGGGACATTCGGGT
R: GGTTGCCCATGTTGCATTCTCT | 62 |
| CD105 | F:
CATCCTTGAAGTCCATGTCCTCTT
R: GCCAGGTGCCATTTTGCTT | 62 |
| Oct-4 | F:
GACAACAATGAGAACCTTCAGGAGA
R: TTCTGGCGCCGGTTACAGAACCA | 62 |
| Sox2 | F:
AACCAAGACGCTCATGAAGAAG
R: GCGAGTAGGACATGCTGTAGGT | 62 |
| Nanog | F:
ATAGCAATGGTGTGACGCAG
R: GATTGTTCCAGGATTGGGTG | 62 |
| GAPDH | F:
GTGGTCTCCTCTGACTTCAACA
R: CTCTTCCTCTTGTGCTCTTGCT | 62 |
| p21 | F:
GCGATGGAACTTCGACTTTG
R: CGTTTTCGACCCTGAGAGAGTC | 60 |
| Dlx5 | F:
ACCATCCGTCTCAGGAATCG
R: ACCTTCTCTGTAATGCGGCC | 60 |
| Runx2 | F:
GACCAGTCTTACCCCTCCTACC
R: CTGCCTGGCTCTTCTTACTGAG | 58 |
| BMP7 | F:
CCAACGTCATCCTGAAGAAATAC
R: GCTTGTAGGATCTTGTTCATTGG | 60 |
| Sox9 | F:
GCCGGGCAAGGCTGACCTGAAG
R: TTCTGGTGGTCGGTGTAGTCGT | 61 |
| PPARG | F:
TCTCTCCGTAATGGAAGACC
R: GCATTATGAGACATCCCCA | 62 |
| CEBPA | F:
CCAAGAAGTCGGTGGACAAGAA
R: TCATTGTCACTGGTCAGCTCCA | 62 |
| IL-6 | F:
ATGAACTCCTTCTCCACAAGC
R: GTTTTCTGCCAGTGCCTCTTTG | 60 |
| IL-10 | F:
ACCTGGTAGAAGTGATGCCCCAGGCA
R: CTATGCAGTTGATGAAGATGTCAA | 58 |
Quantitative PCR (qPCR)
Total RNA was extracted using RiboEx reagent
(GeneAll Biotechnology Co., Ltd.). Isolated RNA was
reverse-transcribed into cDNA using a Maxime™ RT PreMix
(Intron Biotechnology, Inc.), according to the manufacturer's
protocol. qPCR was performed in 96-well plates using SYBR-Green I
Master mix (Roche Applied Science) on a LightCycler 480 System
(Roche Applied Science) using the following cycling conditions:
95°C for 5 min, and 45 cycles of 95°C for 10 sec, 60°C for 20 sec,
and 72°C for 15 sec, followed by a melting curve program. Primers
used are listed in Table I. Gene
expression was normalized to that of GAPDH and analyzed using
advanced relative quantification based on the E-method provided by
Roche Applied Science (23). Data
were expressed as the mean ± SD of three independent
experiments.
Immunostaining
The cells were fixed in 4% paraformaldehyde solution
(Biosesang, Inc.) for 20 min on ice. After washing with PBS
containing 0.1% bovine serum albumin (Sigma-Aldrich; Merck KGaA)
for 10 min, cells were incubated in permeabilization/blocking
solution (PBS containing 0.3% Triton X-100 and 10% FBS) for 10 min
at room temperature. The following primary antibodies were then
added at 4°C for 24 h: Human MT1 (1:200; Santa Cruz Biotechnology,
Inc.; cat. no. sc-390328) and human MT2 (1:200; Abcam; cat. no.
ab203346). After washing for 10 min, fluorescently-labeled
secondary antibodies were added as follows: Anti-mouse Ig
immunoglobulin (Ig) G Alexa Fluor 488 and anti-rabbit IgG Alexa
Fluor 594 (cat nos. A11029 and A11012 respectively; both 1:400;
Thermo Fisher Scientific, Inc.) at room temperature for 30 min.
After washing for 10 min, cells were stained with DAPI (1:1,000;
Sigma-Aldrich; Merck KGaA) for 5 min. Fluorescence images were
obtained using a confocal laser scanning microscope (Carl-Zeiss LSM
700 Exciter; Zeiss GmbH). One representative image of three
independent experiments is shown.
Colony-forming efficiency (CFE)
assay
To evaluate the stemness of AdMSCs, CFE assays were
performed. Approximately 2×103 cells at passage 5-10
were seeded in 100 mm dishes (Corning Inc.), and the cells were
cultured for 2 weeks. Whenever the media were replaced, melatonin
and luzindole were added to the test group. After 2 weeks, the
media were removed and the cells were washed twice in PBS. Then,
the cells were stained with 3% crystal violet (Sigma-Aldrich; Merck
KGaA) in methanol for 10 min at room temperature, and stained
colonies (>50 cells) were counted. Data were expressed as the
mean ± SD of three independent experiments.
β-galactosidase (gal) assay
β-gal assays were performed when cells exhibited
typical senescent phenotypes, with an enlarged and flattened cell
morphology after 15 passages. To identify β-gal activity in
senescent cells, a senescence detection kit was used (BioVision,
Inc.), according to the manufacturer's instructions. After washing
with PBS, fixed cells were incubated with β-gal staining reagent
overnight at 37°C. The number of β-gal-stained cells (blue color)
was counted under a light microscope (IX71; Olympus Corporation).
Data were expressed as the mean ± SD of three independent
experiments.
Adipogenic differentiation
To differentiate AdMSCs into adipocytes, cells at
passage 5-10 were induced in adipogenic differentiation medium
(Lonza Group, Ltd.) for 3 weeks. The media were changed every 3-4
days. Melatonin and/or luzindole were added to the tested cells
whenever the media were replaced. The differentiated cells were
washed with PBS, then incubated with 10% formalin (Sigma-Aldrich;
Merck KGaA) for 30 min at room temperature. After washing, 60%
isopropanol was dispensed. Oil Red O solution in distilled water
was added to the cells for 10 min. After washing with tap water,
images of the red-stained cells were obtained using an inverted
light phase-contrast microscope (IX71; Olympus Corporation). To
obtain quantitative data regarding adipogenesis, absorbance was
measured at 500 nm after destaining with isopropanol. Data were
expressed as the mean ± SD of three independent experiments.
T cell proliferation assay
To investigate the effects of melatonin on the
suppression of T cell proliferation, 1×105 human
peripheral blood mononuclear cells (obtained from the same donors)
were co-cultured with 1×104 AdMSCs in 96-well plates.
Then, 10 µM of melatonin and 5 µM of luzindole were
added to the cells, and 10 µg/ml phytohaemagglutinin
(Sigma-Aldrich; Merck KGaA) was applied for activation of T cells.
After 3 days, the inhibition of T cells was determined using the
EZ-cytox proliferation assay kit, according to the manufacturer's
instructions. Data were expressed as the mean ± SD of three
independent experiments.
Statistical analysis
Quantitative data were expressed as means ± SD.
