Introduction
Colon cancer is a life-threatening and lethal
disease with the highest incidence among solid tumors globally,
which is also associated with cancer-related financial stress
(1,2). Similar to other malignancies, the
development and progression of colon cancer may be caused by
mutations in certain key genes (3,4).
Therefore, investigating the underlying molecular mechanisms and
identifying new targets for the diagnosis and treatment of colon
cancer are of utmost importance. These findings will provide novel
therapeutic approaches which are beneficial to the patients, and
may reduce the harmful effects of the disease.
At present, microRNAs (miRNAs or miRs), types of
small non-coding RNAs of 20–24 nucleotides in length, have gained
increasing attention due to their regulatory effects on the
transcription of their target genes (5). A large number of studies have
suggested that miRNAs regulate fundamental biological processes,
while their dysfunction may affect the balance of the human body,
which in turn induces tissue/organ injuries (5,6).
Among the miRNA families, the present study aimed to investigate
the role of miR-181b in colon cancer. Several studies have
demonstrated the effects of miR-181b on the regulation of cell
proliferation, invasion and apoptosis in different types of cancer,
such as colorectal (7), ovarian
(8) and cervical cancer (9). Furthermore, miR-181 has been shown
to be involved in the regulation of tumor growth and the immune
system by engaging factors involved in signaling pathways, such as
mitogen-activated protein kinase 1 (MAPK1) (10) and nuclear factor-κB (NF-κB)
(11). However, the biological
functions of miR-181b in regulating the NF-κB signaling pathway in
colon cancer remain largely unclear.
The NF-κB pathway regulates the inflammatory
responses associated with tumorigenesis (12). Furthermore, the NF-κB signaling
pathway is constitutively activated in a number of types of cancer
and performs a variety of pro-tumorigenic functions. In thyroid
cancer, NF-κB has been shown to regulate the proliferative and
anti-apoptotic process (13). The
termination of the transcriptional activity of NF-κB is mainly
achieved by the upregulation of the expression of inhibitors of the
IκB family, particularly IκBα (14). In addition, the negative
regulatory factors of the NF-κB pathway are also upregulated,
including cylindromatosis (CYLD) (15). CYLD, a K63-specific
deubiquitinase, has been shown to inhibit the NF-κB signaling
pathway particularly by deconjugating the K63-linked polyubiquitin
chains (16). However, the
function of miR-181b in regulating CYLD and NF-κB in colon cancer
has not yet been fully elucidated; therefore, further studies on
this matter are required.
In the present study, in order to gain insight into
the role of miR-181b in regulating the NF-κB signaling pathway in
colon cancer, its expression levels and functions were determined
in colon cancer cells. Furthermore, the association between
miR-181b and CYLD was verified.
Materials and methods
Cell culture and treatment
The human colonic cancer cell lines, SW480, HCT116
and LoVo, and the human colonic epithelial HCoEpiC cell line were
obtained from the Shanghai Institute of Cell Research. Cells were
incubated in a 5% CO2 incubator (Thermo Fisher
Scientific, Inc.) at 37°C in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin solution (Gibco; Thermo Fisher Scientific,
Inc.). Cells were passaged every 2–3 days, and when they reached
the log phase, they were used for further experiments.
The expression of miR-181b was analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
levels of miR-181b in the human colon cancer cell lines were
significantly higher compared with those in the HCoEpiC cells. In
particular, the expression of miR-181b was the highest in the SW480
cells among all the colon cancer cells. The SW480 cells were
selected for use in subsequent experiments. The human colonic SW480
cancer cells were passaged and cultured in 6-well plates 24 h prior
transfection. Transfection was performed using lentiviral particles
according to the manufacturer's instructions. The cells were then
divided into the following groups: The blank control group (BC),
where cells were not treated; the miR-181b overexpression negative
control group (NC1), where cells were transfected with miR-181b
scramble; the miR-181b overexpression group (miR-181b), where cells
were transfected with miR-181b mimics; the miR-181b silencing
negative control group (NC2), where cells were transfected with
miR-181b inhibitor negative control; and the miR-181b silencing
group (si-miR), where cells were transfected with miR-181b
inhibitor. Subsequently, RT-qPCR analysis was performed to detect
the miR-181b expression levels in transfected cells.
For further experiments, the SW480 cells were
divided into the following groups: The blank control (BC) group;
miR-181b silencing (si-miR) group; CYLD overexpression negative
control (NC3) group, in which cells were treated with control
adenovirus vector containing non-targeting (AdNT, HBAD-GFP, Hanbio
Biotechnology Co., Ltd.); CYLD overexpression (CYLD) group, in
which cells were treated with adenovirus vector for CYLD (AdCYLD,
HBAD-h-CYLD-3*flag-GFP, Hanbio Biotechnology Co., Ltd.); miR-181b
silencing + CYLD silencing negative control (NC4) (si-miR + NC4)
group, in which cells were transfected with miR-181b inhibitor and
scramble control siRNA duplexes using Lipofecamine 2000
transfection reagent; and miR-181b silencing + CYLD silencing
(si-C) siRNA (si-miR + si-C) group, in which cells were transfected
with miR-181b inhibitor and CYLD specific small-interfering RNA
(siRNA) duplexes. RT-qPCR was used to detect the expression of CYLD
mRNA in the transfected cells.
Mimic control (5′-UUCUCCGAACGUGUCACGUTT-3′),
miR-181b mimic (5′-AACAUUCAUUGCUGUCGGUGGGU-3′), inhibitor control,
miR-181b inhibitor (5′-ACCCACCGACAGCAAUGAAUGUU-3′), scramble
control siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′), siCYLD
(5′-GAUUCUGCCUGGCUCUUCUUUGACA-3′) (GenePharma) were transfected
into the SW480 cells using Lipofectamine 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. All concentrations used were 50 nM.
After 24 h, the transfection mixture was removed and fresh complete
medium was added. The HBAD-GFP and HBAD-h-CYLD-3*flag-GFP were
trans-fected into 293-T cells. The supernatant titer was determined
to be 1x108 TU/ml. Subsequently, the lentivirus was used
to infect SW480 cells.
