Introduction
The blindness rate due to cataracts is decreasing
every year; however, cataracts remain the leading cause of
blindness worldwide, with a high prevalence rate from 1990 to 2010,
especially in developing countries (1). Surgery is the only effective cure
for cataracts (2). Although the
cataract surgery rate is increasing, it needs more cost to achieve
improved visual quality through the multifocal lens, and more
ophthalmologists are required to perform cataract surgery (3-5).
Studies are ongoing, but there are currently no drugs to treat or
reverse the formation of cataracts. Therefore, it is urgent and
necessary to study the pathogenesis of cataracts and seek effective
preventive measures.
Human lens epithelial cells (HLECs) consist of a
single layer of epithelial cells located on the anterior surface of
the lens (6). HLECs are widely
used to investigate the pathogenesis of cataracts (7,8)
and were therefore used in the present study. Ion channels are
involved in LEC processes, including proliferation, differentiation
and apoptosis (9-11). Chloride channels have been widely
studied in the apoptosis of various types of cells, such as
cardiomyocytes and nasopharyngeal carcinoma cells (12,13). Chloride channels are associated
with ER stress and oxidative stress. For example, chloride channels
promote ER stress and induce apoptosis in cardiomyocytes and
preadipocytes (14,15). Additionally, chloride channels
promote oxidative stress and induce apoptosis in nasopharyngeal
carcinoma cells and cardiomyocytes (12,16). However, to the best of our
knowledge, there are no studies involving HLECs. Therefore, the
role of chloride channels in LECs requires further study.
Endoplasmic reticulum (ER) stress induces epithelial-to-mesenchymal
transition, oxidative stress, apoptosis and autophagy in HLECs, and
is an important mechanism of cataract formation (17-19). Additionally, oxidative stress is
a known causative factor in cataract formation (20). On one hand, ER stressors, such as
thapsigargin and tunicamycin, produce significant levels of ROS and
promote apoptosis in colon carcinoma CT26, breast cancer MDA-MB468
cells and neuronal HT22 cells (21,22). On the other hand, ROS induces
apoptosis by activating ER stress in acute myeloid leukemia cells
(23). ER stress and oxidative
stress interact with each other. However, whether chloride channels
affect HLEC apoptosis and the corresponding mechanism have not been
investigated. 5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB)
is a non-specific chloride channel inhibitor, which was used in the
present study to observe the effects and possible mechanisms of
chloride channels in HLECs apoptosis.
Materials and methods
Materials
HLECs were purchased from the American Type Culture
Collection. The following additional reagents were used in the
present study: NPPB (Abmole Bioscience Inc.), DMSO, 4-phenylbutyric
acid (4-PBA; Sigma-Aldrich; Merck KGaA), low-glucose DMEM (HyClone;
Cytiva), FBS (Gibco; Thermo Fisher Scientific, Inc.), Cell Counting
Kit-8 (CCK-8) assays (cat. no. C0040), RIPA lysis buffer (cat. no.
P0013C), SDS-PAGE Sample Loading Buffer 5X (cat. no. P0015),
Ca2+ indicator dye Fluo-4 AM kit (cat. no. S1060),
reactive oxygen species (ROS) Assay kit (cat. no. S0033M),
N-acetylcysteine (NAC; cat. no. ST1546-10g), enhanced bicinchoninic
acid (BCA) protein assay kits (cat. no. P0009), JC-1 probes (cat.
no. C2006) and antibodies against caspase-3 (1:1,000; cat. no.
AC030) (all from Beyotime Institute of Biotechnology), primary
antibodies against protein kinase R-like endoplasmic reticulum
kinase (PERK; 1:2,000; cat. no. 33247), phosphorylated (p)-PERK
(1:1,000; cat. no. 12814), B-cell lymphoma-2 (Bcl-2; 1:500; cat.
no. 48496), Bcl-2-associated X (BAX; 1:1,000; cat. no. 48690),
C/EBP homologous protein (CHOP; 1:1,000; cat. no. 49418),
activating transcription factor 6 (ATF6; 1:1,000; cat. no. 32008),
JNK (1:3,000; cat. no. 48615), caspase-12 (1:1,000; cat. no.
48277), β-actin (1:10,000; cat. no. 21338), HRP-conjugated goat
anti-mouse IgG secondary antibody (1:10,000; cat. no. L3032) and
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:10,000;
cat. no. L3042) (all from Signalway Antibody LLC), immobilon
western chemiluminescent HRP substrate (cat. no. WBKLS0500; EMD
Millipore).
Cell culture
HLECs are located in the inner surface of the lens
capsule and are characterized by a flat and irregular
polygonal-shaped morphology (6).
HLECs were maintained and cultured in DMEM with 10% FBS in a
humidified incubator with 5% CO2 at 37°C.
