Introduction
Ischemic stroke occurs when a blood clot blocks or
narrows an artery, leading to the obstruction of blood supply to
the brain. As the third highest cause of mortality and the most
common cause of invalidity in the Western world, stroke affects 15
million individuals annually worldwide; five million of these
individuals do not survive, and the other five million are
permanently challenged physically (1). Currently, treatment for ischemic
stroke involves the restoration of the blood supply to the ischemic
area as soon as possible following its occurrence in order to
prevent the death of neurons, glial cells and vascular endothelial
cells (2). As regards acute
ischemic stroke, the only effective treatment involves the use of
thrombolysis drugs, such as intravenous tissue plasminogen
activator (3) and endovascular
therapy within 3-4.5 h of symptom onset (4). Since the treatment window is narrow
and there are various hemorrhagic complications (3,5),
the use of endovascular therapy is often limited (6). Mechanical thrombectomy in selected
patients with acute ischemic stroke has also been able to produce
partial or complete arterial recanalization (4). In addition, various combinations of
antiplatelet therapies have been considered for various mechanisms
of ischemia (7,8). However, the development of effective
strategies with which to restore the blood supply in the ischemic
brain tissue continues to pose a major challenge for stroke
treatment (9).
Over the past years, several strategies have been
used in the treatment of ischemic diseases. These treatments aim to
restore the function of neurons, astroglia and oligodendroglia due
to cerebral artery occlusion. For example, embryonic stem cells
(ESCs) derived from pre-implantation embryos have the ability of
unlimited self-renewal and the potential to differentiate into
virtually any cell type. Following implantation into the cortex
with severe focal ischemia in mouse models, mouse ESCs have been
shown to express the surface markers of neurons, astrocytes and
oligodendrocytes, leading to an improved functional recovery
(10). The intrastriatal
transplantation of mouse ESC-derived neuron-like cells has also
been shown to improve dopaminergic function in mice with focal
ischemia (11) and to promote the
functional recovery of injured tissues (12-14). In addition, inducible pluripotent
stem cells (iPSCs) have been found to be able to effectively reduce
the total infarct volume and to markedly improve the behavior of
mice with cerebral ischemia induced by middle cerebral artery
occlusion (MCAO) when transplanted with fibrin glue (15).
Long non-coding RNAs (lncRNAs) are endogenous
molecules without protein-encoding capacity. There is increasing
evidence to suggest that lncRNAs play critical roles in several
aspects of ischemic stroke and may thus be used as therapeutic
targets in ischemic stroke (16).
Cerebral ischemia has been found to alter lncRNA transcriptomic
profiles in the microvascular endothelium, leading to the up- and
downregulation of lncRNAs interacting with transcription factors,
with a potential role in mediating endothelial cell responses to
ischemic stimuli (17). A number
of lncRNAs have been found to be associated with the severity and
prognosis of ischemic stroke, and inflammation (18-20). For example, the levels of lncNEAT1
have been found to be elevated in cells following
ischemia/reperfusion (I/R), inducing cell apoptosis and
inflammation; the knockdown of lncNEAT1 has been found to reduce
I/R-induced apoptosis (21). In
addition, a recent study demonstrated that FOXD3 antisense RNA 1
(FOXD3-AS1) was upregulated in response to ischemic injury in
cardiomyocytes (22). The
knockdown of FOXD3-AS1 was shown to attenuate cerebral I/R injury
by upregulating Bcl2-like 13 expression (23). These findings suggest that both
cell and gene therapies may provide new opportunities with which to
manage ischemic stroke (24).
Several lncRNAs were found to be significantly
upregulated in patients with ischemic stroke, including
dihydrofolate reductase-like 1 (DHFRL1-4), small nucleolar RNA host
gene 15 (SNHG15) and FAM98A-3, and may thus be used as novel
diagnostic markers and therapeutic targets for ischemic stroke
(25,26). For example, the suppression of
SNHG15 has been found to attenuate I/R injury in SH-SY5Y cells
(27). The present study aimed to
investigate the effects on and possible mechanisms of action of
DHFRL1-4 in cerebral I/R injury in order to explore novel
strategies for the treatment of ischemic stroke. For this purpose,
DHFRL1-4 expression was examined in a model of I/R injury and was
knocked down using small inferring (si)RNA to investigate the
pathological consequences. The findings presented herein may
provide a new avenue with which to alleviate cerebral I/R injury
and may also facilitate the translation of gene therapy in ischemic
stroke treatment.
Materials and methods
Animals
A total of 30 male mice (weighing 25-30 g) were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd., and were
exposed to artificial light from 6.00 a.m. to 8.30 p.m. in rooms
with a controlled humidity (50%) and temperature (22°C) for 7 days
before being used in the experiments. The animals were housed under
pathogen-free conditions and were provided with ad libitum
access to a constant pellet diet and water. The experiments were
carried out on mice aged 7 and 10 weeks. Following the completion
of experiments, the mice were euthanized by CO2
asphyxiation at a flow rate of 20% chamber volume per min which was
immediately followed by decapitation. All animal experiments and
animal care were conducted in accordance with the protocols
approved by the Institutional Animal Care and Use Committee of the
Second Xiangya Hospital, Central South University, Changsha, China.
All animal research was performed in accordance with the ARRIVE
guidelines (28).
Reagents, antibodies and instruments
The terminal deoxynucleotidyl transferase (TdT) dUTP
nick-end labeling (TUNEL) assay kit (cat. no. ab66110), antibodies
against Wnt family member 3a (Wnt3a; cat. no. ab219412, 1/1,000),
basic fibroblast growth factor (bFGF; cat. no. ab92337, 1/1,500),
glycogen synthase kinase-3β (GSK-3β; cat. no. ab32391, 1/1,000),
phosphorylated (p-)GSK-3β (cat. no. ab75745, 1/1,000),
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; cat. no. ab8245,
1/1,000) and horseradish peroxidase (HRP)-labeled goat anti rabbit
IgG (cat no. ab205719, 1/2,000) were obtained from Abcam. Rabbit
antibody against vascular endothelial growth factor (VEGF; cat. no.
sc-7269, 1/1,000) was obtained from Santa Cruz Biotechnology, Inc.;
the polyvinylidene fluoride (PVDF) membrane (cat. no. IPVH00010)
was purchased from MilliporeSigma; the Novex™ ECL Chemiluminescent
Substrate Reagent kit (cat. no. WP20005), TRIzol®
reagent (cat. no. 15596026), the RevertAid First Strand cDNA
Synthesis kit (cat. no. K1621), TaqMan Pre-Amp Master Mix (cat. no.
4391128) and Lipofectamine™ RNAiMAX Transfection Reagent (cat. no.
