Introduction
Degenerative temporomandibular joint (TMJ) cartilage
disorders are one of the most common diseases in dental clinical
practice (1). Pathological
changes in the TMJ can result in osteoarthritis (OA), which is
mainly characterized by condylar cartilage degradation (2). Chondrocytes play a role in the
synthesis and degradation of extracellular matrix (ECM) components,
including type II collagen and proteoglycans such as aggrecan
(3). A decrease in the number of
chondrocytes leads to the manifestation of cartilage degradation
(4). All components of the
condylar cartilage ECM can also be regulated by
functionally-balanced matrix metalloproteinases (MMPs), A
disintegrin and metalloprotease with thrombos-pondin motifs
(ADAMTS) and tissue inhibitors of matrix metalloprotease that are
produced by chondrocytes (5).
Gene expression is regulated by both epigenetic and
non-epigenetic mechanisms, leading to the regulation of cell fate,
proliferation and differentiation, not only under physiological
conditions, but also pathological conditions in humans. Epigenetics
is defined as the study of the changes in gene expression without
changes in the DNA coding sequence. The major epigenetic mechanisms
are DNA methylation and histone modifications, which affect gene
expression by changing the chromatin structure (6). The most well-studied histone
modifications are methylation and acetylation. One of the crucial
histone modifications that affect the chromatin structure is the
methylation of histone 3 at lysine 9 (H3K9). There are four known
methylated states of H3K9, namely non-methylated, mono-methylated
(H3K9Me1), di-methylated (H3K9Me2) and tri-methylated (H3K9Me3)
(7), and each modification is
associated with the regulation of gene expression and several
biological responses (8).
The pathogenesis of OA involves multiple etiologies,
including mechanical, biochemical, genetic and epigenetic factors
that contribute to the imbalance in the synthesis and destruction
of articular cartilage (3,9).
Several previous studies have indicated that DNA methylation
influences the expression of anabolic and catabolic factors during
the pathogenesis of cartilage OA (10-12). Furthermore, histone acetylation
mediated by p300/CBP leads to the transcriptional activation of
Sox9, thus indicating that histone acetylation plays a role in
sustaining chondrocyte homeostasis (13). However, the biological
significance of histone methylation in OA is currently not well
understood. The molecular basis of the action of histone
methylation on the regulation of chondrocyte homeostasis also
remains poorly understood.
The effects of histone methylation on the etiologies
of degenerative TMJ cartilage disorders are a subject of growing
interest. The present study examined H3K9 to elucidate the role of
histone methylation in the pathogenesis of degenerative TMJ
cartilage disorders. The histone H3K9 methylation level was
investigated in degenerated condylar articular cartilage in mice.
The effects of treatment with chaetocin, a selective histone H3K9
inhibitor, were also investigated using chondrogenic ATDC5
cells.
Materials and methods
Ethics and mice
All animal experiments were approved and conducted
in accordance with the Animal Care and Use Ethics Committee of
Hokkaido University (Sapporo, Japan) and the National Center for
Geriatrics and Gerontology (Obu, Japan). Eight-week-old female
C57BL/6NCrSlc mice (n=10; weight, ~20 g; Japan SLC, Inc.) and
20-month-old female C57BL/6NCrSlc mice (n=10; weight, ~40 g; Aging
Farm, National Center for Geriatrics and Gerontology) were used in
the present study. All samples from the mice were collected and
analyzed between March 2016 and October 2018 at the Hokkaido
University and National Center for Geriatrics and Gerontology. Mice
used for experiments were anesthetized by intraperitoneal injection
of 0.3 mg/kg medetomidine hydrochloride, 4 mg/kg midazolam and 5
mg/kg butorphanol tartrate. This mixture of three anesthetics
[medetomidine, midazolam and butorphanol (MMB)] was developed as an
alternative to ketamine for use in rodents (14). The depth of anesthesia in the mice
was monitored via the corneal reflex and toe-pinch reflex to verify
a lack of response. MMB anesthesia is known to have several adverse
effects, including hypothermia (15). No side effects associated with
anesthesia were observed in the present study. The mice were
euthanized by cervical dislocation under anesthesia. Following
this, the skin of the heads of the mice was removed and the heads
were dissected into two halves. The TMJs were carefully isolated
with all the attached soft tissues removed using small
scissors.
Tissue preparation and histological
staining
TMJ tissues were fixed in 4% paraformaldehyde for 24
h at 4°C. The tissues were decalcified in 10% EDTA at pH 7.4 for
7-14 days at room temperature and then embedded in paraffin using
conventional methods. By using a microtome (REM-700; Yamato Kohki
Industrial Co., Ltd.), serial sagittal sections (5 µm) were
cut from the blocks of paraffin with embedded TMJ. The serial
sections of each condyle were stained with hematoxylin and eosin
(HE) and 0.1% safranin-O/0.02% fast green, according to the
following protocols, for analysis under a light microscope (Model
Eclipse Ci-S; Nikon Corporation) with a video controller (Digital
Sight, DS-L3; Nikon Corporation): For HE staining, the slides ware
stained with hematoxylin for 10 min, rinsed and stained with Eosin
Y for 5 min at room temperature; for safranin-O fast green
staining, the slides were stained with hematoxylin for 7 min,
rinsed, stained with 0.05% Fast Green for 5 min with a brief rinse
in 1% acetic acid, and stained with 0.1% Safranin-O for 30 min at
room temperature. HE staining was used to assess condylar changes,
and safranin-O staining and fast green staining were performed to
determine the proteoglycan changes.
