According to global cancer statistics, gastric
cancer (GC) ranked fifth in incidence (5.7% of the total cases) and
third in mortality (8.2% of the total cancer deaths) among
malignancies worldwide in 2018 (1). When detected early, GC is usually
curable and has a 5-year survival rate of >90%; however, the
prognosis of patients with advanced GC remains poor (2). The lack of early clinical symptoms
often delays the diagnosis of GC, resulting in the development of
an incurable disease in a number of patients (3). Another reason for the high mortality
is that surgical resection is the only curative treatment for GC
(4). Despite the availability of
a number of novel therapies, including targeted therapy and
immunotherapy (3,5), the treatment of GC remains
unsatisfactory. Therefore, elucidating the underlying mechanisms
underlying tumor progression may help develop more effective
treatments.
The tumor microenvironment (TME) is a complex and
dynamic cellular community composed of cancer cells, endothelial
cells, fibroblasts, immune cells, and mesenchymal stem cells (MSCs)
(6). The TME is formed via the
recruitment of tumor-supporting MSCs and extensive remodeling of
adjacent tissues; thus, the TME differs from normal tissues in
numerous aspects, including the extracellular matrix, blood vessels
and phenotypes of cells (7). The
interaction between tumor cells and the TME serves an important
role in tumor initiation, progression, chemoresistance and immune
escape, and certain molecules present in the TME are prognosis
predictors in various types of cancer, such as pancreatic cancer,
GC and urothelial carcinoma (8-11).
MSCs, which are important components of the TME and serve critical
roles in tumor progression, have been extensively studied.
MSCs, also known as mesenchymal stromal cells, are
multipotent stromal cells with the ability to differentiate into
osteoblasts, adipocytes, chondrocytes and other types of cells
under different conditions (12).
Despite extensive research efforts, the multi-differentiation
potential of MSCs has only been demonstrated in vitro, and
there are few studies describing their characteristics in
vivo (13,14). MSCs can accelerate wound healing
by regulating inflammation (15).
Additionally, MSCs can suppress the function of both innate and
adaptive immune cells, including macrophages and lymphocytes
(15). In cases of weak
inflammatory responses, MSCs can act as antigen-presenting cells
and increase the immune response (15). In cancer, MSCs are critical
components of the TME and promote the progression of several types
of tumor, such as hepatocarcinoma and colorectal cancer (16,17). Tumors are considered to be 'wounds
that never heal', and MSCs can migrate to injured tissues,
supporting the tumor tropism of MSCs and their ability to sense
wound-associated signals (18).
Upon recruitment to tumors, MSCs are converted into
tumor-associated MSCs (TA-MSCs), which can promote tumor
progression more potently (19).
TA-MSCs interact with tumor cells and are involved in the
remodeling of the TME in response to signals from tumor cells and
other stromal cells (19,20). The crosstalk between TA-MSCs and
tumor cells can be mediated by several mechanisms, including
paracrine signals, exosomes and direct contact (21,22). Furthermore, TA-MSCs can
differentiate into cancer-associated fibroblasts, which promote
tumor progression (23).
In the present review, the effect of GC-derived
MSC-like cells (GC-MSCs) on GC progression, metastasis,
chemo-resistance and immune escape are described. Additionally, the
mechanisms by which GC cells, immune cells and other stromal cells
educate MSCs and skew MSCs towards the GC-MSC fate are discussed.
Finally, the therapeutic potential of GC-MSCs as both targets and
biomarkers are summarized.
Studies have revealed that BM-MSCs have tropism to
several types of tumor, such as glioma and breast cancer, by
intravenous or intraperitoneal injection (31,32). CXC-chemokine ligand 16 (CXCL16)
secreted by breast cancer cells binds to its receptor CXC-chemokine
receptor 6 (CXCR6) on BM-MSCs, which in turn produce CXCL10,
thereby promoting the recruitment of BM-MSCs to cancer cells
(33). The combination of CXCL12
and CXCR6 facilitates the recruitment of BM-MSCs to prostate tumors
(34). Other chemokines and
growth factors also participate in the migration of MSCs from
non-cancer to cancer tissues, such as transforming growth factor-β
(TGF-β), platelet-derived growth factor, monocyte chemoattractant
protein-1 and stromal cell-derived factor-1 (35-37). Hypoxia in the TME induces BM-MSCs
tropism to breast cancer (33).
Thus, there are various mechanisms involved in the migration of
MSCs from non-cancer to tumor tissues. In GC, Berger et al
(38) performed a 'plug assay',
in which GC and lung carcinoma cell-derived microvesicles (MVs)
were collected and used as a Matrigel plug, which was implanted
into teratoma tissues; 6-10 days after the injection, the plug was
harvested and subjected to histological analysis or dissociated
into a single cell suspension. The results revealed that MSCs were
present in the plug containing GC cell-derived MVs, whereas the
control group did not exhibit MSCs, suggesting that GC cell-derived
MVs are responsible for the migration of MSCs, although the
underlying mechanism remains unknown (38). To the best of our knowledge, there
are no studies investigating the tropism of MSCs from non-GC to GC
tissues, and additional studies are required to understand this
migration and to identify key cytokines or chemokines.
