1. Introduction
Insulin resistance (IR) is defined as impaired
insulin function, reduced glucose uptake and increased glucose
production (1), which may result
in type II diabetes and metabolic syndrome (2) and may subsequently promote the
development of non-alcoholic fatty liver disease (NAFLD) (3). Numerous previous studies (4-6)
have reported mechanisms through which IR serves a crucial role in
a variety of systemic diseases, including bone metabolic disorders
(7). The etiology of IR is
complex and a possible reason for the increasing incidence of IR is
the aging population, as epidemiological research has reported that
the prevalence of IR and type II diabetes is higher in older
adults. It has also been demonstrated that aging induces IR, and in
turn, hyperinsulinemia and IR may accelerate aging, promoting a
vicious circle (8,9).
Adipose tissue (AT) is an active organ regulating
ATP expenditure, which serves an essential role in regulating
energy homeostasis of the entire body (10). Our previous studies focusing on
the function of AT identified that the dysfunction of AT induced IR
(11,12) and more recent studies (13,14) have also reported a crucial role
for AT in IR during aging. Therefore, an improved understanding of
the effects of aging on AT may prove useful in pioneering novel
therapeutic strategies to target age-related diseases. The present
review aimed to summarize the associative evidence between AT and
age-dependent IR. Notably, the findings suggested that the browning
of white AT (WAT) may be influenced to attain positive therapeutic
outcomes in age-dependent IR and other metabolic complications.
2. AT characteristics
AT can be subdivided into three subtypes, namely
WAT, brown AT (BAT) and beige AT, which all possess distinct
characteristic features that are associated with their function,
localization and composition (15). BAT develops following the
stimulation of anatomical thermogenic sites corresponding with WAT
in a process termed 'browning' (15,16). In mammals, BAT and WAT have
opposing functions. For example, BAT expends energy, while WAT
stores energy. Significant changes are known to occur over the
years in the distribution and composition of AT during aging. The
progressive dysfunction of AT has been proposed to be a
characteristic feature of the aging process (17).
White adipocytes
The majority of AT in the human body is WAT, which
is distinguishable by large unilocular lipid droplets.
Functionally, WAT is subdivided into a stromal vascular fraction,
which is composed of lymphocytes, progenitor and endothelial cells,
preadipocytes and fibroblasts, and the adipocyte fraction, which
primarily contains mature adipocytes (17,18). WAT is dispersed throughout
visceral organs and the surrounding subcutaneous region of the
face. Notably, despite their histological similarities, visceral AT
(VAT) and subcutaneous tissue have been shown to exert distinct
metabolic functions. Subcutaneous WAT has metabolic characteristics
that differ from those of VAT. For example, the metabolic activity
of WAT is regulated by a smaller number of insulin receptors,
β-adrenoceptors (β-AR) and glucocorticoid receptors (19).
Brown adipocytes
BAT is a major source of metabolically active fat
and is located in the retroperitoneal, cervical and mediastinal
regions (20). BAT is named due
to the brown color of the adipocytes and has a high mitochondria
content and vascular supply. BAT can arise from precursor cells
already present in WAT, which display identical unilocular
morphology as WAT during the basal state (21). However, the morphological
characteristic features of BAT are altered upon cold stimulation,
eventually resulting in the expression of typical BAT proteins, and
the transformation from stored fat to small lipid droplets, which
are typical of BAT (22). The
beta-3 adrenergic receptor (β3-AR), type 2 iodothyronine deiodinase
and uncoupling protein 1 (UCP1) are highly expressed in the
adipocytes of BAT (23), in
which UCP1 functions as a ther- moregulator (24).
Beige adipocytes
Beige adipocytes are brown-like adipocytes that
exist within WAT in adult humans and play an essential role in ATP
expenditure (25), which are
characterized by a mix of the features of both brown and white
adipocytes (21). Inactive beige
cells appear morphologically as regular white adipocytes, although
upon stimulation (through adrenergic receptors), oxidative and
mitochondrial biogenesis is enhanced (25). However, it has been suggested
that beige adipocytes may serve a role similar to the role brown
adipocytes play in the expenditure of ATP, which has been
demonstrated in humans (26).
Notably, beige adipocytes have also been found to be
transplantable. Several studies have suggested that beige stem
cells may derive from WAT cells by trans-differentiation, which is
driven by bone morphogenetic protein 7 (21,27). However, there is also evidence to
suggest that beige adipocytes may arise from unique precursor cells
(25). For instance, Wu et
al (25) reported that beige
adipocytes were differentiated from myogenic factor 5
(Myf5)+ progenitor cells. Nevertheless, an ongoing
debate still surrounds the anatomical location,
trans-differentiation and cellular identity of the three adipocyte
subtypes.
3. WAT in age-dependent IR
It has been reported that the accumulation of
senescent cells was increased in the WAT of diabetic patients
(28); thus, the association
between WAT and aging may be of clinical significance, as numerous
classical aging mechanisms have been shown to occur in WAT, which
may be associated with to age-dependent IR (29).
Adipocyte self-renewal
In adults, human WAT is dynamic and can
differentiate into mature lipid-storing adipocytes. In total, ~10%
of adipocytes die and renew every year (30). Renewed progenitor cells were
demonstrated to play a crucial role in insulin sensitivity (IS),
the expansion of AT and lipid handling. Thus, the failure of
progenitor cells to self-renew could lead to the exhaustion of new
adipocytes, resulting in the senescence and death of adipocytes
(31).
It is estimated that 15-50% of cells in WAT are
preadipocytes that are continuously renewed throughout an
individual's life, which results in the varying size and functional
changes observed in older adults compared with younger individuals
(32,33). Thus, determining the association
between fat mass expansion and adipogenesis may be essential for
establishing the pathophysiological role that adipocyte turnover
plays in IR. Notably, the function of progenitor cells, such as
those within the skeletal muscle, also decreases with age
(age-related IR). Thus, the inherent characteristics of progenitor
cells may contribute to the variation in WAT function with aging
(34,35).
The reduced ability of adipocytes to differentiate
during aging may combine with the decreased insulin responsiveness
of WAT when exposed to excess nutrients during old age. Although
very little is known regarding the precise age, percentage and
required threshold, progenitor cells may cause physiological
changes or become dysfunctional (36).
Location of AT
The location of AT is equally as essential as the
absolute amount of AT during reduced IS. Fat redistribution in the
elderly has been associated with an increased risk of IS (37). Subcutaneous WAT and VAT are
diverse with regards to their metabolic effects; for example,
subcutaneous WAT has been associated with increased IS and a lower
risk of cardiovascular disease (CVD) and diabetes, while VAT has
been associated with IR (38).
Furthermore, pear-shaped fat distribution with more gluteal femoral
AT was associated with an increased IS and lower risk of CVD and
diabetes, while apple-shaped fat distribution with an increased
waist circumference was associated with VAT mass and IR.
Individuals with relatively higher amounts of VAT compared with WAT
were also at an increased risk of developing IR (39). The loss of subcutaneous fat is a
well-established feature in aging (38), as the distribution of AT
primarily moves from subcutaneous fatty tissue deposition to
ectopic sites, muscle and visceral deposition, including within the
liver, which are all closely associated with the development of IR
(40).
