Introduction
Based on the 2015 report from the World Health
Organization, cardiovascular disorders, as a serious health
concern, has become a common cause of mortality worldwide (1). In China, consistent with the global
trend, cardiovascular disorder cases are increasing annually
(2). Atherosclerosis, as a common
risk factor for cardiovascular disease, is considered to be a type
of chronic lipid-triggered vessel-wall inflammation (3). Atherosclerosis has also been
revealed to be an important cause of mortality and morbidity in
industrialized countries (4).
The onset of atherosclerosis is mainly induced by
dysfunction and leukocytes of endothelial cell (EC) dysfunction and
leukocyte infiltration (5).
Therefore, injury to ECs is commonly considered an initial event in
the development and progression of atherosclerosis (4). The deposition or accumulation of
modified-lipoproteins within the artery wall can enhance
endothelial permeability and induce the apoptosis of ECs indirectly
(6). Moreover, previous studies
(7,8) have reported that oxidized
low-density lipoprotein (ox-LDL)-triggered oxidative injury is a
critical risk factor for the damage or injury to ECs, resulting in
the formation of atherosclerotic plaques. Clinically, the
application of statins has exhibited greater efficacy in numerous
patients with cardiovascular diseases, but has also led to certain
side-effects (9).
Apoptosis, as a form of programmed cell death, is
mainly characterized by the energy-dependent biochemical functions
and the distinct morphological characteristics (10). Apoptosis has been proven as a
critical component for various processes, such as the development
and functions of the immune system, normal cell turnover, embryonic
development and cell death (10).
In addition, apoptosis has been implicated in a series of
pathological and/or physiological progressions, such as
inflammation, oxidative stress and immune responses in several
disorders (11). Previous studies
have demonstrated that the induction of apoptosis is a promising
therapeutic target for treating atherosclerosis (3,12).
Another study (13) also revealed
that endoplasmic reticulum (ER) stress may lead to apoptosis and
may be involved in ox-LDL-induced damage to ECs. Therefore, the
inhibition of ER stress-associated apoptosis may be a potential
strategy with which to protect ECs against damage caused by ox-LDL
stimulation.
Insulin receptor substrate 1 (IRS-1), as an
insulin-receptor tyrosine kinase ligand, plays important roles in
the progression of coronary artery disorder and type-2 diabetes
mellitus (14). A previous study
(15) demonstrated that the
IRS-1-associated signaling pathway in the endothelium can prevent
the dysfunction of ECs and may participate in the pathological
processes of diabetes and cardiovascular diseases. Moreover, it has
been demonstrated that IRS-1 plays a crucial role in
atherosclerosis (15-17); however, the potential mechanisms
have yet to be fully elucidated. Forkhead box O1 (FoxO1) is
commonly overexpressed in atherosclerotic plaques and participates
in a series of atherogenic signaling pathways in ECs (18). Therefore, FoxO1 may be a potential
therapeutic target for the treatment of atherosclerosis (19).
Based on the aforementioned findings, it was
hypothesized that IRS-1 may prevent ox-LDL-induced injury to ECs by
modulating apoptosis and regulating molecules in the FoxO1
signaling pathway. Therefore, the aims of the present study were to
investigate the effects of IRS-1 expression on ox-LDL-induced
damage to ECs and EC apoptosis, as well as to identify the
potential underlying mechanisms.
Materials and methods
Animals and primary culture of ECs
Sprague-Dawley rats (weighing 200-220 g) were
purchased from the Laboratory Animal Center of Third Military
Medical University, and were used for isolating thoracic aortas and
culturing the ECs. The rats were anaesthetized via an
intraperitoneal injection of 50 mg/kg body weight pentobarbital,
and the thoracic aortas were then isolated. The rats were finally
euthanized by an intraperitoneal injection of 120 mg/kg body weight
pentobarbital. The chest of the rats was opened to expose the
thoracic aorta, which was rapidly removed and placed into D-Hanks
medium (Gibco; Thermo Fisher Scientific, Inc.; Fig. 1A). After washing 3 times with
D-Hanks solution, the thoracic aorta tissues were cut into small
sections (~1 mm3). The tissue sections were placed into
12-well plates containing 10% FBS (ZSGB Bio, Inc.) and cultured for
2 h at 37°C with 5% CO2. The tissue sections were then
cultured in DMEM containing 20% FBS (Gibco; Thermo Fisher
Scientific, Inc.) for 48 h to separate the cells from the tissues.
The cells were digested using 0.25% trypsin (Beyotime Institute of
Biotechnology) supplemented with 0.02% EDTA, cultured for 24 h and
passaged for 2-3 generations for use in the following experiments
(Fig. 1B). For the remaining rats
following all of the experiments, the rats were subjected to
euthanasia by an intraperitoneal injection of 120 mg/kg body weight
pentobarbital.
All animal experiments procedures were approved by
the IACUC of Daping Hospital, Army Medical University, and complied
with the guidelines for the Care and Use of Laboratory Animals.