Statistical comparisons were performed using Student's t-test or
one-way analysis of variance with post hoc Bonferroni correction
using SPSS software v18 (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Melatonin treatment enhances the
proliferation of AdMSCs and induces melatonin receptor
expression
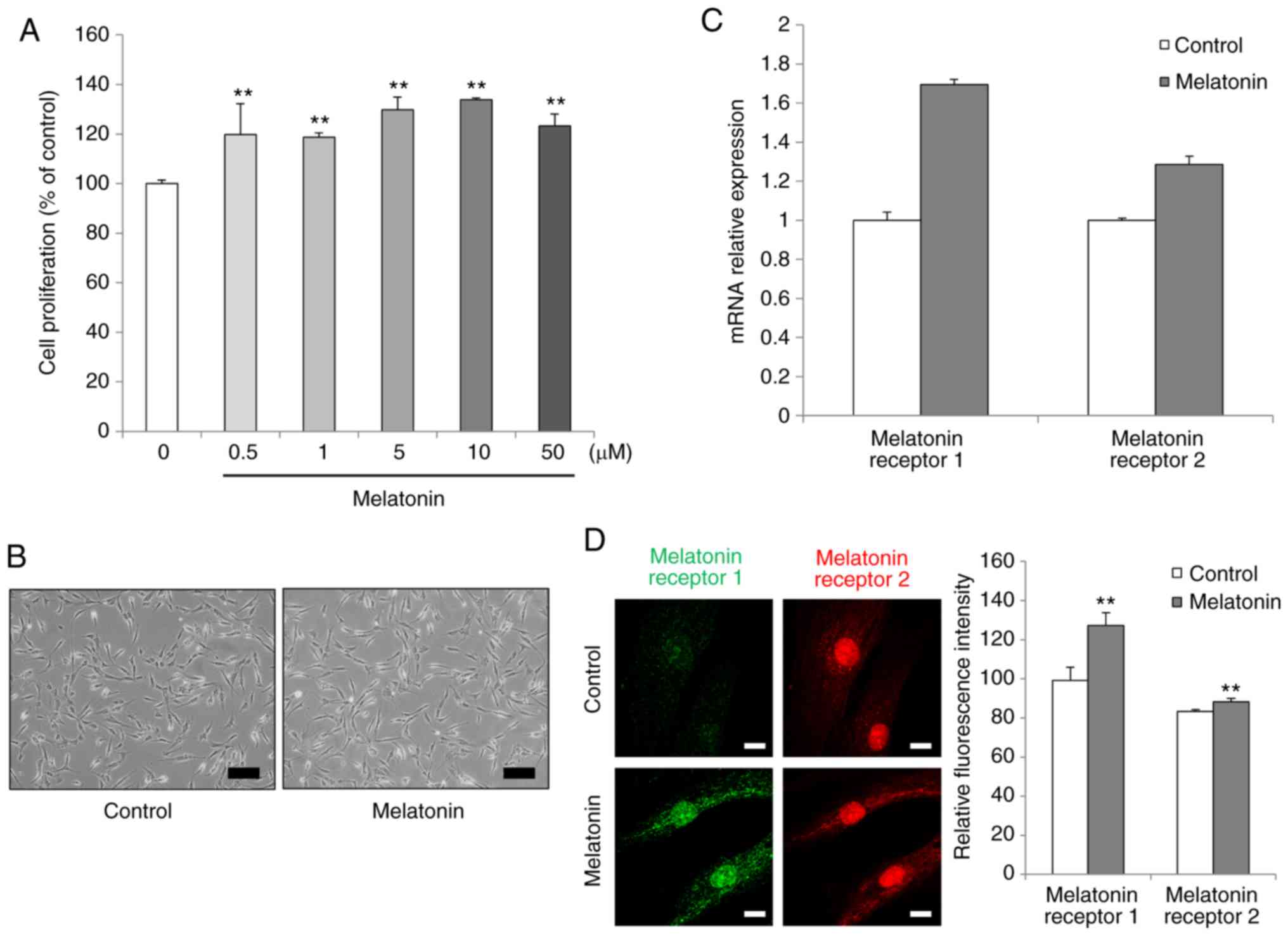
To investigate the effects of melatonin on the
viability and proliferation rates of AdMSCs, a water-soluble
tetrazolium salt in vitro assay system was used. The cell
numbers of AdMSCs were increased following melatonin incubation,
without any reduction in cell viability. After 3 days of
incubation, the dose 10 µM melatonin resulted in the highest
proliferation rate in the treated AdMSCs (Fig. 1A). Therefore, the dose of 10
µM melatonin was selected for subsequent experiments.
Melatonin-treated AdMSCs exhibited a fibroblast-like spindle shape,
similar to that of untreated cells (Fig. 1B).
To identify changes involving the melatonin
receptor, the expression levels of MT1 and MT2 were detected by
RT-qPCR. When AdMSCs were treated with melatonin, the mRNA
expression levels of MT1 and MT2 were upregulated compared with
those in untreated cells, although these changes were not
significant statistically (Fig.
1C). In order to confirm that melatonin treatment induced the
expression levels of both the melatonin receptors, their protein
expression was evaluated by immunofluorescence with specific
antibodies. MT1 and MT2 were strongly expressed in
melatonin-treated cells after 3 days of incubation, compared with
the control untreated cell group (Fig. 1D). These results indicated that
melatonin facilitated AdMSC proliferation and upregulated MT1 and
MT2 expression and abundance.