Increased VAT accumulation was demonstrated to
result in lipotoxicity in old age (>65 years old) (41) and chronic low-grade inflammation
(42). The central accumulation
of VAT during aging has been closely associated with an increase of
inflammatory and metabolic markers compared with peripheral fat
accumulation (43). Similar to
humans, the redistribution of fat in rat and mice models tended to
occur with aging due to insulin-like growth factor-1 and/or growth
hormone deficiencies (44). As
reported by Niu et al (45), diabetes and IR was effectively
reduced in aging rats by antagonizing the age-dependent
accumulation of VAT.
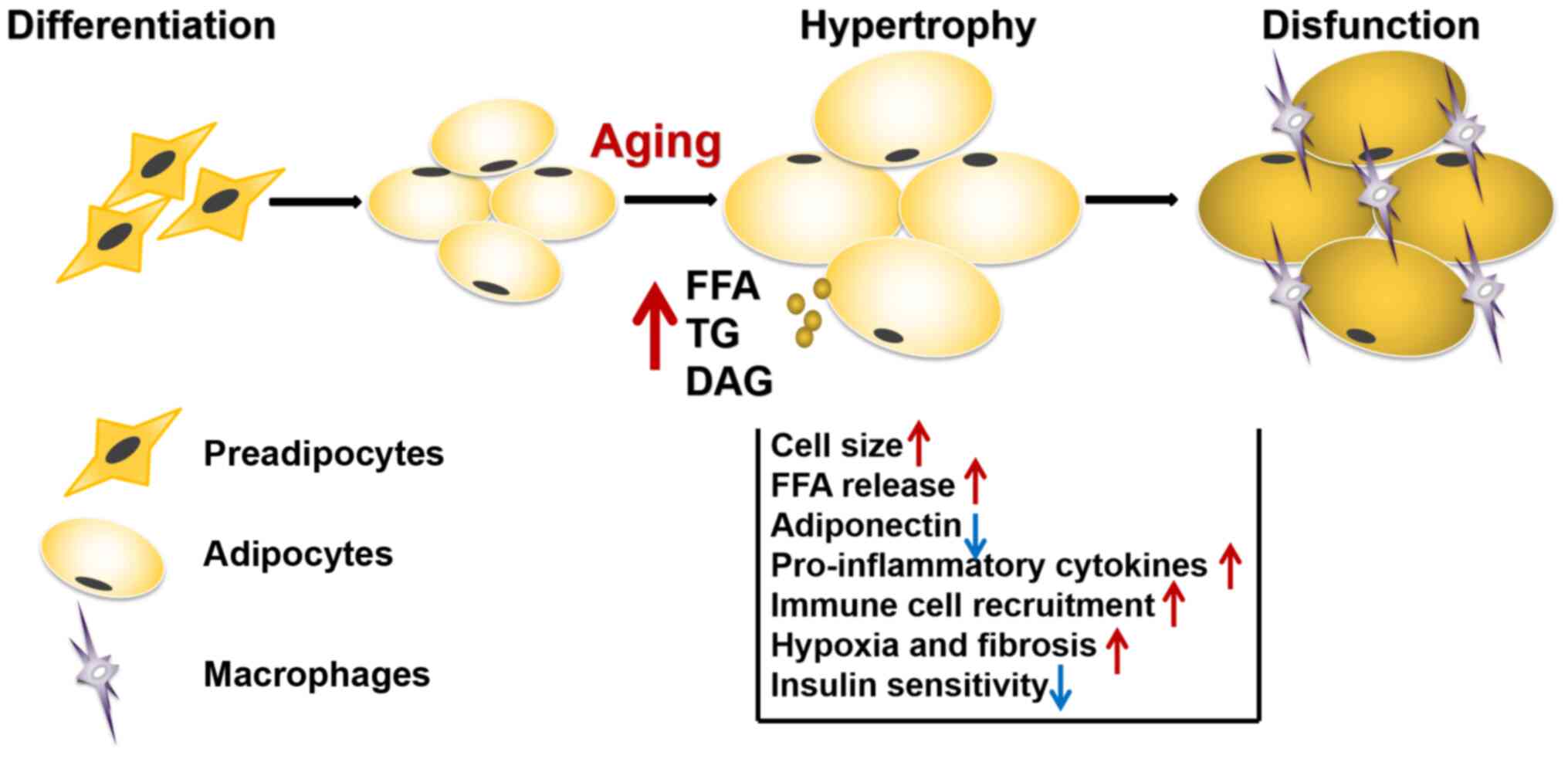
Volume of adipocytes
Body fat mass typically increases by either
hypertrophy or hyperplasia, with the former involving slower rates
of adipogenesis compared with the latter. Hypertrophic adipocytes
were reported to induce hypoxic conditions and have been implicated
in several types of condition, including atherosclerosis, type II
diabetes and IR. The development of hypoxia underpins the
infiltration of macrophages, which subsequently promotes the
initiation and progression of the inflammatory response in AT
(46).
The migration of macrophages results in impaired
insulin signaling, which is known to be central to the development
of IR and metabolic syndrome (Fig.
1). In addition, during hypertrophy, adipocytes present in the
senescent state and secrete a multitude of growth factors,
cytokines, matrix metalloproteinases and chemokines (47). Thus, removing senescent cells in
progeroid INK-ATTAC mice has been found to improve WAT IS (48,49). Free fatty acids (FFAs) are
essential biomedical indicators of lipid metabolic disorders,
whereby the effects of FFAs on IR are dose-dependent, which
adversely affects insulin signaling pathways (45). Plasma FFA levels have been
associated with aging and excess FFA accumulation was found to
induce IR during aging (50).
An increase in FFAs has demonstrated to induce IR at
the cellular level, while increasing very-low-density hepatic
lipoprotein production (44). It
has also been reported that the accumulation of FFAs, triglycerides
(TGs) and diglycerides may promote adipocyte necrosis in obese
mice, which resulted in the polarization of macrophages towards a
standard M1 or activated phenotype in the AT.
MicroRNA (miRNA) processing
miRNAs have been identified in multiple tissues,
including the skeletal muscles, AT, liver and brain (51). The number of miRNAs present in
WAT was found to decrease with age, owing to the downregulation of
the miRNA-processing enzyme known as Dicer15 (52). An experimental study with mice
demonstrated that Dicer15 exhibited a defect in miRNA processing in
AT, prevented the whitening of BAT and resulting in an inadequate
response of WAT to metabolic stress, and promoted lipodystrophy, IR
and inflammation (53). In
addition, AT was recently reported to be an essential source of
circulating exosomal miRNAs, which have been associated with
glucose tolerance; thus, representing a novel target to produce
therapeutics that modulate age-related metabolic diseases and
various aging processes (54).
Adipokines
WAT secretes varying amounts of bioactive molecules
named adipokines, which serve crucial roles in regulating diverse
metabolic processes ranging from food intake to nutrient recycling,
energy homeostasis and IR (55).
It has been hypothesized that age-associated changes may, however,
influence the impact of adipokine secretion (56).
Adiponectin
Adiponectin is an adipose-derived hormone that has
been associated with an improved IS, which also inhibits the
synthesis of proinflammatory cytokines (57). As adipokines secreted from WAT
are involved in the regulation of glucose metabolism, it has been
suggested that adiponectin may mediate IS and possess
anti-inflammatory properties (58). However, the association between
aging and adiponectin remains poorly understood; for example,
several previous studies have reported that plasma adiponectin
levels increased with age (59,60), while other studies reported the
opposite results (61). Overall,
the relative decrease in adiponectin secretion in VAT has been
associated with an increased risk of aging (62).
In addition, lower levels of adiponectin have been
associated with obesity, cigarette smoking and oxidative stress.