RT-qPCR.To determine the mRNA expression
levels of CYLD and miR-181b, RT-qPCR was performed. Briefly, total
RNA was extracted using TRIzol reagent (Takara Bio, Inc.) and was
reverse transcribed into cDNA at 42°C for 50 min using the
appropriate kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The qPCR reactions were carried out in the Mastercycler®
nexus X2 (Eppendorf) under the following conditions: 95°C for 15
sec, 60°C for 1 min and 35 cycles at 72°C for 40 sec. Data were
analyzed using the 2−ΔΔCq method (17), and the mRNA expression of U6 and
β-actin served as internal control for CYLD and miR-181b
expression, respectively. The primer sequences (Shanghai Biotech
Engineering Services) used were the following: miR-181b forward,
5′-GCGGATCATTCATTGCTGTCG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6
forward, 5′-GACCTCTATGCCAACACAGT-3′ and reverse,
5′-AGTACTTGCGCTCAGGAGGA-3′; CYLD forward,
5′-TCTATGGGGTAATCCGTTGG-3′ and reverse, 5′-CAGCCTGCACACTCATCTTC-3′;
and β-actin forward, 5′-CAGAGCCTCGCCTTTGCC-3′ and reverse,
5′-GTCGCCCACATAGGAATC-3′.
Cell Counting kit-8 (CCK-8) assay
Cell proliferation was assessed using a CCK-8 assay
(Tongren Institute of Chemistry, Japan). Briefly, the cells were
plated into 96-well plates, cultured for 24, 48, 72 and 96 h, and
the CCK-8 assay was then performed. Subsequently, the cells were
further cultured at 37°C for an additional 4 h and the absorbance
(OD) at 450 nm was measured in each well using a microplate reader
(ELI0A, BioBase).
Cell clone formation assay
Briefly, the cells were digested, plated into 6-well
plates at a density of 500 cells/well and cultured with DMEM
supplemented with 10% FBS and 1% penicillin-streptomycin solution
at 37°C for 2–3 weeks. The culture medium was replaced every 2
days. The cells were then immobilized with 4% paraformaldehyde for
15 min, and soaked in Giemsa stain (G4640, Beijing Solarbio Science
& Technology Co., Ltd.) for 30 min at room temperature and the
cells were then washed twice with ultra-pure water. Images were
acquired using a camera [DSC-HX90, SONY (China), Co. Ltd.].
Transwell migration and invasion
assays
The cell invasion ability was assessed using a
Transwell assay. Briefly, Matrigel (Beijing Solebao) was diluted
1:1 with pre-chilled DMEM and the solution was evenly spread on the
bottom of the Transwell chamber (Corning, Inc.). The cell
suspension (density, 5x104 cells; volume, 50 μl)
from each group was incubated at 37°C for 4 h. The substratum was
supplemented with 600 μl of DMEM for 72 h. The membrane was
then removed from the chamber and washed twice with PBS. The
transmembrane cells were fixed with 5% glutaraldehyde at 4°C and
stained with 0.1% crystal violet (Beijing Solarbio Science &
Technology Co., Ltd.) for 30 min at room temperature. Images were
acquired under an inverted microscope (Olympus Corporation) and 5
fields (magnification, ×400) were randomly selected to calculate
the average number of invaded cells. The migration assay was
performed as described above without the Matrigel.
Annexin V-FITC staining assay
An Annexin V-FITC staining assay was performed to
determine the cell apoptotic rate. Briefly, following treatment for
24 h, cells were resuspended, 5 μl of Annexin V-FITC and 5
μl of propidium iodide (PI) was added to the cell suspension
and the cells were incubated for 15 and 5 min at 37°C,
respectively. The cell apoptotic rate was assessed using a flow
cytometer (Beckman Coulter, Inc.) and the results were analyzed
using CellQuest software (Version 5.1; BD Biosciences).
Western blot analysis for the detection
of NF-κB signaling- and apoptosis-related proteins
Cells were lysed and the supernatant was isolated
following centrifugation at 850 × g for 20 min at 4°C. The protein
concentration was measured with a bicinchoninic acid kit (BSA;
Beijing Solarbio Science & Technology Co., Ltd.). Total protein
extracts (concentration, 40 μg) were subjected to 8%
SDS-PAGE electrophoretic separation and the resolved proteins were
transferred onto a polyvinylidene difluoride (PVDF) membrane.
Following blocking with 3% bovine serum albumin (BSA) in
tris-buffered saline containing Tween-20 (TBST) solution, the
membrane was incubated with primary antibodies against Bax (1:500,
orb378567), Bcl-2 (1:500, orb10173), cleaved caspase-3 (1:500,
orb106556), p65 (1:500, orb344389), p-p65 (1:500, orb14753), IκBα
(1:500, orb14528), p-IκBα (1:500, orb14868), CYLD (1:500, orb19453)
and β-actin (1:2,000, orb378579; all from Biorbyt Ltd.) overnight
at 4°C. Subsequently, the membrane was incubated with a goat
anti-rabbit IgG (1:1,000; ab6721; Abcam) secondary antibody for 50
min at 37°C. The protein bands were visualized using an ECL
chemiluminescence kit and β-actin was selected as a control to
calculate the relative expression of the target proteins. Protein
expression levels were semi-quantified using ImageJ software
(National Institutes of Health, version 6.0).
Dual luciferase reporter assay
The target gene prediction between miR-181b and CYLD
was performed using TargetScan software (www.targetscan.org). The wild-type (wt) and mutant
(mut) 3'untranslated region (3'UTR) of CYLD were amplified and
subcloned into the pGL3/luciferase vector (Promega Corporation)
downstream the luciferase gene. The constructed luciferase reporter
plasmid (wt-CYLD or mut-CYLD) was separately co-transfected with
miR-181b or NC into SW480 cells using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h. Following
co-transfection, cells were cultured for 48 h with DMEM containing
with 10% FBS and 1% penicillin-streptomycin solution. The
luciferase activity was detected using a dual luciferase reporting
system (Promega Corporation) according to the manufacturer's
instructions. Firefly luciferase activity was normalized to
Renilla luciferase activity for each transfected cell
sample.
Establishment of the subcutaneous tumor
transplantation model and animal grouping
A total of 24 male specific pathogen-free (SPF)
BALB/c nude mice (age, 4 weeks, weight, 18–20 g) were obtained from
Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[SCXK(Jing)20190006]. Mice were bred in a sterile isolation cage
with independent air supply in a room with air laminar flow
apparatus, constant temperature (26±1°C) and constant humidity.
Mice were free to drink autoclaved water and sterilized food. All
animal experiments were conducted in accordance with the National
Institute of Health (NIH) guidelines and were approved by the
Animal Ethics Committee of Yantaishan Hospital (approval no.
20190456-013). Furthermore, cells were digested with 0.25% trypsin
and counted using countess™ cell counting chamber slides (C10228.
