Introduction
Acute myocardial infarction (AMI) and subsequent
heart failure (HF) remain one of the leading causes of mortality
and disability worldwide (1,2).
Mitochondrial dysfunction is a critical determinant of cell death
in acute phases and heart remodeling in chronic phases following
AMI (1). Therefore, targeting
mitochondrial dysfunction may be a promising therapeutic strategy
with which to mitigate cardiac remodeling (3,4).
Fibroblast growth factor (FGF)21 is a peptide
hormone from the FGF protein family and is predominantly secreted
by liver tissue. It plays a crucial role in the regulation of
mitochondrial stress and metabolism by binding to FGF cell surface
receptors and its obligatory coreceptor β-klotho (KLB) (5,6).
There is evidence to suggest that increased FGF21 levels improve
defective mitochondrial function and stress adaptation in muscles
(7). Moreover, it has been shown
that the overexpression of FGF21 significantly reduces
aging-related obesity, adipose tissue hypertrophy and inflammation
(8), indicating appealing
prospects for the clinical translation of FGF21signaling in
aging-associated metabolic diseases.
Patients with AMI manifest elevated serum FGF21
levels compared with healthy individuals, and FGF21 levels are
significantly related to an increased incidence of adverse
cardiovascular events at 30 days post-infarction (9). In addition, mice with diabetic
cardiomyopathy have also been found to have higher circulating
levels of FGF21 than non-diabetic mice (5). Notably, exercise reduces FGF21
levels, while enhancing FGF21 sensitivity by promoting co-receptor
KLB expression in heart tissues, attenuating diabetes-related
cardiac pathological remodeling (5). Therefore, it was hypothesized that
promoting KLB expression rather than excessively increasing FGF21
levels may more effectively mitigate cardiac remodeling
post-myocardial infarction (MI).
Ultrasound-targeted microbubble destruction (UTMD)
is an emerging approach for targeted delivery of drugs and gene
transport to specific tissues (10). Employing low-frequency ultrasound
offers the advantages of enhanced penetration, reduced attenuation
and focalization on deep-seated local tissues (11). Intravenous microbubbles flow in
the bloodstream and oscillate, collapsing within the target tissue
under ultrasound stimulation, causing localized cavitation effects
that enhance the penetration and uptake of carried plasmids
(12). The UTMD technique has
demonstrated efficacy and safety in gene delivery to target organs
and is extensively validated in rodent and primate models (10). Furthermore, UTMD circumvents
ethical and biosafety controversies associated with viral gene
delivery (13), and the clinical
safety of ultrasound microbubbles is widely recognized in
diagnostics and therapy (14,15).
In the present study, it was hypothesized that the
UTMD-targeted delivery of the KLB gene to the heart prevents the
occurrence of HF post-infarction. The KLB gene exerts this
effect by augmenting myocardial KLB expression, enhancing FGF21
sensitivity, and attenuating mitochondrial dysfunction and cardiac
remodeling post-infarction through the FGF21-KLB pathway. These
findings contribute to the advancements of the clinical translation
of the FGF21 pathway as a promising therapeutic avenue within the
cardiovascular field.
Materials and methods
Study participants
A total of 50 patients aged ≥20 years and diagnosed
with HF post-infarction without renal replacement therapy were
enrolled between June and December, 2022 at the Department of
Cardiology of the Qujing First People's Hospital, Qujing, China.
Age- and sex-matched healthy adults from the outpatient department
not diagnosed with cardiovascular diseases were included as the
control group. Fasting serum samples were collected and stored at
-80°C. The Ethics Review Board of Qujing First People's Hospital
approved the study protocols (approval no. QJFH2021-017). All
participants provided written informed consent prior to
enrollment.