One of the most prominent hypotheses regarding the mechanisms
inducing or worsening obesity and consequently, NAFLD, is the
overproduction of reactive oxygen species (ROS) (63).
Leptin
Leptin is a WAT-secreted hormone, which stimulates
fatty acid oxidation and regulates insulin binding and production,
by interacting with regions of the brain involved in controlling
appetite (64). In addition, the
availability of leptin was found to influence lipogenesis and
lipolysis, and the failure of leptin to restore metabolic
homeostasis is termed as a state of leptin resistance (65). Notably, aging has been associated
with a decrease in leptin activity, while youth is associated with
a high sensitivity of leptins; The redistribution of adipose tissue
observed during old age contribute however to an increase in
circulating leptin. However, leptin resistance could not be
overcome by an increase in leptin levels during aging. This
suggested that leptin resistance appears to be an early contributor
to the development of metabolic abnormalities in old age (64,66).
Resistin
Resistin is activated during the process of IR
(insulin antagonism) and is a distinctive cysteine-rich signaling
molecule. In mice, resistin is produced by monocytes and AT, while
in humans, resistin is produced in monocytes and macrophages, but
not in AT (67). Circulating
resistin levels have been reported to increase with age, and
elevated levels of resistin were associated with an increased risk
for coronary intervention and CVDs (68).
Inflammation
Low-grade, chronic inflammation, which is a central
mechanism involved in the aging process, has been established as a
precursor of aging and closely associated with IR (69). The hypotheses surrounding the WAT
inflammatory capacity suggest that immune cells infiltrating WAT
are the primary source of inflammation (70). Adipose resident immune cells,
such as M2 macrophages, which are anti-inflammatory cells, have
been reported to improve IS, whereas M1 macrophages, mast cells,
natural killer cells and neutrophils, which are proinflammatory
cells, promote IR during aging (71,72). Therefore, immune cells and their
participation in the inflammatory response are important
pathophysiological mechanisms of aging WAT (Fig. 2).
 | Figure 2Chronic inflammation is closely
associated with age-dependent IR. The expression levels of
inflammatory factors, such as MCP-1, IL-6, IL-1, TNF-α and leptin
are upregulated, while the expression levels of adiponectin are
downregulated in aging WAT. Subsequently, aging WAT is infiltrated
by macrophages, CD4+ T cells, CD8+ T cells
and Treg cells. Excessive metabolic products flux into
the mitochondria during aging, resulting in the overproduction of
ROS and spin-down of electrons, which can result in oxidative
stress and ultimately, damage the activation of the PI3K/AKT
signaling pathway, thereby inducing IR. MCP-1, monocyte chemotactic
protein 1; WAT, white adipose tissue; Treg, T
regulatory; ROS, reactive oxygen species; IR, insulin resistance;
IRS, insulin receptor substrate. |
AT macrophages (ATMs)
ATMs are induced by aging and secrete chemokines
that attract monocytes to the AT. ATMs consist of two subsets:
Proinflammatory M1 ATMs (which secrete TNF-α) and anti-inflammatory
M2 ATMs (which secrete arginase 1) (73). Proinflammatory ATMs also secrete
their own chemokines once they migrate into the WAT, attracting
additional macrophages and promoting the inflammatory processes
(74).
AT T cells
T cells differentiate into proinflammatory
phenotypes with elevated levels of chemokine receptors and enhanced
chemotaxis potential. Notably, aging has been found to alter T cell
development. T regulatory (Treg) cells, a small subset
of T cells, are an essential defense mechanism of the body against
inappropriate immune responses due to inflammation, autoimmunity,
infection, cancer or allergies. Recently, Treg cells
were identified to be involved in controlling the inflammatory
state of the AT. At birth, subcutaneous AT and VAT contain low
percentages of Treg cells; however, over time, the
percentage of Treg cells increases in the VAT (75).
Adipose-secreted inflammatory
cytokines
Aging has also been associated with higher levels of
proinflammatory cytokines, which act in a paracrine or an autocrine
manner to induce IR by decreasing the expression of insulin
receptor substrate (IRS)-1 (76). For example, TNF-α, expressed
primarily by AT macrophages, induced IR and inhibited insulin
signaling in human adipocytes by affecting the expression of IRS
proteins (77). A previous study
has also shown that inflammatory responses disrupt normal lipid
accumulation. For example, proinflammatory cytokines, such as IL-6,
impaired lipid accumulation by promoting Wnt signaling (78).
Mitochondrial dysfunction
Cumulative molecular damage can result in
mitochondrial impairment (78).
The mitochondria are the primary origin of ROS, which are
inevitable by-products of oxidative phosphorylation. Mitochondrial
dysfunction and oxidative stress in WAT are important factors
leading to IR during the aging process. Mitochondrial ROS were
demonstrated to attenuate insulin action in adipocytes and abolish
insulin-stimulated glucose transporter 4 translocation in mouse
adipocytes. Notably, impaired mitochondrial function was also shown
to increase endoplasmic reticulum stress (78,79). Mitochondria also serve a central
role in cell death; therefore, mitochondrial dysfunction in
adipocytes may trigger cell death in adipocytes to induce AT
inflammation (79). Thus, the
dysfunction of mitochondria highly probably a significant cause of
age-dependent chronic inflammation.
Mitochondrial sirtuins (mtSIRTs) comprise three
members, SIRT3, SIRT4 and SIRT5, which are all involved in
regulating energy metabolism and metabolic homeostasis (80). Among the mtSIRTs, SIRT4
expression levels were found to be upregulated during senescence
and were induced by different stimuli, such as DNA damage (80). SIRT4 functions as an efficient
mitochondrial ADP-ribosyl transferase that inhibits mitochondrial
glutamate dehydrogenase (GDH) activity. GDH is known to convert
glutamate to α-ketoglutarate, which promotes ATP generation and
insulin secretion in pancreatic β cells (81). In vivo experiments
observed a significant increase in insulin secretion from
SIRT4-deficient pancreatic β cells in response to glucose and amino
acids in SIRT4-knockout (KO) mice. More recently, SIRT4 has also
been shown to interact with insulin-degrading enzyme, which
modulates insulin secretion, providing an alternative possible
mechanism by which SIRT4 may impact insulin secretion (82).
4. BAT in age-dependent IR
BAT is a metabolically active tissue that regulates
plasma glucose levels, IR, TG metabolism and hyperlipidemia
(83). Notably, it has recently
emerged as a potential target in the treatment of type II diabetes.
BAT has the capacity to actively clear glucose from the circulation
and has been suggested to serve a role in preventing age-dependent
dysfunction and disease (83).
Abnormalities in BAT
A notable change in AT distribution associated with
aging is due to the loss of BAT. A decline in BAT function with
aging has been described in both rodents and humans (84). In one study, the proportion of
BAT was >50% in younger individuals (<35 years old), but
<10% in older individuals (>65 years old) (84). In addition, metabolic alterations
in subcutaneous WAT harbored a subpopulation of UCP1+
brown adipocytes, thereby promoting IR (85). Indeed, a predominant consequence
of aging in murine subcutaneous WAT is the loss of 'browning'
subcutaneous WAT, and UCP1 expression in subcutaneous WAT was found
to be increasingly downregulated with age in rodents (85). Loss of BAT in older mice was also
inversely correlated with IR development (86). Furthermore, it has been suggested
that BAT itself may become dysfunctional with age (86).
