Introduction
Silicosis is a disease caused by long-term exposure
to a large amounts of free silica dust, characterized by diffuse
nodular fibrosis of the lungs, which eventually damages lung
function, leading to respiratory failure and even death (1). China has the largest population of
patients with silicosis, and the annual direct economic losses
caused by silicosis in the country amounts to >8 billion RMB. In
developed countries, silicosis is also a significant occupational
health concern (2,3). However, the pathogenesis of
silicosis is currently unclear, and there is a lack of clinically
effective therapeutic drugs (4,5).
Therefore, it is crucial to study the pathogenesis of silicosis and
explore novel preventative methods.
Silicosis is a complex chronic inflammatory process
involving multiple cells (alveolar macrophages, alveolar epithelial
cells, fibroblasts and lymphocytes, amongst others), cytokines and
multiple mediators (6). Amongst
these multiple factors, transforming growth factor (TGF)-β1 serves
a key role. An increasing number of studies have demonstrated that
TGF-β1 is a powerful fibrogenic cytokine, which serves an important
regulatory role in cell proliferation, differentiation, migration,
immune regulation and transformation of the extra-cellular matrix
(ECM) in fibrotic diseases, and participates in tissue repair and
fibrosis (7-9). In addition, several studies have
suggested that gaining additional insight into biological events
downstream of TGF-β1 signaling may lead to the identification of
novel molecular targets for the treatment of silicosis and various
other fibrotic lung conditions.
Since there are currently few treatments and
medicines aimed at reversing silicosis or delaying disease
progression, the potential efficacy of traditional Chinese
medicines are increasingly being assessed (10). Astragaloside IV (ASV) is one of
the primary active substances of Astragalus membranaceus,
which has attracted significant attention due to its prominent
immune-regulatory, anti-inflammatory, anti-asthmatic and
anti-fibrotic properties (11).
According to Yu et al (12), ASV can resist bleomycin
(BLM)-induced pulmonary fibrosis by inhibiting oxidative stress and
inflammatory response levels. Wang et al (13) also reported that ASV can reduce
the progression of renal fibrosis by inhibiting the
mitogen-activated protein kinase pathway and TGF-β1/Smad signaling
pathways. However, there is lack of data regarding the effects of
ASV on silica-induced lung silicosis fibroblast fibrosis.
Therefore, the aim of the present study was to investigate the
effects of ASV on silicosis and explore its potential mechanisms
using in vivo experiments, in the hope of identifying a
novel potential target for the treatment of silicosis.
Materials and methods
Animals and groups
A total of 60 male Sprague Dawley rats, weighing
180-200 g were used in the present study. All rats had ad
libitum access to standard rat feed and tap water. The animals
were randomly divided into four groups (n=15 per group): i) Control
group, 1 ml saline and 0.25 ml air; ii) ASV group, intraperitoneal
injection of 20 mg/kg ASV; ii) silicosis model group, 1 ml silica
dust suspension (50 mg/ml) and 0.25 ml air; and iv) silicosis model
group + ASV, 1 ml silica dust suspension (50 mg/ml) and 0.25 ml
air, followed by ASV injected intraperitoneally. The flowchart of
the animal experimental procedures is shown in Fig. 1. The present study was approved by
the Ethics Committee of The Second Hospital of Shandong University
and all procedures were performed in strict accordance with the
recommendations outlined in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health.
Silica-induced rat model
All animals were anesthetized with 1% pentobarbital
sodium (35 mg/kg, Dainippon Sumitomo Pharma) and fixed on a 60°
inclined board with the help of rubber bands. The tongue of the
mice was pulled and held using blunt forceps. Under the head
mirror, endotracheal intubation was performed using an epidural
anesthesia catheter with a connector attaching it to a 1-ml syringe
that was inserted into the trachea to the point of the tracheal
bifurcation. The crystalline particles were suspended in normal
sterile saline and the suspension (50 mg/ml) was vigorously mixed
using a Stuart® vortex shaker (Cole-Palmer) prior to
instillation. For pathological tissue extraction, the rats were
anesthetized with 1% pentobarbital sodium (35 mg/kg, Dainippon
Sumitomo Pharma) and fixed on the plate. After the bronchoalveolar
lavage fluid (BALF) was collected, the rats were euthanized by
bloodletting. Then the pathological organs were collected
immediately after animal euthanasia.
Hematoxylin and eosin (H&E)
staining
Briefly, the lung tissues were fixed in 4%
paraformaldehyde for 24 h at 37°C. Subsequently, they were embedded
in paraffin and cut into 5 µm sections using a microtome.
Dewaxing and dehydration were performed using xylene and ethanol
aqueous solution, followed by H&E staining. The sections were
stained with hematoxylin for 5 min and eosin for 3 min at 37°C.
Masson's trichrome staining
The dewaxed deparaffinized 5-µm lung tissue
sections were fixed in Bouin's solution over-night at 37°C, washed
twice with distilled water, and stained with Mayer's hematoxylin
and acid Ponceau for 5 and 10 min at 37°C, respectively, then
rinsed with distilled water 3 times. Subsequently, the sections
were dissolved in a 1% phosphomolybdic acid aqueous solution. After
10-15 min, the sample was transferred to aniline blue and stained
at 37°C for 20 min. Finally, the sections were rapidly dehydrated
in 95% alcohol, followed by hyaluronic acidification with
dimethylbenzene (DAB).
