Introduction
Multiple myeloma (MM) is a malignant plasma cell
disorder, accounting for the leading cause of mortality in patients
with hematological cancer (1).
Despite recent and extensive progress being made in the treatment
of MM, novel strategies for the more effective management of MM are
urgently required (2-5). Thus, it is necessary to identify
novel prognostic biomarkers and therapeutic regimens for patients
with MM.
MicroRNAs (miRNAs or miRs), endogenous small
(approximately 22 nucleotides in length) non-coding RNAs, act as
unique regulators of gene expression by targeting mRNAs for
translational repression or cleavage (6). Recently, increasing evidence has
unveiled the roles of miRNAs in the development of MM (7-10).
For example, Gupta et al found that circulatory levels of
miR-203 were decreased in the serum of patients with MM, and may be
an independent predictive biomarker for the diagnosis of MM
(11). In addition to diagnosis,
exploiting the therapeutic potentials of miRNAs has been reported
in cancer research. Zhang et al demonstrated the tumor
suppressive ability of miR-29b through the downregulation of Mcl-1,
functioning as a potential therapeutic target for MM (12). Jia et al reported the
antitumor role of miR-26b-5p by targeting JAG1 (13). These data suggest the crucial role
of miRNAs in the progression of MM.
As one of the most well-studied miRNAs, miR-25-3p is
aberrantly expressed and principally functions as an oncogenic
miRNA in the development and progression of human cancers. For
example, Zhang et al found that miR-25-3p promoted
tumorigenesis by regulating CDKN1C expression in glioma (14). In liver cancer, Sanchez-Mejias
et al also reported the oncogenic role of miR-25-3p with the
inhibition of the SOCS5/mammalian target of rapamycin (mTOR)
signaling axis (15). Notably, in
MM, miR-25-3p has been found to be significantly upregulated in the
serum of patients with MM, functioning as a potential diagnostic
target in MM (16,17). Although miR-25-3p has been
reported to be highly expressed in MM, the roles and molecular
mechanisms of miR-25-3p in MM remain unknown.
Thus, the present study aimed to elucidate the
potential involvement of miR-25-3p in MM and to explored the
possible underlying mechanisms. The findings suggest that miR-25-3p
may be a possible biomarker and effective therapeutic target for
MM.
Materials and methods
Human tissue samples
Bone marrow samples from 50 patients with MM were
obtained from the Department of Hematology, the First Affiliated
Hospital of Xinxiang Medical University between June, 2017 and
June, 2018. All patients were diagnosed based on the World Health
Organization diagnostic criteria for MM (18,19). The inclusion criteria were as
follows: i) Patients newly diagnosed with MM according to the World
Health Organization diagnostic criteria for MM; ii) an age >18
years old; iii) patients had to be able to be regularly
followed-up. The exclusion criteria were as follows: i) Patients
with relapsed/refractory MM; ii) patients that had received
chemotherapy, radiotherapy or other systematic treatments prior to
enrollment; iii) patients with a history of solid tumors or
hematological malignancies other than MM. At the same time, 10
healthy volunteers undergoing bone marrow transplant were recruited
as the controls. All patient characteristics are presented in
Table I. Written informed
consents were acquired from all patients and healthy volunteers and
the present study was approved by the Ethics Committee of the First
Affiliated Hospital of Xinxiang Medical University. The samples
were rapidly stored at -80°C until use.
 | Table IAssociation between miR-25-3p and the
pathological characteristics of patients with MM. |
Table I
Association between miR-25-3p and the
pathological characteristics of patients with MM.
| Clinical
feature | Patients (total)
n=50 | miR-25-3p
expression
| P-value |
|---|
| High | Low |
|---|
| Sex | | | | 0.9453 |
| Male | 22 | 10 | 12 | |
| Female | 28 | 13 | 15 | |
| Age (years) | | | | 0.6233 |
| <50 | 17 | 7 | 10 | |
| ≥50 | 33 | 16 | 17 | |
| Anemia | | | | 0.0150a |
| Mild | 20 | 5 | 15 | |
|
Moderate/severe | 30 | 18 | 12 | |
| Impairment of renal
function | | | | 0.0208a |
| Renal
inadequacy | 38 | 24 | 14 | |
| No impairment | 12 | 3 | 9 | |
| ISS staging | | | | 0.0354a |
| Stage I | 21 | 6 | 15 | |
| Stage II-III | 29 | 17 | 12 | |
| D-S staging | | | | 0.0100a |
| Stage I | 13 | 2 | 11 | |
| Stage II-III | 37 | 21 | 16 | |
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the MM tissues and
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). For the detection of miR-25-3p, total RNA was reverse
transcribed into cDNA using the TaqMan™ MicroRNA Reverse
Transcription kit (cat no. 4366596, Thermo Fisher Scientific,
Inc.). qPCR was conducted using the miScript SYBR-Green PCR kit
(cat no. 218073, Qiagen GmbH) in a Light Cycler instrument (Bio-Rad
Laboratories, Inc.). For the detection of phosphatase and tensin
homolog deleted on chromosome 10 (PTEN) mRNA, total RNA was reverse
transcribed into cDNA using a Reverse Transcription kit (cat no.
RR047A, Takara Bio, Inc.). SYBR Premix Ex Taq II (Takara Bio, Inc.,
Tokyo, Japan) was then used for PCR. The thermocycling conditions
were as follows: 50°C for 2 min and 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 10 min. Primers used for
miR-25-3p and PTEN were as follows: miR-25-3p forward, 5′-CAT TGC
ACT TGT CTC GGT CTG A-3′ and reverse, 5′-GCT GTC AAC GAT ACG CTA
CGT AAC G-3′; U6 forward, 5′-GCT TCG GCA GCA CAT ATA CTA AAA T-3′
and reverse, 5′-CGC TTC ACG AAT TTG CGT GTC AT-3′; PTEN forward,
5′-CCA GGA CCA GAG GAA ACC T-3′ and reverse, 5′-GCT AGC CTC TGG ATT
TGA-3′; and GAPDH forward, 5′-GTG GTG AAG ACG CCA GTG GA-3′ and
reverse, 5′-CGA GCC ACA TCG CTC AGA CA-3′. U6 and GAPDH were used
as internal controls for detecting miR-25-3p and PTEN expression,
respectively, and fold changes were calculated through the
2−∆∆Cq method (20).
Cells and cell culture
The NCI-H929 (ATCC® CRL-9068), RPMI-8226
(ATCC® CCL-155) and U266 (ATCC® TIB-196) cell
lines were purchased from the American Type Culture Collection
(ATCC). The MM.1R cell line was purchased from the Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences (cat.
no. SCSP-505). Normal plasma cells (nPCs) were obtained from
Procell Life Science & Technology Co., Ltd. All cells were
cultured in RPMI-1640 medium (Cambrex) supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) at
37°C and 5% CO2.
