Introduction
Hypertension is a prevalent cardiovascular disorder
worldwide, and the kidneys have been identified as primary target
organs in the pathophysiology of hypertension (1,2).
Hypertensive renal injury is characterized by inflammation, the
excessive accumulation of extracellular matrix (ECM) and renal
tubular injury (3). The
renin-angiotensin system (RAS) system performs crucial functions
during hypertension-induced kidney injury development, which is
regulated by an essential factor of the RAS, namely angiotensin II
(Ang II) (4,5). Ang II can promote the release of
inflammation-related factors, such as interleukin-6 (IL-6) and
tumor necrosis factor-α (TNF-α), leading to small renal artery
endothelial cell damage and kidney vascular remodeling (6,7).
In addition, Ang II enhances the accumulation of ECM in smooth
vascular cells, tubular epithelial cells and glomerular mesangial
cells, causing kidney glomerular sclerosis and parenchyma fibrosis
(3). However, the mechanisms of
Ang II-induced kidney damage are complex and unclear.
Previous studies have suggested that various
critical signaling pathways participate in Ang II-induced kidney
damage, including the nuclear factor-κB (NF-κB) and extracellular
signal-regulated kinase (ERK) pathways (8,9).
The NF-κB and ERK signaling pathways have been reported to mediate
the expression of inflammatory and apoptotic mediators (10). Wu et al (11) indicated that NF-κB signaling
plays an important role in Ang II-induced hypertensive renal
damage. Additionally, previous studies have confirmed that the
inhibition of NF-κB or ERK signaling can attenuate the progression
of acute renal damage (10,12). Therefore, the NF-κB and ERK
signaling pathways may be considered as targets for the treatment
of hypertension-induced renal injury.
Sinigrin
(C10H16KNO9S2) is a
natural compound obtained from cruciferous vegetables, including
cauliflower, broccoli, and cabbage, and is usually used for the
treatment of multiple disorders in combination with other herbs.
Sinigrin has been shown to suppress adipocyte differentiation
through the ERK signaling pathway (13). Furthermore, sinigrin can exert
potent anti-atherogenic effects by disrupting the NF-κB and MAPK
pathways (14). Sinigrin can
also significantly inhibit inflammatory responses by suppressing
the NF-κB/MAPK pathway or NLR family pyrin domain containing 3
(NLRP3) inflammasome activation in macrophages (15). However, the effects of sinigrin
on Ang II-induced renal injury and the related molecular mechanisms
remain unclear.
Thus, the present study investigated the effects of
sinigrin on Ang II-induced kidney damage. The findings presented
herein demonstrate that sinigrin can protect against toward Ang
II-induced renal injury by inactivating NF-κB and ERK signaling
in vivo and in vitro.
Materials and methods
Induction of hypertension in rats via Ang
II infusion
To evaluate the function of sinigrin in the
modulation of hypertension-induced kidney damage, a hypertensive
rat model was constructed using Ang II. Briefly, Sprague-Dawley
rats (n=10 per group, weighing 200-225 g, male, SiPeiFu
Biotechnology Co., Ltd.) were subcutaneously administered Ang II
(Sigma-Aldrich; Merck KGaA) for 28 days via low-dose Ang II
infusion from osmotic minipumps (model 2002, ALZET, DURECT
Corporation) in the dorsum of the neck under anesthesia
(pentobarbital sodium, 50 mg/kg i.p.). Beginning from the first day
of Ang II pump implantation, sinigrin (#85440, Sigma-Aldrich; Merck
KGaA) was administered at the indicated doses to the rats once
daily via oral gavage and continued for 28 days. The rats were
assigned to 5 groups as follows: The sham-operated (sham) group
(n=10, infused with normal saline and orally administered normal
saline); Ang II group (n=10, infused with Ang II and orally
administered normal saline); Ang II + sinigrin (10 mg/kg) group
(n=10, infused with Ang II and orally administered 10 mg/kg
sinigrin); Ang II + sinigrin (20 mg/kg) group (n=10, infused with
Ang II and orally administered 20 mg/kg sinigrin); Ang II +
sinigrin (40 mg/kg) group (n=10, infused with Ang II and orally
administered 40 mg/kg sinigrin). At the end of the treatment
period, the rats were housed in individual metabolic cages for 24
h, and provided with water and food ad libitum, and urine
samples were obtained from the rats. Prior to sacrifice by cervical
dislocation, the rats were anesthetized by pentobarbital sodium (50
mg/kg i.p.) and blood samples (5 ml) were harvested from the
abdominal aorta for one time. Subsequently, the kidneys were
excised and collected; one part was placed in 10% formalin and
embedded in paraffin for histopathological analysis, and the other
was snap-frozen in liquid nitrogen for protein evaluation. All
procedures and experimental protocols were approved by the Animal
Ethics Committee of the Third Affiliated Hospital of Shandong First
Medical University (no. LL202001003).
