Introduction
Sepsis may promote systemic inflammatory injury of
the blood vessels, resulting in microvascular endothelial cell
dysfunction and injury (1-3).
Microvascular dysfunction is of great importance in the clinic and
has been associated with increasing mortality when the dysfunction
persists for a long time (4-7).
Endothelial dysfunction is considered to be an early event for a
range of vascular diseases (such as atherosclerosis, hypertension
and myocardial ischemia) and it has been reported that inflammation
is involved in this pathological process (8,9).
Results from clinical and scientific studies have demonstrated that
septic microvascular dysfunction may be mediated by a number of
factors and processes, including the activation of leukocytes
(10), the secretion of
inflammatory cytokines (11) and
the exposure of microvascular cells to harmful leukocyte-derived
molecules (12). In addition, it
has been suggested that the local production of inflammatory
factors in vascular cells may exert a direct and significant effect
on the pathological process of sepsis (13). The expression of several types of
inflammatory cytokines, including interleukin (IL)-1β, IL-6 and
tumor necrosis factor (TNF-α) may be induced by sepsis (14,15). Furthermore, inflammatory cell
infiltration and the oxidative stress-mediated generation of
reactive oxygen species (ROS) may promote blood vessel damage,
activation of mitogen-activated protein kinases (MAPKs) and
translocation of nuclear factor-κB (NF-κB) into the cell nucleus
(16,17). However, the specific mechanism
underlying the sepsis-induced pro-inflammatory responses remains
unclear. Therefore, the present study aimed to determine the
mechanism underlying the sepsis-induced inflammatory injury of
HUVECs by focusing on the effects of ROS, MAPKs and NF-κB.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) was
obtained from HyClone (Cytiva), and fetal bovine serum (FBS) was
purchased from Gibco. TRIzol® was obtained from
Invitrogen (Thermo Fisher Scientific, Inc.). PrimeScript™ 1st
strand cDNA Synthesis kit and SYBR® Premix Ex Taq were
obtained from Takara Biotechnology Co., Ltd. The extracellular
signal regulated kinase 1/2 (ERK 1/2) inhibitor PD98059, the p38
inhibitor SB203580, the antioxidant N-acetylcysteine (NAC) and the
NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) were purchased
from Sigma-Aldrich, Merck KGaA. The following antibodies were used
in the present study: Rabbit monoclonal anti-β-actin (cat. no.
NC021; Zhuangzhi Biotech), anti-NF-κB (cat. no. ab16502; Abcam),
anti-lamin B1 (cat. no. AF1408), anti-NF-κB p65 (cat. no. SN368),
anti-ERK1/2 (cat. no. AF1051), anti-phospho-ERK1/2 (cat. no.
AF5851), anti-JNK (cat. no. AJ518), anti-phospho-JNK cat. no.
AJ516) (all from Beyotime Institute of Biotechnology),
anti-phospho-p38 (cat. no. 9216), anti-p38 (cat. no. 8690) (both
from Cell Signaling Technology, Inc.). The HRP-conjugated
anti-mouse IgG (cat. no. CW0102S) and anti-Rabbit IgG (cat. no.
CW0103S) secondary antibodies were obtained from CW Biotech, Co.,
Ltd. Furthermore, 2′,7′-dichlorodihydrofluo-rorescein diacetate
(H2DCF-DA) was obtained from Beyotime Institute of
Biotechnology, and ELISA kits for detecting human IL-1β (cat. no.
F01220), IL-6 (cat. no. F01310) and TNF-α (cat. no. F02810) were
obtained from Westang. All other chemicals used in the experiments
were of analytical grade.
Isolation of septic serum
Male Sprague-Dawley (SD) rats (age, 6 weeks; n=16;
weight, 150-170 g; purchased from Chengdu Dossy Experimental
Animals Co., Ltd.) were randomly divided into two groups. The
animals were maintained under pathogen-free conditions
(temperature, 25°C; humidity, 50%; 12-h light/dark cycle) and had
free access to food and water. A cecal ligation and
puncture-induced sepsis rat model was established as previously
described (18). Briefly, rats
were fasted overnight (12 h) one day prior surgery (the body weight
loss after fasting was 3-8 g) and anesthetized by intra-peritoneal
injection of 10% chloral hydrate (200 mg/kg body weight). None of
the rats presented with signs of peritonitis following injection.