Identification of ECs
For the isolated and cultured cells,
immunohistochemical staining was conducted to identify ECs,
according to a previously described method (20). The aforementioned cultured cells
were fixed with 4% paraformaldehyde (Sangon Biotech. Co., Ltd.) for
10 min, rinsed in PBS (ZSGB Bio, Inc.) and then fixed using
methanol for 5 min. The cells were treated with 0.1% Triton-X 100
for 5 min and incubated with rabbit anti-rat factor VIII polyclonal
antibody (1:2,000; cat. no. ab236284; Abcam) overnight at 4°C. The
cells were then washed with PBS and treated with horseradish
peroxidase (HRP)-labeled goat anti-rabbit IgG (1:1,000; cat. no.
ab6721; Abcam) in the dark for 1 h at room temperature. The stained
images were viewed and captured under a classic light microscope
(model, DMI3000; Leica Microsystems GmbH).
Synthesis and transfection of IRS-1
overexpression plasmid and IRS-1 small interfering RNA (siRNA)
The complementary DNA (cDNA) sequence of IRS-1 was
synthesized depending on mRNAs extracted from the ECs by Western
Biotech. The obtained IRS-1 sequence was then sub-cloned into the
pcDNA3.1 vector (cat. no. V790-20, Invitrogen; Thermo Fisher
Scientific, Inc.), to generate the pcDNA3.1-IRS-1 plasmid (IRS-1
group), which could overexpress IRS-1. siRNA targeting IRS-1
(accession no. NM_012969) was also synthesized by Western Biotech.
The siRNA sequences were as follows: Sense,
5′-UCCACUUGCAUCCAGAACUCGGUUUUGGCCACUGACUGACCGAGUUCUAUGCAAGUGGA-3′
and antisense,
5′-AGGUGAACGUAGGUCUUGAGCCAAAACCGGUGACUGACUGGCUCAAUACGUUCACCU-3′.
Moreover, a negative control siRNA was synthesized with the
following sequences: Sense,
5′-CCTGTCAGTTCCAGACTTCGGAACCCCAGTCAGTGGCCAA-3′ and antisense,
5′-GGGTTCCGAAGTCTGGAACTGACA-3′. The synthesized pcDNA3.1-IRS-1
plasmid and siIRS-1 or control siRNA were transfected into ECs
using Lipofectamine® 2000 regent (cat. no. 11668-027;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Briefly, the ECs were adjusted to density
of 1x105 cells/well in a 24-well plate, and the plasmids
were transfected when the cells achieved a cell confluence of
70-80%. The ECs were administrated with plasmids at dose of 1.2
µg/well and cultured for 4 h at 37°C. The medium was then
replaced with fresh medium and the ECs were cultured at 37°C for a
further 24 h. Finally, the ECs were collected for use in the
following experiments.
Moreover, in preliminary experiments, the effects of
ox-LDL treatment on the transfection process were evaluated, the
findings of which revealed that there were no marked effects of
ox-LDL treatment on transfection (data not shown). The transfection
efficiency of the plasmids was also determined by reverse
transcription-quantitative PCR (RT-qPCR), which illustrated a high
efficacy (data not shown).
Establishment of EC models of
ox-LDL-induced atherosclerosis and trial grouping
The establishment of EC models of ox-LDL-induced
atherosclerosis was conducted according to a previously described
protocol (21). The ECs were
cultured in DMEM containing ox-LDL (final concentration, 80
µg/ml), supplemented with 10% FBS and 1%
streptomycin-penicillin (Beyotime Institute of Biotechnology) for
24 h at 37°C with 5% CO2. The cells in the EC model
ox-LDL-induced atherosclerosis were divided into following groups:
i) The negative control+ox-LDL group (NC+ox-LDL group); ii) The
group of ECs exposed to ox-LDL and transfected with the blank
pcDNA3.1 plasmid (Vector+ox-LDL group); iii) The group of ECs
exposed to ox-LDL and transfected with the pcDNA3.1-IRS-1 plasmid
(IRS-1+ox-LDL group); and iv) The group of ECs exposed to ox-LDL
and transfected with siIRS-1 (IRS-1-siRNA+ox-LDL group). In order
to compare these groups, ECs not exposed to ox-LDL were also
divided into the negative control group (NC group, transfected with
the negative control siRNA sequence as described above), the Vector
group, the IRS-1 group and the IRS-1-siRNA group.
MTT assay
The proliferative activity of the ECs was evaluated
using MTT assay. Pre-treated ECs were cultured on 96-well plates
for 24 h and then incubated with MTT (Sigma-Aldrich; Merck KGaA) at
a final concentration of 5 mg/ml for 4 h. The supernatants of the
cells were then discarded and the formed formazan crystals were
dissolved with DMSO solution (150 µl; Amresco, Inc.). An
ELISA reader at 490 nm was used to measure the absorbance of the
96-well culture plates. The proliferative activity (the activity of
cell proliferation) of the ECs was calculated as an optical value
ratio of living cells vs. normal wells.
TUNEL analysis
The apoptosis of the ox-LDL-exposed and/or
IRS-1/IRS-1-siRNA-transfected ECs was evaluated by TUNEL assay.
TUNEL assay was conducted using a Roch In Situ Cell Death
kit (cat. no. 11684795899; Roche Diagnostics, Inc.), according to
the manufacturer's protocol. TUNEL-positive cells were assigned as
the levels of TUNEL-positive staining cells vs. total cells from
≥10 random visual fields.