Melatonin suppresses cellular senescence
of AdMSCs and induces SRY-box transcription factor 2 (Sox2)
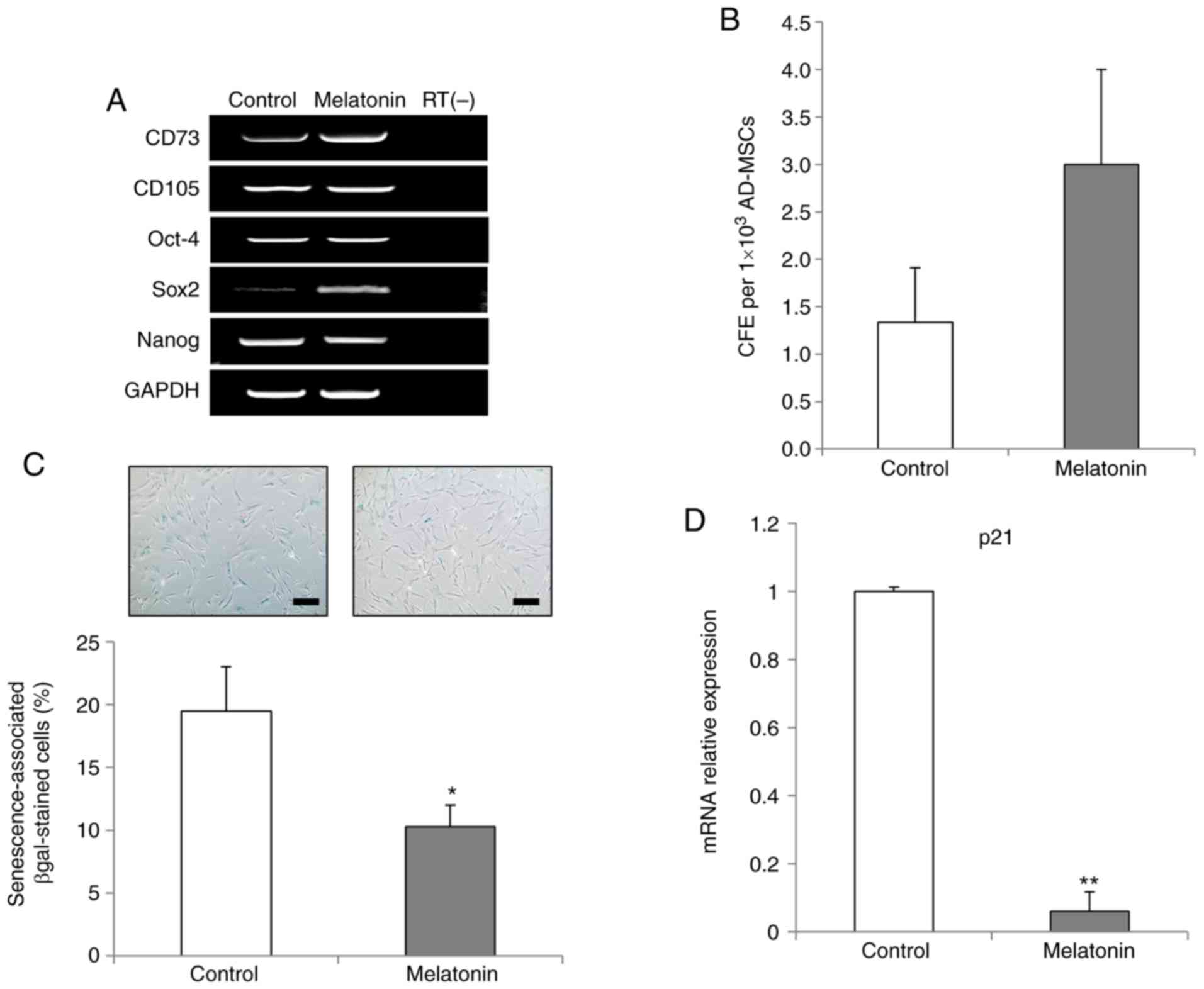
The mRNA expression levels of CD73 (also known as
ecto-5′-nucleotidase) and CD105 (also known as endoglin), which are
known key markers of MSCs, were detected, in order to determine
whether melatonin-treated AdMSCs retained MSC-like properties.
RT-PCR analysis revealed that melatonin-treated AdMSCs retained the
expression of key markers of MSCs (Fig. 1A). Next, the expression levels of
octamer-binding transcription factor 4 (Oct-4), Sox2 and nanog
homeobox (Nanog), which are known key factors for maintenance of
stem cell pluripotency, were analyzed, in order to investigate the
stemness status of AdMSCs following melatonin treatment. Sox2 was
markedly upregulated in the melatonin-treated AdMSCs, while the
expression levels of Oct-4 and Nanog genes remained unaltered
compared with those in the control group (Fig. 2A). Subsequently, the effects of
melatonin were investigated on the colony formation ability of
AdMSCs, and the results demonstrated that melatonin-treated AdMSCs
exhibited increased self-renewal capacity compared with the control
group (Fig. 2B).
Next, the present study investigated whether
melatonin inhibited the replicative senescence of AdMSCs in
long-term culture. Melatonin-treated AdMSCs exhibited a significant
reduction in the proportion of β-gal-stained cells compared with
the control group (Fig. 2C).
Furthermore, the inhibition of cellular senescence was confirmed
RT-qPCR results demonstrating a significant downregulation of the
p21 gene, which is crucial for MSC senescence (24), in the melatonin-treated AdMSCs
(Fig. 2D). Taken together, these
results indicated that melatonin reduced the replicative senescence
of AdMSCs by modulating the expression levels of Sox2 and p21.
Melatonin affects the differentiation
capacity and anti-inflammatory activity of AdMSCs
To investigate whether melatonin affects the in
vitro tri-lineage differentiation capacity of AdMSCs into
osteoblasts, chondrocytes and adipocytes, the mRNA expression
levels of key differentiation marker genes were analyzed. The
expression levels of distal-less homeobox 5 (Dlx5) and runt-related
transcription factor 2 (Runx2), key transcription factors for
osteogenesis, and the expression levels of bone morphogenetic
protein 7 (BMP7) and SRY-box transcription factor 9 (Sox9), markers
of chondrogenesis, remained unaltered following melatonin treatment
(Fig. 3A). Notably, the
expression levels of CCAAT enhancer binding protein α (CEBPA), a
key transcription factor for adipogenesis, were markedly reduced in
the melatonin-treated AdMSCs (Fig.
3A). The expression levels of the peroxisome proliferator
activated receptor γ (PPARG) gene were unchanged. To further
examine the effects of melatonin in adipogenesis of AdMSCs, in
vitro adipogenic differentiation assays were performed. The
results demonstrated that melatonin disrupted AdMSC adipogenesis,
as evidenced by reduced Oil red O staining in the melatonin-treated
AdMSCs (Fig. 3B), indicating that
melatonin exhibited specific anti-adipogenic effects.