Cell viability assay
NPPB was dissolved in DMSO to form a 0.1 M stock
solution. The solution was stored at <−20°C in the dark until
use. NPPB was diluted to concentrations of 10-200 µM in
serum-free DMEM on the day of the experiment. HLECs were
transferred to 96-well plates (100 µl/well) at a density of
0.5×104 cells/well. Cells were incubated for 24 h and
treated with 10, 50, 100 and 200 µM of NPPB for 24 h at
37°C. CCK-8 solution (10 µl) was added to each well for 30
min to 1 h at 37°C. The absorbance was expressed as the optical
density and was measured at 450 nm using an Infinite M200 Pro
microplate reader (Tecan Group, Ltd.).
NAC and 4-PBA with NPPB treatment
protocol
NAC was dissolved in double-distilled H2O
to form a 1 M stock solution. 4-PBA was dissolved in PBS to form a
500 mM stock solution. Cells were incubated for 24 h and were then
treated with 50 µM NPPB with NAC (500 µM) or 4-PBA
(20 µM) for 24 h at 37°C.
Intracellular reactive oxygen species
(ROS) measurement
The intracellular ROS level was determined using a
ROS assay kit and dichloro-dihydro-fluorescein diacetate (DCFH-DA).
According to the manufacturer's protocol, the cells were cultured
in 96-well plates for 24 h at 37°C and were then washed twice with
serum-free medium. Medium containing 10 µM DCFH-DA was
added. The cells were incubated in a cell incubator for 20 min at
37°C. Light was avoided during procedures and incubation. After
incubation, the cells were washed three times with serum-free
medium, then observed and photographed using a fluorescence
microscope (magnification, ×400; Olympus Corporation). The
fluorescence intensity was quantitatively measured using ImageJ
software (v1.51; National Institutes of Health).
Mitochondrial membrane potential (ΔΨm)
analysis
The JC-1 probe was used to measure apoptosis via
mitochondrial depolarization in HLECs. Briefly, cells (80%
confluent) were cultured in 96-well plates after treatment with 50
µM NPPB at 37°C for 24 h. The cells were washed three times
with PBS, and 50 µl medium and 50 µl JC-1 working
solution were added. The cells were incubated at 37°C for 20 min
and washed three times with precooled JC-1 solution. The ΔΨm was
determined by measuring the fluorescence intensity of red and green
fluorescence using a fluorescence microscope (magnification, ×400).
Green fluorescence indicated JC-1 monomers. JC-1 monomers appeared
in the cytosol after mitochondrial membrane depolarization, which
indicated early stage of apoptosis. Red fluorescence indicated JC-1
aggregation and was located on the mitochondria. Mitochondrial
depolarization indicated apoptosis, which was reflected by an
increase in the green/red fluorescence intensity ratio. The
fluorescence intensity was measured using ImageJ software.
Ca2+ measurement
Cytosolic Ca2+ levels were measured using
a calcium ion fluorescent probe (Fluo-4 AM) assay kit. Cells were
cultured in 96-well plates after treatment with 50 µM NPPB
for 24 h at 37°C. The cells were washed three times with PBS, and
Fluo-4 AM (final concentration of 1 µM) was added for 1 h at
37°C and washed three times with PBS. To ensure that the Fluo-4 AM
was completely converted into Fluo-4 in the cells, another 30-min
incubation at 37°C was necessary after washing. The cells were
observed and photographed under a fluorescence microscope
(magnification, ×400) at 488 nm.
Western blot analysis
The cells were washed three times with PBS and
scraped with a rubber spatula. Cells were collected and centrifuged
at 157 × g for 5 min at 4°C. The cells were lysed in an appropriate
amount of RIPA lysis buffer containing 1% phosphatase and protease
inhibitors. The protein concentration was determined by BCA protein
assay kit. A total of 20 µg protein was mixed with SDS-PAGE
sample loading buffer. The samples were boiled for 5 min and kept
at -20°C until use. The proteins were separated via 10% SDS-PAGE
and transferred to polyvinylidene fluoride membranes. The membrane
was blocked with a blocking solution composed of 5% non-fat dry
milk and TBS with 0.1% Tween-20 (TBS-T) for 1 h at 37°C, then
washed in TBS-T three times for 5 min each. The membranes were
incubated with primary antibodies against BAX, Bcl-2, caspase-3,
ATF6, p-PERK, PERK, JNK, CHOP, caspase-12 and β-actin overnight at
4°C. The membranes were washed three times with TBS-T for 10 min
each, and incubated with the corresponding biotinylated secondary
antibodies for 1 h at 37°C. The membranes were washed three times
with TBS-T. After coating with immobilon western chemiluminescent
HRP substrate, the membranes were observed and photographed using a
microscope equipped with a CCD camera (Tanon Science &
Technology Co., Ltd.). The western blotting results were presented
as the ratio of the fluorescence intensity of the target protein to
β-actin or PERK (for p-PERK) calculated using ImageJ software.
