Introduction
Myocardial injury is a common complication of
sepsis, and is independently associated with early, but not late
mortality and post-discharge cardiovascular morbidity (1). The pathological manifestations of
sepsis-induced cardiomyopathy (SIC) are as follows: Spot hemorrhage
on the myocardial surface, extensive myocardial hemorrhage,
inflammatory cell infiltration, interstitial edema and degeneration
and necrosis of some myocardial cells (2-4).
During the process of sepsis, bacterial infection activates
Toll-like receptor (TLR) via pathogen-related molecular patterns,
such as lipopolysaccharide (LPS), thereby inducing MAPK and NF-κB
pathway activation, which promotes the infiltration of inflammatory
cells into myocardial tissue and triggers myocardial inhibition
(5-9). Therefore, it is currently
hypothesized that the inhibition of the NF-κB and MAPK pathways can
effectively reduce the inflammatory response of the septic
myocardium and is one of the strategies used to improve SIC.
Dual specificity phosphatase-1 (DUSP1) is the first
member of the MAP kinase phosphatase (MKP) family, with a size of
1101 bp and encoding 367 amino acids. MKP is a member of the
bidirectional specificity Sue/tyrosine phosphatase family and is
comprised of 25 members, which results in both serine/threonine
phosphorylation and tyrosine phosphorylation (10,11). Studies have demonstrated that
DUSP1 can regulate the MAPK signaling pathway by regulating the
dephosphorylation of threonine/serine and tyrosine residues and
regulating cell proliferation, division and cytokine production
(12,13). Additionally, DUSP1 can act as a
direct target of p53, transcription factor E2F1, c-Jun and cyclic
AMP-dependent transcription factor ATF2 during sepsis, ischemia,
hypoxia, oxidative stress and other environmental effects (14,15).
MicroRNAs (miRs/miRNAs) are a type of endogenous
non-coding single-stranded small RNA with a length of 19-23
nucleotide sequences. They are highly conserved in evolution,
mainly through full or partial matching with the 3′-untranslated
region (3′-UTR) of target mRNAs to regulate physiological or
pathological processes in human genes (16). In recent years, an increasing
number of studies have demonstrated that miRNAs, such as miR-146b
(17), play an important role in
the diagnosis and treatment of SIC. Additionally, miR-101-3p, which
is an active form of miR-101, is widely found in eukaryotic cells
and can be abnormally expressed as a tumor suppressor in gastric
cancer (18) and breast cancer
(19). In non-tumor diseases,
miR-101-3p has been shown to be significantly upregulated in the
plasma of patients with adult onset Still's disease (AOSD) and to
positively correlate with the expression of inflammatory mediators
(20). Moreover, miR-101-3p also
affects other inflammation-related diseases, such as rheumatoid
arthritis (21) and heart
transplant recipients with histologically-verified acute cellular
rejection (22). However, to the
best of our knowledge, there are limited studies available
investigating the effects of miR-101-3p on the inflammatory
response post-SIC.
Therefore, the present study aimed to further
explore the effects of miR-101-3p in SIC and its possible
mechanisms both in vitro and in vivo.
Materials and methods
Patient data
A total of 27 patients with SIC admitted to the
Affiliated Hospital of Southwest Medical University between
January, 2017 to January, 2019 were selected as the study subjects.
Subjects included 16 males and 11 females, aged 21-56 years, with
an average age of 36.4±8.4 years. A total of 15 healthy volunteers
enrolled at the same hospital during the same time period were
included as the control group, including 10 males and 5 females,
aged 21-56 years, with an average age of 35.7±8.3 years. The
exclusion criteria were as follows: Pregnant females, organ
transplant recipients and acquired immunodeficiency syndrome. Prior
to the study, all participants signed informed consent forms and
agreed to the use of their samples in this scientific research.
Venous blood was obtained from all patients at the time of
diagnosis, and cardiac ultrasound was performed to examine cardiac
function within 24 h following diagnosis. The present study was
approved by the Ethics Committee of the Affiliated Hospital of
Southwest Medical University.
Establishment of an animal model of SIC
and drug administration
Sprague-Dawley male rats, weighing 220-250 g, were
purchased from the Nanjing Experimental Animal Center. All rats
were raised in a standard environment, and there were no marked
differences in the age and weight between the rats. The
Sprague-Dawley rats were randomly divided into the sham-operated
(sham) group (n=15) and the LPS group (20 mg/kg group; n=15). The
model of SIC was established according to a previous study
(23). Briefly, the rats in each
group were intraperitoneally injected with the corresponding dose
of LPS (20 mg/kg, Sigma-Aldrich; Merck KGaA) (23) or the same volume of saline. After
6 h, the cardiac function of each group of rats was detected by
ultrasound. The sepsis model was confirmed by the following: i)
Clinical signs, such as featuring malaise, fever, chills,
piloerection, generalized weakness and reduced gross motor
activity; ii) cardiac function examination by echocardiography
showing a reduced ejection fraction [EF (%)] and fractional
shortening [FS (%)]; iii) significantly increased levels of
pro-inflammatory cytokines in serum. miR-101-3p inhibitors were
obtained from Guangzhou RiboBio Co., Ltd. For the downregulation of
miR-101-3p, miR-101-3p inhibitors (10 nmol) or negative controls
(miR-101-3p NC, 10 nmol) in 50 µl PBS were administered to
the rats (n=20 in each group) via the caudal vein once every 3 days
(normal saline was used as a control) prior to LPS stimulation. The
animal experiments were approved by the Ethics Committee of the
Affiliated Hospital of Southwest Medical University. All
experimental procedures were performed in accordance with the
National Institutes of Health Guidelines for the Care and Use of
Laboratory Animals.
Examination of cardiac function
Echocardiography was performed using a Mylab 30 CV
ultrasound system (Esoate, S.P.A.) and a 10 mHz linear ultrasound
sensor. The procedure is briefly described as follows: Following
anesthesia [via an intraperitoneal injection of pentobarbital
sodium (50 mg/kg body weight)], the rats were shaved in the
anterior chest area and placed on a heating plate at 37°C with the
left side up. The probe was applied with a coupling agent and
pressed against the chest wall, and indexes, including EF (%), FS
(%) and heart rate were detected.
