Introduction
Aging is considered the main risk factor for various
diseases (1); as the aging
population increases, the prevalence of several age-related chronic
diseases also increases. This is mainly caused by cumulative damage
in the organs and cells, thereby leading to the on-set of
age-related chronic diseases. Therefore, by exploring the specific
mechanisms of aging, valuable information on age-related diseases
can be obtained and consequently, strategies to delay its early
onset may also be developed.
Oxidative stress is a state in which there are high
levels of reactive oxygen species (ROS) compared to antioxidant
defenses (2), and this is a
hallmark of age-related diseases, including Parkinson's disease
(3), Alzheimer's disease
(4), chronic inflammation
(5), heart failure (6), atherosclerosis (7), kidney disease (8), certain types of cancer (9,10)
and aging itself (11). Among
these, vascular disease is the most common cause of mortality in
the industrialized world (12,13). The normal function and integrity
of vascular endothelial cells are of utmost importance for the
stability of the vascular environment. The dysfunction of the
vascular endothelium, characterized by morphological alterations in
the vascular wall, endothelial cell inflammation and apoptosis, is
considered the initial event of some diseases (14). Oxidative stress is a critical
cause of vascular dysfunction, as ROS can damage cells of the
vascular wall and induce vascular endothelial cell dysfunction
(15). Antioxidants prevent the
damage of vascular endothelial cells and are considered one of the
crucial factors for the prevention of cardiovascular diseases
(16).
Antioxidants are highly effective in the prevention
or treatment of oxidative damage in animal models (17). Commonly used antioxidants include
vitamin C, α-tocopherol and polyphenols (18). Among these, vitamin C, a
recognized antioxidant, is easily accessible in daily life and has
the advantages of low cost, a significant effect, easy
accessibility and hydrophilicity (19). As a potent antioxidant, vitamin C
plays a protective role in oxidative damage by blocking the
production of free radicals (20,21). It also plays a critical role in
certain types of cancer and exerts immunomodulatory effects that
enhance host defenses (22).
Vitamin C deficiency can lead to scurvy, a fatal disease (23). Vitamin C suppresses the expression
of KRAS and BRAF, which inhibit glycolysis and the ensuing energy
crisis by targeting GAPDH in colorectal cancer cells (24). In addition, high levels of vitamin
C have been shown to induce the apoptosis of HT29 colon cancer and
MCF7 breast cancer cells by hindering the energy flux in the
tricarboxylic acid cycle and glycolysis, eventually resulting in
the insufficient production of adenosine triosphosphate (25). Vitamin C can inhibit the death of
human umbilical vein endothelial cells (HUVECs) induced by
oxidative stress (19). Vitamin C
or vitamin C-Na pre-treatment has also been shown to enhance
antioxidant capacity, thus protecting H9C2 cells from heat-induced
damage (26). It also improves
the endothelium-dependent vasodilation of forearm resistance
vessels in patients with hypercholesterolemia (27). Vitamin C reduces lipid
peroxidation and enhances the antioxidant defense system, which
exerts a beneficial effect on the heart by reducing oxidative
stress in patients with cardiovascular disease (28). However, the molecular mechanisms
of vitamin C in vascular-related diseases warrant further
investigation.
MicroRNAs (miRNAs or miRs) are a family of small
non-coding RNA molecules with 18-25 nucleotides that bind to the
3′-untranslated regions of target mRNAs in a sequence-specific
manner, inhibiting their translation or regulating their
degradation (29). The expression
of miRNAs is tissue-specific, and this localized expression is
crucial for their tailored roles in regionalized function and
development (30). miRNAs play
vital roles in the absorption of vitamin C in the intestines by
modulating the post-transcriptional regulation of several vitamin
transporter genes, including that of solute carrier family
(SLC)23A2, SLC19A2, SLC52A3, SLC26A3
and SLC15A1 (31). Vitamin
C can also regulate multiple stem cell-specific signaling pathways,
such as cell adhesion molecules, fatty acid biosynthesis and
hormone signaling pathways by altering miRNA expression (32).
Although vitamin C has been widely studied, the
mechanisms underlying the protective effects of vitamin C on
H2O2-induced HUVECs associated with vascular
diseases warrant further investigation. The present study
demonstrates the potential benefits of vitamin C treatment against
H2O2-induced oxidative damage in HUVECs,
indicating that vitamin C partly exerts protective effects against
H2O2-induced oxidative damage by regulating
the expression of miRNAs.
Materials and methods
Culture and treatment of HUVECs
HUVECs were obtained from the Cell Bank of the
Chinese Academy of Sciences and cultured in complete endothelial
cell growth medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (100 U/ml; BD
Biosciences). HUVECs were digested with 0.25% trypsin and
1x104 cells/ml HUVECs were seeded and cultured at 37°C
under 5% CO2. In the H2O2 +
vitamin C-treated HUVEC group, the HUVECs were cultured in
endothelial cell growth medium containing 100 µM
H2O2 (Sigma-Aldrich; Merck KGaA) for 2 h and
were then cultured in endothelial cell growth medium supplemented
with 200 µM vitamin C (Sigma-Aldrich; Merck KGaA) for 48 h.
In the H2O2-treated HUVEC group, the HUVECs
were cultured in endothelial cell growth medium containing 100
µM H2O2 for 2 h and were then cultured
in endothelial cell growth medium for 48 h. HUVECs not treated with
vitamin C and H2O2 were used as control.
