Introduction
Lung cancer is one of the most common malignant
tumor types worldwide, and non-small cell lung cancer (NSCLC)
accounts for ~80% of all lung cancer types (1). NSCLC includes adenocarcinoma and
squamous cell carcinoma, among which lung adenocarcinoma (LAD) is
the most common pathological type (2). In addition to surgical treatment,
chemotherapy is an effective method to improve the survival rate of
patients with LAD (3). However,
chemotherapeutic resistance is a major barrier to chemotherapy
failure. For instance, drug-resistant tumor cells continue to
metastasize to the distal end, can proliferate quickly and have a
strong invasive ability. Therefore, it is important to understand
the molecular mechanism of cisplatin (DDP) resistance in LAD and to
identify novel targets to prevent the occurrence of DDP
resistance.
Previous studies have reported that long non-coding
RNA (lncRNA) could be involved in the development of tumors. For
instance, Yang et al (4)
revealed that lncRNA MIR4435-2HG was upregulated and promoted the
tumorigenesis of NSCLC, while Zhao et al (5) observed that GMDS-AS1 inhibited the
proliferation of LAD cells and enhanced cell apoptosis. Moreover,
the underlying mechanism of lncRNAs in multiple tumor
chemoresistance is a hot topic of research. It has been
demonstrated that some lncRNAs promote DDP-resistance in multiple
cancer types. For example, Hu et al (6) discovered that colon cancer
associated transcript 1 (CCAT1) enhanced DDP resistance in
esophageal cancer via the microRNA (miRNA/miR)-142/PLK1/BUBR axis.
Furthermore, Yan et al (7)
reported that nuclear enriched abundant transcription 1 decreased
the sensitivity of anaplastic thyroid carcinoma cells to DDP via
the miR-9/c-Jun-amino-terminal kinase-inter-acting protein 4 axis.
Therefore, understanding the molecular mechanism of lncRNA in
DDP-resistant LAD could help identify novel therapeutic targets.
lncRNA FGD5-antisense 1 (FGD5-AS1) is considered as a potential
target in the treatment of various types of cancer, including
colorectal cancer, periodontitis and kidney carcinoma (8-10).
However, the role of FGD5-AS1 in DDP-resistant LAD remains
unknown.
miRNAs have biological characteristics and targeted
specificity, and have the potential to be a marker for tumor
treatment and prognosis (11,12). Liang et al (13) revealed that miR-485-5p suppressed
papillary thyroid cancer development by modulating Raf1. Moreover,
Fan et al (14) suggested
that miR-1281 expression was significantly downregulated in breast
cancer tissues, which was associated with the pathological stage
and poor prognosis of patients with breast cancer. These
characteristics of miRNA provide novel strategies for the treatment
of various malignant tumors.
The present study aimed to elucidate the biological
role and underlying molecular mechanisms of FGD5-AS1 in
DDP-resistant LAD cells. It was demonstrated that FGD5-AS1 enhanced
the resistance of LAD cells to DDP via the miR-142/programmed cell
death 1 ligand 1 (PD-L1) axis, and thus may provide potential
therapeutic strategies for LAD treatment.
Materials and methods
Tumor tissues
A total of 46 LAD tissues, including 25
DDP-resistant LAD tissues and 21 DDP-sensitive LAD tissues, were
obtained from 46 patients with LAD with a mean age of 47 years (age
range, 33-64 years; 31 male patients and 15 female patients)
between April 2015 and August 2017 at The Third Affiliated Hospital
of Soochow University (Changzhou, China). All patients agreed to
cooperate with the present research and provided signed informed
consent. The research was approved by the Ethics Committee of The
Third Affiliated Hospital of Soochow University. Fresh tissues were
immediately frozen in liquid nitrogen and stored at -80°C.
Cell culture
Human LAD cell lines (A549 and HCC827) were
purchased from the American Type Culture Collection. All cell lines
were cultured in RPMI0-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) and supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin at
37°C in a humidified chamber with 5% CO2. To establish
DDP-resistant LAD cells (A549/DDP and HCC827/DDP), A549 and HCC827
cells were incubated at room temperature with increasing
concentrations of DDP (0.5-10 µg/ml; Sigma Aldrich; Merck
KGaA) for >6 months.