Measurement of intracellular reactive
oxygen species (ROS) levels
The production of intracellular ROS was measured
using a flow cytometry assay with an oxidation-specific probe of
2,7-dichlorofuorescin diacetate (DCFH-DA), using a commercial ROS
Assay Staining kit (BD Biosciences) according to the manufacturer's
instructions. Cells were washed 3 times using PBS and stained with
DCFH-DA (10 µM) at 37°C for 60 min. The produced
neutrophil-fluorescence from 10,000 cells was examined using a
FACSCanto II flow cytometer (BD Biosciences). The fluorescence was
measured and analyzed with a professional Cell-Quest Software
(version 3.3, BD Biosciences). The oxidative viability was assigned
as P1 values.
RT-qPCR
RT-qPCR assay was conducted to measure the
expression levels of proliferator-activated receptor γ co-activator
1 α (Ppargcla), phosphoenolpyruvate carboxykinase 1
(Pck1) and glucose-6-phosphatase catalytic subunit
(G6pc) in ox-LDL-exposed and/or IRS-1/IRS-1-siRNA-treated
ECs. Total RNAs of ECs were extracted using TRIzol®
reagent (Beyotime Institute of Biotechnology) and cDNA was
synthesized using a Reverse-Transcription kit (Western Biotech)
following the manufacturer's instructions. qPCR was conducted using
a Eppendorf PCR device (Eppendorf) and a SYBR-Green I PCR kit
(Western Biotech). The following thermocycling conditions were
used: 35 cycles at 94°C for 20 sec, 60°C for 30 sec and 72°C for 30
sec. The specific primers for amplifying the aforementioned genes
are presented in Table I. The
2-ΔΔCq method was utilized to analyze the relative value
of the RT-qPCR data (22).
 | Table IPrimer sequences used for the reverse
transcription-quantitative PCR assay. |
Table I
Primer sequences used for the reverse
transcription-quantitative PCR assay.
| Genes | Sequence
(5′-3′) | Length (bp) |
|---|
|
Ppargc1a | Forward
CAAGTATCTGACCACAAACGATG | 110 |
| Reverse
ACTGCGGTTGTGTATGGGAC |
| Pck1 | Forward
CAGACTCGCCCTATGTGGTG | 157 |
| Reverse
TTGCAGGCCCAGTTGTTG |
| G6pC | Forward
GACTGTGGGCATCAATCTCCTC | 160 |
| Reverse
GGTGACGGGGAACTGTTTTATC |
|
β-actin | Forward
CCCATCTATGAGGGTTACGC | 150 |
| Reverse
TTTAATGTCACGCACGATTTC |
Western blot analysis
Proteins from ox-LDL-exposed and/or
IRS-1/IRS-1-siRNA transfected ECs were extracted using
radioimmunoprecipitation assay (RIPA, Beyotime Institute of
Biotechnology) and the protein concentrations of the extracted
proteins were measured using a BCA Detection kit (Amersham;
Cytiva). A total of 2 µg proteins (for each lane) in cell
lysates were loaded and separated by 12% SDS-PAGE (Sigma-Aldrich;
Merck KGaA) and then electro-transferred onto PVDF membranes
(Amersham; Cytiva). The membranes were then blocked with the 5%
non-fat dried milk in TBST solution at 37°C for 1 h. Subsequently,
the PVDF membranes were incubated with rabbit-derived antibodies,
including anti-IRS-1 (1:2,000; cat. no. ab52167), anti-FoxO1
(1:2,000; cat. no. ab52857), anti-phosphorylated (p-)FoxO1
(1:1,000; phospho S256) (anti-p-FoxO1; 1:2,000; cat. no. ab131339),
anti-78-kDa glucose-regulated protein (GRP78; 1:1,000; cat. no.
ab108615), anti-p-eukaryotic translation initiation factor 2A
(eIF2α; phospho S51) (1:2,000; cat. no. ab32157), anti-CHOP
(1:2,000; cat. no. ab179823), anti-Akt (1:1,000; cat. no. ab10693),
anti-p-Akt (phospho S473) (1:1,000; cat. no. ab81283) and
anti-GAPDH (1:2,000; cat. no. ab9485) at 4°C overnight. The
aforementioned primary antibodies were obtained from Abcam. After
washing 3 times with phosphate-buffered solution Tween-20 (PBST,
Beyotime Institute of Biotechnology), the PVDF membranes were
incubated with HRP-conjugated goat anti-rabbit IgG (1:1,000; cat.
no. AQ132P; Sigma-Aldrich; Merck KGaA) at 37°C for 2 h. The western
blot bands were visualized with an Pierce ECL kit (cat. no.
NCI4106; Thermo Fisher Scientific, Inc.), as described by the
protocol of the manufacturer. For the quantification of visualized
western blot images, protein expression was represented as the
relative value of targeting protein bands normalized to the GAPDH
control. For the quantification of the western blot images,
Labworks Analysis Software (version 4.5, UVP, Inc.) was used.
Immunofluorescence staining for p-FoxO1
nuclear localization
The ECs were cultured in poly-L-lysine pre-treated
cover-slips containing FBS-free DMEM for starvation. The cells were
then fixed using 4% paraformaldehyde (Sangon Biotech. Co., Ltd.)
and stained using rabbit anti-p-FoxO1 antibody (1:1,000; cat. no.
ab131339; Abcam) at 4°C overnight, followed by incubation with goat
anti-rabbit IgG-Alexa Fluor 647 antibody (1:1,000; cat. no.
ab150083; Abcam) at 37°C for 2 h. The stained coverslips were
observed and analyzed with a TCS SP5 confocal-laser scanning
microscope (Leica Microsystems GmbH). The localization of p-FoxO1
in the nucleus was evaluated using ImageJ Software 2.0 (National
Institutes of Health) by analyzing the fluorescence intensity.