Cell culture and treatment
H9C2 human cardiomyocytes were obtained from the
Cell Bank of the Chinese Academy of Sciences. H9C2 cells were
cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with L-glutamine (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin, 100 mg/ml Sinopharm Chemical Reagent
and 10% FBS (Gibco; Thermo Fisher Scientific, Inc.). The cells were
incubated in a humidified incubator at 37°C with 5% CO2.
H9C2 cells were treated with 10 µg/ml LPS (Sigma-Aldrich;
Merck KGaA) for 24 h to establish an in vitro model of
LPS-induced sepsis.
Cell transfection
H9C2 cells were inoculated in a 6-well plate at a
density of 5×105 cells/ml and cultured at 37°C with 5%
CO2 for 24 h prior to transfection. miR-101-3p NC,
miR-101-3p inhibitors and short hairpin RNA (sh)-DUSP1 were
purchased from Guangzhou RiboBio Co., Ltd. The H9C2 cells were
transfected with the above-mentioned vectors (10 nmol/ml) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
transfection efficiency was measured by reverse
transcription-quantitative PCR (RT-qPCR). Following 48 h of
transfection, the cells were further cultured with fresh medium at
37°C and 5% CO2.
RT-qPCR
Total RNA was extracted from the myocardial tissues
and H9C2 cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Reverse transcription was performed using
MMLV reverse transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.) to generate first-strand cDNA. The ABI 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
SYBR premix Ex Taq II (Takara Biotechnology Co., Ltd.) was used to
perform RT-qPCR according to the manufacturer's instructions. The
following thermocycling conditions were used for qPCR:
Pre-denaturation at 95°C for 10 min, and 45 cycles of 95°C for 15
sec and 60°C for 15 sec. Fluorescent signals were obtained at 60°C.
GAPDH was used as the internal reference gene for DUSP1, IL-1β,
IL-6 and TNF-α expression. U6 was used as standardized internal
reference of miR-101-3p expression. Gene expression was calculated
using the 2−ΔΔCq method (24). Each experiment was repeated and
measured 3 times. Primers were designed using Primer 6.0 software
based on the serial number of each gene in GenBank. The following
primer pairs were used for qPCR: α-MHC forward, 5′-GCC TCT CTG ATG
GTT CAG C-3′ and reverse, 5′-TTA GAA TTT GCT TTG GGT G-3′; β-MHC
forward, 5′-TGA TAC GGC CGT GCC TCT -3′ and reverse, 5′-AGC TCT ACG
ATT GTG GCA TGC T-3′; S100A8 forward, 5′-CCG TCA GCC TGG TTG CTC
-3′ and reverse, 5′-CTT CAC CAG TCG ACG ATG -3′; S100A9 forward,
5′-GAT CGC CCT CTC TGT TTA AAG C-3′ and reverse, 5′-ATC CCA CCT TAT
GTG TCC TTG G-3′; DUSP1 forward, 5′-ATA TGT TGG AGG GAG ACG AC-3′
and reverse, 5′-ACA GAA GCC TGT TCC TGG TGT -3′; TNF-α forward,
5′-GAC CCC TTT ACT CTG ACC CC-3′ and reverse, 5′-AGG CTC CAG TGA
ATT CGG AA-3′; IL-1β forward, 5′-ACA GAT GAA GTG CTC CTT CCA -3′
and reverse, 5′-GTC GGA GAT TCG TAG CTG GA-3′; IL-6 forward, 5′-TGT
CTT CCT CAC CGA TTC CT-3′ and reverse, 5′-ACC ACC CGA GCT CTG TCT
TAC TC-3′; GAPDH forward, 5′-TAT GAT GAT ATC AAG AGG GTA GT-3′ and
reverse, 5′-TGT ATC CAA ACT CAT TGT CAT AC-3′; miR-101-3p forward,
5′-GGA CTT TCT TCA TTC ACA CCG -3′ and reverse, 5′-GAC CAC TGA GGT
TAG AGC CA-3′ and U6 forward, 5′-GCT TCG GCA GCA CAT ATA CTA AAA
T-3′ and reverse, 5′-CGC TTC ACG AAT TTG CGT GTC AT-3′.
ELISA
ELISA kits (Wuhan Boster Biological Technology,
Ltd.) were used to detect the levels of the inflammatory factors,
IL-1β, IL-6 and TNF-α, in the serum of patients with SIC or healthy
controls (HCs) according to the manufacturer's protocols. The
measured optical density value (at a wavelength of 450 nm) was
converted to the concentration value through the Multiskan FC
Microplate Reader (Thermo Fisher Scientific, Inc.). The experiment
was repeated 3 times.
Western blot analysis
The undetermined cells were washed 3 times with PBS,
and total protein was extracted using RIPA buffer (Beyotime
Institute of Biotechnology, Inc.) and protease inhibitor PMSF or
cocktail (Roche Diagnostics). The concentration of total protein
was determined by the BSC method (Beyotime Institute of
Biotechnology, Inc.). Proteins were denatured at 100°C for 5 min.
Equal amounts of protein (50 µg) were separated by 10%
SDS-PAGE and transferred to PVDF membranes. Subsequently, PVDF
membranes were blocked with 5% skim milk for 2 h. The membranes
were incubated with anti-MKP-1 (DUSP1) antibody (cat. no. ab1351;
1:1,000; Abcam), anti-p38 antibody (cat. no. ab31828; 1:1,000;
Abcam), anti-phosphorylated-p38 antibody (cat. no. ab178867;
1:1,000; Abcam), anti-NF-κB p65 antibody (cat. no. ab140751;
1:1,000; Abcam), anti-phosphorylated-NF-κB p65 antibody (cat. no.
ab76302; 1:1,000; Abcam) and anti-GAPDH anti-body (cat. no. ab9485;
1:2,000; Abcam) overnight at 4°C. Following washing, membranes were
incubated with corresponding secondary antibodies [goat anti-rabbit
IgG H&L (HRP), cat. no. ab205718, 1:2,000, Abcam; goat
anti-mouse IgG H&L (HRP), cat. no. ab6789, 1:2,000, Abcam] at
room temperature for 2 h. Protein bands were visualized with drip
developer development (GS-700, Bio-Rad Laboratories, Inc.), and
Molecular Analyst (IBM, Inc.) was used for densitometry. The
experiment was repeated 3 times.