After their recruitment to the tumor site, MSCs from
non-cancer tissues are converted into TA-MSCs under the influence
of tumor cells, immune cells, local TA-MSCs and other stromal cells
(34). In GC, tumor cells are
involved in the malignant transition of MSCs through several
mechanisms (Table I). For
example, Shamai et al (39) revealed that GC cells have the
capacity to increase R-spondin expression in GC-MSCs, and GC-MSCs
can in turn upregulate Lgr5 expression in GC cells. Therefore,
tumor cells can alter gene expression in GC-MSCs and GC-MSCs can
induce tumor cell stemness. Exosomes from GC cells regulate the
immunomodulatory properties of adipose-derived MSCs by activating
the NF-κB signaling pathway (40). GC-MSCs exposed to GC cell-derived
exosomes can activate both macrophages and T lymphocytes, thus
maintaining the inflammatory TME and promoting tumor progressio
(40). MicroRNAs (miRNAs/miRs)
are involved in the malignant transition of MSCs from non-GC
tissues. The downregulation of miR-155-5p expression caused by the
activation of NF-κB signaling in GC-MSCs promotes this process, as
demonstrated by the effect of miR-155-5p inhibition on conferring
BM-MSCs a GC-MSC-like phenotype and function (41). Unlike miR-155-5p, miR-374
expression is upregulated in GC-MSCs, and overexpression of miR-374
promotes the proliferation and migration of MSCs from normal
gastric tissues by regulating the Wnt5a/β-catenin signaling pathway
(42,43). Additionally, immune cells can
participate in the malignant transition of MSCs. Macrophages
activate umbilical cord-derived MSCs and confer these MSCs a
pro-inflammatory phenotype, which can promote GC progression partly
through the NF-κB signaling pathway, as demonstrated by in
vitro and in vivo experiments (44). CD4+ T lymphocytes may
also be involved in this process; although the effect of
CD4+ T cells on the malignant transition of MSCs has not
been investigated, these cells upregulate the expression levels of
PD-L1 in GC-MSCs through the p-STAT3 signaling pathway, and GC-MSCs
with positive PDL1 expression protect tumor cells from immune cells
(45). Patients with GC infected
with Helicobacter pylori have a high mortality rate because
this bacterium confers MSCs a pro-inflammatory phenotype through
the activation of the NF-κB signaling pathway (46). Overall, these findings suggest
that GC cells, as well as immune cells and bacteria, can convert
BM-MSCs into GC-MSCs (Fig.
1B).
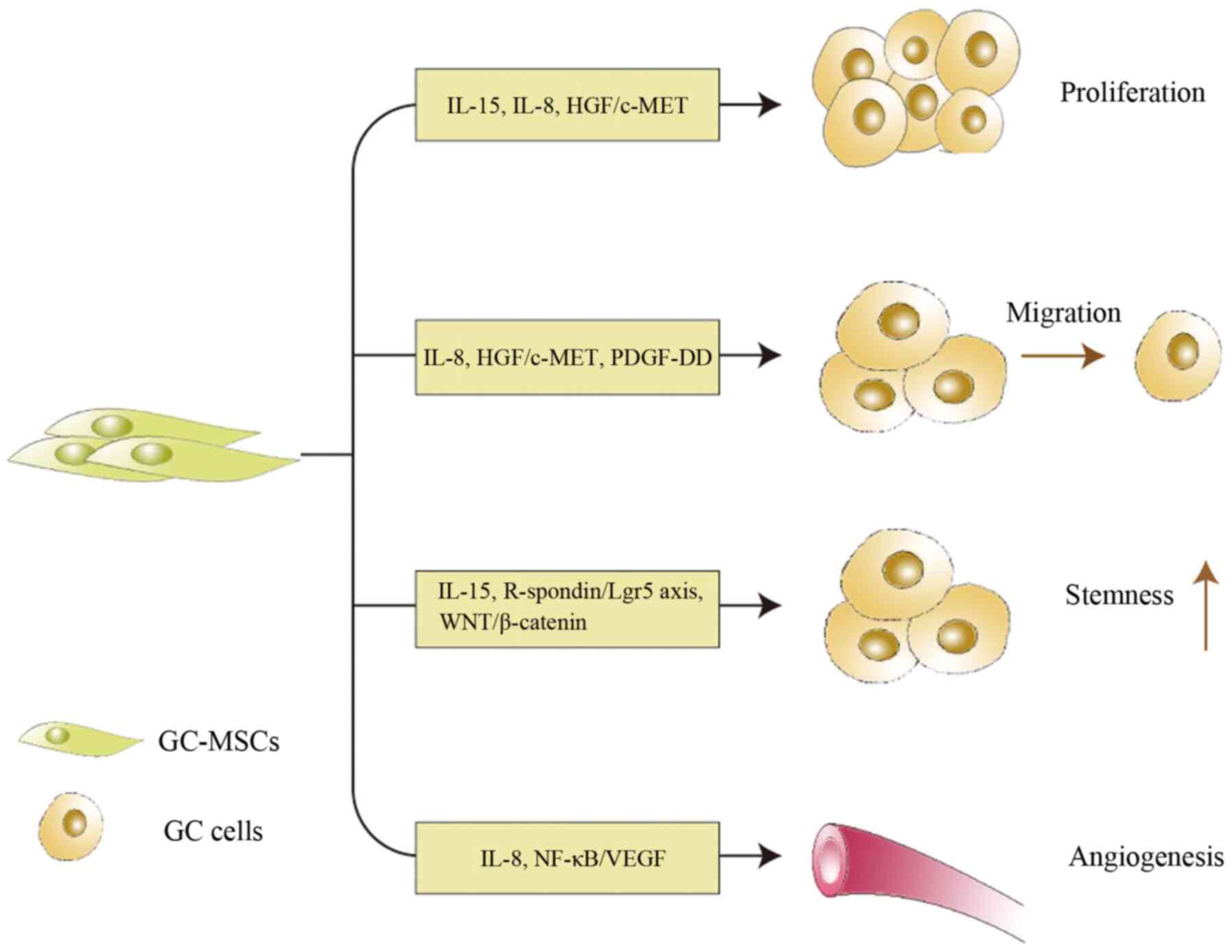
TA-MSCs promote tumor progression by secreting
cytokines, which act directly on tumor cells or other stromal cells
in the TME (47-49). GC-MSCs can promote GC progression
through various mechanisms (Fig.