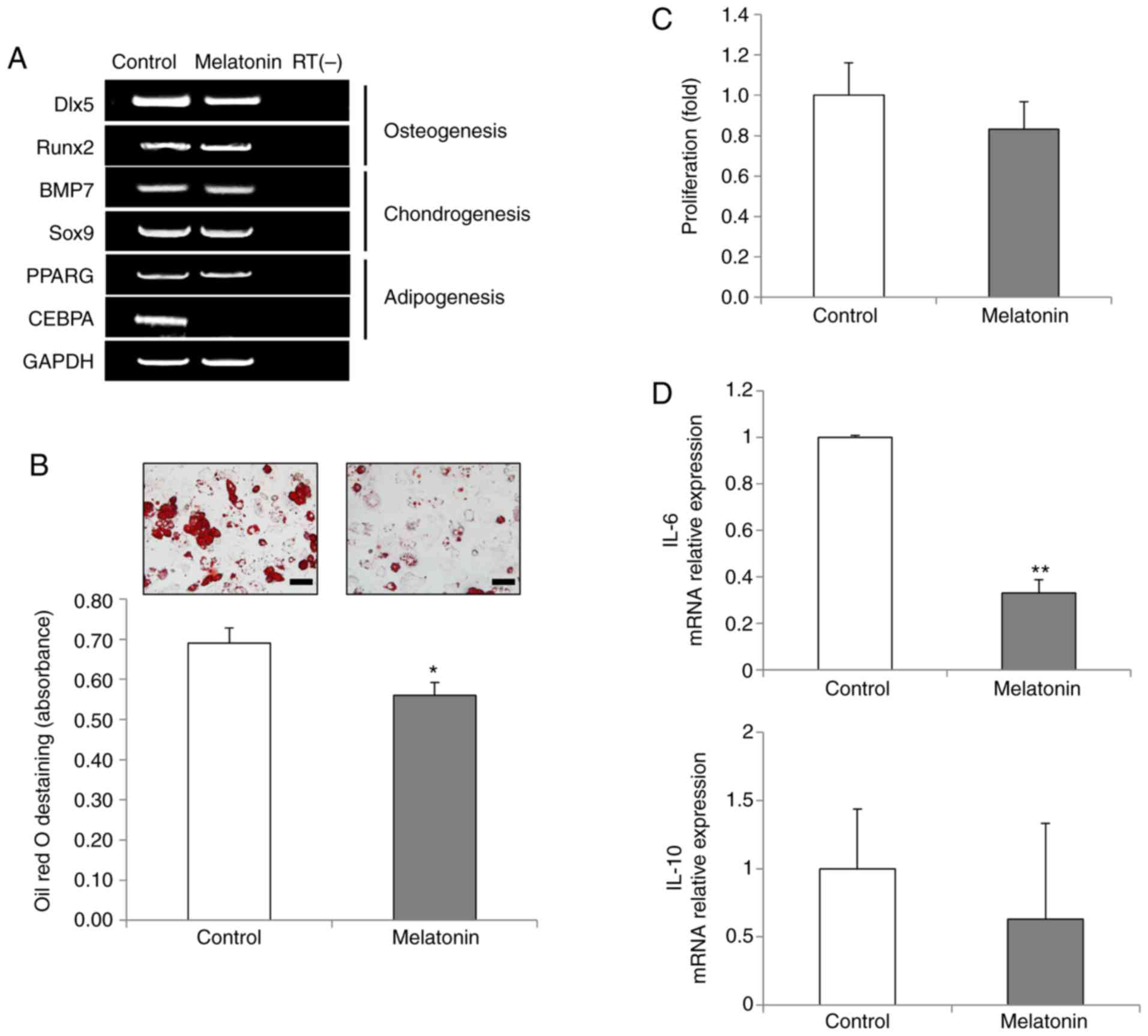 | Figure 3Effects of melatonin on the
differentiation capacity and immunomodulation of AdMSCs. (A)
Reverse transcription-PCR analysis of key transcription factors for
osteogenesis, chondrogenesis and adipogenesis in untreated cells
and in 10 µM melatonin-treated cells. One representative of
three independent experiments is shown. RT(-) indicates a negative
control using water alone as the reaction input. (B) Adipogenic
differentiation capacity was analyzed by Oil Red O staining (scale
bar, 50 µm; magnification, ×400). For quantitative analysis,
absorbance was measured at 500 nm after destaining. (C) Inhibition
of activated mononuclear cells was evaluated through EZ-cytox
assays. Phytohaemagglutinin-induced mononuclear cells were
co-cultured with untreated or melatonin-treated AdMSCs for 3 days.
(D) Relative mRNA expression levels of inflammation-related factors
IL-6 and IL-10 were evaluated by quantitative PCR. Data are
presented as the mean ± SD of three independent experiments.
*P<0.05 and **P<0.01. AdMSCs,
adipose-derived mesenchymal stem cells; IL, interleukin; Dlx5,
distal-less homeobox 5; Runx2, runt-related transcription factor 2;
BMP7, bone morphogenetic protein 7; Sox9, SRY-box transcription
factor 9; PPARG, peroxisome proliferator activated receptor γ;
CEBPA, CCAAT enhancer binding protein α. |
To assess the immunomodulatory effects of
melatonin-treated AdMSCs, a co-culture system of T cells and AdMSCs
was used. T cell proliferation was slightly inhibited in the
presence of melatonin-treated AdMSCs, although this effect was not
statistically significant (Fig.
3C). The immuno-modulation of MSCs is mediated by various
cytokines, such as interleukin (IL)-6, as a pro-inflammatory
cytokine, and IL-10, as an anti-inflammatory cytokine (25). In the present study, a significant
decrease in IL-6 expression was observed following melatonin
treatment, while the mRNA expression levels of IL-10 remained
unchanged (Fig. 3D). These
results indicated that melatonin-treated AdMSCs may have an
anti-inflammatory role via immunomodulation (Fig. 3D).
Effects of melatonin in the presence of
the melatonin receptor inhibitor, luzindole
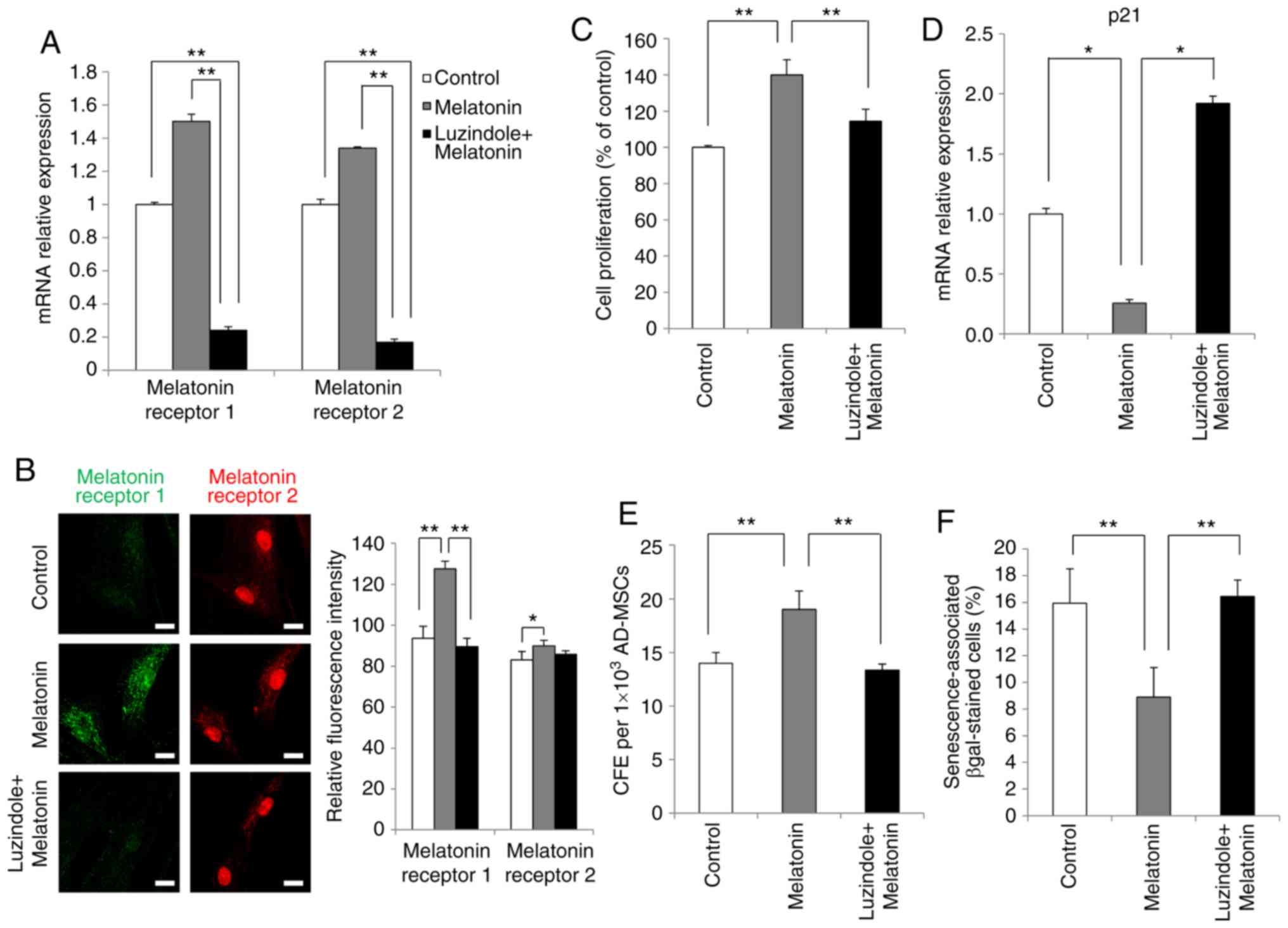
Next, the present study aimed to identify whether
the effects of melatonin on AdMSCs were mediated by the melatonin
receptor pathway. Accumulating evidence from studies using cell or
animal models have suggested that luzindole acts as a potent
inhibitor of melatonin receptors (15,26). Therefore, the effects of melatonin
on AdMSCs were investigated in the presence of the melatonin
receptor antagonist luzindole. Firstly, luzindole was added as a
pretreatment for 24 h to inhibit melatonin receptors, and then the
cells were treated with melatonin. Morphological changes were not
observed despite luzindole treatment during the 3 days of the
experiment (data not shown). The results revealed that the mRNA
expression levels of melatonin type 1 and 2 receptors, otherwise
upregulated by melatonin, were significantly inhibited by luzindole
(Fig. 4A). Furthermore, the
results of immunofluorescence staining confirmed at the protein
level that upregulation of melatonin receptors was inhibited by
luzindole treatment (Fig.