Luciferase reporter gene assay
The coding sequence of human DUSP1 3′-UTR was
obtained from GenBank and analyzed by bioinformatics analysis. The
potential binding sites between miR-101-3p with DUSP1 was analyzed
using TargetScan 6.0 (http://www.targetscan.org). The wild-type DUSP1
(DUSP1-WT) 3′-UTR fragment and the mutant-type DUSP1 (DUSP1-MUT)
3′-UTR fragment were cloned into a pGL3-control vector downstream
(GeneCopoeia, Inc.) of the luciferase coding sequence to obtain
LuDP recombinant plasmids. H9C2 cells were inoculated into 96-well
plates and transfected with miR-101-3p mimics or miR-101-3p NC or
DUSP1-WT plasmids or DUSP1-MUT plasmids (100 nM aliquots),
respectively. Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used as transfection reagent. The
luciferase activity of the cells was measured at 24 h following
transfection using a dual luciferase reporter gene system (Promega
Corporation). The experiment was repeated 3 times, and the blank
group was used for normalization.
Hematoxylin and eosin (H& E) staining
and immunohistochemistry (IHC)
After 6 h of LPS stimulation, the rats in each group
were sacrificed using Nembutal (intraperitoneal sodium
pentobarbital; 80 mg/kg/body weight). Subsequently, the rats
underwent cardiac perfusion with at least 100 ml saline, and heart
tissues were obtained, fixed with paraformaldehyde and embedded in
paraffin.
H&E staining was then performed to assess
pathological changes. Briefly, the tissues were continuously
sectioned into coronary sections of approximately 5 µm in
thickness. The sections were then routinely dewaxed with xylene and
hydrated with gradient alcohol and finally rinsed with distilled
water. They sections were then immersed in hematoxylin dye solution
for 3-8 min and washed at room temperature, and then differentiated
by 1% hydrochloric acid alcohol for several seconds, then washed
with water. After washing for 10-15 min using a small water flow,
the slices were placed into eosin dye solution for 1-3 min at room
temperature. The slices were then dehydrated and transparent by
alcohol and xylene for 5 min each, and the slices were then dried
and sealed with neutral gum. A microscope (Zeiss AxioImager Z1) was
used for observation and image analyses were conducted.
Additionally, IHC was performed to detect caspase
3-and TUNEL-labeled apoptotic cells and DUSP1 expression in heart
tissues according to a previous study (25). Briefly, the tissues were
continuously sectioned into coronary sections of approximately 5
µm in thickness. Subsequently, the sections were routinely
dewaxed with xylene and hydrated with gradient alcohol. After the
sections were blocked with 3% H2O2 for 10
min, the endogenous peroxidase was inactivated, and 0.01 mol/l
sodium citrate buffer was used for microwave repair (pH 6.0, 15
min). After blocking with 5% bovine serum albumin (BSA) for 20 min,
the primary antibodies were added and incubated with the sections
overnight at 4°C. On the following day, goat anti-rabbit IgG
H&L (HRP) (ab6721, 1:100, Abcam) was added and incubated with
the sections at room temperature for 60 min, and the sections were
then developed with DAB after being washed with PBS. Following
hematoxylin counterstaining at room temperature for 5-8 min, the
sections were dehydrated and transparentized, and mounted for
microscopic examination (Zeiss AxioImager Z1). Image-Pro Plus image
analysis software (MediaCybemetics, Inc.) was employed to analyze
the number of positive cells. The following primary antibodies were
used: Anti-caspase 3 (cat. no. ab4051; 1:200; Abcam) and anti-MKP-1
(DUSP1; cat. no. ab1351; 1:100; Abcam). The TUNEL Assay kit was
also used (cat. no. ab206386; Abcam).
Statistical analysis
SPSS 21.0 was used for statistical analysis (IBM
Corp.). Data are expressed as the means ± SD. A Student's t-test
was used to compare differences between 2 groups, and one-way ANOVA
was used for the analysis of multiple groups, followed by Tukey's
post hoc test. Pearson's correlation analysis was performed to
determine the correlation between cytokine levels and miR-101-3p
expression in tissues from different patients or rats. P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-101-3p expression is increased in
patients with SIC
To preliminarily assess he expression of miR-101-3p
in SIC, the expression levels of miR-101-3p in the serum of
patients with SIC and HCs were detected. The results revealed that
miR-101-3p expression in the serum of patients with SIC was
significantly upregulated (Fig.
1A). Moreover, the expression of the pro-inflammatory factors,
IL-1β, IL-6, and TNF-α, in serum was detected by EILSA. The results
revealed that the expression levels of the pro-inflammatory
factors, IL-1β, IL-6, and TNF-α, were all significantly upregulated
in the serum of patients with SIC compared with the HCs (Fig. 1B). Further analysis revealed that
there was a significant positive correlation between miR-101-3p,
and IL-1β, IL-6 and TNF-α expression (P<0.05; Fig. 1C-E). These data indicated that
miR-101-3p may play a role in SIC by modulating inflammation.
Upregulation of miR-101-3p expression in
the myocardial tissue of rats administered LPS
To further investigate the role of miR-101-3p in
sepsis-induced myocardial injury, a rat model of SIC was
established. Ultrasound was used to evaluate the cardiac function
of the rats. The results revealed that the EF (%) and FS (%) of the
rats in the LPS group were significantly lower compared with those
of the rats in the sham group (P<0.05; Fig. 2A-C). Moreover, the results of
H&E staining and IHC indicated that there was significant
myocardial cell injury, cell apoptosis and inflammatory cell
infiltration in the rats in the LPS group (Fig. 2D-F). These data confirmed that the
model of SIC was successfully established. Furthermore, miR-101-3p
expression in the rat myocardial tissues was detected. The results
revealed that compared with the sham group, the miR-101-3p levels
in the LPS group were significantly increased (P<0.05; Fig. 2G). Therefore, the aforementioned
data indicated that miR-101-3p plays a role in the process of
sepsis-induced myocardial injury.