2). For example, IL-15 secreted by GC-MSCs induces GC cell
epithelial-mesenchymal transition and promotes GC cell migration,
which are associated with tumor growth and metastasis, respectively
(28). Consistently, IL-15 levels
are higher in patients with GC than in healthy donors in both serum
and tissues in association with lymph node metastasis (28). In addition, GC-MSCs produce high
levels of IL-8, which serves a role in tumor cell proliferation and
migration, and in angiogenesis (30). However, the tumor-promoting
ability of IL-8 has only been demonstrated in vitro and the
underlying mechanisms remain unknown. In addition to cytokines,
other molecules serve roles in the tumor-promoting effect of
GC-MSCs. The interaction between GC cells and GC-MSCs maintains GC
cell stemness through the activation of the R-spondin/Lgr5 axis and
WNT/β-catenin signaling pathway (39). Hepatocyte growth factor (HGF)
exclusively secreted by GC-MSCs promotes the proliferation and
migration of GC cells through the activation of the HGF/c-MET
signaling pathway (38).
Furthermore, platelet-derived growth factor-DD (PDGF-DD) secreted
by GC-MSCs increases the phosphorylation of PDGF receptor-β in GC
cells, thus promoting GC cell proliferation and migration;
recombinant PDGF-DD can mimic the tumor-promoting effect of GC-MSCs
conditioned medium (CM) on GC cell proliferation and migration
(50). GC-MSCs induce VEGF
expression in GC cells in vitro and in vivo, and
contribute to GC-MSC-mediated angiogenesis by activating the
NF-κB/VEGF signaling pathway (51). Since tumor neovascularization is
indispensable for continuous tumor growth, this pathway may be a
potential target to inhibit tumor growth. Notably, lung carcinoma
cell proliferation is independent of lung carcinoma MSCs, whereas
GC cell proliferation is critically dependent on the presence of
their counterparts GC-MSCs (38).
This phenomenon suggests that tumor growth does not always depend
on the counterpart TA-MSCs. The interaction between tumor cells and
TA-MSCs is specific not only for the requirements of the tumor
cells, but also their capacity to recruit MSCs and educate them to
further promote tumor progression (38). Overall, these results demonstrate
that GC-MSCs serve essential roles in GC progression.
Chemotherapy is the first-line treatment for
patients with advanced GC; pre- or post-operative chemotherapy with
5-fluorouracil (5-FU) and cisplatin has improved the survival rates
of patients with GC (52-54). However, the response rate for this
therapy is limited by the development of chemoresistance (55,56). Therefore, it is urgent to
investigate the mechanisms underlying GC cell chemoresistance. The
TME can protect tumors from chemo-therapy through physical barriers
and metabolites, exosomes and other substances secreted by tumor
stromal cells (57). One of the
key components of the TME, TA-MSCs, induces tumor cell
chemoresistance through various mechanism, such as elevating tumor
cell stemness and secreting certain molecules, such as interleukins
and chemokines (58-60). Cancer stem cells (CSCs), also
known as cancer initiation cells, were identified based on the
observation of histological heterogeneity in tumors and on the fact
that a single mouse tumor cell can form a new tumor (61). Although the definition of CSC is
contentious, these cells have self-renewal and differentiation
capacities and sustain the growth of tumors (62,63), which is associated with tumor cell
chemoresistance. In GC, He et al (64) suggested that MSCs promote GC cell
stemness and chemoresistance through fatty acid oxidation (FAO)
based on in vitro and in vivo experiments.
Mechanistically, TGF-β1 secreted by MSCs activates SMAD2/3 through
TGF-β recep-tors, which then induces lncRNA MACC1-AS1 expression in
GC cells and promotes FAO-dependent stemness and chemoresistance by
antagonizing miR-145-5p (64).
GC-MSCs express high levels of the ATP-binding cassette subfamily B
member 1 transporter, which results in decreased drug accumulation
in chemoresistant cells (39).
Exosomes secreted by GC-MSCs induce GC cell resistance to 5-FU by
activating calcium/calmodulin-dependent protein kinases and the
Raf/MEK/ERK kinase cascade, thus upregulating the expression levels
of multi-drug resistance-associated proteins (65). The aforementioned studies indicate
that GC-MSCs can induce GC cell chemoresistance by secreting
cytokines and exosomes, although there may be other undiscovered
mechanisms that may help develop strategies to overcome
chemoresistance.
The number and types of inflammatory factors in the
TME can alter the immune responses to tumors, although the
underlying mechanisms remain obscure. MSCs modulate immune cells
and can suppress the immune response; however, they can also
promote immune responses when inflammatory conditions are not
enough (66). Additionally,
GC-MSCs can modulate antitumor immunity by interacting with immune
cells, such as macro-phages, neutrophils and T lymphocytes.
Macrophages are associated with a poor prognosis of GC and are used
as prognostic indicators (67).