Measurement of systolic blood pressure
(SBP) and diastolic blood pressure (DBP)
SBP and DBP were recorded and analyzed using the
CODA Monitor (Kent Scientific) with a tail-cuff non-invasive method
according to the instructions of the manufacturer. Prior to the
measurement, the rats were placed in the holder for 5 min, and the
SBP and DBP were recorded three times for each rat at 0, 7, 14, 21
and 28 days of Ang II infusion.
Analysis of kidney damage markers
The levels of serum creatinine (SCR) and blood urea
nitrogen (BUN) were measured using an automatic biochemical
analyzer (Beckman Coulter, Inc.) as described in a previous study
(16). The levels of urinary
protein were examined using a BCA Protein Assay kit (#P0010,
Beyotime Institute of Biotechnology).
Histological analysis of kidney
tissues
For the histological analysis of kidney tissues, the
kidneys were perfused with physiological salt solution, treated
with formalin (10%) and longitudinally sectioned, followed by
fixation in formalin (10%) overnight. Subsequently, the kidney
tissues were paraffin-embedded and sectioned as previously
described (17). Glomerular
basement membrane thickness was analyzed by periodic acid-Schiff
(PAS) staining (Beyotime Institute of Biotechnology). Briefly, the
sections were treated with periodic acid at room temperature for 5
min, and then incubated with Schiff at room temperature for 15
min.
Cells and cell culture
Human HK-2 proximal tubule epithelial cell lines
were maintained at the Central Laboratory of the Third Affiliated
Hospital of Shandong First Medical University and incubated at 37°C
with 5% CO2 in Dulbecco's modified Eagle medium (Cytiva)
containing fetal bovine serum (15%, Gibco; Thermo Fisher
Scientific, Inc.), streptomycin (0.1 mg/ml; Beijing Solarbio
Science & Technology Co., Ltd.) and penicillin (100 units/ml;
Beijing Solarbio Science & Technology Co., Ltd.). To induce
HK-2 cell injury, the cells were exposed to Ang II (Sigma-Aldrich;
Merck KGaA) at a concentration of 1 µM. Subsequently, the
cells were treated with sinigrin at1, 10 and 100 µg/ml.
MTT assays
The viability of the HK-2 cells was evaluated by MTT
assays. In brief, ~2×104 cells were plated in 96-well
plates and incubated for 12 h at room temperature. Following the
indicated treatments, the cells were mixed with MTT solution (5
mg/ml, 10 µl) and incubated for 4 h at room temperature. The
medium was then removed, and DMSO (150 µl) was added to the
cells. Cell viability was determined by measuring the absorbance at
570 nm using an ELISA browser (Bio-Tek EL 800; BioTek Instruments,
Inc.).
Analysis of cell apoptosis
HK-2 cells (~2×105) were plated in 6-well
plates. Cell apoptosis was assessed using the Annexin V-FITC
Apoptosis Detection kit (#6592, Cell Signaling Technology, Inc.)
according to the manufacturer's instructions. In brief,
~2×106 cells collected and washed cells were resuspended
in binding buffer, dyed with propidium iodide at 25°C, and
subjected to flow cytometric analysis (FACSCalibur; BD
Biosciences).
Analysis of oxidative stress-related
enzymes
Oxidative stress-related enzymes, including
superoxide dismutase (SOD), malondialdehyde (MDA) and catalase
(CAT), were analyzed in vivo and in vitro. The
activity of SOD was measured using a SOD assay kit (#706003; Cayman
Chemical Company). The activity of SOD was analyzed using a
microplate reader (BioTek Instruments, Inc.) at 450 nm. The
activity of MDA was measured using a MDA assay kit (#700870; Cayman
Chemical Company). The activity of MDA was analyzed using a
microplate reader (BioTek Instruments, Inc.) at 405±414 nm. The
activity of CAT was measured using a CAT assay kit (#707002, Cayman
Chemical Company). The activity of CAT was analyzed using a
microplate reader (BioTek Instruments, Inc.) at 340 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). First-strand cDNA was
manufactured using the PrimeScript™ II 1st Strand cDNA Synthesis
kit (#6210A; Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. The qPCR assays were performed using
Premix Ex Taq SYBR-Green (#RR820A; Takara Biotechnology Co., Ltd.).