Once the rats appeared unconscious with normal breath, the lower
abdomen was incised, and the cecum was ligated with 2-0 surgical
silk, pierced with an 18-gauge needle, gently compressed until
fecal matter was extruded, and returned to the abdominal cavity.
Finally, the abdomen was completely closed with 2-0 surgical silk.
Animals in the sham group underwent exactly the same procedure
without the cecal puncture. After 24 h, the rats were euthanized
with intraperitoneal injection of pentobarbital sodium (200 mg/kg);
death was confirmed by the occurrence of cardio-respiratory arrest,
and ~10 ml of serum was collected from the abdominal aorta. In the
current study, symptoms such as pain, weight loss, loss of appetite
or weakness were set as humane endpoints; however, no animal was
sacrificed prior the completion of the experiments due to reaching
these endpoints. All experimental procedures in animals were
carried out according to international, national and institutional
regulations, and were approved by the Shaanxi University of Chinese
Medicine (approval no. 201801115).
Cell culture and treatment
HUVECs were obtained from Cobioer Biosciences Co.,
Ltd. (lot no. CBP60340) and cultured in DMEM supplemented with 10%
FBS at 37°C with 5% CO2. Prior to treatment, cells
(1-1.5×107) were cultured in serum-free medium for an
additional 12 h. HUVECs in the septic serum-treatment group were
cultured in DMEM supplemented with 10% septic serum for 12 or 24 h,
whereas those in the control group were cultured in DMEM with 10%
control serum. For the cell signaling pathway investigation, the
cells were pre-treated with specific inhibitors for 1 h, followed
by treatment with 10% septic serum. The concentrations of the
specific inhibitors were as follows: 20 µM ERK 1/2 inhibitor
PD98059; 10 µM p38 inhibitor SB203580; 20 µM JNK
inhibitor SP600125; 10 µM antioxidant NAC; and 10 µM
NF-κB inhibitor PDTC, as previously described (19).
Cell viability assay
Following treatment, HUVEC viability was assessed
using a Cell Counting Kit (CCK-8; cat. no. C0037; Beyotime
Institute of Biotechnology). Briefly, cells (1-1.5×105)
were seeded into a 96-well plate, and 10 µl CCK-8 solution
was added into each well, followed by incubation at 37°C for an
additional 4 h. Subsequently, the optical density (OD) of each well
was measured at 450 nm using a microplate reader (Molecular
Devices, LLC). The mean OD value from six wells was obtained, and
the cell viability was calculated as the percentage relative to the
OD values in the control group.
Cell morphology analysis
Cell morphology was examined using a JEM-101 (Jeol
Electron Inc.) transmission electron microscope (TEM). At 12 h
following treatment, cells were collected by centrifugation (150.3
× g; 5 min), washed three times with PBS and fixed in 1%
paraformaldehyde supplemented with 2% glutaraldehyde for 24 h at
4°C. Fixed cells were further treated with 1% osmium tetroxide for
2 h at 25°C, dehydrated in graded ethanol (50, 70, 80, 90 and 100%
for 10 min/step) and embedded in araldite. Ultrathin sections (70
nm) were cut, stained with uranyl acetate for 30 min at 25°C,
washed three times with double distilled water, and stained with
lead citrate for 10 min at 25°C, followed by washing three times
with double distilled water. Finally, the cell morphology was
observed under a JEM-101 TEM (×8,000 magnification; JEOL Ltd.), and
three fields were observed per sample.
Reverse transcription-qPCR (RT-qPCR)
Following treatment, HUVECs (1×105
cells/well) were washed twice with ice-cold PBS, and total RNA was
extracted using TRIzol® reagent according to the
manufacturer's instructions. The concentration of the total RNA was
determined by measuring the absorbance at 260 nm. Subsequently,
total RNA was reverse-transcribed into cDNA using a PrimeScript™
1st strand cDNA Synthesis Kit. The cDNA of the target genes was
amplified using the SYBR® Premix Ex Taq on the Mx3000P
quantitative PCR system (Stratagene; Agilent Technologies, Inc.).
