Introduction
Balneotherapy is a clinically effective
complementary approach to the treatment of low-grade inflammation
and stress-related conditions (1). The biological mechanisms through
which immersion in mineral-medicinal water and the application of
mineral mud alleviate symptoms of various diseases remain largely
unknown. However, it is known that neuroendocrine and immunological
responses to balneo-therapy, including humoral and cell-mediated
immunity, are involved in these mechanisms, and lead to
anti-inflammatory, analgesic, antioxidant, chondroprotective and
anabolic effects, as well as to neuroendocrine-immune regulation in
various conditions (2,3).
The Campania region of Southern Italy is one of the
richest regions worldwide in terms of thermal and mineral water
resources. Indeed, thanks to their chemical and physical
prop-erties, these waters have been used for therapeutic purposes
since Roman times (4-6). The Nitrodi spring on the Island of
Ischia in the Bay of Naples was known to the Romans 2,000 years
ago, as witnessed by marble votive reliefs dated between the first
century B.C. and the second century A.D. found on the island.
Notably, a School of Medical Hydrology attended by such prominent
physicians as Menippo, Aurelius Monnus, Numerius Fabius, was linked
to the Nitrodi springs. It has been suggested that its name is
derived from the word 'nitro', namely 'soda' as the waters were
believed to be rich in this compound.
Thermal mineral water is classified based on its
chemical composition and temperature in low mineralized, mildly
miner-alized and highly mineralized, and cold (<20°C),
hypothermal (20-30°C), thermal (>30-40°C) and hyperthermal
(>40°C) (7). The Marotta and
Sica classification (8,9), which is the most widely accredited
classification in Italy (10), is
based on temperature, fixed residue and chemical composition
(8,9). According to this classification,
Nitrodi water is classified as follows: 'Medium mineral content,
thermal waters, bicarbonate, sulphate and alkaline, and earthy'
(8,9). Its dry residue at 180°C is
approxi-mately 0.9598 g/l, its source temperature is approximately
28°C, its concentration is approximately 0.4758 g/l
HCO3−, 0.2200 g/l SO4−,
0.1676 g/l NA+ and 0.1182 g/l Ca++ (dry
residue component of 1l water: Na, 0.167 g; Ca++ 0.118
g; SO4−, 0.220 g; CO3−,
0.234 g). Its main components are sodium, calcium, chlorine,
sulphur and carbon (11).
Although the last official analysis of Nitrodi water dates back to
1984, the findings were confirmed by the University of Naples
Federico II in an analysis conducted in 2003, which was recognized
by the Italian Ministry of Health (Decree no. 3509, October 9, 2003
'https://www.fontenin-fenitrodi.com/lafonte/pubblicazioni;),
and by a chemical and chemical-physical analysis in 2018, which
issued a signed report (unpublished data).
Moreover, in October, 2003, the Italian Ministry of
Health recognised the therapeutic properties of Nitrodi water.
Indeed, when ingested or when applied in the form of mud baths, it
has been proven to be effective in the treatment of various
ailments (6,11). For example, in the 1950s, Mancioli
(11) reported that Nitrodi water
promoted and regulated diuresis, improved the functional capacity
of the kidneys, and resolved or greatly alleviated gastritis and
gastroduodenitis. It is also an excellent adjuvant in the treatment
of gastroduodenal ulcers, varicose veins, wounds, fistulas and
pimples, and improves the health and appearance of the skin
(11). However, the molecular
mechanisms underlying the beneficial effects of Nitrodi water have
not yet been elucidated. Thus, the present study evaluated the
molecular basis of the benefits of Nitrodi water on wound healing
and in inflammatory diseases.
Materials and methods
Cell lines and culture
The RKO cell line (ATCC) was used to explore the
molecular basis of the beneficial effects of Nitrodi waters on
'inflammatory diseases' as they overexpress IL-6 and IL-6 receptors
(12), which are the main
cytokines of the inflammatory cascade (13) and several inflammatory diseases
involving the gastrointestinal compartment. The RKO cells were
grown in Eagle's minimum essential medium supplemented with 10%
fetal bovine serum, 100 U/ml penicillin and 100 µg/ml
streptomycin (completed medium). The cells were treated with
phosphate-buffered saline (PBS) prepared with Nitrodi water
(PBS-Nitrodi) for 4 h/day, 5 days a week, for 6 weeks and analysed
as described below. Treatment with PBS alone served as the
control.
Migration assays
Cell migration was evaluated using in vitro
Boyden chamber and wound healing assays as described elsewhere
(14). Briefly, to perform wound
healing assays, the cells treated as described above for 6 weeks
were seeded at 1×104 cells/well in 6-well plates. After
the cells formed a mono-layer (95-100% confluence) cells was rinsed
and then medium without FBS was added overnight (14 h);
subsequently, scratch wounds were made with the tip of a
200-µl pipette, and the scratch was photographed under a
light microscope (Leica Automated Inverted Microscope for Life
Science Research Leica DMI4000 B, Type DMI400B 11888318, serial
number 279034) at ×5 magnification. The cells were again incubated
for 4 h with PBS (RKO-PBS) or PBS-Nitrodi (RKO-Nitrodi), rinsed and
then medium without FBS was added. Finally, after 24 h from the
scratch, cells were photographed a second time. The experiment was
repeated 3 times.
A Boyden chamber assay was performed using the QCM™
24-well colorimetric cell migration assay systems (pore size: 8.0
µm; EMD Millipore). A 300 µl aliquot of a
1×106 cells/ml suspension was resuspended in serum-free
medium and seeded in the upper chamber of a 24-well insert.