Cell transfection
Short hairpin (sh)-RNA targeting FGD5-AS1
(sh-FGD5-AS1; 5′-GCA AUG AUG CGC CAC UAG AUU G-3′) or PD-L1
(sh-PD-L1; 5′-AGC AAA CUG CAC GCC CAG CUG C-3′), miR-142 mimics
(5′-CAU AAA GUA GAA AGC ACU ACU-3′), miR-142 inhibitor (5′-AGT AGT
GCT CCT TTC TAC TTT ATG-3′) and their negative control (NC mimic,
5′-GGU UCG UAC GUA CAC UGU UCA-3′; NC inhibitor, 5′-CCA UCA GUC CCA
AAU CCA-3′) group were purchased from Shanghai GenePharma Co.,
Ltd.. To overexpress FGD5-AS1, FGD5-AS1 was subcloned into pcDNA3.1
by Shanghai GenePharma Co., Ltd., and pcDNA3.1 served as the
control. A549 and HCC827 cells (1×106) were transfected
with sh-FGD5-AS1 (35 nM), sh-PD-L1 (35 nM), sh-NC (35 nM), miR-142
mimics (35 nM), miR-142 inhibitor (35 nM), NC mimic (35 nM), NC
inhibitor (35 nM), pcDNA4.1 (15 nM) or FGD5-AS1 (15 nM) using
Lipofectamine® 2000 reagent (Invitrogen, Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
subsequent experiments were performed 48 h after transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from LAD tissues and cells
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.). The RNA was reverse-transcribed to cDNA using the
PrimeScript™ RT Reagent Kit (Takara Biotechnology Co., Ltd.) at
37°C for 15 min. RT-qPCR was conducted using SYBR-Green PCR Master
Mix kit (Takara Biotechnology Co., Ltd.) with the following
thermocycling conditions: Initial denaturation at 95°C for 3 min,
followed by 40 cycles of denaturation at 95°C for 30 sec, annealing
at 60°C for 30 sec and extension at 72°C for 20 sec, and
subsequently the final extension at 72°C for 5 min. GAPDH or U6 was
used as an internal control. The expression levels of genes were
calculated using the 2−ΔΔCq method (15). The primer sequences were as
follows: FGD5-AS1 forward, 5′-AGA AGC GGA GGG GTG AAA AT-3′ and
reverse, 5′-CCG CCT TAT AGT TGG CCC TC-3′; miR-142-5p forward,
5′-GGC CCA TAA AGT AGA AAG C-3′ and reverse, 5′-TTT GGC ACT AGC ACA
TT-3′; PD-L1 forward, 5′-TAG AAT TCA TGA GGA TAT TTG CTG TCT T-3′
and reverse, 5′-TAG GAT CCT TAC GTC TCC TCC AAA TGT G-3′; GAPDH
forward, 5′-AAC GGA TTT GGT CGT ATT G-3′ and reverse, 5′-GGA AGA
TGG TGA TGG GAT T-3′; and U6 forward, 5′-CTC GCT TCG GCA GCA CA-3′
and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′.
Cell Counting Kit (CCK)-8 assay
To determine the IC50 value, the cells
(1×104 cells/well) were seeded into 96-well plates at
37°C and 5% CO2. The cells were treated with (0, 2, 4,
6, 8 and 10 µg/ml) DDP for 48 h in the medium with 10% FBS.
For the detection of cell viability, the transfected A549/DDP and
HCC827/DDP cells were treated with 4 µg/ml DDP for 48 h.
Then, cells were incubated with 10 µl CCK-8 reagent
(Beyotime Institute of Biotechnology) for another 2 h. The
absorbance at 450 nm wavelength was observed on a micro-plate
reader (Thermo Fisher Scientific, Inc.). The IC50 value
was calculated using the relative survival curve.
RNA immunoprecipitation (RIP) assay
A RIP assay was performed using the EZ-Magna RIP™
RNA-Binding Protein Immunoprecipitation Kit (cat. no. 17-701; EMD
Millipore). Cell lysate was prepared from 1.5×107 cells
and the cell lysate was centrifuged at 513 × g for 15 min at 4°C.
The supernatant was incubated in 100 µl RIP buffer
containing magnetic beads conjugated with the Ago2 antibody (cat.
no. ab32381; 1:1,000; Abcam) or IgG antibody (cat. no. ab150077;
1:1,000; Abcam). Subsequently, the enrichment of FGD5-AS1 and
miR-142 was determined via RT-qPCR.
Transwell assay
The migratory and invasive abilities were assessed
using Transwell chambers (8.0 µm pore size; EMD Millipore)
and Matrigel (Corning, Inc.). For the migration assay, transfected
cells (1×105) were placed in the upper chamber
containing 200 µl RPMI-1640 medium, and 600 µl
RPMI-1640 medium containing 10% FBS was added in the lower chamber.
The cells were cultured for 48 h, and the cells in the lower
chamber were fixed with methanol and stained with crystal violet
(Sigma-Aldrich; Merck KGaA) both for 20 min at room temperature.
For the invasion assay, the insert membranes were coated with
Matrigel for 1 h at room temperature and then cultured under the
same conditions as aforementioned. Migrated and invaded cells were
counted under a light microscope (magnification, ×200) and
imaged.