Li et al (68) suggested
that GC-MSCs may trigger the polarization and generation of M2-like
macro-phages by activating the JAK2/STAT3 signaling pathway via
high secretion of IL-6/IL-8. M2-like macrophages can facilitate the
metastasis and progression of GC by enhancing
epithelial-mesenchymal transition in GC cells (68). Exosomes extracted from the GC AGS
cell line can induce macrophage phagocytosis and promote the
secretion of pro-inflammatory factors, thereby activating CD69 and
CD25 on the surface of T cells through the NF-κB signaling pathway
in MSCs (40). Regarding
neutrophils, there is a reciprocal interaction between GC-MSCs and
neutrophils. GC-MSCs can induce chemotaxis and neutrophil
activation, as well as suppress neutrophil spontaneous apoptosis
through the activation of the STAT3 and ERK1/2 signaling pathways
(29). Neutrophils incubated with
GC-MSCs or GC-MSCs-CM can promote the migration of tumor cells and
induce the formation of tube-like structures in endothelial cells
(29). Furthermore,
GC-MSC-treated neutrophils can in turn convert normal MSCs into
tumor-associated fibroblasts (30). GC-MSCs-CM pretreatment reverses
the inhibitory effect of peripheral blood mononuclear cells and
promotes GC liver metastases by disrupting the balance of
regulatory T cells and T helper 17 cells (69). Both innate and adaptive immune
cells can be affected by GC-MSCs, and they can gain tumor-promoting
abilities or be inhibited.
In past years, immune checkpoints, including
programmed cell death protein 1 (PD-1)/programmed cell death 1
ligand 1 (PD-L1), cytotoxic T-lymphocyte-associated protein 4 and
T-cell immunoglobulin domain and mucin domain-3, have attracted
increasing attention for their ability to weaken the function of T
lymphocytes and induce tumor immune escape. Blocking the
interaction between PD1 and PD-L1 has exhibited promising results
in the treatment of several types of cancer, including breast
cancer and squamous cell carcinoma of the head and neck (70,71). In GC, pembrolizumab, a PD-1
inhibitor, has shown good results in phase I/II trials, with
objective response rates of 11.6-25.8% and low toxicity (72,73), and the combination of nivolumab
and regorafenib, which respectively inhibit PD-1 or VEFGR tyrosine
kinase, also exhibits antitumor activity (74). However, the response to this
therapy cannot be predicted due to a lack of effective biomarkers
(72). GC-MSCs upregulate PD-L1
expression in GC cells by secreting IL-8, which can regulate the
STAT3 and mTOR signaling pathways (75). The role of GC-MSCs in regulating
PD-L1 in GC cells has not been investigated extensively. Further
investigation of the involvement of GC-MSCs in the regulation of
PD-L1 expression and elucidation of the underlying mechanism may
help the development of anti-PD-L1 therapy and may provide novel
biomarkers.
Since MSCs can be isolated from bone marrow and
adipose tissues, they have been used in numerous studies in past
years (76,77). MSCs from non-cancer tissues can
suppress immune responses and have immune evasion properties; they
are therefore stable in an allogeneic environment and display
promise for cell therapy (78).
For instance, exosomes from MSCs from non-GC tissues and MSCs
themselves can serve as vehicles to transport miRNAs or drugs and
cytokines to tumors, thus suppressing tumor progression, according
to laboratory tests and clinic trials (79-81).
Unlike MSCs from non-GC tissues, GC-MSCs cannot be
used as drug carriers due to their critical role in tumor
progression. Instead, they should be specifically targeted to
eliminate their effect on tumor cell proliferation, drug-resistance
and migration. However, GC-MSCs have no specific surface markers,
and therefore they cannot be targeted without affecting other
cells. Only the substances released by GC-MSCs can serve as targets
to suppress their tumor-promoting capability. For example, GC-MSCs
can induce chemoresistance in tumor cells through FAO, and
inhibition of FAO attenuates this phenomenon, suggesting that FAO
may be a potential target to reduce chemoresistance in GC (64). Similarly, GC-MSCs-CM treatment can
promote tumor cell proliferation and migration and increase
pro-angiogenic abilities through the secretion of IL-8; therefore,
the use of an IL-8 neutralizing antibody may suppress the effects
of GC-MSCs (30). In addition,
IL-8 produced by GC-MSCs upregulates PD-L1 expression in tumor
cells and can thus induce tumor cell resistance to CD8+
T cells, and inhibition of IL-8 can eliminate resistance (75). The afore-mentioned studies
indicate that target key modulators in the tumor-promoting process
of GC-MSCs can suppress tumor progression and chemoresistance.
Furthermore, this strategy may be effective in combination with
other therapies, such as chemotherapy and anti-PD-L1 therapy.
Furthermore, miRNAs and cytokines secreted by
GC-MSCs can predict prognosis. For instance, GC-MSCs have higher
expression levels of miR-214, miR-221 and miR-222 than GCN-MSCs,
and high expression levels of miR-221 and miR-222, or miR-214 and
miR-222 in the tissues of patients with GC are positively
associated with lymph node metastasis and serosal invasion,
respectively (82). IL-15 in the
GC microenvironment is mostly derived from GC-MSCs and is
associated with lymph node metastasis (28). GC-MSCs can secrete high levels of
IL-8, which predicts a poor prognosis in patients with GC (30). In summary, GC-MSCs can potentially
be developed into novel therapies and prognostic biomarkers.