Statistical analysis
Data are presented as the means ± standard deviation
(SD) and were analyzed using SPSS statistical software 20.0 (IBM
Corp.). The differences among variables were analyzed using one-way
ANOVA followed by Tukey's post hoc test. All data were obtained
from at least 6 experiments or repeats. P<0.05 was considered to
indicate a statistically significant difference.
Results
IRS-1 enhances the proliferative ability
of ECs in a model of ox-LDL-induced atherosclerosis
Following the successful isolation of thoracic aorta
tissue (Fig. 1A) and the culture
of isolated cells (Fig. 1B),
factor VIII expression was examined to identify the ECs. The
results of immunohistochemistry staining indicated that the
primarily cultured cells positively expressed factor VIII
(expressed both in the cytoplasm and cell nuclei) (Fig. 1C), which suggested that the cells
were ECs. The results of MTT assay demonstrated that there were no
significant differences in the proliferative activities of the ECs
(without ox-LDL exposure) among the NC, Vector and IRS-1 groups at
24 h (Fig. 2A), 48 h (Fig. 2B) and 72 h (Fig. 2C; all P>0.05). However, the
proliferative activities were markedly lower in the IRS-1-siRNA
group compared to other groups without ox-LDL exposure at 24 h
(Fig. 2A), 48 h (Fig. 2B) and 72 h (P<0.05). Moreover,
exposure to ox-LDL significantly decreased the proliferative
activities of the ECs compared with the ECs not exposed to ox-LDL
(Fig. 2; all P<0.05). In the
ECs exposed to ox-LDL, the proliferative activity in the
IRS-1+ox-LDL group was significantly enhanced; however, it was
significantly reduced in the IRS-1-siRNA+ox-LDL group, compared
with the Vector+ ox-LDL group, at 24 h (Fig. 2A), 48 h (Fig. 2B) and 72 h (Fig. 2C; all P>0.05).
IRS-1 inhibits the apoptosis of ECs in
the model of ox-LDL-induced atherosclerosis
To determine the apoptosis of ECs, a TUNEL assay was
performed (Fig. 3A), and the
results suggested that apoptotic rates of the ox-LDL-exposed ECs
were significantly higher compared with those of the ECs without
ox-LDL exposure (Fig. 3B; all
P<0.05). In addition, IRS-1 overexpression significantly
inhibited the cell apoptotic rates, while transfection with
IRS-1-siRNA significantly enhanced apoptosis compared with the
Vector group, both in the ox-LDL-exposed or in the ECs not exposed
to ox-LDL (Fig. 3B; both
P<0.05).
IRS-1 upregulates the phosphorylation of
FoxO1 in ECs in a model of ox-LDL-induced atherosclerosis
Following transfection of the ECs with the IRS-1
plasmid or IRS-1-siRNA, western blot analysis was conducted to
determine IRS-1 expression (Fig.
4A). It was found that IRS-1 expression was significantly
higher in the IRS-1 group (P<0.05) and significantly lower in
the IRS-1-siRNA group (P<0.05) compared with the Vector group
(Fig. 4A). Furthermore, the
p-FoxO1/FoxO1 ratio reflects the phosphorylation of FoxO1 protein;
therefore, the p-FoxO1/FoxO1 ratio was determined. The
p-FoxO1/FoxO1 ratio in the ECs was significantly increased in the
IRS-1 group (P<0.05) and significantly decreased in the
IRS-1-siRNA group (P<0.05) compared with the Vector group
(Fig. 4B). It was demonstrated
that exposure to ox-LDL significantly decreased the p-FoxO1/FoxO1
ratio compared with the ECs not exposed to ox-LDL (Fig. 4B; all P<0.05). In addition, the
p-FoxO1/FoxO1 ratio was upregulated in the IRS-1+ox-LDL group
(P<0.05) and downregulated in the IRS-1-siRNA+ox-LDL group
(P<0.05) compared with the Vector+ox-LDL group (Fig. 4B).