Histological analysis and application of
the modified Mankin scoring system
A modified Mankin scoring system was used to assess
the degree of cartilage degradation (16). The scoring of the articular
cartilage was based on the pericellular and background staining of
safranin-O and fast green, the arrangement of the chondrocytes, and
the structural condition of the cartilage. In this system, the
score for normal articular cartilage was 0 points, whereas the
maximum score for degenerative articular cartilage was 10 points
(16).
Antibodies
The following antibodies were used in the present
study: Anti-H3K9me1 (cat. no. 07-450; rabbit polyclonal),
anti-H3K9me2 (cat. no. 07-441; rabbit polyclonal), and anti-H3K9me3
(cat. no. 07-442; rabbit polyclonal) (all from EMD Millipore).
Immunohistochemistry
Immunohistochemical analysis was performed using
primary antibodies against H3K9me1, H3K9me2 and H3K9me3.
Paraformaldehyde-fixed, paraffin-embedded 5-µm sagittal
sections of the condyles were prepared from 8-week-old and
20-month-old mice. After deparaffinization, antigen retrieval was
performed using Liberate Antibody Binding Solution (Polysciences,
Inc.) for 35 min and washed in PBS with Tween-20 at room
temperature, according to the manufacturer's directions. After
endogenous peroxidase was blocked with 1.4%
H2O2 in methanol for 20 min at room
temperature, the sections were incubated overnight at 4°C with
anti-H3K9me1 antibody (1:100), anti-H3K9me2 antibody (1:100) or
anti-H3K9me3 antibody (1:100). Thereafter, the sections were
incubated with secondary antibodies conjugated to the
peroxidase-labeled polymer, Envision+ Dual Link System-horseradish
peroxidase (cat. no. K4061; Dako; Agilent Technologies, Inc.) for 2
h at room temperature. Color development was performed using
3,3′-diaminobenzidine tetrahydrochloride (Wako Pure Chemical
Industries, Ltd.) for 10 min, and the sections were counterstained
with hematoxylin for 5 sec at room temperature. Images were
captured using a light microscope (Model Eclipse Ci-S; Nikon
Corporation) with a video controller (Digital Sight, DS-L3; Nikon
Corporation) at a magnification of ×200. When necessary,
whole-image adjustments of contrast and brightness were made
uniformly to the original data. The total number of cells and
immunopositive cells in each section were counted, and the
resulting ratio was calculated as the number of immunopositive
cells/total cells.
Cell cultures
ATDC5 mouse chondroprogenitor cells were obtained
from the RIKEN BioResource Research Center Cell Bank (Tsukuba,
Japan) (17). Cells were grown to
confluence in DMEM (Sigma-Aldrich; Merck KGaA) with 100
µg/ml kanamycin (Meiji Seika Kaisha, Ltd.) and 10% FBS
(SAFC; Merck KGaA) at 37°C in a humidified atmosphere of 5%
CO2. At 1 day after the cells reached confluence, the
medium was replaced with or without chaetocin (1, 10 or 100 nM;
Sigma-Aldrich; Merck KGaA) for 24 h. To investigate whether
chaetocin affects Mmp mRNA expression, cells were cultured
by adding interleukin 1β (R&D Systems, Inc.). Confluent ATDC5
cells were preincubated with DMEM containing 1% FBS for 24 h and
then cultured with 10 ng/ml interleukin 1β at the indicated
concentrations of chaetocin for 24 h.
Quantification of gene expression by
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from ATDC5 cells with or
without exposure to chaetocin by using RNAiso Plus (Takara Bio
Inc.), according to the manufacturer's directions. RT was then
performed using a PrimeScript RT Master Mix (Perfect Real Time;
Takara Bio Inc.). The conditions were as follows: RT reaction at
37°C for 15 min, RT heat inactivation at 85°C for 5 sec, and
cooling at 4°C. RT-qPCR was performed using assay-on-demand TaqMan
probes [cat. no. Mm00473485_m1 for Mmp1, cat. no.
Mm00439491_m1 for Mmp13, cat. no. Mm00448840_m1 for
Sox9 and cat. no. Mm01309565_m1 for collagen α1(II)
(Col2a1); Applied Biosystems; Thermo Fisher Scientific,
Inc.] and the StepOne® qPCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The qPCR cycling conditions
included an initial denaturation at 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min. The relative levels
of gene expression were quantified using the ΔΔCq method
(18), with gapdh (cat.
no. Mm99999915_g1; Applied Biosystems; Thermo Fisher Scientific,
Inc.) acting as the endogenous control. The fold-change in the mRNA
level of chaetocin-treated cells was calculated on the basis of the
level in untreated control ATDC5 cells.
Histone isolation and quantification
Total histone proteins were isolated from ATDC5
cells with or without exposure to chaetocin by using an EpiQuik™
Total Histone Extraction kit (Epigentek Group, Inc.), according to
the manufacturer's instructions. Global changes in the levels of
H3K9 mono-, di- and tri-methylation results were determined using
an EpiQuik™ Global Mono-methylation Histone H3K9 Quantification kit
(cat. no. P-3030), an EpiQuik™ Global Di-methylation Histone H3K9
Quantification kit (cat. no. P-3032), and an EpiQuik™ Global
Tri-methylation Histone H3K9 Quantification kit (cat. no. P-3034)
(all Epigentek Group, Inc.), according to the manufacturer's
protocol. Briefly, 50 µl C2 buffer was placed in each well,
and 200 ng isolated histone proteins was then added to each sample
well. Different concentrations of standard histone with H3K9 mono-,
di- and tri-methylation (10, 20, 40, 60, 80 or 100 ng/µl)
processing were added to standard wells. A blank was maintained and
the plate was incubated for 2 h at room temperature. After
incubation, the contents of the plate were discarded and the wells
were washed with 150 µl C1 buffer. A total of 50 µl
C3 buffer containing the secondary antibody was added to each well
and then incubated for 60 min at room temperature on an orbital
shaker. At the end of this incubation period, the wells were washed
with C1 buffer, and 100 µl C4 buffer was added to each well
and subsequently incubated for 2 min at room temperature in the
dark. The enzymatic reaction was stopped via the addition of 50
µl C5 buffer, and the absorbance was read at 450 nm using a
microplate reader (iMark; Bio-Rad Laboratories, Inc.). The
fold-change in the histone methylation level of chaetocin-treated
cells was calculated on the basis of a comparison with that
observed in untreated control ATDC5 cells.
