Introduction
As a primary contributor of sepsis in elderly
patients, ventilator-associated pneumonia (VAP) affects up to 30%
patients in intensive care units (ICUs) with a mortality rate of
~60% (1,2). Although VAP seems to be triggered by
the intubation and related mechanical ventilation procedures
applied to patients in the ICU, that promote the exposure of these
patients to various types of pathogens that are resistant to
ordinary antibiotics, the detailed mechanisms underlying the
pathogenesis of VAP remain unclear (3). In particular, since the genetic
conditions of patients can be affected by a wide range of single
nucleotide polymorphisms (SNPs) in their genes, their defense and
immunity against pathogens, such as viruses, bacteria, fungi and
other harmful microorganisms, can be significantly affected due to
their differential expression of various cytokines, receptors and
pro-inflammatory factors (3).
As a type of short RNA transcript of ~22 nucleotides
in length with no protein encoding abilities, microRNAs (miRNAs or
miRs) can regulate the expression of their target genes at the
post-transcriptional level (4).
miRNAs are involved functionally in the regulation of a wide range
of cellular processes, such as the apoptosis, invasion,
differentiation, growth and proliferation of different types of
cells (5). In addition, miRNAs can
act as either tumor suppressors or oncogenes to affect the onset,
development, prognosis and metastasis of a wide range of malignant
tumors (3,6).
A number of miRNAs can interact with the same mRNA
transcript to play a gene regulatory role at the
post-transcriptional level through a complex network of signaling
pathways (7). For example, SNPs
positioned in the 3-untranslated regions (UTRs) of target genes of
miRNAs can alter the binding affinity between these target mRNAs
and their targeting miRNAs, resulting in the differential
expression of these target genes (8). In particular, the rs1056628 SNP found
in the seed sequence of miR-491 may affect the expression of one of
its targets, matrix metalloproteinase (MMP)-9, due to the
complementary binding between miR-491 and the MMP-9 3′UTR (9). Moreover, the A/C variants of
rs1056628 SNP located in the MMP-9 3′UTR have been shown to
increase the incidence of atherosclerotic cerebral infarction (ACI)
in Chinese patients; in addition, it demonstrated that a
significant association existed between the risk of ACI and a
haplotype of MMP-9, i.e., the combination of three SNPs of rs9509T,
rs1056628C and rs20544C (10).
The concentration, as well as the activity of MMP-9
in the plasma of patients with VAP have been shown to be markedly
increased as compared with a control group of healthy patients free
of VAP (11). Thus, the plasma
level of MMP-9 protein in patients with chronic obstructive
pulmonary disease (COPD) may be used as a potential biomarker to
determine the necessity of antibiotic treatments provided to
decrease the chance of VAP (11).
As a member of the superfamily of zinc-dependent endopeptidases,
the 92-kDa MMP-9, which is also a member of type IV collagenase,
can play a vital role in the initialization of immune responses
(12–14). MMP-9 can be generated by a wide
range of cells, such as monocytes, leukocytes, macrophages,
keratinocytes, as well as malignant tumor cells (15). In addition, MMP-9 can play an
essential role in inflammation by promoting the synthesis and
release of reactive oxygen species from neutrophils (16).
It has been found that the plasma matrix MMP-9 level
is associated with the severity of VAP (11). The rs1056629 SNP situated at the
3′UTR of MMP-9 has been found to increase the expression of MMP-9
by interrupting the interaction between MMP-9 and miR-491 (9). In the present study, patients with
COPD who developed VAP were enrolled and the effects of rs1056629
on the expression of MMP-9 and the severity of VAP were
examined.
Materials and methods
Patients and sample collection
Peripheral blood samples were collected from a total
of 96 patients with COPD hospitalized at the ICU of Qinghai Red
Cross Hospital from September, 2015 to August, 2017 for clinical
analysis. Although all patients treated with mechanical ventilation
were eligible for the screening of the study, all enrolled patients
must have experienced at least one episode of VAP. Peripheral blood
samples were collected from all subjects to isolate their monocytes
and to determine their genotypes of rs1056629 SNP. The isolation
process was accomplished using the Human Peripheral Blood
Mononuclear Cell Isolation and Viability kit (ab234628; Abcam)
following the instructions of the manufacturer. Subsequently, based
on the results of rs1056629 SNP genotyping, all the subjects were
divided into 3 groups as follows: The CC group (n=18), the CA group
(n=33) and the AA group (n=45). The protocol of the study, as well
as the template of informed consent form (ICF) was reviewed and
approved by the Clinical Ethics Committee of our Qinghai Red Cross
Hospital for the retrospective use of these blood samples, and
written informed consent was obtained from all subjects or their
family members prior to the initialization of the study.