Subsequently, 500 µl of free medium (prepared according to
the manufacturer's instructions) was added to the lower well of the
migration plate. Finally, the cells were removed from the top of
the membrane, the migrated cells were stained for 10 min at room
temperature with crystal violet solution (Sigma-Aldrich; Merck
KGaA), the stain was extracted and optical density was measured at
wavelength of 560 nm according to the manufacturer's instructions
(BioTek Synergy Microplate Reader; BioTek Instruments, Inc.). The
results represent the median of 4 experiments.
Cell viability assay
Cell viability was analysed by 3-(4,
5-dimeth-ylthiazol-2-yl)-5-(3-carboxymethxyphenyl)-2-(4-sulfophenyl)-2H
tetrazolium (MTT) assay as previously described (15). Cell suspensions (500 µl)
from the RKO-PBS and RKO-Nitrodi cells, containing 3×104
viable cells, were plated in 24 multi-well plates. To measure MTT
reduction by colorimetric assay, the cells were washed and
incubated for 3 h in 100 µl DMEM without phenol red (D2429,
Sigma-Aldrich; Merck KGaA), and supplemented with 0.45 mg/ml MTT.
The medium was then replaced by 100 µl 0.1 M HCl in
isopropanol and the cells were incubated at 37°C for 30 min for
lysis. The insoluble formazan was resuspended and optical densities
were measured at a wavelength of 570 nm using a microplate reader
(BioTek Synergy Microplate Reader; BioTek Instruments, Inc.),
according to the MTT manufacturer's protocol. The results represent
the mean of 3 experiments, each performed in duplicate.
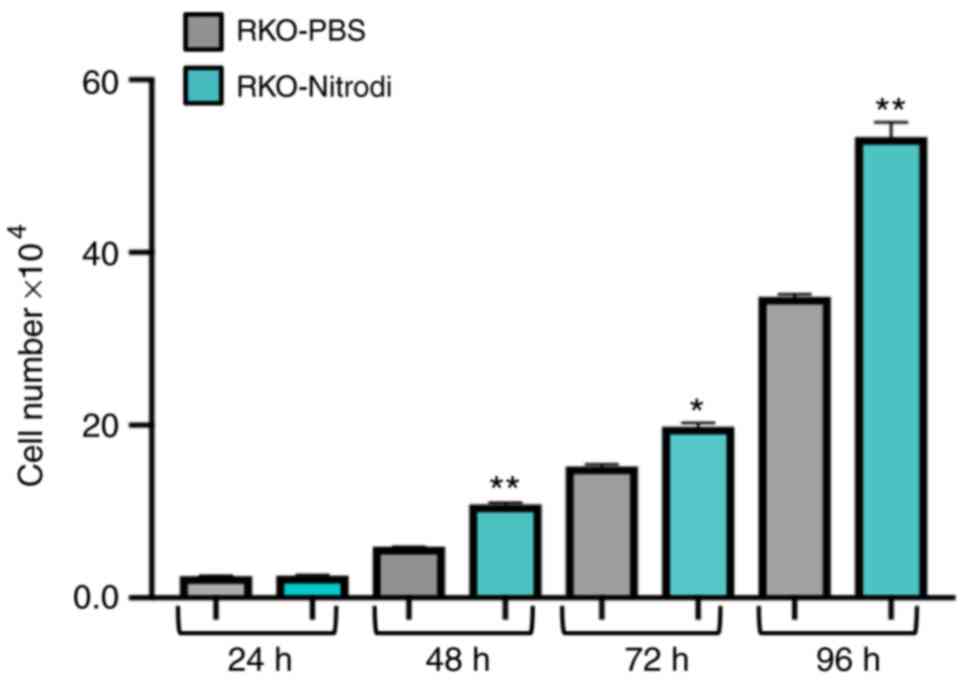
Cell growth analysis
To analyse cell growth, 2×104 cells were
seeded in a 24-well culture plate, trypsinized and counted every 24
h, i.e., 24 (T24 h), 48 (T48 h), 72 (T72 h) and 96 h (T96 h) after
seeding, using a Burker chamber apparatus. The experiments were
carried out in triplicate for each experimental point.
Western blot analysis
Total protein extracts were isolated from the RKO
cells treated with PBS or PBS-Nitrodi for 6 weeks as previously
described (16). Concentrations
were determined by using a protein assay kit adopting bovine serum
albumin standards, according to the manufacturer's instructions
(Bio-Rad Laboratories, inc.). Proteins were separated by
SDS-polyacrylamide gel electrophoresis at a 12% concentration, and
the blots were prepared as previously described (17). Nitrocellulose membranes were
stained with a Ponceau solution 0.1% (w/v) in 5% acetic acid
(P7170, Sigma-Aldrich; Merck KGaA) for 5 min at room temperature.
After blocking, the membranes were incubated in a solution
containing the primary antibody overnight at 4°C. Primary antibody
against cyclooxygenase (COX)-2 (rabbit polyclonal anti-human;
ab15191; dilution 1:1,000) was from Abcam; MMP2 antibody (rabbit
polyclonal l anti-human; NB200-193, diluition 1:10,000) was from
Novus Biologicals. The anti-GAPDH (mouse mono-clonal anti-human;
sc-69778; diluition 1:50,000) antibody was from Santa Cruz
Biotechnology, Inc. The membranes were probed with
peroxidase-conjugated secondary antibodies against rabbit (rabbit
monoclonal anti-human; #7074S; Cell Signaling Technology, Inc.;
dilution, 1:3,000) or mouse IgG (monoclonal anti-mouse; #7076S;
Cell Signaling Technology, Inc.; dilution, 1:3,000) for 1 h at room
temperature and immunoreactive bands were detected using the
enhanced chemiluminescence HRP Substrate Immobilon Western (EMD
Millipore). The experiment was repeated 3 times. Densitometry was
performed using ImageJ software 1.45s.