Statistical analysis
All experiments were repeated 3 times independently.
The data are expressed as the mean ± standard deviation. The data
were analysed by unpaired Student's t-test and one-way ANOVA
followed by Tukey's post-hoc test and Bonferroni's correction. SPSS
statistic software 25.0 (IBM Corp.) was used to analyse the data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of NPPB on the viability of
HLECs
Cells were treated with different concentrations of
NPPB (0, 10, 50, 100 and 200 µM) for 24 h. Cell viability
was determined via CCK-8 assays. Fig. 1 shows that NPPB significantly
inhibited the viability of HLECs in a dose-dependent manner, with
an IC50 value of 53.36±0.1% (P<0.01 vs. 0 µM).
These results suggested that NPPB decreased cell survival rate and
inhibited the viability of HLECs. A concentration of 50 µM
NPPB was used in subsequent experiments.
Effect of NPPB on mitochondrial apoptosis
of HLECs
Red fluorescence indicated that JC-1 accumulated in
mitochondria and the cells were alive, while green fluorescence
indicated apoptosis. Exposure of HLECs to NPPB (50 µM) for
24 h resulted in the dissipation of the ΔΨm. Green fluorescence
increased after JC-1 staining, and the ratio of green to red
fluorescence increased, indicating the toxicity of NPPB in
mitochondria (Fig. 2). JC-1
aggregated in the mitochondria in the control group, with a ratio
of 0.15±0.027, while the NPPB-treated group exhibited a
significantly higher ratio (0.59±0.080) since the monomeric form of
JC-1 was present in the cytosol. (P<0.001; Fig. 2). Additionally, the expression
levels of apoptosis-associated proteins were detected by western
blot analysis. NPPB-induced apoptosis was accompanied by a decrease
in Bcl-2 expression (0.51±0.07 vs. 0.80±0.16; P<0.05) and a
significant increase in BAX (0.92±0.19 vs. 0.57±0.08; P<0.05)
and cleaved caspase-3 expression (1.77±0.47 vs. 0.49±0.21;
P<0.05) compared with the vehicle group (Fig. 3).
Effect of NPPB on ROS in HLECs
NPPB induced ROS generation in HLECs. NPPB (50
µM) significantly increased intracellular ROS levels
(3.33±0.71 vs. 0.88±0.17; P<0.01) compared with the vehicle
group (Fig. 4).
Effect of NPPB on ER stress and
caspase-dependent apoptosis in HLECs
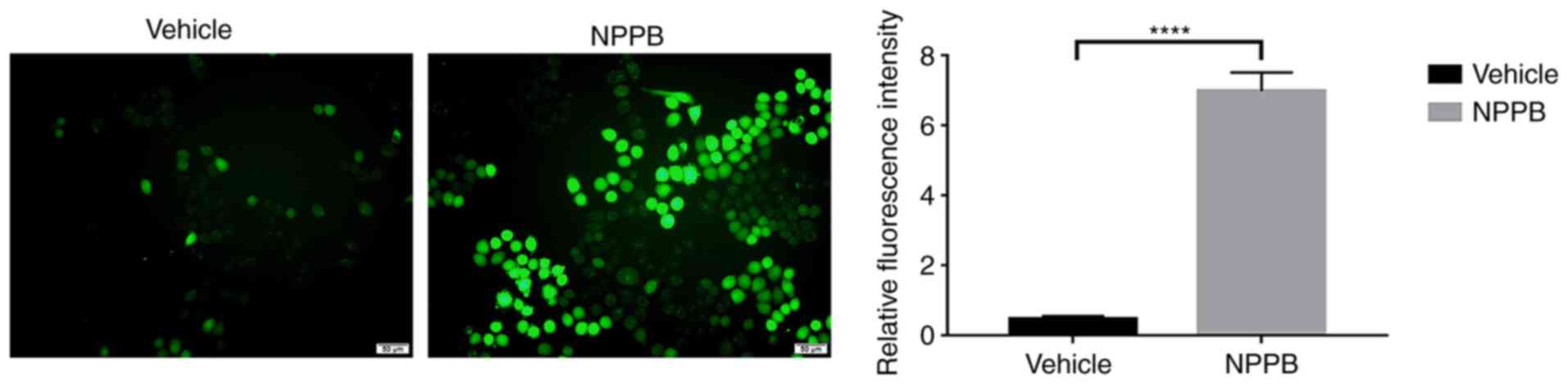
Mitochondria and the ER are major reservoirs of
intracellular Ca2+. The imbalance of calcium ion is
closely associated with mitochondrial apoptosis and ER stress
(24). Therefore, whether NPPB
induced apoptosis and ER stress by perturbing intracellular
Ca2+ homeostasis was further explored. NPPB stimulation
significantly increased Ca2+ levels in the cytosol in
HLECs (7.0±0.53 vs. 0.46±0.09; P<0.0001) compared with the
vehicle group (Fig. 5).