 | Figure 2Expression of miR-101-3p in
myocardial tissues in a rat model of sepsis-induced MI. LPS (20
mg/kg) was administered to rats to establish a model of
sepsis-induced MI. (A-C) Ultrasound was used to evaluate rat
cardiac functions. The EF (%) and FS (%) were measured. (D and E)
H&E staining, IHC and RT-qPCR were used to detect the (D)
histopathological changes of cardiac tissues, and (E) caspase 3-
and (F) TUNEL-labeled cardiac apoptotic cells. (G) miR-101-3p
expression in the myocardial tissue of rats was detected by
RT-qPCR. **P<0.01 and ***P<0.001. miR,
microRNA; MI, myocardial injury; LPS, lipopolysaccharide; EF,
ejection fraction; FS, fractional shortening; H&E, hematoxylin
and eosin; IHC, immunohistochemistry; RT-qPCR, reverse
transcription-quantitative PCR. |
Inhibition of miR-101-3p significantly
attenuates LPS-induced myocardial injury
To evaluate the effects of miR-101-3p on the cardiac
function of rats with sepsis-induced myocardial injury, the rats
were treated with miR-101-3p inhibitors to inhibit miR-101-3p
expression. The results of RT-qPCR revealed that miR-101-3p
expression was significantly downregulated following treatment with
miR-101-3p inhibitors compared with the LPS + saline or miR-101-3p
NC group (Fig. 3A). Further
assessment of the cardiac function of the rats revealed that the
inhibition of miR-101-3p significantly enhanced the EF (%) and FS
(%) compared with the LPS + saline or miR-101-3p NC group (Fig. 3B and C). Histopathological
analysis revealed that the downregulation of miR-101-3p suppressed
cardiac cell damage, inflammatory cell infiltration and apoptosis
(Fig. 3D-F). In addition, RT-qPCR
was used to detect changes in the expression of a-myosin heavy
chain (α-MHC) and β-MHC in myocardial tissues. Following the
addition of miR-101-3p inhibitors, the α-MHC and β-MHC levels were
significantly upregulated, while those of S100A8 and S100A9 were
significantly downregulated compared with the LPS + saline or
miR-101-3p NC groups (P<0.05; Fig.
3G and H). ELISA was also used to detect the expression of
IL-1β, IL-6 and TNF-α. The expression levels of IL-1β, IL-6 and
TNF-α were significantly downregulated following the addition of
miR-101-3p inhibitor compared with the LPS + saline or miR-101-3p
NC groups (P<0.05; Fig. 3I).
Taken together, these data indicated that the downregulation of
miR-101-3p exerted protective effects against sepsis-induced
myocardial injury by mitigating inflammation and apoptosis.
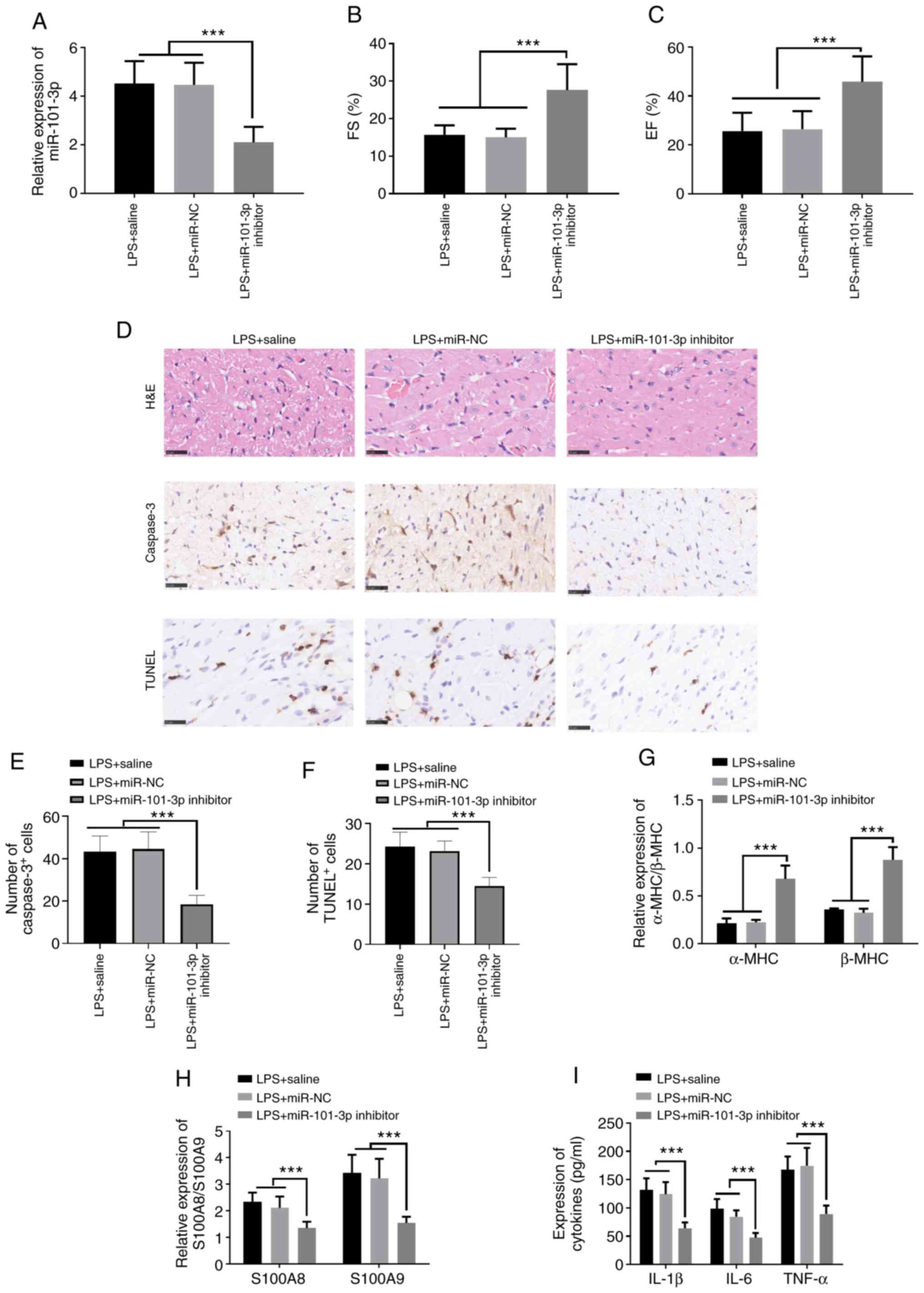 | Figure 3Regulatory effects of miR-101-3p on
cardiac function in rats with LPS-induced SIC. miR-101-3p
inhibitors (10 nmol) or negative controls (miR-101-3p NC; 10 nmol)
in 50 µl PBS were administered into the caudal veins of rats
once for 3 days, and were then treated with LPS (20 mg/kg) for 6 h.