The primer sequences were as follows: TNF-α forward, 5′-CCC AGG CAG
TCA GAT CAT CTT C-3′ and reverse, 5′-GCT TGA GGG TTT GCT ACA ACA
TG-3′; IL-6 forward, 5′-TCA GGA AAT TTG CCT ATT GAA AAT TT-3′ and
reverse, 5′-GCT TTG TCT TTC TTG TTA TCT TTT AAG TTG T-3′; monocyte
chemoattractant protein-1 (MCP-1) forward, 5′-CAT AGC AGC CAC CTT
CAT TCC -3′ and reverse, 5′-TCT CCT TGG CCA CAA TGG TC-3′; GAPDH
forward, 5′-AAC GGA TTT GGT CGT ATT GGG -3′ and reverse, 5′-CCT GGA
AGA TGG TGA TGG GAT -3′. The RT-qPCR reaction conditions were as
follows: 95°C, 10 sec (denaturation); 55°C, 30 sec (annealing);
72°C, 30 sec (extension) for 40 cycles. The relative expression
levels were calculated using the 2−ΔΔCq method (18).
Western blot analysis
Total protein was isolated from the cells using RIPA
buffer (Cell Signaling Technology, Inc.) and quantified using the
BCA Protein Quantification kit (Abbkine Scientific Co., Ltd.).
Protein samples were subjected to 10% SDS-PAGE and transferred to
PVDF membranes (EMD Millipore), followed by blocking with 5%
skimmed milk at room temperature and incubating with primary
antibodies at 4°C overnight. The horseradish peroxidase-conjugated
goat anti-rabbit IgG (1:5,000, cat. no. BA1039; Wuhan Boster
Biological Technology, Ltd.) were used to incubate the membranes
for 1 h at room temperature, followed by visualization by using a
chemiluminescence detection kit (Beyotime Institute of
Biotechnology, Inc.). The primary antibodies used in the present
study were the following: Collagen I (#ab260043, 1:500; Abcam),
collagen IV (1:500, cat. no. ab236640; Abcam), fibronectin
(1:1,000, cat. no. ab268020; Abcam), ERK (1:500, cat. no. 4695;
Cell Signaling Technology, Inc.), p65 (1:1,000, cat. no. 8242; Cell
Signaling Technology, Inc.), IκB α (1:500, cat. no. 8412; Cell
Signaling Technology, Inc.), p-ERK (1:300, cat. no. 4370; Cell
Signaling Technology, Inc.), p-p65 (1:500, cat. no. 3033; Cell
Signaling Technology, Inc.), p-IκBα (1:300, cat. no. 2859; Cell
Signaling Technology, Inc.) and GAPDH (1:2,000, cat. no. ab181602;
Abcam). The density of the protein bands was quantified using
ImageJ software (version V1.8.0; National Institutes of
Health).
Statistical analysis
Data are expressed as the mean ± SD, and statistical
analysis was conducted using GraphPad Prism 7 (GraphPad Software,
Inc.). One-way ANOVA followed by Tukey's post hoc test was used to
assess the differences between the groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Sinigrin alleviates Ang II-induced kidney
dysfunction in vivo
To investigate the role of sinigrin in the
modulation of hypertension-induced kidney damage, a spontaneously
hypertensive rat model was constructed using Ang II. It was
observed that SBP and DBP were increased in the Ang II-challenged
rats, and sinigrin treatment attenuated this increase in a
dose-dependent manner (Fig. 1A and
B). The levels of BUN and SCR were enhanced by Ang II in the
rats, and sinigrin reversed this effect in a dose-dependent manner
(Fig. 1C and D). Moreover, the
Ang II-induced increase in urinary protein levels was inhibited by
sinigrin treatment in the rats (Fig.
1E). These results suggest that sinigrin may alleviate Ang
II-induced kidney dysfunction in vivo.
Sinigrin attenuates Ang II-induced kidney
injury in vivo
The present study then explored the influence of
sinigrin on Ang II-induced kidney injury in a rat model. The
results revealed that renal glomerular basement membrane (GBM)
thickness was increased in the Ang II-challenged rats, which was
reduced by treatment with sinigrin in a dose-dependent manner
(Fig. 2A). Moreover, the
expression of fibronectin, collagen IV and I was increased by Ang
II in the rats, and sinigrin reversed this effect in a
dose-dependent manner (Fig.
2B-E). These results indicate that sinigrin may alleviate Ang
II-induced kidney injury in vivo.
Sinigrin reduces Ang II-induced
inflammation and oxidative stress in vivo
Given that oxidative stress and inflammatory
processes are involved in hypertension-related kidney damage, the
present study further explored the effects of sinigrin on
associated markers in the rat model. It was found that the
expression of TNF-α, IL-6 and MCP-1 was upregulated by Ang II and
treatment with sinigrin attenuated this upregulation in the rats
(Fig. 3A-D). Moreover, the
levels of SOD and CAT were decreased, whereas the levels of MDA
were increased in the Ang II-challenged rats; these effects were
reversed by treatment with sinigrin (Fig. 3E-G). Taken together, the results
suggest that sinigrin may reduce Ang II-induced inflammation and
oxidative stress in vivo.
 | Figure 3Sinigrin attenuates Ang II-induced
inflammation and oxidative stress in vivo. (A-G) The
hypertensive rat model was constructed by challenge with Ang II and
the rats were then treated with sinigrin at the indicated doses.