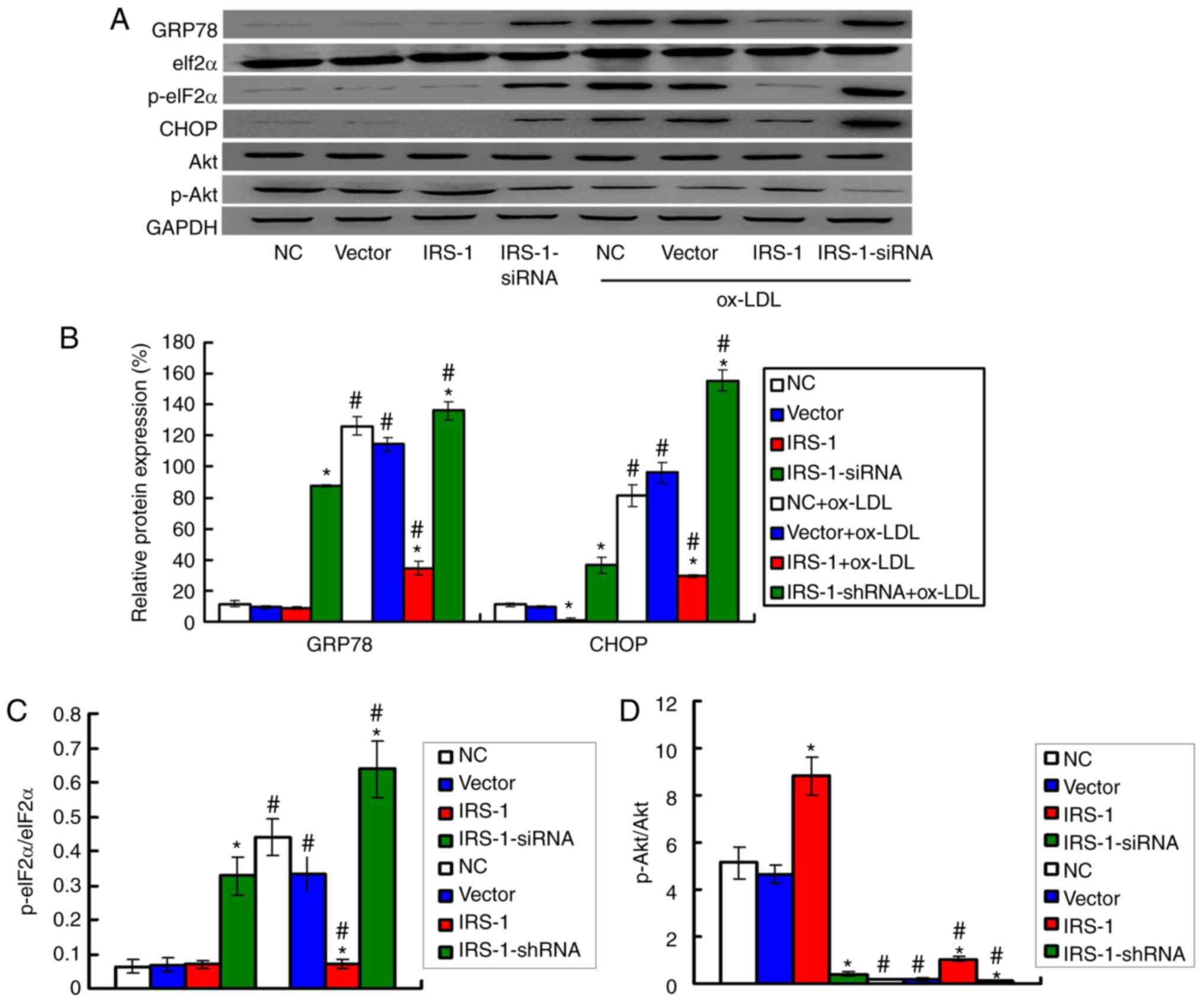
IRS-1 downregulates the expression ER
stress biomarkers in ECs in a model of ox-LDL-induced
atherosclerosis
The levels of the ER stress biomarkers, including
GRP78, CHOP and p-eIF2α, were examined by western blot analysis
(Fig. 5A). The results
demonstrated that exposure to ox-LDL significantly upregulated the
expression levels of GRP78 and CHOP (Fig. 5B) and increased the p-eIF2α/eIF2α
ratio (Fig. 5C) compared with the
ECs not exposed to ox-LDL (all P<0.05). Moreover, the expression
levels of GRP78 and CHOP (Fig.
5B) and the p-eIF2α/eIF2α ratio (Fig. 5C) were significantly lower in the
IRS-1+ox-LDL group and higher in the IRS-1-siRNA+ox-LDL group
compared with the Vector+ox-LDL group (all P<0.05).
IRS-1 promotes the phosphorylation of Akt
in ECs in a model of ox-LDL-induced atherosclerosis
Based on the effect of IRS-1 on ROS production, the
levels of the oxidative stress molecules, Akt and p-Akt, were
measured by western blot analysis (Fig. 5A). The results demonstrated that
exposure to ox-LDL significantly reduced the p-Akt/Akt ratio
compared with the ECs not exposed to ox-LDL (Fig. 5D; all P<0.05). However, IRS-1
overexpression significantly reversed the suppressive effects of
ox-LDL on the p-Akt/Akt ratio (Fig.
5D; P<0.05), which suggested that IRS-1 promoted the
p-Akt/Akt ratio. It was found that transfection with IRS-1-siRNA
significantly reduced the p-Akt/Akt ratio in ox-LDL-exposed ECs,
compared with the Vector+ox-LDL group (Fig. 5D; P<0.05).
IRS-1 reduces intracellular ROS levels in
ECs in a model of ox-LDL-induced atherosclerosis
A flow cytometric assay (Fig. 6A) was conducted to determine ROS
production in the EC model of ox-LDL-induced atherosclerosis. For
the ECs not exposed to ox-LDL, intracellular ROS production (P1
value) was significantly lower in the IRS-1 group and significantly
higher in the IRS-1-siRNA group compared with the Vector group
(Fig. 6B; both P<0.05). The
results also indicated that exposure to ox-LDL significantly
increased ROS production (P1 value) compared with the ECs not
exposed to ox-LDL (Fig. 6B; all
P<0.05). In addition, the ox-LDL-induced upregulation of ROS
production (P1 value) was significantly suppressed following
transfection with IRS-1 plasmid (IRS-1+ox-LDL group) and was
further significantly enhanced following transfection with
IRS-1-siRNA (IRS-1-siRNA+ox-LDL group) compared with the
Vector+ox-LDL group (Fig. 6B;
both P<0.05).