Luciferase reporter assay
StarBase version 2.0 (http://star-base.sysu.edu.cn) was used to predict the
binding sites between FGD5-AS1 and miR-142, and TargetScan 7.2
(http://www.targetscan.org) was used to
predict the binding sites between miR-142 and 3′-untranslated
region (UTR) of PD-L1. A luciferase reporter assay was performed to
verify the binding ability between RNAs. The wild-type (WT) and
mutant-type (Mut) of the FGD5-AS1 sequences (or PD-L1 3′-UTR) were
cloned into firefly luciferase gene reporter vectors pmirGLO
(Promega Corporation). These constructed vectors were
co-transfected with NC mimics or miR-142 mimics into LAD cells
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h, the relative luciferase activity was
determined using the Dual-Luciferase® Reporter Assay
System (Promega Corporation) according to the manufacturer's
instructions. Firefly luciferase activity was normalized to
Renilla luciferase (Promega Corporation) gene activity.
Western blot analysis
Proteins were extracted from transfected LAD cells
using RIPA buffer (Thermo Fisher Scientific, Inc.). Protein
concentration was measured using the bicinchoninic acid assay
(Beyotime Institute of Biotechnology). A total of 10 µg
protein/lane were separated using 10% SDS-PAGE (EMD Millipore), and
then transferred to PVDF membranes (Bio-Rad Laboratories, Inc.).
After blocking with 5% skimmed milk for 2 h at room temperature,
membranes were probed with primary antibodies against PD-L1
(1:1,000; cat. no. ab205921; Abcam) and anti-GAPDH (1:1,000; cat.
no. sc-47724; Santa Cruz Biotechnology, Inc.) overnight at 4°C.
Subsequently, membranes were incubated with horse-radish
peroxidase-conjugated goat anti-mouse IgG (cat. no. ab205719) and
goat anti-rabbit IgG (cat. no. ab205718) secondary antibodies
(1:1,000; Abcam) at room temperature for 2 h. The protein bands
were visualized using an enhanced chemiluminescence kit (Bio-Rad
Laboratories, Inc.) and semi-quantified by densitometric analysis
of protein signals using ImageJ (version 1.49; National Institutes
of Health). GAPDH served as the loading control.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for statistical
analysis and each experiment was repeated ≥3 times. Comparisons of
parameters between two groups were analyzed using a paired
Student's t-test. Comparisons among multiple groups were performed
using one-way ANOVA followed by Tukey's test. Pearson's correlation
analysis was used for analyzing the correlation between FGD5-AS1
and miR-142. P<0.05 was considered to indicate a statistically
significant difference.
Results
lncRNA FGD5-AS1 is upregulated in
DDP-resistant LAD tissues and cells
RT-qPCR results demonstrated that FGD5-AS1
expression was significantly upregulated in DDP-resistant LAD
tissues compared with that in DDP-sensitive LAD tissues (Fig. 1A). To investigate whether
DDP-resistant LAD cells were successfully established, the
IC50 of DDP was measured using a CCK-8 assay. The
results indicated that the IC50 of DDP in DDP-resistant
LAD (A549/DDP and HCC827/DDP) cells was significantly enhanced
compared with their parental cells (Fig. 1B and C). In addition, RT-qPCR
identified that FGD5-AS1 expression was significantly upregulated
in A549/DDP and HCC827/DDP cells compared with their counterparts
(Fig. 1D). These results
suggested that FGD5-AS1 was highly expressed in DDP-resistant LAD
tissues and cells.
FGD5-AS1 knockdown decreases DDP
resistance in DDP-resistant LAD cells
To evaluate the role of FGD5-AS1 in DDP-resistant
LAD cells, sh-FGD5-AS1 was transfected into HCC827/DDP and A549/DDP
cells. RT-qPCR confirmed that the expression of FGD5-AS1 decreased
in the FGD5-AS1 knockdown group compared with the control group
(Fig. 2A). CCK-8 assay results
demonstrated that cell viability was suppressed after the knockdown
of FGD5-AS1 in DDP-resistant LAD cells treated with 4 µg/ml
DDP (Fig. 2B). Moreover, FGD5-AS1
knockdown decreased the invasion and migration of HCC827/DDP and
A549/DDP cells treated with 4 µg/ml DDP (Fig. 2C and D). Thus, the results
indicated that FGD5-AS1 knockdown enhanced the DDP sensitivity of
DDP-resistant LAD cells.
FGD5-AS1 interacts with miR-142 in LAD
cells
lncRNAs have been reported to promote cancer
progression by acting as competing endogenous RNA (ceRNA). Using
StarBase, miR-142 was predicted as a candidate target of FGD5-AS1
(Fig. 3A). RT-qPCR showed that
miR-142 expression was significantly increased in A549 and HCC827
cells transfected with miR-142 mimics (Fig. 3B). Subsequently, the luciferase
reporter assay results identified that overexpression of miR-142
significantly weakened the luciferase activity of the FGD5-AS1-WT
group, while it had no effect on the FGD5-AS1-Mut reporter group
(Fig. 3C). The RIP assay revealed
that miR-142 and FGD5-AS1 were significantly enriched with the
anti-ago2 antibody compared with anti-IgG, which further indicated
that FGD5-AS1 may be associated with miR-142 (Fig. 3D).