13778030) were all purchased from Themo Fisher Scientific, Inc.;
the ultrasensitive chemiluminescence imaging system (Chemi Doc™
XRS+) and fluorescence quantitative PCR (CFX Connect™) instruments
were obtained from Bio-Rad Laboratories, Inc.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Brain tissue obtained from the mice was homogenized
using an electric homogenizer and the total RNA was extracted using
TRIzol® reagent (Themo Fisher Scientific, Inc.)
according to the supplier's protocols. cDNA was then reverse
transcribed from 1 µg total RNA using the RevertAid First
Strand cDNA Synthesis kit according to the manufacturer's
instructions. The reverse transcription reactions were carried out
under the following conditions: 42°C for 60 min; 25°C for 5 min;
and 70°C for 5 min. RT-DHFRL1-4 was quantified by PCR reactions
carried out under the following cycling conditions: Denaturing for
10 min at 95°C, followed by 18 cycles, each one consisting of 15
sec at 95°C and 4 min at 60°C, on a CFX Connect PCR system. The
qPCR mixtures had a final volume of 20 µl and contained 1
µl of template cDNA, 0.5 µl of each sense (5′-TTA CCC
AAA TAA AGT ATA) and antisense primer (5′-ATG GGT GTT GAG CTT GAA),
10 µl of TaqMan pre-amp master mix, and 8 µl of
diethyl pyrocarbonate (DEPC)-treated water. β-actin was used as an
internal control with (forward primer, 5′-GAC GTT GAC ATC CGT AAA
GAC C; and reverse primer, 5′-CTA GGA GCC AGG GCA GTA ATC T. Each
reaction was repeated for at least three times, independently.
Relative mRNA levels were calculated using the 2−ΔΔCt
method (29).
Animal grouping
The mice were randomly divided into the control (no
treatment, n=6), sham-operated (sham; subjected to a procedure
similar to DMCAO, although the carotid arteries were not ligated
with a nylon suture, n=6) and the I/R model (subjected to DMCAO and
carotid artery ligation, n=18) group. The I/R model group was
further divided into the si-NC (I/R model infected with si-NC RNA,
n=6) and the si-DHFRL1-4 (I/R model infected with si-DHFRL1-4 RNA,
n=6) groups.
Transfection
si-NC (5′-TUC UCU TGC TUG UCA UAC UTT-3′) and
si-DHFRL1-4 (5′-CCU CCU GUC UUG UUC UAC UTT-3′) were obtained from
Nanjing KeyGen Biotech Co., Ltd. to knockdown DHFRL1-4. Mice (n=6)
were anesthetized with pentobarbital sodium (30 mg/kg, Roiland
Dingchang Technology, Co., Ltd.) and fixed on a 69100 Rotational
Digital Stereotaxic Instrument (RWD Life Science Co., Ltd.) to
inject RNA. Before the injection, 50 µl lentivirus si-NC or
si-DHFRL1-4 (109 units/ml) were mixed with an equal
volume of Lipofectamine RNAiMAX transfection reagent according to
the manufacturer's instructions and injected into the lateral
ventricle (0.2 mm posterior to the bregma, 1.0 mm lateral to the
midline and 1.5 mm below the brain surface) using a microinjector
equipped with the stereotaxic instrument at an injection rate of 10
µl/min. After 24 h later, the mice were used to construct
the model of I/R.
Cerebral I/R model
The cerebral I/R model was constructed as previously
described (30). Briefly, the
mice were placed on heating pads to maintain their core body
temperature at 37°C after being anesthetized with pentobarbital
sodium (30 mg/kg) for the surgery. Distal MCAO (DMCAO) was used to
generate focal cerebral ischemia. To perform DMCAO, a smooth
incision was made along the middle line of the neck to bluntly
separate the left sternocleidomastoid and cervical muscles and to
expose the right common carotid artery. The blood vessels were
pulled out to the trigeminal nerve to expose the carotid arteries.
The right common carotid artery and the proximal end of external
carotid artery were ligated. A small incision was made on the right
common carotid artery at a distance of 1 cm from the trigeminal
point and a silicon-coated nylon suture (cat. no. 602156PK5Re,
Doccol Corporation) was inserted into the carotid artery to block
the blood flow. The blood flow was monitored using a RFLSI III
Laser Speckle Contrast Imaging System (RWD Life Science Co., Ltd.).
After 2 h, the suture was withdrawn to restore the blood
reperfusion for 24 h. Cefazolin antibiotics (25 mg/kg;
MilliporeSigma) were subcutaneously administered immediately
following surgery, and slow-release buprenorphine (1 mg/kg;
ZooPharm) was subcutaneously administered 24 h following surgery.
The mice in the sham-operated (sham) group were treated in an
identical manner, except that DMCAO was not performed with a
silicon-coated nylon suture.
At 24 h after reperfusion, the neurologic deficit
was carefully scored by a neurologist (one of the authors, WM) who
was blinded to the animal treatments according to a previously
described method (31). Briefly,
the mice were held up gently by the tail one meter above the floor
and observed for forelimb flexion. The mice that extended both
forelimbs toward the floor and had no other neurological deficit
were assigned grade 0, mice with any amount of consistent forelimb
flexion and no other abnormality were graded as 1. Mice with
decreased resistance to lateral push without and with circling were
graded as 2 and 3.
2,3,5-Triphenyltetrazolium chloride (TTC)
staining
TTC staining was used to visualize the infarct area.
On day 7, the mice were deeply anesthetized by an intraperitoneal
injection of 200 µl of sodium pentobarbital and were
transcardially perfused with ice-cold PBS with 0.1%
diethylpyrocarbonate (MilliporeSigma) for 3 min. The sodium
pentobarbital method was used only for extracting brain tissue for
TTC staining, as this was milder procedure and the aim was to
maintain the brain tissue unaltered for TTC staining. The mice were
decapitated and the brains were frozen at -80°C for 60 min to
dissect the cerebellum, brain stem and olfactory bulb. The brain
tissues were sliced using a matrix device (Zivic Instruments) into
2-mm-thick coronal sections. The sections were incubated in 2%
(TTC; MilliporeSigma) solution in the dark at 37°C for 15 min.
During this period, the brain slices were turned round every 5 min.
The TTC-stained brain sections were imaged using a digital camera
(Sony HDR-PJ790, Sony Corporation) for infarct size analyses. The
infarct areas were traced using ImageJ software (version 1.2.1,
National Institutes of Health) as previously described (32).
Hematoxylin and eosin (H&E)
staining
H&E staining was used to visualize tissue damage
as previously described (33).
Briefly, the brain tissues were dehydrated through an ethanol
gradient from 70 to 100% and cleared with three changes of xylene
(1 min for each change). The dehydrated tissues were embedded in
paraffin, sectioned to a thickness of 20 µm, dewaxed in
xylene overnight and subsequently passed through 3×10 min 99%
ethanol and 2×10 min 96% ethanol and rehydrated. The sections were
immersed in an aqueous hematoxylin solution (cat. no. H9627,
MilliporeSigma) for 3 min at 25°C, differentiated with hydrochloric
acid for 15 sec, rinsed briefly and counterstained with eosin (cat.
no. 318906, MilliporeSigma) for 3 min at 25°C. After rinsing
thoroughly in distilled water and dehydrated as described above,
the slides were cleared with xylene, sealed and examined under a
microscope (Olympus CX33, Olympus Corporation).