(A) The expression levels of miR-101-3p was detected using RT-qPCR.
(B and C) Ultrasound was used to evaluate rat cardiac function. The
EF (%) and FS (%) were measured. (D and E) H&E staining, IHC
and RT-qPCR were used to detect (D) the histopathological changes
of cardiac tissues and (E and F) the apoptosis of cardiac cells. (G
and H) Differential expression of α-MHC, β-MHC, S100A8 and S100A9
in the myocardial tissues of different groups of rats detected by
RT-qPCR. (I) Differential expression of IL-1β, IL-6, and TNF-α in
the myocardial tissues of rats in different groups detected using
ELISA. ***P<0.001. miR, microRNA; LPS,
lipopolysaccharide; SIC, sepsis-induced cardiomyopathy; EF,
ejection fraction; FS, fractional shortening; H&E, hematoxylin
and eosin; IHC, immunohistochemistry; RT-qPCR, reverse
transcription-quantitative PCR; MHC, myosin heavy chain. |
DUSP1 is a functional target of
miR-101-3p
To verify the specific mechanisms through which
miR-101-3p improves LPS-induced myocardial function in rats, the
downstream targets of miR-101-3p were analyzed using TargetScan 7.1
(http://www.targetscan.org/vert_71/).
The results revealed that DUSP1 was one of the targets of
miR-101-3p (Fig. 4A). To further
validate the targeting association between miR-101-3p and DUSP1, a
luciferase activity assay was performed. The results demonstrated
that compared with the control group, the luciferase activity of
the DUSP1 wild-type (DUSP1-WT) group was significantly decreased
following co-transfection with miR-101-3p mimics, while the
luciferase activity of the DUSP1 mutant (DUSP1-MT) group exhibited
no significant change (Fig. 4B).
Furthermore, RT-qPCR, IHC and western blot analysis were performed
to detect the levels of DUSP1, MAPK-p38 and NF-κB in cardiac
tissues. Compared with the sham group, DUSP1 expression was
significantly downregulated in the LPS group (Fig. 4C-E), while the levels of
phosphorylated MAPK-p38 and NF-κB were upregulated (Fig. 4E). However, the inhibition of
miR-101-3p enhanced the expression of DUSP1, while downregulating
the expression of phosphorylated MAPK-p38 and NF-κB (Fig. 4C-E). These data indicated that
miR-101-3p may exerted its effects in SIC by targeting DUSP1.
Downregulation of DUSP1 activates the
MAPK-p38 and NF-κB pathways
MAPK-p38 and NF-κB are important signaling pathways
associated with myocardial cell injury (6). In the present study, to further
verify the function of DUSP1, a cell model using H9C2 cells in
which DUSP1 was downregulated was constructed (Fig. 5A). Furthermore, the H9C2 cells
were stimulated with LPS. The results revealed that compared with
the control group, both the LPS and sh-DUSP1 groups exhibited a
significantly increased expression of inflammatory cytokines,
increased levels of phosphorylated MAPK-p38 and NF-κB, and
decreased levels of DUSP1 (Fig. 5B
and C). Moreover, compared with the LPS group, the levels of
inflammatory cytokines and phosphorylated MAPK-p38 and NF-κB were
further enhanced in the LPS + sh-DUSP1 group. Hence, DUSP1 also
plays a role in controlling the inflammatory response in H9C2 cells
by modulating the MAPK-p38 and NF-κB pathways.
Downregulation of miR-101-3p protects
against LPS-induced myocardial cell injury dependently via
DUSP1
To further examine the effects of miR-101-3p on
targeting the DUSP1/MAPK-p38/NF-κB pathway in myocardial injury,
the H9C2 cells transfected with sh-DUSP1 and/or miR-101-3p were
stimulated with LPS. The results revealed that miR-101-3p
expression was significantly suppressed by miR-101-3p inhibitors
with or without LPS stimulation (P<0.05; Fig. 6A and B). RT-qPCR was then
performed to detect the expression levels of IL-1β, IL-6, TNF-α,
α-MHC, β-MHC, S100A8 and S100A9. The results revealed that compared
with the control group, the expression of IL-1β, IL-6, TNF-α,
S100A8 and S100A9 in the LPS group was significantly upregulated,
while the levels α-MHC and β-MHC were significantly downregulated
(P<0.05; Fig. 6C-G).
Additionally, compared with the LPS group, the levels of IL-1β,
IL-6, TNF-α, S100A8 and S100A9 were downregulated, while those of
α-MHC and β-MHC were significantly upregulated following
transfection with miR-101-3p inhibitors (P<0.05; Fig. 6C-G). However, the expression
levels of IL-1β, IL-6, TNF-α, S100A8, S100A9, α-MHC and β-MHC in
the LPS + sh-DUSP1 + miR-101-3p inhibitor group did not
significantly differ compared with those in the LPS + sh-DUSP1
group (Fig. 6C-G). These data
suggest that miR-101-3p exerts its effects dependently by
suppressing DUSP1.
 | Figure 6Downregulation of miR-101-3p protects
against LPS-induced myocardial cell injury dependently via DUSP1.