Cell transfection
The miR-25-3p mimics, mimics negative control
(mimics NC), miR-25-3p inhibitor and inhibitor NC were purchased
from RiboBio Co., Ltd. PTEN overexpression vector pcDNA-PTEN and
pcDNA vector were purchased from GenePharma, Co. Ltd. In addition,
PTEN siRNA (si-PTEN, 5′-CAU CAG CAU AUG GAA AGC UUC AUU U-3′) and
si-Scramble (5′-CAU ACG UAU AGG GAA UUC ACC AUU U-3′) were also
obtained from RiboBio Co., Ltd. RPMI-8226 and U266 cells
(8.0×105/well) in 6-well plates were grown to
approximately 80% confluency, and then respectively transfected
with miR-25-3p inhibitor (20 nM), inhibitor NC (20 nM), si-PTEN (50
nM), or 2 µg pcDNA-PTEN using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). After 24 h, the cells
were collected, and RT-qPCR was performed to ensure silencing was
achieved.
Cell proliferation
For the detection of cell proliferation, RPMI-8226
and U266 cells (5×103/well) were seeded in 96-well
plates for 24 h, and then transfected with 20 nM miRNA inhibitor
and 50 nM si-PTEN, or 2 µg pcDNA-PTEN as described above. At
1, 2 and 3 days post-transfection, 10 µl CCK-8 solution
(Dojindo Molecular Technologies, Inc.) were added and the cells
were cultured at 37°C for an additional 2 h. The OD absorbance at
450 nm was then detected using an iMark microplate reader (Bio-Rad
Laboratories, Inc.).
Cell apoptosis assay
For the detection of cell apoptosis, the Annexin
V-FITC/PI apoptosis detection kit (cat no. ab14085, Abcam) was
applied according to the manufacturer's instructions. Briefly, 48 h
following transfection, cells were centrifuged at 300 × g at 4°C
for 10 min and washed with PBS, and stained with Annexin V and
propidium iodide (PI) for 15 min at room temperature in the dark.
Apoptosis was then measured on a FACScan flow cytometer (Beckman
Coulter, Inc.) and then analyzed using FlowJo 8.7.1 software
(FlowJo, LLC). The results revealed healthy viable cells in the
lower left quadrant (Q4) on the scatter plot as
(FITC−/PI−); the lower right quadrant (Q3)
represented early-stage apoptotic cells
(FITC+/PI−); the upper right quadrant (Q2)
represented necrotic cells and late-stage apoptotic cells
(FITC+/PI+). The rate of apoptosis was
calculated as follows: Apoptotic rate=percentage of early stage
apoptotic cells (Q3) + percentage of late stage apoptotic cells
(Q2). The experiment was repeated 3 times independently.
Luciferase assay
PicTar (http://pictar.mdc-berlin.de/) and TargetScan
(http://www.targetscan.org) were used to
search for the putative targets of miR-25-3p. The luciferase
reporter plasmids (wt-PTEN-UTR-pGL3 or mut-PTEN-UTR-pGL3) were
synthesized by GenePharma, Co., Ltd. 293T cells (8×104)
(ATCC® CRL-11268) were co-transfected with the
luciferase reporter along with miR-25-3p mimics/inhibitor using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). At
48 h post-transfection, the luciferase activity was measured using
the dual-luciferase reporter assay system (Promega Corporation).
Renilla luciferase was used to normalize the cell number at
48 h following transfection.
Western blot analysis
Proteins from the transfected cells were extracted
using RIPA lysis buffer (EMD Millipore) containing protease
inhibitors and phosphatase inhibitors. The protein concentration
was determined using a bicinchoninic acid (BCA) assay (cat no.
P6651, Beyotime Institute of Biotechnology). Briefly, the protein
samples (40 µg/lane) were separated by 12% SDS-PAGE gel and
transferred to polyvinylidene difluoride membranes (EMD Millipore).
The membranes were then blocked with 5% skim milk for 2 h at room
temperature, followed by incubation with PTEN anti-body (cat no.
9559; 1:2,000), p-AKT antibody (cat no. 4060; 1:1,000), AKT
antibody (cat no. 4685; 1:1,000), p-mTOR antibody (cat no. 5536;
1:1,000 dilution), mTOR antibody (cat no. 2983; 1:2,000), PCNA (cat
no. 13110; 1:2,000) and β-actin antibody (cat no. 3700, 1:2,000) at
4°C overnight. Anti-mouse IgG (H + L; cat no. 8887, 1:2,000) were
used as the secondary antibodies for 1 h at room temperature. All
antibodies were obtained from Cell Signaling Technology, Inc.
Detection was performed using an enhanced chemiluminescence kit
(cat no. 34096; GE Healthcare; Cytiva) and the densities of protein
bands was analyzed using ImageJ software (version 1.46; National
Institutes of Health).
Statistical analysis
The SPSS 19.0 software package (SPSS, Inc.) was
applied to analyze the data. All data are presented as the means ±
S.D. Differences between 2 groups were analyzed using an unpaired
Student's t-test. Multiple comparisons were performed using one-way
analysis of variance followed by Tukey's post hoc test. When only 2
groups were compared, a Student's t-test was conducted. The
Chi-squared test was used to evaluate the difference of categorical
variables in Table I. The
differences in overall survival were assessed by Kaplan-Meier
survival analysis and the log rank test. Patients were divided into
a low expression group and high expression group by the cut-off of
the median expression value of miR-25-3p. Pearson's correlation
coefficient was used for correlation analysis between miRNA and
mRNA levels. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of miR-25-3p is upregulated in
MM and is associated with patient clinicopathologic parameters
To evaluate the expression status of miR-25-3p in
MM, the relative expression of miR-25-3p was first assessed in 50
bone marrow samples from patients with MM and in 10 bone marrow
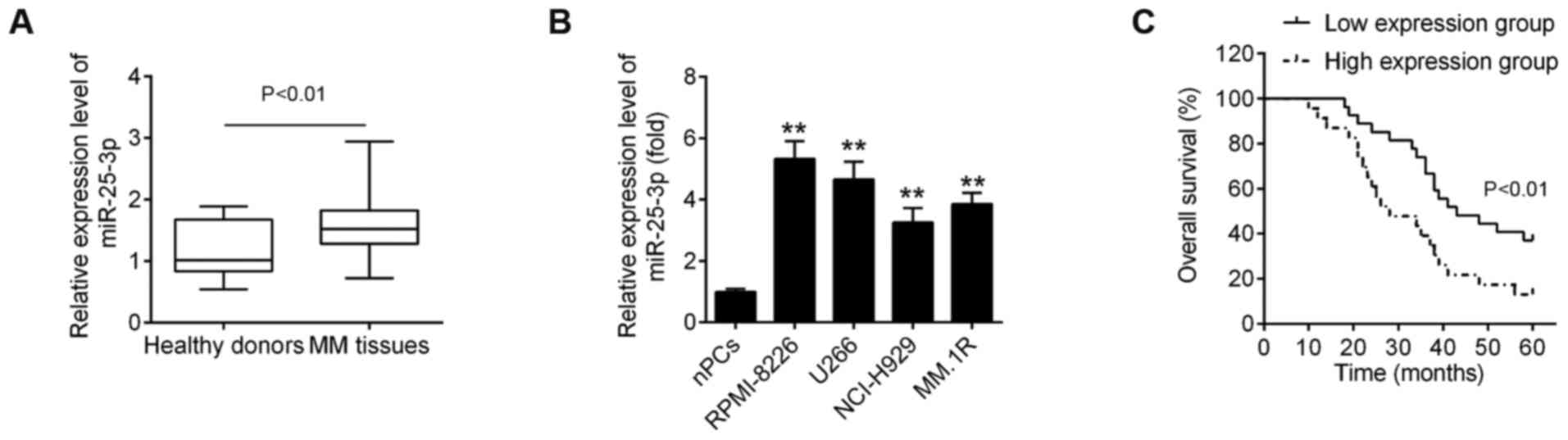
samples from healthy donors. As shown in Fig. 1A, miR-25-3p expression was
significantly upregulated in MM tissues compared with healthy
donors. In addition, the levels of miR-25-3p expression were
measured in 4 MM cell lines relative to the nPCs. Compared with the
nPCs, miR-25-3p expression was upregulated, with differential
expression levels, in the 4 MM cell lines (Fig. 1B), particularly in the RPMI-8226
and U266 cells.