IRS-1 suppresses the transcription of
atherosclerosis-associated genes in ECs in a model of
ox-LDL-induced atherosclerosis
The results of RT-qPCR (Fig. 7) revealed that IRS-1
overexpression significantly suppressed the transcription of
atherosclerosis-associated genes, including Ppargcla,
Pck1 and G6pC, compared with the Vector group, in
both the ox-LDL-exposed or unexposed ECs (Fig. 7; all P<0.05). Furthermore,
transfection with IRS-1-siRNA significantly increased the
transcription levels of the Ppargcla, Pck1 and
G6pC genes, compared with both the Vector group and
Vector+ox-LDL group (Fig. 7; all
P<0.05).
IRS-1 increases the cytoplasmic
localization of p-FoxO1 in ECs in a model of ox-LDL-induced
atherosclerosis
In order to assess the cytoplasmic localization of
p-FoxO1 in ECs, immunofluorescence staining was conducted (Fig. 8A). The results indicated that
exposure to ox-LDL significantly inhibited the cytoplasmic
localization of p-FoxO1 compared with the ECs not exposed to ox-LDL
(Fig. 8B; all P<0.05). In
addition, transfection with IRS-1 plasmid (IRS-1+ox-LDL group)
significantly increased the cytoplasmic localization of p-FoxO1
compared with tje Vector+ox-LDL group (Fig. 8B; P<0.05). It was demonstrated
that transfection with IRS-1-siRNA (IRS-1-siRNA+ox-LDL group)
significantly decreased the cytoplasmic localization of p-FoxO1
compared with the Vector+ox-LDL group (Fig. 8B; P<0.05).
Discussion
ox-LDL-induced injury to ECs can mimic the oxidative
damage subjected to ECs, which is considered the initial process of
atherosclerosis (23). IRS-1, as
an insulin-receptor tyrosine kinase, modulates atherosclerosis and
other cardiovascular diseases (14-17). To the best of our knowledge, the
present study was the first to investigate the protective effects
of IRS-1 in an ox-LDL-induced atherosclerosis EC model. The results
demonstrated that IRS-1 protected the ECs against injury by
inhibiting oxidative stress and ER stress-associated apoptosis.
Based on a previous study (24), ECs were pre-transfected with IRS-1
overexpression plasmid and IRS-1-siRNA, and subsequently stimulated
using ox-LDL at a concentration of 80 µg/ml. Initially, the
protective effects of IRS-1 on ECs were determined by both MTT and
TUNEL assays. The results indicated that IRS-1 attenuated the
ox-LDL-induced death and apoptosis of ECs, which suggested that
IRS-1 plays a cytoprotective role in ox-LDL-induced injury to ECs.
According to a previous study, the dysfunction of ECs can be
triggered by ROS-associated oxidative damage (25). Therefore, in the present study,
intracellular ROS production was examined by flow cytometry, and
the findings indicated that IRS-1 overexpression significantly
reduced intracellular ROS production in the EC model of
ox-LDL-induced atherosclerosis. Thus, it was hypothesized that the
antioxidant effets of IRS-1 may serve a critical function in
protecting ECs against ox-LDL-induced apoptosis.
In recent years, the function of ER stress-mediated
apoptosis in atherosclerosis pathological processes of
atherosclerosis has been receiving increasing attention (26,27). ER stress-induced apoptosis mainly
mediates a protective mechanism in response to intracellular
stimuli (28). The findings of
the present study indicated that exposure to ox-LDL significantly
increased the expression levels of the ER stress biomarkers, GRP78,
CHOP and the p-eIF2α/eIF2α ratio, in ECs. However, ISR-1
overexpression significantly decreased the expression levels of
GRP78 and CHOP, and the ratio of p-eIF2α/eIF2α in the ECs exposed
to ox-LDL. These results suggested that the mechanism responsible
for the anti-apoptotic effects of ISR-1 may involve the suppression
of ER stress in ox-LDL-exposed ECs.
A recent study (29) reported that FoxO1 was closely
associated with ER stress-induced apoptosis. Moreover, oxidative
stress can induce the deacetylation of FoxO1 in ECs, which acts as
a risk factor for atherosclerosis (18,30). Therefore, the present study
examined FoxO1 expression in ox-LDL-exposed ECs, by determining
both the FoxO1 and p-FoxO1 expression levels and analyzing
p-FoxO1/FoxO1 ratios (reflects the phosphorylation of FoxO1
protein). It was found that IRS-1 promoted the phosphorylation of
Akt (enhanced the p-Fox/FoxO1 ratio) in an EC model of
ox-LDL-induced atherosclerosis. Furthermore, IRS-1 increased the
cytoplasmic localization of p-FoxO1 in an EC model of
ox-LDL-induced atherosclerosis. Therefore, it was indicated that
IRS-1 exerts its protective effects by activating FoxO1 and
inhibiting ER stress-mediated apoptosis by targeting the
cytoplasmic localization of p-FoxO1. The results of the present
study are consistent with those of a previous study (19), which revealed that
autophagy-mediated apoptosis targeted nuclear FoxO1. Thus, based on
the aforementioned results, FoxO1 maybe an important target that
mediates IRS-1-induced protective effects against the apoptosis of
ox-LDL-exposed ECs.