Cell viability assay
The tetrazolium-based colorimetric TetraColor One
assay (Seikagaku Corporation) was used to quantitate cell
viability. Briefly, ATDC5 cells were seeded into 96-well plates and
cultured until confluence, under the aforementioned conditions. At
1 day post-confluence, the medium was replaced with or without
chaetocin for 24 h. Thereafter, the substrate WST-8 [composed of
2-(2-methoxy-4-nitrophenyl)-3-(4-nitro
phenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] was
added to each well. After incubation for an additional 2 h, the
absorbance at 450 nm was measured with a microplate reader (iMark;
Bio-Rad Laboratories, Inc.). The fold-change in the viability of
chaetocin-treated cells was then calculated on the basis of a
comparison with that in untreated ATDC5 cells.
Measurement of caspase-3/7 activity
The cellular enzymatic activities of caspase-3/7
were determined using a caspase colorimetric assay (Caspase-Glo 3/7
Assay Systems; Promega, Corporation), as described previously
(19). For each reaction, the
cells were lysed and incubated with a luminogenic substrate
containing the DEVD sequence, which is cleaved by activated
caspase-3/7. After incubation at room temperature for 1 h,
luminescence was quantified using a Mini Lumat LB 9506 luminometer
(Berthold Technologies GmbH & Co. KG). The fold-change in the
level of caspase-3/7 activity of chaetocin-treated cells was then
calculated on the basis of that present in untreated ATDC5
cells.
Statistical analysis
The quantitative data for modified Mankin score and
immunopositive cell ratios of H3K9 methylation are expressed as
ranges in box plots, and the differences between two groups were
assessed via the Mann-Whitney U test using the statistical program
file 'ystat 2008' (Igakutosho Shuppan, Ltd.) developed for
Microsoft Excel 2011 (Microsoft Corporation). The quantitative data
for RT-qPCR were recorded as the mean ± SD, and comparisons between
multiple groups were performed via one-way analysis of variance
followed by the application of Dunnett's multiple comparison test
(software provided by Osaka University, Japan; http://www.gen-info.osaka-u.ac.jp/MEPHAS/dunnett-e.html).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histone H3K9 methylation is reduced in
the articular cartilage of the degraded TMJ (the resorptive
condyle)
The condylar cartilage of 8-week-old and
20-month-old C57BL/6NCrSlc female mice was examined to elucidate
the differences in site-specific histone modifications associated
with TMJ cartilage damage. The surface of the condylar cartilage
was intact and smooth in 8-week-old mice (Fig. 1A). The condylar cartilage is