TUNEL assay
TUNEL assay was carried to examine apoptosis in the
brain tissue as previously described (34). Briefly, paraffin-embedded tissue
sections were baked at 65°C for 2 h and rehydrated by passing an
ethanol serial gradient and incubated in 1 µg/ml proteinase
K (cat. no. 25530049, Thermo Fisher Scientific, Inc.) in a 10 mM
Tris solution (7.5 µl of 20 µg/µl proteinase K
in 150 ml 10 mM Tris, pH 7.4-8.0) at room temperature for 15 min.
The slides were then rinsed with 1X PBS three times and blot-dried.
Subsequently, 100 µl TUNEL reaction mixture were applied to
each slide and the slides were incubated at 37°C in the dark for 1
h in a humid chamber according to the supplier's protocols. The
slides were then washed three times with PBS, immersed in 100 mM
Tris buffer (pH 8.2) at room temperature for 5 min, followed by the
addition of 100 µl substrate solution and incubation in the
dark at room temperature for 10 min. The nuclei were counterstained
with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI;
cat. no. MBD0015, MilliporeSigma) at 25°C for 1 h. The slides were
with then sealed with quenching solution and observed under a
fluorescence microscope (Olympus BX51, Olympus Corporation).
Western blot analysis
For 10 mg tissue, 300 µl ice-cold
radioimmunoprecipitation assay (RIPA) buffer (MilliporeSigma) were
added and the tissue was homogenized using an electric homogenizer
and agitated for 2 h at 4°C. The lysates were centrifuged at 16,000
× g for 20 min at 4°C and the proteins in the supernatant were
quantified using the BCA protein assay kit (Thermo Fisher
Scientific, Inc.) according to manufacturer's instructions. After
denaturing by boiling at 100°C for 5 min, ~50 µg proteins
were loaded onto a 12% sodium dodecyl sulfate polyacrylamide gel
and separated by electrophoresis at a constant voltage of 50 V. The
proteins were then transferred to PVDF membranes by electroblotting
in an ice bath at a constant voltage of 24 V. The PVDF membranes
were then blocked with 5% non-fat milk in 1X Tris-buffered saline
with 0.1% Tween-20 (TBST) for 4 h at room temperature and incubated
overnight with primary antibodies against VEGF, Wnt3a, bFGF, GSK3β,
p-GSK3β and GAPDH at 4°C. The membranes were washed six times with
Tris-buffered saline buffer, then incubated in HRP-conjugated goat
anti-rabbit IgG (H+L) solution at room temperature for 2 h. The
membranes were washed six times with PBS at room temperature and
immunoreactive bands were visualized using the Novex ECL
chemiluminescent substrate reagent kit in the dark according to the
supplier's protocols. To quantify the proteins, the gray values of
the bands were analyzed using Quantity One (version 4.62) analysis
software (General Electric).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean (SEM) and were obtained from at least three independent
assays. One-way analysis of variance with Tukey's post hoc test was
used to investigate the differences among the various groups.
Non-parametric ordinal data obtained for the neurologic deficit
scores were analyzed using the Kruskal-Wallis test followed by
Dunn's post hoc tests. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
DHFRL1-4 expression is upregulated after
I/R modeling and downregulated after DHFRL1-4 knockdown
The present study first assessed the expression of
DHFRL1-4 in brain tissue after I/R modeling. The RT-qPCR data
revealed that the mRNA level of DHFRL1-4 was significantly
upregulated following in the I/R model group as compared with the
control and sham groups (Fig. 1).
However, following the injection of si-DHFRL1-4, the mRNA level of
DHFRL1-4 was significantly decreased, although it was still
slightly higher (although not significantly) than that of the
control and sham groups. On the other hand, si-NC and sham
operation did not markedly affect the DHFRL1-4 mRNA level (Fig. 1).
DHFRL1-4 knockdown reduces the
I/R-induced infarcted area and the neurologic deficit score
TTC staining revealed that following I/R modeling
using the DMCAO procedure, the infarct area was significantly
greater in the I/R group as compared to the control and sham
groups, which di not exhibit an unstained area (Fig. 2). The injection of si-DHFRL1-4
before the I/R modelling significantly reduced the infarcted area
as compared to the model group without the injection (P<0.05).
On other hand, the injection of si-NC did not reduce the infarct
area as compared to the model group. Similarly, I/R modelling
significantly deteriorated nerve function, leading to higher
neurological deficit scores in the I/R model group as compared to
the control and sham groups (Fig.
2B); however, the knockdown of DHFRL1-4 significantly improved
nerve function (Fig. 2B). Of
note, no improvement was observed when si-NC was used (P<0.05;
Fig. 2B).
 | Figure 2Cerebral infarct area and
neurological deficit scores after I/R modeling and DHFRL1-4
knockdown in mice. (A) TTC staining of infarct area. Right panel,
TTC staining results; left panel, infarct area.
*P<0.05 and **P<0.01. (B) Neurological
deficit score. *P<0.05, #P<0.05 and and
@P<0.05 compared to the sham, IR/model and si-NC
groups, respectively. DHFRL1-4, dihydrofolate reductase-like 1;
I/R, ischemia/reperfusion; si-DHFRL1-4, siRNA lentivirus targeting
DHFRL1-4; si-NC, negative control siRNA; TTC,
2,3,5-triphenyltetrazolium chloride. |
DHFRL1-4 knockdown reduces the
I/R-induced degeneration and necrosis of nerve cells
The present study then assessed brain tissue injury
using H&E staining. The results revealed that in the control
and sham groups, the cells were arranged in a regular manner, with
a relatively uniform size and intact membranes. The nuclei and
nucleoli were clear and visible (Fig.
3). In the model group however, some necrosis was observed in
the nerve fibers, and the fibers were swollen and had edema. The
number of nerve cells was less in the I/R model group, than that in
the control and sham groups with large cell-cell gaps. However,
following DHFRL1-4 knockdown, there was markedly less swelling and
edema as compared to the model group; the brain tissue also
exhibited less liquefaction and degeneration, and the distribution
of nerve cells was relatively uniform (Fig. 3).