4B).
Next, the effects of luzindole treatment on AdMSC
proliferation were investigated. The results demonstrated that
proliferation of AdMSCs, accelerated by melatonin, was reduced
following luzindole treatment (Fig.
4C). In addition, treatment of AdMSCs with luzindole for 3 days
significantly increased the mRNA expression levels of p21 (Fig. 4D). Subsequently, the present study
investigated whether luzindole reduced the self-renewal capacity of
melatonin-treated AdMSCs, using the colony formation assay. AdMSCs
cultured with melatonin exhibited a significant increase in the
number of colonies, while this effect was significantly revered
following luzindole treatment (Fig.
4E). Finally, as presented in Fig. 4F, the percentage of β-gal-positive
cells was decreased by melatonin treatment, whereas this effect was
reversed in the presence of luzindole. These results demonstrated
that the enhanced proliferation of melatonin-treated AdMSCs was
mediated by melatonin receptor signaling.
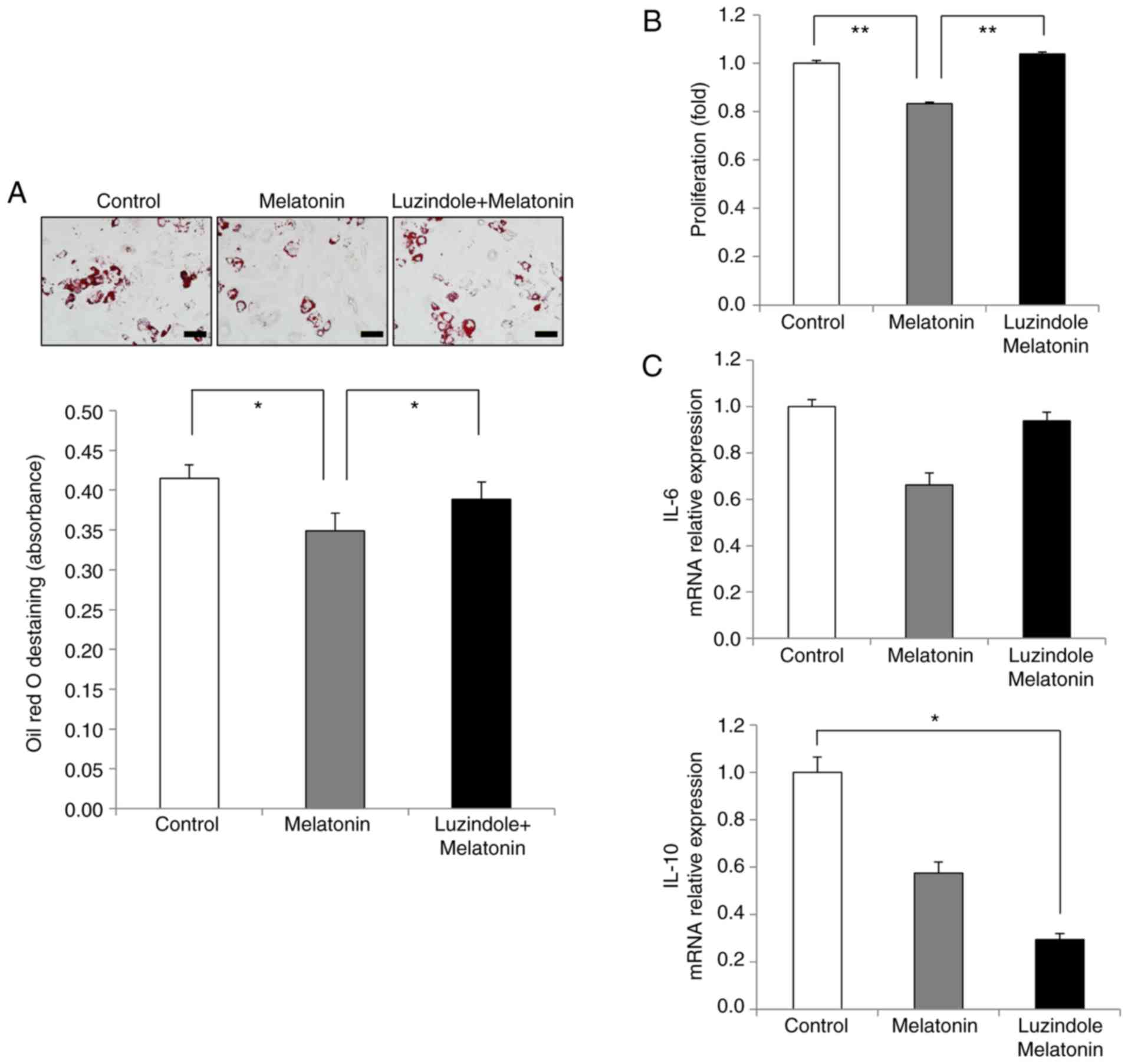
The present results revealed that melatonin
inhibited AdMSC adipogenesis (Fig.
3B). During in vitro adipogenic differentiation, the
amount of lipid accumulation in melatonin-treated cells was less
compared to that in the control group, while the lipid content in
luzindole-treated cells was equivalent to that in control cells
(Fig. 5A). Quantification of the
staining results confirmed the effects of melatonin and luzindole
during adipogenesis of AdMSCs (Fig.