composed of fibrous, proliferative and hypertrophic layers, above a
layer of subchondral bone. In 8-week-old mice, safranin-O and fast
green staining showed that the condylar cartilage exhibited a rich
and even distribution of proteoglycans, particularly in the deep
layers of the cartilage (Fig.
1B). By contrast, the fibrocartilage layer was lost, and the
proliferative cartilage layer was exposed in 20-month-old mice,
thus indicating that the articular cartilage layer was damaged
(Fig. 1B). Furthermore, in the
aged mice, the articular cartilage appeared thinner, and the number
of chondrocytes appeared decreased. A pronounced loss of
proteoglycans was also observed in these mice (Fig. 1B). An analysis of histological
changes in the condyles using the modified Mankin scoring system
indicated that the degradation of the articular cartilage was
significantly higher in 20-month-old mice than in 8-week-old mice
(Fig. 1C). Thereafter, H3K9me was
examined in sections of the condyles by using specific antibodies
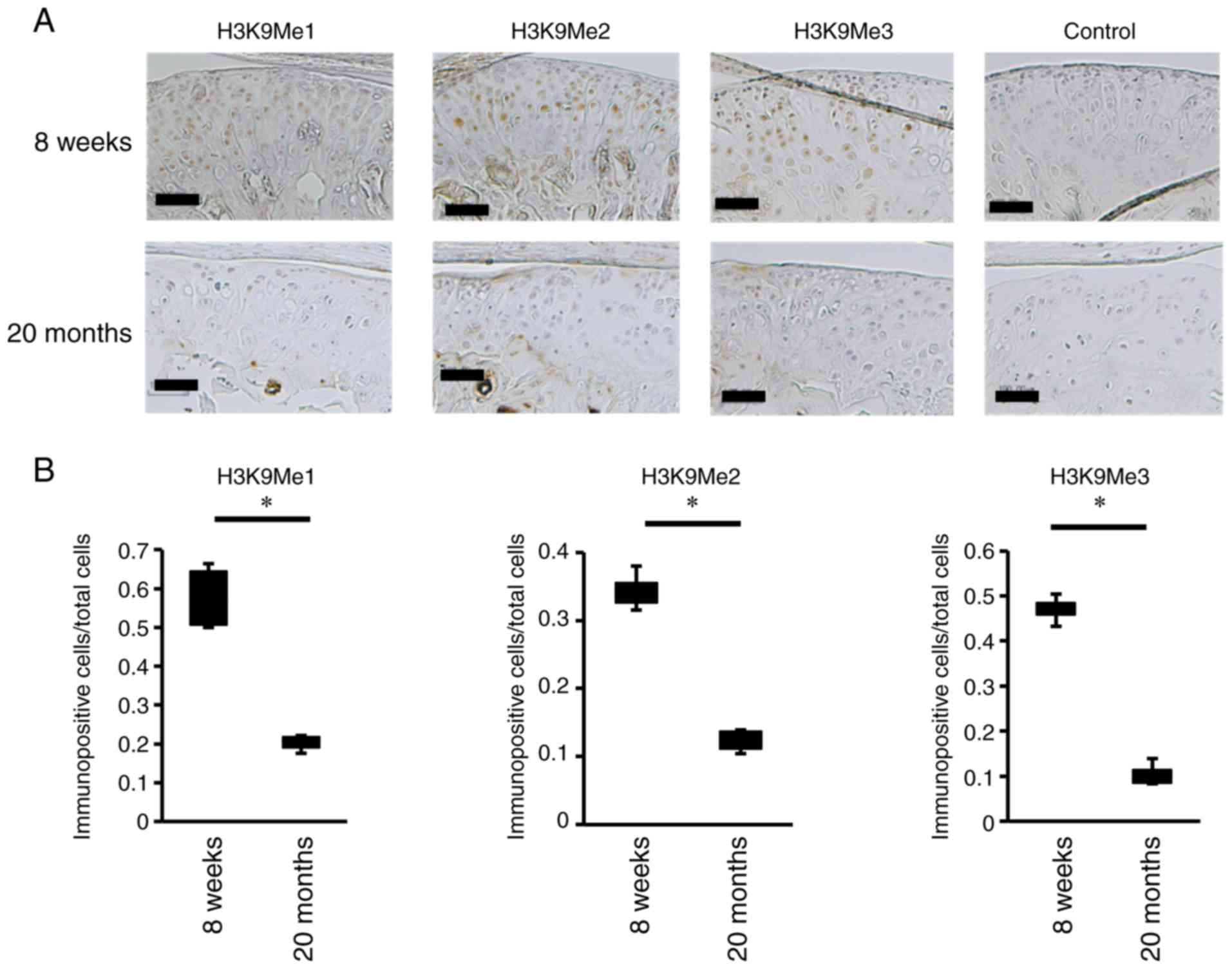
for H3K9Me1, H3K9Me2 and H3K9Me3. H3K9Me1-, H3K9Me2- and
H3K9Me3-positive chondrocytes were detected in the intact mouse
condyles and were localized in the hypertrophic cartilage layer
(Fig. 2A). However, in the
condyles of cartilage-damaged mice, decreases in H3K9me1-, H3K9Me2-
and H3K9Me3-positive cells were observed in the articular cartilage
layer (Fig. 2A). Furthermore, the
ratio of H3K9Me-positive cells in the condyles of cartilage-damaged
mice was lower than that in intact mice (Fig. 2B).
Inhibition of histone H3K9 methylation
reduces cell viability and induces apoptosis in ATDC5 cells
To elucidate the effects of histone H3K9 methylation
on the proliferation and differentiation of chondrocytes, ATDC5
cells were used for in vitro studies. The ATDC5 cell line is
derived from mouse teratocarcinoma cells, and characterized as a
chondrogenic cell line that exhibits a multistep sequential process
analogous to chondrogenic differentiation during endochondral bone
formation (20). Chaetocin, a
thiodioxopiperazine produced by Chaetomium spp.,
specifically inhibits suppressor of variegation 3-9 homolog 1 (a
histone H3K9-specific methyltransferase), thus resulting in a
reduction in H3K9 methylation (21). Treatment with chaetocin decreased
the methylation levels of H3K9Me1, H3K9Me2 and H3K9Me3 in ATDC5
cells (Fig. 3A). The number of
viable ATDC5 cells was also reduced by way of chaetocin treatment
(Fig. 3B). Furthermore,
caspase-3/7 activity in the cells was promoted by chaetocin
(Fig. 3C). These data indicated
that the inhibition of H3K9 methylation reduced cell viability and
induced apoptosis in ATDC5 cells.