Fluorometric detection of
S-nitrosothiols
The fluorometric method reported by Wink et
al (18) was used to detect
S-nitrosolthiols. Briefly, the RKO cells were treated with PBS or
Nitrodi-PBS as described above. Following total protein extraction,
100 µg proteins were reacted with 100 µM
2,3-diaminonaphthalene in the presence of 100 µM of
HgCl2, for each sample, and incubated in the dark for 30
min at room temperature. The generated fluorescent compound
2,3-napththyltrazole was then measured at an excitation wavelength
of 375 nm and an emission wavelength of 450 nm, with a microplate
reader (BioTek Synergy Microplate Reader; BioTek Instruments,
Inc.).
Detection of protein S-nitrosocysteine
post-translational modifications
Protein S-nitrosocysteine post-translational
modifications were detected using the Pierce™ S-Nitrosylation
Western Blot assay according to the manufacturer's instructions
(Pierce; Thermo Fisher Scientific, Inc.). In brief, unmodified
cysteines were first blocked using a sulfhydryl-reactive compound,
the methyl methanethiosulfonate. S-nitrosylated cysteines were then
selectively reduced with ascorbate in HENS Buffer for specific
labeling with iodoTMTzero reagent, which irreversibly binds to the
cysteine thiol that was S-nitrosylated. The detection of the TMT
reagent-modified proteins is facilitated using an anti-TMT antibody
provided by the assay.
Statistical analysis
All data were obtained from at least 3 independent
experiments and are reported as the means ± standard error of the
mean (SEM). The Student's t-test was used to evaluate differences
between 2 groups. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
3 times as biological replicates that produced the same
results.
Results
Nitrodi spring water promotes cell
migration and viability
To investigate the effects of Nitrodi spring water
on cell migration in vitro, wound healing and Boyden chamber
migration assays were performed, as described in 'Materials and
methods', and it was found that Nitrodi water promoted cell
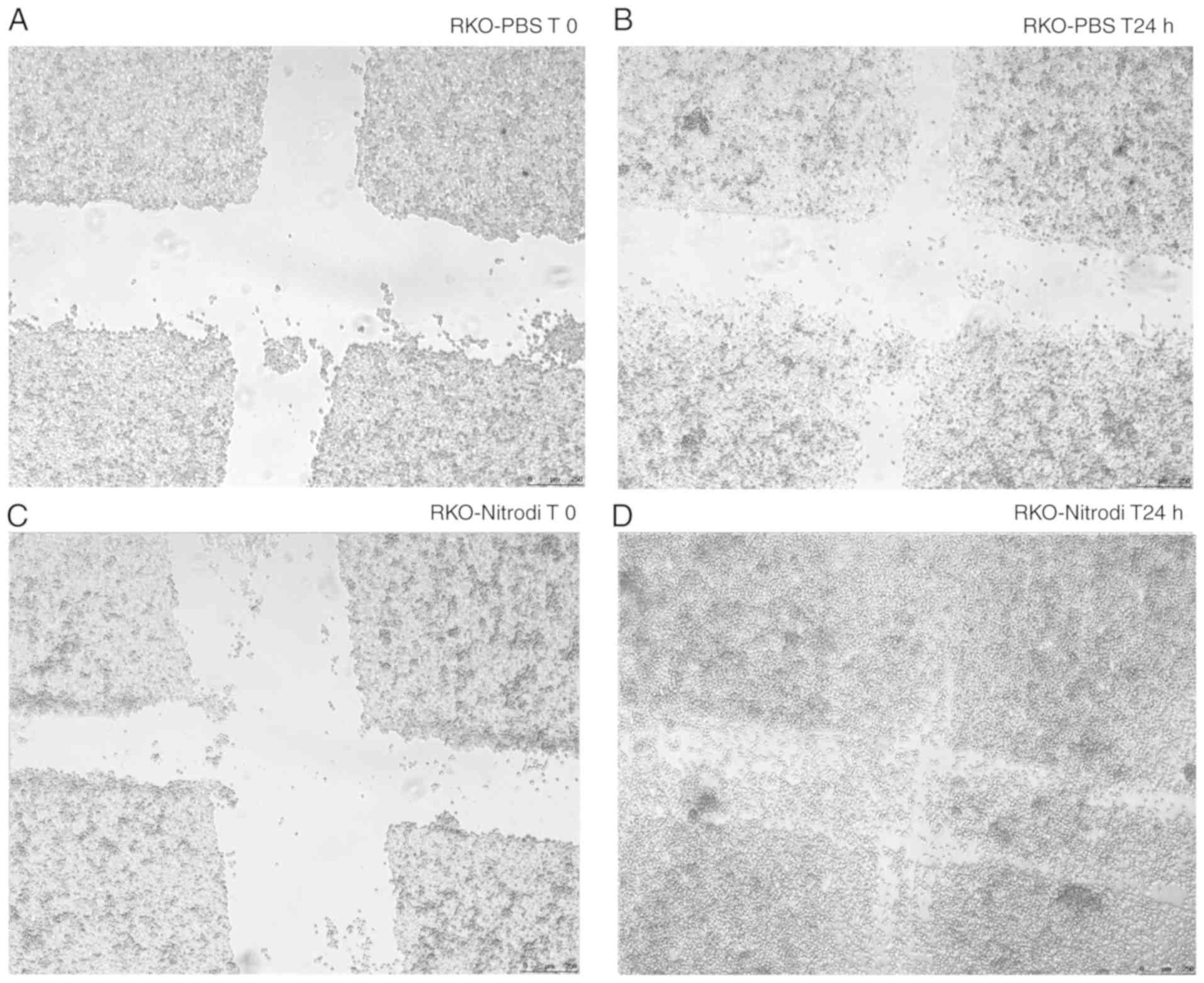
migration. In fact, as shown in Fig.