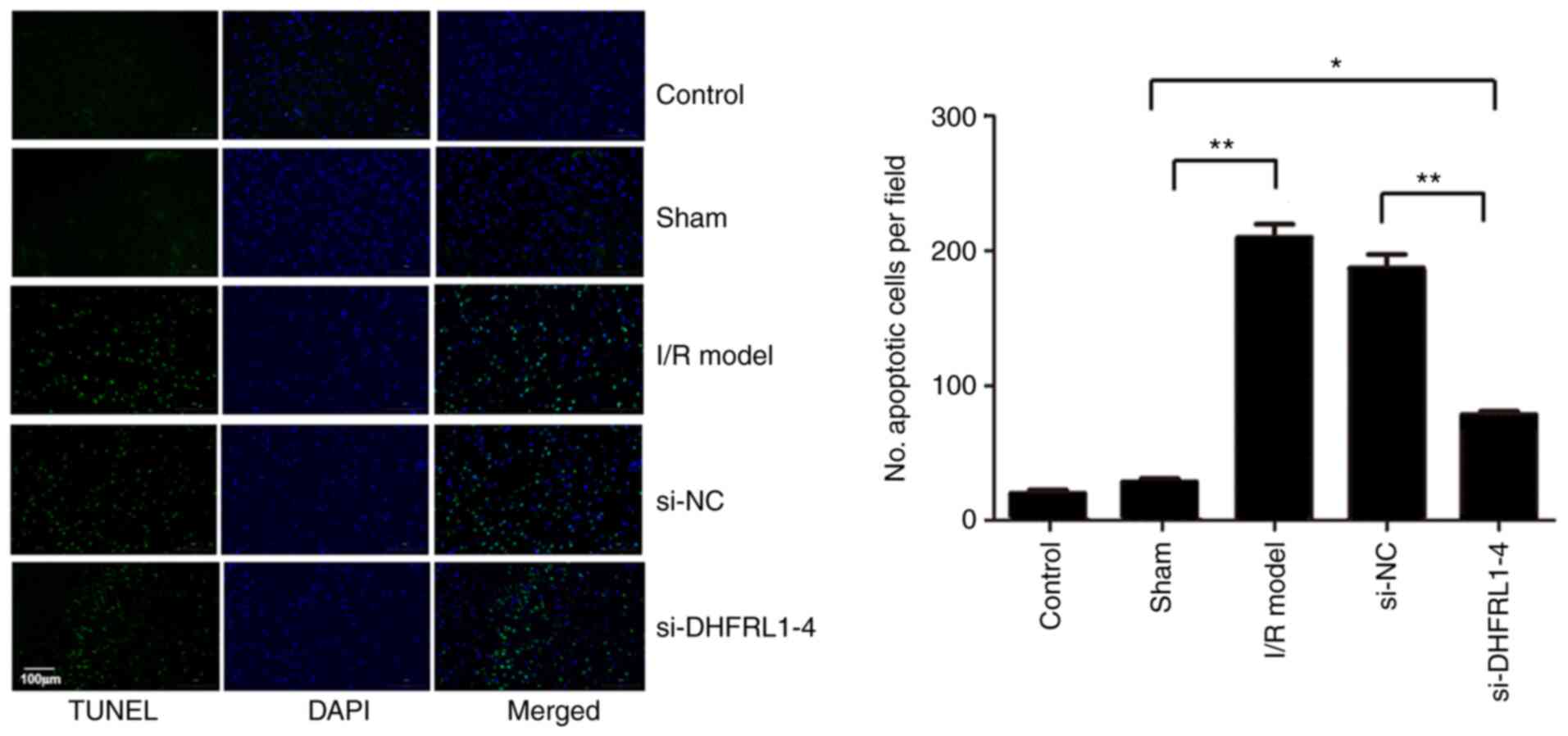
DHFRL1-4 knockdown reduces I/R-induced
apoptosis
To further investigate the mechanisms underlying the
reduction of I/R injury by the knockdown of DHFRL1-4, apoptosis in
the brain tissue was assessed using TUNEL assay. Compared with the
control and sham groups, there were significantly more apoptotic
cells after I/R modelling (P<0.05). However, apoptosis was
significantly reduced when the mice were injected with si-DHFRL1-4
before I/R modelling compared with the model group, although this
was still higher than that in the control and sham groups
(P<0.05). On the other hand, si-NC did not reduce apoptosis
(P>0.05; Fig. 4).
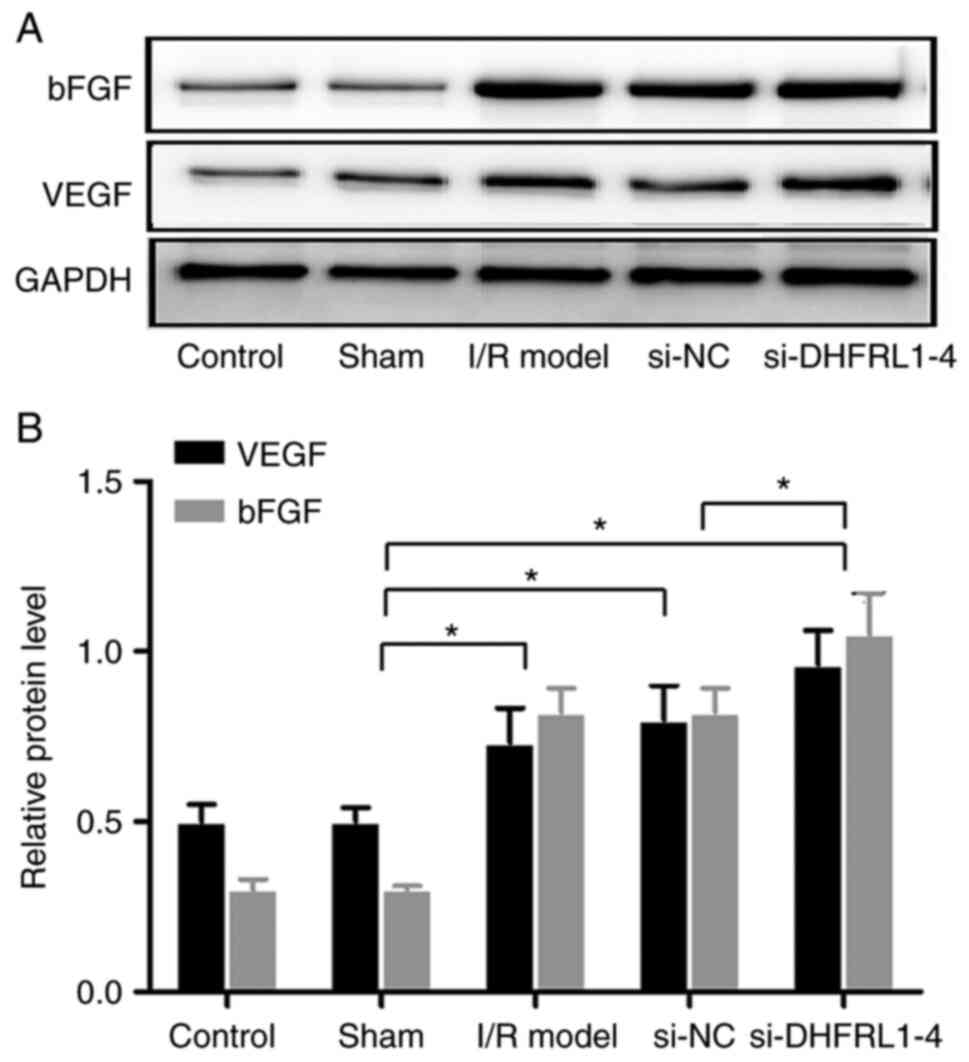
DHFRL1-4 knockdown upregulates bFGF and
VEGF expression
Since bFGF and VEGF are key growth factors related
to angiogenesis, the expression of these two genes was examined in
the brain tissue. Western blot analysis revealed that the
expression levels of bFGF and VEGF were significantly increased
after I/R modelling (P<0.05). DHFRL1-4 knockdown further
upregulated these expression levels (P<0.05); however, si-NC did
not alter bFGF and VEGF expression after I/R modelling (P>0.05;
Fig. 5).