Bronchoalveolar lavage fluid (BALF) and
inflammatory cell counting
A tracheal tube was inserted into the trachea with a
sufficient volume of Hank's Balanced Salt Solution (1 ml each time)
to collect BALF. Following BALF collection, one part of the
re-suspended BALF cells were used for differential counting of
inflammatory cells, including neutrophils, lymphocytes and
macrophages, using Giemsa staining (15 min at 37°C). The remaining
re-suspended BALF cells were later used for calculating the total
cell count using a hemocytometer.
Measurement of TNF-a, interleukin (IL)-1β
and IL-6 levels by ELISA
Concentrations of IL-6 (cat. no. ab178013; Abcam),
IL-1β (cat. no. ab214025; Abcam) and TNF-a (cat. no. ab181421;
Abcam) in the lung tissues were assessed using commercially
available ELISA kits (USCN Business Co., Ltd.) according to the
manufacturer's protocol (14).
Evaluation of oxidative stress in
tissues
Lung samples were homogenized in ice-cold 250 mM
sucrose solution to determine the levels of malondialdehyde (MDA),
reactive oxygen species (ROS), superoxide dismutase (SOD) and
glutathione peroxidase (GSH-px). For the detection of MDA, the
tissue was homogenized in 1 ml PBS. MDA was determined using the
thiobarbituric acid reactive substance method, and catalase as
described previously (15). For
the detection of ROS, intra-cellular reactive levels were evaluated
using dihydroethidium staining, as described previously (16). For the detection of SOD, nitroblue
tetrazolium, riboflavin and tetrametyletylene-diamine were used in
accordance with the Beauchamp and Fridovich method (17). For the detection of GSH-px, the
tissue antioxidant capacity was measured as described previously by
Moron et al (18).
Immunohistochemical analysis
Briefly, endogenous peroxidase activity within the
sections was quenched by incubating the sections with 3%
H2O2 for 10 min after dewaxing and hydration.
Lung tissues were incubated in a humidified chamber with primary
antibodies directed against Collagen I (1:100; cat. no. AF7001;
Affbiotech), fibronectin (1:100; cat. no. WL00712a; Wanleibio) and
α-SMA (1:100; cat. no. WL02510; Wanleibio) overnight at 4°C. On the
following day, the lung tissues were washed with PBS and incubated
with a IgG antibody (1:100; cat. no. ab150077; Abcam) at 37°C for
45 min. In the negative controls, the primary antibody was replaced
with PBS. Subsequently, tissues were counterstained with DAB.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated from lung tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific
Inc.) and then the extracted RNA was reverse transcribed to cDNA
using Sensiscript RT kit (Thermo Fisher Scientific, Inc.). The
reverse transcription temperature protocol was denaturation at 70°C
for 10 min followed by reverse transcription at 42°C for 15 min.
Subsequently, qPCR was performed using BeyoFast™ SYBR Green qPCR
mix (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol (19) The
thermocycling conditions were as follows: 95°C for 5 min; followed
by 30 cycles of 95°C for 30 sec, 56°C for 30 sec and extension step
at 72°C for 1 min. The sequences of the primers are presented in
Table I.
 | Table ISequences of the primers. |
Table I
Sequences of the primers.
| Gene | Forward, 5′-3′ | Reverse, 5-3′ |
|---|
| Collagen I |
TCCTGCCGATGTCGCTATCC |
TCGTGCAGCCATCCACAAGC |
| Fibronectin |
TCGCTTTGACTTCACCACCA |
TGAGACCCAGGAGACCACG |
| α-SMA |
GGGCATCCACGAAACCACCT |
GAGCCGCCGATCCAGACAGA |
| TGF-β1 |
AACAATTCCTGGCGTTACCT |
GCCCTGTATTCCGTCTCCTT |
| TGFβ1RI |
GACCTTTGCCGATGCTTTCT |
GACCTTTGCCGATGCTTTCT |
| TGFβ1RII |
TGTGAGAAGCCGCAGGAAGT |
CAGAGTGAAGCCGTGGTAGGT |
| Smad2 |
TTTGCCGAGTGCCTAAGTGA |
AGGTTACAGCCTGGTGGGAT |
| Smad3 |
AGGGCTTTGAGGCTGTCTACC |
CCCATTCAGGTGTAGCTCGAT |
| Smad7 |
ACTGGTGCGTGGTGGCATACT |
CCGATCTTGCTCCTCACTTTCTG |
| β-actin |
CACTGTGCCCATCTACGAGG |
TAATGTCACGCACGATTTCC |
Western blotting
According to the manufacturer's protocol (20), proteins were extracted using RIPA
Lysis Buffer (Beyotime Institute of Biotechnology) and
concentration was measured using a bicinchoninic acid assay.