1, at 24 h after scratching, the wounds were almost completely
healed in the cells treated with PBS-Nitrodi (Fig. 1C and D), whereas the size of the
wounds in the cells incubated with PBS alone remained essentially
unaltered (Fig. 1A and B).
Similar results were obtained in the Boyden chamber assay (Fig. 2). Nitrodi water promoted the
migration of the RKO cells, as shown by the crystal violet staining
images of the PBS-control cells (Fig.
2A) and Nitrodi water-treated cells (Fig. 2B). Indeed, the crystal violet
optical density of the migrated cells, measured at a wavelength of
540 nm, was 0.416 and 0.594 nm in the PBS and PBS-Nitrodi-treated
cells, respectively (Fig. 2C).
Moreover, as shown in Fig. 3A and
B, cell viability was approximately 62% greater in the cells
treated with Nitrodi water than in those treated with PBS alone.
The mean optical density values, measured at a wave-length of 570
nm, were 0.0474 and 0.0758 nm in the PBS and PBS-Nitrodi-treated
cells, respectively (Fig. 3B).
Furthermore, the Nitrodi-treated cells grew at a significantly
faster rate than the PBS-control cells (Fig. 4).
Nitrodi spring water exerts
anti-inflammatory effects in vitro
To investigate whether COX-2 enzyme regulation is
involved in the therapeutic effects exerted by Nitrodi spring water
on inflammatory diseases (11),
the expression of the COX-2 enzyme and that of its direct target,
metalloprotease-2 (MMP2), were examined in the present study. To
this aim, a western blot analysis of the total protein extract from
RKO-PBS or RKO-Nitrodi cells was performed. As shown in Fig. 5A, following immunostaining and
autoradiography, the COX-2 antibody recognized two signals of
approximately 70 and 72 kDa, respectively. It was considered that
these 2 signals correspond to a light form (COX-L) and to the
S-nitrosylated active form (COX-H). As shown in Fig. 5A and B, COX-H expression was
higher in the RKO-PBS cells than in the RKO-Nitrodi cells, whereas
the expression of the COX-L isoform was significantly higher in the
RKO-Nitrodi cells. To verify the hypothesis that Nitrodi water
induces COX-2 inactivation, an immunostaining assay against MMP2
was performed, which is a COX-2 downstream target. As shown in
Fig. 5, MMP2 protein expression
was markedly downregulated in the RKO-Nitrodi cells compared with
the RKO-PBS control cells.
Nitrodi spring water downregulates
protein S-nitrosylation
To investigate whether Nitrodi water downregulates
protein S-nitrosylation, the fluorometric detection of
S-nitrosothiol and S-nitrosylation western blot analysis were
performed. The specific reaction between 2,3-diaminonaphthalene and
HgCl2, performed to detect S-nitrosothiols, generates
the fluorescent compound 2,3-napththyltrazole that emits light at a
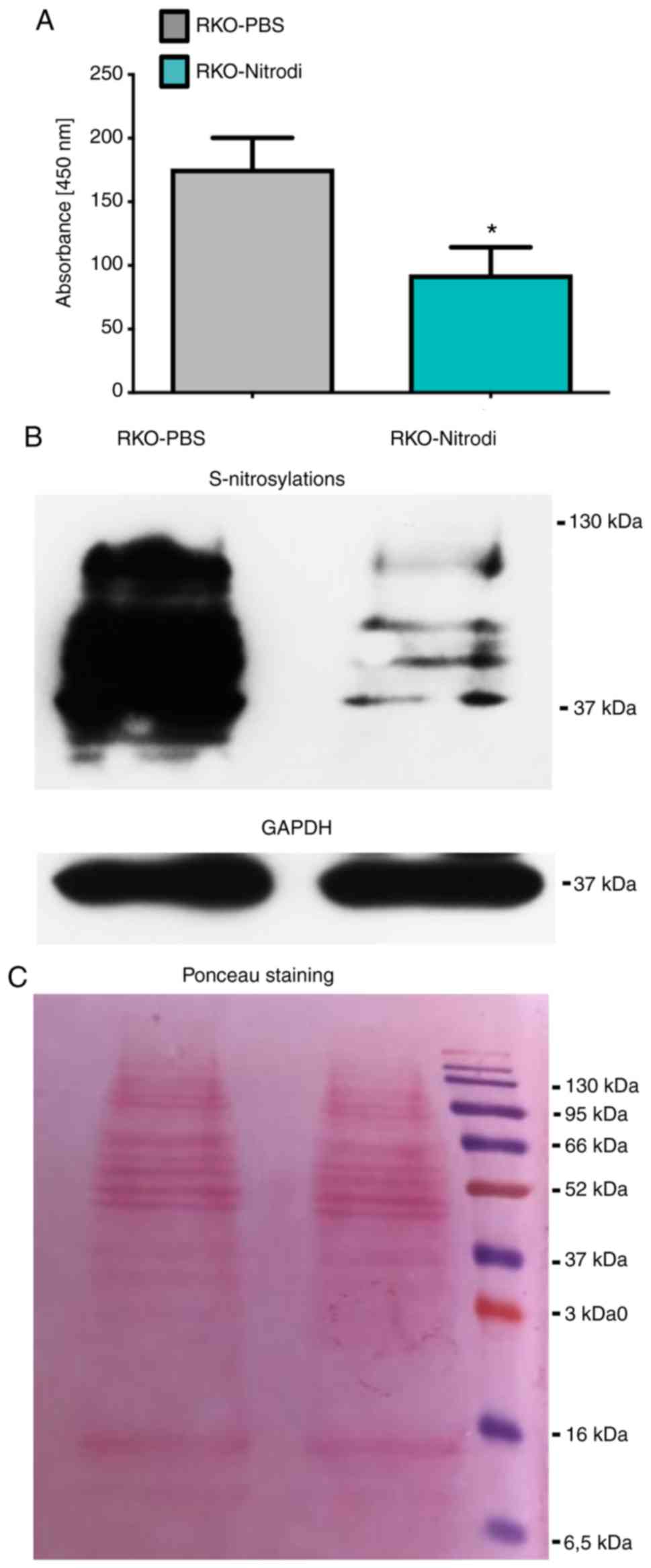
wave-length of 450 nm. As shown in Fig. 6A, the measured emitted light at a
wavelength of 450 nm, was approximately 50% lower in the
RKO-Nitrodi cells than in the RKO-PBS control cells. The mean OD
values were 176 in the RKO-PBS and 93 and in the RKO-Nitrodi cells.
These results were confirmed by an S-nitrosylation western blot
analysis that revealed the marked downregulation of protein
S-nitrosylation in the RKO-Nitrodi cells (Fig. 6B). Ponceau staining and GAPDH
protein served as internal controls (Fig. 6C).
Discussion
Balneotherapy and the assumption of thermal mineral
water have long been used in the treatment of diseases such as
atopic dermatitis, psoriasis, rheumatoid arthritis and