DHFRL1-4 knockdown downregulates Wnt3a
and GSK-3β expression
In addition, western blot analysis revealed that the
protein levels of Wnt3a, GSK-3β and p-GSK-3β were significantly
upregulated after I/R modelling as compared with the control and
sham groups (P<0.05 and P<0.01). After DHFRL1-4 knockdown,
the Wnt3a and GSK-3β levels were reduced; however, the p-GSK-3β
level was not altered, resulting in an increased p-GSK-3β/GSK-3β
ratio (P<0.01; Fig. 6). On the
other hand, si-NC did not affect the protein levels compared with
the I/R model group (P>0.05; Fig.
6).
Discussion
Despite recent advances being made in innovative
treatment strategies, ischemic stroke remains one of the leading
causes of mortality and disability worldwide. The present study
attempted to explore whether lncRNAs can be used to attenuate
I/R-induced brain injury in a mouse model. It was found that the
expression of DHFRL1-4 was upregulated in the model of I/R and
DHFRL1-4 knockdown reduced I/R injury, degeneration and the
necrosis of brain cells and apoptosis and improved neurological
function. Molecular analysis revealed that following DHFRL1-4
knockdown, bFGF and VEGF expression levels were upregulated, and
Wnt3a and GSK-3β expression levels were downregulated. These
findings suggest that DHFRL1-4 may be further explored as a
potential treatment target and avenue for ischemic stroke.
There is mounting evidence to indicate that lncRNAs
play crucial roles in the occurrence and progression of solid
tumors, atherosclerosis, blinding retinopathy and other diseases
(35,36). A number of lncRNAs, including
DHFRL1-4, have been found to be related the prognosis of ischemic
stroke (18-20). Recent studies have demonstrated
that several lncRNAs are upregulated in patients following ischemic
stroke (25,26); however, whether the manipulation
of lncRNA expression can be used to affect disease remains largely
unclear. In the present study, it was found that following I/R, the
expression of DHFRL1-4, which was upregulated in ischemic stroke
patients (25), was significantly
upregulated. This is in accordance with the findings of a previous
study (25). Therefore, the
present study attempted to knockdown the expression of DHFRL1-4 to
examine its biological impact on I/R-induced injury using
si-DHFRL1-4. The experimental data revealed that the DHFRL1-4
expression level was significantly decreased after the mice were
injected with si-DHFRL1-4; however, it remained unaltered after the
injection with si-NC, indicating that the knockdown was
DHFRL1-4-specific. The examination of the infarct size revealed
that there was significantly less injury after DHFRL1-4 knockdown
with si-DHFRL1-4 as compared to the controls (si-NC), suggesting
that the knockdown of DHFRL1-4 could attenuate I/R-induced injury.
Furthermore, the neurological tests demonstrated that the mice
injected with si-DHFRL1-4 behaved more normally as compared with
the controls, suggesting that si-DHFRL1-4 could prevent damage to
the nervous system. Cellular analysis revealed that I/R modelling
resulted in the occurrence of necrosis and apoptosis in brain
cells. However, in the mice injected with si-DHFRL1-4, there were
significantly less necrotic cells and less apoptotic cells as
compared with the control group. Taken together, these findings
indicated that the knockdown of DHFRL1-4 significantly alleviated
I/R-induced injury and may be further explored for ischemic stroke
management.
Angiogenesis occurs within 4 to 7 days following
cerebral ischemia in the border of the ischemic core and periphery.
Therefore, it plays a crucial role in ischemic stroke and may
facilitate functional recovery following ischemic stroke (37); thus, it may be one of the
mechanisms underlying the observed protective role by DHFRL1-4
knockdown in the present study. The angiogensis of endothelial
cells is regulated by a number of angiogenic genes, including bFGF
(38) and VEGF (39,40). bFGF is one of the earliest, most
extensively studied and most widely used fibroblast growth factors
in the FGF family. It has potent angiogenesis-promoting activity.
In the case of tissue injury, it can promote angiogenesis, provide
nutrition for wound healing, and enhances the proliferation,
migration and survival of endothelial cells (41,42). VEGF facilitates the proliferation
and migration of endothelial cells after binding with kinase insert
domain-containing receptor kinase in vascular endothelial cells
(43,44). The present study demonstrated that
the expression of bFGF and VEGF was significantly increased
following I/R compared with the controls and was further
upregulated following DHFRL1-4 knockdown; this suggests that one of
the mechanisms underlying DHFRL1-4 mediated-protection may be
associated with increased angiogenesis. However, the exact
mechanisms through which DHFRL1-4 interacts with these
angiogenesis-related factors remain to be elucidated.
A number signaling pathways have been implicated in
I/R-induced injury and ischemic stroke, such as the PI3K/Akt
pathway (45), the Notch
signaling pathway (46) and the
Wnt signaling pathway (47). The
Wnt signaling pathway is a well-studied signaling pathway, which is
involved in the proliferation, differentiation and axon formation
of neural stem cells, and plays a critical role in cerebral
vascular regeneration and remodeling (48). Previous research has demonstrated
that Wnt/β-catenin signaling is involved in the mesenchymal stem
cell-induced increase in the survival and neuronal differentiation
of neuronal progenitor cells, and plays a main role in injury
repair and neurovascular remodeling in ischemic stroke (49). It was also previously demonstrated
that the Wnt signaling pathway was activated in rats when ischemic
stroke occurred, leading to increased GSK-β phosphorylation
(50,51). GSK-3β has been implicated in
various diseases, including inflammation, neurodegenerative
disease, diabetes and cancers, and is involved in several signal
pathways, including the Wnt/β-catenin signaling pathway (52). The present study also found that
the levels of Wnt3, GSK-β and p-GSK-β were upregulated after I/R
modelling. However, the levels of Wnt3 and GSK-β were downregulated
following DHFRL1-4 knockdown, suggesting that DHFRL1-4 regulated
the expression of the Wnt/β-catenin signaling pathway. In a
previous study, the accumulation of p-GSK-β/GSK-β was observed in
murine models of cerebral ischemia following treatment with
tanshinol borneol ester, a synthetic therapeutic agent for ischemic
stroke (53) and the activation
of GSK3β/Nrf-2 signaling was associated with improved cerebral
I/R-mediated injury (54). These
results, however, are in contrast with the observations of the
present study that Wnt3 and GSK-β expression levels were
downregulated after DHFRL1-4 knockdown, in spite of improved I/R
injury. Therefore, further studies are required to elucidate this
discrepancy.