(A-D) The expression of TNF-α, IL-6, MCP-1, and GAPDH was measured
by western blot analysis and the results were quantified using
ImageJ software. (E-G) The levels of MDA, SOD, and CAT were
determined in the rats. **P<0.01 vs. sham group;
#P<0.05, ##P<0.01 vs. Ang II group. Ang
II, angiotensin II; TNF-α, tumor necrosis factor α; IL-6,
interleukin 6; MCP-1, monocyte chemoattractant protein-1; MDA,
malondialdehyde; SOD, superoxide dismutase; CAT, catalase. |
Sinigrin inhibits ERK and NF-κB signaling
in the Ang II-induced spontaneously hypertensive rat model
The present study then investigated the underlying
mechanisms of sinigrin-mediated hypertensive kidney damage.
Notably, the phosphorylation of ERK, p65 and IκBα was stimulated in
the Ang II-challenged rats; however, this was reduced by sinigrin
treatment (Fig. 4), indicating
that sinigrin may attenuate hypertension-induced kidney damage by
inactivating ERK and NF-κB signaling in vivo.
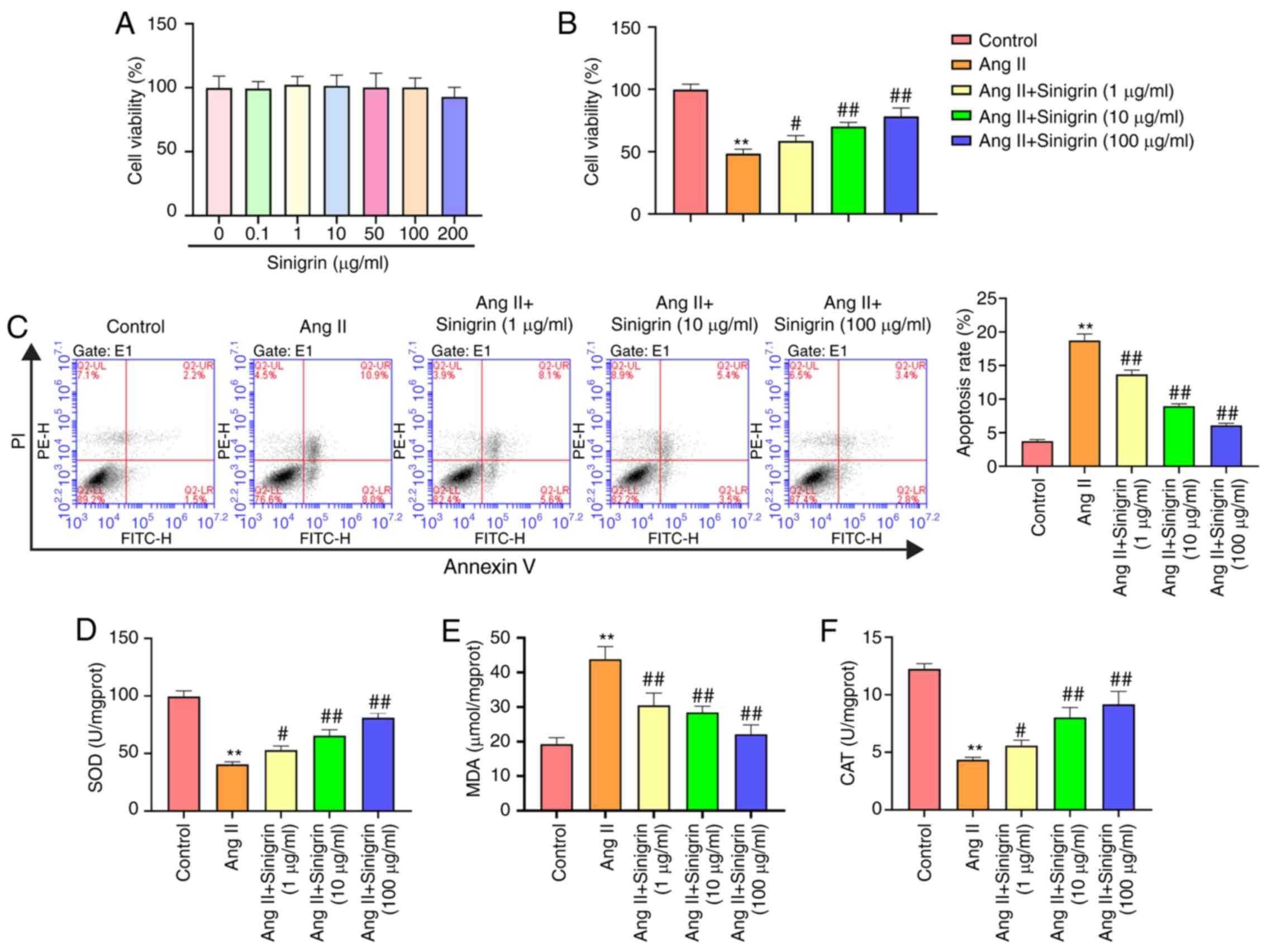
Sinigrin reduces Ang II-induced HK-2 cell
injury in vitro
Ang II is a vasoconstrictive peptide that modulates
blood pressure homeostasis and can cause hypertensive renal
inflammation in renal tubular epithelial cells (19,20). Therefore, in the present study,
the Ang II-induced injury model using HK-2 cells, a human renal
tubular epithelial cell line, was used to determine the function of
sinigrin in vitro. HK-2 cells were treated with sinigrin at
the indicated concentrations, which did not exhibit cytotoxicity to
the cells (Fig. 5A). The
viability of the Ang II-exposed HK-2 cells was reduced however, and
this was attenuated by by sinigrin treatment in a
concentration-dependent manner (Fig.