Inhibition of histone H3K9 methylation
regulates chondrocyte mRNA expression in ATDC5 cells
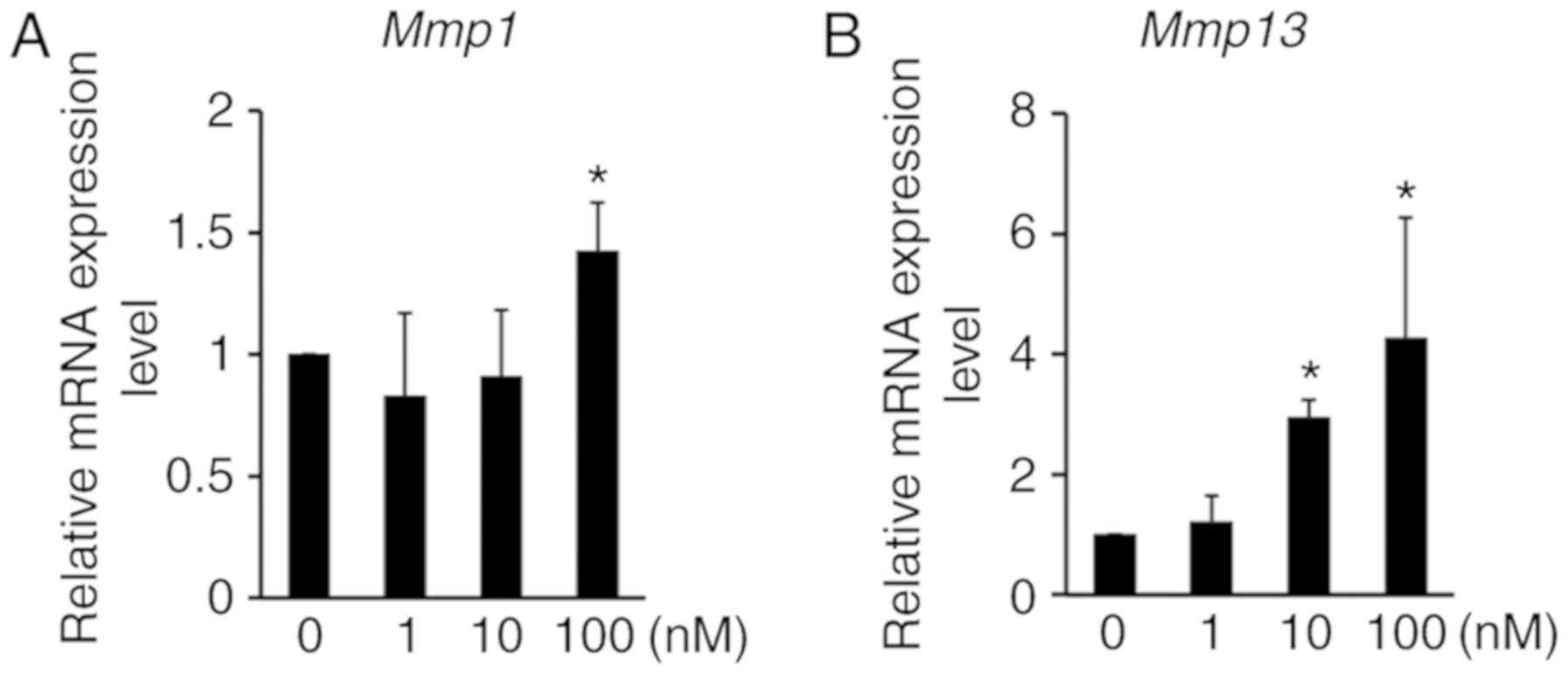
To investigate the relationship between H3K9
methylation and cartilage degradation, the expression of MMPs was
analyzed by RT-qPCR. The expression of Mmp1 and Mmp13
mRNA was significantly induced by chaetocin treatment in ATDC5
cells (Fig. 4), thus suggesting
that H3K9 methylation regulates not only chondrocyte proliferation
and apoptosis, but also cartilage ECM degradation. Thereafter,
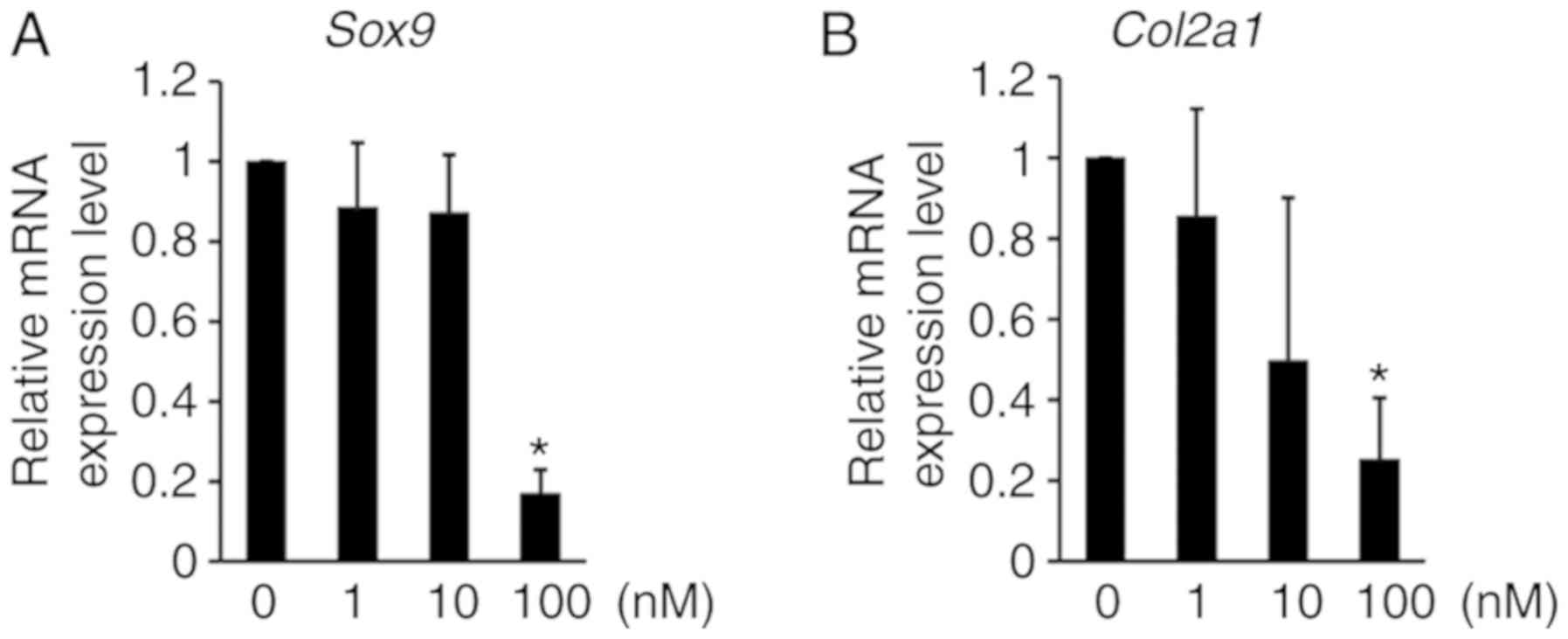
RT-qPCR analysis was performed to examine how the inhibition of
H3K9 methylation affects anabolic gene expression in ATDC5 cells.