Thermo Fisher Scientific, Inc.). SW480 cells were transfected with
miR-181b inhibitor, CYLD siRNA and AdCYLD, respectively. A total of
4 groups were included, blank control (BC), miR-181b silencing
(si-miR), CYLD overexpression (CYLD) and miR-181b silencing + CYLD
silencing (si-miR + si-C). The cell concentration was adjusted to
2.5x106 cells/ml with sterile normal saline. Finally,
0.2 ml of the cell suspension were subcutaneously inoculated into
the back of the right forelimbs of the nude mice. A total of 24
nude mice were randomly divided into the following groups: The
blank control (BC), miR-181b silencing (si-miR), CYLD
overexpression (CYLD) and miR-181b silencing + CYLD silencing
(si-miR + si-C) groups (n=6 per group). A Vernier caliper was used
to measure the long and short diameter of the tumor once a week and
the tumor volume was calculated using the following formula: Tumor
volume (V)=long diameter × short diameter2/2. The animal
health and behaviors were monitored each day, including diet,
weight, mental states and none of the mice died before the end of
the experiment. There was no death before the end of the
experiment. After 28 days, the nude mice were anesthetized with
0.6% sodium pentobarbital (40 mg/kg) by intraperitoneal injection,
and sacrificed by decapitation. Cardiac arrest for 5 min confirmed
death. The tumor was obtained and weighed. In addition, the tumor
tissues were immobilized in 4% paraformaldehyde and embedded in
paraffin. A tumor tissue sample was placed in a cryopreservation
tube and stored in a refrigerator at −80°C.
Detection of Ki67 using
immunohistochemistry
Tissue slices were deparaffinized in xylene,
rehydrated in gradient ethanol solution and treated with 3%
H2O2. To retrieve the antigen, slices were
heated at high temperature in a citrate buffer. Slices were then
incubated with a rabbit anti-human Ki67 antibody (1:200, orb389335
Biorbyt) and a secondary goat anti-rabbit IgG (1:1,000, ab6721;
Abcam) labeled with horseradish peroxidase. The sections were then
dehydrated in gradient ethanol, transparently treated with xylene
and sealed with neutral gum. The sections were then observed under
a light microscope (CX22, Olympus Corporation) and the Aperio
ImageScope software (version 11.1; Leica Microsystems GmbH) was
used to count cells.
TUNEL assay
The tumor tissue slices were treated with xylene and
gradient ethanol, and cell apoptosis was determined using a TUNEL
assay kit (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.).
Apoptotic cells were counted in 5 randomly selected fields under a
light microscope (CX22; Olympus Corporation). The apoptotic cells
were detected by the presence of tan or brown particles in the
cytoplasm and the morphological characteristics of apoptotic cells.
The apoptotic rate was calculated as follows: Apoptotic
index=(number of positive cells/total number of cells) x100%.
Statistical analysis
SPSS 19.0 software was used to perform the
statistical analyses, and data are expressed as the means ±
standard deviation (SD). One-way analysis of variance (ANOVA) with
a Tukey's post-hoc test was used to analyze the statistical
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of miR-181b on human colon cancer
cell proliferation
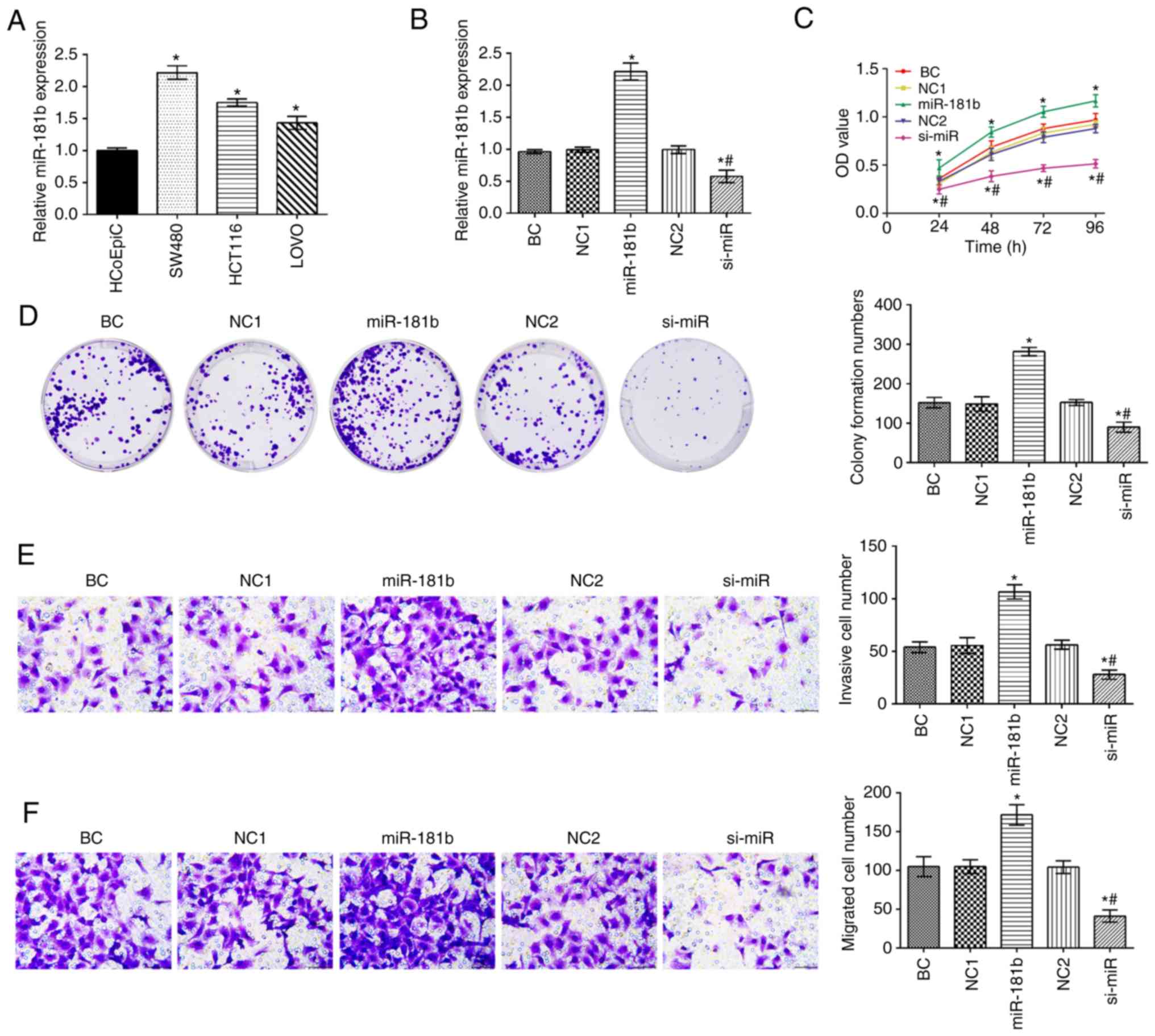
The expression of miR-181b in human colon cancer
cell lines was significantly higher compared with that in HCoEpiC
cells (P<0.05; Fig. 1A). The
expression of miR-181b was the highest in the SW480 cell among
colon cancer cells. The SW480 cells were thus selected to assess
the expression of miR-181 in the different groups (Fig. 1B). Compared with the BC group, the
expression of miR-181b, and the cell proliferative, invasive and
migratory capabilities were increased in the miR-181b group. By
contrast, miR-181b was downregulated in the si-miR group and this
was followed by a decreased cell proliferative, invasive and
migratory ability (P<0.05; Fig.