osteoarthritis, as well as metabolic and psychological diseases.
This treatment, alone or in combination with orthodox medical
treatments, has exhibited a renaissance over the past decade
(19). Balneotherapy exerts three
types of effects: mechanical or hydrostatic effects, thermal
effects depending on the temperature of the water, and chemical
effects depending the composition of the water that can be salty,
sulfurous, bicarbonated, carbonic, and/or enriched in other
specific elements. A limit of balneotherapy is that the mechanisms
through which each element contributes to the therapeutic effects
are unknown. Moreover, the biological mechanisms through which
mineral-medicinal water alleviates the symptoms of diseases remain
largely obscure. However, it has been well documented that
balneotherapy exerts anti-inflammatory, analgesic and antioxidant
effects together with neuroendocrine-immune regulation in various
disorders (2,20-22).
The aim of the present study was to shed light on
the molecular mechanisms sustaining the therapeutic efficacy of
Nitrodi spring water and to provide a robust scientific basis that
can improve its application in balnelogical therapy and hydroponic
therapy. To this aim, RKO cells were treated with PBS-Nitrodi water
or PBS alone as control, for 4 h/day, 5 days/week, for a total of 6
weeks. This in vitro protocol mimics the in vivo
treatment offered to patients and analyses the effects of Nitrodi
water on cell features and metabolism. Using this strategy, it was
demonstrated that Nitrodi spring water promotes the motility and
viability of RKO cells and induces the downregulation of protein
S-nitrosylation.
S-nitrosylation is an important post-translational
modification induced by nitric oxide (NO), and consists in the
coupling of the NO molecule to a reactive cysteine thiol to form an
S-nitrosothiol (23-28). S-Nitrosylation is implicated in
the regulation of carbohydrate and lipid metabolism, and, moreover,
aberrant S-nitrosylation of proteins is associated to
cardiovascular, pulmonary, musculoskeletal diseases, to
neurological dysfunction and to cancer (29-33). Accumulating evidence suggests that
NO production and S-nitrosylation dysregulation are key events in
the beginning of neurode-generation (34) and neuronal dysfunction in
Alzheimer's disease (35). NO is
also a key messenger in the pathogenesis of inflammation and exerts
this effect by activating innate and adaptive immunity (36).
The S-nitrosylation of COX-2 negatively regulates
its enzymatic activity (37,38). In the present study, following
immunostaining and autoradiography, the COX-2 antibody recognized a
light form of approximately 70 kDa (COX-L) and another isoform of
approximately 72 kDa attributable to the S-nitrosylated active form
(COX-H). The difference in mass may be compatible with a different
nitrosylation level of COX-2 cysteine residues; however, molecular
mass measured by western blot analysis is not very precise. In
accordance with this finding and with the therapeutic effects of
Nitrodi water, higher levels of un-nitrosylated total proteins were
found in cells treated with PBS-Nitrodi water than in PBS-treated
control cells. Notably, these findings suggest that the levels of
the un-nitrosylated isoform of the COX-2 enzyme were higher in the
RKO-Nitrodi cells than in the RKO-PBS cells. In accordance with
this hypothesis, the downstream COX-2 target MMP2 was also
downregulated following exposure to Nitrodi water.