In past years, the interaction between MSCs and
tumors has gained increasing attention. The present review focused
on the transformation of MSCs from non-GC tissues into GC-MSCs and
the role of GC-MSCs in tumor progression, chemoresistance and
immune escape. In addition to GC cells, immune cells and bacteria
can be involved in the malignant transformation of MSCs into
GC-MSCs. GC-MSCs can in turn promote tumor progression, induce
chemoresistance and confer immune cells a tumor-promoting
phenotype. Regarding their therapeutic potential, the upstream or
downstream modulators of GC-MSCs can serve as targets to weaken
their effect on tumor progression. However, the interaction between
other stromal cells and GC-MSCs, and the underlying mechanisms
require further investigation, including the roles of natural
killer cells and endothelial cells. In addition, specific surface
markers of GC-MSCs remain to be identified, which may facilitate
the specific targeting of GC-MSCs without affecting other cells.
The association between GC-MSCs and PD-L1 should be investigated
further, as it may provide new insight into PD-L1 co-therapy.
Despite numerous advances in the understanding of the effect of
GC-MSCs on tumor progression, elucidating the function and
underlying mechanisms of GC-MSCs may provide valuable information
to improve the treatment of GC.
The present study was supported by the National
Science Foundation of China (grant nos. 81972313, 81972822 and
81772641), the Wu Jieping Medical Foundation (grant no.
320.6750.19060) and the Bethune Charitable Foundation (grant no.
G-X-2019-0101-12).
Not applicable.
JS and WZ reviewed the literature on the role of
GC-MSCs in tumor progression and the tumor microenvironment, and
wrote most of the review. WZ discussed the role of GC-MSCs in GC
treatment. Both authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki H, Oda I, Abe S, Sekiguchi M, Mori
G, Nonaka S, Yoshinaga S and Saito Y: High rate of 5-year survival
among patients with early gastric cancer undergoing curative
endoscopic submucosal dissection. Gastric Cancer. 19:198–205. 2016.
View Article : Google Scholar
|
|
3
|
Hsu A, Chudasama R, Almhanna K and Raufi
A: Targeted therapies for gastroesophageal cancers. Ann Transl Med.
8:11042020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hunt RH, Camilleri M, Crowe SE, El-Omar
EM, Fox JG, Kuipers EJ, Malfertheiner P, McColl KE, Pritchard DM,
Rugge M, et al: The stomach in health and disease. Gut.
64:1650–1668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dolcetti R, De Re V and Canzonieri V:
Immunotherapy for Gastric Cancer: Time for a personalized approach?
Int J Mol Sci. 19:16022018. View Article : Google Scholar :
|
|
6
|
Cheng HS, Lee JXT, Wahli W and Tan NS:
Exploiting vulnerabilities of cancer by targeting nuclear receptors
of stromal cells in tumor microenvironment. Mol Cancer. 18:512019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou ZX and Lu ZR: Molecular imaging of
the tumor microen-vironment. Adv Drug Deliver Rev. 113:24–48. 2017.
View Article : Google Scholar
|
|
8
|
Denton AE, Roberts EW and Fearon DT:
Stromal Cells in the Tumor Microenvironment. Adv Exp Med Biol.
1060:99–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhan HX, Zhou B, Cheng YG, Xu JW, Wang L,
Zhang GY and Hu SY: Crosstalk between stromal cells and cancer
cells in pancreatic cancer: New insights into stromal biology.
Cancer Lett. 392:83–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen DX, Chen G, Jiang W, Fu M, Liu W, Sui
J, Xu S, Liu Z, Zheng X, Chi L, et al: Association of the collagen
signature in the tumor microenvironment with lymph node metastasis
in early gastric cancer. JAMA Surg. 154:e1852492019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hodgson A, Xu B, Satkunasivam R and Downes
MR: Tumour front inflammation and necrosis are independent
prognostic predictors in high-grade urothelial carcinoma of the
bladder. J Clin Pathol. 71:154–160. 2018. View Article : Google Scholar
|
|
12
|
Patil S and Singh N: Spatially controlled
functional group grafting of silk films to induce osteogenic and
chondrogenic differentiation of human mesenchymal stem cells. Mater
Sci Eng C Mater Biol Appl. 91:796–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghaneialvar H, Soltani L, Rahmani HR,
Lotfi AS and Soleimani M: Characterization and classification of
mesenchymal stem cells in several species using surface markers for
cell therapy purposes. Indian J Clin Biochem. 33:46–52. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schafer R and Bieback K: Characterization
of mesenchymal stem or stromal cells: Tissue sources,
heterogeneity, and function. Transfusion. 56:S2–S5. 2016.
View Article : Google Scholar
|
|
15
|
Wang Y, Chen X, Cao W and Shi Y:
Plasticity of mesenchymal stem cells in immunomodulation:
Pathological and therapeutic implications. Nat Immunol.
15:1009–1016. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zong C, Zhang HJ, Yang X, Gao L, Hou J, Ye
F, Jiang J, Yang Y, Li R, Han Z and Wei L: The distinct roles of
mesenchymal stem cells in the initial and progressive stage of
hepatocarcinoma. Cell Death Dis. 9:3452018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Hu FY, Li G, Li G, Yang X, Liu L,
Zhang R, Zhang B and Feng Y: Human colorectal cancer-derived
mesenchymal stem cells promote colorectal cancer progression
through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 9:252018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Ma L, Zhang N, Zhu Y, Zhang K, Xu
Z and Wang Q: Mesenchymal stem cells promote tumor progression via
inducing stroma remodeling on rabbit VX2 bladder tumor model. Int J
Biol Sci. 14:1012–1021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao T, Yu Y, Cong Q, Wang Y, Sun M, Yao L,
Xu C and Jiang W: Human mesenchymal stem cells in the tumour
micro-environment promote ovarian cancer progression: The role of
platelet-activating factor. BMC Cancer. 18:9992018. View Article : Google Scholar
|
|
21
|
Shojaei S, Hashemi SM, Ghanbarian H,
Salehi M and Mohammadi-Yeganeh S: Effect of mesenchymal stem
cells-derived exosomes on tumor microenvironment: Tumor progression
versus tumor suppression. J Cell Physiol. 234:3394–3409. 2019.