Apoptosis assays
The HUVECs from each group were washed twice with
cold phosphate-buffered saline before being resuspended in 1X
Binding Buffer at a concentration of 1×106 cells/ml. The
cells were stained with FITC-conjugated anti-Annexin V antibody and
propidium iodide (PI) in the apoptosis kit (cat. no. 556547, BD
Biosciences) for 15 min at room temperature and dark, and apoptotic
cells were quantified using a FACSCalibur flow cytometer (BD
Biosciences).
Measurement of ROS generation
According to the manufacturer's protocol, the ROS
assay kit (S0033; Beyotime Institute of Biotechnology, Inc.) was
used to measure intracellular ROS levels using the probe
2′,7′-dichlorofluorescin diacetate (DCFH-DA). Briefly, cells
treated with 100 µM H2O2 for 2 h or
100 µM H2O2 for 2 h + 200 µM
vitamin C for 48 h were washed twice with PBS. The cells were then
incubated with 10 µM DCFH-DA at 37°C for 30 min. After
washing with PBS twice, the fluorescence of the cells was imaged
using a confocal microscope (Carl Zeiss AG) with an excitation of
488 nm/emission of 529 nm. The fluorescence intensity was measured
using ImageJ_v1.8.0 software.
Analysis of the expression of miRNAs
miRNAs were isolated using a miRNA isolation kit
(Guangzhou Forevergen Biosciences Co., Ltd.) that specifically
captures small RNAs with lengths of <200 nucleotides. The
quality of the RNA samples was examined on an Agilent 2100
BioAnalyzer (Agilent Technologies, Inc.) and the yield of the RNA
samples was determined using the ABI Step One Plus Real-Time PCR
System (Applied Biosystems). The miRNA profile was analyzed for
hierarchical clustering to produce heatmaps.
Gene ontology (GO) and pathway enrichment
analyses of differentially expressed miRNAs
GO and pathway enrichment analyses were performed to
identify biological processes that were potentially regulated by
the differentially expressed miRNAs based on the GO (http://geneontology.org) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathways (http://www.genome.jp/kegg/pathway.html) databases. The
P-value for each GO term was calculated using the right-sided
hypergeometric tests. The Benjamin-Hochberg adjustment was used for
multiple test correction (33,34). Those terms with a P-value <0.05
were considered to be significantly enriched. Simultaneously, the
target mRNAs associated with apoptosis or oxidative metabolic
signaling pathways of the top 10 differentially expressed miRNAs
were predicted using TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do). The top 10
differentially expressed miRNAs and their target mRNAs associated
with cell apoptosis or involved in oxidative metabolism were
integrated, and regulatory networks were constructed using
Cytoscape 3 software.
Reverse transcription-quantitative PCR
(RT-qPCR) validation analyses of miRNAs
Total RNA was extracted from the cells and gene
expression was detected by RT-qPCR. According to the manufacturer's
protocol, cDNA was synthesized from total RNA using M-MLV Reverse
Transcriptase (Promega Corporation). The GoTaq qPCR Master Mix
(Promega Corporation) was used for qPCR. The ABI 7500 system
(Applied Biosystems) was used to perform the PCR amplification,
while U6 was used as an internal control. The primer information of
the miRNAs is presented in Table
SI.
Transient transfection
Negative control (NC) mimics, hsa-miR-323a-5p mimics
and hsa-miR-3928-5p mimics were purchased from GenePharma. The
sequences of the mimics were as follows: hsa-miR-323a-3p mimics
sense, 5′-CAC AUU ACA CGG UCG ACC UCU-3′ and antisense, 5′-AGG UCG
ACC GUG UAA UGU GUU-3′; hsa-miR-3928-5p mimics sense, 5′-UGA AGC
UCU AAG GUU CCG CCU GC-3′ and antisense, 5′-AGG CGG AAC CUU AGA GCU
UCA UU-3′; NC mimics sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and
antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′. HUVECs were
transfected with 50 nM hsa-miR-323a-3p mimics, 50 nM
hsa-miR-3928-5p mimics, or 50 nM NC mimics using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Following transfection for 48 h, the cells
were incubated with 100 µM H2O2 for 2
h and harvested for western blot analysis.
Western blot analysis
Cells were lysed using the RIPA buffer (R0010;
Solarbio) to separate the protein. The BCA working solution was
used to detect the protein concentration at 562 nm using BCA
Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.).
Subsequently, 10% SDS-PAGE was used to isolate the target protein.
The isolated target protein was transferred to a PVDF membrane.
After sealing with 5% non-fat milk at room temperature for 1 h,
primary antibodies, including anti-inter-leukin (IL)10 (1:1,000;
ab34843, Abcam), anti-cAMP-response element binding protein (CREB;
1:1,000; ab31387, Abcam), anti-p-CREB (1:1,000; CST9198, Cell
Signaling Technology, Inc.), anti-caspase (CASP)3 (1:1,000;
CST9662, Cell Signaling Technology, Inc.), anti-cleaved-CASP3
(1:1,000; CST9662, Cell Signaling Technology, Inc.),
anti-mitogen-activated protein kinase (MAPK)9 (1:1,000; CST4672,
Cell Signaling Technology, Inc.), anti-matrix metalloproteinase
(MMP)2 (1:1,000; ab37150, Abcam) and anti-GAPDH (1:1,000;
60004-1-1g, Proteintech) were incubated with the PVDF membrane
overnight at a temperature 4°C. HRP-labeled secondary antibody
(1:5,000; ab150117, Abcam) was then used to incubate the membrane
for 2 h at room temperature. GAPDH was used as an internal control.