Mitochondrial mass in BAT
In addition to TG stores in multilocular lipid
droplets, BAT has high mitochondrial density. During aging, there
is a significant decline of UCP1 activity and an age-dependent
accumulation of point mutations in mitochondrial DNA (87). Cumulative molecular damage was
found to result in mitochondrial functional decline and impairment
(88), which are both associated
with an age-dependent increase in the incidence of
neurodegenerative diseases and metabolic disorders (89).
Inflammation in BAT
As aforementioned, chronic inflammation increases
with age. It was previously demonstrated that BAT is resistant to
macrophage infiltration, as shown by the downregulated expression
of immune cell-enriched genes, which indicates the
anti-inflammatory properties of BAT (90). However, during the inflammatory
response in mice, a novel chemokine (C-X-C chemokine ligand 14) was
observed to be secreted by thermogenically activated BAT (91). In addition, several
proinflammatory cytokines reduce UCP1 gene expression in BAT. For
instance, TNF-α induces the apoptosis of WAT and downregulates the
expression of UCP1 via Toll-like receptor activation, thereby
decreasing thermogenesis in BAT (92).
Hormonal regulation of BAT function
The age-dependent decline of BAT activity has been
suggested to be associated with changes in circulating levels of
hormones. Ghrelin signaling is an essential thermogenic regulator
during aging (93). Obestatin,
which upregulates UCP1 expression, and Ghrelin, which downregulates
UCP1 expression, are both derived from the same pre-proghrelin
gene. During aging, following no change in plasma obestatin
expression, increased growth hormone secretagogue receptor (GHS-R)
expression and plasma ghrelin expression in BAT was demonstrated to
promote thermogenic regulatory imbalance in middle-aged and old
mice. Moreover, the knockdown of the ghrelin receptor and/or GHS-R
decreased the risk of age-associated IR in
Ghsr−/−mice (94). Thyroid hormones, such as
triiodothyronine, were also demonstrated to be regulators of
thermogenesis; therefore, a decrease in thyroid hormones may
significantly contribute to the dysfunction of BAT during aging
(95).
5. Beige AT in age-dependent IR
Numerous molecular mechanisms have been reported to
be involved in the browning of WAT during aging. The origin of
beige adipocytes may result from a white-to-brown
trans-differentiation and/or the de novo differentiation of
specific precursor cells. For example, progenitor cells and/or
adipose stem cells of WAT may differentiate into white or beige
adipocytes (96). Among them, a
distinct subpopulation of WAT resident progenitors expressing CD137
and transmembrane protein 26 (TMEM26) are more able to
differentiate into beige adipocytes (25). Thus, the age-related lose of
beige AT may be explained by the loss of CD137/TMEM26+
progenitors. In addition, several regulators were demonstrated to
serve important roles in the browning of WAT. For example,
peroxisome proliferator activated receptor (PPAR)γ and PR/SET
domain 16 (PRDM16) are not only be involved in the induction of
BAT-associated genes, but also in the repression of WAT-associated
genes. PRDM16 upregulated the expression levels of BAT-specific
genes by associating with the transcriptional co-activators, PPARγ
coactivator 1 (PGC)-1α and PGC-1β (97). SIRT1, an important target in
adipose biology, facilitated the browning of AT via PPARγ and
PRDM16 (98) and enhanced beige
adipocyte differentiation ability via the senescence-associated
p53/p21 signaling pathway (99),
which is downregulated during aging. Irisin was also reported to
upregulate the expression of UCP1 and promote the WAT browning
process, which is a novel hormonal factor that converts WAT into
BAT (100). Notably, irisin
also induced a significant increase in total body energy
expenditure (101). In
conclusion, the combined effect of the aforementioned factors have
been suggested to participate in the browning process.
6. Therapeutic value of beige adipocytes and
acquisition of brown-like properties in WAT
In a previous study, BAT transplantation in mice
improved whole-body energy expenditure and IS, suggesting a key
role of WAT browning in improving whole body energy metabolism and
IS (102). Thus, it may be
important to shift beige adipose cells from a WAT phenotype to a
BAT phenotype under specific stimuli (Fig. 3).
 | Figure 3Possible mechanisms involved in the
process of WAT browning. Under cold exposure, thermogenic brown and
beige adipocytes produce heat by oxidizing fatty acids.
Liver-derived lipid metabolites and bile acids stimulate
thermogenesis in BAT. With cold exposure, the levels of bile acid
were elevated. β-adrenergic receptors are activated by the release
of noradrenaline, and the resultant Ca2+ influx causes
AMPK-dependent SIRT1 activation, which deacetylates PPARγ and
PRDM-16. A PPARγ/PRDM-16 interaction results in the browning of
inguinal WAT. WAT, white adipose tissue; BAT, brown adipose tissue;
AMPK, 5′AMP-activated protein kinase; SIRT1, sirtuin 1; PPARγ,
peroxisome proliferator activated receptor γ; PRDM-16, PR/SET
domain family-16; UCP-1, uncoupling protein 1; SIRT1,
sirtuin-1. |
Cold exposure
Cold exposure is a strong beige adipocyte inducer.
Significant metabolism and gene expression changes in WAT were
found to be induced by chronic cold exposure (103). For example, PGC-1α expression
levels, a co-activator of PPARγ, were upregulated upon cold
exposure. In primary human subcutaneous WAT, the overexpression of
PGC-1α promoted polarization to a BAT phenotype, which was
accompanied by the upregulated expression levels of fatty acid
oxidation enzymes, respiratory chain proteins and UCP1 (104). In addition, chronic cold
exposure was also observed to stimulate the differentiation of
precursors into beige adipocytes within one week of exposure
(105).
Physical exercise
Physical exercise has been reported to be associated
with a reduction in lipid content, increased mitochondrial activity
and the modified secretion of adipokines (106). However, the effects of physical
exercise in the AT of the elderly, particularly in BAT, remain
unknown. To date, the findings of previous studies investigating
the effect of exercise on BAT in the elderly remain controversial.
Recent studies revealed that endurance exercise training could
facilitate brown-like adipocyte recruitment within WAT by
activating cytokines such as irisin and IL-6 in rodents (107). Furthermore, strength and
aerobic training induced an increase in BAT mitochondrial activity
in old rats (108). However,
another study revealed that chronic endurance exercise was not
associated with the recruitment of brown and beige adipocytes
(109). Thus, further studies
are required to investigate the complex association between
different types of exercise and exercise-induced WAT and BAT
adaptations.
Diet
Previous studies have reported that several food
ingredients may stimulate thermogenesis and browning of the WAT.
For example, in a human model, a significant increase in
insulin-induced fluorodeoxyglucose uptake in BAT was observed,
suggesting that BAT may influence postprandial energy metabolism
(110). In addition, capsaicin
and capsinoids, which are alkaloids extracted from Capsicum
genus, simulated the heat burning sensation (111). The chronic oral administration
of capsinoids each day also triggered a significant increase in BAT
activity (111). Fucoxanthin
(FX), which was found to be present in abundance in the diatom
Phaeodactylum tricornutum, significantly downregulated the
expression levels and activity of lipogenic enzymes, upregulated
the expression levels of AR-β3 in adipocytes and the fatty acid
oxidation rate (112). In both
WAT and BAT, the administration of a high-fat diet mixed with 0.2%
FX upregulated the expression levels of UCP1 in mice. Carotenoids
have a large spectrum of isoprenoids, such as β-carotene and
retinoic acid, which regulate thermogenesis and energy expenditure
in both WAT and BAT (113).