In the present study, it was found that VEGF, bFGF
and pGSK were expressed in all treatment groups, including the
controls. It is likely that these proteins have different levels of
background expression and can be regulated by various biological
and mechanical stimuli. For example, p-GSK is expressed in rat
intestinal epithelial cells (55)
and in an I/R model of ischemic stroke (56). As a potent stimulator of
angiogenesis, VEGF is expressed in multiple cell types and its
expression is regulated developmentally (57) and by a number of factors, such as
metallothionein-III (58),
osteoarthritis (59) and can also
be upregulated by vascular damage and in wounds (60,61). bFGF is also expressed in non-wound
tissue, and its expression can be upregulated during the wound
healing process by treatment with collagen extract (62) and kefir (63), and even by surgical debridement
(64). As a lncRNA, DHFRL1-4 may
regulate the expression of these genes via its interaction with
microRNAs, or serving as a scaffold for modifying protein complexes
(65). It may also alter the
chromatin conformation of spatially related genes to regulate their
expression (66). However, the
exact mechanisms through which DHFRL1-4 regulates the expression of
VEGF, bFGF and pGSK remain to be investigated.
In conclusion, the present study demonstrated that
DHFRL1-4 was upregulated in I/R-damaged brain tissue and DHFRL1-4
knockdown reduced I/R-induced apoptosis, and attenuated
neurological deficits and the degeneration of brain cells. This
attenuation may be partially attributed to the upregulation of
angiogenesis-related genes and the downregulation of the Wnt
signaling pathway, although specific underlying mechanisms remain
to be elucidated.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, DH and ML designed the study. YZ, DH, YC, MW, WM
and ZJ performed the experiments. YZ, DH, YC, MW, WM, ZJ and ML
collected the data and performed the analyses. YZ, DH, YC, MW, WM,
ZJ and ML drafted the manuscript. YZ, DH and ML confirm the
authenticity of all the raw data. All authors have read and
approved the final version of manuscript.
Ethics approval and consent to
participate
All animal experiment protocols were approved by the
Institutional Animal Care and Use Committee of the Second Xiangya
Hospital, Central South University, Changsha, China (approval no.
2020438).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was performed with the regular operation
budget of the Department of Neurosurgery, the Second Xiangya
Hospital, Central South University, Changsha, China.
Abbreviations:
|
DHFRL1-4
|
dihydrofolate reductase-like 1
|
|
Wnt3a
|
Wnt family member 3a
|
|
GSK-3β
|
glycogen synthase kinase-3β
|
|
I/R
|
ischemia/reperfusion
|
|
lncRNA
|
long non-coding RNA
|
|
TTC
|
2,3,5-triphenyltetrazolium
chloride
|
|
DMCAO
|
distal middle cerebral artery
occlusion
|
|
H&E
|
hematoxylin and eosin
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
(TdT) dUTP nick-end labeling
|
|
bFGF
|
basic fibroblast growth factor
|
|
VEGF
|
vascular endothelial growth factor
|
|
ESC
|
embryonic stem cell
|
|
iPSC
|
inducible pluripotent stem cell
|
|
MCAO
|
middle cerebral artery occlusion
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
HRP
|
horseradish peroxidase
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
References
|
1
|
WHO publishes definitive atlas on global
heart disease and stroke epidemic. Indian J Med Sci. 58:405–406.
2004.
|
|
2
|
Jena I, Nayak SR, Behera S, Singh B, Ray
S, Jena D, Singh S and Sahoo SK: Evaluation of ischemia-modified
albumin, oxidative stress, and antioxidant status in acute ischemic
stroke patients. J Nat Sci Biol Med. 8:110–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Institute of Neurological
Disorders and Stroke rt-PA Stroke Study Group: Tissue plasminogen
activator for acute ischemic stroke. N Engl J Med. 333:1581–1587.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prabhakaran S, Ruff I and Bernstein RA:
Acute stroke intervention: A systematic review. JAMA.
313:1451–1462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomalla G, Sobesky J, Köhrmann M, Fiebach
JB, Fiehler J, Zaro Weber O, Kruetzelmann A, Kucinski T, Rosenkranz
M, Röther J and Schellinger PD: Two tales: Hemorrhagic
transformation but not parenchymal hemorrhage after thrombolysis is
related to severity and duration of ischemia: MRI study of acute
stroke patients treated with intravenous tissue plasminogen
activator within 6 h. Stroke. 38:313–318. 2007. View Article : Google Scholar
|
|
6
|
Yoshimura S, Sakai N, Uchida K, Yamagami
H, Ezura M, Okada Y, Kitagawa K, Kimura K, Sasaki M, Tanahashi N,
et al: Endovascular therapy in ischemic stroke with acute
large-vessel occlusion: Recovery by endovascular salvage for
cerebral ultra-acute embolism Japan registry 2. J Am Heart Assoc.
7:e0087962018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
SPS3 Investigators; Benavente OR, Hart RG,
McClure LA, Szychowski JM, Coffey CS and Pearce LA: Effects of
clopidogrel added to aspirin in patients with recent lacunar
stroke. N Engl J Med. 367:817–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong KS, Lee SH, Kim EG, Cho KH, Chang DI,
Rha JH, Bae HJ, Lee KB, Kim DE, Park JM, et al: Recurrent ischemic
lesions after acute atherothrombotic stroke: Clopidogrel plus
aspirin versus aspirin alone. Stroke. 47:2323–2330. 2016.
View Article : Google Scholar
|
|
9
|
Moussouttas M and Papamitsakis NIH:
Critique on the use of early short-term dual antiplatelet therapy
following minor acute cerebral ischemic events. Cerebrovasc Dis.
49:237–243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei L, Cui L, Snider BJ, Rivkin M, Yu SS,
Lee CS, Adams LD, Gottlieb DI, Johnson EM Jr, Yu SP and Choi DW:
Transplantation of embryonic stem cells overexpressing Bcl-2
promotes functional recovery after transient cerebral ischemia.
Neurobiol Dis. 19:183–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yanagisawa D, Qi M, Kim DH, Kitamura Y,
Inden M, Tsuchiya D, Takata K, Taniguchi T, Yoshimoto K, Shimohama
S, et al: Improvement of focal ischemia-induced rat dopaminergic
dysfunction by striatal transplantation of mouse embryonic stem
cells. Neurosci Lett. 407:74–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdelwahid E, Siminiak T, Guarita-Souza
LC, Teixeira de Carvalho KA, Gallo P, Shim W and Condorelli G: Stem
cell therapy in heart diseases: A review of selected new
perspectives, practical considerations and clinical applications.