Subsequently, to explore the clinical and
pathological role of miR-25-3p in MM, the association between
miR-25-3p expression and the clinicopathological characteristics of
the patients with MM we analyzed. As shown in Table I, a high expression of miR-25-3p
was closely associated with anemia, renal function impairment, the
international staging system (ISS) staging and Durie-Salmon (D-S)
staging (Table I). However, other
clinicopathological parameters, such as sex and age exhibited no
significant association with miR-25-3p. It was also found that the
overall survival (OS) rate of patients with MM was longer in the
miR-25-3p low expression group compared with the miR-25-3p high
expression group (Fig. 1C).
Collectively, these data indicate that miR-25-3p expression was
indeed upregulated in MM and was mainly associated with disease
progression.
Knockdown of miR-25-3p suppresses cell
proliferation and induces cell apoptosis
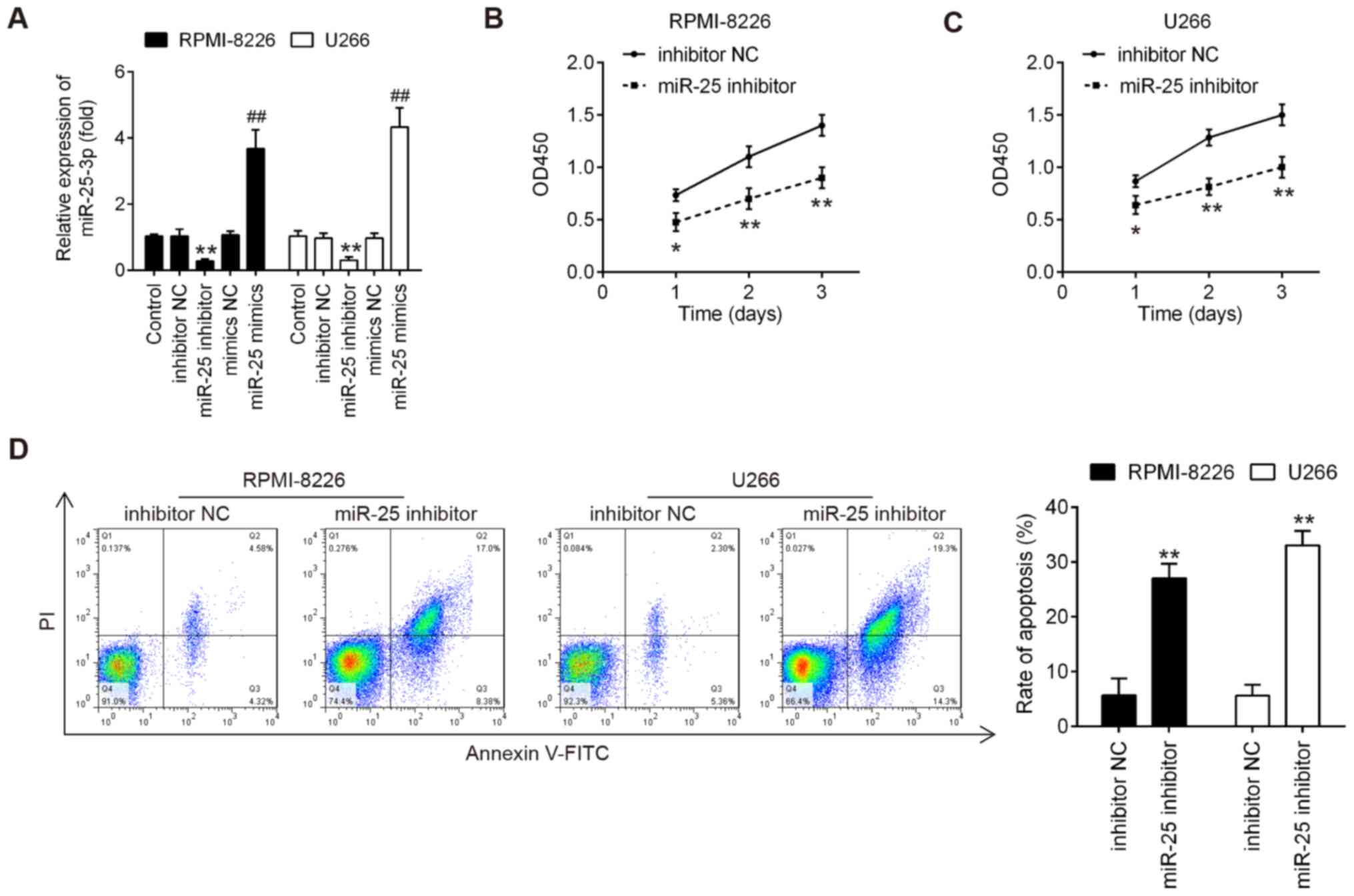
To determine the role of miR-25-3p in MM cells, both
the RPMI-8226 and U266 cells were transfected with miR-25-3p
inhibitor/mimics or mimics/inhibitor NC. As was expected, miR-25-3p
expression was notably increased or decreased following
transfection with mimics or inhibitor, respectively (Fig. 2A), which confirmed the
transfection efficiency. According to the results of CCK-8 assay,
miR-25-3p knockdown markedly inhibited cell proliferation at
varying degrees in both the RPM-I8226 and U266 cells (Fig. 2B and C). The present study then
determined whether the effects of miR-25-3p on cell proliferation
may be associated with apoptosis. To determine this, these treated
cells were subjected to FITC-Annexin V/PI staining followed by flow
cytometric analysis. As shown in Fig.
2D, miR-25-3p knockdown resulted in a significant increase in
the apoptotic portion of both the RPMI-8226 and U266 cells compared
to the inhibitor NC. These results suggested that miR-25-3p
knockdown inhibited cell proliferation by promoting cell apoptosis
in vitro.