Animal models
A total of 100 healthy male Sprague-Dawley rats
(weighing 220-250 g; 8 weeks old) were purchased from the Animal
Center of Kunming Medical University and kept at ~22°C and a 12-h
light-dark cycle with free access to food and water. The study
protocols were in accordance with the Guide for the Care and Use of
Laboratory Animals and approved by the Animal Care and Use
Committee of Kunming Medical University (Kunming, China). All
relevant animal welfare considerations were taken, including
efforts to minimize suffering and distress, the use of analgesics
or anesthetics or special housing conditions, particularly for the
AMI and UTMD procedures.
The left anterior descending coronary artery
ligation was performed to induce AMI and establish a model of MI in
rats, as previously described (16). An intraperitoneal injection of
ML385 (30 mg/kg, HY-100523, MedChemExpress) was administered to
inhibit nuclear factor erythroid 2-related factor 2 (Nrf2)
activity, as previously described (17). All animals were randomly divided
into the following experimental groups: The sham-operated (sham)
group (n=8), the AMI group (n=36), the GFP + UTMD group (n=5), the
AMI + FGF21 group (n=12), the AMI + UTMD@Vector group (n=3), the
AMI + UTMD@ KLB group (n=12), the AMI + UTMD@KLB +
FGF21 (n=12) group and the AMI + UTMD@KLB + FGF21 + ML385
group (n=12). During the surgery, 5, 3, 2, 3 and 3 rats died in the
AMI, AMI + FGF21, AMI + UTMD@KLB, AMI + UTMD@ KLB +
FGF21 and AMI + UTMD@KLB + FGF21 + ML385 groups,
respectively, due to sudden cardiac death and severe heart failure.
The mortality rate as was expected according to previous studies
using rats following left anterior descending coronary artery (LAD)
occlusion (18-20). Animal health and behavior were
monitored every day for the first week, then once every 2 days. The
pre-designed humane endpoints in the present study mainly were
severe heart failure, loss of appetite and fragility for >24 h
and an agonal stage. A total of 3 rats reached the humane endpoints
and were therefore euthanized before the end of the study and were
thus recorded as deaths due to heart failure.
In brief, the rats were placed in the supine
position for endotracheal intubation and anesthetized by the
inhalation of 2-3% isoflurane (induction, 3%; maintenance, 2%)
using a small animal anesthesia machine (Harvard Apparatus). All
animals received buprenorphine hydrochloride (0.1 mg/kg,
subcutaneous injection) for pain management when establishing the
model of MI. The surgical procedure involved a left thoracotomy to
access the heart. A 5-0 Prolene suture with a needle was used to
ligate the LAD. The ligation was performed ~2 mm below the left
atrial appendage. The model of MI model was validated by observing
distinct changes in the heart anatomy, and the whitening of the
left ventricular anterior wall and apex. The rats in the sham group
were only subjected to drilling underneath the LAD coronary artery
but were not ligated. The rats were administered an intraperitoneal
injection of the vehicle (0.9% saline, ST341, Beyotime Institute of
Biotechnology) or FGF21 at 0.5 mg/kg/day. Recombinant human FGF21
(CSB-AP002471HU, Cusabio Technology, LLC) was expressed in E.
coli and harvested by ion exchange and hydrophobic interaction
chromatography, as previously described (21). In accordance with the AVMA
Guidelines for the Euthanasia of Animals, the rats were euthanized
by cervical dislocation under anesthetization with isoflurane
inhalation (induction, 3%; maintenance, 2%). Animal death was
verified by respiratory cessation and muscular tension
disappearance. Subsequently, ~3 ml blood was rapidly obtained from
the heart cavity to extract serum for use in enzyme-linked
immunosorbent assay (ELISA). The heart tissues were then harvested
for use in subsequent experiments. The duration of the animal
experiment was 4 weeks, including the establishment of the model of
AMI, the UTMD procedure and subsequent observation until
euthanasia.