The COX-2 enzyme plays a key role in the arachidonic
acid conversion to eicosanoids, thereby promoting inflammation and
tumour progression. The present study suggested that the molecular
mechanisms through which Nitrodi spring water exerts its
anti-inflammatory effects may involve COX-2 inactivation through
the downregulation of protein S-Nitrosylation. Given the crucial
role that both the S-nitrosylation of proteins and the inflammatory
cascade play in cell signalling, cell function and diseases
pathogenesis (23-33), the findings of the present study
support the application of Nitrodi spring water in the treatment of
diseases, such as osteoarthritis. Notably, it has recently been
demonstrated that osteoarthritis is caused by the inflammatory
response to high fluid shear stress (39). High fluid shear stress induces
COX-2 activation that, in turn, activates the expression of
prostaglandins, MMPs and pro-inflammatory cytokines (40). Furthermore, patients affected by
rheumatoid arthritis, psoriatic arthritis, reactive arthritis or
osteoarthritis also have high levels of PGF2α (41). These observations are in agreement
with the well-known beneficial therapeutic effects of Nitrodi water
on inflammatory diseases, such as osteoarthritis (2,3).
The data of the present study coincide with the
finding that mineral waters exert a positive therapeutic effect on
the anti-oxidant system, particularly sulphurous mineral water
(42,43). It has also been demonstrated that
hydrogen sulphide, released from sulphur, increased the release of
anti-inflammatory cytokines (44). It is conceivable that a similar
mechanism of action could also be responsible for the
anti-inflammatory proper-ties of Nitrodi spring water, and that
other mineral spring waters could act in a similar manner or by
modifying other general molecular and biochemical cell mechanisms,
such as microRNA expression (45).
However, cell biology studies on the molecular basis
of the properties of thermal waters worldwide are limited. To our
knowledge, Italy represents a unique case in that it is
particularly rich in springs that share the same therapeutic
effects and that have similar salt and thermal features to the
Nitrodi spring water, probably since they have the same
hydro-geological origin. For example, the Lepoldine water of
Montecatini Terme in Tuscany exerts anti-inflammatory effects
(46), and is also effective in
the treatment of atopic dermatitis, seborrhoea and psoriasis
(47). Research on the possible
therapeutic effects of other hot springs with comparable salt
content and thermal features worldwide should be encouraged.
Although Nitrodi water promoted the mobility and
proliferation of the colon cancer RKO cell line, it also induced
the downregulation of MMP2 expression, probably via COX2
inactivation, both molecules involved in cancer progression,
metastases and inflammation (48,49). It has recently been demonstrated
that MMP inhibitor is able to suppress the meta-static progression
of cancer (50). Furthermore,
Nitrodi water downregulates protein S-nitrosylation which modulates
cell signalling towards inflammation (51). It was hypothesized that these
effects, which could act synergistically, are the molecular basis
of the beneficial effects that Nitrodi water could exert on several
diseases (11).
The present study provides preliminary results that
should be verified in in vitro studies performed on
non-cancerous cell lines and in vivo. Additional experiments
are required to confirm the role of Nitrodi water on COX-2
S-nitrosylation, using the S-nitrosylation biotin switch assay to
precipitate nitrosylated protein and specific COX-2 detection by
western blot analysis (52).
Taking into consideration the association between NO and
S-nitrosylation (53), inducible
nitric oxide synthase inhibitors, such as N-nitro-L-arginine-methyl
ester (L-NAME), need to be used in the future to perform a negative
experimental control. Furthermore, future perspectives in thermal
water research may also aim to evaluate, in greater depth, the
protective effects that Nitrodi water exerts against inflammatory
stimulation, such as Il-6, in non-cancerous cells and to explore
the cytokine expression pattern following in vitro
incubation in Nitrodi water.
Funding
The present study was funded by a grant from
FORST-Fondazione per la Ricerca Termale-2018.
Availability of data and materials
All data generated or analysed during this study are
included in this published article. No datasets were generated or
analysed during the current study.
Authors' contributions
PI, ADP, MDR and FWR participated in the conception
and design of the study. FC and AA performed the cellular and
molecular experiments. MDR performed the statistical analysis of
the data. PI and ADP coordinated the work. MDR and FWR contributed
to data interpretation and wrote the manuscript. PI and ADP
critically revised the manuscript. PI provided funding. All authors
edited and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Jean Ann Gilder
(Scientific Communication srl, Naples, Italy) for editing the text.
The authors would also like to Giuseppe Di Meglio (Fonti delle
NinfeNitrodi, via pendio Nitrodi, 80070 Barano d'Ischia; termedinitrodi@gmail.com) for
supplying the Nitrodi water.
References
|
1
|
Matsumoto S: Evaluation of the role of
balneotherapy in rehabilitation medicine. J Nippon Med Sch.
85:196–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gálvez I, Torres-Piles S and Ortega-Rincón
E: Balneotherapy, immune system, and stress response: A hormetic
strategy? Int J Mol Sci. 19:16872018. View Article : Google Scholar :
|
|
3
|
Huang A, Seité S and Adar T: The use of
balneotherapy in dermatology. Clin Dermatol. 36:363–368. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andreassi L and Flori L: Mineral water and
spas in Italy. Clin Dermatol. 14:627–632. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ricciardi E, Ricciardi CA and Ricciardi B:
Treatment of kidney diseases in the thermal springs of Pithecusa
during the XVIII Century. G Ital Nefrol. 33(Suppl 66):
33.S66.262016.PubMed/NCBI
|
|
6
|
Forti L: Rilievi dedicati alle ninfe
nitrodi. Rendiconti dell'Accademia di Archeologia. (Lettere e Belle
Arti-Napoli, New Series 26). pp. 161–191. 1951
|
|
7
|
Nasermoaddeli A and Kagamimori S:
Balneotherapy in medicine: A review. Environ Health Prev Med.