5B). Sinigrin also reversed the Ang II-induced apoptosis of
HK-2 cells in a concentration-dependent manner (Fig. 5C). In addition, the levels of SOD
and CAT were reduced, whereas the levels of MDA were enhanced the
Ang II-exposed HK-2 cells; these effects reversed by treatment with
sinigrin (Fig. 5D-F). Taken
together, these results suggest that sinigrin attenuates Ang
II-induced HK-2 cell injury in vitro.
Sinigrin alleviates Ang II-induced
inflammation and ECM degradation in HK-2 cells
It was demonstrated that the expression of TNF-α,
IL-6 and MCP-1 was upregulated by Ang II in the HK-2 cells, and
treatment with sinigrin abrogated this effect (Fig. 6A-C). Furthermore, the expression
of fibronectin, collagen IV and I was elevated by Ang II in the
cells, and this increase was reversed by sinigrin treatment in a
concentration-dependent manner (Fig.
6D-G). These results indicate that sinigrin may alleviate Ang
II-induced inflammation and ECM degradation in HK-2 cells.
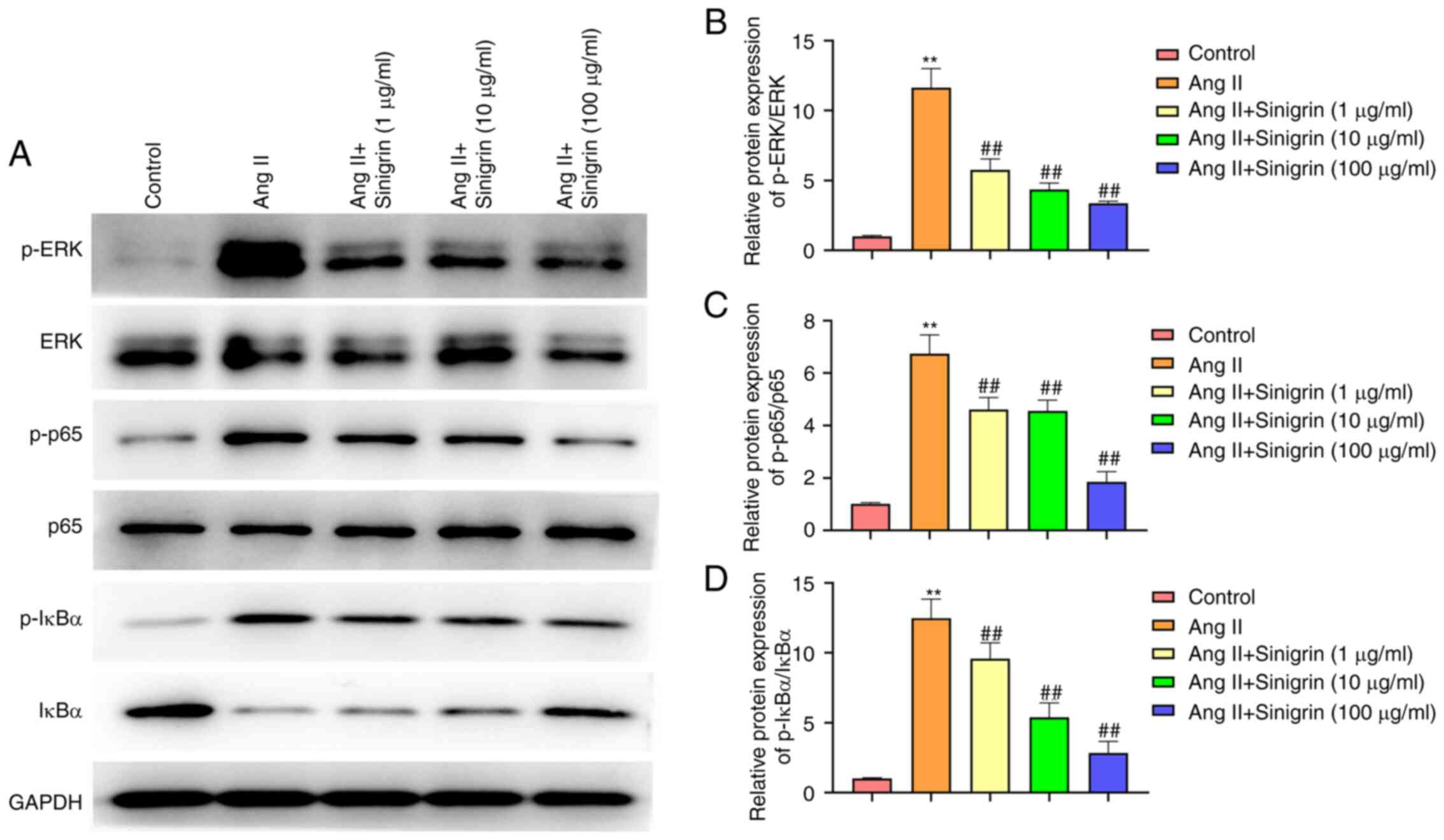
Sinigrin inactivates ERK and NF-κB
signaling in Ang II-exposed HK-2 cells
The results revealed that the phosphorylation of
ERK, p65 and IκBα was enhanced in Ang II-exposed HK-2 cells, and
sinigrin treatment reduced this effect in a concentration-dependent
manner (Fig. 7). Therefore,
sinigrin may attenuate hypertension-induced kidney damage by
inactivating ERK and NF-κB signaling in vitro.
Discussion
Kidney damage is a prevailing complication of
hypertension, which induces severe inflammatory response, oxidative
stress and ECM degradation (21). Sinigrin is a natural compound
extracted from cruciferous vegetables, which has anti-inflammatory
and antioxidant activities (15,22). Nevertheless, the effects of
sinigrin on hypertension-induced kidney damage remain elusive. In
the present study, it was found that sinigrin attenuated Ang
II-induced renal injury by inactivating ERK and NF-κB signaling
in vivo and in vitro.
With the emergence of natural compounds in plants
for the treatment of various disorders, several natural compounds
have been identified to regulate hypertension-induced kidney
damage. Brazilian red propolis has been reported to alleviate