The primer sequences used were as follows: Human IL-1β forward,
5′-CAT TGA GCC TCA TGC TCT GTT-3′ and reverse, 5′-CGC TGT CTG AGC
GGA TGA A-3′; human IL-6 forward, 5′-TTC GGT CCA GTT GCC TTC TC-3′
and reverse, 5′-TCA CCA GGC AAG TCT CCT CA-3′; human TNF-α forward,
5′-GCT GCA CTT TGG AGT GAT CG-3′ and reverse, 5′-GCT TGA GGG TTT
GCT ACA ACA-3′; and human GAPDH forward, 5′-TGT GGG CAT CAA TGG ATT
TGG-3′ and reverse, 5′-ACA CCA TGT ATT CCG GGT CAA T-3′. The
expression levels of the target mRNAs were normalized to those of
GAPDH. All samples were run in triplicate and analyzed using the
2−ΔΔCq method as previously described (20).
ELISA
The current knowledge of the pathophysiology of
sepsis suggests that patients present with hyperinflammation, and
excessive production of inflammatory markers (such as IL-1β, IL-6
and TNF-α) occurs throughout the course of sepsis (21). In the current study, HUVECs
(1-1.5×105) were cultured in 96-well plates and
stimulated with septic serum for 12 h. Subsequently, the
supernatant was collected by centrifugation (900 × g; 10 min at
4°C), and the serum levels of IL-1β, IL-6 and TNF-α were evaluated
using specific ELISA kits according to the manufacturer's
instructions. Subsequently, the optical density (OD) of each well
was measured at 490 nm using a microplate reader (Molecular
Devices, LLC).
Measurement of superoxide anion
generation
HUVECs (1-1.5×107) were cultured in
6-well plates and treated with septic serum for 12 h. Subsequently,
the cells were supplemented with 10 µM H2DCF-DA
for 1 h and washed with ice-cold PBS three times. Fluorescence
images were acquired at an excitation wavelength of 488 nm and an
emission wavelength of 525 nm under a fluorescence microscope (×200
magnification; Olympus Corporation), and six fields were observed
per well. Fluorescence intensity was calculated and analyzed from
the fluorescence images with the Image-pro plus software (Version
X; Media Cybernetics, Inc.). The relative fluorescence intensity
was calculated as the mean value of six independent
experiments.
Western blotting
Following treatment with septic or normal serum in
6-well plates, cells were washed twice with ice-cold PBS and lysed
using a lysis buffer (100 µl/well; Beyotime Institute of
Biotechnology) supplemented with a protease inhibitor cocktail and
phosphatase inhibitors (Roche Diagnostics). The nuclear proteins
were extracted using a NE-PER Nuclear Cytoplasmic Extraction
Reagent kit (Pierce; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Briefly, treated cells were lysed
with 200 µl cytoplasmic extraction reagent I followed by the
addition of 11 µl cytoplasmic extraction reagent II for 5
sec. Subsequently, the cells were incubated on ice for 1 min,
centrifuged (10,000 × g) at 4°C for 5 min, and the supernatant
fractions (cytoplasmic extracts) were transferred into a prechilled
tube. The insoluble pellet fraction was resuspended in 100
µl nuclear extraction reagent followed by vortexing for 15
sec, incubation on ice for 10 min and centrifugation (12,000 × g)
at 4°C for 10 min. The resulting supernatant was used for
subsequent experiments. Protein concentration was determined with a
BCA protein assay kit (Bio-Rad Laboratories, Inc.). Equal amounts
of protein extracts (30 µg) were separated by 10% SDS-PAGE
and transferred to polyvinylidene fluoride membranes (pore size,
0.45 µm; Cytiva). The membranes were incubated with
antibodies against β-actin (1:2,000), NF-κB (1:2,500), lamin B1
(1:1,500), JNK (1:1,500), phospho-JNK (1:800), p38 (1:1,000),
phospho-p38 (1:500), ERK1/2 (1:1,000) or phospho-ERK1/2 (1:800)
overnight at 4°C. Following washing with TBS + 0.25% Tween-20 three
times, the membranes were incubated with the corresponding
secondary antibody (1:2,500) for 3 h at 25°C, and the immune
complexes were enhanced by chemiluminescence (Merck Life Science
UK, Ltd.). The intensity of the bands was determined by scanning
and quantification using the Bio-Rad Gel Doc™ 2000 imaging system
(Bio-Rad Laboratories, Inc.).