The expression levels of ER stress-associated
proteins were detected by western blot analysis, including p-PERK
(1.37±0.03 vs. 1.01±0.16; P<0.05), ATF6 (1.09±0.14 vs.
0.86±0.03; P<0.05), JNK (1.13±0.28 vs. 0.63±0.03; P<0.05),
CHOP (1.04±0.04 vs. 0.80±0.04; P<0.01) and caspase-12 (1.07±0.02
vs. 0.87±0.08; P<0.05) were upregulated following NPPB (50
µM) treatment compared with the vehicle group (Fig. 6). Quantification of the western
blotting results indicated that NPPB significantly induced ER
stress and the unfolded protein response (UPR) in HLECs.
 | Figure 6Effect of NPPB on ER
stress-associated markers. ER stress markers and ER
stress-associated caspase-dependent apoptosis markers ATF6, JNK,
CHOP, caspase-12, PERK and p-PERK were measured via western
blotting. β-actin was used as an internal control. The results are
presented as the mean ± standard deviation (n=3).
*P<0.05; **P<0.01. HLEC, human lens
epithelial cell; NPPB, 5-nitro-2-(3-phenylpropylamino) benzoic
acid; ER, endoplasmic reticulum; ATF6, activating transcription
factor 6; CHOP, C/EBP homologous protein; p-PERK, phosphorylated
protein kinase R-like endoplasmic reticulum kinase. |
Effects of NAC and 4-PBA in NPPB-treated
HLECs
The ROS scavenger NAC (500 µM) and the ER
stress inhibitor 4-PBA (20 µM) were used to co-treat cells
with 50 µM NPPB at 37°C for 24 h. NAC and 4-PBA
significantly decreased the levels of the green-to-red fluorescence
ratio in JC-1 staining compared with NPPB alone (NAC + NPPB,
4.53±0.70 and 4-PBA + NPPB, 3.47±0.25 vs. NPPB, 8.81±2.10; both
P<0.01; Fig. 7A).
Additionally, compared with NPPB alone, NAC and 4-PBA with NPPB
decreased the expression levels of BAX (NAC + NPPB, 1.34±0.07 and
4-PBA + NPPB, 1.47±0.12 vs. NPPB, 1.75±0.11; P<0.01 and
P<0.05, respectively) and cleaved caspase-3 (NAC+NPPB, 0.69±0.20
and 4-PBA + NPPB, 0.69±0.24 vs. NPPB, 1.77±0.47; both P<0.01)
and increased the expression levels of Bcl-2 (NAC + NPPB, 1.16±0.11
and 4-PBA + NPPB, 1.14±0.13 vs. NPPB, 0.82±0.12; both P<0.05)
compared with the NPPB group (Fig.
7B). Furthermore, NAC and 4-PBA significantly decreased
NPPB-induced ROS (NAC + NPPB, 1.21±0.19 and 4-PBA + NPPB, 1.10±0.21
vs. NPPB, 12.32±1.62; both P<0.0001; Fig. 7C), Ca2+ levels (NAC +
NPPB, 4.53±0.70 and 4-PBA + NPPB, 3.47±0.25 vs. NPPB, 8.81±2.10;
both P<0.05; Fig. 7D) in the
cytosol and the level of ER stress, including the levels of p-PERK
(NAC + NPPB, 1.19±0.20 and 4-PBA + NPPB, 1.19±0.12 vs. NPPB,
2.00±0.41; both P<0.05), ATF6 (NAC + NPPB, 0.89±0.03 and
4-PBA+NPPB, 0.85±0.11 vs. NPPB, 1.70±0.07; both P<0.0001,), JNK
(NAC + NPPB, 2.74±0.15 and 4-PBA + NPPB, 2.80±0.16 vs. NPPB,
3.50±0.17; both P<0.05), CHOP (NAC+NPPB, 1.85±0.13 and 4-PBA +
NPPB, 1.72±0.04 vs. NPPB, 2.25±0.19; P<0.05 and P<0.01,
respectively) and caspase-12 (NAC + NPPB, 1.91±0.06 and 4-PBA +
NPPB, 1.75±0.07 vs. NPPB, 2.29±0.08; P<0.05 and P<0.01,
respectively) (Fig. 7E).