Clinical pulmonary infection score
(CPIS) evaluation
The CPIS of each subject was assessed using an
established method as described in a previous study (17).
Genotyping by TaqMan assay
First, peripheral blood samples were collected under
fasting conditions from each subject using EDTA blood collection
tubes to isolate mononuclear cells. The genomic DNA in each sample
of mononuclear cells was isolated from archived pellets utilizing a
QIAamp genomic DNA extraction assay kit (Qiagen, Inc.) following
the instructions provided with the assay kit. The genotypes of
rs1056629 SNP in the genomic DNA isolated from the mononuclear
cells of each subject were determined using quantitative PCR
(qPCR), which was carried out using a TaqMan genotyping assay kit
(cat. no. 4381657; Applied Biosystems; Thermo Fisher Scientific,
Inc.) on a 7900HT fast real-time PCR machine (Applied Biosystems;
Thermo Fisher Scientific, Inc.) following a standard protocol
provided by the manufacturer. For the purpose of quality control,
both negative control wells, which contained blank samples free of
DNA, and positive control wells, which contained genomic DNA
samples of a known genotype of rs1056629 SNP, were set up in each
microtiter plate of the qPCR reaction.
RNA isolation and reverse
transcription-qPCR (RT-qPCR)
Peripheral blood samples collected from each subject
and the A549 and H1299 cells (described below) were subjected to
treatment with a mirVana assay kit (Ambion; Thermo Fisher
Scientific, Inc.) following the instructions provided by the kit
manufacturer to collect and purify the total RNA content in each
sample. Subsequently, the integrity and concentration of each
purified RNA sample were evaluated utilizing an Agilent Bioanalyzer
(Model 2100, Agilent Technologies, Inc.). Reverse transcription was
then performed utilizing a MiScript Reverse Transcription kit (cat.
no. 218160; Qiagen GmbH) to produce cDNA templates, which were then
subjected to qPCR utilizing specific TaqMan probes and Universal
TaqMan Master Mix (cat. no. 4304437; Applied Biosystems; Thermo
Fisher Scientific, Inc.) in accordance with the instructions
provided by the manufacturer. All qPCR reactions were carried out
in triplicate wells of a 384-well qPCR plate, which was then loaded
into a 7900HT fast real-time PCR machine (Applied Biosystems;
Thermo Fisher Scientific, Inc.) for operation. The thermocycling
conditions were 95°C for 15 min (initial activation), 94°C for 15
sec (denaturation), 55°C for 30 sec (annealing), and 72°C for 60
sec (extension). Finally, the relative expression of miR-491 and
MMP-9 mRNA in each sample was calculated by normalization vs. the
expression of the U6 (for miR-491) and GAPDH (for MMP-9 mRNA)
housekeeping genes using the 2−ΔΔCq method, respectively
(18). The primers used for PCR
were as follows: miR-491 forward, 5′-AGTGGGGAACCCTTCC-3′ and
reverse, 5′-GAACATGTCTGCGTATCTC-3′; MMP-9 forward,
5′-GCCACTACTGTGCCTTTGAGTC-3′ and reverse,
5′-CCCTCAGAGAATCGCCAGTACT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH forward,
5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse,
5′-ACCACCCTGTTGCTGTAGCCAA-3′.