5A). In addition, the effect of luzindole was assessed on the
immunomodulation-related anti-inflammatory ability of AdMSCs.
Melatonin-treated cells inhibited the proliferation of T cells
compared with that of the control group (Fig. 5B). However, luzindole treatment
reversed the inhibitory effects of melatonin on the growth of T
cells (Fig. 5B). Next, the
effects of luzindole were analyzed on the anti-inflammatory
properties of melatonin-treated AdMSCs. Compared with the control
group, the expression levels of IL-6 decreased in the
melatonin-treated cells, whereas IL-6 expression in activated T
cells was increased after luzindole treatment (Fig. 5C). Of note, a significant
reduction in IL-10 expression was observed in cells treated with
melatonin and luzindole (Fig.
5C). Taken together, these results indicated that the
activation of melatonin receptors type 1 and 2 was involved in
mediating the positive effects of melatonin on the proliferative
activity, self-renewal capacity and immunomodulation of AdMSCs.
Discussion
Melatonin has been reported to have critical roles
in various physiological and pathological processes in vitro
and in vivo in stem cell-based regenerative medicine
(16). In numerous experimental
reports, melatonin displayed anti-inflammatory effects by
modulating the balance between pro-inflammatory and
anti-inflammatory cytokines in stem cells (27,28). A recent study showed that the
injection of melatonin-treated MSCs into ischemic rat brain
significantly enhances the survival of MSCs and improves their
therapeutic efficacy (15). In
addition, melatonin has been demonstrated to influence the
proliferative activity of a variety of cell types, including neural
stem cells and MSCs (13,29,30); however, the mechanisms through
which the biological actions of AdMSCs are influenced by melatonin
have not been extensively studied to date.
Melatonin, known as an activator of the ERK pathway,
increases cell proliferation (31). However, the optimal concentration
of melatonin depends on the cell type (32,33). In the current study, melatonin
treatment increased the cell proliferation rate in all dose groups
(0.5, 1, 5, 10 and 50 µM). The maximal effect was observed
at 10 µM melatonin. Notably, melatonin exhibited maximal
effects after daily exposure, because it has a short half-life.
Thus, it was speculated that the exposure period of melatonin is
possibly an important factor contributing to its effectivess.
Melatonin acts via the G protein-coupled receptors MT1 and MT2, and
interacts with intracellular proteins (34). Treatment with 10 µM
melatonin upregulated the expression levels of MT1 and MT2.
Furthermore, the results confirmed, at the mRNA and the protein
level, that melatonin acted through both melatonin receptors. MT1
is normally localized on the cell membrane. However, in the present
study MT1 expression was observed both in the cytoplasm and the
nucleus. The experiments for the detection of specific localization
in cell culture may vary by the protocol or type of reagents.
Future investigations are underway to examine the localization of
melatonin receptors in MSCs in detail.
Replicative senescence of MSCs during in
vitro expansion for clinical application reduces therapeutic
efficacy of the cells by decreasing their viability (20). Previously, Yoon et al
(35) demonstrated that
activation of Sox2 significantly improved the multi-potentiality
and proliferation of MSCs. Melatonin is known to act as an
anti-aging agent, as reported in numerous studies (36,37). It has also been recently reported
that treatment with melatonin during long-term in vitro
expansion preserved >80% of the self-renewal capacity of MSCs by
retaining their stemness (16).
The present results suggested that melatonin inhibited replicative
senescence by upregulating Sox2 expression. MSCs, at late passage,
exhibited increased expression of p21 and decreased proliferative
activity (38). p21, a key
molecule that regulates the cell cycle, is known to serve a
critical role in mediating cellular senescence of MSCs (24). The present results demonstrated
that inhibition of p21 expression by melatonin reversed the
senescent phenotype of AdMSCs. In addition, pro-inflammatory
cytokines, such as IL-6, tumor necrosis factor (TNF)-α and IL-1β,
are well-known to induce cellular senescence in diverse cell types,
including MSCs (39). In the
present study, melatonin-treated AdMSCs had significantly decreased
IL-6 mRNA expression levels. The current data suggested that
melatonin may be considered as a critical factor for the expansion
of AdMSCs by inhibiting cellular senescence. These findings are
consistent with results of previous studies (16).
Zhang et al (30) suggested that melatonin inhibits
adipogenesis and enhances the osteogenesis of MSCs by suppressing
PPARγ and enhancing Runx2 gene expression. The present data
demonstrated that melatonin treatment inhibited adipo-genic
differentiation in AdMSCs by suppressing CEBPA gene expression,
which is a key transcription factor in adipogenesis, whereas
osteogenic differentiation was induced regardless of exposure to
melatonin. This result indicated that CEBPA may represent a key
factor that regulates adipogenesis. We postulated that the effect
of melatonin on pluripotency of AdMSCs may depend on the specific
cell type involved and the culture conditions. For example, a
previous study reported that melatonin, at low concentrations,
significantly promoted 3T3-L1 cell growth, whereas in another
study, melatonin exhibited no significant effects on the
proliferation of bovine intramuscular preadipocytes (40,41). Several studies have suggested that
stimulation of differentiation potential by melatonin involves
melatonin receptor and signaling pathways, including Wnt/catenin,
transforming growth factor and mitogen-activated protein kinases,
to regulate the expression of critical differentiation factors
(42,43). However, the exact mechanisms of
the melatonin-mediated regulation of differentiation remain
unclear. Obesity is considered to be a result of dysfunctional
adipogenesis involving deregulation of adipocyte size and number,
and may be associated with differentiation processes affecting stem
cell-derived pre-adipocytes (44). Activation of 5′AMP-activated
protein kinase (AMPK) inhibits adipogenesis and promotes lipolysis,
by suppressing adipogenesis-related gene expression (45). The present study confirmed that
melatonin treatment enhanced the activity of AMPK in AdMSCs,
suggesting that it acted as an activator of AMPK (data not shown).
Thus, adipogenic regulation of AdMSCs by melatonin may serve as a
novel therapeutic application against fat deposition.
Melatonin, as one of the immunomodulatory factors
involved in immune responses, exerts anti-inflammatory effects via
the regulation of inflammation-related cytokines (46). Biological effects of melatonin on
immune regulation have been validated in vivo and in
vitro (47). A previous study
has shown that the administration of melatonin decreased
inflammatory responses by mediating the levels of immunomodulatory
factors, including IL-1β, TNF-α and IL-6 (48). The present study demonstrated that
treatment with melatonin effectively inhibited T cell
proliferation. This inhibition was associated with decreased levels
of IL-6 and IL-10, indicating that their expression may be related
to the inhibition of T cell activity. However, the possible
interactions of other immunomodulatory factors and immune cells
cannot be excluded. In the present study, the melatonin receptor
antagonist luzindole was used to block the effects of melatonin on
AdMSCs. The expression levels of melatonin receptors MT1 and MT2 at
the mRNA and protein levels could be detected in melatonin-treated
AdMSCs, whereas luzindole strongly inhibited the activities of
melatonin receptors. Luzindole was demonstrated to completely
reverse the positive effects of melatonin on proliferation,
self-renewal capacity and immunomodulation, indicating that the
beneficial effects of melatonin were receptor-mediated.