Protein samples (30 µg) were loaded on an 8% SDS-gel,
resolved using SDS-PAGE and transferred to a PVDF membrane. After
blocking with 5% non-fat dry milk for 2.5 h at 37°C, the PVDF
membrane was incubated with primary antibodies: TGF-β1 (1:500; cat.
no. WL01076a; Wanleibio), TGFβ1RI (1:400; cat. no. WL03150;
Wanleibio), TGFβ1RII (1:500; cat. no. A1415; ABclonal), Smad2
(1:500; cat. no. WL02286; Wanleibio), phospho-(p-)Smad2 (1:1,000;
cat. no. E-AB-21129; Elabscience), Smad3 (1:500; cat. no. ab52903;
Abcam), p-Smad3 (1:1,000; cat. no. ab52903; Abcam) or Smad7
(1:1,000; cat. no. WL02975; Wanleibio) overnight at 4°C. The
following day, the protein samples were incubated with an IgG
antibody (1:500; cat. no. ab254262,; Abcam) at room temperature for
45 min. Signals were visualized using ECL reagent, and densitometry
analysis was performed using Gel-Pro-Analyzer version 4.0 (Media
Cybernetics, Inc.).
Statistical analysis
SPSS version 19.0 (IBM Corp.) was used to analyze
all the data. Data are presented as the mean ± standard deviation.
Differences amongst multiple groups were statistically analyzed
using a one-way ANOVA with a post hoc Bonferroni test. P<0.05
was considered to indicate a statistically significant
difference.
Results
ASV alleviates silica-induced pulmonary
fibrosis in silicosis rats
H&E staining was performed to examine the
pathological changes in the lung. As shown in Fig. 2A, compared with the control group,
severe inflammatory damage was observed in the silicosis group,
including infiltration of inflammatory cells, fused alveolar walls,
as well as injury and thickening of the bronchial epithelial cell
lining. However, when treated with ASV, these symptoms were
significantly alleviated. Masson's trichrome staining was used to
observe the changes in collagen fibers and ECM secretion in lung
tissue (Fig. 2B). In the disease
model group, notable fibrosis and strong collagen deposition were
observed, and these pathological changes were alleviated in the ASV
treated group.
ASV treatment reduces the expression of
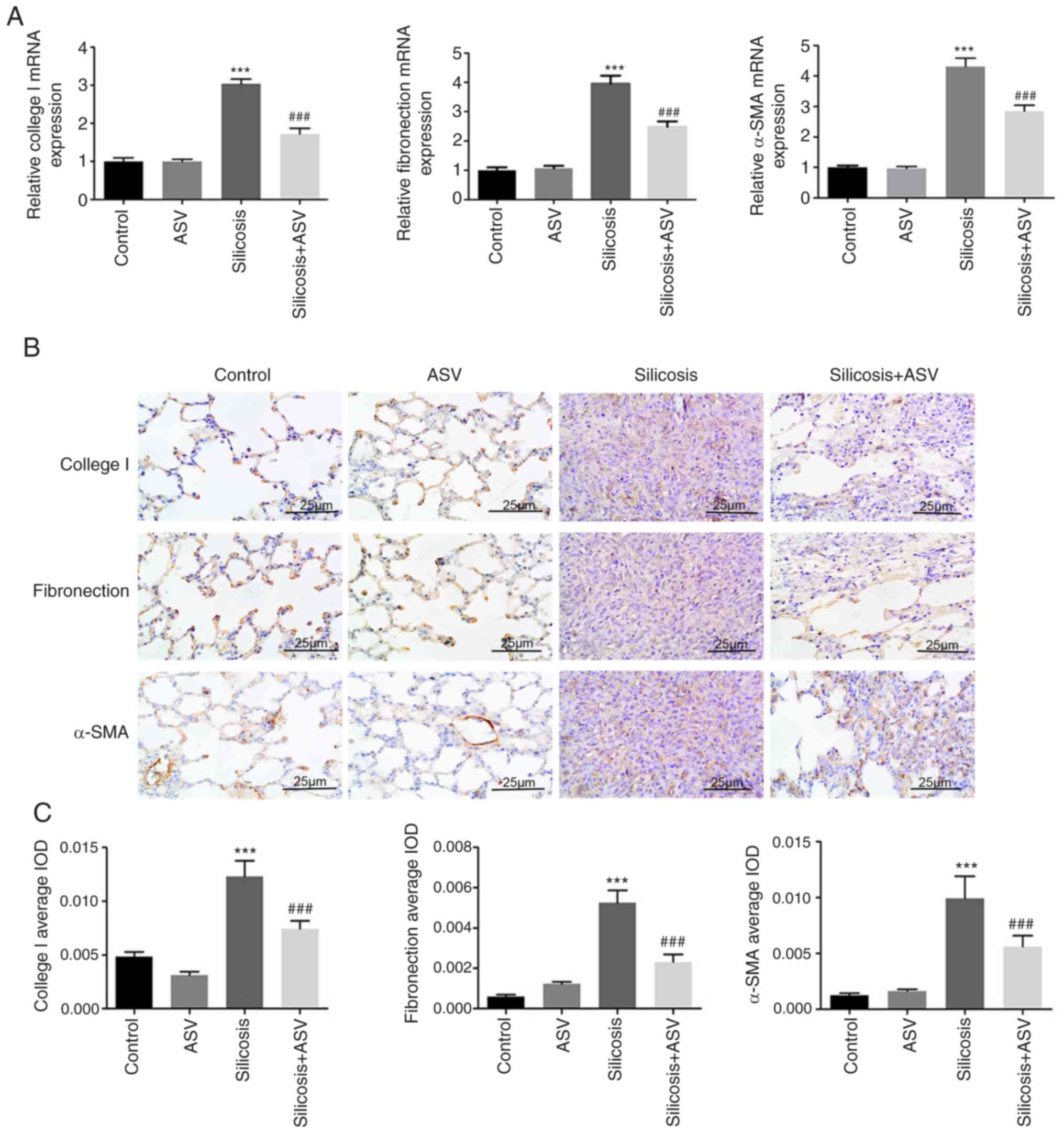
Collagen I, fibronectin and α-SMA
To evaluate the role of ASV on silica-induced
fibroblast fibrosis, the changes in the expression of Collagen I,
fibronectin and α-SMA were determined. As shown in Fig. 3A, increased mRNA expression levels
of Collagen I, fibronectin and α-SMA were observed in the silicosis
group compared with the control group (all P<0.05). Notably, ASV
treatment significantly reduced the mRNA expression levels of these