The target protein levels were visualized by an enhanced
chemiluminescence reagent (Vazyme Biotech Co., Ltd.). ImageJ v1.8.0
software (National Institutes of Health) was used for the
semi-quantitative analysis of protein expression.
Statistical analyses
The results are shown as the means ± standard
deviation from at least 3 independent experiments. Statistical
signifcance was determined using one-way analysis of variance
(ANOVA) followed by Bonferroni post hoc test evaluations for
multiple comparisons with SPSS 19.0 statistical software. A
P<0.05 was considered to indicate a statistically significant
difference.
Results
Vitamin C significantly reduces the
H2O2-induced apoptosis of HUVECs
As a potent first-line antioxidant, vitamin C
attenuates the production of free radicals and reduces oxidative
damage (20,21). In the present study, to further
investigate the antioxidant function of vitamin C, vitamin C was
used to treat H2O2-induced HUVECs. The
percentage of apoptotic HUVECs induced with
H2O2 was approximately 6%, while vitamin C
significantly reduced the percentage of apoptotic HUVECs induced by
H2O2 (Fig. 1A
and B). In addition, ROS assay revealed that vitamin C reduced
the green fluorescence intensity in HUVECs induced with
H2O2 (Fig. 1C
and D). These results demonstrated that vitamin C attenuated
H2O2-induced apoptosis and oxidative damage
in H2O2-induced HUVECs.
Vitamin C modulates miRNA profiles in
H2O2-induced HUVECs
To identify differentially expressed miRNAs due to
vitamin C treatment, a comprehensive miRNA microarray analysis of
samples from H2O2-induced HUVECs with or
without vitamin C treatment was conducted. These miRNAs in HUVECs
were isolated after 2 h of H2O2 exposure and
48 h of vitamin C treatment. The miRNAs exhibited a significant
(P<0.05) 1.5-fold difference in expression following treatment
with H2O2 + vitamin C compared with the
control group and H2O2 exposure group,
respectively. The results revealed that there was a significant
change in the expression of 287 miRNAs, including 70 upregulated
miRNAs and 217 miRNAs that were downregulated in the
H2O2 exposure group compared with the control
group (Fig. 2A and Table SII). In addition, in the
H2O2 + vitamin C treatment group, 710
(including 706 upregulated and only 4 downregulated) miRNAs were
differentially expressed compared with the
H2O2 treatment group (Fig. 2B and Table SIII). The analyses revealed that
there were 42 identical miRNAs among the differentially expressed
miRNAs induced in the HUVEC group and H2O2 +
vitamin C-treated group compared to the
H2O2-treated group, including 41 upregulated
miRNAs and 1 downregulated miRNAs (Fig. 2C and Table SIV). Hierarchical cluster
analysis using the normalized miRNA expression data confirmed that
the expression of miRNAs in the H2O2 or
H2O2 + vitamin C-treated HUVECs could be
clearly distinguished from that in the control group (Fig. 2).
GO analysis and KEGG pathway
prediction
Categorizing these miRNAs may enhance our
understanding of the cellular components and biological processes
regulated by the miRNAs with an altered expression in the treated
HUVECs. GO enrichment analyses demonstrated that these
differentially expressed miRNAs were associated with cellular
components and biological processes, including cell, organelle,
membrane, protein-containing complex, membrane-enclosed lumen,
catalytic activity, binding, localization, metabolic processes,
developmental processes, multicellular organismal processes, immune
system processes, positive and negative regulation biological
processes, and cellular proliferation (Fig. 3A). Pathway enrichment analyses
revealed significant enrichment in apoptosis, MAPK signaling
pathway, PI3KAkt signaling pathway and oxidative phosphorylation
(Fig. 3B).