1C-F). Additionally, in contrast to the miR-181b group, the
expression of miR-181b and the cell proliferation, invasion and
migration rates were significantly decreased in the si-miR
group.
Effects of miR-181b on human colon cancer
cell apoptosis and the NF-κB signaling pathway
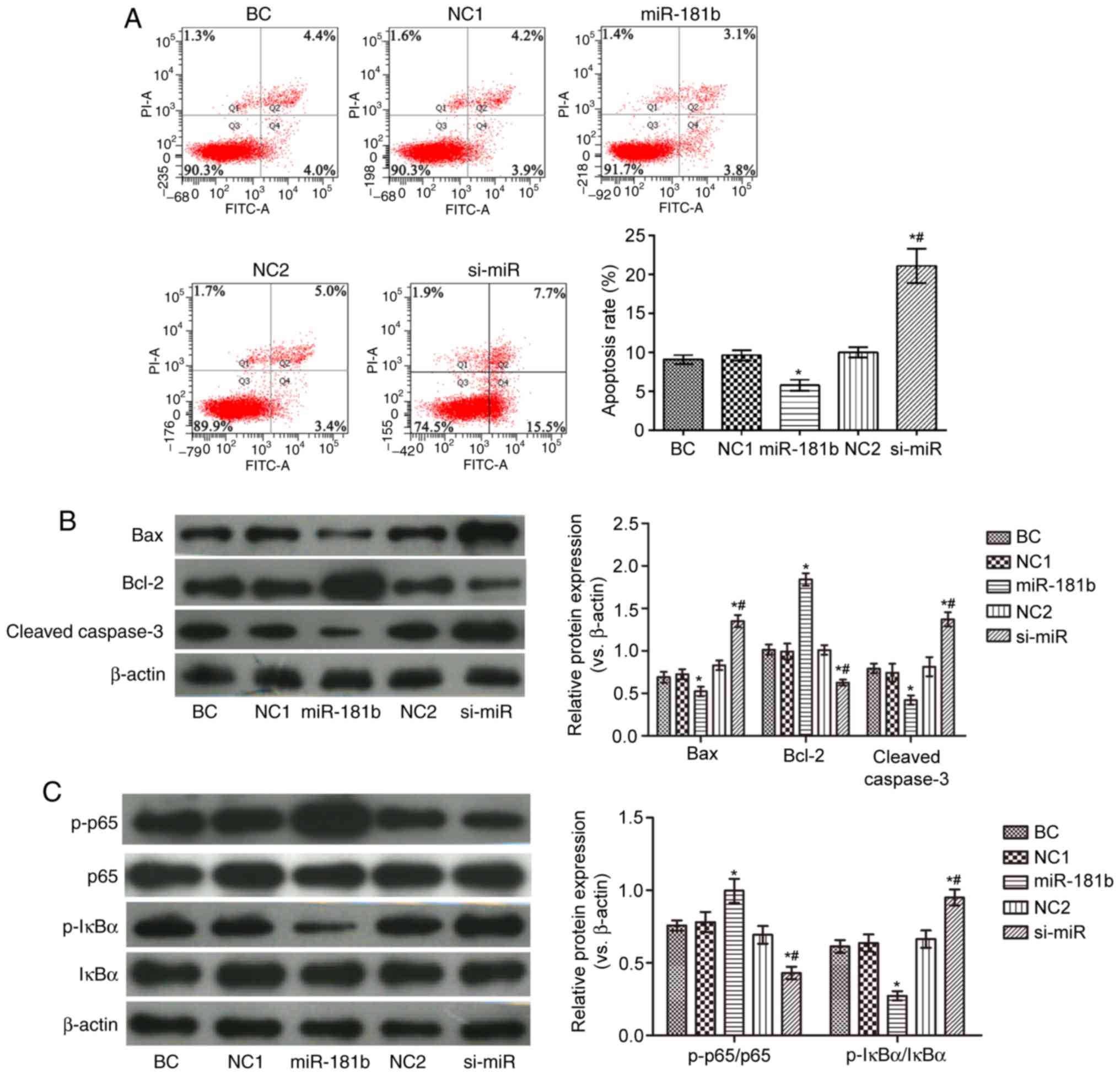
As shown in Fig.
2, compared with the BC group, the proportion of apoptotic
cells in the miR-181b group was observably reduced. In addition,
the expression levels of Bax, cleaved caspase-3 and p-IκBα/IκBα
were decreased, while those of Bcl-2 and p-p65/p65 were markedly
increased in the miR-181b group. In the si-miR group, the apoptotic
rate and the expression levels of Bax, cleaved caspase-3 and
p-IκBα/IκBα were notably increased, while those of Bcl-2 and
p-p65/p65 were markedly decresaed, compared with the BC group
(P<0.05). Furthermore, compared with the miR-181b group, the
proportion of apoptotic cells and the expression of Bax, cleaved
caspase-3 and p-IκBα/IκBα were increased, and the expression of
Bcl-2 and p-p65/p65 was notably decreased in the si-miR group
(P<0.05).