genes (P<0.05). Furthermore, immunohistochemistry was performed
to verify these results, and the results showed that the staining
was notably stronger in the silicosis group and weaker in the
silicosis + ASV group (Fig. 3B).
Accordingly, the mean density of Collagen I, fibronectin and α-SMA
in the lung tissue increased significantly in the silicosis group
when compared with that in the control group (P<0.05; Fig. 3C).
ASV-mediates an anti-pulmonary fibrosis
response via reduction in inflammation
As shown in Fig.
4A, the cytological characteristics of BALF were affected by
the administration of silica. Specifically, the total cell,
neutrophil, lymphocyte and macrophage counts in the silicosis group
were notably increased compared with the control group (all
P<0.05). By contrast, the addition of ASV significantly
decreased the total cell, neutrophil, lymphocyte and macrophage
counts when compared with the silicosis group (all P<0.05).
 | Figure 4ASV-mediates an anti-pulmonary
fibrosis response via a reduction in inflammation. (A) Cytological
BALF parameters, including total cell, neutrophil, lymphocyte and
macrophage counts, were significantly increased in the Silicosis
group compared with the control group. ASV treatment significantly
decreased the levels of these parameters. (B) ASV decreased the
levels of inflammatory cytokines: TNF-α, IL-1β and IL-6.
***P<0.001 vs. Control; #P<0.05,
##P<0.01, ###P<0.001 vs. Silicosis. ASV,
astragaloside IV; BALF, bronchoalveolar lavage fluid; TNF-α, tumor
necrosis factor-α; IL, interleukin. |
Furthermore, the levels of TNF-α, IL-1β and IL-6 in
lung tissues were quantified using ELISA. As shown in Fig. 4B, compared with the control group,
silica exposure significantly increased the levels of TNF-α, IL-1β
and IL-6 in lung tissues (all P<0.05). However, treatment with
ASV resulted in a significant downregulation in the levels of these
cytokines (all P<0.05).
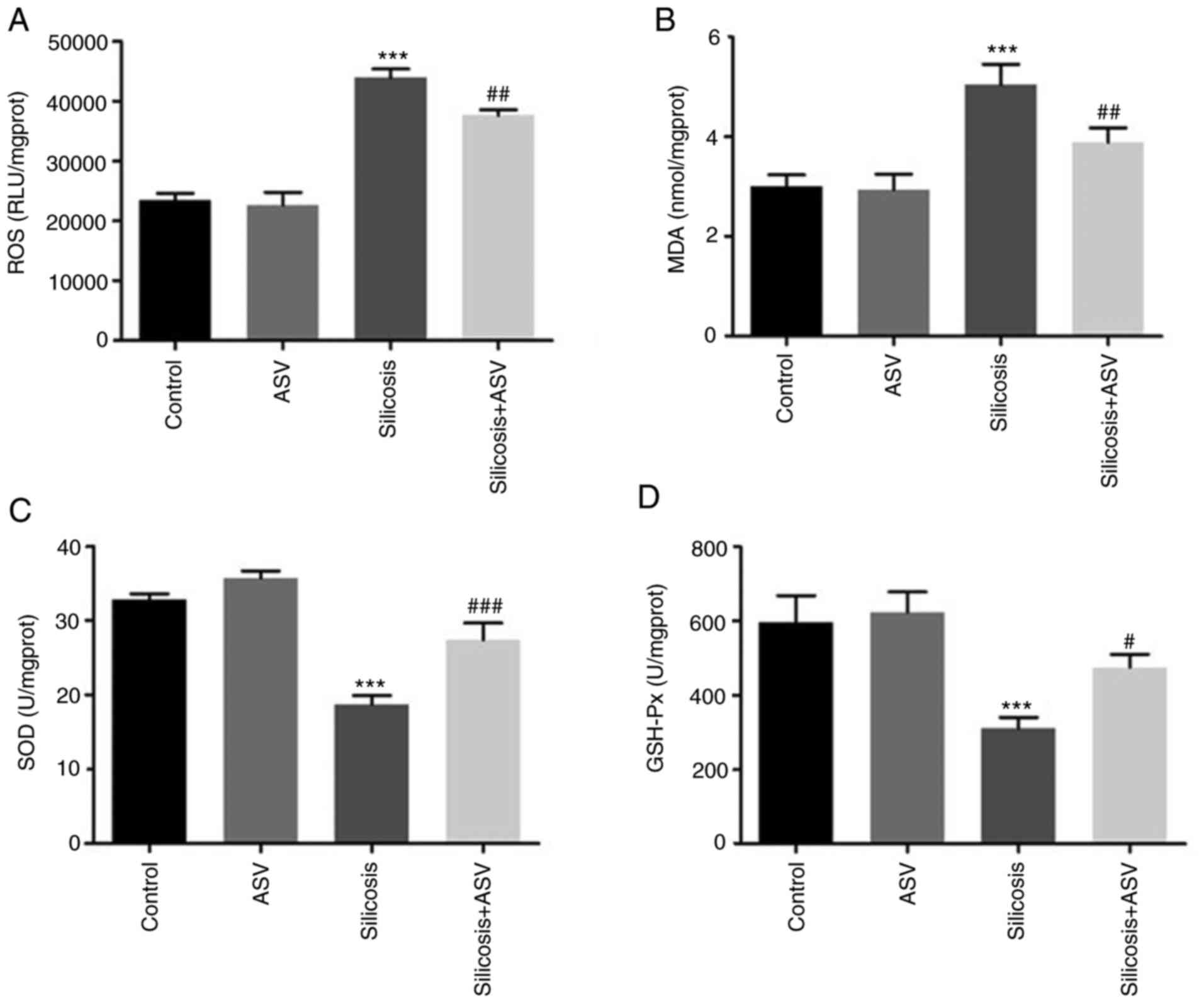
ASV-mediates an anti-pulmonary fibrosis
response via reduction of oxidative stress
As shown in Fig. 5A
and B, the ROS levels and MDA concentration in the silicosis
group were significantly higher compared with the control group,
and treatment with ASV significantly decreased the ROS and MDA
levels when compared with the silicosis group (all P<0.05).
Furthermore, there was a significant decrease in the SOD and GSH-px
concentrations in the silicosis group compared with the control
group (P<0.05). However, following treatment with ASV, an