Cell culture and transfection
The human lung adenocarcinoma cell line, A549 (cat.
no. CRM-CCL-185™), and the human lung carcinoma cell line, H1299
(cat. no. CRL-5803), were obtained from the American Type Culture
Collection and incubated in a Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 2
mM L-glutamine, 10% heat inactivated FBS and 100 U/ml
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
The culture conditions were 95% air, 5% CO2, saturated
humidity and a temperature of 37°C. These cell lines were selected
due to the fact that they exhibited good growth conditions and were
easier to obtain during the study. When the cells reached the
logarithmic growth, they were divided into 3 groups as follows: The
NC group, the 50 nM miR-491 mimics (Thermo Fisher Scientific, Inc.)
group and the 100 nM miR-491 mimics (Thermo Fisher Scientific,
Inc.) group, and transfected with either a scramble miRNA control
sequence (5′-UGGGCGUAUAGACGUGUUACAC-3′) or the corresponding
concentrations of miR-491 mimics (Thermo Fisher Scientific, Inc.)
using Lipofectamine 3000® (Invitrogen; Thermo Fisher
Scientific, Inc.) at 4°C for 48 h following a standard transfection
protocol provided by the manufacturer. Subsequent observations were
performed at 48 h post-transfection.
Vector construction and luciferase
assay
To determine the regulatory association between
miR-491 and MMP-9, as well as the effect of different genotypes of
rs1056629 SNP on the binding affinity between miR-491 and MMP-9
3′UTR, the 3′UTR of MMP-9 containing the rs1056629-A or rs1056629-C
allele in its miR-491 binding site were respectively sub-cloned
into pcDNA luciferase vectors (Promega Corporation). The A549 and
H1299 cells were then co-transfected with different vectors of
MMP-9 3′UTR in conjunction with 20 pmol miR-491 mimics or a
scramble control for 24 h at 4°C using Lipofectamine
3000®, followed by the detection of luciferase activity
of the transfected cells at 48 h following the start of
transfection using a Bright-Glo luciferase assay kit (Promega
Corporation) following the protocol provided with the kit. The
relative luciferase activity was normalized to the Renilla
luciferase activity.
Western blot analysis
The collected clinical samples, as well as the
cultured cell samples were first lysed in a RIPA lysis buffer (pH
7; Cell Signaling Technology, Inc.) containing 0.5% sodium
deoxycolate, 10 mM EDTA, 0.5% NP-40, 100 mM NaCl, 100 mM Tris, and
a cocktail of phosphatase and protease inhibitors (Cell Signaling
Technology, Inc.). The supernatant of each sample was then
collected via 30 min of centrifugation at 4°C and 14,000 × g,
followed by the quantification of the protein concentration using a
BCA assay kit (Pierce; Thermo Fisher Scientific, Inc.).
Subsequently, the protein was resolved using 10% SDS-PAGE and
transferred onto a PVDF membrane, which was then blocked with 5%
skim milk, incubated at 4°C for 12 h with primary anti-MMP-9
antibody (1:1,000; ab38898; Abcam) and subsequently incubated at
room temperature for 1 h with corresponding HRP-labeled secondary
antibody (1:2,000; ab6721; Abcam) consecutively, developed using an
enhanced chemiluminescence reagent (Amersham; Cytiva), visualized
using a Bio-Rad imager (Bio-Rad Laboratories, Inc.) and processed
using ImageJ software (V1.4.1; National Institutes of Health) to
determine the relative expression of MMP-9 proteins utilizing
β-actin as the internal reference.
ELISA
The levels of TNF-α and IL-6 in monocytes isolated
from the peripheral blood samples of all subjects were determined
using commercial ELISA kits (E-EL-H0109 for TNF-α, E-EL-H0102 for
IL-6, Elabscience) following the kit manuals, and the absorbance
values were measured utilizing a Multiskan GO microplate reader
(Thermo Fisher Scientific, Inc.).
Statistical analysis
All statistical analyses were carried out utilizing
SPSS 16.0 statistical software (SPSS, Inc.). Continuous parameters
are presented as the mean ± SD, and inter-group comparisons were
carried out using one-way ANOVA with the Student-Newman-Keuls post
hoc test. All statistical tests were bilateral and P-values
<0.05 were considered to indicate statistically significant
differences.
Results
Patients with COPD with the AC and CC
genotypes of rs1056629 have a higher risk of developing VAP
All 96 patients with COPD in the present study were
genotyped for their rs1056629 SNP, which was located within the
3′UTR of MMP-9 mRNA. As shown in Fig.
1, carriers of either one or two C alleles had a significantly
shorter time to develop VAP when compared to the carriers of the
wild-type AA (Fig. 1A).