View Article : Google Scholar
|
|
22
|
Worner PM, Schachtele DJ, Barabadi Z,
Srivastav S, Chandrasekar B, Izadpanah R and Alt EU: Breast tumor
micro-environment can transform naive mesenchymal stem cells into
tumor-forming cells in nude mice. Stem Cells Dev. 28:341–352. 2019.
View Article : Google Scholar
|
|
23
|
Shi Y, Du L, Lin L and Wang Y:
Tumour-associated mesenchymal stem/stromal cells: Emerging
therapeutic targets. Nat Rev Drug Discov. 16:35–52. 2017.
View Article : Google Scholar
|
|
24
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kine. 3:393–403. 1970.
|
|
25
|
Mushahary D, Spittler A, Kasper C, Weber V
and Charwat V: Isolation, cultivation, and characterization of
human mesen-chymal stem cells. Cytometry A. 93:19–31. 2018.
View Article : Google Scholar
|
|
26
|
Xu X, Zhang X, Wang S, Qian H, Zhu W, Cao
H, Wang M, Chen Y and Xu W: Isolation and comparison of mesenchymal
stem-like cells from human gastric cancer and adjacent
non-cancerous tissues. J Cancer Res Clin. 137:495–504. 2011.
View Article : Google Scholar
|
|
27
|
Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H,
Zhang X and Xu X, Li J, Chen Z and Xu X: Mesenchymal stem cell-like
cells derived from human gastric cancer tissues. Cancer Lett.
274:61–71. 2009. View Article : Google Scholar
|
|
28
|
Sun L, Wang Q, Chen B, Zhao Y, Shen B,
Wang X, Zhu M, Li Z, Zhao X, Xu C, et al: Human gastric cancer
mesenchymal stem cell-derived IL15 contributes to tumor cell
epithelial-mesenchymal transition via upregulation tregs ratio and
PD-1 expression in CD4 + T cell. Stem Cells Dev.
27:1203–1214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan
X, Mao F, Wang M, Zhu W, Qian H and Xu W: The IL-6-STAT3 axis
mediates a reciprocal crosstalk between cancer-derived mesenchymal
stem cells and neutrophils to synergistically prompt gastric cancer
progression. Cell Death Dis. 5:e12952014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W, Zhou Y, Yang J, Zhang X, Zhang H,
Zhang T, Zhao S, Zheng P, Huo J and Wu H: Gastric cancer-derived
mesenchymal stem cells prompt gastric cancer progression through
secretion of interleukin-8. J Exp Clin Cancer Res. 34:522015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao M, Mao J, Duan X, Lu L, Zhang F, Lin
B, Chen M, Zheng C, Zhang X and Shen J: In vivo tracking of the
tropism of mesenchymal stem cells to malignant gliomas using
reporter gene-based MR imaging. Int J Cancer. 142:1033–1046. 2018.
View Article : Google Scholar
|
|
32
|
Chen Y, He Y, Wang X, Lu F and Gao J:
Adiposederived mesenchymal stem cells exhibit tumor tropism and
promote tumorsphere formation of breast cancer cells. Oncol Rep.
41:2126–2136. 2019.PubMed/NCBI
|
|
33
|
Chaturvedi P, Gilkes DM, Takano N and
Semenza GL: Hypoxia-inducible factor-dependent signaling between
triple-negative breast cancer cells and mesenchymal stem cells
promotes macrophage recruitment. Proc Natl Acad Sci USA.
111:E2120–E2129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung YH, Kim JK, Shiozawa Y, Wang J,
Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, et al:
Recruitment of mesenchymal stem cells into prostate tumours
promotes metastasis. Nat Commun. 4:17952013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Camorani S, Hill BS, Fontanella R, Greco
A, Gramanzini M, Auletta L, Gargiulo S, Albanese S, Lucarelli E,
Cerchia L and Zannetti A: Inhibition of bone marrow-derived
mesenchymal stem cells homing towards triple-negative breast cancer
micro-environment using an Anti-PDGFRβ Aptamer. Theranostics.
7:3595–3607. 2017. View Article : Google Scholar :
|
|
36
|
Barcellos-de-Souza P, Comito G,
Pons-Segura C, Taddei ML, Gori V, Becherucci V, Bambi F, Margheri
F, Laurenzana A, Del Rosso M and Chiarugi P: Mesenchymal stem cells
are recruited and activated into carcinoma-associated fibroblasts
by prostate cancer microenvironment-derived TGF-β1. Stem Cells.
34:2536–2547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pavon LF, Sibov TT, de Souza AV, da Cruz
EF, Malheiros SMF, Cabral FR, de Souza JG, Boufleur P, de Oliveira
DM, de Toledo SRC, et al: Tropism of mesenchymal stem cell toward
CD133+ stem cell of glioblastoma in vitro and promote
tumor proliferation in vivo. Stem Cell Res Ther. 9:3102018.