 | Figure 3FGD5-AS1 interacts with miR-142 in LAD
cells. (A) Bioinformatics prediction of the binding site of miR-142
on FGD5-AS1. (B) RT-qPCR showed the expression of miR-142 in A549
and HCC827 cells transfected with NC mimics and miR-142 mimics. The
interaction between miR-142 and FGD5-AS1 was verified by (C)
luciferase reporter and (D) RNA immunoprecipitation assays. (E)
RT-qPCR showed the relative miR-142 expression in DDP-sensitive
(n=21) and DDP-resistant (n=25) LAD tissues. RT-qPCR showed the
relative miR-142 expression in (F) A549 and A549/DDP cells, and (G)
HCC827 and HCC827/DDP cells. RT-qPCR showed that FGD5-AS1 knockdown
upregulated miR-142 expression in (H) A549/DDP and (I) HCC827/DDP
cells, whereas miR-142 reversed this effect. (J and K) The
correlation between FGD5-AS1 and miR-142 in DDP-sensitive (n=21)
and DDP-resistant (n=25) LAD tissues was assessed by Pearson's
correlation analysis. The data are presented as the mean ± SD.
*P<0.05. FGD5-AS1, FGD5-antisense 1; DDP, cisplatin;
LAD, lung adenocarcinoma; NC, negative control; miR, microRNA;
RT-qPCR, reverse transcription-quantitative PCR; WT, wild-type;
Mut, mutant; sh-, short hairpin RNA. |
The RT-qPCR results identified that miR-142
expression was significantly upregulated in DDP-sensitive tissues
compared with that in DDP-resistant LAD tissues (Fig. 3E). Moreover, RT-qPCR indicated a
decrease in miR-142 expression in A549/DDP and HCC827/DDP cells
(Fig. 3F and G). It was found
that transfection with the miR-142 inhibitor abolished the
promotive effect of sh-FGD5-AS1 on miR-142 expression in
DDP-resistant LAD cells (Fig. 3H and
I). In addition, miR-142 expression was negatively correlated
with FGD5-AS1 expression in DDP-resistant (Fig. 3J) and DDP-sensitive LAD tissues
(Fig. 3K). Taken together, the
results suggested that FGD5-AS1 functioned as a sponge for miR-142,
and FGD5-AS1 inhibited miR-142 expression in DDP-resistant LAD
cells.
FGD5-AS1 enhances DDP resistance via
miR-142 in DDP-resistant LAD cells
Rescue experiments were performed by transfecting
A549/DDP cells with NC mimics, miR-142 mimics, miR-142 mimics +
pcDNA3.1 or miR-142 mimics + FGD5-AS1. RT-qPCR showed that FGD-AS1
expression was upregulated in A549 cells transfected with the
FDG5-AS1 overexpression plasmid (Fig.
4A). CCK-8 results identified that miR-142 mimics decreased the
viability of A549/DDP cells treated with 4 µg/ml DDP
compared with the NC mimics group, which was reversed by FGD5-AS1
overexpression (Fig. 4B).
Moreover, Transwell assay results indicated that miR-142-attenuated
invasion and migration of A549/DDP cells treated with 4
µg/ml DDP were reversed by the overexpression of FGD5-AS1
(Fig. 4C and D). These data
demonstrated that FGD5-AS1 increased DDP resistance by modulating
miR-142.
PD-L1 is directly targeted by
miR-142
TargetScan analysis was performed and it was found
that miR-142 possessed a binding site for PD-L1 (Fig. 5A). miR-142 mimics significantly
suppressed the relative luciferase activity of the PD-L1-WT group,
but had no effect on the PD-L1-Mut group (Fig. 5B). In addition, RT-qPCR results
identified that PD-L1 expression was significantly increased in
DDP-resistant LAD tissues (Fig.
5C). RT-qPCR and western blot assays also revealed that the
mRNA and protein expression levels of PD-L1 were significantly
increased in A549/DDP and HCC827/DDP cells (Fig. 5D and E). It was found that miR-142
mimics decreased PD-L1 expression, whereas transfection with the
miR-142 inhibitor increased PD-L1 expression (Fig. 5F and G). Thus, miR-142 may exert
its biological function via PD-L1.