Consistently, the CPIS was notably increased in both the CC and CA
groups (Fig. 1B).
Genotypes of rs1056629 SNP are
associated with the differential expression of TNF-α, IL-6 and
MMP-9
ELISA was carried out to evaluate the expression of
TNF-α and IL-6 in the monocytes of the 3 groups of patients. The
expression of TNF-α and IL-6 was evidently elevated in patients
with the CC and AC genotypes than in those with the AA genotype
(Fig. 2). Furthermore, the
expression of miR-491 and MMP-9 mRNA was also analyzed using
RT-qPCR, and no marked differences in the expression of miR-491
were found between all 3 groups (Fig.
3A). However, the expression of MMP-9 mRNA was significantly
increased in the CC and AC groups (Fig. 3B).
miR-491 inhibits the expression of
MMP-9 by directly binding to the 3′UTR of MMP-9
The screening of potential binding sites of miR-491
identified a miR-491 binding site at the 3′UTR of MMP-9 mRNA.
Subsequently, luciferase reporter plasmids of MMP-9 3′UTR
containing different genotypes of rs1056629 SNP were constructed,
and were then co-transfected with miR-491 into A549 cells (Fig. 4A). The luciferase activity of
wild-type rs1056629-A, but not that of mutant rs1056629-C SNP was
markedly inhibited by miR-491 (Fig.
4B). Furthermore, the inhibitory effect of miR-491 on MMP-9
expression was also confirmed in H1299 cells (Fig. 4C). Thus, these results indicated
that miR-491 suppressed the expression of MMP-9 by binding to its
3′UTR.
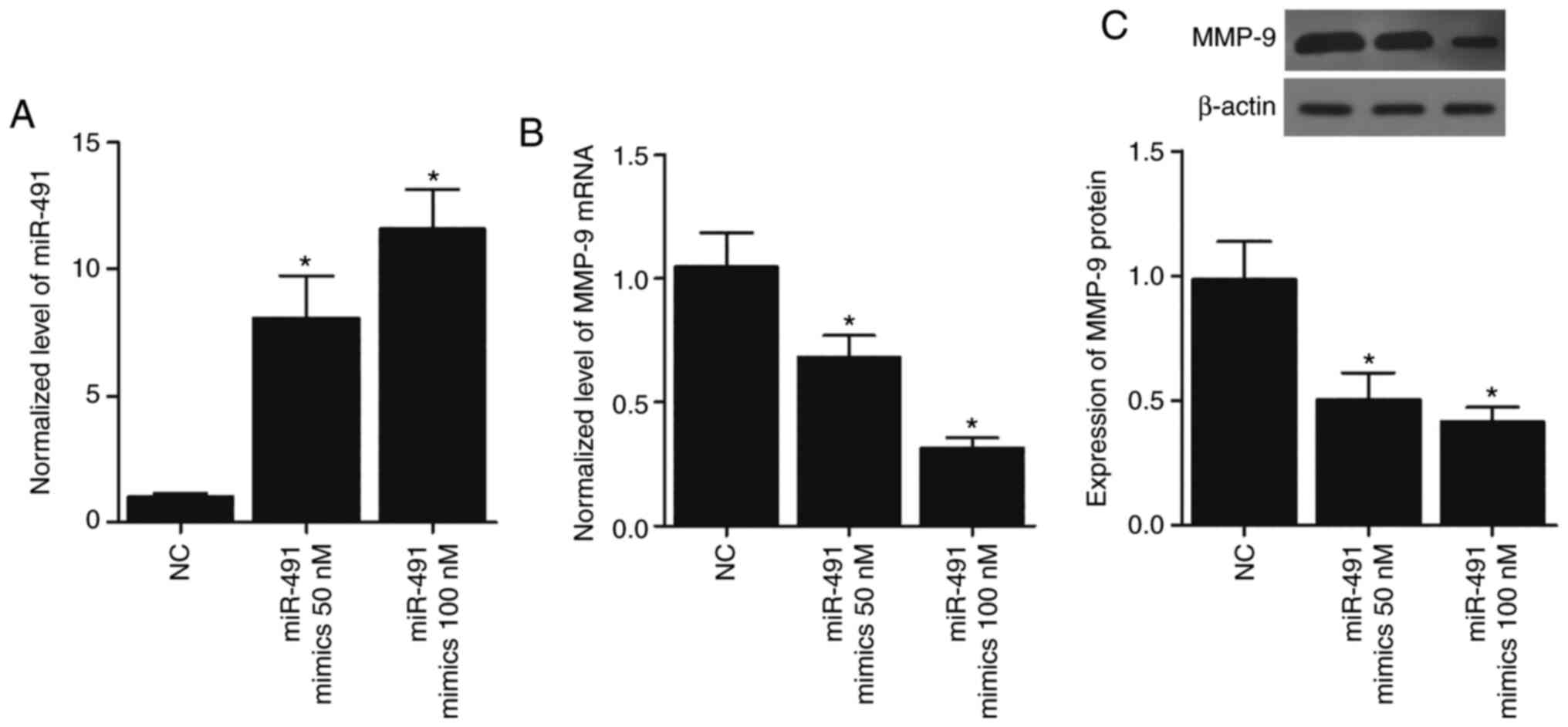
Overexpression of miR-491 in A549 and
H1299 cells suppresses the expression of MMP-9
To further confirm the inhibitory role of miR-491 in
MMP-9 expression, 50 and 100 nM miR-491 mimics were transfected
into the A549 and H1299 cells, and this led to the overexpression
of miR-491 (Figs. 5A and 6A) in a concentration-dependent manner.
MMP-9 mRNA expression was also significantly reduced in the A549
(Fig. 5B) and H1299 cells
(Fig. 6B) transfected with miR-491
in a concentration-dependent manner. Similarly, the expression of
MMP-9 protein in the A549 (Fig.
5C) and H1299 (Fig. 6C) cells
transfected with miR-491 was also decreased in a
concentration-dependent manner. Collectively, these results
suggested that miR-491 inhibited MMP-9 expression in a
concentration-dependent manner.
Discussion
As a type of severe infectious disease, VAP can be
easily observed in patients in the ICU who have been treated using