Statistical analysis
The data are presented as the mean ± standard error
of the mean. The normality and homogeneity of these data were
tested, and the differences among groups were assessed with one-way
ANOVA followed by Dunnett's or non-parametric Kruskal-Wallis
analysis followed by Dunn's test using GraphPad Prism 8.3 software
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Septic serum attenuates HUVEC
viability
In the present study, cell viability was assessed to
evaluate the toxic effects of septic serum. As demonstrated in
Fig. 1, the viability of HUVECs
was significantly decreased following treatment with septic serum
for 12 and 24 h. This suggested that sepsis exerted harmful effects
on vascular endothelial cells; according to these results, 12 h was
selected the treatment time for subsequent experiments.
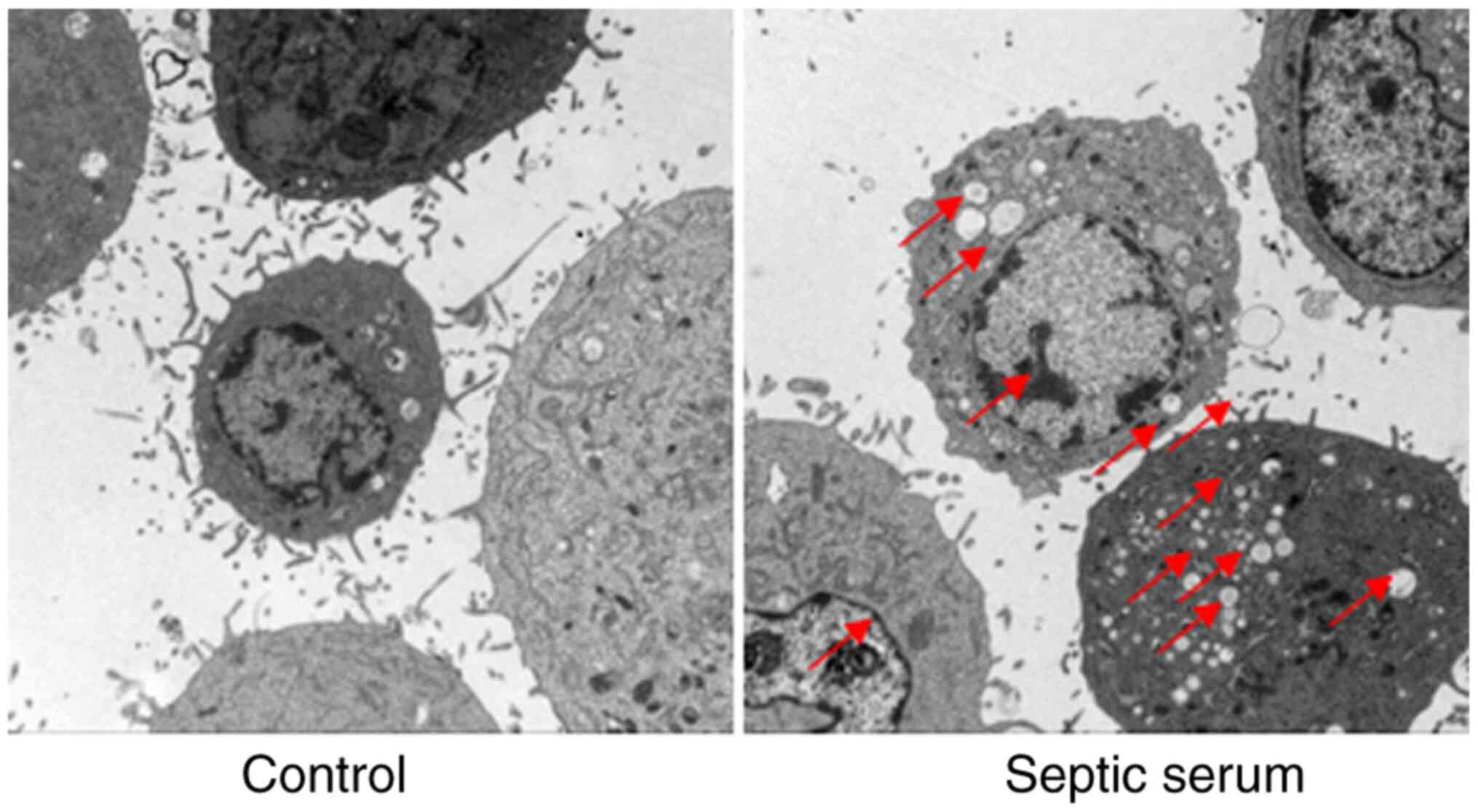
Septic serum induces HUVEC injury
To evaluate the effects of septic serum on
endothelial cell injury, HUVECs were observed under TEM following
treatment with septic serum. As presented in Fig. 2, cells treated with the normal
serum exhibited normal morphology. However, when cells were treated
with septic serum for 12 h, harmful morphological changes were
identified, including chromatin condensation, mitochondrial
vacuolization and endoplasmic reticulum degranulation. These
results further demonstrated that septic serum mediated HUVEC
injury.