miR-181b directly targets CYLD
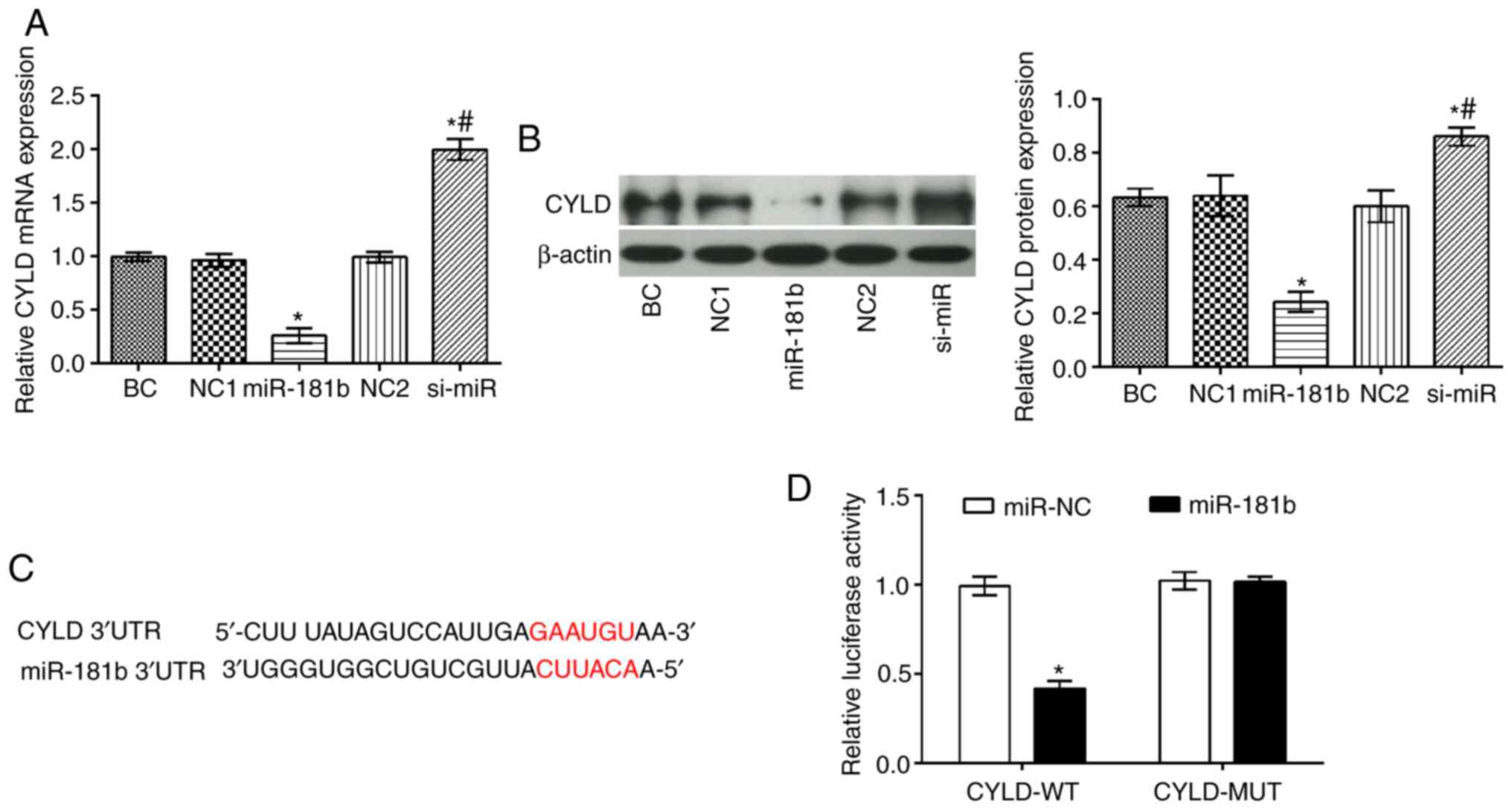
The CYLD mRNA and protein levels were significantly
decreased in the miR-181b group compared with the BC group
(P<0.05), while the opposite findings were obtained in the
si-miR group (P<0.05; Fig. 3).
In addition, compared with the miR-181b group, it was apparent that
the mRNA and protein expression levels of CYLD were markedly
increased in the si-miR group (P<0.05) (Fig. 3A and B). Furthermore, TargetScan
(http://www.targetscan.org) software
predicted that CYLD was a direct target of miR-181b (Fig. 3C). To further confirm this
interaction a dual luciferase assay was performed (Fig. 3C). Therefore, in cells
co-transfected with miR-181b mimic and the wt-CYLD 3'UTR, the
luciferase activity was markedly attenuated. However, the
luciferase activity was unaltered in the cells co-transfected with
miR-181b mimic and the mut-CYLD 3'UTR.
Effect of CYLD on human colon cancer cell
proliferation
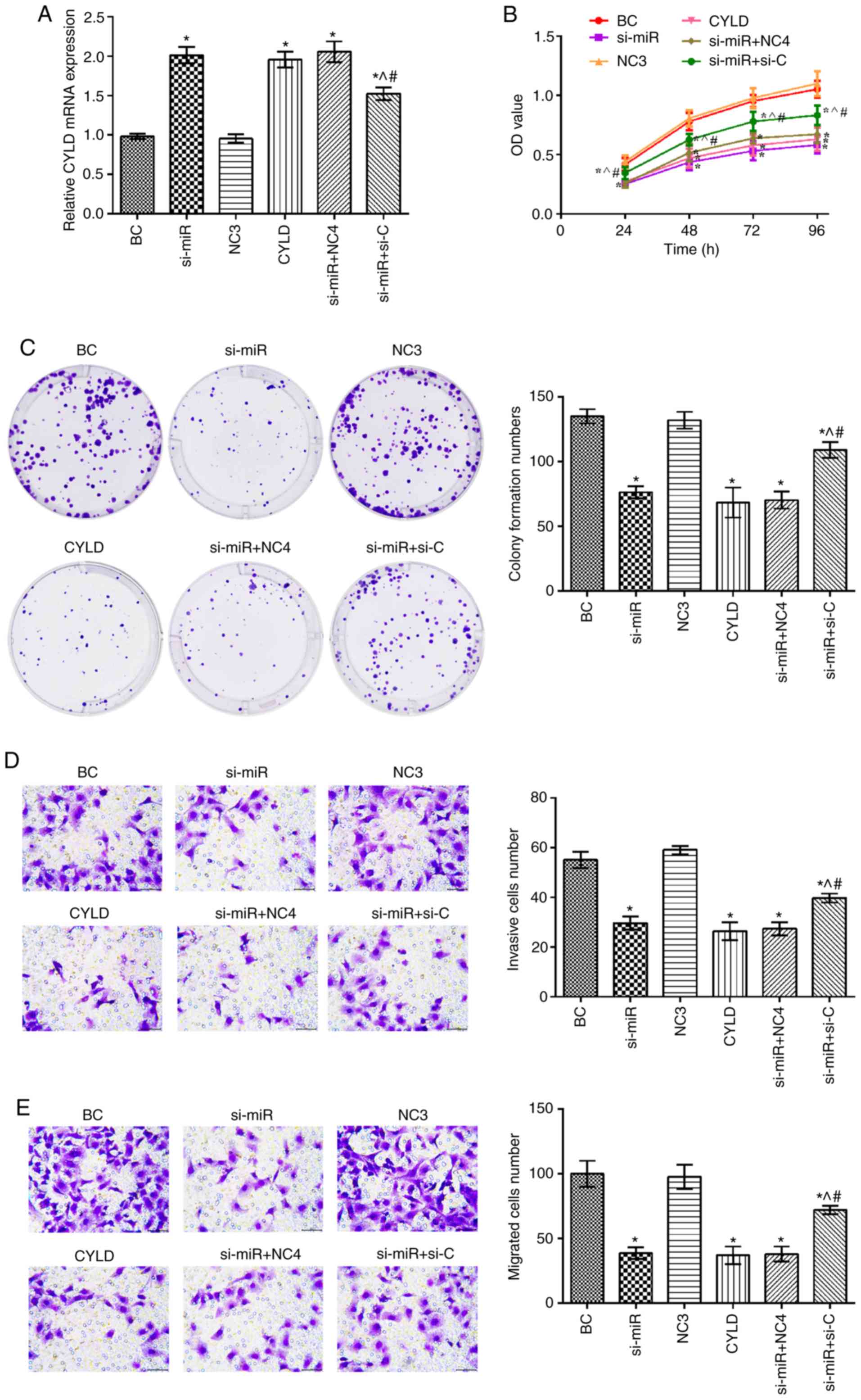
CYLD expression was upregulated in the si-miR, CYLD,
si-miR + NC4 and si-miR + si-C groups compared with the BC group
(P<0.05; Fig. 4). In addition,
the mRNA expression levels of CYLD in the si-miR + si-C group were
significantly decreased compared with the si-miR and CYLD groups.