Cationic microbubble (CMB)
generation
CMBs were synthesized using the thin-film
hydration-sonic vibration method, as previously described (22). A mixture of chemicals, including
dipalmitoyl-phosphatidylcholine (cat. no. 850355P-1G-A-3214, Avanti
Polar Lipids, Inc.),
1,2-distearoyl-sn-glycerol-3-phosphoethanolamine-N-maleimide
(DSPE-PEG, cat. no. 880160P-200MG-A-047, Avanti Polar Lipids, Inc.)
and 3β-[N-(N',N'-dimethyl-aminoethane)-carbamoyl] cholesterol
(DC-Chol, cat. no. 700001P-200MG-B-021, Avanti Polar Lipids, Inc.),
was prepared and processed to form a thin lipid film (mass ratio,
5:2:0.5) (10). Glycerol
solution (cat. no. 56-81-5, MillliporeSigma) was added, and
octafluoropropane (C3F8, Foshan Huate Gas Co. Ltd.) gas was bubbled
through the solution to generate gas-filled CMBs, widely used in
clinical practice with favorable safety and performance (23). The CMBs were diluted to
1×109/ml with PBS (C0221A, Beyotime Institute of
Biotechnology), treated with 60Co-γ irradiation (BFT-II
Cobalt-60 gamma-ray Irradiation Device, MDS Nordion) and stored at
4°C. The particle size distribution and surface potential were
determined using the Zetasizer Nano ZS system (Malvern Panalytical
Ltd.) (10).
KLB@CMB generation
Plasmids (80 μg, Hanbio Biotechnology Co.,
Ltd.) expressing the GFP or KLB gene were incubated in a
100-μl CMB suspension (0.5×109 MBs) for 15 min.
The suspension was centrifuged at 400 × g for 5 min under 4°C to
obtain plasmid-bound CMBs (10).
The percentage of bound plasmid DNA and the payload mass of plasmid
DNA in CMBs were calculated as follows: Percentage of bound plasmid
DNA in
CMBs=(DNAtotal-DNAfree)/DNAtotal
×100%; payload mass of plasmid DNA in
CMBs=(DNAtotal-DNAfree)/CMB number (10). The plasmid DNA was stained by 10
μg/ml propidium iodide solution (C0080 Beijing Solarbio
Science & Technology Co., Ltd.) for 10 min at room temperature.
The combination of plasmid DNA and CMBs was validated using a
fluorescence microscope (DMI3000, Leica Microsystems GmbH) and a
flow cytometer (BD Biosciences).
UTMD technology
All animals were anesthetized via isoflurane
inhalation (induction, 3%; maintenance, 2%) for the UTMD procedure.
In brief, the rats were administered intravenous injections of
KLB@CMBs (1.0-1.2 ml/h) via the tail vein, as previously
reported (10). An M3S
transducer (S5-1 probe, GE Healthcare) with a low 1-5 MHz frequency
range was used due to the known 1-3 MHz resonant frequency of
microbubbles (10,12,24). The parameters of the second
harmonic mode were used and set as transmit 1.6 MHz and receive 3.2
MHz, mechanical index (MI, low 0.30; flash, 1.31), and -16 dB
output power (10). The
KLB delivery to the heart was repeated three time at 1-day
intervals at 1, 3, and 5 days at 1 week following the induction of
AMI (25).
Rat cardiomyoblast H9C2 cell culture and
treatment
H9C2 rat cardiomyoblasts (purchased from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences)
were cultured in DMEM containing 10% (v/v) fetal bovine serum (cat.
no. 0500, ScienCell Research Laboratories, Inc.) in a humidified
incubator with 5% CO2 and at 37°C. When the cell density
reached 60%, the cells were transfected with a plasmid containing
the KLB gene using Lipofectamine 3000® reagent
(L3000008, Invitrogen; Thermo Fisher Scientific, Inc.) following
the manufacturer's protocols. The cells were cultured with or
without FGF21 (2 μg/ml, CSB-AP002471HU, Cusabio Technology,
LLC) for 24 h. Subsequently they were treated under hypoxic
conditions (5% CO2 and 1% O2 at 37°C) to
mimic AMI for 4 h and collected for further analysis.