Septic serum stimulates the expression of
inflammatory cytokines by HUVECs
In the present study, the mRNA and protein levels of
IL-1β, IL-6 and TNF-α were determined by RT-qPCR and ELISA,
respectively. The results demonstrated that the mRNA expression
levels of IL-1β, IL-6 and TNF-α were significantly increased
following treatment of HUVECs with septic serum compared with those
in the normal serum-treated cells (Fig. 3A-C). Consistent with these
results, ELISA demonstrated that the protein secretion levels of
IL-1β, IL-6 and TNF-α in the culture medium were significantly
increased following treatment of HUVECs with septic serum compared
with those in the medium collected from cells treated with normal
serum (Fig. 3D-F).
Septic serum promotes ROS generation,
ERK1/2 and p38 phosphorylation, and the translocation of NF-κB in
HUVECs
As demonstrated in Fig. 4A, the intracellular ROS generation
was notably enhanced in HUVECs treated with septic serum
compared with that in the control cells. Furthermore, western
blotting results demonstrated that the levels of phosphorylation of
ERK1/2, p38 and JNK, and the protein levels of NF-κB p65 in the
cell nuclei were markedly increased after cell stimulation with
septic serum for 12 h compared with those in the control group
(Fig. 4B-E). Immunofluorescence
staining also revealed that septic serum promoted NF-κB
translocation into HUVEC nuclei (Fig.
4F). Additionally, ELISA results demonstrated that the
secretion levels of IL-1β, IL-6 and TNF-α were significantly
decreased in the culture medium isolated from HUVECs pre-treated
with the ERK1/2 inhibitor PD98059, the p38 inhibitor SB203580, the
JNK inhibitor SP610025, the NF-κB inhibitor PDTC or the antioxidant
NAC for 1 h compared with those in cells treated with septic serum
alone (Fig. 5). These results
indicated that ROS, MAPKs and the NF-κB signaling pathway may be
involved in septic serum-induced inflammation in HUVECs.
 | Figure 4Septic serum increases the generation
of ROS, activates MAPKs and promotes NF-κB transduction in HUVECs.
(A) The cells were subjected to septic serum treatment for 12 h,
and ROS generation was determined by fluorescence microscopy.
Magnification, ×200. (B-E) Protein phosphorylation levels of (B)
ERK1/2, (C) p38, (D) JNK and (E) NF-κB were assayed by western
blotting. (F) The NF-κB level in the nuclei of HUVECs was detected
by immunofluo-rescence staining. Red, NF-κB subunit p65; blue,
nucleus. Data are expressed as the mean ± SEM from three
independent experiments. *P<0.05 and
**P<0.01. DCF, 2′,7′-dichlorodihydrofluororescein
diacetate; ROS, reactive oxygen species; p-, phosphorylated;
HUVECs, human umbilical vein endothelial cells. |
Discussion
Endothelial cells are considered to be a crucial
link between the cardiovascular and immune systems, and an
essential and active component of the immune response (22). In sepsis, multiple organ
dysfunction is partially caused by systemic inflammation-mediated
microvascular endothelial cell injury (23-25). Furthermore, it has been revealed
that the high levels of circulating endothelial cells and soluble
markers associated with endothelial cell damage may indicate
vascular injury, and are highly associated with severe sepsis and
high mortality (26). In the
current study, a sepsis rat model was established to investigate
the effects and mechanism of sepsis on endothelial cell injury. The
results demonstrated that treatment of HUVECs with septic serum
induced the expression of IL-1β, IL-6 and TNF-α, suggesting that
the in vitro model mimicked the in vivo
processes.