Therefore, NPPB activated ROS production and induced the UPR
pathway, which likely promoted apoptosis.
 | Figure 7NAC and 4-PBA inhibit the effects of
NPPB in HLECs. NPPB-treated cells were co-treated with NAC (500
µM) and 4-PBA (20 µM). (A) Relative green/red
fluorescence intensity was determined using ImageJ software. (B)
Expression levels of apoptosis markers BAX, Bcl-2 and cleaved
caspase-3 were determined by western blotting. β-actin was used as
an internal control. (C) Reactive oxygen species were stained with
a dichloro-dihydro-fluorescein diacetate fluorescent probe and
examined using ImageJ software. (D) Cytosolic Ca2+ was
measured with Fluo-4 AM, a Ca2+ indicator dye, and
examined using ImageJ software. (E) ER stress markers and ER
stress-associated caspase-dependent apoptosis markers ATF6, JNK,
CHOP, PERK and p-PERK were measured by western blotting. β-actin
was used as an internal control. Scale bar, 50 µm. The
results are presented as the mean ± standard deviation (n=3).
*P<0.05; **P<0.01;
***P<0.001; ****P<0.0001. HLEC, human
lens epithelial cell; NPPB, 5-nitro-2-(3-phenylpropylamino) benzoic
acid; Bcl-2, B-cell lymphoma-2; BAX, Bcl-2-associated X; ER,
endoplasmic reticulum; ATF6, activating transcription factor 6;
CHOP, C/EBP homologous protein; p-PERK, phosphorylated protein
kinase R-like endoplasmic reticulum kinase; NAC, N-acetylcysteine;
4-PBA, 4-phenylbutyric acid. |
Discussion
Investigation of LEC apoptosis is the main method to
study the pathogenesis of cataracts (25). Chloride channels serve important
roles in numerous cellular aspects, especially apoptosis (26-29). The non-specific inhibitor of
chloride channels NPPB is commonly used to inhibit the chloride
current and to study the function of voltage-gated chloride
channels (30). Voltage-gated
chloride channels are involved in the occurrence of some diseases,
such as cardiovascular and nervous system diseases and
nasopharyngeal carcinoma (29,31,32). However, their role in cataracts
has not been widely discussed. Therefore, NPPB was used in the
present study to investigate whether chloride channels were
involved in the pathogenesis of cataracts. If NPPB was associated
with the apoptosis of LECs, further studies may examine which
voltage-gated chloride channels may be involved in the formation of
cataracts. NPPB is widely used to study apoptosis mechanisms
(30,33,34). Souktani et al (35) demonstrated that NPPB promoted
cardiomyocyte apoptosis. However, Malekova et al (30) indicated that NPPB served a
protective role in H2O2-induced cardiomyocyte
apoptosis. To the best of our knowledge, the mechanism of chloride
channel mediation of LEC apoptosis has not been previously studied.
Therefore, NPPB was used in the present study to investigate the
role of chloride channels in LEC apoptosis. The current results
revealed that NPPB inhibited cell viability in a concentration-
dependent manner. The results of CCK-8 assays indicated that the
IC50 of NPPB in LECs at 24 h was ~50 µM.
Therefore, a concentration of 50 µM was used in subsequent
experiments. NPPB increased the number of JC-1 monomers and the
expression levels of BAX and cleaved caspase-3, and decreased Bcl-2
expression. Therefore, it was concluded that NPPB induced HLEC
apoptosis via a mitochondrial-dependent pathway. Similar results
were obtained in basilar artery smooth muscle cells and human
bronchial epithelium (36,37). The effects of chloride channels
on apoptosis are distinct in different cell types. Chloride
channels have been shown to be cardioprotective in ischemic
preconditioning of isolated hearts; however, the inhibitor of
chloride channels NPPB prevented the appearance of
H2O2-induced apoptosis of pheochromocytoma
cells (38,39). Chloride channel activation in
intestinal epithelial cells leads to apoptosis via activation of
caspase 3 (40). The reason for
the different effects may be associated with the various
experimental conditions and models used.
Cataract pathogenesis is closely associated with
oxidative stress (41). To the
best of our knowledge, the present study revealed for the first
time that NPPB induced oxidative stress in LECs and increased the
production of intracellular ROS, and that NAC inhibited
NPPB-induced production of ROS. ROS promote apoptosis in a variety
of ways, such as via ER stress, PI3K/AKT signalling, the
Foxo3a/TRIM69/p53 regulatory network and the
calmodulin-like-protein 3-dependent JNK1/2 and ERK1/2 signalling
pathways (17,42-44). In the present study, 4-PBA also
inhibited the production of NPPB-induced ROS, suggesting that
oxidative stress and ER stress interacted and influenced each
other.
The ER is a central organelle used in a series of
important biological processes to maintain the stability of the
intracellular environment. The present study revealed that NPPB
increased intracellular Ca2+ and the levels of ER
stress-associated proteins, such as p-PERK, PERK, ATF6, JNK and
CHOP, which indicated that NPPB significantly induced ER stress in
HLECs. Additionally, NPPB increased the expression levels of
caspase-12, which indicated that NPPB significantly induced
apoptosis via ER stress and the mitochondrial pathway. NAC and
4-PBA decreased intracellular Ca2+ and the expression
levels of ER stress-associated proteins, therefore attenuating
NPPB-induced ER stress in HLECs. The homeostasis of Ca2+
in HLECs is very important since its balance maintains clarity of
the lens (45). When ER stress
occurs, the ER releases Ca2+, increasing the cytosolic
Ca2+ levels (46).