miRNA target prediction by bioinformatics
analysis
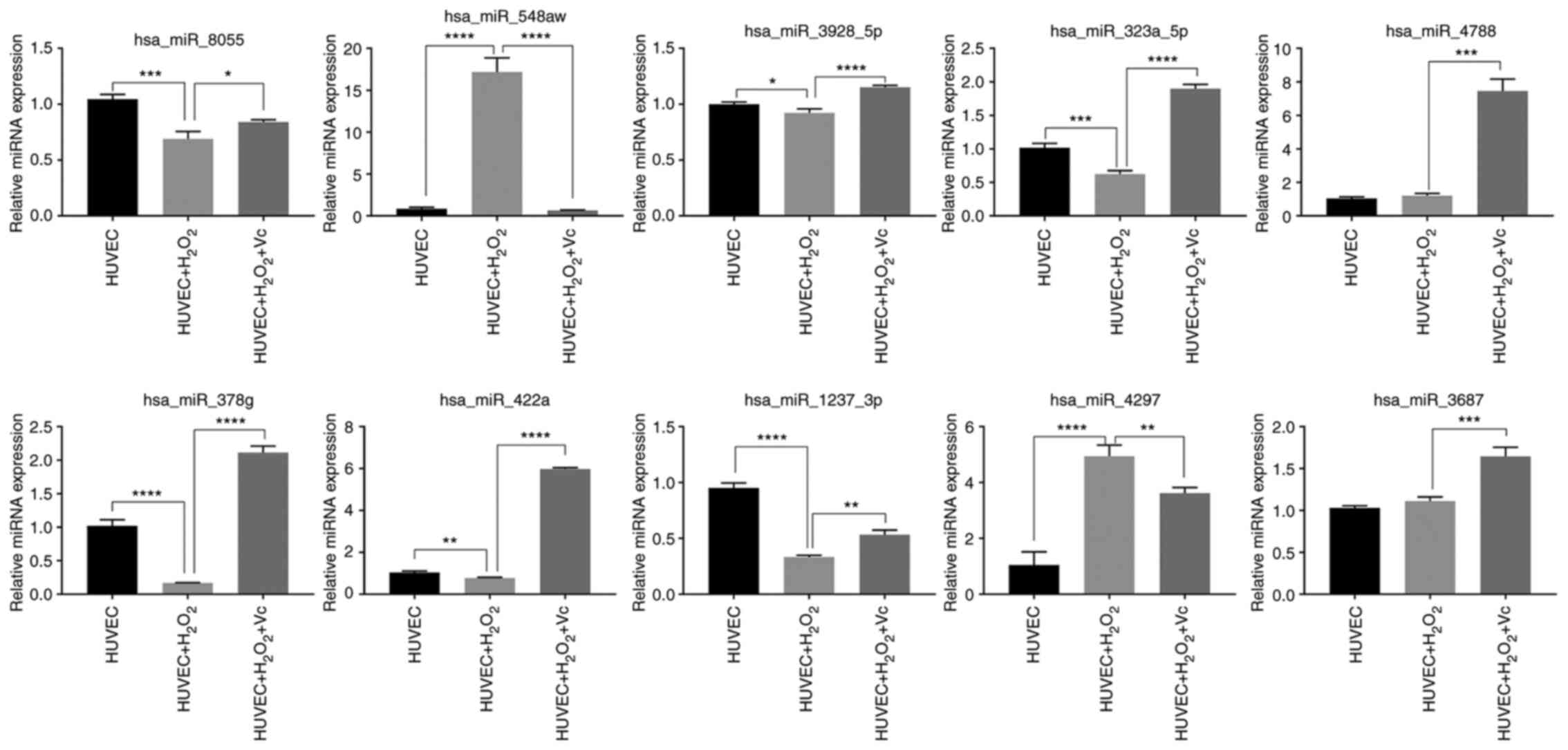
The top 10 miRNAs with the most evident differences
in expression were has-miR-323a-5p, hsa-miR-378g, hsa-miR-4788,
hsa-miR- 4297, hsa-miR- 422a, hsa-miR-3928-5p, hsa-miR-3687,
hsa-miR-1237-3p, hsa-miR-8055 and hsa-miR-548aw. The target mRNAs
associated with apoptosis or oxidative metabolic signaling pathways
of the top 10 differentially expressed miRNAs were predicted using
TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do). The network
of miRNA-targeted mRNAs was constructed according to their
regulatory association. hsa-miR-323a-5p can target CASP3, CASP6,
CASP9, MAPK9 and other mRNAs to regulate apoptosis; hsa-miR-422a
may be associated with apoptosis by regulating PIK3CA, E2F2, FAS,
or SMAD4; hsa-miR-8055 may target BCL2, FAS, TNF and other mRNAs to
regulate apoptosis (Fig. 4 and
Table SV). hsa-miR-3928-5p may
be involved in regulating oxidative stress by targeting IL10, MMP2,
or CREB; hsa-miR-378g may target ATG12, ANPEP, MAPK3, or EREG to
mediate oxidative stress; hsa-miR-1237-3p may target AKT2, AKT3 and
MAPK to regulate oxidative stress (Fig. 5 and Table SV).
Validation of differentially expressed
miRNAs
To confirm the miRNA microarray data, RT-qPCR was
performed to examine the expression levels of the top 10 miRNAs
from the H2O2 or H2O2 +
vitamin C-treated HUVEC groups. The expression levels of
hsa-miR-8055, hsa-miR-3928-5p, has-miR-323a-5p, has-miR-378g,
has-miR-422a and has-miR-1237-3p detected by RT-qPCR were
downregulated in the HUVECs induced by H2O2
compared to those in the control HUVECs, and were upregulated in
the HUVECs treated with vitamin C and H2O2
compared to those in the HUVECs exposed to
H2O2; these findings were consistent with
those of the microarray analyses (Fig. 6 and Table SIV). However, the has-miR-548aw
and has-miR-4297 expression levels were upregulated in the HUVECs
induced by H2O2 compared to those in the
control HUVECs, and were downregulated in the HUVECs treated with
vitamin C and H2O2 compared to those in the
HUVECs treated with H2O2; the has-miR-4788
and has-miR-3687 expression levels were upregulated in HUVECs
induced by H2O2 compared to those in HUVECs;
these findings were inconsistent with the results of microarray
analysis. Overall, the results of RT-qPCR very-fication of the
selected miRNAs were mostly consistent with the results of
sequencing analysis.