Sepsis is characterized by the activation of
inflammation via several mechanisms, including severe oxidative
stress-induced endothelial cell damage (27-29), and ROS-associated molecular
signature predicts survival in patients with sepsis (30). ROS generation occurs during the
onset of the inflammatory cascade (31). The results of the current study
demonstrated that ROS generation was involved in the inflammatory
effects of septic serum, as high levels of ROS were detected in
HUVECs following treatment with septic serum, whereas pre-treatment
with NAC significantly attenuated the sepsis-mediated expression of
the inflammatory factors IL-1β, IL-6 and TNF-α. It has been
suggested that ROS serves a crucial role in the activation of
proinflammatory mediators such as MAPKs, NF-κB and NLRP3
inflammasomes (32,33). Dysregulation of ROS generation or
insufficient ROS scavenging may result in the oxidation of a range
of biomolecules, such as hypoxia-inducible factor 1α, AMPK and
NF-κB inducing kinase, and the structural modification of proteins
triggering signaling cascades, including the MAPK, NF-κB and
PI3K/AKT signaling pathways, leading to the progression of
inflammatory diseases (34,35). In the present study, increased
levels of phosphorylated ERK1/2 and p38, and translocation of NF-κB
to the nucleus were observed following stimulation of HUVECs with
septic serum compared with those observed in the control cells.
MAPKs and the NF-κB signaling serve a pivotal role in inflammation
(17,36-40), whereas the activation of NF-κB
regulates the expression of a number of inflammatory cytokines
(41,42). In the present study, treatment of
HUVECs with selective ERK1/2, p38 MAPK and NF-κB inhibitors
significantly suppressed the septic serum-induced secretion of
inflammatory factors.
In conclusion, the results of the present study
demonstrated that septic serum mediated endothelial cell injury via
increasing ROS generation, activating MAPKs and promoting NF-κB
translocation (Fig. 6). These
results may provide a novel strategy for vascular protection and
the development of new types of antioxidants, as well as MAPK and
NF-κB inhibitors for the treatment of sepsis.
Funding
The present study was supported by the National
Training Program of Innovation and Entrepreneurship for Students of
China (grant no. 201910716019), the Research Project of the Shaanxi
University of Chinese Medicine (grant no. 2020GP19), the Scientific
Research Fund Project of Shaanxi Province Department of Education
(grant no. 19JK0228) and the Subject Innovation Team of Shaanxi
University of Chinese Medicine (grant no. 2019-QN07/132041933).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX and JZ made substantial contributions to the
conception and design of the study. YY, ZY and JX acquired,
analyzed and interpreted the data. BQ, JL, ZZ and YH interpreted
the data, drafted the article and revised it critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Institutional Animal Care and Use Committee of the Shaanxi
University of Chinese medicine (approval no. 201801115; Xianyang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Colbert JF, Schmidt EP, Faubel S and Ginde
AA: Severe sepsis outcomes among hospitalizations with inflammatory
bowel disease. Shock. 47:128–131. 2017. View Article : Google Scholar
|
|
2
|
Cepinskas G and Wilson JX: Inflammatory
response in micro-vascular endothelium in sepsis: Role of oxidants.
J Clin Biochem Nutr. 42:175–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Blasi RA, Palmisani S, Alampi D,
Mercieri M, Romano R, Collini S and Pinto G: Microvascular
dysfunction and skeletal muscle oxygenation assessed by
phase-modulation near-infrared spectroscopy in patients with septic
shock. Intensive Care Med. 31:1661–1668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990-2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar V: Sepsis roadmap: What we know,
what we learned, and where we are going. Clin Immunol.
210:1082642020. View Article : Google Scholar
|
|
6
|
Martin JB and Badeaux JE: Interpreting
Laboratory tests in infection: Making sense of biomarkers in sepsis
and systemic inflammatory response syndrome for intensive care unit
patients. Crit Care Nurs Clin North Am. 29:119–130. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edul VK, Ferrara G and Dubin A:
Microcirculatory dysfunction in sepsis. Endocr Metab Immune Disord
Drug Targets. 10:235–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bro-Jeppesen J, Johansson PI, Kjaergaard
J, Wanscher M, Ostrowski SR, Bjerre M and Hassager C: Level of
systemic inflammation and endothelial injury is associated with
cardio-vascular dysfunction and vasopressor support in post-cardiac
arrest patients. Resuscitation. 121:179–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iba T and Levy JH: Inflammation and
thrombosis: Roles of neutrophils, platelets and endothelial cells
and their interactions in thrombus formation during sepsis. J
Thromb Haemost. 16:231–241. 2018. View Article : Google Scholar
|
|
10
|
Uchimido R, Schmidt EP and Shapiro NI: The
glycocalyx: A novel diagnostic and therapeutic target in sepsis.