Echocardiography assessment
As previously described, cardiac function was
evaluated using an M-mode echocardiography system (S12-4 probe,
EPIQ 5; Koninklijke Philips N.V.) 4 weeks following the induction
of AMI (16). The rats were
lightly anesthetized via isoflurane inhalation (induction, 3%;
maintenance, 1.5%) for the echocardiography assessment. Left
ventricular end-systolic diameter (LVESD) and left ventricular
end-systolic diameter (LVESD) were measured to calculate the left
ventricular ejection fraction (LVEF) and left ventricular
fractional shortening (LVFS) using computer algorithms. Each
diameter was obtained from at least four consecutive cardiac cycles
and averaged.
Cell death assessment
To evaluate the apoptosis of H9C2 cells or that of
cells in the heart sections, terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) staining (cat.
no. A321, Elabscience) was used, as previously described (3). The apoptotic index was calculated
by determining the ratio of TUNEL-positive nuclei to the total
nuclei counts in five randomly selected fields per sample, as
previously described (10).
Assessment of reactive oxygen species
(ROS) generation
For the assessment of ROS generation DHE staining
was performed on fresh heart tissues (10). The heart sections were
pre-treated with cold PBS for 10 min and exposed to 10
μmol/l DHE solution (cat. no. S0063, Beyotime Institute of
Biotechnology) at 37°C in a dark environment, as previously
described (10). Following a
30-min incubation at 37°C, the heart sections were subjected to
triple PBS washes and were maintained in a wet condition until
examination through a fluorescence microscope (Leica Microsystems
GmbH).
Assessment of mitochondrial membrane
potential
Mitochondrial membrane potential was determined
using the JC-1 staining assay kit (cat. no. C2005, Beyotime
Institute of Biotechnology), as previously described (26). JC-1, a fluorescent dye,
accumulated in the mitochondrial matrix in two forms: Aggregates
(red fluorescence) in polarized mitochondria and monomers (green
fluorescence) in depolarized mitochondria (10). The heart sections were treated
with 10 μg/ml JC-1 solution at 37°C for 0.5 h in a
light-protected environment, as previously described (27). Following three washes with PBS,
the fluorescence intensity was observed using a fluorescence
microscope (Leica Microsystems GmbH). To quantify the mitochondrial
membrane potential, five random images were captured per sample and
the fluorescence intensity of both the J-monomers and the
J-aggregates was examined.
Assessment of mitochondrial respiratory
function
As previously described (28), the H9C2 cells were intervened and
digested with pancreatic enzymes, and the cell suspension was then
collected for analysis. The mitochondrial respiratory function was
assessed using an XF24 Extracellular Flux Analyzer (Seahorse
Bioscience) with a sequential titration of oligomycin, carbonyl
cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), rotenone and
antimycin A (XF Cell Mito Stress Test kit, Seahorse Bioscience), as
previously described (28).
Assessment of antioxidant capacity
Total antioxidant capacity was measured using a
commercial kit based on the ferric-reducing ability of plasma
method (S0116, Beyotime Institute of Biotechnology) according to
the product manual. The capacity was quantified by measuring the
absorbance at 593 nm using a microplate reader (Infinite F500,
Tecan Group, Ltd.) and estimated as a percentage of the combined
ferric reducing/antioxidant potency of the antioxidants in
protein.
Pathological analysis
The heart tissues were fixed with 4%
paraformaldehyde (cat. no. P0099, Beyotime Institute of
Biotechnology) at room temperature for 72 h and subsequently sliced
into sections measuring 5 μm in thickness. These sections
were subjected to staining with hematoxylin and eosin [H&E;
Right Tech, China] for 2 min at room temperature, and Masson's
Trichrome Stain with celestine blue staining (cat. no. G1346,
Beijing Solarbio Science & Technology Co., Ltd.) for 3 min at
room temperature and Ponceau-acid magenta staining (cat. no. C0189,
Beyotime Institute of Biotechnology) for 10 min at room temperature
to observe and analyze the cardiac fibrosis morphology using
microscope (Olympus IX70, Olympus Corporation) according to the
manufacturer's instructions.