Overexpression of miR-25-3p promotes the
proliferation and inhibits the apoptosis of MM cells
The effects of miR-25-3p overexpression on cell
proliferation and apoptosis in vitro were then evaluated.
The RPMI-8226 and U266 cells were transfected with miR-25-3p mimics
or mimics NC. As shown in Fig. 3A and
B, miR-25-3p upregulation markedly promoted the proliferation
of both RPMI-8226 and U266 cells. The results of western blot
analysis revealed that compared with the mimics NC group, the
expression levels of PCNA in both the RPMI-8226 and U266 cells were
significantly increased in the miR-25-3p mimics group (Fig. 3C). In addition, compared with the
mimics NC group, the apoptotic portion of cells in the miR-25-3p
mimics group was significantly decreased (Fig. 3D). All results suggest that
miR-25-3p overexpression promoted cell proliferation by inhibiting
apoptosis in vitro.
PTEN was a direct target of
miR-25-3p
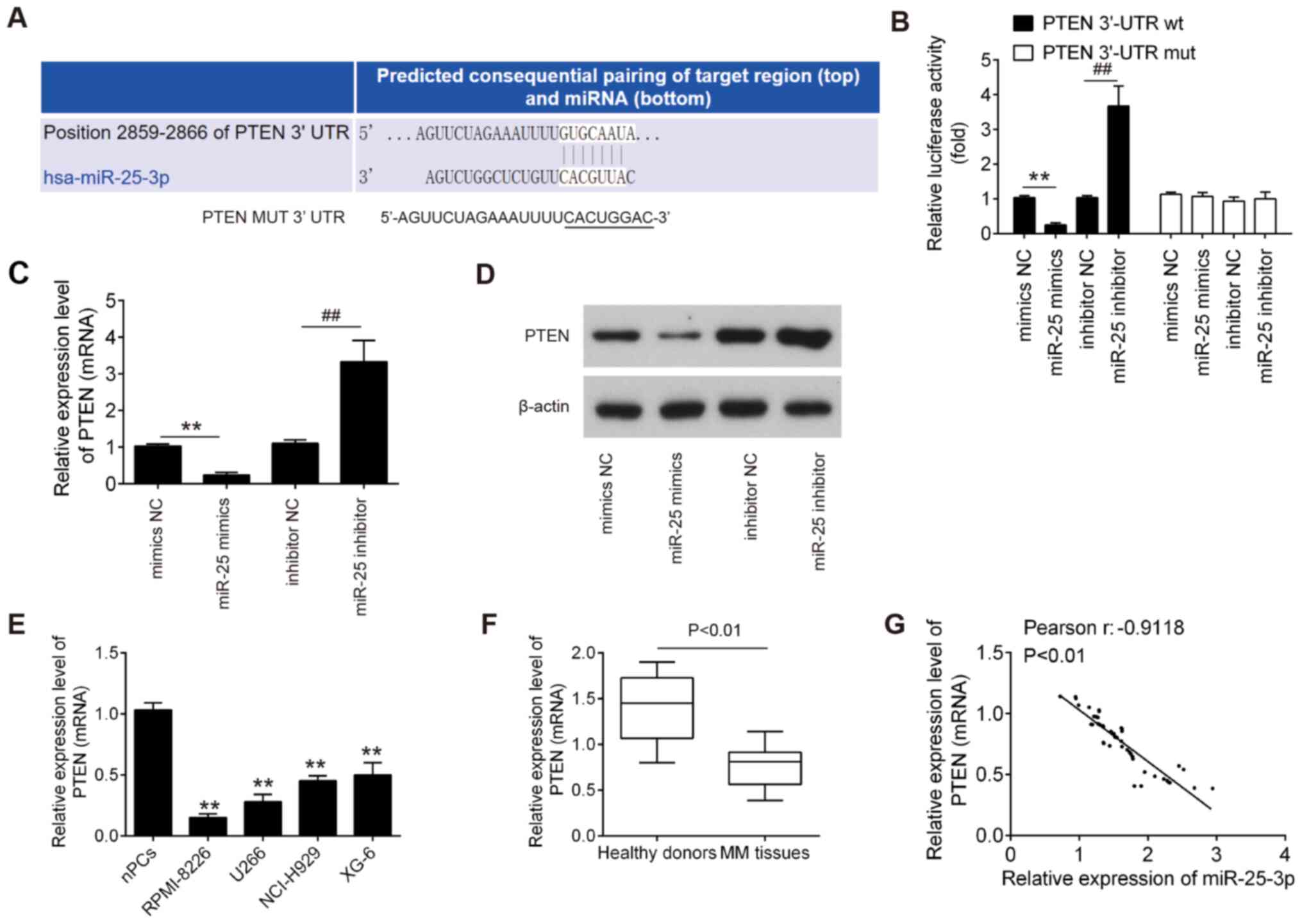
To examine the molecular mechanism by which
miR-25-3p functions in MM, candidate target genes of miR-25-3p were
computationally screened using the online database PicTar and
TargetScan. Among several predicted target genes, PTEN was
identified as a potential target of miR-25-3p (Fig. 4A). To verify whether miR-25-3p
directly binds to PTEN, a dual luciferase reporter assay was
conducted. It was found that miR-25-3p upregulation markedly
inhibited, whereas miR-25-3p inhibition promoted the luciferase
activity of the PTEN-2-3′UTR wt reporter; however, co-transfection
with mutant vector did not lead to any changes in luciferase
activity (Fig. 4B). To further
confirm whether PTEN is regulated by miR-25-3p, the mRNA and
protein expression levels of PTEN in the RPMI-8226 and U266 cells
were measured by RT-qPCR and western blot analysis, respectively.
It was found that PTEN expression was significantly decreased
following transfection with miR-25-3p mimics, while it was
increased following miR-25-3p inhibitor transfection (Fig. 4C and D). Subsequently, the
expression levels of PTEN in MM cell lines and the above-mentioned
clinical samples were detected by RT-qPCR. As shown in Fig. 4E and F, PTEN expression was
significantly downregulated in MM cell lines and MM tissues,
compared with the nPCs and healthy donor tissues. In addition, an
evident inverse correlation was observed between the PTEN and
miR-25-3p expression levels in tumor tissues (Fig. 4G, r=−0.9118; P<0.01). These
results indicate that PTEN may be a downstream target of
miR-25-3p.