H9C2 cells transfected with sh-DUSP1 and/or miR-101-3p were treated
with LPS (10 µg/ml). (A-G) Reverse
transcription-quantitative PCR was used to detect the expression of
(A and B) miR-101-3p, (C) IL-1β, (D) IL-6, (E) TNF-α, (F) α-MHC and
β-MHC, (G) S100A8 and S100A9. n.s, not significant (P>0.05);
***P<0.001 vs. con. #P<0.05;
##P<0.01 and ###P<0.001 vs. LPS. DUSP1, dual
specificity phosphatase 1; LPS, lipopolysaccharide; sh, short
hairpin RNA; MHC, myosin heavy chain; miR, microRNA. |
Discussion
The present study demonstrated that miR-101-3p
expression was significantly elevated in SIC and was positively
associated with the degree of systemic inflammatory response.
Further experiments suggested that miR-101-3p activated the
MAPK-p38 and NF-κB pathways by inhibiting DUSP1 in cardiomyocytes,
thereby aggravating sepsis-induced myocardial injury.
Septic shock is a common and critical illness in
clinical practice with a high mortality rate. To date, there is
still a lack of clinical treatments with high efficacy and minimal
side-effects. Bacterial infection is the most common cause of
septic shock. As a bacterial product, LPS can directly or
indirectly stimulate monocytes, polymorphonuclear neutrophils,
endothelial cells and other myocardial cells to initiate the
inflammatory process, produce proinflammatory cytokines and
increase inflammatory response (25,26). Cardiomyocytes also respond to
sepsis during the early stage, which is closely associated with
disease prognosis. In particular, SIC, which is a highly monitored
disease in the intensive care unit, is characterized by myocardial
contractile function impairment, cardiac enlargement, decreased
ejection fraction, poor response to volumetric load contraction and
decreased peak contraction pressure/end-systolic volume ratio
(27,28). Therefore, the pathophysiological
mechanism of SIC has been the focus of studies.
Studies have indicated that miRNAs play a crucial
role in the regulation of the SIC inflammatory response,
accompanied by dynamic changes in miRNA expression. miRNAs, as
novel biomarkers of sepsis, can be used for severity grading, early
diagnosis, treatment response and prognosis evaluation of SIC.
However, the effects of miRNAs on SIC have not been systematically
studied (29). Wang et al
demonstrated that miR-21-3p expression is increased in animal
models of LPS-induced SIC, whereas inhibitors of miR-21-3p can
significantly improve cardiac function and survival in SIC
(30). However, knowledge of the
role of miR-101-3p in sepsis is limited. The results of the present
study demonstrated that miR-101-3p expression was significantly
increased in patients with SIC and positively correlated with the
expression of IL-1β, IL-6 and TNF-α, and other inflammatory
factors, but was negatively associated with cardiac function. This
suggests that miR-101-3p plays a role in promoting disease
progression in SIC and may also be an indicator for SIC diagnosis
and prognosis.
As a mature form of miR-101, decreased levels of
miR-101-3p have been shown to exert tumor suppressor functions in
numerous malignant tumors, and to regulate proliferation, invasion
and metastasis (20,21). However, to the best of our
knowledge, the role of miR-101-3p in inflammatory diseases has been
rarely reported. For instance, Qin et al reported that in a
rat model of ischemia-reperfusion injury, zinc exerted protective
effects by suppressing miR-101-3p, which promoted oxidative stress
by activating nuclear factor erythroid 2-related factor 2 and NF-κB
levels in TM3 cells (31). In
another study, miR-101-3p was shown to reduce joint swelling and
the arthritic index in rats with rheumatoid arthritis by
downregulating prostaglandin G/H synthase 2 (21). Nevertheless, miR-101-3p expression
has been shown to be significantly higher in heart transplant
recipients with histologically verified acute cellular rejection
(22), as well as in plasma of
patients with AOSD compared with HCs (20). Thus, the effect of miR-101-3p in
various diseases may differ. The results of the presents study
demonstrated that miR-101-3p exerted significant protective effects
against sepsis-induced myocardial injury by suppressing
inflammation and apoptosis, suggesting that miR-101-3p may be a
valuable therapeutic target for sepsis-induced myocardial
injury.
DUSP1 exerts a significant effect on the
anti-inflammatory environment. For example, DUSP1 can regulate the
pro-inflammatory environment of head and neck squamous cell
carcinoma, thereby preventing cancer progression; it can also act
on dexamethasone to improve the anti-inflammatory effects of drugs
(32). Moreover, a previous study
demonstrated that miR-127 can mediate the polarization of
macrophages and facilitate lung inflammation and injury by
activating the JNK pathway and downregulating Bcl6 or DUSP1
expression (33). Additionally,
MKP-1 knockdown re-phosphorylates p38 and restores the capacity to
translate TNF-α and IL-6 mRNAs (34). These results indicate that DUSP1
exerts anti-inflammatory effects by phosphorylating and
inactivating members of the MAPK family. The present study found
that miR-101-3p can directly bind to the 3′-UTR region of DUSP1,
thereby suppressing DUSP1 in cardiac cells. Moreover, the
downregulation of miR-101-3p significantly upregulated the DUSP1
levels and suppressed the activation of the MAPK-p38/NF-κB pathway.
Furthermore, the present in vitro experiments indicated that
the downregulation of DUSP1 led to a significant increase in the
inflammatory response and MAPK-p38/NF-κB pathway activation.
Furthermore, the protective effects of miR-101-3p inhibition were
attenuated following the decreased expression of DUSP1. Therefore,
the inhibition of miR-101-3p inhibits the inflammatory response of
cardiomyocytes and reduces myocardial injury in SIC dependently by
enhancing DUSP1 expression.
The two MHC isoforms identified in mammalian
ventricles, α-MHC and β-MHC, contribute to a large difference in
heart rate (35). Moreover, α-MHC
is mainly found in embryonic cardiomyocytes, while β-MHC is mostly
expressed in mature cardiomyocytes. The β-MHC protein content
reflects the differentiation of MSCs into cardiomyocytes to a
certain extent, and its gene expression level is one of the
important indicators for observing the differentiation of MSCs into
mature cardiomyocytes (36). A
previous study found that the upregulation of α-MHC and β-MHC
promoted the cardiac differentiation of mouse embryonic stem cells
(37). Furthermore, in a model of
LPS-induced sepsis, α-MHC and β-MHC were shown to both be
significantly downregulated (38), which was also identified in the
present study. The present study also found that the inhibition of
miR-101-3p promoted the α-MHC and β-MHC levels in the myocardial
tissues and cardiomyocytes.