View Article : Google Scholar
|
|
38
|
Berger L, Shamai Y, Skorecki KL and
Tzukerman M: Tumor specific recruitment and reprogramming of
mesenchymal stem cells in tumorigenesis. Stem Cells. 34:1011–1026.
2016. View Article : Google Scholar
|
|
39
|
Shamai Y, Alperovich DC, Yakhini Z,
Skorecki K and Tzukerman M: Reciprocal reprogramming of cancer
cells and associated mesenchymal stem cells in gastric cancer. Stem
Cells. 37:176–189. 2019. View Article : Google Scholar
|
|
40
|
Shen Y, Xue C, Li X, Ba L, Gu J, Sun Z,
Han Q and Zhao RC: Effects of gastric cancer cell-derived exosomes
on the immune regulation of mesenchymal stem cells by the NF-κB
signaling pathway. Stem Cells Dev. 28:464–476. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu M, Wang M, Yang F, Tian Y, Cai J, Yang
H, Fu H, Mao F, Zhu W, Qian H and Xu W: miR-155-5p inhibition
promotes the transition of bone marrow mesenchymal stem cells to
gastric cancer tissue derived MSC-like cells via NF-κ B p65
activation. Oncotarget. 7:16567–16580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji RB, Zhang X, Qian H, Gu H, Sun Z, Mao
F, Yan Y, Chen J, Liang Z and Xu W: miR-374 mediates the malignant
transformation of gastric cancer-associated mesenchymal stem cells
in an experimental rat model. Oncol Rep. 38:1473–1481. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun Z, Chen J, Zhang J, Ji R, Xu W, Zhang
X and Qian H: The role and mechanism of miR-374 regulating the
malignant transformation of mesenchymal stem cells. Am J Transl
Res. 10:3224–3232. 2018.PubMed/NCBI
|
|
44
|
Yang T, Zhang X, Wang M, Zhang J, Huang F,
Cai J, Zhang Q, Mao F, Zhu W, Qian H and Xu W: Activation of
mesenchymal stem cells by macrophages prompts human gastric cancer
growth through NF-κ B pathway. PLoS One. 9:e975692014. View Article : Google Scholar
|
|
45
|
Xu R, Zhao X, Zhao Y, Chen B, Sun L, Xu C,
Shen B, Wang M, Xu W and Zhu W: Enhanced gastric cancer growth
potential of mesenchymal stem cells derived from gastric cancer
tissues educated by CD4+ T cells. Cell Prolif.
51:e123992018. View Article : Google Scholar
|
|
46
|
Zhang Q, Ding J, Liu J, Wang W, Zhang F,
Wang J and Li Y: Helicobacter pylori-infected MSCs acquire a
pro-inflammatory phenotype and induce human gastric cancer
migration by promoting EMT in gastric cancer cells. Oncol Lett.
11:449–457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vafaei R, Nassiri SM and Siavashi V:
3-Adrenergic regulation of EPC features through manipulation of the
bone marrow MSC niche. J Cell Biochem. 118:4753–4761. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang Y, Bucan V, Baehre H, Von der Ohe J,
Otte A and Hass R: Acquisition of new tumor cell properties by
MSC-derived exosomes. Int J Oncol. 47:244–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Whiteside TL: Exosome and mesenchymal stem
cell cross-talk in the tumor microenvironment. Semin Immunol.
35:69–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang F, Wang M, Yang T, Cai J, Zhang Q,
Sun Z, Wu X, Zhang X, Zhu W, Qian H and Xu W: Gastric
cancer-derived MSC-secreted PDGF-DD promotes gastric cancer
progression. J Cancer Res Clin. 140:1835–1848. 2014. View Article : Google Scholar
|
|
51
|
Huang F, Yao Y, Wu J, Liu Q, Zhang J, Pu
X, Zhang Q and Xia L: Curcumin inhibits gastric cancer-derived
mesenchymal stem cells mediated angiogenesis by regulating
NF-κB/VEGF signaling. Am J Transl Res. 9:5538–5547. 2017.
|
|
52
|
Sun J, Song Y, Wang Z, Chen X, Gao P, Xu
Y, Zhou B and Xu H: Clinical significance of palliative gastrectomy
on the survival of patients with incurable advanced gastric cancer:
A systematic review and meta-analysis. BMC Cancer. 13:5772013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kwee RM and Kwee TC: Role of imaging in
predicting response to neoadjuvant chemotherapy in gastric cancer.
World J Gastroenterol. 20:1650–1656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shitara K: Chemotherapy for advanced
gastric cancer: Future perspective in Japan. Gastric Cancer.
20:102–110. 2017. View Article : Google Scholar
|
|
56
|
Roberto M, Romiti A, Onesti CE, Zullo A,
Falcone R and Marchetti P: Evolving treatments for advanced gastric
cancer: Appraisal of the survival trend. Expert Rev Anticancer
Ther. 16:717–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
McMillin DW, Negri JM and Mitsiades CS:
The role of tumour-stromal interactions in modifying drug response:
Challenges and opportunities. Nat Rev Drug Discov. 12:217–228.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zeng J, Chen S, Li C, Ye Z, Lin B, Liang
Y, Wang B, Ma Y, Chai X, Zhang X, et al: Mesenchymal stem/stromal
cells-derived IL-6 promotes nasopharyngeal carcinoma growth and
resistance to cisplatin via upregulating CD73 expression. J Cancer.