The expression levels of Sox9 and Col2a1 mRNA, which
are well-known to be anabolic factors for chondrogenic
differentiation, were decreased by chaetocin treatment in ATDC5
cells (Fig. 5), thus indicating
that the inhibition of H3K9 methylation regulates the mRNA
expression of anabolic factors that induce chondrocyte
differentiation.
Discussion
Histone methylation and demethylation play crucial
roles in transcriptional control, thereby affecting gene expression
(6). H3K9 methylation is a
generally epigenetic mark of heterochromatin formation and
transcriptional silencing (22).
Previous studies have reported that histone H3K9 methylation at a
specific gene promoter is associated with the pathogenesis of OA. A
variety of changes in the Sox9 (the master transcriptional
factor that regulates chondrogenesis) gene promoter increase H3K9
methylation in the cartilage in advanced hip OA (23). The induction of microsomal
prostaglandin E synthase-1 (mPges-1) expression is
correlated with decreased levels of H3K9Me1 and H3K9Me2 at the
mPges-1 gene promoter in human OA chondrocytes (24). These observations indicate that
H3K9 methylation in specific gene promoters manages the expression
of several genes related to OA pathogenesis. The present study
demonstrated a decrease in global histone H3K9 methylation in a
mouse model of TMJOA. Histone methylation is regulated by histone
methyltransferases (HMTs) and histone demethylases (HDMs) (7). An increase in H3K9 methylation
appears to be associated with a reduced level of lysine-specific
demethylase 3A (25). The
conditional knockout of SET domain bifurcated histone lysine
methyltransferase 1 (ESET; an ERG-associated protein with a SET
domain; also called SETDB1), one of the SET domain-containing
lysine-specific HMTs, results in hypertrophy, apoptosis and the
terminal differentiation of articular chondrocytes at the knee
joint in C57BL/6 mice, thus indicating that HMTs may be implicated
in joint diseases, such as OA (26). However, there is a paucity of data
available related to the changes in HMTs and HDMs during the
pathogenesis of OA. It may be speculated that the regulation of the
activity of these HMTs and/or HDMs is associated with the
pathogenesis of TMJOA.
OA is associated with structural damage and
functional failure in articular cartilage, and cartilage
homeostasis is maintained by chondrocytes. OA chondrocytes are
characterized by accelerated catabolic processes and the
suppression of anabolic processes (27). Matrix-degrading enzymes, including
MMPs and ADAMTS, break down the ECM of cartilage (28,29). The promoters of genes encoding
catabolic enzymes such as MMP3, MMP9, MMP13 and ADAMTS4 are
demethylated, thus resulting in the increased expression of these
enzymes under OA-related pathogenic conditions in chondrocytes
(10). The results of the present
study indicated that treatment with chaetocin, a selective H3K9
methylation inhibitor, increased the expression of the Mmp1
and Mmp13 genes in ATDC5 cells. These observations suggest a
link between the epigenetic regulation of DNA methylation and
histone modification, including H3K9 methylation, in regulating the
gene expression of catabolic enzymes in OA chondrocytes. This
relationship may lead to cartilage degradation.
During the pathogenesis of OA, the expression of
anabolic factors, including ECM components, is suppressed in OA
chondrocytes. DNA methylation and histone modification have been
implicated by prior research in the regulation of the expression
levels of anabolic factors in OA (11,12), such as the methylation of collagen
type IX α1 chain (Col9a1) enhancer, which causes the
transcriptional repression of Col9a1 (30). A study involving human
chondrocytes found that histone methyltransferase Set7/9 elevated
trimethylated H3K4 in the Col2a1 promoter, thus resulting in
increased Col2a1 expression (31). Under OA-related pathogenic
conditions, the promoter of Sox9 exhibits elevated
tri-methylation levels of H3K9 and 27, thus leading to the
transcriptional repression of Sox9 (21). The present results showed that the
selective inhibition of H3K9 methylation suppressed anabolic
Sox9 and Col2a1 expression in ATDC5 cells. It is
assumed that the methylation of H3K9 regulates the expression of
anabolic and catabolic genes in ATDC5 cells, and may be related to
TMJOA pathogenesis. Although the status of histone methylation has
been broadly investigated in the development of cancer (32,33), it has not been as widely reported
in relation to the pathogenesis of OA, particularly TMJOA.
Many studies have shown that there are very low
levels of proliferative activity and apoptotic cell death in
chondrocytes in OA cartilage (4,34,35). In the present study, histone H3K9
demethylation by chaetocin decreased the number of viable cells and
induced caspase-3/7 activity in ATDC5 cells. The present data are
in agreement with those of another study showing that HMT and HDM
levels in cancer cells result in regulated proliferation and
apoptosis (36). For example,
lysine demethylase 4A regulates cell proliferation and apoptosis in
colon cancer cells (37).