Role of has-miR-3928-5p and
has-miR-323a-5p in oxidation and apoptosis of HUVECs
To further analyze the molecular mechanisms of
miRNAs in apoptosis and oxidation, western blot analysis was used
to analyze the expression level of mRNAs regulated by
hsa-miR-3928-5p and hsa-miR-323a-5p. The results revealed that
H2O2 upregulate the IL10, MMP2, CREB and
p-CREB protein expression levels, and the ratio of p-CREB/CREB in
HUVECs; however, the IL10, MMP2, CREB and p-CREB protein expression
levels and the ratio of p-CREB/CREB in the HUVECs transfected with
has-miR-3928-5p and induced by H2O2 were
significantly downregulated, compared to those of HUVECs induced by
H2O2 (Fig. 7A
and B). Simultaneously, the results also revealed that
H2O2 upregulated the MAPK9, CASP3 and
cleaved-CASP3 protein expression levels and the ratio of
cleaved-CASP3/CASP3 in HUVECs (Fig.
7C and D). Moreover, the MAPK9, CASP3 and cleaved-CASP3 protein
expression levels and the ratio of cleaved-CASP3/CASP3 in HUVECs
transfected with hsa-miR-323a-5p and induced by
H2O2 were significantly downregulated,
compared to those of HUVECs induced by H2O2
(Fig. 7C and D). It could thus be
inferred that vitamin C may regulate the oxidation and apoptosis of
HUVECs through the above-mentioned molecular mechanism.
Discussion
Vitamin C is known to be a potent antioxidant that
quenches ROS and has also been demonstrated to ease vascular
endothelium dysfunction in conditions, such as
hyperhomocysteinemia, diabetes, hypercholesterolemia, coronary
artery disease, and renovascular hypertension (27,35-38). Vitamin C induces the pluripotent
differentiation of mouse embryonic stem cells via the modulation of
miRNA expression (39). High
Vitamin C levels result in enhanced anti-atherosclerotic and
anti-senescence effects by regulating anti-inflammatory miRNAs
(40,41). At present, the role of vitamin
C-dependent miRNAs in the regulation of the antioxidant and
antiapoptotic activities of endothelial cell remains to be fully
determined. Hence, the identification of vitamin C-induced
differentially expressed miRNAs is crucial for the further
understanding of the specific mechanisms underlying endothelial
dysfunction. In the present study, a list of differentially
expressed miRNAs were identified following vitamin C treatment and
it was revealed that vitamin C attenuated the apoptosis and
oxidative damage of H2O2-induced HUVECs.
Simultaneously, GO analysis demonstrated that
cellular components and biological processes were clearly critical
to the H2O2-induced oxidation stress and
apoptosis of HUVECs. Moreover, KEGG annotation demonstrated that
apoptosis, the MAPK signaling pathway, PI3K/Akt signaling pathway
and oxidative phosphorylation were involved in the anti-oxidative
and anti-apoptotic effects of vitamin C in
H2O2-induced HUVECs. Research has indicated
that all MAPK inhibitors increase the O2•-
levels in H2O2-induced A549 cells (42). The PI3K/Akt signaling pathway is
related to survival, angiogenesis and oxidative stress under
pathophysiologic conditions in ischemia (43). Klotho has been shown to weaken
oxidized low-density lipoprotein (ox-LDL)-induced oxidative stress
in HUVECs via the upregulation of oxidative scavengers by
suppressing lectin-like ox-LDL receptor expression and activating
the PI3K/Akt/eNOS pathway (44).
To further investigate the potential roles of miRNAs involved in
HUVECs following exposure to vitamin C, the present study analyzed
the predicted target mRNAs of selected miRNAs. The present study
focused on the top 10 miRNAs, including hsa-miR-323a-5p,
has-miR-378g, has-miR-4788, has-miR-4297, hsa-miR-422a,
hsa-miR-3928-5p, hsa-miR-3687, hsa-miR-1237-3p, hsa-miR-8055 and
hsa-miR-548aw, with the most evident differences in expression. It
was revealed that these miRNAs target mRNAs that regulate cell
apoptosis and oxidative metabolism signaling pathways.
miR-422a can inhibit the migration and proliferation
of gastric cancer cells, and can promote the metabolic transition
from aerobic glycolysis to oxidative phosphorylation (45). miR-323a-3p can attenuate the
apoptosis of 16HBE14o-cells stimulated with staurosporine or
tunicamycin by inhibiting the CASP3 expression level (46). CASP-9/3 plays a critical role in
apoptotic signaling in the mitochondria (47). The activation of CASP-9/3 mediates
cell apoptosis in various cell types (48-50). In addition,
1,25-dihydroxyvitamin-D3 induces neutrophil apoptosis in
periodontitis with type 2 diabetes mellitus patients via the
p38/MAPK pathway (51). In this
study, western blot showed that hsa-miR-323a-5p downregu-lated the
protein expression levels of CASP3, Cleaved-CASP3, and MAPK9 and
the ratio of the Cleaved-CASP3/CASP3 in HUVECs induced by
H2O2, suggesting that hsa-miR-323a-5p
obtained the antiapoptotic effects of Vitamin C by targeting CASP3
and MAPK9. In oxidative stress, MMP2 knockdown has been shown to
prevent the protective effects of miR-125 inhibitor on H9C2 cells
(52). MMPs play important roles
in anti-inflammation; the activity of MMP-2 is increased by
oxidative stress in early hypertension (53). The administration of anthocyanin
suppresses the generation of ROS and attenuates naproxen-induced
suppression of MMP-2 (54). IL10,
a human cytokine influencing immunoregulation and inflammation, has
anti-inflammatory properties and plays an important role in
limiting immune responses to pathogens and oxidative stress
(55). The activation of the
Akt/CREB axis by stressin-1 can counteract the adverse effects of
various cell stresses (56). In
the present study, the results of western blot analysis revealed
that hsa-miR-3928-5p downregulated the protein expression levels of
IL10, MMP2, CREB and p-CREB, and the ratio of p-CREB/CREB in HUVECs
induced by H2O2, suggesting that
hsa-miR-3928-5p mediated the anti-inflammatory effects of vitamin C
by targeting IL10, MMP2 and CREB.