Overexpression of PTEN inhibits the
proliferation and promotes the apoptosis of MM cells
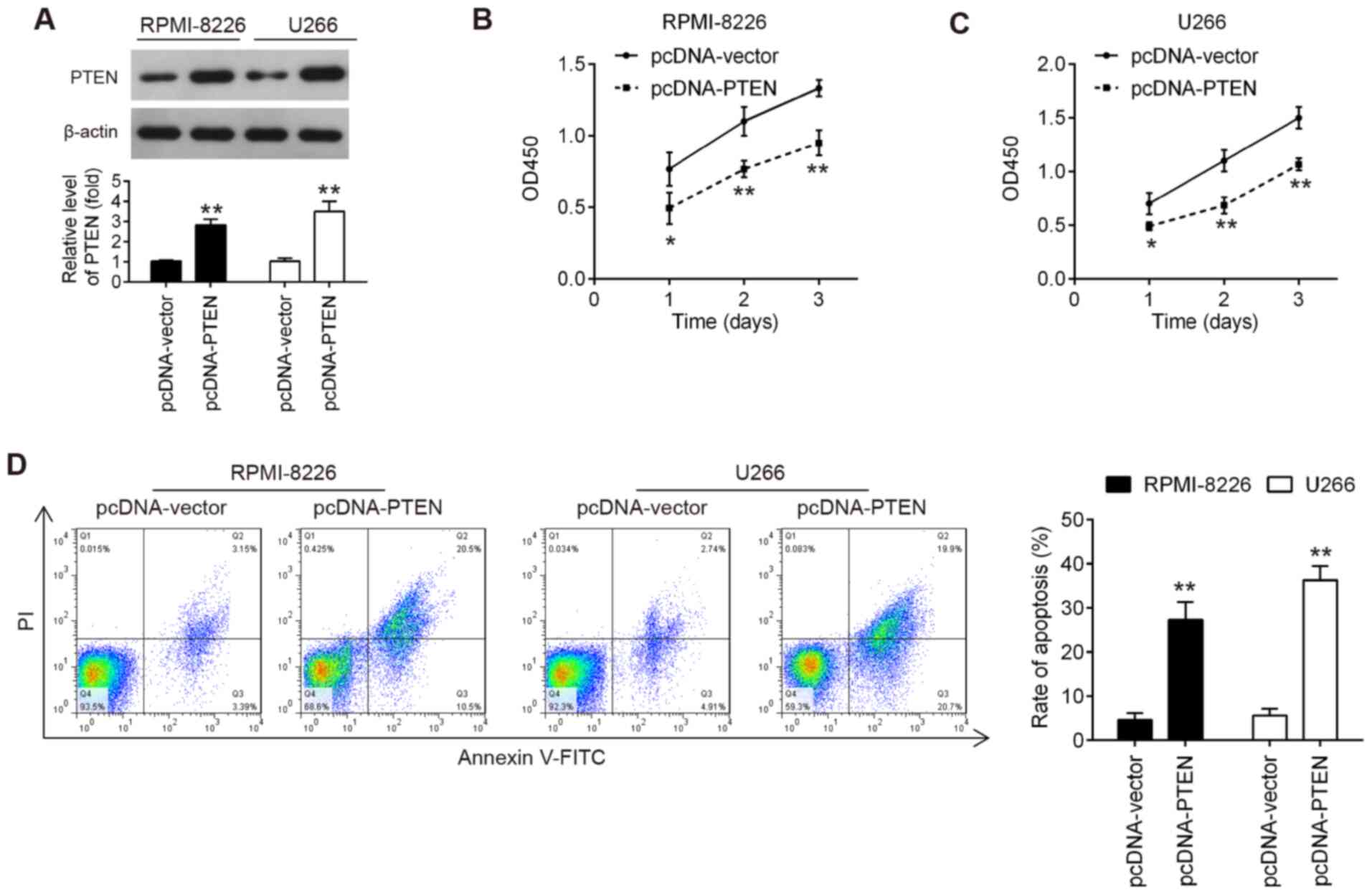
The results mentioned above demonstrated that
miR-25-3p inhibited cell proliferation in vitro and
miR-25-3p directly targeted PTEN. The present study then wished to
determine whether the upregulation of PTEN exerted a similar effect
to that of miR-20a knockdown. To address this question, pcDNA-PTEN
plasmid was transfected into RPMI-8226 and U266 cells. As shown in
Fig. 5A, the PTEN protein level
was notably increased in the RPMI-8226 and U266 cells transfected
with the PTEN overexpression plasmid. Cell proliferation and
apoptosis were then estimated. As shown by the results of CCK-8
assay, PTEN upregulation significantly suppressed the proliferation
of RPMI-8226 and U266 cells compared with the pcDNA-vector group
(Fig. 5B and C). It was also
found that PTEN upregulation markedly promoted the apoptosis of
RPMI-8226 and U266 cells, compared with the pcDNA-vector group
(Fig. 5D). Consequently, PTEN
upregulation exerted similar effects to those of miR-25-3p
knockdown in MM cells.
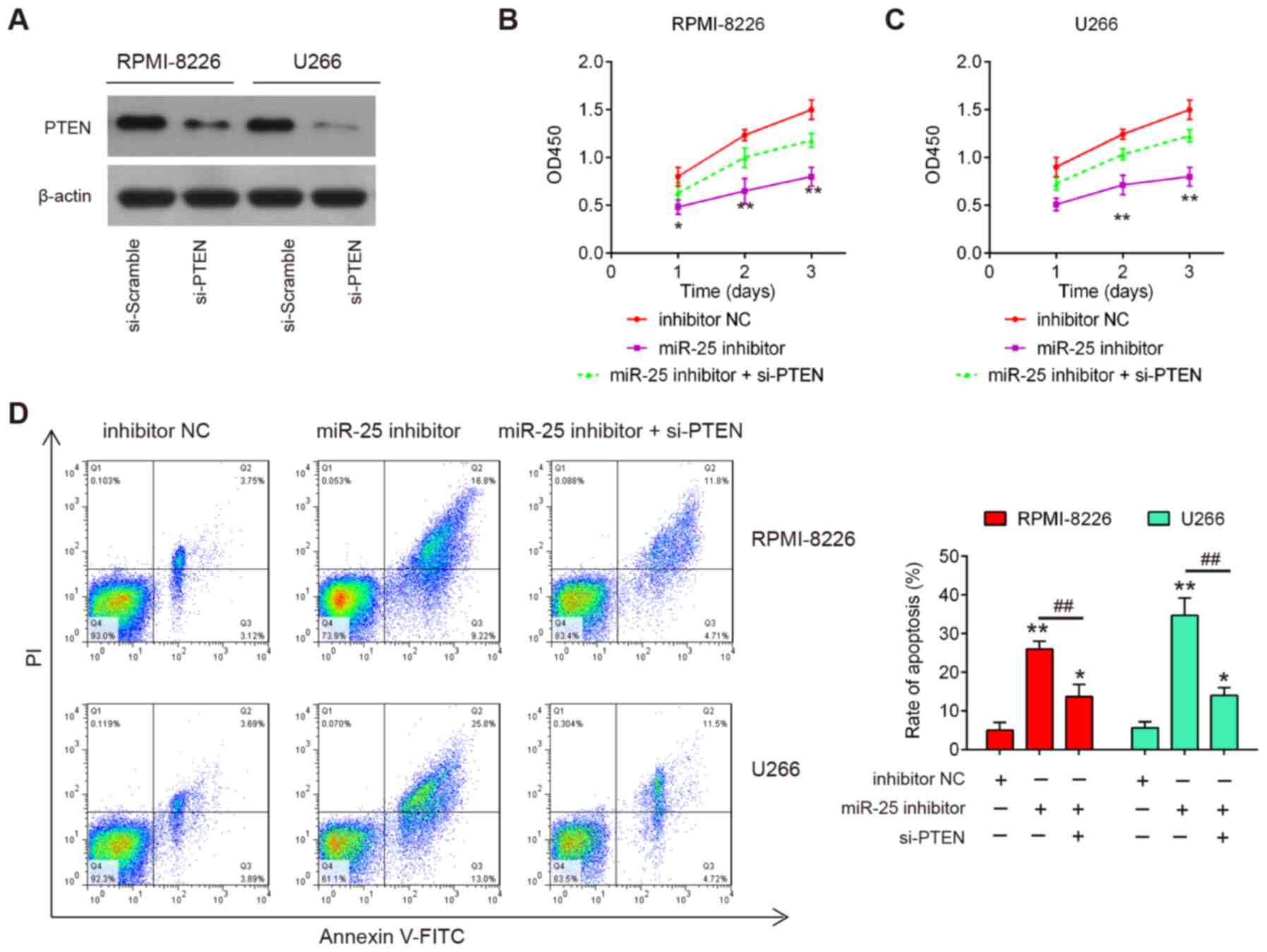
PTEN inhibition reverses the effects of
miR-25-3p on the proliferation and apoptosis of MM cells
To verify whether PTEN mediates the inhibitory
effects of miR-25-3p on cell proliferation and cell apoptosis,
rescue experiments were performed by transfecting si-PTEN with
miR-25-3p inhibitor into RPMI-8226 and U266 cells. As shown in
Fig. 6A, the protein expression
levels of PTEN were notably decreased in the RPMI-8226 and U266
cells following si-PTEN transfection. The results of CCK8 assay
revealed that PTEN knockdown partially reversed the inhibitory
effects of miR-25-3p inhibitor on cell proliferation (Fig. 6B and C). Moreover, the increased
apoptotic portion induced by miR-25-3p inhibitor was significantly
reduced by PTEN knockdown in the RPMI-8226 and U266 cells (Fig. 6D). Taken together, these findings
demonstrated that miR-25-3p inhibited cell proliferation and