hypertension-induced kidney damage (23). Boldine can inhibit
hypertension-related renal injury by repressing TGF-β expression
(24). Moreover, previous
studies have demonstrated that sinigrin can inhibit inflammatory
responses. Sinigrin has been reported to exhibit anti-inflammatory
and neuroprotective activities by inhibiting NF-κB/TNF-α signaling
(25). Sinigrin can suppress
inflammatory mediator production by reducing NF-κB/MAPK signaling
or NLRP3 inflammatory activation in macrophages (15). Sinigrin has also been
demonstrated to exhibit antioxidant activity in previous studies
(22,26). In addition, it has been found
that sinigrin can regulate cell viability and apoptosis under
pathological conditions (27).
In the present study, it was demonstrated that sinigrin was able to
alleviate Ang II-induced kidney dysfunction, kidney injury,
inflammation and oxidative stress in vivo and in
vitro. The findings demonstrated the critical role of sinigrin
in the inhibition of hypertension-related renal damage, and shed
light on a novel effect of sinigrin on Ang II-induced renal
injury.
ERK and NF-κB signaling contributes to the
progression of hypertension-induced kidney damage, and the
inhibition of ERK and NF-κB signaling can attenuate the adverse
effects of hypertension-related renal injury. It has been reported
that quercetin can ameliorate sodium fluoride-induced hypertension
by reducing oxidative stress through ERK/PPARγ signaling modulation
(28). Angiotensin has been
found to attenuate hypertension-related renal fibrosis by
inhibiting mTOR/ERK signaling in an apolipoprotein E-deficient
mouse model (29). Nebivolol can
inhibit profibrotic and pro-oxidant responses during the vascular
remodeling of renovascular hypertension by regulating ERK signaling
(30). Exercise training can
relieve hypertension by targeting NF-κB signaling in the
hypothalamic paraventricular nucleus (31). Rutin has been reported to
ameliorate sodium fluoride-induced hypertension by regulating
NF-κB/Nrf2 signaling in rats (32). The present study revealed that
the phosphorylation of ERK, p65 and IκBα was stimulated by Ang II,
and sinigrin treatment reduced this effect in a
concentration-dependent manner in vivo and in vitro.