Furthermore, compared with the BC group, the cell proliferation,
invasion and migration rates were notably suppressed in the si-miR,
CYLD, si-miR + NC4 and si-miR + si-C groups (P<0.05). Notably,
these rates were elevated in the si-miR + si-C group compared with
the si-miR and CYLD groups (P<0.05).
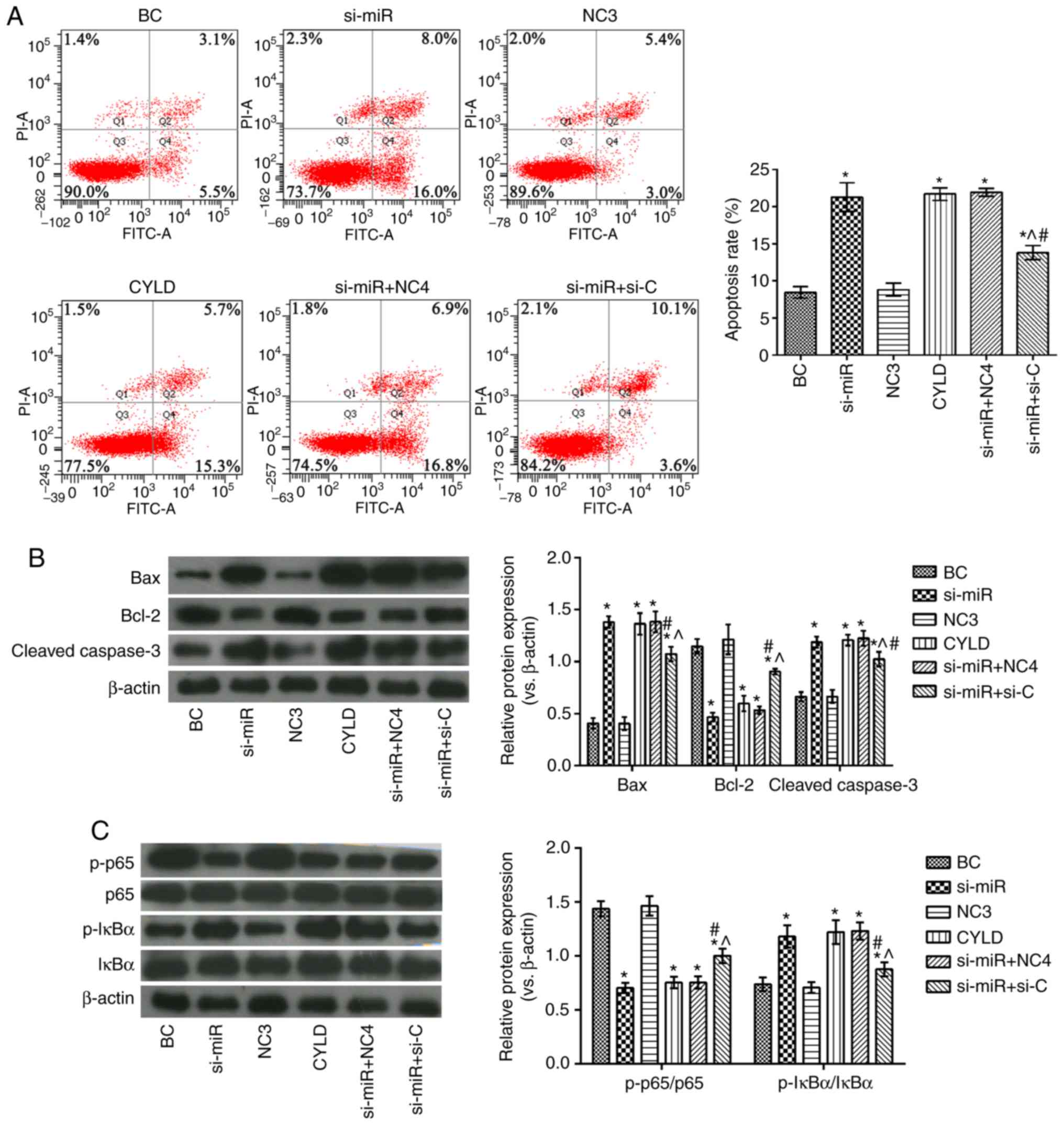
Effects of miR-181b targeting CYLD on
human colon cancer cell apoptosis and the NF-κB signaling
pathway
As shown in Fig.
5, the si-miR, CYLD, si-miR + NC4 and si-miR + si-C groups
exhibited significantly higher apoptotic rates, as well as
increased Bax, cleaved caspase-3 and p-IκBα/IκBα expression levels,
and decreased Bcl-2 and p-p65/p65 levels compared with the BC
group. However, compared with the si-miR and CYLD groups, the
proportion of apoptotic cells was evidently decreased in the si-miR
+ si-C group. In addition, the expression levels of Bax, cleaved
caspase-3 and p-IκBα/IκBα were evidently decreased and those of
Bcl-2 and p-p65/p65 were notably elevated in the same groups.