promotes apoptosis by directly targeting PTEN expression in MM
cells.
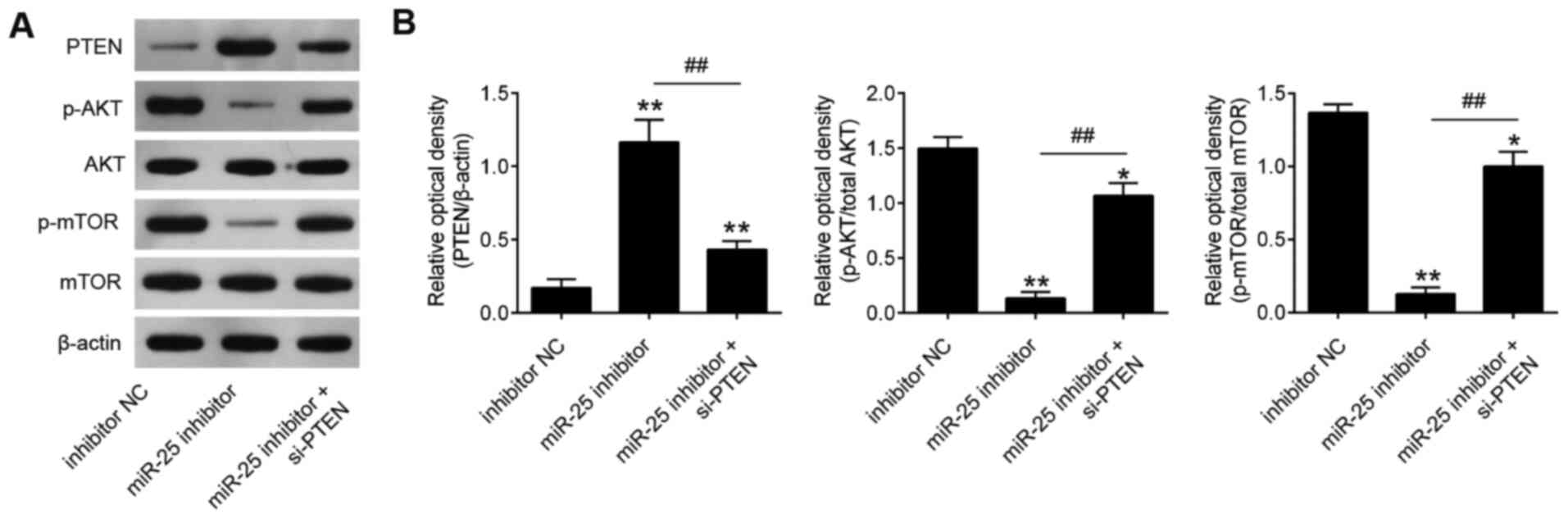
Knockdown of miR-25-3p blocks the
activity of PTEN/PI3K/AKT pathway
It has been well recognized that PTEN negatively
regulates the activity of the Akt pathway, and the Akt pathway is
closely associated with cell growth and cell apoptosis in human
cancers (21-23). To investigate whether miR-25-3p
affects the activity of the PI3K/Akt signaling pathway, the
expression levels of downstream proteins in the PI3K/Akt signaling
pathway, namely AKT, p-AKT, mTOR and p-mTOR, were evaluated in
RPMI-8226 and U266 cells. The results revealed that miR-25-3p
inhibition elevated PTEN expression and reduced p-AKT and p-mTOR
expression levels relative to the control group (Fig. 7). These data suggest that
miR-25-3p knockdown blocked the activity of PTEN/PI3K/AKT pathway
in MM cells.
Discussion
In the present study, it was found that miR-25-3p
expression was upregulated in MM tissues and cell lines; high
levels of miR-25-3p were closely associated with the
clinicopathological characteristics of patients with MM. Moreover,
miR-25-3p knockdown suppressed cell proliferation and promoted cell
apoptosis by blocking the activity of the PTEN/PI3K/AKT pathway.
All these findings suggest that miR-25-3p may prove to be a novel
biomarker and therapeutic target for MM.
Previous studies have demonstrated that miR-25-3p
expression is upregulated in MM tissues and functions as a
non-invasive prognosis biomarker (16,17). Consistent with these studies, the
present study found miR-25-3p expression was significantly
upregulated in both MM tissues and MM cell lines. Taken together,
these results suggest that an increased miR-25-3p expression is a
frequent event in human MM tissues and may be involved in
carcinogenesis as a tumor oncogene. Moreover, its high expression
is predictive of a poor prognosis of patients with MM. In view of
the above, it was hypothesized that miR-25-3p may participate in
the progression of MM.