This finding suggests that sinigrin may inactivate ERK and NF-κB
signaling.
In conclusion, the present study demonstrated that
sinigrin alleviated Ang II-induced renal injury by inactivating ERK
and NF-κB signaling. Sinigrin may thus be a potential candidate for
the treatment of hypertension-induced kidney damage.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CC made substantial contributions to conception and
design, and revised the manuscript. XY, YH and LT performed the
research. WC and YW analyzed the data. LT wrote and revised the
manuscript. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The experimental protocol of the present study was
performed in accordance with the Guide for the Care and Use of
Laboratory Animals and approved by The Third Affiliated Hospital of
Shandong First Medical University (Affiliated Hospital of Shandong
Academy of Medical Sciences) (no. LL202001003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Manosroi W and Williams GH: Genetics of
human primary hypertension: Focus on hormonal mechanisms. Endocr
Rev. 40:825–856. 2019. View Article : Google Scholar
|
|
2
|
Suneja M and Sanders ML: Hypertensive
emergency. Med Clin North Am. 101:465–478. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong D, Hu W, Ye ST and Tan YS:
Isoliquiritigenin alleviated the Ang II-induced hypertensive renal
injury through suppressing inflammation cytokines and oxidative
stress-induced apoptosis via Nrf2 and NF-κB pathways. Biochem
Biophys Res Commun. 506:161–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escobales N, Nuñez RE and Javadov S:
Mitochondrial angiotensin receptors and cardioprotective pathways.
Am J Physiol Heart Circ Physiol. 316:H1426–H1438. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li XC, Zhang J and Zhuo JL: The
vasoprotective axes of the renin-angiotensin system: Physiological
relevance and therapeutic implications in cardiovascular,
hypertensive and kidney diseases. Pharmacol Res. 125:21–38. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weber GJ, Pushpakumar SB and Sen U:
Hydrogen sulfide alleviates hypertensive kidney dysfunction through
an epigenetic mechanism. Am J Physiol Heart Circ Physiol.
312:H874–H885. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aroor AR, McKarns S, Demarco VG, Jia G and
Sowers JR: Maladaptive immune and inflammatory pathways lead to
cardiovascular insulin resistance. Metabolism. 62:1543–1552. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JK, Müller DN, Mervaala EM, Dechend
R, Fiebeler A, Schmidt F, Bieringer M, Schäfer O, Lindschau C,
Schneider W, et al: Cerivastatin prevents angiotensin II-induced
renal injury independent of blood pressure- and
cholesterol-lowering effects. Kidney Int. 58:1420–1430. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Henke N, Schmidt-Ullrich R, Dechend R,
Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, et
al: Vascular endothelial cell-specific NF-kappaB suppression
attenuates hypertension-induced renal damage. Circ Res.
101:268–276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, He S, Wang Y, Zhu X, Shao W, Xu Q
and Cui Z: miRNA-20a suppressed lipopolysaccharide-induced HK-2
cells injury via NFκB and ERK1/2 signaling by targeting CXCL12. Mol
Immunol. 118:117–123. 2020. View Article : Google Scholar
|
|
11
|
Wu SJ, Shi ZW, Wang X, Ren FF, Xie ZY, Lei
L and Chen P: Activation of the cholinergic anti-inflammatory
pathway attenuated angiotension II-dependent hypertension and renal
injury. Front Pharmacol. 12:5936822021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Liu N, Xue X and Zhou S: The
effect of overexpression of the enhancer of zeste homolog 1 (EZH1)
gene on aristolochic acid-induced injury in HK-2 human kidney
proximal tubule cells in vitro. Med Sci Monit. 25:801–810. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HW, Rhee DK, Kim BO and Pyo S:
Inhibitory effect of sinigrin on adipocyte differentiation in
3T3-L1 cells: Involvement of AMPK and MAPK pathways. Biomed
Pharmacother. 102:670–680. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jang YJ, Park B, Lee HW, Park HJ, Koo HJ,
Kim BO, Sohn EH, Um SH and Pyo S: Sinigrin attenuates the
progression of atherosclerosis in ApoE−/− mice fed a
high-cholesterol diet potentially by inhibiting VCAM-1 expression.
Chem Biol Interact. 272:28–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HW, Lee CG, Rhee DK, Um SH and Pyo S:
Sinigrin inhibits production of inf lammatory mediators by
suppressing NF-κB/MAPK pathways or NLRP3 inflammasome activation in
macrophages. Int Immunopharmacol. 45:163–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang LL, Pan C, Yu TT, Guo K, Wang XH,
Zhang JY, Wang HZ and Gao S: Benefical therapeutic effect of
Chinese Herbal Xinji'erkang formula on hypertension-induced renal
injury in the 2-kidney-1-clip hypertensive rats. Afr J Tradit
Complement Altern Med. 11:16–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou S, Guo J, Zhao L, Liao Y, Zhou Q, Cui
Y, Hu W, Chen J, Ren X, Wei Q, et al: ADAMTS13 inhibits oxidative
stress and ameliorates progressive chronic kidney disease following
ischaemia/reperfusion injury. Acta Physiol (Oxf). 231:e135862021.