mechanical ventilators for at least 48 h (19). As a result, the development of
effective measures with the potential to reduce the risk of VAP has
become a great challenge (20).
The rs1056629 SNP found in both the seed sequence of
miR-491, as well as the 3′UTR domain of MMP-9 is suspected to
increase the risk of atherosclerotic cerebral infarction (10). By playing an essential role in the
degradation and decomposition of type V and type IV collagens,
MMP-9 expression is increased in patients suffering from acute
ischemic stroke, as well as atherosclerosis (21,22).
MMP-9 can also play an important role in the onset of vascular
remodeling, atherosclerosis, as well as in the formation of
arterial plaques (12,23,24).
The 3′UTR domain of mRNAs is crucial for maintaining the stability
of their host mRNAs while serving as a primary target in the
functioning of miRNAs. Nevertheless, a few studies have tried to
investigate the regulatory association between the risk of ACI and
the presence of various SNPs in MMP-9 3′UTR, although in
vitro experiments have provided some evidence supporting the
aforementioned association (10).
In the present study, 96 patients with COPD who developed VAP were
enrolled and divided into different groups according to their
genotypes of rs1056629 SNP in the 3′UTR of MMP-9. It was found that
carriers of rs1056629-C SNP exhibited a significantly accelerated
development of VAP. Furthermore, ELISA was performed to evaluate
the expression of TNF-α and IL-6 in the monocytes of these patients
with VAP, and an elevated expression of TNF-α and IL-6 was observed
in patients carrying the rs1056629-C allele. In addition, RT-qPCR
was used to assess the differential expression of miR-491 and MMP-9
in the monocytes of patients with VAP carrying different genotypes
of rs1056629, and no difference in the expression of miR-491 was
observed among the different groups of patients. However, MMP-9
expression was increased in patients carrying the rs1056629-C
allele.
Multiple inflammatory diseases, such as ulcerative
colitis, have been shown to display an abnormal level of MMP-9
activity and expression (25–29).
As a frequently recurring autoimmune disease of the colon,
ulcerative colitis is featured not only by a significantly elevated
level of MMP-9 proteins, but also a significantly elevated level of
proteolytic activity, which may be caused by the excessive
expression of certain inflammatory factors, including IL1-α and
TNF-α (29,30–33).