In conclusion, the present study may be an important
step in obtaining a greater understanding of the mechanisms through
which vitamin C exerts anti-apoptotic and antioxidant effects via
miRNA signaling networks, thereby revealing the potential molecular
mechanisms of vitamin C as regards the antioxidation and apoptosis
of HUVEC induced by H2O2.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Guangdong
Basic and Applied Basic Research Fund (Key project of
Guangdong-Foshan Joint Fund) (2019B1515120044) and Science and
Technology Innovation Project from Foshan, Guangdong
(FS0AA-KJ218-1301-0006 and FS0AA-KJ218- 1301-0010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, YHu and YHuang conceived and designed the
experiments. JW, JingjingL, MLi, MLin, LM, XH, JianqiuL performed
the experiments. JW and JingjingL analyzed the data and wrote the
manuscript. JianqiuL and YHuang revised the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ruano L, Portaccio E, Goretti B, Niccolai
C, Severo M, Patti F, Cilia S, Gallo P, Grossi P, Ghezzi A, et al:
Age and disability drive cognitive impairment in multiple sclerosis
across disease subtypes. Mult Scler. 23:1258–1267. 2017. View Article : Google Scholar
|
|
2
|
Sies H: Oxidative stress: Oxidants and
antioxidants. Exp Physiol. 82:291–295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou C, Yong H and Przedborski S:
Oxidative stress in Parkinson's disease: A mechanism of pathogenic
and therapeutic significance. Ann N Y Acad Sci. 1147:93–104. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markesbery WR: Oxidative stress hypothesis
in Alzheimer's disease. Free Radic Biol Med. 23:134–147. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Federico A, Morgillo F, Tuccillo C,
Ciardiello F and Loguercio C: Chronic inflammation and oxidative
stress in human carcinogenesis. Int J Cancer. 121:2381–2386. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsutsui H, Kinugawa S and Matsushima S:
Oxidative stress and heart failure. Am J Physiol Heart Circ
Physiol. 301:H2181–H2190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palade F, Alexa ID, Azoicăi D, Panaghiu L
and Ungureanu G: Oxidative stress in atherosclerosis. Rev Med Chir
Soc Med Nat Iasi. 107:502–511. 2003.In Romanian.
|
|
8
|
Kubrak OI, Husak VV, Rovenko BM, Poigner
H, Mazepa MA, Kriews M, Abele D and Lushchak VI: Tissue specificity
in nickel uptake and induction of oxidative stress in kidney and
spleen of goldfish carassius auratus, exposed to waterborne nickel.
Aquat Toxicol. 118-119:88–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and Lleonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar
|
|
10
|
Fiaschi T and Chiarugi P: Oxidative
stress, tumor microenvironment, and metabolic reprogramming: A
diabolic liaison. Int J Cell Biol. 2012:7628252012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halliwell B and Gutteridge J: Free
radicals in biology and medicine. J Free Radical Biol Med.
1:331–332. 2007. View Article : Google Scholar
|
|
12
|
Gibbons GH and Dzau VJ: Molecular
therapies for vascular diseases. Science. 272:689–693. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang X, Luo YX, Chen HZ and Liu DP:
Mitochondria, endothelial cell function, and vascular diseases.
Front Physiol. 5:1752014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schinzari F, Tesauro M and Cardillo C:
Endothelial and perivascular adipose tissue abnormalities in
obesity-related vascular dysfunction: Novel targets for treatment.
J Cardiovasc Pharmacol. 69:360–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang M and Vousden KH: Serine and
one-carbon metabolism in cancer. Nat Rev Cancer. 16:650–662. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Xia L, Zhang F, Zhu D, Xin C,
Wang H, Zhang F, Guo X, Lee Y, Zhang L, et al: A novel mechanism of
diabetic vascular endothelial dysfunction:
Hypoadiponectinemia-induced NLRP3 inflammasome activation. Biochim
Biophys Acta Mol Basis Dis. 1863:1556–1567. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li S, Tan HY, Wang N, Zhang ZJ, Lao L,
Wong CW and Feng Y: The role of oxidative stress and antioxidants
in liver diseases. Int J Mol Sci. 16:26087–26124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmad KA, Yuan Yuan D, Nawaz W, Ze H, Zhuo
CX, Talal B, Taleb A, Mais E and Qilong D: Antioxidant therapy for
management of oxidative stress induced hypertension. Free Radic
Res. 51:428–438. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen AY, Lü JM, Yao Q and Chen C:
Entacapone is an antioxidant more potent than vitamin C and vitamin
E for scavenging of hypochlorous acid and peroxynitrite, and the
inhibition of oxidative stress-induced cell death. Med Sci Monit.