Ca2+ dysregulation further promotes apoptotic cell death
(47,48). Therefore, chloride channels serve
important roles in maintaining lens transparency, inhibiting ER
stress and protecting lens cells.
In conclusion, the present study demonstrated that
NPPB induced HLEC apoptosis. NPPB inhibited cell viability and
induced ROS, ER stress and apoptosis. The current findings provide
strong evidence that chloride channels may serve an important role
in the pathogenesis of cataracts.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors designed the present study. LN, JZ, KL
and YW performed the experiments. LN, XL, JZ and YZ confirmed the
authenticity of the data. LN, YW, XL, KL and YL analysed the data
and prepared the figures. LN, XL, JZ and YS drafted the initial
manuscript. XL, JZ and YZ reviewed and revised the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Referencves
|
1
|
Khairallah M, Kahloun R, Bourne R, Limburg
H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K,
et al: Number of people blind or visually impaired by cataract
worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis
Sci. 56:6762–6769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ibrahim N, Pozo-Martin F and Gilbert C:
Direct non-medical costs double the total direct costs to patients
undergoing cataract surgery in Zamfara state, Northern Nigeria: A
case series. BMC Health Serv Res. 15:1632015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lundström M, Goh PP, Henry Y, Salowi MA,
Barry P, Manning S, Rosen P and Stenevi U: The changing pattern of
cataract surgery indications: A 5-year study of 2 cataract surgery
databases. Ophthalmology. 122:31–38. 2015. View Article : Google Scholar
|
|
4
|
Lin JC and Yang MC: Cost-effectiveness
comparison between monofocal and multifocal intraocular lens
implantation for cataract patients in Taiwan. Clin Ther.
36:1422–1430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Resnikoff S, Felch W, Gauthier TM and
Spivey B: The number of ophthalmologists in practice and training
worldwide: A growing gap despite more than 200,000 practitioners.
Br J Ophthalmol. 96:783–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rabsilber TM and Auffarth GU: Lens
epithelial cells. Encyclopedia of Ophthalmology. Schmidt-Erfurth U
and Kohnen T: Springer; Berlin, Heidelberg: pp. 1–2. 2016
|
|
7
|
Lu B, Christensen IT, Yu T, Wang C, Yan Q
and Wang X: SUMOylation evoked by oxidative stress reduced lens
epithelial cell antioxidant functions by increasing the stability
and transcription of TP53INP1 in age-related cataracts. Oxid Med
Cell Longev. 2019:78980692019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong Y, Sun Y, Rong X, Li D, Lu Y and Ji
Y: Exosomes from adipose-derived stem cells attenuate UVB-induced
apoptosis, ROS, and the Ca2+ level in HLEC cells. Exp
Cell Res. 396:1123212020. View Article : Google Scholar
|
|
9
|
Nebe B, Kunz F, Peters A, Rychly J, Noack
T and Beck R: Induction of apoptosis by the calcium antagonist
mibefradil correlates with depolarization of the membrane potential
and decreased integrin expression in human lens epithelial cells.
Graefes Arch Clin Exp Ophthalmol. 242:597–604. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meissner A and Noack T: Proliferation of
human lens epithelial cells (HLE-B3) is inhibited by blocking of
voltage-gated calcium channels. Pflugers Arch. 457:47–59. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chimote AA, Adragna NC and Lauf PK: Ion
transport in a human lens epithelial cell line exposed to
hyposmotic and apoptotic stress. J Cell Physiol. 223:110–122.
2010.PubMed/NCBI
|
|
12
|
Wang L, Shen M, Guo X, Wang B, Xia Y, Wang
N, Zhang Q, Jia L and Wang X: Volume-sensitive outwardly rectifying
chloride channel blockers protect against high glucose-induced
apoptosis of cardiomyocytes via autophagy activation. Sci Rep.