β-carotene modulates the expression levels of UCP1 and retinoic
acid upregulates the expression levels of catabolic and thermogenic
proteins that increase mitochondrial biogenesis. Long-chain ω-3
polyunsaturated fatty acids (LCPUFAs), in particular
eicosapentaenoic acid (EPA), upregulated the expression levels of
mitochondrial oxidative-and thermogenic-associated genes, in
addition to increasing 5′AMP-activated protein kinase (AMPK)
phosphorylation and carnitine palmitoyltransferase 1 expression in
mice, suggesting that the treatment with EPA may improve energy
expenditure, thermogenesis and oxidation in subcutaneous adipocytes
(114). Polyphenols, which are
secondary metabolites of plants, were also found to recruit new
brown adipocytes, and increase metabolic activity, including WAT
browning (115).
Autophagy
Autophagy, a cell-protective dynamic process,
rearranges subcellular membranes to segregate the cytoplasm and
organelles for transmission to lysosomes, and promotes cell death
via the excessive degradation of cellular constituents, which may
have a role in regulating the browning of WAT. After knocking down
the expression of autophagy-related 7 (ATG7) in aP2+
adipocytes to impair autophagy, a BAT phenotype was evidenced by an
increased WAT and BAT mass (116). Concurrently, brown adipocyte
differentiation and function were specifically disrupted by
suppressing ATG7 in Myf5+ precursor cells (117). In addition, mTOR is an
essential negative regulator of autophagy. Previous studies have
shown that the inhibition of mTOR blocked the cold-induced browning
of WAT (118). The
β-AR-dependent upregulation of UCP1 expression and increase in the
number of beige adipocytes in WAT were observed in mice with mTOR
complex 1 impairment (119).
Another previous study suggested that the tumor suppressor
folliculin (FLCN) regulated WAT browning via a transcription factor
binding the IGHM enhancer 3 (TFE3) and mTOR. mTOR-dependent
cytoplasmic retention of TFE3 was relieved by the adipose-specific
deletion of FLCN, resulting in the activation of the PGC-1α
transcriptional co-activator function, suggesting that adipose
browning was suppressed by mTOR (120).
7. Conclusion
As there are multiple different mechanisms to induce
the WAT browning process, it is plausible that the browning process
may represent a potential target to achieve efficacious therapeutic
outcomes in aging-dependent IR. Numerous molecules that induce BAT
activation or WAT browning may represent potential targets to treat
various disease types associated with IR, including type II
diabetes, metabolic syndrome and bone diseases.
Availability of data and materials
Not applicable.
Authors' contributions
RS designed the concept of the review and its
structure. CW wrote and revised the manuscript. PY was involved in
the writing of the review. All authors agree to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant no. 81802169) and
Shanghai Sailing Program (grant no. 18YF1413900).
References
|
1
|
Ikemura M, Nishikawa M, Hyoudou K,
Kobayashi Y, Yamashita F and Hashida M: Improvement of insulin
resistance by removal of systemic hydrogen peroxide by PEGylated
catalase in obese mice. Mol Pharm. 7:2069–2076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo S: Insulin signaling, resistance, and
the metabolic syndrome: Insights from mouse models into disease
mechanisms. J Endocrinol. 220:T1–T23. 2014. View Article : Google Scholar
|
|
3
|
Tarantino G, Citro V and Capone D:
Nonalcoholic fatty liver disease: A challenge from mechanisms to
therapy. J Clin Med. 9:152019. View Article : Google Scholar
|
|
4
|
Czech MP: Insulin action and resistance in
obesity and type 2 diabetes. Nat Med. 23:804–814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y,
Wang L, Zhang Y, Liang X, Wang L, et al: Gut microbiota-bile
acid-interleukin-22 axis orchestrates polycystic ovary syndrome.
Nat Med. 25:1225–1233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petersen MC and Shulman GI: Mechanisms of
insulin action and insulin resistance. Physiol Rev. 98:2133–2223.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Napoli N, Chandran M, Pierroz DD,
Abrahamsen B and Schwartz AV: Mechanisms of diabetes
mellitus-induced bone fragility. Nat Rev Endocrinol. 13:208–219.
2017. View Article : Google Scholar
|
|
8
|
Chow HM, Shi M, Cheng A, Gao Y, Chen G,
Song X, So RWL, Zhang J and Herrup K: Age-related hyperinsulinemia
leads to insulin resistance in neurons and cell-cycle-induced
senescence. Nat Neurosci. 22:1806–1819. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barzilai N and Ferrucci L: Insulin
resistance and aging: A cause or a protective response? J Gerontol
A Biol Sci Med Sci. 67:1329–1331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosen ED and Spiegelman BM: Adipocytes as
regulators of energy balance and glucose homeostasis. Nature.
444:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun R, Wu Y, Hou W, Sun Z, Wang Y, Wei H,
Mo W and Yu M: Bromodomain-containing protein 2 induces insulin
resistance via the mTOR/Akt signaling pathway and an inflammatory
response in adipose tissue. Cell Signal. 30:92–103. 2017.
View Article : Google Scholar
|
|
12
|
Zong J, Li S, Wang Y, Mo W, Sun R and Yu
M: Bromodomain-containing protein 2 promotes lipolysis via ERK/HSL
signalling pathway in white adipose tissue of mice. Gen Comp
Endocrinol. 281:105–116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bapat SP, Myoung Suh J, Fang S, Liu S,
Zhang Y, Cheng A, Zhou C, Liang Y, LeBlanc M, Liddle C, et al:
Depletion of fat-resident Treg cells prevents age-associated
insulin resistance. Nature. 528:137–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sierra Rojas JX, Garcia-San Frutos M,
Horrillo D, Lauzurica N, Oliveros E, Carrascosa JM,
Fernández-Agulló T and Ros M: Differential development of
inflammation and insulin resistance in different adipose tissue
depots along aging in wistar rats: Effects of caloric restriction.
J Gerontol A Biol Sci Med Sci. 71:310–322. 2016. View Article : Google Scholar
|
|
15
|
Park A, Kim WK and Bae KH: Distinction of
white, beige and brown adipocytes derived from mesenchymal stem
cells. World J Stem Cells. 6:33–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giralt M and Villarroya F: White, brown,
beige/brite: Different adipose cells for different functions?
Endocrinology. 154:2992–3000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palmer AK and Kirkland JL: Aging and
adipose tissue: Potential interventions for diabetes and
regenerative medicine. Exp Gerontol. 86:97–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madonna R and De Caterina R: In vitro
neovasculogenic potential of resident adipose tissue precursors. Am
J Physiol Cell Physiol. 295:C1271–1280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chon SH and Pappas A: Differentiation and
characterization of human facial subcutaneous adipocytes.
Adipocyte. 4:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frontini A and Cinti S: Distribution and
development of brown adipocytes in the murine and human adipose
organ. Cell Metab. 11:253–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cedikova M, Kripnerová M, Dvorakova J,
Pitule P, Grundmanova M, Babuska V, Mullerova D and Kuncova J:
Mitochondria in white, brown, and beige adipocytes. Stem Cells Int.
2016:60673492016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye L, Wu J, Cohen P, Kazak L, Khandekar
MJ, Jedrychowski MP, Zeng X, Gygi SP and Spiegelman BM: Fat cells
directly sense temperature to activate thermogenesis. Proc Natl
Acad Sci USA. 110:12480–12485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cypess AM, White AP, Vernochet C, Schulz
TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et
al: Anatomical localization, gene expression profiling and
functional characterization of adult human neck brown fat. Nat Med.