11:2068–2079. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Le Naour A, Prat M, Thibault B, Mével R,
Lemaitre L, Leray H, Joubert MV, Coulson K, Golzio M, Lefevre L, et
al: Tumor cells educate mesenchymal stromal cells to release
chemoprotective and immunomodulatory factors. J Mol Cell Biol.
12:202–215. 2020. View Article : Google Scholar :
|
|
60
|
Pasquier J, Gosset M, Geyl C,
Hoarau-Véchot J, Chevrot A, Pocard M, Mirshahi M, Lis R, Rafii A
and Touboul C: CCL2/CCL5 secreted by the stroma induce IL-6/PYK2
depen-dent chemoresistance in ovarian cancer. Mol Cancer.
17:472018. View Article : Google Scholar
|
|
61
|
Wang X: Stem cells in tissues, organoids,
and cancers. Cell Mol Life Sci. 76:4043–4070. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Polejaeva IA, Chen SH, Vaught TD, Page RL,
Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, et al:
Cloned pigs produced by nuclear transfer from adult somatic cells.
Nature. 407:86–90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
He W, Liang B, Wang C, Li S, Zhao Y, Huang
Q, Liu Z, Yao Z, Wu Q, Liao W, et al: MSC-regulated lncRNA
MACC1-AS1 promotes stemness and chemoresistance through fatty acid
oxidation in gastric cancer. Oncogene. 38:4637–4654. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan
Y, Wang M, Zhu W, Qian H and Xu W: Exosomes derived from human
mesenchymal stem cells confer drug resistance in gastric cancer.
Cell Cycle. 14:2473–2483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chaudhary D, Trivedi RN, Kathuria A,
Goswami TK, Khandia R and Munjal A: In vitro and in vivo
immunomodulating properties of mesenchymal stem cells. Recent Pat
Inflamm Allergy Drug Discov. 12:59–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Su CY, Fu XL, Duan W, Yu PW and Zhao YL:
High density of CD68+ tumor-associated macrophages
predicts a poor prognosis in gastric cancer mediated by IL-6
expression. Oncol Lett. 15:6217–6224. 2018.PubMed/NCBI
|
|
68
|
Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H,
Zheng P and Zhao S: Gastric cancer-derived mesenchymal stromal
cells trigger M2 macrophage polarization that promotes metastasis
and EMT in gastric cancer. Cell Death Dis. 10:9182019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang M, Chen B, Sun XX, Zhao XD, Zhao YY,
Sun L, Xu CG, Shen B, Su ZL, Xu WR and Zhu W: Gastric cancer
tissue-derived mesenchymal stem cells impact peripheral blood
mononuclear cells via disruption of Treg/Th17 balance to promote
gastric cancer progression. Exp Cell Res. 361:19–29. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kamath SD, Kalyan A and Benson A:
Pembrolizumab for the treatment of gastric cancer. Expert Rev
Anticancer Ther. 18:1177–1187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bang YJ, Kang YK, Catenacci DV, Muro K,
Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, et al:
Pembrolizumab alone or in combination with chemotherapy as
first-line therapy for patients with advanced gastric or
gastroesophageal junction adenocarcinoma: Results from the phase II
nonrandomized KEYNOTE-059 study. Gastric Cancer. 22:828–837. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fukuoka S, Hara H, Takahashi N, Kojima T,
Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et
al: Regorafenib plus nivolumab in patients with advanced gastric or
colorectal cancer: An open-label, dose-escalation, and
dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol.
38:2053–2061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun L, Wang Q, Chen B, Zhao Y, Shen B,
Wang H, Xu J, Zhu M, Zhao X, Xu C, et al: Gastric cancer
mesenchymal stem cells derived IL-8 induces PD-L1 expression in
gastric cancer cells via STAT3/mTOR-c-Myc signal axis. Cell Death
Dis. 9:9282018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Song Y, Du H, Dai C, Zhang L, Li S, Hunter
DJ, Lu L and Bao C: Human adipose-derived mesenchymal stem cells
for osteoarthritis: A pilot study with long-term follow-up and
repeated injections. Regen Med. 13:295–307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ahn SY, Chang YS, Sung SI and Park WS:
Mesenchymal stem cells for severe intraventricular hemorrhage in
preterm infants: Phase I dose-escalation clinical trial. Stem Cells
Transl Med. 7:847–856. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lin W, Huang L, Li Y, Fang B, Li G, Chen L
and Xu L: Mesenchymal stem cells and cancer: Clinical challenges
and opportunities. Biomed Res Int. 2019:28208532019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pakravan K, Babashah S, Sadeghizadeh M,
Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N and Javan M:
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes
suppresses in vitro angiogenesis through modulating the
mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol
(Dordr). 40:457–470. 2017. View Article : Google Scholar
|
|
80
|
Zhang J, Hou L, Wu X, Zhao D, Wang Z, Hu
H, Fu Y and He J: Inhibitory effect of genetically engineered
mesenchymal stem cells with Apoptin on hepatoma cells in vitro and
in vivo. Mol Cell Biochem. 416:193–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shojaati G, Khandaker I, Funderburgh ML,
Mann MM, Basu R, Stolz DB, Geary ML, Dos Santos A, Deng SX and
Funderburgh JL: Mesenchymal stem cells reduce corneal fibrosis and
inflammation via extracellular vesicle-mediated delivery of miRNA.
Stem Cells Transl Med. 8:1192–1201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang M, Zhao C, Shi H, Zhang B, Zhang L,
Zhang X, Wang S, Wu X, Yang T, Huang F, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
Novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|