Cell viability assay
The MTT Cell Proliferation Assay kit (cat. no.
C0009, Beyotime Institute of Biotechnology) was used to assess cell
viability. The H9C2 cells (0.5×104 per well) were
cultured in 96-well plates and treated as needed (e.g., plasmid
transfection, FGF21 treatment and hypoxia-induced stress), and were
then incubated with MTT reagent for 4 h at 37°C (10). Additionally, lactate
dehydrogenase (LDH) activity was measured in the serum or medium
supernatant using a commercially available assay kit (cat. no.
A020, Jiancheng Bioengineering Institute). A total of 50 μl
diluted serum was added to 96-well plates and combined with 50
μl reaction mix [the mixed solution of reagent I and II
(v/v: 5:1)] per well (10). The
absorbance was then measured at 450 nm using a spectrophotometer
(Evolution 60S, Thermo Fisher Scientific, Inc.), and LDH activity
was calculated following the manufacturer's instructions (10).
ELISA
The serum levels of creatine kinase-myocardial band
(CK-MB; E-EL-R1327c, Elabscience Biotechnology Co., Ltd.), cardiac
troponin T (cTnT; E-EL-R0151c Elabscience Biotechnology Co., Ltd.),
KLB (ml028418, Mlbio) and FGF21 (CSB-EL008627RA, Cusabio
Biotechnology Co., Ltd.) were measured according to the
instructions provided with the kits.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol reagent (cat.
no. B0201, HaiGene) and converted into cDNA using the RT Easy II
First Strand cDNA Synthesis kit (cat. no. 04379012001, Roche
Diagnostics), as previously described (16). Followinh incubation for 60 min at
42°C, the reactions were terminated by heating at 70°C for 10 min.
qPCR was performed using the Real-Time PCR Easy (HY-K0501SYBR-Green
I, MedChemExpress), with the following procedures: Denaturation at
95°C for 10 min, amplification by 40 cycles of 95°C for 10 sec and
60°C for 30 sec. Gene expression was normalized to β-actin and
calculated using the 2-ΔΔcq method (29). The primer sequences are presented
in Table SI.
Western blot analysis
Total proteins were extracted from the H9C2 cells or
heart tissues using RIPA buffer containing protease (P0013C,
Beyotime Institute of Biotechnology) and phosphatase inhibitors
(P1045, Beyotime Institute of Biotechnology). The protein
concentration was determined using BCA assay. Specifically, ~30
μg proteins per lane were loaded into 10% SDS-PAGE gels and
transferred onto 0.22 μm PVDF membranes (MilliporeSigma).
The membranes were blocked using 5% BSA (ST025, Beyotime Institute
of Biotechnology) for 1 h at room temperature, and incubated using
corresponding primary antibodies overnight at 4°C and HRP-linked
secondary antibody for 1 h at room temperature (all antibodies used
are listed in Table SII). Due
to the close molecular weights of the target proteins, the protein
expression signals on different membranes were detect with the same
loading amount, and all other western blotting conditions for each
protein were also the same excepting the primary antibody used. The
density of immunoreactivity was assessed using a chemiluminescence
ECL kit using a chemiluminescence system (Tanon 5200, Tanon Science
& Technology Co., Ltd.) (30). ImageJ software (version v1.52a,
National Institutes of Health) was used for the semi-quantification
of protein expression.
Statistical analyses
Unless stated otherwise, quantitative variables are
expressed as the mean ± standard deviations (SD). Statistical
analyses and data visualization were conducted using SPSS 20.0
software (IBM Corp.) and GraphPad Prism (version 6.0; Dotmatics).