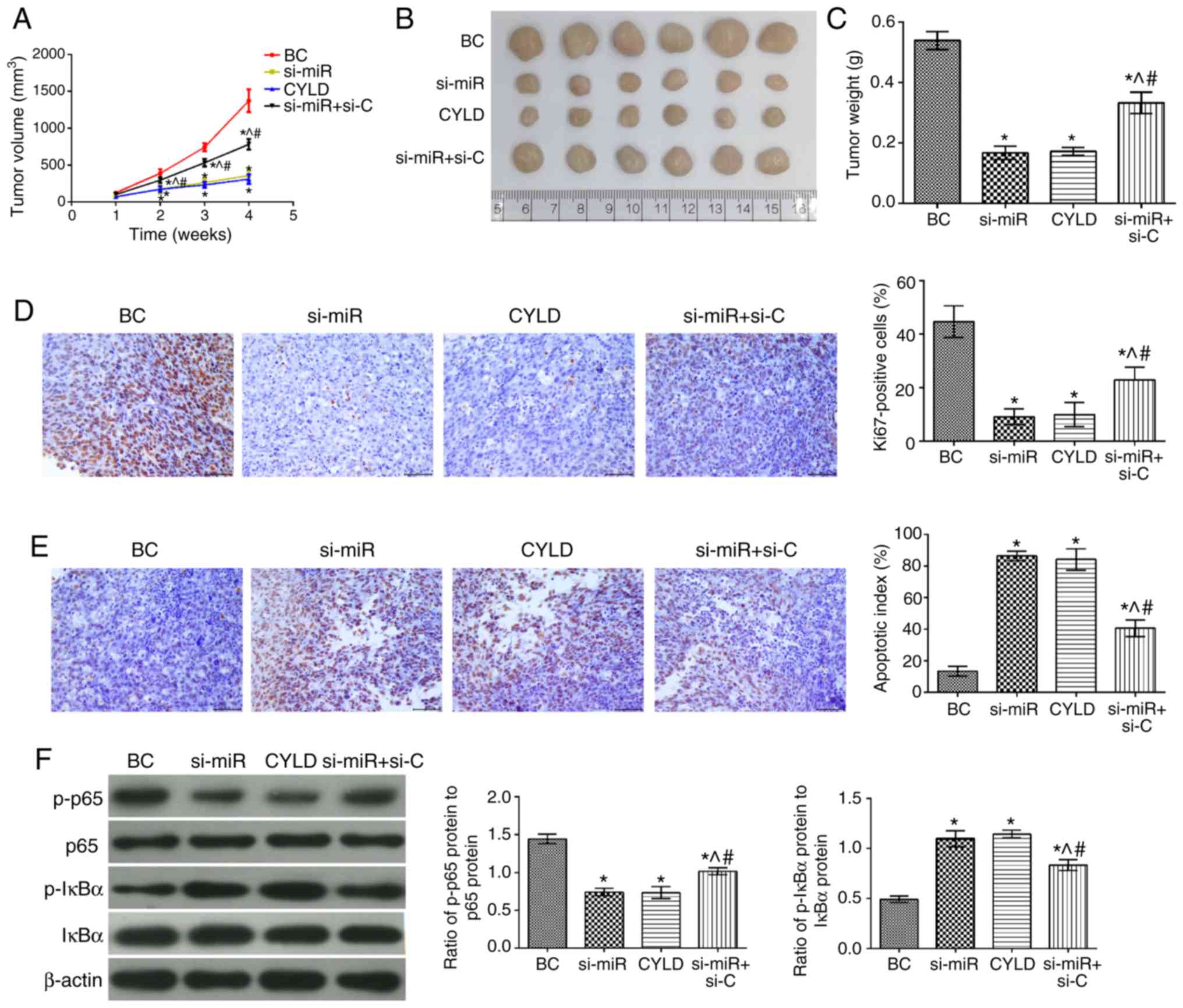
Effects of miR-181b on human colon cancer
xenografts
To determine the effects of miR-181b in vivo,
colon cancer xeno-grafts were established and evaluated. The tumor
growth rate, tumor volume and mass in all groups were significantly
attenuated compared with the BC group (Fig. 6). In addition, the number of
Ki67-positive cells was also decreased in the tumor tissues, while
the apoptotic index was significantly increased. Furthermore, the
levels of p-p65/p65 and p-IκBα/IκBα were significantly
downregulated and upregulated, respectively (P<0.05). The tumor
growth rate, volume and mass were notably higher in the si-miR +
si-C group compared with the si-miR and CYLD groups. Moreover, the
number of Ki67-positive cells and the expression levels of
p-p65/p65 were also increased. Notably, the cell apoptotic rate and
the expression levels of p-IκBα/IκBα were markedly attenuated in
the tumor tissues (P<0.05).
Discussion
The present study provided a mechanistic insight
underlying the miR-181b-mediated development of colon cancer.
Firstly, miR-181b was overexpressed and downregulated in the SW480
cells and the results confirmed that miR-181b regulated the cell
proliferative, invasive and migratory abilities. Furthermore, the
present study also demonstrated that miR-181b downregulation
promoted CYLD upregulation and attenuated the activity of the NF-κB
signaling pathway, thereby inhibiting cell growth and promoting
cell apoptosis both in vitro and in vivo.
The roles of miR-181b in different types of cancer
have been widely explored in several studies. It has been reported
that the expression levels of miR-181b are associated with the
response to chemorapy in non-small cell lung cancer (NSCLC), while
miR-181b downregulation is significantly associated with the
overall survival of patients with NSCLC (18). Xu et al demonstrated that
miR-181b regulated the proliferation of esophageal cancer stem-like
cells via the CYLD pathway and that CYLD was directly targeted by
miR-181b (19). In colon cancer,
a positive feedback loop between miR-181b and STAT3 was identified,
which regulated the Warburg effect and xenograft tumor growth
(20). It has been well
documented that the expression of miR-181b in glioblastoma, where
it acts as an onco-suppressor gene, is downregulated via the NF-κB
pathway (5). The NF-κB signaling
pathway plays a key role in colon cancer cell proliferation and
apoptosis (14). The present
study revealed that miR-181b was overexpressed in colon cancer
cells, while its silencing attenuated colon cancer cell
proliferation, invasion and migration abilities. Furthermore, the
expression levels of Bax, cleaved caspase-3 and p-IκBα were
increased following miR-181b downregulation. These findings
suggested that miR-181b played a negative role in the progression
of colon cancer and activated the NF-κB signaling pathway.
In unstimulated cells, NF-κB is conjugated to IκBα,
which keeps the first in an inactive state in the cytosol (21). Different physiological or
pathological stimuli may activate and promote the phosphorylation
of IκBα. CYLD is a negative regulatory factor, which inhibits
inflammation via the NF-κB pathway (15). Emerging evidence has indicated
that the effect of CYLD on targeting NF-κB factors is dependent on
the type of cells and activated receptors (22). Moreover, CYLD loss-of-function is
involved in NF-κB-associated inflammation, which in turn promotes
the development of different types of cancer (23,24). However, the association between
CYLD and NF-κB signaling in colon cancer remains unclear. In the
present study, CYLD overexpression inhibited cell growth and
promoted cell apoptosis in colon cancer cells, while the expression
levels of p-p65/p65 were decreased and those of p-IκBα/IκBα were
increased. These results indicated that CYLD may be involved in the
regulation of the NF-κB signaling pathway in colon cancer.