7:442652017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou C, Tang X, Xu J, Wang J, Yang Y, Chen
Y, Chen L, Wang L, Zhu L and Yang H: Opening of the CLC-3 chloride
channel induced by dihydroartemisinin contributed to early
apoptotic events in human poorly differentiated nasopharyngeal
carcinoma cells. J Cell Biochem. 119:9560–9572. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen M, Wang L, Wang B, Wang T, Yang G,
Shen L, Wang T, Guo X, Liu Y, Xia Y, et al: Activation of
volume-sensitive outwardly rectifying chloride channel by ROS
contributes to ER stress and cardiac contractile dysfunction:
Involvement of CHOP through Wnt. Cell Death Dis. 5:e15282014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang YY, Huang XQ, Zhao LY, Sun FY, Chen
WL, Du JY, Yuan F, Li J, Huang XL, Liu J, et al: ClC-3 deficiency
protects preadipocytes against apoptosis induced by palmitate in
vitro and in type 2 diabetes mice. Apoptosis. 19:1559–1570. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Gao H, Yang X, Liang X, Tan Q,
Chen Z, Zhao C, Gu Z, Yu M, Zheng Y, et al: The apoptotic effect of
Zoledronic acid on the nasopharyngeal carcinoma cells via ROS
mediated chloride channel activation. Clin Exp Pharmacol Physiol.
45:1019–1027. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou S, Yang J, Wang M, Zheng D and Liu Y:
Endoplasmic reticulum stress regulates epithelial-mesenchymal
transition in human lens epithelial cells. Mol Med Rep. 21:173–180.
2020.
|
|
18
|
Ma TJ, Lan DH, He SZ, Ye Z, Li P, Zhai W,
Chen WQ, Huang Y, Fu Y, Sun A, et al: Nrf2 protects human lens
epithelial cells against H2O2-induced
oxidative and ER stress: The ATF4 may be involved. Exp Eye Res.
169:28–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Smith AJ, Ball SS, Bowater RP, Wang
N and Michael Wormstone I: Sulforaphane promotes ER stress,
autophagy, and cell death: Implications for cataract surgery. J Mol
Med (Berl). 95:553–564. 2017. View Article : Google Scholar
|
|
20
|
Braakhuis AJ, Donaldson CI, Lim JC and
Donaldson PJ: Nutritional strategies to prevent lens cataract:
Current status and future strategies. Nutrients. 11:11862019.
View Article : Google Scholar :
|
|
21
|
Verfaillie T, Rubio N, Garg AD, Bultynck
G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A
and Agostinis P: PERK is required at the ER-mitochondrial contact
sites to convey apoptosis after ROS-based ER stress. Cell Death
Differ. 19:1880–1891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song Q, Gou WL and Zhang R: FAM3A
attenuates ER stress-induced mitochondrial dysfunction and
apoptosis via CHOP-Wnt pathway. Neurochem Int. 94:82–89. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Wang G, Wu W, Yao S, Han X, He D,
He J, Zheng G, Zhao Y, Cai Z and Yu R: Camalexin induces apoptosis
via the ROS-ER stress-mitochondrial apoptosis pathway in AML cells.
Oxid Med Cell Longev. 2018:74269502018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Wang C, Meng Q, Liu Z, Huo X, Sun
P, Sun H, Ma X, Peng J and Liu K: Targeting P-glycoprotein and
SORCIN: Dihydromyricetin strengthens anti-proliferative efficiency
of adriamycin via MAPK/ERK and Ca2+-mediated apoptosis
pathways in MCF-7/ADR and K562/ADR. J Cell Physiol. 233:3066–3079.
2018. View Article : Google Scholar
|
|
25
|
Ohtsubo M, Theodoras AM, Schumacher J,
Roberts JM and Pagano M: Human cyclin E, a nuclear protein
essential for the G1-to-S phase transition. Mol Cell Biol.
15:2612–2624. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng JW, Wang XG, Ma MM, Lv XF, Liu J,
Zhou JG and Guan YY: Integrin β3 mediates cerebrovascular
remodelling through Src/ClC-3 volume-regulated Cl(-) channel
signalling pathway. Br J Pharmacol. 171:3158–3170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang W, Huang L, Zhao D, He JZ, Sharma P,
Liu J, Gramolini AO, Ward ME, Cho HC and Backx PH:
Swelling-activated Cl- currents and intracellular CLC-3 are
involved in proliferation of human pulmonary artery smooth muscle
cells. J Hypertens. 32:318–330. 2014. View Article : Google Scholar
|
|
28
|
Zhang H, Li H, Yang L, Deng Z, Luo H, Ye
D, Bai Z, Zhu L, Ye W, Wang L and Chen L: The ClC-3 chloride
channel associated with microtubules is a target of paclitaxel in
its induced-apoptosis. Sci Rep. 3:26152013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L, Zhang S, Fan H, Zhong Z, Li X, Jin X
and Chang Q: ClC-3 chloride channel in hippocampal neuronal
apoptosis. Neural Regen Res. 8:3047–3054. 2013.
|
|
30
|
Malekova L, Tomaskova J, Novakova M,
Stefanik P, Kopacek J, Lakatos B, Pastorekova S, Krizanova O,
Breier A and Ondrias K: Inhibitory effect of DIDS, NPPB, and
phloretin on intracellular chloride channels. Pflugers Arch.