The S100 subfamily which belongs to the EF-hand
family has been found to play a vital role in modulating heart
functions (39). Notably, several
S100 members, including S100A8, S100A9, S100A11 and S100A12 are
proved as biomarkers in sepsis (40,41). In addition, the downregulation of
the S100 subfamily can exert protective effects against sepsis. For
instance, pentamidine has been shown to ameliorate cecal ligation
and puncture (CLP)-induced brain damage by mitigating gliosis and
S100B/RAGE/NF-κB pathway activation (42). Alamandine has been shown to
attenuate sepsis-induced S100A8 and S100A9 expression by
suppressing MAPKs (38). The
present study also found that S100A8 and S100A9 were overexpressed
in the heart tissues and cardiomyocytes subjected to LPS. However,
miR-101-3p inhibition markedly suppressed S100A8 and S100A9 and
MAPK-p38 activation. Hence, the data further elucidated the
underlying mechanisms of the protective effects of the inhibition
of miR-101-3p against sepsis-induced heart injury.
In conclusion, the present study demonstrated that
the inhibition of the miR-101-3p pathway can effectively inhibit
the inflammatory response and myocardial cell damage in SIC,
improve the cardiac function of SIC and play a role in myocardial
protection. The mechanism involved may that the inhibition of
miR-101-3p suppresses the SIC-induced inflammatory response by
targeting the MAPK-p38/NF-κB pathway via DUSP1. These findings also
suggested that the inhibition of miR-101-3p may be a potential
important target for the treatment and diagnosis of SIC.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FH had full access to all of the data in the study
and all authors take responsibility for the integrity of the data
and the accuracy of the data analysis. YX and LT were involved in
the acquisition, analysis, or interpretation of the data. YX, JC
and DC conducted RT-qPCR, H&E staining and the related data
analysis, and they were involved in the drafting of the manuscript.
YX and WW were involved in performing the statistical analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Southwest Medical
University. Prior to the study, all participants signed informed
consent forms and agreed to the use of their samples in this
scientific research. The animal experiments were approved by the
Ethics Committee of the Affiliated Hospital of Southwest Medical
University. All experimental procedures were performed in
accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Frencken JF, Donker DW, Spitoni C,
Koster-Brouwer ME, Soliman IW, Ong DSY, Horn J, van der Poll T, van
Klei WA, Bonten MJM and Cremer OL: Myocardial injury in patients
with sepsis and its association with long-term outcome. Circ
Cardiovasc Qual Outcomes. 11:e0040402018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lv X and Wang H: Pathophysiology of
sepsis-induced myocardial dysfunction. Mil Med Res.
3:302016.PubMed/NCBI
|
|
3
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao C, Zhang Y, Chai Y, Wang L, Yin C,
Shou S and Jin H: Attenuation of sepsis-induced cardiomyopathy by
regulation of microRNA-23b is mediated through targeting of
MyD88-mediated NF-κB activation. Inflammation. 42:973–986. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ouyang MZ, Zhou D, Zhu Y, Zhang M and Li
L: The inhibition of MyD88 and TRIF signaling serve equivalent
roles in attenuating myocardial deterioration due to acute severe
inflammation. Int J Mol Med. 41:399–408. 2018.
|
|
6
|
Zeng M, Zhang B, Li B, Kan Y, Wang S, Feng
W and Zheng X: Adenosine attenuates LPS-induced cardiac dysfunction
by inhibition of mitochondrial function via the ER pathway. Evid
Based Complement Alternat Med. 2019:18320252019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S and Fan B: Myricetin protects
cardiomyocytes from LPS-induced injury. Myricetin schützt
Kardiomyozyten vor LPS-induzierten Verletzungen. Herz. 43:265–274.
2018. View Article : Google Scholar
|
|
8
|
Zheng Z, Ma H, Zhang X, Tu F, Wang X, Ha
T, Fan M, Liu L, Xu J, Yu K, et al: Enhanced glycolytic metabolism
contributes to cardiac dysfunction in polymicrobial sepsis. J
Infect Dis. 215:1396–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopolysaccharide (LPS) aggravates high glucose- and
hypoxia/reoxygenation-induced injury through activating
ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019:81518362019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wancket LM, Frazier WJ and Liu Y:
Mitogen-activated protein kinase phosphatase (MKP)-1 in immunology,
physiology, and disease. Life Sci. 90:237–248. 2012. View Article : Google Scholar
|
|
11
|
Boutros T, Chevet E and Metrakos P:
Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase
regulation: Roles in cell growth, death, and cancer. Pharmacol Rev.
60:261–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Low HB and Zhang Y: Regulatory roles of
MAPK phosphatases in cancer. Immune Netw. 16:85–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoppstädter J and Ammit AJ: Role of
dual-specificity phosphatase 1 in glucocorticoid-driven
anti-inflammatory responses. Front Immunol. 10:14462019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kristiansen M, Hughes R, Patel P, Jacques
TS, Clark AR and Ham J: Mkp1 is a c-Jun target gene that
antagonizes JNK-dependent apoptosis in sympathetic neurons. J
Neurosci. 30:10820–10832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rastogi R, Jiang Z, Ahmad N, Rosati R, Liu
Y, Beuret L, Monks R, Charron J, Birnbaum MJ and Samavati L:
Rapamycin induces mitogen-activated protein (MAP) kinase
phosphatase-1 (MKP-1) expression through activation of protein
kinase B and mitogen-activated protein kinase kinase pathways. J
Biol Chem. 288:33966–33977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Catalanotto C, Cogoni C and Zardo G:
MicroRNA in control of gene expression: An overview of nuclear
functions. Int J Mol Sci. 17:17122016. View Article : Google Scholar :
|
|
17
|
Wang X and Yu Y: MiR-146b protect against
sepsis induced mice myocardial injury through inhibition of Notch1.