View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Nair AR, Ebenezer PJ, Saini Y and Francis
J: Angiotensin II-induced hypertensive renal inflammation is
mediated through HMGB1-TLR4 signaling in rat tubulo-epithelial
cells. Exp Cell Res. 335:238–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen D, Xiong XQ, Zang YH, Tong Y, Zhou B,
Chen Q, Li YH, Gao XY, Kang YM and Zhu GQ: BCL6 attenuates renal
inflammation via negative regulation of NLRP3 transcription. Cell
Death Dis. 8:e31562017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Cai W, Xi W, Sun W, Shen W, Wei T,
Chen X, Sun L, Zhou H, Sun Y, et al: MicroRNA-31 regulates
immunosuppression in Ang II (Angiotensin II)-induced hypertension
by targeting Ppp6C (protein phosphatase 6c). Hypertension.
73:e14–e24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Awasthi S and Saraswathi NT: Sinigrin, a
major glucosinolate from cruciferous vegetables restrains
non-enzymatic glycation of albumin. Int J Biol Macromol.
83:410–415. 2016. View Article : Google Scholar
|
|
23
|
Teles F, da Silva TM, da Cruz Júnior FP,
Honorato VH, de Oliveira Costa H, Barbosa AP, de Oliveira SG,
Porfírio Z, Libório AB, Borges RL and Fanelli C: Brazilian red
propolis attenuates hypertension and renal damage in 5/6 renal
ablation model. PLoS One. 10:e01165352015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gómez GI and Velarde V: Boldine improves
kidney damage in the goldblatt 2K1C model avoiding the increase in
TGF-β. Int J Mol Sci. 19:18642018. View Article : Google Scholar
|
|
25
|
Subedi L, Venkatesan R and Kim SY:
Neuroprotective and anti-inflammatory activities of Allyl
isothiocyanate through attenuation of JNK/NF-κB/TNF-α signaling.
Int J Mol Sci. 18:14232017. View Article : Google Scholar
|
|
26
|
Mays JR, Weller Roska RL, Sarfaraz S,
Mukhtar H and Rajski SR: Identification, synthesis, and enzymology
of non-natural glucosinolate chemopreventive candidates.
Chembiochem. 9:729–747. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith TK, Mithen R and Johnson IT: Effects
of Brassica vegetable juice on the induction of apoptosis and
aberrant crypt foci in rat colonic mucosal crypts in vivo.
Carcinogenesis. 24:491–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oyagbemi AA, Omobowale TO, Ola-Davies OE,
Asenuga ER, Ajibade TO, Adejumobi OA, Arojojoye OA, Afolabi JM,
Ogunpolu BS, Falayi OO, et al: Quercetin attenuates hypertension
induced by sodium fluoride via reduction in oxidative stress and
modulation of HSP 70/ERK/PPARγ signaling pathways. Biofactors.
44:465–479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen LJ, Xu YL, Song B, Yu HM, Oudit GY,
Xu R, Zhang ZZ, Jin HY, Chang Q, Zhu DL and Zhong JC:
Angiotensin-converting enzyme 2 ameliorates renal fibrosis by
blocking the activation of mTOR/ERK signaling in apolipoprotein
E-deficient mice. Peptides. 79:49–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ceron CS, Rizzi E, Guimaraes DA,
Martins-Oliveira A, Gerlach RF and Tanus-Santos JE: Nebivolol
attenuates prooxidant and profibrotic mechanisms involving TGF-β
and MMPs, and decreases vascular remodeling in renovascular
hypertension. Free Radic Biol Med. 65:47–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi J, Yu XJ, Fu LY, Liu KL, Gao TT, Tu JW,
Kang KB, Shi XL, Li HB, Li Y and Kang YM: Exercise training
attenuates hypertension through TLR4/MyD88/NF-κB signaling in the
hypothalamic paraventricular nucleus. Front Neurosci. 13:11382019.
View Article : Google Scholar
|
|
32
|
Oyagbemi AA, Omobowale TO, Ola-Davies OE,
Asenuga ER, Ajibade TO, Adejumobi OA, Afolabi JM, Ogunpolu BS,
Falayi OO, Ayodeji F, et al: Ameliorative effect of Rutin on sodium
fluoride-induced hypertension through modulation of
Kim-1/NF-κB/Nrf2 signaling pathway in rats. Environ Toxicol.
33:1284–1297. 2018. View Article : Google Scholar : PubMed/NCBI
|