Based on a previous study (31), which demonstrated that the FoxO1
was suppressed by the phosphorylation of Akt and that oxidative
stress was associated with Akt and p-Akt, the present study
examined the Akt and p-Akt expression levels. The results
demonstrated that IRS-1 overexpression significantly enhanced the
phosphorylation of Akt (increased the ratio of p-Akt/Akt), which
suggested that the IRS-1/Akt/FoxO1 signaling pathway may play a
role in the protective effects of IRS-1 in ECs. Moreover, the
atherosclerosis-associated genes, including Ppargcla,
Pck1 and G6pC were also measured in ox-LDL-exposed
ECs. The results indicated that IRS-1 over-expression significantly
suppressed the transcription of the aforementioned
atherosclerosis-associated genes, which also demonstrated that
IRS-1 overexpression protects ECs against injury in atherosclerosis
models.
However, the present study also has a few
limitations. Firstly, the specific localization or distribution of
ECs biomarker factor VIII has not been clearly demonstrated at the
cytoplasm or cell nuclei. Secondarily, fluorescence staining is
also a method for identifying biomarkers for ECs, which has not
been performed in the present study. Thirdly, there was also a
limitation as regards the statistical analysis. Based on the design
of the study, a mixed design ANOVA may have been more appropriate
for comparing the differences among different groups, as well as
between ox-LDL and no ox-LDL exposure.
In conclusion, to the best of our knowledge the
present study was the first to demonstrate the protective effects
of IRS-1 on ox-LDL-induced EC injury and identify its associated
mechanisms. It was found that IRS-1 exerted protective effects
against the ox-LDL-induced injury to ECs by inhibiting ER
stress-mediated apoptosis and activating the IRS-1/Akt/FoxO1
signaling pathway. Therefore, these findings indicated a novel
function of IRS-1 in improving the apoptotic process and enhancing
the proliferative activity of ECs, which may prove to be a
promising therapeutic approach against atherosclerosis.
Funding
The present study was supported by the fund of
Chongqing Science and Technology Commission (grant no.
cstc2016j-cyjAX0021).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
JL and ZX conceived and designed the experiments.
JL, XY, YT and YW performed the experiments and analyzed the data.
ZX contributed as regards the reagents/materials/analysis tools. JL
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments procedures were approved by
the IACUC of Daping Hospital, Army Medical University, and complied
with the guidelines for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Kishore SP, Blank E, Heller DJ, Patel A,
Peters A, Price M, Vidula M, Fuster V, Onuma O, Huffman MD and
Vedanthan R: Modernizing the world health organization list of
essential medicines for preventing and controlling cardiovascular
diseases. J Am Coll Cardiol. 71:564–574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Y, Benjamin EJ and MacMahon S:
Prevention and control of cardiovascular disease in the rapidly
changing economy of China. Circulation. 133:2545–2560. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen L, Yang W, Guo Y, Chen W, Zheng P,
Zeng J and Tong W: Exosomal lncRNA GAS5 regulates the apoptosis of
macrophages and vascular endothelial cells in atherosclerosis. PLoS
One. 12:e01854062017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bano A, Chaker L, Mattace-Raso FU, van der
Lugt A, Ikram MA, Franco OH, Peeters RP and Kavousi M: Thyroid
function and the risk of atherosclerotic cardiovascular morbidtiy
and mortality: The rotterdam study. Circ Res. 121:1392–1400. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Novák J, Bienertová-Vašků J, Kára T and
Novák M: MicroRNA involved in the lipid metabolism and their
possible implications for atherosclerosis development and
treatment. Mediators Inflamm. 2014:2758672014. View Article : Google Scholar
|
|
7
|
Takahashi Y, Zhu H and Yoshimoto T:
Essential roles of lipoxygenases in LDL oxidation and development
of atherosclerosis. Antioxid Redox Signal. 7:425–431. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zi Y, Yi-An Y, Bing J, Yan L, Jing T,
Chun-Yu G, Fan P, Hao L, Jia-Ni T, Han-Jin H, et al: Sirt6-induced
autophagy restricted TREM-1 mediated pyroptosis with ox-LDL treated
endothelial cells: Relevance to prognostication of patients with
acute myocardial infarction. Cell Death Discov. 5:882019.
View Article : Google Scholar
|
|
9
|
Mitchell JD, Fergestrom N, Cage BF,
Paisley R, Moon P, Novak E, Cheezum M, Shaw LJ and Villines TC:
Impact of Statins on cardiovascular outcomes following coronary
artery calcium scoring. J Am Coll Cardiol. 72:3233–3242. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis of disease. Aging
(Albany NY). 4:330–349. 2012.
|
|
12
|
Wei DH, Jia XY, Liu YH, Guo FX, Tang ZH,
Li XH, Wang Z, Liu LS, Wang GX, Jian ZS and Ruan CG: Cathepsin L
stimulates autophagy and inhibits apoptosis of ox-LDL induced
endothelial cells: Potential role in atherosclerosis. Int J Mol
Med. 31:400–406. 2013.
|
|
13
|
Hong D, Bai YP, Gao HC, Wang X, Li LF,
Zhang GG and Hu CP: Ox-LDL induces endothelial cell apoptosis via
the LOX-1-dependent endoplasmic reticulum stress pathway.