22:687–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryan MJ, Dudash HJ, Docherty M, Geronilla
KB, Baker BA, Haff GG, Cutlip RG and Always SE: Vitamin E and C
supple-mentation reduces oxidative stress, improves antioxidant
enzymes and positive muscle work in chronically loaded muscles of
aged rats. Exp Gerontol. 45:882–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monacelli F, Acquarone E, Giannotti C,
Borghi R and Nencioni A: Vitamin C, aging and Alzheimer's disease.
Nutrients. 9:6902017.
|
|
22
|
Linster CL and Van Schaftingen E: Vitamin
C. Biosynthesis, recycling and degradation in mammals. FEBS J.
274:1–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carr AC and Maggini S: Vitamin C and
immune function. Nutrients. 9:12112017. View Article : Google Scholar :
|
|
24
|
Yun J, Mullarky E, Lu C, Bosch KN,
Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, et
al: Vitamin C selectively kills KRAS and BRAF mutant colorectal
cancer cells by targeting GAPDH. Science. 350:1391–1396. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uetaki M, Tabata S, Nakasuka F, Soga T and
Tomita M: Metabolomic alterations in human cancer cells by vitamin
C-induced oxidative stress. Sci Rep. 5:138962015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin B, Tang S, Sun J, Zhang X, Xu J, Di L,
Li Z, Hu Y and Bao E: Vitamin C and sodium bicarbonate enhance the
antioxidant ability of H9C2 cells and induce HSPs to relieve heat
stress. Cell Stress Chaperones. 23:735–748. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ting HH, Timimi FK, Haley EA, Roddy MA,
Ganz P and Creager MA: Vitamin C improves endothelium-dependent
vasodilation in forearm resistance vessels of humans with
hyper-cholesterolemia. Circulation. 95:2617–2622. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karajibani M, Hashemi M, Montazerifar F
and Dikshit M: Effect of vitamin E and C supplements on antioxidant
defense system in cardiovascular disease patients in Zahedan,
southeast Iran. J Nutr Sci Vitaminol (Tokyo). 56:436–440. 2010.
View Article : Google Scholar
|
|
29
|
Bagga S, Bracht J, Hunter S, Massirer K,
Holtz J, Eachus R and Pasquinelli AE: Regulation by let-7 and lin-4
miRNAs results in target mRNA degradation. Cell. 122:553–563. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Y, Schug J, McKenna LB, Le Lay J,
Kaestner KH and Greenbaum LE: Tissue-specific regulation of mouse
microRNA genes in endoderm-derived tissues. Nucleic Acids Res.
39:454–463. 2011. View Article : Google Scholar :
|
|
31
|
Xie Q, Chen C, Li H, Xu J, Wu L, Yu Y, Ren
S, Li H, Hua X, Yan H, et al: miR-3687 overexpression promotes
bladder cancer cell growth by inhibiting the negative effect of
FOXP1 on cyclin E2 transcription. Mol Ther. 27:1028–1038. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kolhe R, Mondal AK, Pundkar C,
Periyasamy-Thandavan S, Mendhe B, Hunter M, Isales CM, Hill WD,
Hamrick MW and Fulzele S: Modulation of miRNAs by vitamin C in
human bone marrow stromal cells. Nutrients. 10:1862018. View Article : Google Scholar :
|
|
33
|
Xu C, Chen Y, Zhang H, Chen Y, Shen X, Shi
C, Liu Y and Yuan W: Integrated microRNA-mRNA analyses reveal OPLL
specific microRNA regulatory network using high-throughput
sequencing. Sci Rep. 6:215802016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee S, Woo J, Kim YS and Im HI: Integrated
miRNA-mRNA analysis in the habenula nuclei of mice intravenously
self-administering nicotine. Sci Rep. 5:129092015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chambers JC, McGregor A, Jean-Marie J,
Obeid OA and Kooner JS: Demonstration of rapid onset vascular
endothelial dysfunction after hyperhomocysteinemia: An effect
reversible with vitamin C therapy. Circulation. 99:1156–1160. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heitzer T, Finckh B, Albers S, Krohn K,
Kohlschütter A and Meinertz T: Beneficial effects of alpha-lipoic
acid and ascorbic acid on endothelium-dependent, nitric
oxide-mediated vasodilation in diabetic patients: Relation to
parameters of oxidative stress. Free Radic Biol Med. 31:53–61.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang J, Agus DB, Winfree CJ, Kiss S, Mack
WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, et al:
Dehydroascorbic acid, a blood-brain barrier transportable form of
vitamin C, mediates potent cerebroprotection in experimental
stroke. Proc Natl Acad Sci USA. 98:11720–11724. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Higashi Y, Sasaki S, Nakagawa K, Matsuura
H, Oshima T and Chayama K: Endothelial function and oxidative
stress in reno-vascular hypertension. N Engl J Med. 346:1954–1962.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao Y, Han Z, Li Q, Wu Y, Shi X, Ai Z, Du
J, Li W, Guo Z and Zhang Y: Vitamin C induces a pluripotent state
in mouse embryonic stem cells by modulating microRNA expression.