In conclusion, the findings of the present study
demonstrated that melatonin treatment effectively promoted the
proliferation and prevented the replicative senescence of AdMSCs
via the melatonin receptors MT1 and MT2. In addition, it was
confirmed that melatonin treatment inhibited the expression of the
CEBPA gene, a key transcription factor of adipogenic
differentiation in MSCs. There was no effect on osteogenic and
chondrogenic differentiation. The present study focused
predominantly on the examination of adipogenesis of MSCs by
melatonin treatment in an AdMSC culture model. The results
suggested that melatonin-induced MSCs might be considered in the
development of anti-obesity therapy. To improve the therapeutic
efficacy of MSCs, several studies have suggested that
pre-conditioning of MSCs by hypoxia, natural molecules and growth
factors enhances the activity of MSCs (49,50). The greatest advantage of
melatonin, a natural small molecule, is that it has no reported
side effects. It is, therefore, suggested that melatonin could be
used for treating various diseases, including inflammation;
however, the mechanisms underlying the beneficial effects of
melatonin need to be elucidated in future studies. Future
investigations are also needed to explore further the role of
melatonin in improving cell therapy using AdMSCs in disease
models.
Acknowledgments
Not applicable.
Funding
This study was supported by a Jungwon University
Research Grant (grant no. 2017-050).
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Authors' contributions
SGL and JK planned the project and wrote the
manuscript. JSH, SP and JYL performed the experiments, and analysed
the data. DWY, BYK and JHK participated in the stem cell
experiments and analysed the data. GJK provided stem cells and
analysed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of CHA General Hospital, Seoul, Korea. Written informed
consent was obtained from the tissue donors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rivera-Cruz CM, Shearer JJ, Figueiredo
Neto M and Figueiredo ML: The immunomodulatory effects of
mesenchymal stem cell polarization within the tumor
microenvironment niche. Stem Cells Int. 2017:40150392017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X
and Hui Y: Adipose-derived stem cells: Sources, potency, and
implications for regenerative therapies. Biomed Pharmacother.
114:1087652019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heo JS, Choi Y, Kim HS and Kim HO:
Comparison of molecular profiles of human mesenchymal stem cells
derived from bone marrow, umbilical cord blood, placenta and
adipose tissue. Int J Mol Med. 37:115–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Badimon L, Oñate B and Vilahur G:
Adipose-derived mesenchymal stem cells and their reparative
potential in ischemic heart disease. Rev Esp Cardiol (Engl Ed).
68:599–611. 2015. View Article : Google Scholar
|
|
5
|
Fernández O, Izquierdo G, Fernández V,
Leyva L, Reyes V, Guerrero M, León A, Arnaiz C, Navarro G, Páramo
MD, et al: Adipose-derived mesenchymal stem cells (AdMSC) for the
treatment of secondary-progressive multiple sclerosis: A triple
blinded, placebo controlled, randomized phase I/II safety and
feasibility study. PLoS One. 13:e01958912018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang JJ, Meng X, Li Y, Zhou Y, Xu DP, Li
S and Li HB: Effects of melatonin on liver injuries and diseases.
Int J Mol Sci. 18:2017.
|
|
7
|
Arendt J: Melatonin, circadian rhythms,
and sleep. N Engl J Med. 343:1114–1116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Zhang Y, Liu S, Li F, Wang B, Wang
J, Cao L, Xia T, Yao Q, Chen H, et al: Melatonin enhances
proliferation and modulates differentiation of neural stem cells
via autophagy in hyperglycemia. Stem Cells. 37:504–515. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Veneroso C, Tuñón MJ, González-Gallego J
and Collado PS: Melatonin reduces cardiac inflammatory injury
induced by acute exercise. J Pineal Res. 47:184–191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brydon L, Petit L, Delagrange P, Strosberg
AD and Jockers R: Functional expression of MT2 (Mel1b) melatonin
receptors in human PAZ6 adipocytes. Endocrinology. 142:4264–4271.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolden-Hanson T, Mitton DR, McCants RL,
Yellon SM, Wilkinson CW, Matsumoto AM and Rasmussen DD: Daily
melatonin administration to middle-aged male rats suppresses body
weight, intraabdominal adiposity, and plasma leptin and insulin
independent of food intake and total body fat. Endocrinology.
141:487–497. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alonso-Vale MI, Peres SB, Vernochet C,
Farmer SR and Lima FB: Adipocyte differentiation is inhibited by
melatonin through the regulation of C/EBPbeta transcriptional
activity. J Pineal Res. 47:221–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato H, Tanaka G, Masuda S, Ogasawara J,
Sakurai T, Kizaki T, Ohno H and Izawa T: Melatonin promotes
adipogenesis and mitochondrial biogenesis in 3T3-L1 preadipocytes.
J Pineal Res. 59:267–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Radio NM, Doctor JS and Witt-Enderby PA:
Melatonin enhances alkaline phosphatase activity in differentiating
human adult mesenchymal stem cells grown in osteogenic medium via
MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J
Pineal Res. 40:332–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Y, Cai B, Yuan F, He X, Lin X, Wang
J, Wang Y and Yang GY: Melatonin pretreatment improves the survival
and function of transplanted mesenchymal stem cells after focal
cerebral ischemia. Cell Transplant. 23:1279–1291. 2014. View Article : Google Scholar
|
|
16
|
Shuai Y, Liao L, Su X, Yu Y, Shao B, Jing
H, Zhang X, Deng Z and Jin Y: Melatonin treatment improves
mesenchymal stem cells therapy by preserving stemness during
long-term in vitro expansion. Theranostics. 6:1899–1917. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mias C, Trouche E, Seguelas MH, Calcagno
F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L,
Bianchi P, Calise D, et al: Ex vivo pretreatment with melatonin
improves survival, proangiogenic/mitogenic activity, and efficiency
of mesenchymal stem cells injected into ischemic kidney. Stem
Cells. 26:1749–1757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan
G, Cui W, Luo ZP, Pei M, Yang H and He F: Melatonin reverses
H2O2-induced premature senescence in
mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal
Res. 59:190–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones DL and Wagers AJ: No place like
home: Anatomy and function of the stem cell niche. Nat Rev Mol Cell
Biol. 9:11–21. 2008. View
Article : Google Scholar
|
|
20
|
Heo JS, Kim HO, Song SY, Lew DH, Choi Y
and Kim S: Poly-L-lysine prevents senescence and augments growth in
culturing mesenchymal stem cells ex vivo. Biomed Res Int.
2016:81960782016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heo JS, Lee SG and Kim HO: The flavonoid
glabridin induces OCT4 to enhance osteogenetic potential in
mesenchymal stem cells. Stem Cells Int. 2017:69217032017.
View Article : Google Scholar
|
|
22
|
Shaw J, Dale I, Hemsley P, Leach L, Dekki
N, Orme JP, Talbot V, Narvaez AJ, Bista M, Martinez Molina D, et
al: Positioning high-throughput CETSA in early drug discovery
through screening against B-Raf and PARP1. SLAS Discov. 24:121–132.