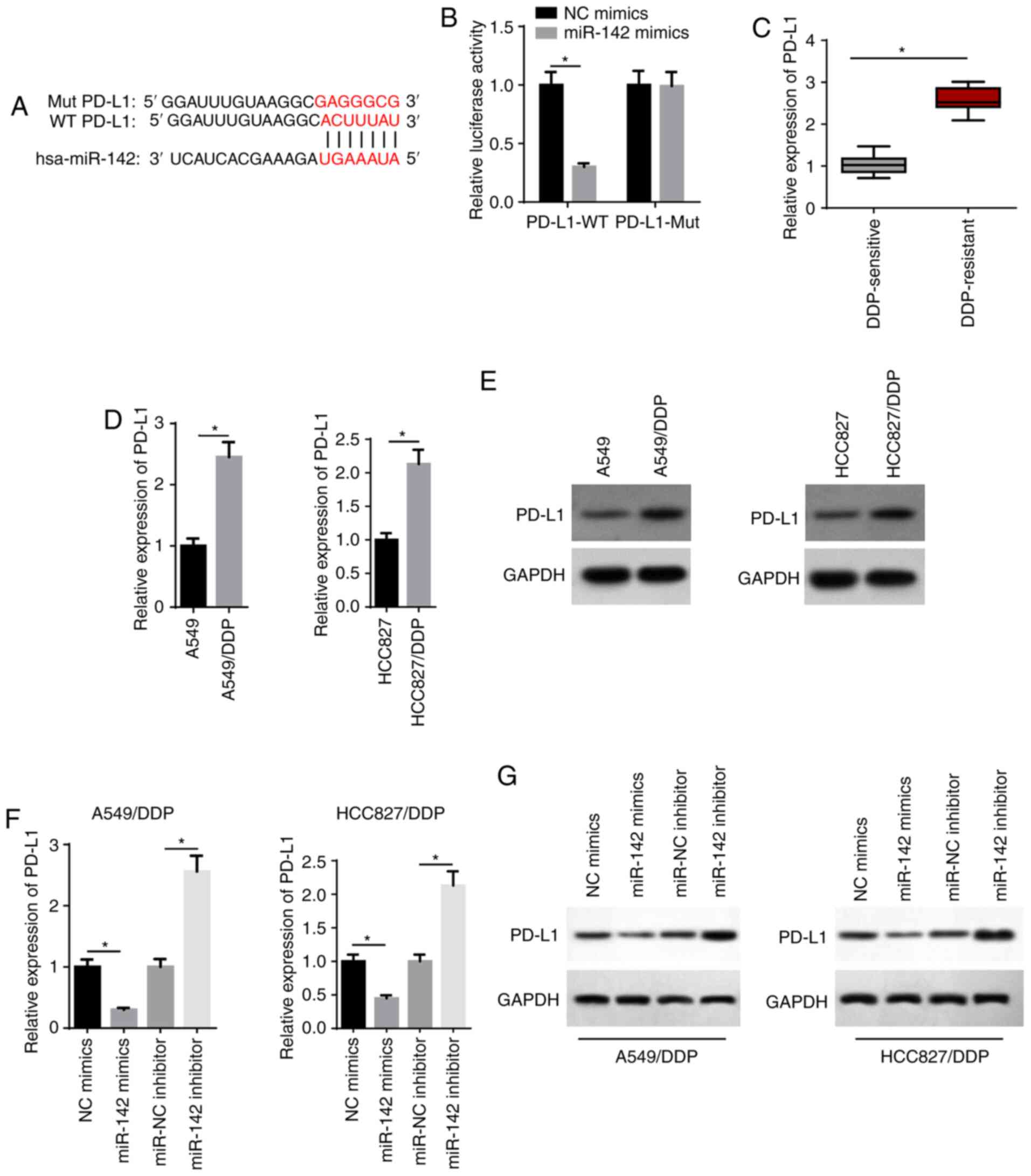 | Figure 5PD-L1 is directly targeted by
miR-142. (A) Bioinformatics prediction of the binding site of
miR-142 on PD-L1. (B) The interaction between miR-142 and PD-L1 was
verified by a luciferase reporter assay. (C) RT-qPCR showed the
relative PD-L1 expression in DDP-sensitive (n=21) and DDP-resistant
(n=25) lung adenocarcinoma tissues. (D) RT-qPCR and (E) western
blotting showed the relative PD-L1 expression in A549, A549/DDP,
HCC827 and HCC827/DDP cells. (F) RT-qPCR and (G) western blotting
showed the relative PD-L1 expression in A549/DDP and HCC827/DDP
cells transfected with NC mimics, miR-142 mimics, miR-NC inhibitor
and miR-142 inhibitor. The data are presented as the mean ± SD.
*P<0.05. PD-L1, programmed cell death 1 ligand 1;
miR, microRNA; RT-qPCR, reverse transcription-quantitative PCR;
DDP, cisplatin; NC, negative control; WT, wild-type; Mut,
mutant. |
FGD5-AS1 decreases the chemosensitivity
of DDP-resistant LAD cells via the miR-142/PD-L1 axis
RT-qPCR analysis demonstrated that the expression of
miR-142 was decreased in A549/DDP cells following transfection with
the miR-142 inhibitor (Fig. 6A).