Euchromatic histone lysine methyl-transferase 2 is also involved in
the proliferation, apoptosis and cell invasion of neuroblastoma
cells (38). This is due to the
modification of H3K9 methylation, which alters the chromatin
structure, cell cycle and apoptosis, and is correlated with the
onset of OA and pathological cartilage degradation (39).
The results of the present study demonstrated that
H3K9 demethylation occurs in TMJOA mouse cartilage, and that H3K9
demethylation suppresses cell proliferation and negatively
regulates chondrocyte homeostasis. TMJ cartilage degradation is a
late-onset, complex disease (40). The results of the present study
suggested that the epigenetic process of histone methylation may
play a crucial role in the pathogenesis of TMJOA. If the molecular
mechanisms connecting histone methylation and TMJOA can be
clarified by further investigations, histone methylation may be a
promising treatment method for TMJOA.
Funding
This work was supported in part by Grants-in-Aid for
Young Scientific Research (B) from the Japan Society for the
Promotion of Science (grant no. 17K1714707). This work was in part
also supported by the Akiyama Life Science Foundation (grant no.
FY2017).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MU conceived the project conducted most of the
experiments. KM conducted the animal experiments on 20-month-old
C57BL/6NCrSlc female mice. TY and MT provided technical and
conceptual support and advice. MU and MT wrote the manuscript. All
authors have read and approved the final version of this manuscript
for publication.
Ethics approval and consent to
participate
Ethical protocols for all animal experiments were
approved by the Institutional Animal Care Committee and conducted
in accordance with the Animal Care and Use Ethics Committee of
Hokkaido University (Sapporo, Japan) and the National Center for
Geriatrics and Gerontology (Obu, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Shigeru Takahashi
(Department of Oral Functional Anatomy, Faculty of Dental Medicine
and Graduate School of Dental Medicine, Hokkaido University,
Sapporo, Japan), Mr. Yoshiyuki Honma and Ms. Tomomi Takahashi
(Support Section for Education and Research, Faculty of Dental
Medicine and Graduate School of Dental Medicine, Hokkaido
University) for their consistent support throughout the project.
The authors would also like to thank Dr Tadahiro Iimura (Department
of Pharmacology, Faculty of Dental Medicine and Graduate School of
Dental Medicine, Hokkaido University) for valuable suggestions.
References
|
1
|
Schiffman E, Ohrbach R, Truelove E, Look
J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo
F, et al: Diagnostic Criteria for Temporomandibular Disorders
(DC/TMD) for Clinical and Research Applications: Recommendations of
the International RDC/TMD Consortium Network* and Orofacial Pain
Special Interest Group†. J Oral Facial Pain Headache. 28:6–27.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ibi M: Inflammation and temporomandibular
joint degradation. Biol Pharm Bull. 42:538–542. 2019. View Article : Google Scholar
|
|
3
|
Malemud CJ: Biologic basis of
osteoarthritis: State of the evidence. Curr Opin Rheumatol.
27:289–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun MM and Beier F: Chondrocyte
hypertrophy in skeletal development, growth, and disease. Birth
Defects Res C Embryo Today. 102:74–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang CY, Chanalaris A and Troeberg L:
ADAMTS and ADAM metalloproteinases in osteoarthritis-looking beyond
the 'usual suspects'. Osteoarthritis Cartilage. 25:1000–1009. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blum R: Stepping inside the realm of
epigenetic modifiers. Biomol Concepts. 6:119–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Black JC, Van Rechem C and Whetstine JR:
Histone lysine methylation dynamics: Establishment, regulation, and
biological impact. Mol Cell. 48:491–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh K, O'Neil K and Capell BC: Histone
modifiers: Dynamic regulators of the cutaneous transcriptome. J
Dermatol Sci. 89:226–232. 2018. View Article : Google Scholar :
|
|
9
|
Zhang M, Egan B and Wang J: Epigenetic
mechanisms underlying the aberrant catabolic and anabolic
activities of osteoarthritic chondrocytes. Int J Biochem Cell Biol.
67:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roach HI, Yamada N, Cheung KS, Tilley S,
Clarke NM, Oreffo RO, Kokubun S and Bronner F: Association between
the abnormal expression of matrix-degrading enzymes by human
osteoarthritic chondrocytes and demethylation of specific CpG sites
in the promoter regions. Arthritis Rheum. 52:3110–3124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramos YF and Meulenbelt I: The role of
epigenetics in osteoarthritis: Current perspective. Curr Opin
Rheumatol. 29:119–129. 2017. View Article : Google Scholar
|
|
12
|
Simon TC and Jeffries MA: The epigenomic
landscape in osteoarthritis. Curr Rheumatol Rep. 19:302017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furumatsu T, Tsuda M, Yoshida K, Taniguchi
N, Ito T, Hashimoto M, Ito T and Asahara H: Sox9 and p300
cooperatively regulate chromatin-mediated transcription. J Biol
Chem. 280:35203–35208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawai S, Takagi Y, Kaneko S and Kurosawa
T: Effect of three types of mixed anesthetic agents alternate to
ketamine in mice. Exp Anim. 60:481–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsukamoto A, Serizawa K, Sato R, Yamazaki
J and Inomata T: Vital signs monitoring during injectable and
inhalant anesthesia in mice. Exp Anim. 64:57–64. 2015. View Article : Google Scholar :
|
|
16
|
Xu L, Polur I, Lim C, Servais JM, Dobeck
J, Li Y and Olsen BR: Early-onset osteoarthritis of mouse
temporomandibular joint induced by partial discectomy.