Crit Care. 23:162019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Houschyar KS, Pyles MN, Rein S,
Nietzschmann I, Duscher D, Maan ZN, Weissenberg K, Philipps HM,
Strauss C, Reichelt B and Siemers F: Continuous hemoadsorption with
a cytokine adsorber during sepsis-a review of the literature. Int J
Artif Organs. 40:205–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farley KS, Wang LF, Law C and Mehta S:
Alveolar macrophage inducible nitric oxide synthase-dependent
pulmonary micro-vascular endothelial cell septic barrier
dysfunction. Microvasc Res. 76:208–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berger C, Rossaint J, Van Aken H, Westphal
M, Hahnenkamp K and Zarbock A: Lidocaine reduces neutrophil
recruitment by abolishing chemokine-induced arrest and
transendothelial migration in septic patients. J Immunol.
192:367–376. 2014. View Article : Google Scholar
|
|
14
|
Lambertucci F, Motino O, Villar S, Rigalli
JP, de Luján Alvarez M, Catania VA, Martín-Sanz P, Carnovale CE,
Quiroga AD, Francés DE and Ronco MT: Benznidazole, the trypanocidal
drug used for Chagas disease, induces hepatic NRF2 activation and
attenuates the inflammatory response in a murine model of sepsis.
Toxicol Appl Pharmacol. 315:12–22. 2017. View Article : Google Scholar
|
|
15
|
Li HR, Liu J, Zhang SL, Luo T, Wu F, Dong
JH, Guo YJ and Zhao L: Corilagin ameliorates the extreme
inflammatory status in sepsis through TLR4 signaling pathways. BMC
Complement Altern Med. 17:182017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Top AP, Ince C, de Meij N, van Dijk M and
Tibboel D: Persistent low microcirculatory vessel density in
nonsurvivors of sepsis in pediatric intensive care. Crit Care Med.
39:8–13. 2011. View Article : Google Scholar
|
|
17
|
O'Sullivan AW, Wang JH and Redmond HP:
NF-kappaB and p38 MAPK inhibition improve survival in endotoxin
shock and in a cecal ligation and puncture model of sepsis in
combination with antibiotic therapy. J Surg Res. 152:46–53. 2009.
View Article : Google Scholar
|
|
18
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Xu SZ and Liu J: Fibrinopeptide A
induces C-reactive protein expression through the
ROS-ERK1/2/p38-NF-κB signal pathway in the human umbilical vascular
endothelial cells. J Cell Physiol. 234:13481–13492. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Kyriazopoulou E, Leventogiannis K,
Norrby-Teglund A, Dimopoulos G, Pantazi A, Orfanos SE, Rovina N,
Tsangaris I, Gkavogianni T, Botsa E, et al: Macrophage
activation-like syndrome: An immunological entity associated with
rapid progression to death in sepsis. BMC Med. 15:1722017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sturtzel C: Endothelial Cells. Adv Exp Med
Biol. 1003:71–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garrean S, Gao XP, Brovkovych V, Shimizu
J, Zhao YY, Vogel SM and Malik AB: Caveolin-1 regulates NF-kappaB
activation and lung inflammatory response to sepsis induced by
lipopolysaccharide. J Immunol. 177:4853–4860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCuskey RS, Nishida J, McDonnell D, Baker
GL, Urbaschek R and Urbaschek B: Effect of immunoglobulin G on the
hepatic microvascular inflammatory response during sepsis. Shock.
5:28–33. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orfanos SE, Kotanidou A, Glynos C,
Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos
S, Armaganidis A, Papapetropoulos A and Roussos C: Angiopoietin-2
is increased in severe sepsis: Correlation with inflammatory
mediators. Crit Care Med. 35:199–206. 2007. View Article : Google Scholar
|
|
26
|
Yoo JW, Moon JY, Hong SB, Lim CM, Koh Y
and Huh JW: Clinical significance of circulating endothelial cells
in patients with severe sepsis or septic shock. Infect Dis (Lond).