Moreover, RT-qPCR and western blot assays indicated that the
expression of PD-L1 was downregulated in A549/DDP cells after PD-L1
knockdown (Fig. 6B and C). PD-L1
knockdown significantly inhibited the viability of A549/DDP cells
treated with 4 µg/ml DDP compared with the sh-NC group,
whereas transfection with the miR-142 inhibitor reversed the effect
(Fig. 6D). Moreover, rescue
experiments suggested that the miR-142 inhibitor abolished the
inhibitory effect of PD-L1 knockdown on the invasion and migration
of A549/DDP cells treated with 4 µg/ml DDP (Fig. 6E and F). It was also observed that
FGD5-AS1 knockdown downregulated the expression of PD-L1, whereas
the miR-142 inhibitor partially reversed this expression (Fig. 6G and H). Collectively, these data
supported the hypothesis that FGD5-AS1 promoted LAD cell viability,
invasion and migration, and DDP resistance via the miR-142/PD-L1
axis.
 | Figure 6FGD5-AS1 decreases the
chemosensitivity of DDP-resistant lung adenocarcinoma cells via the
miR-142/PD-L1 axis. (A) RT-qPCR showed the relative miR-142
expression in A549/DDP cells transfected with NC inhibitor and
miR-142 inhibitor. (B) RT-qPCR and (C) western blotting showed the
relative PD-L1 expression in A549/DDP cells transfected with sh-NC
and sh-PD-L1. (D) Cell Counting Kit-8 assay showed the viability of
DDP-treated A549/DDP cells transfected with sh-NC, sh-PD-L1,
sh-PD-L1 + NC inhibitor, sh-PD-L1 + miR-142 inhibitor. Transwell
assays showed the (E) invasion and (F) migration of DDP-treated
A549/DDP cells in different transfection groups (magnification,
×200). (G and H) RT-qPCR and western blotting showed PD-L1
expression in A549/DDP cells transfected with sh-NC, sh-FGD5-AS1,
shFGD5-AS1 + NC inhibitor, shFGD5-AS1 + miR-142 inhibitor. The data
are presented as the mean ± SD. *P<0.05. FGD5-AS1,
FGD5-antisense 1; DDP, cisplatin; PD-L1, programmed cell death 1
ligand 1; miR, microRNA; NC, negative control; RT-qPCR, reverse
transcription-quantitative PCR; sh-, short hairpin RNA. |
Discussion
LAD is a malignant tumor type that is a threat to
human health worldwide (16). In
addition to surgical treatment, DDP-based chemotherapy is the
primary therapeutic strategy for LAD cancer therapy. As a result of
DDP resistance in tumor cells, chemotherapy suffers from the
bottleneck phenomenon, whereby tumor cell populations decrease for
a brief period of time but then quickly repopulate. It was
previously reported that lncRNA serves a vital regulatory role in
the chemo-resistance of various cancer types (17). The present study demonstrated that
lncRNA FGD5-AS1 was highly expressed in DDP-resistant LAD cells
compared with parental cells, and FGD5-AS1 knockdown decreased the
DDP resistance of A549/DDP and HCC827/DDP cells.
A ceRNA network exerts its regulatory function in
human cancer, including LAD (18,19). For example, Wang et al
(20) revealed that CCAT1
knockdown promoted chemical sensitivity in DDP-induced ovarian
cancer cells by sponging downstream miR-454, while Qu et al
(21) observed that LINC00461
promoted DDP resistance of rectal cancer by targeting miR-593.
Similarly, Wu et al (22)
found that lncRNA MIAT modulated proliferation and promoted DDP
resistance in NSCLC cells by targeting miR-184. The present study
indicated that FGD5-AS1 could function as a ceRNA to inhibit
miR-142 expression.
miR-142 has been identified to exert an inhibitory
effect in several cancer types, including squamous cell carcinoma,
gastric cancer and ovarian cancer (23-25). In addition, it has been
demonstrated that miR-142 inhibits chemoresistance of various
tumors, such as ovarian cancer and osteosarcoma (26,27). The present results suggested that
miR-142 was downregulated in LAD, and the overexpression of miR-142
contributed to chemosensitivity in DDP-resistant LAD cells.
PD-L1 may promote tumor cell proliferation and
differentiation by creating an imbalance between immune cells and
the cellular environment (28,29). PD-L1 is also reported to be
involved in the regulation of tumor development. For example, PD-L1
is upregulated in human osteosarcoma tissues and increases cell
invasion (30). Moreover, high
expression of PD-L1 affects the survival and prognosis of patients
with ovarian cancer (31). Zuo
et al (32) reported that
PD-L1 silencing suppressed chemoresistance of DDP-resistant ovarian
cancer cells to DDP, as evidenced by inhibited proliferation,
G1-phase cell cycle arrest and promotion of apoptosis. To the best
of our knowledge, the present study provided the first evidence
that PD-L1 could directly interact with miR-142 via a specific
binding site, and that PD-L1 knockdown suppressed tumor progression
and enhanced chemosensitivity of DDP-resistant LAD cells.