Since MMP-9 is apparently associated with an
increased risk of developing pneumonia, it has been hypothesized
that an elevated level of MMP-9 may be an important contributor for
the onset of VAP (14). In the
present study, luciferase assays were used to explore the
inhibitory effects of miR-491 on MMP-9 expression. MMP-9 expression
was markedly suppressed by miR-491 overexpression, which bound to
the 3′UTR of MMP-9.
The activity of MMP-9 is apparently increased in
patients with pneumonia (34). In
addition, the level of MMP-9 has been shown to be markedly
increased in patients with bronchoalveolar lavage fluid (BALF)
along with an obviously increased number of apoptotic neutrophils
(35,36). Furthermore, the plasma level of
MMP-9 proteins in patients with VAP is much higher than that in
patients free of VAP, while the application of appropriate
treatments significantly decreases the level of MMP-9 (11). In the present study, A549 and H1299
cells were transfected with miR-491 mimics, and the mRNA and
protein expression of MMP-9 was then analyzed by RT-qPCR and
western blot analysis, respectively. It was found that MMP-9
expression in the A549 and H1299 cells was markedly decreased by
miR-491 overexpression.
MMP-9 can be a main contributor to the induction of
inflammatory responses by acting to cleave pro-inflammatory
factors, such as IL-1β (37–40).
In addition, MMP-9 can also promote the undocking of activated TNF
from the cell plasma membrane to facilitate the induction of
inflammation (41–43). During the pathogenesis and
development of myocardial infarction, MMP-9 is released by both
macrophages and neutrophils to participate in the degradation of
the extracellular matrix, as well as the regulation of TGF-β
functions, which have been shown to play an essential role in the
formation of collagenous scars, as well as in the pathogenesis of
myocardial fibrosis (44,45).
In conclusion, the present study demonstrated that
the rs1056629 SNP located in MMP-9 3′UTR affected the risk of
developing VAP. The possible mechanisms underlying such
observations are that the C-to-A substitution of rs1056629 SNP
affects the binding of miR-491 to MMP-9 3′UTR, leading to MMP-9
overexpression and a higher risk of developing VAP. Thus, the
rs1056629 SNP of MMP-9 may be used as an important biomarker to
determine the susceptibility to VAP.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the Qinghai
Provincial Fourth People's Hospital Research Fund (Project no.
2017-14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WM and XL designed the study. XC, WS and LZ
performed the literature search for the study. All authors (WM, XC,
WS, LZ, BF, SZ, HL, HW, WW and XL) performed the experiments and
collected the data. XC, BF, SZ and HL analyzed the data and
prepared the figures. WW, WM, XC and XL composed the manuscript.
HW, WW and WS corrected the language. WM and XL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The protocol of the study, as well as the template
of informed consent form (ICF) was reviewed and approved by the
Clinical Ethics Committee of our Qinghai Red Cross Hospital for the
retrospective use of these blood samples, and written informed
consent was obtained from all subjects or their family members
prior to the initialization of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VAP
|
ventilator-associated pneumonia
|
|
CPIS
|
clinical pulmonary infection score
|
|
PBMCs
|
peripheral blood monocytes
|
References
|
1
|
Hunter J, Annadurai S and Rothwell M:
Diagnosis, management and prevention of ventilator-associated
pneumonia in the UK. Eur J Anaesthesiol. 24:971–977. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent JL: Ventilator-associated
pneumonia. J Hosp Infect. 57:272–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Namath A and Patterson AJ: Genetic
polymorphisms in sepsis. Crit Care Clin. 25835–856. (x)2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabriely G, Wurdinger T, Kesari S, Esau
CC, Burchard J, Linsley PS and Krichevsky AM: MicroRNA 21 promotes
glioma invasion by targeting matrix metalloproteinase regulators.
Mol Cell Biol. 28:5369–5380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan G, He Z, Cao L, Shi X, Wu S and Zhou
G: miR-139 inhibits osteosarcoma cell proliferation and invasion by
targeting ROCK1. Front Biosci (Landmark Ed). 24:1167–1177. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deveci M, Catalyürek UV and Toland AE:
mrSNP: Software to detect SNP effects on microRNA binding. BMC
Bioinformatics. 15:732014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian X and Zhang X: A Single nucleotide
polymorphism (rs1056629) in 3′-UTR of MMP-9 is responsible for a
decreased risk of metastatic osteosarcoma by compromising its
interaction with microRNA-491-5p. Cell Physiol Biochem.