Curr Cardiol Rev. 7:201–212. 2011. View Article : Google Scholar
|
|
13
|
Gutiérrez-Fernández M, Rodríguez-Frutos B,
Ramos-Cejudo J, Otero-Ortega L, Fuentes B and Díez-Tejedor E: Stem
cells for brain repair and recovery after stroke. Expert Opin Biol
Ther. 13:1479–1483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee EJ, Park HW, Jeon HJ, Kim HS and Chang
MS: Potentiated therapeutic angiogenesis by primed human
mesenchymal stem cells in a mouse model of hindlimb ischemia. Regen
Med. 8:283–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SJ, Chang CM, Tsai SK, Chang YL, Chou
SJ, Huang SS, Tai LK, Chen YC, Ku HH, Li HY and Chiou SH:
Functional improvement of focal cerebral ischemia injury by
subdural transplantation of induced pluripotent stem cells with
fibrin glue. Stem Cells Dev. 19:1757–1767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Liu X and Zhu R: Long noncoding
RNAs as diagnostic and therapeutic targets for ischemic stroke.
Curr Pharm Des. 25:1115–1121. 2019. View Article : Google Scholar
|
|
17
|
Zhang J, Yuan L, Zhang X, Hamblin MH, Zhu
T, Meng F, Li Y, Chen YE and Yin KJ: Altered long non-coding RNA
transcriptomic profiles in brain microvascular endothelium after
cerebral ischemia. Exp Neurol. 277:162–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren H, Wu F, Liu B, Song Z and Qu D:
Association of circulating long non-coding RNA MALAT1 in diagnosis,
disease surveillance, and prognosis of acute ischemic stroke. Braz
J Med Biol Res. 53:e91742020. View Article : Google Scholar :
|
|
19
|
Na L, Ding H, Xing E, Gao J, Liu B, Wang
H, Yu J and Yu C: Lnc-MEG3 acts as a potential biomarker for
predicting increased disease risk, systemic inflammation, disease
severity, and poor prognosis of sepsis via interacting with miR-21.
J Clin Lab Anal. 34:e231232020. View Article : Google Scholar
|
|
20
|
Zhang Y and Niu C: The correlation of long
non-coding RNA intersectin 1-2 with disease risk, disease severity,
inflammation, and prognosis of acute ischemic stroke. J Clin Lab
Anal. 34:e230532020.
|
|
21
|
Wang L, Qu P, Yin W and Sun J: Lnc-NEAT1
induces cell apoptosis and inflammation but inhibits proliferation
in a cellular model of hepatic ischemia/reperfusion injury. J Int
Med Res. 49:3000605198872512021.PubMed/NCBI
|
|
22
|
Tong G, Wang Y, Xu C, Xu Y, Ye X, Zhou L,
Zhu G, Zhou Z and Huang J: Long non-coding RNA FOXD3-AS1 aggravates
ischemia/reperfusion injury of cardiomyocytes through promoting
autophagy. Am J Transl Res. 11:5634–5644. 2019.
|
|
23
|
Lu Y, Han Y, He J, Zhou B, Fang P and Li
X: LncRNA FOXD3-AS1 knockdown protects against cerebral
ischemia/reperfusion injury via miR-765/BCL2L13 axis. Biomed
Pharmacother. 132:1107782020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamashita T, Deguchi K, Nagotani S, Kamiya
T and Abe K: Gene and stem cell therapy in ischemic stroke. Cell
Transplant. 18:999–1002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng QW, Li S, Wang H, Sun HL, Zuo L, Gu
ZT, Lu G, Sun CZ, Zhang HQ and Yan FL: Differential long noncoding
RNA expressions in peripheral blood mononuclear cells for detection
of acute ischemic stroke. Clin Sci (Lond). 132:1597–1614. 2018.
View Article : Google Scholar
|
|
26
|
Montaner J, Ramiro L, Simats A, Tiedt S,
Makris K, Jickling GC, Debette S, Sanchez JC and Bustamante A:
Multilevel omics for the discovery of biomarkers and therapeutic
targets for stroke. Nat Rev Neurol. 16:247–264. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen Y, Zhang X, Liu X, Huo Y, Gao Y and
Yang Y: Suppression of lncRNA SNHG15 protects against cerebral
ischemia-reperfusion injury by targeting miR-183-5p/FOXO1 axis. Am
J Transl Res. 12:6250–6263. 2020.PubMed/NCBI
|
|
28
|
Kilkenny C, Browne WJ, Cuthill I, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Doyle KP, Fathali N, Siddiqui MR and
Buckwalter MS: Distal hypoxic stroke: A new mouse model of stroke
with high throughput, low variability and a quantifiable functional
deficit. J Neurosci Methods. 207:31–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar
|
|
32
|
Murtha LA, Beard DJ, Bourke JT, Pepperall
D, McLeod DD and Spratt NJ: Intracranial pressure elevation 24 h
after ischemic stroke in aged rats is prevented by early, short
hypothermia treatment. Front Aging Neurosci. 8:1242016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008. pp. pdb.prot49862008
|
|
34
|
Kyrylkova K, Kyryachenko S, Leid M and
Kioussi C: Detection of apoptosis by TUNEL assay. Methods Mol Biol.
887:41–47. 2012. View Article : Google Scholar
|
|
35
|
Zhao Z, Sun W, Guo Z, Zhang J, Yu H and
Liu B: Mechanisms of lncRNA/microRNA interactions in angiogenesis.
Life Sci. 254:1169002020. View Article : Google Scholar
|
|
36
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hatakeyama M, Ninomiya I and Kanazawa M:
Angiogenesis and neuronal remodeling after ischemic stroke. Neural
Regen Res. 15:16–19. 2020. View Article : Google Scholar :
|
|
38
|
Wilkins JR, Pike DB, Gibson CC, Kubota A
and Shiu YT: Differential effects of cyclic stretch on bFGF- and
VEGF-induced sprouting angiogenesis. Biotechnol Prog. 30:879–888.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shima DT, Gougos A, Miller JW, Tolentino
M, Robinson G, Adamis AP and D'Amore PA: Cloning and mRNA
expression of vascular endothelial growth factor in ischemic
retinas of Macaca fascicularis. Invest Ophthalmol Vis Sci.