However, some limitations remain to be addressed in
future studies. Other miRNAs or downstream effectors that are
crucial to FGD5-AS1-regulated DDP resistance of LAD cells need to
be identified in the future. In addition, the expression of
FGD5-AS1 was detected using limited sample sizes. Therefore,
further studies are required to confirm the expression of FGD5-AS1
in a large sample size and to further assess the clinical
significance.
In conclusion, the present results suggested that
the FGD5-AS1/miR-142/PD-L1 axis contributed to DDP resistance in
LAD. These findings provided a novel insight into the issue of DDP
resistance.
Funding
This study was funded by the Scientific Research
Project of Wuxi Municipal Health Commission (grant no.
M202009).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ and XiaonanS designed the present study. RN and
XiaoliangS performed the experiments, analyzed the data and
prepared the figures. FZ and XiaonanS drafted the initial
manuscript. XiaonanS reviewed and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The research was approved by the Ethics Committee of
The Third Affiliated Hospital of Soochow University (Changzhou,
China) and all patients provided signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Tang H, Han X, Li M, Li T and Hao Y:
Linc00221 modulates cisplatin resistance in non-small-cell lung
cancer via sponging miR-519a. Biochimie. 162:134–143. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang T, Li J, Zhang C, Hong Q, Jiang D,
Ye M and Duan S: Distinguishing lung adenocarcinoma from lung
squamous cell carcinoma by two hypomethylated and three
hypermethylated genes: A meta-analysis. PLoS One. 11:e01490882016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu R, Mao Y, Chen K, He W, Shi W and Han
Y: The long noncoding RNA ANRIL acts as an oncogene and contributes
to paclitaxel resistance of lung adenocarcinoma A549 cells.
Oncotarget. 8:39177–39184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang M, He X, Huang X, Wang J, He Y and
Wei L: LncRNA MIR4435-2HG-mediated upregulation of TGF-β1 promotes
migration and proliferation of nonsmall cell lung cancer cells.
Environ Toxicol. 35:582–590. 2020. View Article : Google Scholar
|
|
5
|
Zhao M, Xin XF, Zhang JY, Dai W, Lv TF and
Song Y: LncRNA GMDS-AS1 inhibits lung adenocarcinoma development by
regulating miR-96-5p/CYLD signaling. Cancer Med. 9:1196–1208. 2020.
View Article : Google Scholar
|
|
6
|
Hu M, Zhang Q, Tian XH, Wang JL, Niu YX
and Li G: lncRNA CCAT1 is a biomarker for the proliferation and
drug resistance of esophageal cancer via the miR-143/PLK1/BUBR1
axis. Mol Carcinog. 58:2207–2217. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan P, Su Z, Zhang Z and Gao T: LncRNA
NEAT1 enhances the resistance of anaplastic thyroid carcinoma cells
to cisplatin by sponging miR-9-5p and regulating SPAG9 expression.
Int J Oncol. 55:988–1002. 2019.PubMed/NCBI
|
|
8
|
Chen Z, Ju H, Zhao T, Yu S, Li P, Jia J,
Li N, Jing X, Tan B and Li Y: hsa_circ_0092306 targeting miR-197-3p
promotes gastric cancer development by regulating PRKCB in MKN-45
cells. Mol Ther Nucleic Acids. 18:617–626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Lan Z, Li Q and Li Y: Abnormal
expression of long noncoding RNA FGD5-AS1 affects the development
of periodontitis through regulating miR-142-3p/SOCS6/NF-κB pathway.
Artif Cells Nanomed Biotechnol. 47:2098–2106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu H, Lu J, Zhao H, Chen Z, Cui Q, Lin Z,
Wang X, Wang J, Dong H, Wang S and Tan J: Functional long noncoding
RNAs (lncRNAs) in clear cell kidney carcinoma revealed by
reconstruction and comprehensive analysis of the lncRNA-miRNA-mRNA
regulatory network. Med Sci Monit. 24:8250–8263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Liu X, Pan B, Hu X, Zhu Y, Su Y,
Guo Z, Zhang G, Xu M, Xu X, et al: Serum exosomal miR-122 as a
potential diagnostic and prognostic biomarker of colorectal cancer
with liver metastasis. J Cancer. 11:630–637. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin Y, Zhou X, Huang C, Li L, Liu H, Liang
N, Chen Y, Ma D, Han Z, Xu X, et al: Serum miR-342-3p is a novel
diagnostic and prognostic biomarker for non-small cell lung cancer.