19:635–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mailloux RJ and Harper ME: Uncoupling
proteins and the control of mitochondrial reactive oxygen species
production. Free Radic Biol Med. 51:1106–1115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu J, Boström P, Sparks LM, Ye L, Choi JH,
Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al:
Beige adipocytes are a distinct type of thermogenic fat cell in
mouse and human. Cell. 150:366–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pyrzak B, Demkow U and Kucharska AM: Brown
adipose tissue and browning agents: Irisin and FGF21 in the
development of obesity in children and adolescents. Adv Exp Med
Biol. 866:25–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okla M, Ha JH, Temel RE and Chung S: BMP7
drives human adipogenic stem cells into metabolically active beige
adipocytes. Lipids. 50:111–120. 2015. View Article : Google Scholar :
|
|
28
|
Palmer AK, Tchkonia T, LeBrasseur NK,
Chini EN, Xu M and Kirkland JL: Cellular senescence in type 2
diabetes: A therapeutic opportunity. Diabetes. 64:2289–2298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zoico E, Rubele S, De Caro A, Nori N,
Mazzali G, Fantin F, Rossi A and Zamboni M: Brown and beige adipose
tissue and aging. Front Endocrinol (Lausanne). 10:3682019.
View Article : Google Scholar
|
|
30
|
Spalding KL, Arner E, Westermark PO,
Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J,
Näslund E, Britton T, et al: Dynamics of fat cell turnover in
humans. Nature. 453:783–787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eckel-Mahan K, Ribas Latre A and Kolonin
MG: Adipose stromal cell expansion and exhaustion: Mechanisms and
consequences. Cells. 9:8632020. View Article : Google Scholar :
|
|
32
|
Tchkonia T, Morbeck DE, Von Zglinicki T,
Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD and
Kirkland JL: Fat tissue, aging, and cellular senescence. Aging
Cell. 9:667–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caso G, McNurlan MA, Mileva I, Zemlyak A,
Mynarcik DC and Gelato MC: Peripheral fat loss and decline in
adipogenesis in older humans. Metabolism. 62:337–340. 2013.
View Article : Google Scholar
|
|
34
|
Bukowska J, Frazier T, Smith S, Brown T,
Bender R, McCarthy M, Wu X, Bunnell BA and Gimble JM: Bone marrow
adipocyte developmental origin and biology. Curr Osteoporos Rep.
16:312–319. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahmed AS, Sheng MH, Wasnik S, Baylink DJ
and Lau KW: Effect of aging on stem cells. World J Exp Med. 7:1–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kirkland JL, Tchkonia T, Pirtskhalava T,
Han J and Karagiannides I: Adipogenesis and aging: Does aging make
fat go MAD? Exp Gerontol. 37:757–767. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gill LE, Bartels SJ and Batsis JA: Weight
management in older adults. Curr Obes Rep. 4:379–388. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sepe A, Tchkonia T, Thomou T, Zamboni M
and Kirkland JL: Aging and regional differences in fat cell
progenitors-a mini-review. Gerontology. 57:66–75. 2011. View Article : Google Scholar
|
|
39
|
Pischon T, Boeing H, Hoffmann K, Bergmann
M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG,
Tjønneland A, et al: General and abdominal adiposity and risk of
death in Europe. N Engl J Med. 359:2105–2120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Preis SR, Massaro JM, Robins SJ, Hoffmann
U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D'Agostino RB Sr,
O'Donnell CJ and Fox CS: Abdominal subcutaneous and visceral
adipose tissue and insulin resistance in the Framingham heart
study. Obesity (Silver Spring). 18:2191–2198. 2010. View Article : Google Scholar
|
|
41
|
Paradis ME, Hogue MO, Mauger JF, Couillard
C, Couture P, Bergeron N and Lamarche B: Visceral adipose tissue
accumulation, secretory phospholipase A2-IIA and atherogenecity of
LDL. Int J Obes (Lond). 30:1615–1622. 2006. View Article : Google Scholar
|
|
42
|
Giorgino F: Adipose tissue function and
dysfunction: Organ cross talk and metabolic risk. Am J Physiol
Endocrinol Metab. 297:E975–E976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Capurso C and Capurso A: From excess
adiposity to insulin resistance: The role of free fatty acids.
Vascul Pharmacol. 57:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ibrahim MM: Subcutaneous and visceral
adipose tissue: Structural and functional differences. Obes Rev.
11:11–18. 2010. View Article : Google Scholar
|
|
45
|
Niu Z, Lin N, Gu R, Sun Y and Feng Y:
Associations between insulin resistance, free fatty acids, and
oocyte quality in polycystic ovary syndrome during in vitro
fertilization. J Clin Endocrinol Metab. 99:E2269–E2276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chait A and den Hartigh LJ: Adipose tissue
distribution, inflammation and its metabolic consequences,
including diabetes and cardiovascular disease. Front Cardiovasc
Med. 7:222020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz
DP, Goldstein J, Nelson PS, Desprez PY and Campisi J:
Senescence-associated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS Biol. 6:2853–2868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu M, Palmer AK, Ding H, Weivoda MM,
Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze
N, et al: Targeting senescent cells enhances adipogenesis and
metabolic function in old age. Elife. 4:e129972015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu M, Tchkonia T, Ding H, Ogrodnik M,
Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera
V, et al: JAK inhibition alleviates the cellular
senescence-associated secretory phenotype and frailty in old age.
Proc Natl Acad Sci USA. 112:E6301–E6310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park SS and Seo YK: Excess accumulation of
lipid impairs insulin sensitivity in skeletal muscle. Int J Mol
Sci. 21:19492020. View Article : Google Scholar :
|
|
51
|
Pincus Z, Smith-Vikos T and Slack FJ:
MicroRNA predictors of longevity in caenorhabditis elegans. PLoS
Genet. 7:e10023062011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mori MA, Raghavan P, Thomou T, Boucher J,
Robida-Stubbs S, Macotela Y, Russell SJ, Kirkland JL, Blackwell TK
and Kahn CR: Role of microRNA processing in adipose tissue in
stress defense and longevity. Cell Metab. 16:336–347. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mori MA, Thomou T, Boucher J, Lee KY,
Lallukka S, Kim JK, Torriani M, Yki-Järvinen H, Grinspoon SK,
Cypess AM and Kahn CR: Altered miRNA processing disrupts
brown/white adipocyte determination and associates with
lipodystrophy. J Clin Invest. 124:3339–3351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mori MA, Ludwig RG, Garcia-Martin R,
Brandão BB and Kahn CR: Extracellular miRNAs: From biomarkers to
mediators of physiology and disease. Cell Metab. 30:656–673. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fontes-Carvalho R, Fontes-Oliveira M,
Sampaio F, Mancio J, Bettencourt N, Teixeira M, Rocha Gonçalves F,
Gama V and Leite-Moreira A: Influence of epicardial and visceral
fat on left ventricular diastolic and systolic functions in
patients after myocardial infarction. Am J Cardiol. 114:1663–1669.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mancuso P and Bouchard B: The impact of
aging on adipose function and adipokine synthesis. Front Endocrinol
(Lausanne). 10:1372019. View Article : Google Scholar
|
|
57
|
Chandrasekar B, Boylston WH, Venkatachalam
K, Webster NJ, Prabhu SD and Valente AJ: Adiponectin blocks
interleukin-18-mediated endothelial cell death via APPL1-dependent
AMP-activated protein kinase (AMPK) activation and
IKK/NF-kappaB/PTEN suppression. J Biol Chem. 283:24889–24898. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jura M and Kozak LP: Obesity and related
consequences to ageing. Age (Dordr). 38:232016. View Article : Google Scholar
|
|
59
|
Koh KK, Quon MJ, Han SH, Lee Y, Ahn JY,
Kim SJ, Koh Y and Shin EK: Simvastatin improves flow-mediated
dilation but reduces adiponectin levels and insulin sensitivity in
hypercholesterolemic patients. Diabetes Care. 31:776–782. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Isobe T, Saitoh S, Takagi S, Takeuchi H,
Chiba Y, Katoh N and Shimamoto K: Influence of gender, age and
renal function on plasma adiponectin level: The Tanno and Sobetsu
study. Eur J Endocrinol. 153:91–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Takenouchi Y, Kobayashi T, Matsumoto T and
Kamata K: Gender differences in age-related endothelial function in
the murine aorta. Atherosclerosis. 206:397–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li JB, Nishida M, Kaimoto K, Asakawa A,
Chaolu H, Cheng KC, Li YX, Terashi M, Koyama KI, Amitani H, et al:
Effects of aging on the plasma levels of nesfatin-1 and
adiponectin. Biomed Rep. 2:152–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nigro E, Scudiero O, Monaco ML, Palmieri
A, Mazzarella G, Costagliola C, Bianco A and Daniele A: New insight
into adiponectin role in obesity and obesity-related diseases.