455:349–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ganapathi SB, Wei SG, Zaremba A, Lamb FS
and Shears SB: Functional regulation of ClC-3 in the migration of
vascular smooth muscle cells. Hypertension. 61:174–179. 2013.
View Article : Google Scholar
|
|
32
|
Wang L, Ma W, Zhu L, Ye D, Li Y, Liu S, Li
H, Zuo W, Li B, Ye W and Chen L: ClC-3 is a candidate of the
channel proteins mediating acid-activated chloride currents in
nasopharyngeal carcinoma cells. Am J Physiol Cell Physiol.
303:C14–C23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun L, Dong Y, Zhao J, Yin Y, Tong B,
Zheng Y and Xin H: NPPB modulates apoptosis, proliferation,
migration and extra- cellular matrix synthesis of conjunctival
fibroblasts by inhibiting PI3K/AKT signaling. Int J Mol Med.
41:1331–1338. 2018.
|
|
34
|
Myssina S, Lang PA, Kempe DS, Kaiser S,
Huber SM, Wieder T and Lang F: Cl- channel blockers NPPB and
niflumic acid blunt Ca(2+)-induced erythrocyte 'apoptosis'. Cell
Physiol Biochem. 14:241–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Souktani R, Ghaleh B, Tissier R,
d'Anglemont de Tassigny A, Aouam K, Bedossa P, Charlemagne D,
Samuel J, Henry P and Berdeaux A: Inhibitors of swelling-activated
chloride channels increase infarct size and apoptosis in rabbit
myocardium. Fundam Clin Pharmacol. 17:555–561. 2003. View Article : Google Scholar
|
|
36
|
Qian Y, Du YH, Tang YB, Lv XF, Liu J, Zhou
JG and Guan YY: ClC-3 chloride channel prevents apoptosis induced
by hydrogen peroxide in basilar artery smooth muscle cells through
mitochondria dependent pathway. Apoptosis. 16:468–477. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng G, Shao Z, Chaudhari B and Agrawal
DK: Involvement of chloride channels in TGF-beta1-induced apoptosis
of human bronchial epithelial cells. Am J Physiol Lung Cell Mol
Physiol. 293:L1339–L1347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bozeat ND, Xiang SY, Ye LL, Yao TY, Duan
ML, Burkin DJ, Lamb FS and Duan DD: Activation of volume regulated
chloride channels protects myocardium from ischemia/reperfusion
damage in second-window ischemic preconditioning. Cell Physiol
Biochem. 28:1265–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zuo W, Zhu L, Bai Z, Zhang H, Mao J, Chen
L and Wang L: Chloride channels involve in hydrogen
peroxide-induced apoptosis of PC12 cells. Biochem Biophys Res
Commun. 387:666–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Claud EC, Lu J, Wang XQ, Abe M, Petrof EO,
Sun J, Nelson DJ, Marks J and Jilling T: Platelet-activating
factor-induced chloride channel activation is associated with
intracellular acidosis and apoptosis of intestinal epithelial
cells. Am J Physiol Gastrointest Liver Physiol. 294:G1191–G1200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Periyasamy P and Shinohara T: Age-related
cataracts: Role of unfolded protein response, Ca2+
mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1
dependent cytoprotection. Prog Retin Eye Res. 60:1–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Li H and Liu Y: MicroRNA-378a
regulates the reactive oxygen species (ROS)/phosphatidylinositol
3-kinases (PI3K)/AKT signaling pathway in human lens epithelial
cells and cataract. Med Sci Monit. 25:4314–4321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rong X, Rao J, Li D, Jing Q, Lu Y and Ji
Y: TRIM69 inhibits cataractogenesis by negatively regulating p53.
Redox Biol. 22:1011572019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jia Y, Qin Q, Fang CP, Shen W, Sun TT,
Huang YL, Li WJ and Deng AM: UVB induces apoptosis via
downregulation of CALML3-dependent JNK1/2 and ERK1/2 pathways in
cataract. Int J Mol Med. 41:3041–3050. 2018.PubMed/NCBI
|
|
45
|
Duncan G and Jacob TJ: Calcium and the
physiology of cataract. Ciba Found Symp. 106:132–152.
1984.PubMed/NCBI
|
|
46
|
Hofer AM, Curci S, Machen TE and Schulz I:
ATP regulates calcium leak from agonist-sensitive internal calcium
stores. FASEB J. 10:302–308. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bahar E, Kim H and Yoon H: ER
Stress-mediated signaling: Action potential and Ca(2+) as key
players. Int J Mol Sci. 17:15582016. View Article : Google Scholar
|
|
48
|
Krebs J, Agellon LB and Michalak M: Ca(2+)
homeostasis and endoplasmic reticulum (ER) stress: An integrated
view of calcium signaling. Biochem Biophys Res Commun. 460:114–121.
2015. View Article : Google Scholar : PubMed/NCBI
|