37:1334–1340. 1996.PubMed/NCBI
|
|
40
|
Cantaluppi V, Biancone L, Figliolini F,
Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R,
Salizzoni M, Tetta C, et al: Microvesicles derived from endothelial
progenitor cells enhance neoangiogenesis of human pancreatic
islets. Cell Transplant. 21:1305–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar
|
|
42
|
Zhang W, Bai X, Zhao B, Li Y, Zhang Y, Li
Z, Wang X, Luo L, Han F, Zhang J, et al: Cell-free therapy based on
adipose tissue stem cell-derived exosomes promotes wound healing
via the PI3K/Akt signaling pathway. Exp Cell Res. 370:333–342.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kutikhin AG, Sinitsky MY, Yuzhalin AE and
Velikanova EA: Shear stress: An essential driver of endothelial
progenitor cells. J Mol Cell Cardiol. 118:46–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kumar VV, Heller M, Götz H, Schiegnitz E,
Al-Nawas B and Kämmerer PW: Comparison of growth & function of
endothelial progenitor cells cultured on deproteinized bovine bone
modified with covalently bound fibronectin and bound vascular
endothelial growth factor. Clin Oral Implants Res. 28:543–550.
2017. View Article : Google Scholar
|
|
45
|
Liang S, Wang Y and Liu Y: Dexmedetomidine
alleviates lung ischemia-reperfusion injury in rats by activating
PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 23:370–377.
2019.PubMed/NCBI
|
|
46
|
Jin Z, Guo P, Li X, Ke J, Wang Y and Wu H:
Neuroprotective effects of irisin against cerebral
ischemia/reperfusion injury via Notch signaling pathway. Biomed
Pharmacother. 120:1094522019. View Article : Google Scholar
|
|
47
|
Yang M, Kong DY and Chen JC: Inhibition of
miR-148b ameliorates myocardial ischemia/reperfusion injury via
regulation of Wnt/β-catenin signaling pathway. J Cell Physiol.
234:17757–17766. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kahn M: Wnt signaling in stem cells and
cancer stem cells: A tale of two coactivators. Prog Mol Biol Transl
Sci. 153:209–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Oh SH, Kim HN, Park HJ, Shin JY and Lee
PH: Mesenchymal stem cells increase hippocampal neurogenesis and
neuronal differentiation by enhancing the Wnt signaling pathway in
an Alzheimer's disease model. Cell Transplant. 24:1097–1109. 2015.
View Article : Google Scholar
|
|
50
|
Chen S, Sun YY, Zhang ZX, Li YH, Xu ZM and
Fu WN: Transcriptional suppression of microRNA-27a contributes to
laryngeal cancer differentiation via GSK-3β-involved Wnt/β-catenin
pathway. Oncotarget. 8:14708–14718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X, Liu Y, Shao R and Li W:
Cdc42-interacting protein 4 silencing relieves pulmonary fibrosis
in STZ-induced diabetic mice via the Wnt/GSK-3β/β-catenin pathway.
Exp Cell Res. 359:284–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin J, Song T, Li C and Mao W: GSK-3β in
DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy
of cancer. Biochim Biophys Acta Mol Cell Res. 1867:1186592020.
View Article : Google Scholar
|
|
53
|
Liao S, Wu J, Liu R, Wang S, Luo J, Yang
Y, Qin Y, Li T, Zheng X, Song J, et al: A novel compound DBZ
ameliorates neuroinflammation in LPS-stimulated microglia and
ischemic stroke rats: Role of Akt(Ser473)/GSK3β(Ser9)-mediated Nrf2
activation. Redox Biol. 36:1016442020. View Article : Google Scholar
|
|
54
|
Xu B, Xu J, Cai N, Li M, Liu L, Qin Y, Li
X and Wang H: Roflumilast prevents ischemic stroke-induced neuronal
damage by restricting GSK3β-mediated oxidative stress and
IRE1α/TRAF2/JNK pathway. Free Radic Biol Med. 163:281–296. 2021.
View Article : Google Scholar
|
|
55
|
Zhou W, Yuan Y, Li J, Yuan WM, Huang LG
and Zheng SW: Effect of Bifidobacterium on the mRNA expression
levels of TRAF6, GSK-3β, and microRNA-146a in LPS-stimulated rat
intestinal epithelial cells. Genet Mol Res. 14:10050–10056. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Duan J, Cui J, Yang Z, Guo C, Cao J, Xi M,
Weng Y, Yin Y, Wang Y, Wei G, et al: Neuroprotective effect of
Apelin 13 on ischemic stroke by activating AMPK/GSK-3β/Nrf2
signaling. J Neuroinflammation. 16:242019. View Article : Google Scholar
|
|
57
|
Virgintino D, Errede M, Robertson D,
Girolamo F, Masciandaro A and Bertossi M: VEGF expression is
developmentally regulated during human brain angiogenesis.
Histochem Cell Biol. 119:227–232. 2003. View Article : Google Scholar
|
|
58
|
Kim HG, Hwang YP and Jeong HG:
Metallothionein-III induces HIF-1alpha-mediated VEGF expression in
brain endothelial cells. Biochem Biophys Res Commun. 369:666–671.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Takano S, Uchida K, Inoue G, Matsumoto T,
Aikawa J, Iwase D, Mukai M, Miyagi M and Takaso M: Vascular
endothelial growth factor expression and their action in the
synovial membranes of patients with painful knee osteoarthritis.
BMC Musculoskelet Disord. 19:2042018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhuang H, Shi S, Yuan Z and Chang JY:
Bevacizumab treatment for radiation brain necrosis: Mechanism,
efficacy and issues. Mol Cancer. 18:212019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kubo H, Hayashi T, Ago K, Ago M, Kanekura
T and Ogata M: Temporal expression of wound healing-related genes
in skin burn injury. Leg Med (Tokyo). 16:8–13. 2014. View Article : Google Scholar
|
|
62
|
Elbialy ZI, Atiba A, Abdelnaby A,
Al-Hawary II, Elsheshtawy A, El-Serehy HA, Abdel-Daim MM, Fadl SE
and Assar DH: Collagen extract obtained from Nile tilapia
(Oreochromis niloticus L.) skin accelerates wound healing in rat
model via up regulating VEGF, bFGF, and α-SMA genes expression. BMC
Vet Res. 16:3522020. View Article : Google Scholar
|
|
63
|
Oryan A, Alemzadeh E and Eskandari MH:
Kefir accelerates burn wound healing through inducing fibroblast
cell migration in vitro and modulating the expression of IL-1ß,
TGF-ß1, and bFGF genes in vivo. Probiotics Antimicrob Proteins.
11:874–886. 2019. View Article : Google Scholar
|
|
64
|
Zou Q, Wang W, Li Q, Liu J, Xu T and Zhao
X: Effect of ultra-sound debridement on serum inflammatory factors
and bFGF, EGF expression in wound tissues. J Coll Physicians Surg
Pak. 29:222–225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dykes IM and Emanueli C: Transcriptional
and post-transcriptional gene regulation by long non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ariel F, Lucero L, Christ A, Mammarella
MF, Jegu T, Veluchamy A, Mariappan K, Latrasse D, Blein T, Liu C,
et al: R-loop mediated trans action of the APOLO long noncoding
RNA. Mol Cell. 77:1055–1065.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|