Biomed Res Int. 2014:6589132014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Carter S, Caron A, Richard D and Picard F:
Role of leptin resistance in the development of obesity in older
patients. Clin Interv Aging. 8:829–844. 2013.PubMed/NCBI
|
|
65
|
Katsiki N, Mikhailidis DP and Banach M:
Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta
Pharmacol Sin. 39:1176–1188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Doherty GH: Obesity and the ageing brain:
Could leptin play a role in neurodegeneration? Curr Gerontol
Geriatr Res. 2011:7081542011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lehrke M, Reilly MP, Millington SC, Iqbal
N, Rader DJ and Lazar MA: An inflammatory cascade leading to
hyperresistinemia in humans. PLoS Med. 1:e452004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gencer B, Auer R, de Rekeneire N, Butler
J, Kalogeropoulos A, Bauer DC, Kritchevsky SB, Miljkovic I,
Vittinghoff E, Harris T and Rodondi N: Association between resistin
levels and cardiovascular disease events in older adults: The
health, aging and body composition study. Atherosclerosis.
245:181–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rea IM, Gibson DS, McGilligan V, McNerlan
SE, Alexander HD and Ross OA: Age and age-related diseases: Role of
inflammation triggers and cytokines. Front Immunol. 9:5862018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
de Heredia FP, Gómez-Martinez S and Marcos
A: Obesity, inflammation and the immune system. Proc Nutr Soc.
71:332–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lumeng CN, Liu J, Geletka L, Delaney C,
Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A,
Garg S and Yung RL: Aging is associated with an increase in T cells
and inflammatory macrophages in visceral adipose tissue. J Immunol.
187:6208–6216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lumeng CN, Bodzin JL and Saltiel AR:
Obesity induces a phenotypic switch in adipose tissue macrophage
polarization. J Clin Invest. 117:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Russo L and Lumeng CN: Properties and
functions of adipose tissue macrophages in obesity. Immunology.
155:407–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fang W, Deng Z, Benadjaoud F, Yang D, Yang
C and Shi GP: Regulatory T cells promote adipocyte beiging in
subcutaneous adipose tissue. FASEB J. 34:9755–9770. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Schenk S, Saberi M and Olefsky JM: Insulin
sensitivity: Modulation by nutrients and inflammation. J Clin
Invest. 118:2992–3002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lumeng CN, Deyoung SM and Saltiel AR:
Macrophages block insulin action in adipocytes by altering
expression of signaling and glucose transport proteins. Am J
Physiol Endocrinol Metab. 292:E166–E174. 2007. View Article : Google Scholar
|
|
78
|
Gustafson B and Smith U: Cytokines promote
Wnt signaling and inflammation and impair the normal
differentiation and lipid accumulation in 3T3-L1 preadipocytes. J
Biol Chem. 281:9507–9516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Woo CY, Jang JE, Lee SE, Koh EH and Lee
KU: Mitochondrial dysfunction in adipocytes as a primary cause of
adipose tissue inflammation. Diabetes Metab J. 43:247–256. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
van de Ven RAH, Santos D and Haigis MC:
Mitochondrial sirtuins and molecular mechanisms of aging. Trends
Mol Med. 23:320–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tao Y, Huang C, Huang Y, Hong L, Wang H,
Zhou Z and Qiu Y: SIRT4 suppresses inflammatory responses in human
umbilical vein endothelial cells. Cardiovasc Toxicol. 15:217–223.
2015. View Article : Google Scholar
|
|
82
|
Argmann C and Auwerx J: Insulin secretion:
SIRT4 gets in on the act. Cell. 126:837–839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bartelt A, Bruns OT, Reimer R, Hohenberg
H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H,
Waurisch C, et al: Brown adipose tissue activity controls
triglyceride clearance. Nat Med. 17:200–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yoneshiro T, Aita S, Matsushita M,
Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M and
Saito M: Age-related decrease in cold-activated brown adipose
tissue and accumulation of body fat in healthy humans. Obesity
(Silver Spring). 19:1755–1760. 2011. View Article : Google Scholar
|
|
85
|
Tan CY, Virtue S, Bidault G, Dale M, Hagen
R, Griffin JL and Vidal-Puig A: Brown adipose tissue thermogenic
capacity is regulated by Elovl6. Cell Rep. 13:2039–2047. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rogers NH, Landa A, Park S and Smith RG:
Aging leads to a programmed loss of brown adipocytes in murine
subcutaneous white adipose tissue. Aging Cell. 11:1074–1083. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Valle A, Guevara R, Garcia-Palmer FJ, Roca
P and Oliver J: Caloric restriction retards the age-related decline
in mitochondrial function of brown adipose tissue. Rejuvenation
Res. 11:597–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Detmer SA and Chan DC: Functions and
dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol.
8:870–879. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lin AL, Coman D, Jiang L, Rothman DL and
Hyder F: Caloric restriction impedes age-related decline of
mitochondrial function and neuronal activity. J Cereb Blood Flow
Metab. 34:1440–1443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fitzgibbons TP, Kogan S, Aouadi M,
Hendricks GM, Straubhaar J and Czech MP: Similarity of mouse
perivascular and brown adipose tissues and their resistance to
diet-induced inflammation. Am J Physiol Heart Circ Physiol.
301:H1425–H1437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Villarroya F, Cereijo R, Villarroya J,
Gavaldà-Navarro A and Giralt M: Toward an understanding of how
immune cells control brown and beige adipobiology. Cell Metab.
27:954–961. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lorenzo M, Fernández-Veledo S, Vila-Bedmar
R, Garcia-Guerra L, De Alvaro C and Nieto-Vazquez I: Insulin
resistance induced by tumor necrosis factor-alpha in myocytes and
brown adipocytes. J Anim Sci. 86:E94–E104. 2008. View Article : Google Scholar
|
|
93
|
Amitani M, Amitani H, Cheng KC, Kairupan
TS, Sameshima N, Shimoshikiryo I, Mizuma K, Rokot NT, Nerome Y,
Owaki T, et al: The role of ghrelin and ghrelin signaling in aging.