Atherosclerosis. 235:310–317. 2014.PubMed/NCBI
|
|
14
|
Zhang D, Zhang X, Liu D, Liu T, Cai W, Yan
C and Han Y: Association between insulin receptor substrate 1
polymorhpisms and high platelet reactivity with clopidogrel therapy
in coronary artery disease patients with type 2 diabetes mellitus.
Cardiovasc Diabetol. 15:502016.
|
|
15
|
Park K, Mima A, Li Q, Rask-Madsen C, He P,
Mizutani K, Katagiri S, Maeda Y, Wu IH, Khamaisi M, et al: Insulin
decreases atherosclerosis by inducing endothelin receptor B
expression. JCI Insight. 1:e865742016.PubMed/NCBI
|
|
16
|
Galkina EV, Butcher M, Keller SR, Goff M,
Bruce A, Pei H, Sarembock IJ, Sanders JM, Nagelin MH, Srinivasan S,
et al: Accelerated atherosclerosis in apoe-/- mice heterozygous for
the insulin receptor and the insulin receptor substrate-1.
Arterioscler Thromb Vasc Biol. 32:247–256. 2012.
|
|
17
|
Xi G, Wai C, White MF and Clemmons DR:
Down-Regulation of insulin receptor substrate 1 during
hyperglycemia induces vascular smooth muscle cell
dedifferentiation. J Biol Chem. 292:2009–2020. 2017.
|
|
18
|
Kedenko L, Lamina C, Kedenko I, Kollerits
B, Kiesslich R, Iglseder B, Kronenberg F and Paulweber B: Genetic
polymorphisms at SIRT1 and FoxO1 are associated with carotid
atherosclerosis in the SAPHIR cohort. BMC Med Genet.
15:1122014.PubMed/NCBI
|
|
19
|
Luo Y, Meng X, Zhou P, Lu S, Qin M, Xu X,
Sun G and Sun X: Elatoside C protects against ox-LDL induced HUVECs
injury by FoxO1-mediated autophagy induction. Biochim Biophys Acta
Mol Basis Dis. 1863:1654–1665. 2017.PubMed/NCBI
|
|
20
|
Piao X, Liu B, Sui X, Li S, Niu W, Zhang
Q, Shi X, Cai S and Fan Y: Picroside II improves severe acute
pancreatitis-induced intestinal barrier injury by inactivating
oxidative and inflammatory TLR4-dependent PI3K/AKT/NF-κB signaling
and improving gut microbiota. Oxid Med Cell Longev.
2020:35894972020.
|
|
21
|
Zhong X, Ma X, Zhang L, Li Y, Li Y and He
R: MIAT promotes proliferation and hinders apoptosis by modulating
miR-181b/STAT3 axis in ox-LDL-induced atherosclerosis cell models.
Biomed Pharmacother. 97:1078–1085. 2018. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Chen J, Mehta JL, Haider N, Zhang X,
Narula J and Li D: Role of caspases in ox-LDL-induced apoptotic
cascade in human coronary artery endothelial cells. Circ Res.
94:370–376. 2004. View Article : Google Scholar
|
|
24
|
Qin M, Luo Y, Meng Xb, Wang M, Wang Hw,
Song Sy, Ye Jx, Pan Rl, Yao F, Wu P, et al: Myricitrin attenuates
endothelial cell apoptosis to prevent atherosclerosis: An insight
into PI3K/Akt activation and STAT3 signaling pathway. Vascul
Pharmacol. 70:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cahill-Smith S and Li JM: Oxidative
stress, redox signalling and endothelial dysfunction of
ageing-related neurodegenerative diseases: A role of NADPH oxidase
2. Br J Clin Pharmacol. 78:441–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue Z, Yuan W, Li J, Zhou H, Xu L, Weng J,
Li X, Zhang X, Wang Z and Yan J: Cyclophilin A mediates the ox-LDL
induced activation and apoptosis of macrophages with autophagy. Int
J Cardiol. 230:142–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han X, Han X, Wang Z, Shen J and Dong Q:
HDAC9 regulates ox-LDL-induced endothelial apoptosis by
participating in inflammatory reactions. Front Biosci (Landmark
Ed). 21:907–17. 2016. View
Article : Google Scholar
|
|
28
|
Glab JA, Doerflinger M, Nedeva C, Jose I,
Mbogo GW, Paton JC, Paton AW, Kueh AJ, Herold MJ, Huang DC, et al:
DR5 and caspase 8 are dispensable in ER stress induced apoptosis.
Cell Death Differ. 24:944–950. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kishino A, Hayashi K, Hidai C, Masuda T,
Nomura Y and Oshima T: XBP1-FoxO1 interaction regulates ER stress
induced autophagy in auditory cells. Sci Rep. 7:44422017.
View Article : Google Scholar
|
|
30
|
Tanaka J, Qiang L, Banks AS, Welch CL,
Matsumoto M, Kitamura T, Ido-Kitamura Y, DePinho RA and Accili D:
FoxO1 links hyperglycemia to LDL oxidation and endothelial nitric
oxide synthase dysfunction in vascular endothelial cells. Diabetes.
58:2344–2354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Ren M, Wang X, Cui X, Zhao H, Zhao
C, Zhou J, Guo Y, Hu Y, Yan C, et al: Glutaredoxin 1 mediates the
protective effect of steady laminar flow on endotelial cells
against oxidative stress-induced apoptosis via inhibiting bim. Sci
Rep. 7:155392017. View Article : Google Scholar
|