38:1415–1424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan M, Zhan Q, Duan X, Song B, Zeng S,
Chen X, Yang Q and Xia J: A functional polymorphism at miR-491-5p
binding site in the 3′-UTR of MMP-9 gene confers increased risk for
atherosclerotic cerebral infarction in a Chinese population.
Atherosclerosis. 226:447–452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li YT, Wang YC, Lee HL, Lu MC and Yang SF:
Elevated plasma matrix metalloproteinase-9 and its correlations
with severity of disease in patients with ventilator-associated
pneumonia. Int J Med Sci. 13:638–645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atkinson JJ and Senior RM: Matrix
metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol.
28:12–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiang TY, Tsao SM, Yeh CB and Yang SF:
Matrix metalloproteinases in pneumonia. Clin Chim Acta.
433:272–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown GM, Brown DM, Donaldson K, Drost E
and MacNee W: Neutrophil sequestration in rat lungs. Thorax.
50:661–667. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Z, Shao X, Dou X, Zhang X, Wang Y,
Zhu C, Hao C, Fan M, Ji W and Yan Y: Role of the mycoplasma
pneumoniae/interleukin-8/neutrophil axis in the pathogenesis of
pneumonia. PLoS One. 11:e01463772016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou XY, Ben SQ, Chen HL and Ni SS: A
comparison of APACHE II and CPIS scores for the prediction of
30-day mortality in patients with ventilator-associated pneumonia.
Int J Infect Dis. 30:144–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan Y, Gao F, Wu Y, Zhang J, Zhu M and
Xiong L: Does ventilator-associated event surveillance detect
ventilator-associated pneumonia in intensive care units? A
systematic review and meta-analysis. Crit Care. 20:3382016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Craven DE, Hudcova J and Lei Y: Diagnosis
of ventilator-associated respiratory infections (VARI):
Microbiologic clues for tracheobronchitis (VAT) and pneumonia
(VAP). Clin Chest Med. 32:547–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosell A, Cuadrado E, Ortega-Aznar A,
Hernández-Guillamon M, Lo EH and Montaner J: MMP-9-positive
neutrophil infiltration is associated to blood-brain barrier
breakdown and basal lamina type IV collagen degradation during
hemorrhagic transformation after human ischemic stroke. Stroke.
39:1121–1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lakhan SE, Kirchgessner A, Tepper D and
Leonard A: Matrix metalloproteinases and blood-brain barrier
disruption in acute ischemic stroke. Front Neurol. 4:322013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boroujerdi A, Welser-Alves JV and Milner
R: Matrix metalloproteinase-9 mediates post-hypoxic vascular
pruning of cerebral blood vessels by degrading laminin and
claudin-5. Angiogenesis. 18:255–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen F, Eriksson P, Hansson GK, Herzfeld
I, Klein M, Hansson LO and Valen G: Expression of matrix
metalloproteinase 9 and its regulators in the unstable coronary
atherosclerotic plaque. Int J Mol Med. 15:57–65. 2005.PubMed/NCBI
|
|
25
|
Hu J, Van den Steen PE, Sang QX and
Opdenakker G: Matrix metalloproteinase inhibitors as therapy for
inflammatory and vascular diseases. Nat Rev Drug Discov. 6:480–498.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ram M, Sherer Y and Shoenfeld Y: Matrix
metalloproteinase-9 and autoimmune diseases. J Clin Immunol.
26:299–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahrens D, Koch AE, Pope RM,
Stein-Picarella M and Niedbala MJ: Expression of matrix
metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid
arthritis. Arthritis Rheum. 39:1576–1587. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang YH, Lin IL, Tsay GJ, Yang SC, Yang
TP, Ho KT, Hsu TC and Shiau MY: Elevated circulatory MMP-2 and
MMP-9 levels and activities in patients with rheumatoid arthritis
and systemic lupus erythematosus. Clin Biochem. 41:955–959. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lakatos G, Sipos F, Miheller P, Hritz I,
Varga MZ, Juhasz M, Molnar B, Tulassay Z and Herszenyi L: The
behavior of matrix metalloproteinase-9 in lymphocytic colitis,
collagenous colitis and ulcerative colitis. Pathol Oncol Res.