Previous studies have described the oncogenic role
of miR-25-3p in several types of cancer (24,25). For example, in renal tumors,
miR-25-3p expression is elevated in renal cancer tissues, and is
associated with the growth of renal cancer cells (26). In melanoma, miR-25 directly
targets DKK3, and thereby reduces its downstream signaling, the
WNT/β-catenin pathway to promote melanoma cell proliferation
(27). In gastric cancer,
miR-25-3p knockdown has been shown to suppress tumor xenograft
growth in mice (28). Liu et
al found that the upregulation of miR-25-3p was closely
associated with the progression and a poor prognosis of patients
with cholangio-carcinoma (29).
Although previous studies have demonstrated its high expression in
MM tissue samples, the biological function of miR-25-3p in MM
remains unknown. Herein, it was found that miR-25-3p knockdown
suppressed the cell proliferation by promoting cell apoptosis,
while miR-25-3p overexpression exerted an opposite effect on
RPMI-8226 and U266 cells. Therefore, these data indicate that
miR-25-3p may function as an oncogenic miRNA in the progression of
MM.
As one of the most well-studied miRNAs, miR-25-3p is
aberrantly expressed and principally functions as an oncogenic
miRNA in cancer development and progression. For example, miR-25-3p
has been shown to be significantly upregulated in triple-negative
breast cancer (TNBC) tissues and cell lines, and may serve as a
novel diagnostic and therapeutic target for TNBC (30). Wan et al also found that
miR-25-3p was upregulated in retinoblastoma tissues and cell lines,
and the enforced expression of miR-25-3p increased cell growth,
cell migration and invasion in vitro, as well as promoted
tumor xenograft growth in vivo (31). The increased expression of
miR-25-3p has also been reported to be associated with liver,
prostate and stomach cancers (32-35). Notably, previous studies
demonstrated that miR-25-3p expression was significantly
upregulated in MM tissues, suggesting that it may be considered as
a potential prognostic and diagnostic marker in MM (16,17). Taken together, these results
suggest that an increased miR-25-3p expression may be associated
with the progression of MM.
One of the best strategies with which to understand
the fundamental function of miRNAs is via the elucidation of their
functional targets. In the present study, through the online
data-bases, PicTar and TargetScan, PTEN was identified as a target
gene of miR-25-3p. PTEN is a well-known tumor suppressor gene in
various types of tumors, and plays an important role in regulating
cell proliferation and cell apoptosis (36). A previous study by Jiang et
al demonstrated that PTEN was expressed in low levels in MM
(37). Wan et al found
that PTEN was a direct target of miR-25-3p in retinoblastoma
(31). However, whether PTEN is
regulated by miR-25-3p in MM cells has not yet been determined, at
least to the best of our knowledge. In the present study, the
results revealed that miR-25-3p knockdown exhibited tumor
suppressive roles in MM cells by targeting PTEN.
PTEN is a key upstream of the PI3K/Akt signaling
pathway (36,38) and activated PI3K/Akt signaling
contributes to cell growth and apoptosis in a number of tumors,
including MM (37). In MM,
PI3K/Akt is constitutively active and contributes to the the
proliferation of MM cells, which suggests that the inhibition of
this pathway may be a potential therapeutic approach for MM
(39-42). Jiang et al demonstrated
that miR-20a inhibited the proliferation, migration and apoptosis
of MM cells, and that these effects were associated with PI3K/AKT
signaling pathway inactivation (37). The results of the present study
demonstrated that miR-25-3p knockdown markedly enhanced the
expression levels of PTEN, leading to the subsequent downregulation
of Akt and mTOR phosphorylation. This may explain why miR-25-3p
inhibition suppressed the proliferation and promoted the apoptosis
of MM cells.
In conclusion, the present study found that
miR-25-3p expression was frequently increased in MM tissues and may
serve as a prognostic biomarker in patients with MM. Mechanically,
the data indicated that miR-25-3p knockdown suppressed cell
proliferation through the PTEN/PI3K/AKT pathway. The findings may
provide a potential target for the prevention and treatment of
MM.
Funding
This study was supported by the Key Projects of
Science and Technology of Henan Province (grant no. 182102311141)
and the Research Project of Medical Education in Henan Province
(grant no. Wjlx2017083).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
YZi conceived and designed the experiments. YZh, YW,
LZ, RY and YH performed the experiments and analyzed the data. YZi
contributed reagents/materials/analysis tools and wrote the
manuscript. All authors have read and agreed to the final version
of manuscript.
Ethics approval and consent to
participate
Written informed consents were acquired from all
patients and healthy volunteers. The present study was approved by
the Ethics Committee of the First Affiliated Hospital of Xinxiang
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Terpos E, Ntanasis-Stathopoulos I,
Gavriatopoulou M and Dimopoulos MA: Pathogenesis of bone disease in
multiple myeloma: From bench to bedside. Blood Cancer J. 8:72018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nahi H, Våtsveen TK, Lund J, Heeg BM,
Preiss B, Alici E, Møller MB, Wader KF, Møller HE, Grøseth LA, et
al: Proteasome inhibitors and IMiDs can overcome some high-risk
cytogenetics in multiple myeloma but not gain 1q21. Eur J Haematol.
96:46–54. 2016. View Article : Google Scholar
|
|
3
|
Harding T, Baughn L, Kumar S and Van Ness
B: The future of myeloma precision medicine: Integrating the
compendium of known drug resistance mechanisms with emerging tumor
profiling technologies. Leukemia. 33:863–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velez R, Turesson I, Landgren O,
Kristinsson SY and Cuzick J: Incidence of multiple myeloma in Great
Britain, Sweden, and Malmö, Sweden: The impact of differences in
case ascertainment on observed incidence trends. BMJ Open.
6:e0095842016. View Article : Google Scholar
|
|
5
|
Chen D, Zhou D, Xu J, Zhou R, Ouyang J and
Chen B: Prognostic value of 1q21 gain in multiple myeloma. Clin
Lymphoma Myeloma Leuk. 19:e159–e164. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Handa H, Murakami Y, Ishihara R,
Kimura-Masuda K and Masuda Y: The role and function of microRNA in
the pathogenesis of multiple myeloma. Cancers (Basel). 11:13782019.
View Article : Google Scholar
|
|
8
|
Adamia S, Abiatari I, Amin SB, Fulciniti
M, Minvielle S, Li C, Moreau P, Avet-Loiseau H, Munshi NC and
Anderson KC: The effects of MicroRNA deregulation on pre-RNA
processing network in multiple myeloma. Leukemia. 34:167–179. 2020.