increase in SOD and GSH-px levels was observed (Fig. 5C and D).
ASV suppresses silica-induced lung
fibrosis via the TGF-β1-Smads signaling pathway
The TGF-β/Smads signaling pathway is
well-established as the primary pathway under-lying pulmonary
fibrosis (21). To further
explore whether ASV exerted its anti-fibrotic effect via this
signaling pathway, the mRNA and protein expression levels of genes
associated with this pathway were assessed. At the transcriptional
level, the expression of TGF-β1, TGFβ1RI and TGFβ1RII increased
significantly following exposure to silica (Fig. 6A). Amongst the downstream genes,
there were no statistically significant differences in Smad2 and
Smad3 in any of the groups; however, the mRNA expression levels of
Smad7 were significantly downregulated (P<0.05). Interestingly,
the administration of ASV reversed the changes in the expression of
the above genes (Fig. 6B).
As shown in Fig.
7A-E, the protein expression levels of TGF-β1, TGFβ1RI,
TGFβ1RII, p-Smad2/Smad2 and p-Smad3/Smad3 were significantly
increased in the Silicosis group compared with the Control group,
whereas the Smad7 protein expression levels were decreased
significantly (P<0.05). Treatment with ASV significantly
decreased the protein expression levels of TGF-β1, TGFβ1RI,
TGFβ1RII, p-Smad2/Smad2 and p-Smad3/Smad3, and increased the
expression of Smad7 protein when compared the Silicosis group.
Discussion
A large body of data suggests that activated
fibroblasts serve a critical role in driving the development of
pulmonary fibrosis (22,23). Fibroblasts express high levels of
α-SMA, fibronectin and collagen, andpromote wound healing and
fibrosis remodeling. In addition, fibroblasts can cause excessive
deposition of ECM (collagen I and collagen III) (24). In the present study, it was shown
that exposure to silica caused severe pathological damage to lung
tissues, including increased formation of lung nodules, increased
infiltration of inflammatory cells, and thickening of the alveolar
walls and bronchial epithelial cells. However, treatment with ASV
alleviated these symptoms. Furthermore, it was shown that ASV
treatment reduced the expression of Collagen I, fibronectin and
α-SMA, consistent with a previous study, in which it was shown that
a-SMA, Collagen I, Collagen III, FSP-1, fibronectin and Vimentin
ablation attenuated fibrosis in lung tissue samples from mice
(25). Collectively, the above
data showed that ASV delayed silica-induced pulmonary fibrosis and
that it possesses anti-silicosis fibrosis effects.