J Mol Histol. 49:411–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu X, Zhou J, Wu Z, Chen C, Liu J, Wu G,
Zhai J, Liu F and Li G: miR-101-3p suppresses HOX transcript
antisense RNA (HOTAIR)-induced proliferation and invasion through
directly targeting SRF in gastric carcinoma cells. Oncol Res.
25:1383–1390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CY, Xiong DD, Huang CQ, He RQ, Liang
HW, Pan DH, Wang HL, Wang YW, Zhu HW and Chen G: Clinical value of
miR-101-3p and biological analysis of its prospective targets in
breast cancer: A study based on the cancer genome atlas (TCGA) and
bioinformatics. Med Sci Monit Med Sci Monit. 23:1857–1871. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu Q, Gong W, Gu J, Geng G, Li T, Tian R,
Yang Z, Zhang H, Shao L, Liu T, et al: Plasma microRNA profiles as
a potential biomarker in differentiating adult-onset still's
disease from sepsis. Front Immunol. 9:30992019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei Q, Lv F, Zhang H, Wang X, Geng Q,
Zhang X, Li T, Wang S, Wang Y and Cui Y: MicroRNA-101-3p inhibits
fibroblast-like synoviocyte proliferation and inflammation in
rheumatoid arthritis by targeting PTGS2. Biosci Rep.
40:BSR201911362020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sukma Dewi I, Hollander Z, Lam KK, McManus
JW, Tebbutt SJ, Ng RT, Keown PA, McMaster RW, McManus BM, Gidlöf O
and Öhman J: Association of serum MiR-142-3p and MiR-101-3p levels
with acute cellular rejection after heart transplantation. PLoS
One. 12:e01708422017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Liu P, Feng X and Ma C:
Salidroside suppressing LPS-induced myocardial injury by inhibiting
ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol
Med. 21:3178–3189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Jiang Q, Chen J, Long X, Yao X, Zou X,
Yang Y, Huang G and Zhang H: Phillyrin protects mice from traumatic
brain injury by inhibiting the inflammation of microglia via PPARγ
signaling pathway. Int Immunopharmacol. 79:1060832020. View Article : Google Scholar
|
|
26
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care. 4:222016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan Y, Chen S, Zhong J, Ren J and Dong M:
Mitochondrial injury and targeted intervention in septic
cardiomyopathy. Curr Pharm Des. 25:2060–2070. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Kock I, Van Daele C and Poelaert J:
Sepsis and septic shock: Pathophysiological and cardiovascular
background as basis for therapy. Acta Clin Belg. 65:323–329. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li HM, Li KY, Xing Y, Tang XX, Yang DM,
Dai XM, Lu DX and Wang HD: Phenylephrine attenuated sepsis-induced
cardiac inflammation and mitochondrial injury through an effect on
the PI3K/Akt signaling pathway. J Cardiovasc Pharmacol. 73:186–194.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Bei Y, Shen S, Huang P, Shi J,
Zhang J, Sun Q, Chen Y, Yang Y, Xu T, et al: miR-21-3p controls
sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol
Cell Cardiol. 94:43–53. 2019. View Article : Google Scholar
|
|
31
|
Qin Z, Zhu K, Xue J, Cao P, Xu L, Xu Z,
Liang K, Zhu J and Jia R: Zinc-induced protective effect for
testicular ischemia-reperfusion injury by promoting antioxidation
via microRNA-101-3p/Nrf2 pathway. Aging (Albany NY). 11:9295–9309.
2019. View Article : Google Scholar
|
|
32
|
Zhang X, Hyer JM, Yu H, D'Silva NJ and
Kirkwood KL: DUSP1 phosphatase regulates the proinflammatory milieu
in head and neck squamous cell carcinoma. Cancer Res. 74:7191–7197.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu
Z, Wang H, Xu F and Shi L: MiR-127 modulates macrophage
polarization and promotes lung inflammation and injury by
activating the JNK pathway. J Immunol. 194:1239–1251. 2015.
View Article : Google Scholar
|
|
34
|
Brudecki L, Ferguson DA, McCall CE and El
Gazzar M: Mitogen-activated protein kinase phosphatase 1 disrupts
proinflammatory protein synthesis in endotoxin-adapted monocytes.
Clin Vaccine Immunol. 20:1396–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawai M, Karam TS, Michael JJ, Wang L and
Chandra M: Comparison of elementary steps of the cross-bridge cycle
in rat papillary muscle fibers expressing α- and β-myosin heavy
chain with sinusoidal analysis. J Muscle Res Cell Motil.
37:203–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Q, Liu Y, Wang Y, Wang W, Yang Z, Li T,
Tian Y, Chen P, Ma K, Jia Z and Zhou C: Rapamycin efficiently
promotes cardiac differentiation of mouse embryonic stem cells.
Biosci Rep. 37:BSR201605522017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li P, Chen XR, Xu F, Liu C, Li C, Liu H,
Wang H, Sun W, Sheng YH and Kong XQ: Alamandine attenuates
sepsis-associated cardiac dysfunction via inhibiting MAPKs
signaling pathways. Life Sci. 206:106–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiao X, Yang C, Qu SL, Shao YD, Zhou CY,
Chao R, Huang L and Zhang C: S100 proteins in atherosclerosis. Clin
Chim Acta. 502:293–304. 2020. View Article : Google Scholar
|
|
40
|
Huang H and Tu L: Expression of S100
family proteins in neonatal rats with sepsis and its significance.
Int J Clin Exp Pathol. 8:1631–1639. 2015.PubMed/NCBI
|
|
41
|
Tosson AMS, Glaser K, Weinhage T, Foell D,
Aboualam MS, Edris AA, El Ansary M, Lotfy S and Speer CP:
Evaluation of the S100 protein A12 as a biomarker of neonatal
sepsis. J Matern Fetal Neonatal Med. 33:2768–2774. 2020. View Article : Google Scholar
|
|
42
|
Huang L, Zhang L, Liu Z, Zhao S, Xu D, Li
L, Peng Q and Ai Y: Pentamidine protects mice from cecal ligation
and puncture-induced brain damage via inhibiting S100B/RAGE/NF-κB.
Biochem Biophys Res Commun. 517:221–226. 2019. View Article : Google Scholar : PubMed/NCBI
|




