Int J Mol Sci. 18:15112017. View Article : Google Scholar :
|
|
94
|
Lee JY, Takahashi N, Yasubuchi M, Kim YI,
Hashizaki H, Kim MJ, Sakamoto T, Goto T and Kawada T:
Triiodothyronine induces UCP-1 expression and mitochondrial
biogenesis in human adipocytes. Am J Physiol Cell Physiol.
302:C463–C472. 2012. View Article : Google Scholar
|
|
95
|
Weiner J, Hankir M, Heiker JT, Fenske W
and Krause K: Thyroid hormones and browning of adipose tissue. Mol
Cell Endocrinol. 458:156–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gustafson B, Hedjazifar S, Gogg S,
Hammarstedt A and Smith U: Insulin resistance and impaired
adipogenesis. Trends Endocrinol Metab. 26:193–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kajimura S, Seale P, Tomaru T,
Erdjument-Bromage H, Cooper MP, Ruas JL, Chin S, Tempst P, Lazar MA
and Spiegelman BM: Regulation of the brown and white fat gene
programs through a PRDM16/CtBP transcriptional complex. Genes Dev.
22:1397–1409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Becerril S, Gómez-Ambrosi J, Martin M,
Moncada R, Sesma P, Burrell MA and Frühbeck G: Role of PRDM16 in
the activation of brown fat programming. Relevance to the
development of obesity. Histol Histopathol. 28:1411–1425.
2013.PubMed/NCBI
|
|
99
|
Khanh VC, Zulkifli AF, Tokunaga C,
Yamashita T, Hiramatsu Y and Ohneda O: Aging impairs beige
adipocyte differentiation of mesenchymal stem cells via the reduced
expression of Sirtuin 1. Biochem Biophys Res Commun. 500:682–690.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Boström P, Wu J, Jedrychowski MP, Korde A,
Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al: A
PGC1-α-dependent myokine that drives brown-fat-like development of
white fat and thermogenesis. Nature. 481:463–468. 2012. View Article : Google Scholar
|
|
101
|
Niranjan SB, Belwalkar SV, Tambe S,
Venkataraman K and Mookhtiar KA: Recombinant irisin induces weight
loss in high fat DIO mice through increase in energy consumption
and thermogenesis. Biochem Biophys Res Commun. 519:422–429. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shankar K, Kumar D, Gupta S, Varshney S,
Rajan S, Srivastava A, Gupta A, Gupta AP, Vishwakarma AL, Gayen JR
and Gaikwad AN: Role of brown adipose tissue in modulating adipose
tissue inflammation and insulin resistance in high-fat diet fed
mice. Eur J Pharmacol. 854:354–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang W and Seale P: Control of brown and
beige fat development. Nat Rev Mol Cell Biol. 17:691–702. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
He L, Tang M, Xiao T, Liu H, Liu W, Li G,
Zhang F, Xiao Y, Zhou Z, Liu F and Hu F: Obesity-associated
miR-199a/214 cluster inhibits adipose browning via PRDM16-PGC-1α
transcriptional network. Diabetes. 67:2585–2600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yao L, Cui X, Chen Q, Yang X, Fang F,
Zhang J, Liu G, Jin W and Chang Y: Cold-inducible SIRT6 regulates
thermogenesis of brown and beige fat. Cell Rep. 20:641–654. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gollisch KS, Brandauer J, Jessen N, Toyoda
T, Nayer A, Hirshman MF and Goodyear LJ: Effects of exercise
training on subcutaneous and visceral adipose tissue in normal- and
high-fat diet-fed rats. Am J Physiol Endocrinol Metab.
297:E495–E504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Knudsen JG, Murholm M, Carey AL, Biensø
RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen
JB and Pilegaard H: Role of IL-6 in exercise training- and
cold-induced UCP1 expression in subcutaneous white adipose tissue.
PLoS One. 9:e849102014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Thirupathi A, da Silva Pieri BL, Queiroz
JAMP, Rodrigues MS, de Bem Silveira G, de Souza DR, Luciano TF,
Silveira PCL and De Souza CT: Strength training and aerobic
exercise alter mitochondrial parameters in brown adipose tissue and
equally reduce body adiposity in aged rats. J Physiol Biochem.
75:101–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Vosselman MJ, Hoeks J, Brans B,
Pallubinsky H, Nascimento EB, van der Lans AA, Broeders EP,
Mottaghy FM, Schrauwen P and van Marken Lichtenbelt WD: Low brown
adipose tissue activity in endurance-trained compared with lean
sedentary men. Int J Obes (Lond). 39:1696–1702. 2015. View Article : Google Scholar
|
|
110
|
Orava J, Nuutila P, Lidell ME, Oikonen V,
Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S
and Virtanen KA: Different metabolic responses of human brown
adipose tissue to activation by cold and insulin. Cell Metab.
14:272–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nirengi S, Homma T, Inoue N, Sato H,
Yoneshiro T, Matsushita M, Kameya T, Sugie H, Tsuzaki K, Saito M,
et al: Assessment of human brown adipose tissue density during
daily ingestion of thermogenic capsinoids using near-infrared
time-resolved spectroscopy. J Biomed Opt. 21:0913052016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kim SM, Jung YJ, Kwon ON, Cha KH, Um BH,
Chung D and Pan CH: A potential commercial source of fucoxanthin
extracted from the microalga Phaeodactylum tricornutum. Appl
Biochem Biotechnol. 166:1843–1855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bonet ML, Ribot J, Galmés S, Serra F and
Palou A: Carotenoids and carotenoid conversion products in adipose
tissue biology and obesity: Pre-clinical and human studies. Biochim
Biophys Acta Mol Cell Biol Lipids. 1865:1586762020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hilgendorf KI, Johnson CT, Mezger A, Rice
SL, Norris AM, Demeter J, Greenleaf WJ, Reiter JF, Kopinke D and
Jackson PK: Omega-3 fatty acids activate ciliary FFAR4 to control
adipogenesis. Cell. 179:1289–1305.e21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jiménez-Aranda A, Fernández-Vázquez G,
Campos D, Tassi M, Velasco-Perez L, Tan DX, Reiter RJ and Agil A:
Melatonin induces browning of inguinal white adipose tissue in
Zucker diabetic fatty rats. J Pineal Res. 55:416–423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang Y, Goldman S, Baerga R, Zhao Y,
Komatsu M and Jin S: Adipose-specific deletion of autophagy-related
gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl
Acad Sci USA. 106:19860–19865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Martinez-Lopez N, Athonvarangkul D, Sahu
S, Coletto L, Zong H, Bastie CC, Pessin JE, Schwartz GJ and Singh
R: Autophagy in Myf5+ progenitors regulates energy and
glucose homeostasis through control of brown fat and skeletal
muscle development. EMBO Rep. 14:795–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tran CM, Mukherjee S, Ye L, Frederick DW,
Kissig M, Davis JG, Lamming DW, Seale P and Baur JA: Rapamycin
blocks induction of the thermogenic program in white adipose
tissue. Diabetes. 65:927–941. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu D, Bordicchia M, Zhang C, Fang H, Wei
W, Li JL, Guilherme A, Guntur K, Czech MP and Collins S: Activation
of mTORC1 is essential for β-adrenergic stimulation of adipose
browning. J Clin Invest. 126:1704–1716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wada S, Neinast M, Jang C, Ibrahim YH, Lee
G, Babu A, Li J, Hoshino A, Rowe GC, Rhee J, et al: The tumor
suppressor FLCN mediates an alternate mTOR pathway to regulate
browning of adipose tissue. Genes Dev. 30:2551–2564. 2016.
View Article : Google Scholar : PubMed/NCBI
|