Int J Clin Exp Pathol. 11:2742–2748. 2018.PubMed/NCBI
|
|
13
|
Liang ZX, Liu HS, Wang FW, Xiong L, Zhou
C, Hu T, He XW, Wu XJ, Xie D, Wu XR and Lan P: LncRNA RPPH1
promotes colorectal cancer metastasis by interacting with TUBB3 and
by promoting exosomes-mediated macrophage M2 polarization. Cell
Death Dis. 10:8292019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan LY, Shi KY, Xu D, Ren LP, Yang P,
Zhang L, Wang F and Shao GL: LncRNA GIHCG regulates microRNA-1281
and promotes malignant progression of breast cancer. Eur Rev Med
Pharmacol Sci. 23:10842–10850. 2019.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Yang QS, Li B, Xu G, Yang SQ, Wang P, Tang
HH and Liu YY: Long noncoding RNA LINC00483/microRNA-144 regulates
radiosensitivity and epithelial-mesenchymal transition in lung
adenocarcinoma by interacting with HOXA10. J Cell Physiol.
234:11805–11821. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao J, Lv Y, Jin F, Liu Y, Ma Y, Xiong Y,
Liu L, Zhang S, Sun Y, Tipoe GL, et al: LncRNA HANR promotes
tumorigenesis and increase of chemoresistance in hepatocellular
carcinoma. Cell Physiol Biochem. 43:1926–1938. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Xu C, Gao Y, Wang Y, Ding Z,
Zhang Y, Shen W, Zheng Y and Wan Y: A novel long non-coding RNA,
MSTRG.51053.2 regulates cisplatin resistance by sponging the
miR-432-5p in non-small cell lung cancer cells. Front Oncol.
10:2152020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng F and Xu R: CircPVT1 contributes to
chemotherapy resistance of lung adenocarcinoma through
miR-145-5p/ABCC1 axis. Biomed Pharmacother. 124:1098282020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang DY, Li N and Cui YL: Long non-coding
RNA CCAT1 sponges miR-454 to promote chemoresistance of ovarian
cancer cells to cisplatin by regulation of surviving. Cancer Res
Treat. 52:798–814. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qu W, Huang W, Yang F, Ju H and Zhu G:
Long noncoding RNA LINC00461 mediates cisplatin resistance of
rectal cancer via miR-593-5p/CCND1 axis. Biomed Pharmacother.
124:1097402020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu L, Liu C and Zhang Z: Knockdown of
lncRNA MIAT inhibits proliferation and cisplatin resistance in
non-small cell lung cancer cells by increasing miR-184 expression.
Oncol Lett. 19:533–541. 2020.PubMed/NCBI
|
|
23
|
Dai D, Feng XD, Zhu WQ and Bao YN: LncRNA
BLACAT1 regulates the viability, migration and invasion of oral
squamous cell carcinoma cells by targeting miR-142-5p. Eur Rev Med
Pharmacol Sci. 23:10313–10323. 2019.PubMed/NCBI
|
|
24
|
Li M, Cai O and Tan S: LOXL1-AS1 drives
the progression of gastric cancer via regulating miR-142-5p/PIK3CA
axis. Onco Targets Ther. 12:11345–11357. 2019. View Article : Google Scholar
|
|
25
|
Liu H, Chen R, Kang F, Lai H and Wang Y:
KCNQ1OT1 promotes ovarian cancer progression via modulating
MIR-142-5p/CAPN10 axis. Mol Genet Genomic Med.
8:e10772020.PubMed/NCBI
|
|
26
|
Li X, Chen W, Jin Y, Xue R, Su J, Mu Z, Li
J and Jiang S: miR-142-5p enhances cisplatin-induced apoptosis in
ovarian cancer cells by targeting multiple anti-apoptotic genes.
Biochem Pharmacol. 161:98–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Zhao G, Ji S, Yuan Q and Zhou H:
Downregulated long non-coding RNA MSC-AS1 inhibits osteosarcoma
progression and increases sensitivity to cisplatin by binding to
MicroRNA-142. Med Sci Monit. 26:e9215942020.PubMed/NCBI
|
|
28
|
Kalim M, Iqbal Khan MS and Zhan J:
Programmed cell death ligand-1: A dynamic immune checkpoint in
cancer therapy. Chem Biol Drug Des. 95:552–566. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang M, Hu Z, Yue R, Yang L, Zhang B and
Chen Y: The efficacy and safety of qiming granule for dry eye
disease: A systematic review and meta-analysis. Front Pharmacol.
11:5802020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Chou X, Zhuang M, Zhu C, Hu Y,
Cheng D and Liu Z: LINC00657 activates PD-L1 to promote
osteosarcoma metastasis via miR-106a. J Cell Biochem.
121:4188–4195. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue C, Xu Y, Ye W, Xie Q, Gao H, Xu B,
Zhang D and Jiang J: Expression of PD-L1 in ovarian cancer and its
synergistic antitumor effect with PARP inhibitor. Gynecol Oncol.
157:222–233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuo Y, Zheng W, Liu J, Tang Q, Wang SS and
Yang XS: MiR-34a-5p/PD-L1 axis regulates cisplatin chemoresistance
of ovarian cancer cells. Neoplasma. 67:93–101. 2020. View Article : Google Scholar
|




