10:171–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petraccia L, Liberati G, Masciullo SG,
Grassi M and Fraioli A: Water, mineral waters and health. Clin
Nutr. 25:377–85. 2006. View Article : Google Scholar
|
|
9
|
Marotta and Sica: Classificazione italiana
delle acque minerali. Annuali di Chimica Applicata. 19:1929.
|
|
10
|
Marotta and Sica: Classificazione italiana
delle acque minerali. Annuali di Chimica Applicata. 23:1933.
|
|
11
|
Mancioli M: Le proprietà terapeutiche
delle acque Nitrodi e Olmitello. Li Causi Editore; Bologna:
1984
|
|
12
|
Yuan H, Liddle FJ, Mahajan S and Frank DA:
IL-6-induced survival of colorectal carcinoma cells is inhibited by
butyrate through down-regulation of the IL-6 receptor.
Carcinogenesis. 25:2247–2255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Unver N and McAllister F: IL-6 family
cytokines: Key inflammatory mediators as biomarkers and potential
therapeutic targets. Cytokine Growth Factor Rev. 41:10–17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turano M, Costabile V, Cerasuolo A,
Duraturo F, Liccardo R, Delrio P, Pace U, Rega D, Dodaro CA, Milone
M, et al: Characterisation of mesenchymal colon tumour-derived
cells in tumourspheres as a model for colorectal cancer
progression. Int J Oncol. 53:2379–2396. 2018.PubMed/NCBI
|
|
15
|
Cammarota F, Conte A, Aversano A, Muto P,
Ametrano G, Riccio P, Turano M, Valente V, Delrio P, Izzo P, et al:
Lithium chloride increases sensitivity to photon irradiation
treatment in primary mesenchymal colon cancer cells. Mol Med Rep.
21:1501–1508. 2020.PubMed/NCBI
|
|
16
|
Galatola M, Paparo L, Duraturo F, Turano
M, Rossi GB, Izzo P and De Rosa M: Beta catenin and cytokine
pathway dysregulation in patients with manifestations of the ʻPTEN
hamartoma tumor syndrome'. BMC Med Genet. 13:282012. View Article : Google Scholar
|
|
17
|
Costabile V, Duraturo F, Delrio P, Rega D,
Pace U, Liccardo R, Rossi GB, Genesio R, Nitsch L, Izzo P and De
Rosa M: Lithium chloride induces mesenchymal to epithelial
reverting transition in primary colon cancer cell cultures. Int J
Oncol. 46:1913–1923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wink DA, Kim S, Coffin D, Cook JC,
Vodovotz Y, Chistodoulou D, Jourd'heuil D and Grisham MB: Detection
of S-nitrosothiols by fluorometric and colorimetric methods. In
Methods Enzymol. 301:201–211. 1999. View Article : Google Scholar
|
|
19
|
Nasermoaddeli A and Kagamimori S:
Balneotherapy in medicine: A review. Environ Health Prev Med.
10:171–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ortega E, Gálvez I, Hinchado MD, Guerrero
J, Martín-Cordero L and Torres-Piles S: Anti-inflammatory effect as
a mechanism of effectiveness underlying the clinical benefits of
pelotherapy in osteoarthritis patients: Regulation of the altered
inflammatory and stress feedback response. Int J Biometeorol.
61:1777–1785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galvez I, Torres-Piles S, D Hinchado M,
Alvarez-Barrientos A, Torralbo-Jimenez P, Guerrero J,
Martin-Cordero L and Ortega E: Immune-neuroendocrine dysregulation
in patients with osteoarthritis: A revision and a pilot study.
Endocr Metab Immune Disord Drug Targets. 17:78–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matz H, Orion E and Wolf R: Balneotherapy
in dermatology. Dermatol Ther. 16:132–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ehrenfeld P, Cordova F, Duran WN and
Sanchez FA: S-nitrosylation and its role in breast cancer
angiogenesis and metastasis. Nitric Oxide. 87:52–59. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwakiri Y, Satoh A, Chatterjee S, Toomre
DK, Chalouni CM, Fulton D, Groszmann RJ and Sessa WC: Nitric oxide
synthase generates nitric oxide locally to regulate
compartmentalized protein S-nitrosylation and protein trafficking.
Proc Natl Acad Sci USA. 103:19777–19782. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marín N, Zamorano P, Carrasco R, Mujica P,
González FG, Quezada C, Meininger CJ, Boric MP, Durán WN and
Sánchez FA: S-Nitrosation of β-catenin and p120 catenin: A novel
regulatory mechanism in endothelial hyperpermeability. Circ Res.
111:553–563. 2012. View Article : Google Scholar
|
|
26
|
Guequén A, Carrasco R, Zamorano P,
Rebolledo L, Burboa P, Sarmiento J, Boric MP, Korayem A, Durán WN
and Sánchez FA: S-nitrosylation regulates VE-cadherin
phosphorylation and internalization in microvascular permeability.
Am J Physiol Heart Circ Physiol. 310:H1039–H1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stamler JS, Simon DI, Jaraki O, Osborne
JA, Francis S, Mullins M, Singel D and Loscalzo J: S-nitrosylation
of tissue-type plasminogen activator confers vasodilatory and
antiplatelet properties on the enzyme. Proc Natl Acad Sci USA.