Accumulating evidence has indicated that silica
induces inflammatory responses, including the secretion of
inflammatory factors (26) and
infiltration of inflammatory cells (27). In the present study, it was shown
that in the silicosis group, total cell counts, as well as the
neutrophil, lymphocyte and macrophage counts were high.
Interestingly, ASV treatment reduced the expression of these
cytological markers of lung injury. Additionally, the potential
role of androgens in targeting neutrophils, lymphocytes and
macrophages was determined, which is evident in the reduction of
inflammatory cell infiltration. A study by Hou et al
(28) showed that exposure to
crystalline silica particles increased TNF-α, IL-1β and IL-6,
levels, amongst other cytokines. TNF-α can accelerate EMT and
up-regulates TGF-β1 expression in primary mouse lung fibroblasts
(29) IL-1β can stimulate
collagen expression and notably induces tissue destruction,
accompanied by increased inflammation and collagen deposition
(30). Consistent with the in
vivo experiments, silicosis rats given ASV exhibited decreased
levels of TNF-α, IL-1β and IL-6 in BALF, suggesting that ASV can
delay the progression of pulmonary fibrosis by reducing the
inflammatory response in lung tissues.
Oxidative stress is one of the key features of
silica induced pulmonary fibrosis. Related studies have shown that
the contact between alveolar macrophages and silica during the
inflammatory process can produce oxidase, leading to the production
of high levels of ROS, thereby further promoting inflammation in a
feed-forward loop (31,32). Continued ROS production can cause
phagocytic cell death, inflammatory cell recruitment and silica
deposition, and cause irreversible lung damage (33). MDA is an indicator of oxidative
stress. It reflects the quantities of oxidized free radicals in the
body to a certain extent, and indirectly reflects the degree of
oxidative stress, which is negatively correlated with lung function
(34), and may lead to
irreversible lung injury (27).
Antioxidants, such as GSH-px and SOD, can not only resist the
direct damage caused by oxidants, but also alters the course of
inflammatory events associated with chronic lung diseases (27). In the present study, following
exposure to silica, there was a significant decrease in GSH-px and
SOD levels, as well as a significant increase in the levels of ROS
and MDA in silicotic patients. Conversely, ASV reversed the
expression of the above factors, suggesting that ASV could inhibit
pulmonary fibrosis by reducing oxidative stress.
There is evidence that TGF-β1 can regulate
epithelial cell apoptosis, fibroblast proliferation, myofibroblast
differentiation and collagen synthesis, thus it may be essential in
the progression of lung fibrosis in mice (35). In addition, TGF-β1 is upregulated
in lung tissues of patients with pulmonary fibrosis and
overexpression of active TGF-β1 induces prolonged and severe
interstitial lung fibrosis in rats (36). TGF-β1 is known to serve its
pro-fibrotic role by activating downstream mediators, including
Smad2 and Smad3, and is negatively regulated by Smad7 expression
(7,8). Specifically, TGF-β1 initially binds
to TGF-βII phosphorylating it to activate TGFβRI. Subsequently,
Smad2 and Smad3 are phosphorylated and activated by TGFβRI. The
phosphorylated Smad2 and Smad3 binds to Smad4 to form the Smads
complex, which is transferred to the nucleus by cytoplasmic nuclear
transporters and acts on the TGF-β1 target gene to regulate
transcription of target genes. When the external stimulus inducing
the signal ends, p-Smads are rapidly dephosphorylated, and the
Smads protein in the cytoplasm and nucleus is rapidly metabolized
by the ubiquitin-proteasome system, returning the cytoplasmic
levels to resting levels (29).
Under pathological conditions, the p-Smad2/Smad2 ratio and
p-Smad3/Smad3 ratio are increased. Chang et al (37) showed that following exposure to
100 µg/ml nano NiO, the phosphorylation levels of Smad2 and
Smad3 genes and proteins in A549 cells both increased, suggesting
that NiO leads to abnormal changes in Smad2 and Smad3. Consistent
with previous studies, silica increased the expression of TGF-β1,
TGF-βRI, TGF-βII, p-Smad2/Smad2 and p-Smad3/Smad3, and decreased
Smad7 levels in the present study. Interestingly, the addition of
ASV reversed the expression of these genes to a certain extent.
Collectively, the data indicated that ASV can inhibit
silica-induced pulmonary fibrosis by promoting negative feedback of
the TGF-β1/Smads signaling pathway and inhibiting its positive
feedback (Fig. 8).
In conclusion, ASV elicits its anti-pulmonary
fibrotic effect by decreasing silica-induced pulmonary fibrosis
inflammation and oxidative stress, and the mechanism underlying the
effects of ASV may involve the TGF-β1/Smads pathway. Thus, ASV may
serve as a promising treatment for the management of silicosis.