FEBS J. 282:685–699. 2015. View Article : Google Scholar
|
|
40
|
Kim SM, Lim SM, Yoo JA, Woo MJ and Cho KH:
Consumption of high-dose vitamin C (1250 mg per day) enhances
functional and structural properties of serum lipoprotein to
improve anti-oxidant, anti-atherosclerotic, and anti-aging effects
via regulation of anti-inflammatory microRNA. Food Funct.
6:3604–3612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim YJ, Ku SY, Rosenwaks Z, Liu HC, Chi
SW, Kang JS, Lee WJ, Jung KC, Kim SH, Choi YM, et al: MicroRNA
expression profiles are altered by gonadotropins and vitamin C
status during in vitro follicular growth. Reprod Sci. 17:1081–1089.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park WH: MAPK inhibitors, particularly the
JNK inhibitor, increase cell death effects in
H2O2-treated lung cancer cells via increased
superoxide anion and glutathione depletion. Oncol Rep. 39:860–870.
2018.
|
|
43
|
Samakova A, Gazova A, Sabova N, Valaskova
S, Jurikova M and Kyselovic J: The PI3k/Akt pathway is associated
with angiogenesis, oxidative stress and survival of mesenchymal
stem cells in pathophysiologic condition in ischemia. Physiol Res.
68(Suppl 2): S131–S138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yao Y, Wang Y, Zhang Y and Liu C: Klotho
ameliorates oxidized low density lipoprotein (ox-LDL)-induced
oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways.
Lipids Health Dis. 16:772017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He Z, Li Z, Zhang X, Yin K, Wang W, Xu Z,
Li B, Zhang L, Xu J, Sun G, et al: MiR-422a regulates cellular
metabolism and malignancy by targeting pyruvate dehydrogenase
kinase 2 in gastric cancer. Cell Death Dis. 9:5052018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ge L, Habiel DM, Hansbro PM, Kim RY,
Gharib SA, Edelman JD, Königshoff M, Parimon T, Brauer R, Huang Y,
et al: miR-323a-3p regulates lung fibrosis by targeting multiple
profibrotic pathways. JCI Insight. 1:e903012016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang JK, Guo Q, Zhang XW, Wang LC, Liu Q,
Tu PF, Jiang Y and Zeng KW: Aglaia odorata Lour. extract inhibit
ischemic neuronal injury potentially via suppressing
p53/Puma-mediated mitochondrial apoptosis pathway. J
Ethnopharmacol. 248:1123362020. View Article : Google Scholar
|
|
48
|
Cai ZJ, Lee YK, Lau YM, Ho JC, Lai WH,
Wong NL, Huang D, Hai JJ, Ng KM, Tse HF, et al: Expression of
Lmna-R225X nonsense mutation results in dilated cardiomyopathy and
conduction disorders (DCM-CD) in mice: Impact of exercise training.
Int J Cardiol. 298:85–92. 2020. View Article : Google Scholar
|
|
49
|
Ibrahim RY, Mansour SM and Elkady WM:
Phytochemical profile and protective effect of Ocimum basilicum
aqueous extract in doxorubicin/irradiation-induced testicular
injury. J Pharm Pharmacol. 72:101–110. 2020. View Article : Google Scholar
|
|
50
|
Hirai T, Konishi Y, Mizuno S, Rui Z, Sun Y
and Nishiwaki K: Differential effects of sevoflurane on the growth
and apoptosis of human cancer cell lines. J Anesth. 34:47–57. 2020.
View Article : Google Scholar
|
|
51
|
Tang Y, Liu J, Yan Y, Fang H, Guo C, Xie R
and Liu Q: 1-25-dihydroxyvitamin-D3 promotes neutrophil apoptosis
in periodontitis with type 2 diabetes mellitus patients via the
p38/MAPK pathway. Medicine (Baltimore). 97:e139032018. View Article : Google Scholar
|
|
52
|
Li L, Zhang M, Chen W, Wang R, Ye Z, Wang
Y, Li X and Cai C: LncRNA-HOTAIR inhibition aggravates oxidative
stress-induced H9c2 cells injury through suppression of MMP2 by
miR-125. Acta Biochim Biophys Sin (Shanghai). 50:996–1006. 2018.
View Article : Google Scholar
|
|
53
|
Blascke de Mello MM, Parente JM, Schulz R
and Castro MM: Matrix metalloproteinase (MMP)-2 activation by
oxidative stress decreases aortic calponin-1 levels during
hypertrophic remodeling in early hypertension. Vascul Pharmacol.
116:36–44. 2019. View Article : Google Scholar
|
|
54
|
Kim SJ, Park YS, Paik HD and Chang HI:
Effect of anthocyanins on expression of matrix metalloproteinase-2
in naproxen-induced gastric ulcers. Br J Nutr. 106:1792–1801. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Iyer SS and Cheng G: Role of interleukin
10 transcriptional regulation in inflammation and autoimmune
disease. Crit Rev Immunol. 32:23–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Herkel J, Schrader J, Erez N, Lohse AW and
Cohen IR: Activation of the Akt-CREB signalling axis by a
proline-rich heptapeptide confers resistance to stress–induced cell
death and inflammation. Immunology. 151:474–480. 2017. View Article : Google Scholar : PubMed/NCBI
|