The Shapiro-Wilk test was used to evaluate data distribution, and
P>0.05 was considered as passing the normality test. As the
clinical data in Fig. 1A did not
meet the normal distribution (P<0.001 in the Shapiro-Wilk test),
the non-parametric Mann-Whitney U test was adopted for the
comparison of two groups here. Following normality tests, a
two-tailed unpaired Student's t test was conducted for the
comparisons of two groups, and one-way ANOVA with Tukey's post hoc
test were used for the comparisons of multiple groups. The
correlation between FGF21, KLB and myocardial injury biomarkers was
examined using Spearman's correlation analysis. A P-value <0.05
was considered to indicate a statistically significant
difference.
Results
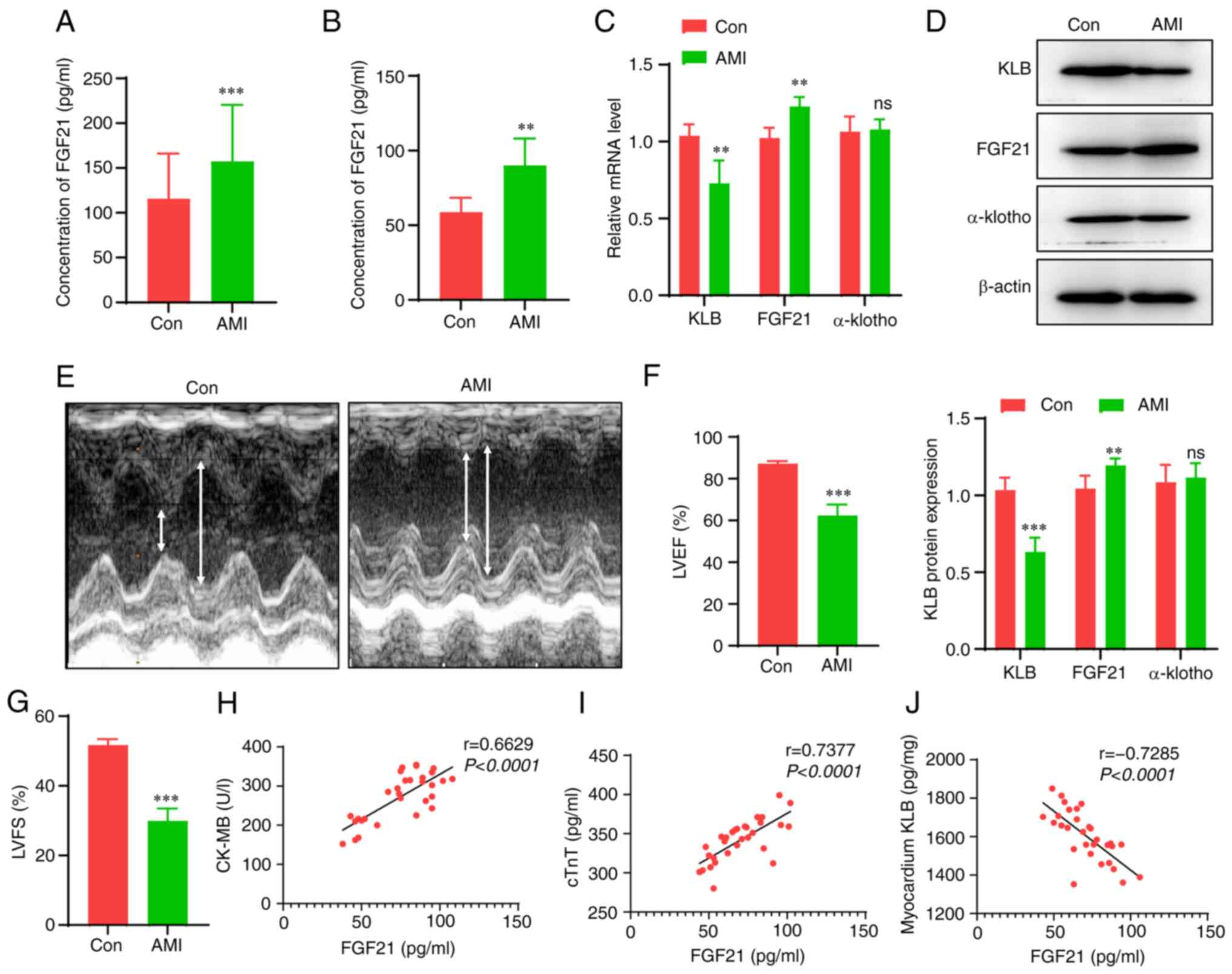
FGF21 levels are increased, but FGF21
sensitivity is decreased in the heart post-infarction
The serum levels of FGF21 were measured in patients
with HF following MI and in age- and sex-matched healthy
participants. The serum levels of FGF21 in the patients with HF
were significantly increased compared with those in the healthy
participants (128 vs. 111 pg/ml, Fig. 1A). Subsequently, AMI was induced
in rats to provoke HF and the FGF21 serum levels were measured.