Funding
This study was supported by the general project of
National Natural Science foundation of China (grant no. 81973630)
and a grant from the Tai'an City Technology Development Program
(grant nos. 2019NS195 and 2018NS0170).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW, NNL and KW conceived and designed the present
study. FFF obtained the study materials and collected the patient
data. NNL and LW performed the experiments. NNL and XZ analyzed and
interpreted the data. All authors participated in writing the
manuscript. All authors have read and approved the final
manuscript. NNL and WW confirmed the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Hospital of Shandong University. All
procedures adhered to the recommendations described in the Guide
for the Care and Use of Laboratory Animals of the National
Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Zhao JQ, Li JG and Zhao CX: Prevalence of
pneumoconiosis among young adults aged 24-44 years in a heavily
industrialized province of China. J Occup Health. 61:73–81. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrante P: Asbestosis and silicosis
hospitalizations in Italy (2001-2015): Results from the national
hospital discharge registry. Eur J Public Health. 29:876–882. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reilly MJ, Timmer SJ and Rosenman KD: The
burden of silicosis in michigan: 1988-2016. Ann Am Thorac Soc.
15:1404–1410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Q, Liu Y, Pan H, Xu T, Li Y, Yuan J, Li
P, Yao W, Yan W and Ni C: Aberrant expression of miR-125a-3p
promotes fibroblast activation via Fyn/STAT3 pathway during
silica-induced pulmonary fibrosis. Toxicology. 414:57–67. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou X, Summer R, Chen Z, Tian Y, Ma J, Cui
J, Hao X, Guo L, Xu H, Wang H and Liu H: Lipid uptake by alveolar
macrophages drives fibrotic responses to silica dust. Sci Rep.
9:3992019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Y, He Z, Gao Y, Zheng R, Zhang X,
Zhao L and Tan M: Induced pluripotent stem cells inhibit
bleomycin-induced pulmonary fibrosis in mice through suppressing
TGF-β1/Smad-mediated epithelial to mesenchymal transition. Front
Pharmacol. 7:4302016. View Article : Google Scholar
|
|
7
|
Li PF, He RH, Shi SB, Li R, Wang QT, Rao
GT and Yang B: Modulation of miR-10a-mediated TGF-β1/Smads
signaling affects atrial fibrillation-induced cardiac fibrosis and
cardiac fibroblast proliferation. Biosci Rep. 39:BSR201819312019.
View Article : Google Scholar
|
|
8
|
Lu Y, Zhang T, Shan S, Wang S, Bian W, Ren
T and Yang D: MiR-124 regulates transforming growth factor-β1
induced differentiation of lung resident mesenchymal stem cells to
myofibroblast by repressing Wnt/β-catenin signaling. Dev Biol.
449:115–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellaye PS, Shimbori C, Upagupta C, Sato
S, Shi W, Gauldie J, Ask K and Kolb M: Lysyl oxidase-like 1 protein
deficiency protects mice from adenoviral transforming growth
factor-β1-induced pulmonary fibrosis. Am J Respir Cell Mol Biol.
58:461–470. 2018. View Article : Google Scholar
|
|
10
|
Li LC and Kan LD: Traditional Chinese
medicine for pulmonary fibrosis therapy: Progress and future
prospects. J Ethnopharmacol. 198:45–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng LQ, Tang JW, Wang Y, Zhao JR, Shang
MY, Zhang M, Liu SY, Qu L, Cai SQ and Li XM: Astragaloside IV
synergizes with ferulic acid to inhibit renal tubulointerstitial
fibrosis in rats with obstructive nephropathy. Br J Pharmacol.
162:1805–1818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu WN, Sun LF and Yang H: Inhibitory
effects of astragaloside IV on bleomycin-induced pulmonary fibrosis
in rats via attenuation of oxidative stress and inflammation.
Inflammation. 39:1835–1841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Chi YF, Yuan ZT, Zhou WC, Yin PH,
Zhang XM, Peng W and Cai H: Astragaloside IV inhibits renal
tubulointerstitial fibrosis by blocking TGF-β/Smad signaling
pathway in vivo and in vitro. Exp Biol Med (Maywood).
239:1310–1324. 2014. View Article : Google Scholar
|
|
14
|
Sangomla S, Saifi MA, Khurana A and Godugu
C: Nanoceria ameliorates doxorubicin induced cardiotoxicity:
Possible mitigation via reduction of oxidative stress and
inflammation. J Trace Elem Med Biol. 47:53–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsang CK, Liu Y, Thomas J, Zhang Y and
Zheng XF: Superoxide dismutase 1 acts as a nuclear transcription
factor to regulate oxidative stress resistance. Nat Commun.
5:34462014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Voicu SN, Balas M, Stan MS, Trică B,
Serban AI, Stanca L, Hermenean A and Dinischiotu A: Amorphous
silica nanoparticles obtained by laser ablation induce inflammatory
response in human lung fibroblasts. Materials (Basel). 12:10262019.
View Article : Google Scholar
|
|
18
|
Moron MS, Depierre JW and Mannervik B:
Levels of glutathione, glutathione reductase and glutathione
S-transferase activities in rat lung and liver. Biochim Biophys
Acta. 582:67–78. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Ni G, Liu Y, Han Y, Jia L and Wang
Y: Tanshinone IIA promotes axonal regeneration in rats with focal
cerebral ischemia through the inhibition of
nogo-A/NgR1/RhoA/ROCKII/MLC signaling. Drug Des Devel Ther.