18:85–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peterson JT: The importance of estimating
the therapeutic index in the development of matrix
metalloproteinase inhibitors. Cardiovasc Res. 69:677–687. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ben David D, Reznick AZ, Srouji S and
Livne E: Exposure to pro-inflammatory cytokines upregulates MMP-9
synthesis by mesenchymal stem cells-derived osteoprogenitors.
Histochem Cell Biol. 129:589–597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ben-David D, Livne E and Reznick AZ: The
involvement of oxidants and NF-kB in cytokine-induced MMP-9
synthesis by bone marrow-derived osteoprogenitor cells. Inflamm
Res. 61:673–688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ordas I, Eckmann L, Talamini M, Baumgart
DC and Sandborn WJ: Ulcerative colitis. Lancet. 380:1606–1619.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schaaf B, Liebau C, Kurowski V, Droemann D
and Dalhoff K: Hospital acquired pneumonia with high-risk bacteria
is associated with increased pulmonary matrix metalloproteinase
activity. BMC Pulm Med. 8:122008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El Solh AA, Akinnusi ME, Wiener-Kronish
JP, Lynch SV, Pineda LA and Szarpa K: Persistent infection with
pseudomonas aeruginosa in ventilator-associated pneumonia. Am J
Respir Crit Care Med. 178:513–519. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El-Solh AA, Amsterdam D, Alhajhusain A,
Akinnusi ME, Saliba RG, Lynch SV and Wiener-Kronish JP: Matrix
metalloproteases in bronchoalveolar lavage fluid of patients with
type III Pseudomonas aeruginosa pneumonia. J Infect. 59:49–55.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ito A, Mukaiyama A, Itoh Y, Nagase H,
Thogersen IB, Enghild JJ, Sasaguri Y and Mori Y: Degradation of
interleukin 1beta by matrix metalloproteinases. J Biol Chem.
271:14657–14660. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schönbeck U, Mach F and Libby P:
Generation of biologically active IL-1 beta by matrix
metalloproteinases: A novel caspase-1-independent pathway of IL-1
beta processing. J Immunol. 161:3340–3346. 1998.PubMed/NCBI
|
|
39
|
Amantea D, Russo R, Certo M, Rombola L,
Adornetto A, Morrone LA, Corasaniti MT and Bagetta G:
Caspase-1-independent maturation of IL-1β in ischemic brain injury:
Is there a role for gelatinases? Mini Rev Med Chem. 16:729–737.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wittmann M, Kingsbury SR and McDermott MF:
Is caspase 1 central to activation of interleukin-1? Joint Bone
Spine. 78:327–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saren P, Welgus HG and Kovanen PT:
TNF-alpha and IL-1beta selectively induce expression of 92-kDa
gelatinase by human macrophages. J Immunol. 157:4159–4165.
1996.PubMed/NCBI
|
|
42
|
Gearing AJ, Beckett P, Christodoulou M,
Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA,
Gilbert R, Gordon JL, et al: Processing of tumour necrosis
factor-alpha precursor by metalloproteinases. Nature. 370:555–557.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu Q and Stamenkovic I: Cell
surface-localized matrix metalloproteinase-9 proteolytically
activates TGF-beta and promotes tumor invasion and angiogenesis.
Genes Dev. 14:163–176. 2000.PubMed/NCBI
|
|
44
|
Rainer PP, Hao S, Vanhoutte D, Lee DI,
Koitabashi N, Molkentin JD and Kass DA: Cardiomyocyte-specific
transforming growth factor β suppression blocks neutrophil
infiltration, augments multiple cytoprotective cascades, and
reduces early mortality after myocardial infarction. Circ Res.
114:1246–1257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dayer C and Stamenkovic I: Recruitment of
matrix metalloproteinase-9 (MMP-9) to the fibroblast cell surface
by lysyl hydroxylase 3 (LH3) triggers transforming growth factor-β
(TGF-β) activation and fibroblast differentiation. J Biol Chem.
290:13763–13778. 2015. View Article : Google Scholar : PubMed/NCBI
|