Osteoarthritis Cartilage. 17:917–922. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Atsumi T, Miwa Y, Kimata K and Ikawa Y: A
chondrogenic cell line derived from a differentiating culture of
AT805 teratocarcinoma cells. Cell Differ Dev. 30:109–116. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iizuka S, Oridate N, Nashimoto M, Fukuda S
and Tamura M: Growth inhibition of head and neck squamous cell
carcinoma cells by sgRNA targeting the cyclin D1 mRNA based on TRUE
gene silencing. PLoS One. 9:e1141212014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao Y and Wang Y: ATDC5: An excellent in
vitro model cell line for skeletal development. J Cell Biochem.
114:1223–1229. 2013. View Article : Google Scholar
|
|
21
|
Greiner D, Bonaldi T, Eskeland R, Roemer E
and Imhof A: Identification of a specific inhibitor of the histone
methyltransferase SU(VAR)3-9. Nat Chem Biol. 1:143–145. 2005.
View Article : Google Scholar
|
|
22
|
Martin C and Zhang Y: The diverse
functions of histone lysine methylation. Nat Rev Mol Cell Biol.
6:838–849. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim KI, Park YS and Im GI: Changes in the
epigenetic status of the SOX-9 promoter in human osteoarthritic
cartilage. J Bone Miner Res. 28:1050–1060. 2013. View Article : Google Scholar
|
|
24
|
El Mansouri FE, Nebbaki SS, Kapoor M, Afif
H, Martel-Pelletier J, Pelletier JP, Benderdour M and Fahmi H:
Lysine-specific demethylase 1-mediated demethylation of histone H3
lysine 9 contributes to interleukin 1β-induced microsomal
prostaglandin E synthase 1 expression in human osteoarthritic
chondrocytes. Arthritis Res Ther. 16:R1132014. View Article : Google Scholar
|
|
25
|
Verrier L, Vandromme M and Trouche D:
Histone demethylases in chromatin cross-talks. Biol Cell.
103:381–401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lawson KA, Teteak CJ, Zou J, Hacquebord J,
Ghatan A, Zielinska-Kwiatkowska A, Fernandes RJ, Chansky HA and
Yang L: Mesenchyme-specific knockout of ESET histone
methyltransferase causes ectopic hypertrophy and terminal
differentiation of articular chondrocytes. J Biol Chem.
288:32119–32125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldring MB and Marcu KB: Cartilage
homeostasis in health and rheumatic diseases. Arthritis Res Ther.
11:2242009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pardo A and Selman M: MMP-1: The elder of
the family. Int J Biochem Cell Biol. 37:283–288. 2005. View Article : Google Scholar
|
|
29
|
Little CB, Barai A, Burkhardt D, Smith SM,
Fosang AJ, Werb Z, Shah M and Thompson EW: Matrix metalloproteinase
13-deficient mice are resistant to osteoarthritic cartilage erosion
but not chondrocyte hypertrophy or osteophyte development.
Arthritis Rheum. 60:3723–3733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imagawa K, de Andrés MC, Hashimoto K, Itoi
E, Otero M, Roach HI, Goldring MB and Oreffo RO: Association of
reduced type IX collagen gene expression in human osteoarthritic
chon-drocytes with epigenetic silencing by DNA hypermethylation.
Arthritis Rheumatol. 66:3040–3051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oppenheimer H, Kumar A, Meir H, Schwartz
I, Zini A, Haze A, Kandel L, Mattan Y, Liebergall M and
Dvir-Ginzberg M: Set7/9 impacts COL2A1 expression through binding
and repression of SirT1 histone deacetylation. J Bone Miner Res.
29:348–360. 2014. View Article : Google Scholar
|
|
32
|
Eissenberg JC and Shilatifard A: Histone
H3 lysine 4 (H3K4) methylation in development and differentiation.
Dev Biol. 339:240–249. 2010. View Article : Google Scholar
|
|
33
|
Ho L and Crabtree GR: Chromatin
remodelling during development. Nature. 463:474–484. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Loeser RF: Aging and osteoarthritis: The
role of chondrocyte senescence and aging changes in the cartilage
matrix. Osteoarthritis Cartilage. 17:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hwang HS and Kim HA: Chondrocyte apoptosis
in the pathogenesis of osteoarthritis. Int J Mol Sci.
16:26035–26054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang GG, Allis CD and Chi P: Chromatin
remodeling and cancer, Part I: Covalent histone modifications.
Trends Mol Med. 13:363–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim TD, Shin S, Berry WL, Oh S and
Janknecht R: The JMJD2A demethylase regulates apoptosis and
proliferation in colon cancer cells. J Cell Biochem. 113:1368–1376.
2012. View Article : Google Scholar
|
|
38
|
Lu Z, Tian Y, Salwen HR, Chlenski A,
Godley LA, Raj JU and Yang Q: Histone-lysine methyltransferase
EHMT2 is involved in proliferation, apoptosis, cell invasion, and
DNA methylation of human neuroblastoma cells. Anticancer Drugs.
24:484–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goldring MB and Marcu KB: Epigenomic and
microRNA-mediated regulation in cartilage development, homeostasis,
and osteoarthritis. Trends Mol Med. 18:109–118. 2012. View Article : Google Scholar :
|
|
40
|
Kalladka M, Quek S, Heir G, Eliav E,
Mupparapu M and Viswanath A: Temporomandibular joint
osteoarthritis: Diagnosis and long-term conservative management: A
topic review. J Indian Prosthodont Soc. 14:6–15. 2014. View Article : Google Scholar : PubMed/NCBI
|