14:2775–2787. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Chen Y, Hou Y, Song P, Zhou M, Nie
M and Liu X: Modulation of proliferation and differentiation of
gingiva-derived mesenchymal stem cells by concentrated growth
factors: Potential implications in tissue engineering for dental
regeneration and repair. Int J Mol Med. 44:37–46. 2019.PubMed/NCBI
|
|
21
|
Li N, Feng F, Wu K, Zhang H, Zhang W and
Wang W: Inhibitory effects of astragaloside IV on silica-induced
pulmonary fibrosis via inactivating TGF-β1/Smad3 signaling. Biomed
Pharmacother. 119:1093872019. View Article : Google Scholar
|
|
22
|
Bagnato G and Harari S: Cellular
interactions in the pathogenesis of interstitial lung diseases. Eur
Respir Rev. 24:102–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Yao W, Hou JY, Zhang L, Bao L, Chen
HT, Wang D, Yue ZZ, Li YP, Zhang M and Hao CF: Crystalline silica
promotes rat fibrocyte differentiation in vitro, and fibrocytes
participate in silicosis in vivo. Biomed Environ Sci. 30:649–660.
2017.PubMed/NCBI
|
|
24
|
Phillips RJ, Burdick MD, Hong K, Lutz MA,
Murray LA, Xue YY, Belperio JA, Keane MP and Strieter RM:
Circulating fibrocytes traffic to the lungs in response to CXCL12
and mediate fibrosis. J Clin Invest. 114:438–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng F, Shen Y, Mohanasundaram P,
Lindström M, Ivaska J, Ny T and Eriksson JE: Vimentin coordinates
fibroblast proliferation and keratinocyte differentiation in wound
healing via TGF-β-Slug signaling. Proc Natl Acad Sci USA.
113:E4320–E4327. 2016. View Article : Google Scholar
|
|
26
|
Stan MS, Sima C, Cinteza LO and
Dinischiotu A: Silicon-based quantum dots induce inflammation in
human lung cells and disrupt extracellular matrix homeostasis. FEBS
J. 282:2914–2929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang H, Chen M, Liu F, Wu H, Wang J, Chen
J, Liu M and Li X: N-acetylcysteine tiherapeutically protects
against pulmonary fibrosis in a mouse model of silicosis. Biosci
Rep. 39:BSR201906812019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou J, Ma T, Cao H, Chen Y, Wang C, Chen
X, Xiang Z and Han X: TNF-α-induced NF-κB activation promotes
myofibroblast differentiation of LR-MSCs and exacerbates
bleomycin-induced pulmonary fibrosis. J Cell Physiol.
233:2409–2419. 2018. View Article : Google Scholar
|
|
29
|
Zheng ZC, Zhu W, Lei L, Liu XQ and Wu YG:
Wogonin ameliorates renal inflammation and fibrosis by inhibiting
NF-κB and TGF-β1/Smad3 signaling pathways in diabetic nephropathy.
Drug Des Devel Ther. 14:4135–4148. 2020. View Article : Google Scholar :
|
|
30
|
Song C, He L, Zhang J, Ma H, Yuan X, Hu G,
Tao L, Zhang J and Meng J: Fluorofenidone attenuates pulmonary
inflammation and fibrosis via inhibiting the activation of NALP3
inflammasome and IL-1β/IL-1R1/MyD88/NF-κB pathway. J Cell Mol Med.
20:2064–2077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robinson JM: Reactive oxygen species in
phagocytic leukocytes. Histochem Cell Biol. 130:281–297. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
33
|
Lopes-Pacheco M, Ventura TG, de Oliveira
HD, Monção-Ribeiro LC, Gutfilen B, de Souza SAL, Rocco PRM,
Borojevic R, Morales MM and Takiya CM: Infusion of bone marrow
mono-nuclear cells reduces lung fibrosis but not inflammation in
the late stages of murine silicosis. PLoS One. 9:e1099822014.
View Article : Google Scholar
|
|
34
|
Kluchová Z, Petrásová D, Joppa P, Dorková
Z and Tkácová R: The association between oxidative stress and
obstructive lung impairment in patients with COPD. Physiol Res.
56:51–56. 2007.
|
|
35
|
Liu H, Fang S, Wang W, Cheng Y, Zhang Y,
Liao H, Yao H and Chao J: Macrophage-derived MCPIP1 mediates
silica-induced pulmonary fibrosis via autophagy. Part Fibre
Toxicol. 13:552016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo J, Fang Y, Jiang F, Li L, Zhou H, Xu X
and Ning W: Neohesperidin inhibits TGF-β1/Smad3 signaling and
alleviates bleomycin-induced pulmonary fibrosis in mice. Eur J
Pharmacol. 864:1727122019. View Article : Google Scholar
|
|
37
|
Chang X, Tian M, Zhang Q, Gao J, Li S and
Sun Y: Nano nickel oxide promotes epithelial-mesenchymal transition
through transforming growth factor β1/smads signaling pathway in
A549 cells. Environ Toxicol. 35:1308–1317. 2020. View Article : Google Scholar : PubMed/NCBI
|