2019.
|
|
23
|
Soong R, Beyser K, Basten O, Kalbe A,
Rueschoff J and Tabiti K: Quantitative reverse
transcription-polymerase chain reaction detection of cytokeratin 20
in noncolorectal lymph nodes. Clin Cancer Res. 7:3423–3429.
2001.PubMed/NCBI
|
|
24
|
Gu Z, Jiang J, Tan W, Xia Y, Cao H, Meng
Y, Da Z, Liu H and Cheng C: p53/p21 Pathway involved in mediating
cellular senescence of bone marrow-derived mesenchymal stem cells
from systemic lupus erythematosus patients. Clin Dev Immunol.
2013:1342432013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang M, Yuan Q and Xie L: Mesenchymal stem
cell-based immunomodulation: Properties and clinical application.
Stem Cells Int. 2018:30576242018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang J, Yan Y, Teng X, Wen X, Li N, Peng
S, Liu W, Donadeu FX, Zhao S and Hua J: Melatonin prevents
senescence of canine adipose-derived mesenchymal stem cells through
activating NRF2 and inhibiting ER stress. Aging (Albany NY).
10:2954–2972. 2018. View Article : Google Scholar
|
|
27
|
Hu C and Li L: Melatonin plays critical
role in mesenchymal stem cell-based regenerative medicine in vitro
and in vivo. Stem Cell Res Ther. 10:132019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Chen S, Li Y and Liu Y: Melatonin
as a promising agent of regulating stem cell biology and its
application in disease therapy. Pharmacol Res. 117:252–260. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Gong Y, Xiong K, Ye Y, Xiong Y,
Zhuang Z, Luo Y, Jiang Q and He F: Melatonin mediates protective
effects on inflammatory response induced by interleukin-1 beta in
human mesenchymal stem cells. J Pineal Res. 55:14–25. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Su P, Xu C, Chen C, Liang A, Du
K, Peng Y and Huang D: Melatonin inhibits adipogenesis and enhances
osteogenesis of human mesenchymal stem cells by suppressing PPARγ
expression and enhancing Runx2 expression. J Pineal Res.
49:364–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roberts P: Heroes for the past and
present: A century of remembering Amundsen and Scott. Endeavour.
35:142–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bai C, Gao Y, Zhang X, Yang W and Guan W:
Melatonin promotes self-renewal of nestin-positive pancreatic stem
cells through activation of the MT2/ERK/SMAD/nestin axis. Artif
Cells Nanomed Biotechnol. 46:62–74. 2018. View Article : Google Scholar
|
|
33
|
Lee JH, Han YS and Lee SH: Potentiation of
biological effects of mesenchymal stem cells in ischemic conditions
by melatonin via upregulation of cellular prion protein expression.
J Pineal Res. 62:2017. View Article : Google Scholar
|
|
34
|
Ekmekcioglu C: Melatonin receptors in
humans: Biological role and clinical relevance. Biomed
Pharmacother. 60:97–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoon DS, Kim YH, Jung HS, Paik S and Lee
JW: Importance of Sox2 in maintenance of cell proliferation and
multipotency of mesenchymal stem cells in low-density culture. Cell
Prolif. 44:428–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hardeland R: Melatonin and the theories of
aging: A critical appraisal of melatonin's role in antiaging
mechanisms. J Pineal Res. 55:325–356. 2013.PubMed/NCBI
|
|
37
|
Armstrong SM and Redman JR: Melatonin: A
chronobiotic with anti-aging properties? Med Hypotheses.
34:300–309. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yew TL, Chiu FY, Tsai CC, Chen HL, Lee WP,
Chen YJ, Chang MC and Hung SC: Knockdown of p21(Cip1/Waf1) enhances
proliferation, the expression of stemness markers, and osteogenic
potential in human mesenchymal stem cells. Aging Cell. 10:349–361.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bakker AD, Silva VC, Krishnan R, Bacabac
RG, Blaauboer ME, Lin YC, Marcantonio RA, Cirelli JA and
Klein-Nulend J: Tumor necrosis factor alpha and interleukin-1beta
modulate calcium and nitric oxide signaling in mechanically
stimulated osteocytes. Arthritis Rheum. 60:3336–3345. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang W, Tang K, Wang Y, Zhang Y and Zan L:
Melatonin promotes triacylglycerol accumulation via MT2 receptor
during differentiation in bovine intramuscular preadipocytes. Sci
Rep. 7:150802017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zwirska-Korczala K, Jochem J,
Adamczyk-Sowa M, Sowa P, Polaniak R, Birkner E, Latocha M, Pilc K
and Suchanek R: Influence of melatonin on cell proliferation,
antioxidative enzyme activities and lipid peroxidation in 3T3-L1
preadipocytesan in vitro study. J Physiol Pharmacol. 56(Suppl 6):
S91–S99. 2005.
|
|
42
|
Sethi S, Radio NM, Kotlarczyk MP, Chen CT,
Wei YH, Jockers R and Witt-Enderby PA: Determination of the minimal
melatonin exposure required to induce osteoblast differentiation
from human mesenchymal stem cells and these effects on downstream
signaling pathways. J Pineal Res. 49:222–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luchetti F, Canonico B, Bartolini D,
Arcangeletti M, Ciffolilli S, Murdolo G, Piroddi M, Papa S, Reiter
RJ and Galli F: Melatonin regulates mesenchymal stem cell
differentiation: A review. J Pineal Res. 56:382–397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cawthorn WP, Scheller EL and MacDougald
OA: Adipose tissue stem cells meet preadipocyte commitment: Going
back to the future. J Lipid Res. 53:227–246. 2012. View Article : Google Scholar :
|
|
45
|
Lo Furno D, Graziano AC, Avola R,
Giuffrida R, Perciavalle V, Bonina F, Mannino G and Cardile V: A
citrus bergamia extract decreases adipogenesis and increases
lipolysis by modulating PPAR levels in mesenchymal stem cells from
human adipose tissue. PPAR Res. 2016:45638152016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mauriz JL, Collado PS, Veneroso C, Reiter
RJ and González-Gallego J: A review of the molecular aspects of
melatonin's anti-inflammatory actions: Recent insights and new
perspectives. J Pineal Res. 54:1–14. 2013. View Article : Google Scholar
|
|
47
|
Cardinali DP, Esquifino AI, Srinivasan V
and Pandi-Perumal SR: Melatonin and the immune system in aging.
Neuroimmunomodulation. 15:272–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mishra A, Paul S and Swarnakar S:
Downregulation of matrix metalloproteinase-9 by melatonin during
prevention of alcohol-induced liver injury in mice. Biochimie.
93:854–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Han YS, Lee JH, Yoon YM, Yun CW, Noh H and
Lee SH: Hypoxia-induced expression of cellular prion protein
improves the therapeutic potential of mesenchymal stem cells. Cell
Death Dis. 7:e23952016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu KH, Mo XM, Han ZC and Zhou B: Stem cell
engraftment and survival in the ischemic heart. Ann Thorac Surg.
92:1917–1925. 2011. View Article : Google Scholar : PubMed/NCBI
|