89:8087–8091. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rizi BS, Achreja A and Nagrath D: Nitric
oxide: The forgotten child of tumor metabolism. Trends in cancer.
3:659–672. 2017. View Article : Google Scholar
|
|
29
|
Foster MW, Hess DT and Stamler JS: Protein
S-nitrosylation in health and disease: A current perspective.
Trends Mol Med. 15:391–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakamura T and Lipton SA: 'SNO'-storms
compromise protein activity and mitochondrial metabolism in
neurodegenerative disorders. Trends Endocrinol Metab. 28:879–892.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Narne P, Pandey V and Phanithi PB: Role of
nitric oxide and hydrogen sulfide in ischemic stroke and the
emergent epigenetic underpinnings. Mol Neurobiol. 56:1749–1769.
2019. View Article : Google Scholar
|
|
32
|
Plenchette S: Role of S-nitrosylation in
the extrinsic apoptotic signalling pathway in cancer. Redox Biol.
5:4152015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei W, Li B, Hanes MA, Kakar S, Chen X and
Liu L: S-nitrosylation from GSNOR deficiency impairs DNA repair and
promotes hepatocarcinogenesis. Sci Transl Med. 2:19ra132010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu C, Liang MC and Soong TW: Nitric
oxide, iron and neurode-generation. Front Neurosci. 13:1142019.
View Article : Google Scholar
|
|
35
|
Spiers JG, Chen HJC, Bourgognon JM and
Steinert JR: Dysregulation of stress systems and nitric oxide
signaling underlies neuronal dysfunction in Alzheimer's disease.
Free Radic Biol Med. 134:468–483. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
García-Ortiz A and Serrador JM: Nitric
oxide signaling in T cell-mediated immunity. Trends Mol Med.
24:412–427. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim SF, Huri DA and Snyder SH: Inducible
nitric oxide synthase binds, S-nitrosylates, and activates
cyclooxygenase-2. Science. 310:1966–1970. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alexanian A and Sorokin A: Cyclooxygenase
2: Protein-protein interactions and posttranslational
modifications. Physiol Genomics. 49:667–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guan PP, Ding WY and Wang P: The roles of
prostaglandin F2 in regulating the expression of matrix
metalloproteinase-12 via an insulin growth factor-2-dependent
mechanism in sheared chondrocytes. Signal Transduct Target Ther.
3:272018. View Article : Google Scholar :
|
|
40
|
Goldring MB: Osteoarthritis and cartilage:
The role of cytokines. Curr Rheumatol Rep. 2:459–465. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Basu S, Whiteman M, Mattey D and Halliwell
B: Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha)
in different rheumatic diseases. Ann Rheum Dis. 60:627–631. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fioravanti A, Karagülle M, Bender T and
Karagülle MZ: Balneotherapy in osteoarthritis: Facts, fiction and
gaps in knowledge. Eur J Integr Med. 9:148–150. 2017. View Article : Google Scholar
|
|
43
|
Jantz MA and Antony VB: Pathophysiology of
the Pleura. Respiration. 75:121–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prandelli C, Parola C, Buizza L, Delbarba
A, Marziano M, Salvi V, Zacchi V, Memo M, Sozzani S, Calza S, et
al: Sulphurous thermal water increases the release of the
anti-inflammatory cytokine IL-10 and modulates antioxidant enzyme
activity. Int J Immunopathol Pharmacol. 26:633–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Karagülle MZ, Karagülle M, Kılıç S, Sevinç
H, Dündar C and Türkoğlu M: In vitro evaluation of natural thermal
mineral waters in human keratinocyte cells: A preliminary study.
Int J Biometeorol. 62:1657–1661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsoureli-Nikita E, Menchini G, Ghersetich
I and Hercogova J: Alternative treatment of psoriasis with
balneotherapy using leopoldine spa water. J Eur Acad Dermatol
Venereol. 16:260–262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Augustin M, Alvaro-Gracia JM, Bagot M,
Hillmann O, van de Kerkhof PCM, Kobelt G, Maccarone M, Naldi L and
Schellekens H: A framework for improving the quality of care for
people with psoriasis. J Eur Acad Dermatol Venereol. 26(Suppl 4):
S1–S16. 2012. View Article : Google Scholar
|
|
48
|
De Jager SCA and Hoefer IE: Beyond the
matrix: MMP2 as critical regulator of inflammation-mediated
vascular dysfunction. Cardiovasc Res. 113:1705–1707. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fingleton B: Matrix metalloproteinases as
regulators of inflammatory processes. Biochim Biophys Acta Mol Cell
Res. 1864:2036–2042. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lyu Y, Xiao Q, Yin L, Yang L, Wei H and He
W: Potent delivery of an MMP inhibitor to the tumor
microenvironment with thermosensitive liposomes for the suppression
of metastasis and angiogenesis. Signal Transduct Target Ther.
4:262019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dasgupta S, Gomez JJ, Singh I and Khan M:
S-Nitrosylation in regulation of inflammation and cell damage. Curr
Drug Targets. 19:1831–1838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Forrester MT, Foster MW, Benhar M and
Stamler JS: Detection of protein S-nitrosylation with the
biotin-switch technique. Free Radical Bio Med. 46:119–126. 2009.
View Article : Google Scholar
|
|
53
|
Martínez-Ruiz A, Cadenasa S and Lamas S:
Nitric oxide signaling: Classical, less classical, and nonclassical
mechanisms. Free Radical Bio Med. 51:17–29. 2011. View Article : Google Scholar
|