View Article : Google Scholar
|
|
9
|
Xu YY, Song YQ, Huang ZM, Zhang HB and
Chen M: MicroRNA-26a inhibits multiple myeloma cell growth by
suppressing cyclin-dependent kinase 6 expression. Kaohsiung J Med
Sci. 35:277–283. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian F, Zhan Y, Zhu W, Li J, Tang M, Chen
X and Jiang J: MicroRNA-497 inhibits multiple myeloma growth and
increases susceptibility to bortezomib by targeting Bcl-2. Int J
Mol Med. 43:1058–1066. 2019.
|
|
11
|
Gupta N, Kumar R, Seth T, Garg B, Sati HC
and Sharma A: Clinical significance of circulatory microRNA-203 in
serum as novel potential diagnostic marker for multiple myeloma. J
Cancer Res Clin Oncol. 145:1601–1611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang YK, Wang H, Leng Y, Li ZL, Yang YF,
Xiao FJ, Li QF, Chen XQ and Wang LS: Overexpression of microRNA-29b
induces apoptosis of multiple myeloma cells through down regulating
Mcl-1. Biochem Biophys Res Commun. 414:233–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia CM, Tian YY, Quan LN, Jiang L and Liu
AC: miR-26b-5p suppresses proliferation and promotes apoptosis in
multiple myeloma cells by targeting JAG1. Pathol Res Pract.
214:1388–1394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Gong X, Tian K, Chen D, Sun J,
Wang G and Guo M: miR-25 promotes glioma cell proliferation by
targeting CDKN1C. Biomed Pharmacother. 71:7–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez-Mejias A, Kwon J, Chew XH, Siemens
A, Sohn HS, Jing G, Zhang B, Yang H and Tay Y: A novel
SOCS5/miR-18/miR-25 axis promotes tumorigenesis in liver cancer.
Int J Cancer. 144:311–321. 2019. View Article : Google Scholar
|
|
16
|
Navarro A, Diaz T, Tovar N, Pedrosa F,
Tejero R, Cibeira MT, Magnano L, Rosiñol L, Monzó M, Bladé J and
Fernández de Larrea C: A serum microRNA signature associated with
complete remission and progression after autologous stem-cell
transplantation in patients with multiple myeloma. Oncotarget.
6:1874–1883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiang T, Hu AX, Sun P, Liu G, Liu G and
Xiao Y: Identification of four potential predicting miRNA
biomarkers for multiple myeloma from published datasets. PeerJ.
5:e28312017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A report from international myeloma working
group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
International Myeloma Working Group:
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: A report of the
international myeloma working group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Franke TF: PI3K/Akt: Getting it right
matters. Oncogene. 27:6473–6488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X and Jiang X: PTEN: A default
gate-keeping tumor suppressor with a versatile tail. Cell Res.
18:807–816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Razumilava N, Bronk SF, Smoot RL, Fingas
CD, Werneburg NW, Roberts LR and Mott JL: miR-25 targets
TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and
promotes apoptosis resistance in cholangiocarcinoma. Hepatology.
55:465–475. 2012. View Article : Google Scholar
|
|
25
|
Feng S, Pan W, Jin Y and Zheng J: MiR-25
promotes ovarian cancer proliferation and motility by targeting
LATS2. Tumour Biol. 35:12339–12344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boguslawska J, Rodzik K, Poplawski P,
Kędzierska H, Rybicka B, Sokół E, Tański Z and Piekiełko-Witkowska
A: TGF-beta1 targets a microRNA network that regulates cellular
adhesion and migration in renal cancer. Cancer Lett. 412:155–169.
2018. View Article : Google Scholar
|
|
27
|
Huo J, Zhang Y, Li R, Wang Y, Wu J and
Zhang D: Upregulated MicroRNA-25 mediates the migration of melanoma
cells by targeting DKK3 through the WNT/β-catenin pathway. Int J
Mol Sci. 17:11242016. View Article : Google Scholar
|
|
28
|
Ning L, Zhang M, Zhu Q, Hao F, Shen W and
Chen D: miR-25-3p inhibition impairs tumorigenesis and invasion in
gastric cancer cells in vitro and in vivo. Bioengineered. 11:81–90.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu H, Ma L and Wang J: Overexpression of
miR-25 is associated with progression and poor prognosis of
cholangiocarcinoma. Exp Ther Med. 18:2687–2694. 2019.PubMed/NCBI
|
|
30
|
Chen H, Pan H, Qian Y, Zhou W and Liu X:
MiR-25-3p promotes the proliferation of triple negative breast
cancer by targeting BTG2. Mol Cancer. 17:42018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wan W, Wan W, Long Y, Li Q, Jin X, Wan G,
Zhang F, Lv Y, Zheng G, Li Z and Zhu Y: MiR-25-3p promotes
malignant phenotypes of retinoblastoma by regulating PTEN/Akt
pathway. Biomed Pharmacother. 118:1091112019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peng G, Yang C, Liu Y and Shen C:
miR-25-3p promotes glioma cell proliferation and migration by
targeting FBXW7 and DKK3. Exp Ther Med. 18:769–778. 2019.PubMed/NCBI
|
|
33
|
Kim YK, Yu J, Han TS, Park SY, Namkoong B,
Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK and Kim VN: Functional links
between clustered microRNAs: Suppression of cell-cycle inhibitors
by microRNA clusters in gastric cancer. Nucleic Acids Res.
37:1672–1681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Tan W, Neo TW, Aung MO, Wasser S,
Lim SG and Tan TM: Role of the miR-106b-25 microRNA cluster in
hepatocellular carcinoma. Cancer Sci. 100:1234–1242. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Poliseno L, Salmena L, Riccardi L, Fornari
A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele G, et
al: Identification of the miR-106b~25 microRNA cluster as a
proto-oncogenic PTEN-targeting intron that cooperates with its host
gene MCM7 in transformation. Sci Signal. 3:ra292010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang Y, Chang H and Chen G: Effects of
microRNA-20a on the proliferation, migration and apoptosis of
multiple myeloma via the PTEN/PI3K/AKT signaling pathway. Oncol
Lett. 15:10001–10007. 2018.PubMed/NCBI
|
|
38
|
Qu L, Gao Y, Sun H, Wang H, Liu X and Sun
D: Role of PTEN-Akt-CREB signaling pathway in nervous system
impairment of rats with chronic arsenite exposure. Biol Trace Elem
Res. 170:366–372. 2016. View Article : Google Scholar
|
|
39
|
Aziz AUR, Farid S, Qin K, Wang H and Liu
B: PIM kinases and their relevance to the PI3K/AKT/mTOR pathway in
the regulation of ovarian cancer. Biomolecules. 8:72018. View Article : Google Scholar :
|
|
40
|
Waniczek D, Śnietura M, Lorenc Z,
Nowakowska-Zajdel E and Muc-Wierzgon M: Assessment of PI3K/AKT/PTEN
signaling pathway activity in colorectal cancer using quantum
dot-conjugated antibodies. Oncol Lett. 15:1236–1240.
2018.PubMed/NCBI
|
|
41
|
Yu T, Li L, Liu W, Ya B, Cheng H and Xin
Q: Silencing of NADPH oxidase 4 attenuates hypoxia resistance in
neuroblastoma cells SH-SY5Y by inhibiting PI3K/Akt-dependent
glycolysis. Oncol Res. 27:525–532. 2019. View Article : Google Scholar
|
|
42
|
Xu H, Li J and Zhou ZG: NEAT1 promotes
cell proliferation in multiple myeloma by activating PI3K/AKT
pathway. Eur Rev Med Pharmacol Sci. 22:6403–6411. 2018.PubMed/NCBI
|