To verify whether CYLD was a target of miR181b in
colon cancer cells, a luciferase reporter assay was performed.
Therefore, SW480 cells were co-transfected with plasmids containing
wt-CYLD or mut-CYLD 3'UTR and miR-181b or NC mimic. As was
expected, miR-181b repressed CYLD expression by directly targeting
its 3'UTR. Taken together, the present study confirmed that
miR-181b downregulation not only inhibited SW480 cell
proliferation, but also promoted apoptosis, partly by targeting
CYLD.
In conclusion, the present study suggests that colon
cancer may be inhibited through miR-181b downregulation to promote
CYLD expression, which in turn attenuates NF-κB activity. At the
same time, the present study provides a research basis for the role
of miR-181b in colon cancer, indicating that miR-181b may be a
novel therapeutic target for patients with colon cancer.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
XY and YS carried out the experimental work and the
data collection and interpretation. XY and SH participated in the
design and coordination of the experimental work, and acquisition
of the data. YS and YZ participated in the study design, data
collection, analysis of the data and preparation of the manuscript.
XY and YS carried out the study design, the analysis and
interpretation of data and drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the National Institute of Health (NIH) guidelines and were
approved by the Animal Ethics Committee of Yantaishan Hospital
(approval no. 20190456-013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Orangio GR: The economics of colon cancer.
Surg Oncol Clin N Am. 27:327–347. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Labianca R, Beretta GD, Kildani B, Milesi
L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, et
al: Colon cancer. Crit Rev Oncol Hematol. 74:106–133. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang H, Wu J, Zhang J, Yang Z, Jin W, Li
Y, Jin L, Yin L, Liu H and Wang Z: Integrated bioinformatics
analysis of key genes involved in progress of colon cancer. Mol
Genet Genomic Med. 7:e005882019. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sadun RE, Sachsman SM, Chen X, Christenson
KW, Morris WZ, Hu P and Epstein AL: Immune signatures of murine and
human cancers reveal unique mechanisms of tumor escape and new
targets for cancer immunotherapy. Clin Cancer Res. 13:4016–4025.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Indrieri A, Carrella S, Carotenuto P,
Banfi S and Franco B: The pervasive role of the miR-181 family in
development, neurode-generation, and cancer. Int J Mol Sci.
21:20922020. View Article : Google Scholar
|
|
6
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao LD, Zheng WW, Wang GX, Kang XC, Qin
L, Ji JJ and Hao S: Epigenetic silencing of miR-181b contributes to
tumorigenicity in colorectal cancer by targeting RASSF1A. Int J
Oncol. 48:1977–1984. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia Y and Gao Y: MicroRNA-181b promotes
ovarian cancer cell growth and invasion by targeting LATS2. Biochem
Biophys Res Commun. 447:446–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Wang YL, Liu S, Zhang PP, Chen Z,
Liu M and Tang H: miR-181b promotes cell proliferation and reduces
apoptosis by repressing the expression of adenylyl cyclase 9 (AC9)
in cervical cancer cells. FEBS Lett. 588:124–130. 2014. View Article : Google Scholar
|
|
10
|
Rezaei T, Amini M, Hashemi ZS, Mansoori B,
Rezaei S, Karami H, Mosafer J, Mokhtarzadeh A and Baradaran B:
microRNA-181 serves as a dual-role regulator in the development of
human cancers. Free Radic Biol Med. 152:432–454. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin Z, Li D, Cheng W, Wu J, Wang K and Hu
Y: MicroRNA-181 functions as an antioncogene and mediates NF-κB
pathway by targeting RTKN2 in ovarian cancers. Reprod Sci.
26:1071–1081. 2019. View Article : Google Scholar
|
|
12
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
15
|
Sanches JGP, Xu Y, Yabasin IB, Li M, Lu Y,
Xiu X, Wang L, Mao L, Shen J, Wang B, et al: miR-501 is upregulated
in cervical cancer and promotes cell proliferation, migration and
invasion by targeting CYLD. Chem Biol Interact. 285:85–95. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Abdel-Mageed AB, Mondal D and Kandil
E: The nuclear factor kappa-B signaling pathway as a therapeutic
target against thyroid cancers. Thyroid. 23:209–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Wang X, Meng Q, Qiao W, Ma R, Ju W, Hu J,
Lu H, Cui J, Jin Z, Zhao Y and Wang Y: miR-181b/Notch2 overcome
chemoresis-tance by regulating cancer stem cell-like properties in
NSCLC. Stem Cell Res Ther. 9:3272018. View Article : Google Scholar
|
|
19
|
Xu DD, Zhou PJ, Wang Y, Zhang L, Fu WY,
Ruan BB, Xu HP, Hu CZ, Tian L, Qin JH, et al: Reciprocal activation
between STAT3 and miR-181b regulates the proliferation of
esophageal cancer stem-like cells via the CYLD pathway. Mol Cancer.
15:402016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan X, Feng J, Zhu Z, Yao L, Ma S, Hao B
and Zhang G: A positive feedback loop between miR-181b and STAT3
that affects Warburg effect in colon cancer via regulating PIAS3
expression. J Cell Mol Med. 22:5040–5049. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Huang W and Xu Y, Zhou L, Liang Y,
Gao C, Long Y and Xu Y: CYLD deubiquitinase negatively regulates
high glucose-induced NF-κB inflammatory signaling in mesangial
cells. Biomed Res Int. 2017:39829062017. View Article : Google Scholar
|
|
22
|
Xu RX, Liu RY, Wu CM, Zhao YS, Li Y, Yao
YQ and Xu YH: DNA damage-induced NF-κB activation in human
glioblastoma cells promotes miR-181b expression and cell
proliferation. Cell Physiol Biochem. 35:913–925. 2015. View Article : Google Scholar
|
|
23
|
Zehavi L, Schayek H, Jacob-Hirsch J, Sidi
Y, Leibowitz-Amit R and Avni D: MiR-377 targets E2F3 and alters the
NF-kB signaling pathway through MAP3K7 in malignant melanoma. Mol
Cancer. 14:682015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk
ML and Struhl K: STAT3 activation of miR-21 and miR-181b-1 via PTEN
and CYLD are part of the epigenetic switch linking inflammation to
cancer. Mol Cell. 39:493–506. 2010. View Article : Google Scholar : PubMed/NCBI
|