47:393–398. 2015. View Article : Google Scholar
|
|
27
|
Constantino L, Gonçalves RC, Giombelli VR,
Tomasi CD, Vuolo F, Kist LW, de Oliveira GM, de Bittencourt
Pasquali MA, Bogo MR, Mauad T, et al: Regulation of lung oxidative
damage by endogenous superoxide dismutase in sepsis. Intensive Care
Med Exp. 2:172014. View Article : Google Scholar
|
|
28
|
Schwalm MT, Pasquali M, Miguel SP, Dos
Santos JP, Vuolo F, Comim CM, Petronilho F, Quevedo J, Gelain DP,
Moreira JC, et al: Acute brain inflammation and oxidative damage
are related to long-term cognitive deficits and markers of
neurodegeneration in sepsis-survivor rats. Mol Neurobiol.
49:380–385. 2014. View Article : Google Scholar
|
|
29
|
Simon F and Fernández R: Early
lipopolysaccharide-induced reactive oxygen species production
evokes necrotic cell death in human umbilical vein endothelial
cells. J Hypertens. 27:1202–1216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bime C, Zhou T, Wang T, Slepian MJ, Garcia
JG and Hecker L: Reactive oxygen species-associated molecular
signature predicts survival in patients with sepsis. Pulm Circ.
6:196–201. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Forrester SJ, Kikuchi DS, Hernandes MS, Xu
Q and Griendling KK: Reactive oxygen species in metabolic and
inflammatory signaling. Circ Res. 122:877–902. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Minutoli L, Puzzolo D, Rinaldi M, Irrera
N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, et
al: ROS-mediated NLRP3 inflammasome activation in brain, heart,
kidney, and testis ischemia/reperfusion injury. Oxid Med Cell
Longev. 2016:21830262016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tejero J, Shiva S and Gladwin MT: Sources
of vascular nitric oxide and reactive oxygen species and their
regulation. Physiol Rev. 99:311–379. 2019. View Article : Google Scholar :
|
|
35
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
36
|
Ronco MT, Manarin R, Francés D, Serra E,
Revelli S and Carnovale C: Benznidazole treatment attenuates liver
NF-κB activity and MAPK in a cecal ligation and puncture model of
sepsis. Mol Immunol. 48:867–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song GY, Chung CS, Chaudry IH and Ayala A:
Immune suppression in polymicrobial sepsis: Differential regulation
of Th1 and Th2 responses by p38 MAPK. Benznidazole treatment
attenuates liver NF-κB activity and MAPK in a cecal ligation and
puncture model of sepsis. J Surg Res. 91:141–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song GY, Chung CS, Jarrar D, Chaudry IH
and Ayala A: Evolution of an immune suppressive macrophage
phenotype as a product of P38 MAPK activation in polymicrobial
sepsis. Shock. 15:42–48. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song GY, Chung CS, Jarrar D, Cioffi WG and
Ayala A: Mechanism of immune dysfunction in sepsis: Inducible
nitric oxide-meditated alterations in p38 MAPK activation. J
Trauma. 53:276–282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Y, Li YH, Wu XX, Zheng W, Guo ZH, Li
Y, Chen T, Hua ZC and Xu Q: Ethanol extract from Artemisia vestita,
a traditional Tibetan medicine, exerts anti-sepsis action through
down-regulating the MAPK and NF-κB pathways. Int J Mol Med.
17:957–962. 2006.PubMed/NCBI
|
|
41
|
Kim WH, An HJ, Kim JY, Gwon MG, Gu H, Lee
SJ, Park JY, Park KD, Han SM, Kim MK and Park KK: Apamin inhibits
TNF-α- and IFN-γ-induced inflammatory cytokines and chemokines via
suppressions of NF-κB signaling pathway and STAT in human
keratinocytes. Pharmacol Rep. 69:1030–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thoma A and Lightfoot AP: NF-κB and
inflammatory cytokine signalling: Role in skeletal muscle atrophy.
Adv Exp Med Biol. 1088:267–279. 2018. View Article : Google Scholar
|