Consistent with the results obtained in the patients, the rats with
HF had higher serum levels of FGF21 compared with the control rats
(Fig. 1B). However, the cardiac
expression of the FGF21 receptor, KLB, not α-klotho, was
significantly decreased in the rats with AMI compared with the
sham-operated rats (Fig. 1C and
D). The successful establishment of the murine model of MI was
verified by measuring the decreased LVEF and LVFS according to the
echocardiography (Fig. 1E-G).
Furthermore, the correlation between the severity of myocardial
injury and FGF21 or KLB levels was assessed (Fig. 1H-J). Although the levels of the
markers of myocardial injury, CK-MB and cTnT positively correlated
with the serum levels of FGF21 (r=0.663 and 0.738, respectively),
there was an inverse correlation between the cardiac KLB content
and the serum levels of FGF21 (r=-0.729). These data suggest that
although the FGF21 levels significantly increase following AMI, the
levels of its downstream KLB co-receptor in heart tissue are
markedly decreased, which may be a critical factor that decreases
the cardiac response to FGF21. Hence, promoting FGF21 sensitivity
may be a potential strategy with which to enhance the therapeutic
efficacy of FGF21.
Overexpression of KLB enhances the
protective effects of FGF21 treatment on hypoxia-induced
cardiomyocyte injury in vitro
There is evidence to indicate that FGF21 activates
intracellular protection signals against hypoxia-induced cellular
injury (9). However, our
previous observation suggests that the insufficiency of the cardiac
KLB co-receptor may impair the pharmacological action of FGF21
(Fig. 1C and D). Further
experiments were performed. First, the transfection of plasmid
containing the KLB gene significantly induced KLB
overexpression in H9C2 cells under normal conditions or hypoxia, as
verified by measuring its mRNA and protein levels (Fig. S1). As was expected, the cellular
activity of H9C2 cells decreased after 3 h under hypoxic conditions
in vitro (Fig. 2A).
However, FGF21 treatment or KLB overexpression alone could not
substantially reverse the decreased cellular activity of H9C2 cells
under hypoxic conditions. When the cells overexpressing KLB
were treated with FGF21, cellular injury was reduced (Fig. 2A). The present study also
assessed oxidative stress, LDH leakage, and cell death that reflect
hypoxia-induced myocardial injury. The overexpression of KLB
enhanced the effects of FGF21, reducing ROS generation and cell
injury induced by hypoxia (Fig.
2B-D). As shown by JC-1 staining, the hypoxia-induced decrease
in the mitochondrial membrane potential was partly attenuated by
FGF21 treatment, and KLB overexpression significantly enhanced the
protective effects of FGF21 on the mitochondria (Fig. 2E). In summary, these findings
suggest that KLB overexpression optimizes the effects of FGF21 on
cardiomyocytes in response to hypoxia in vitro.
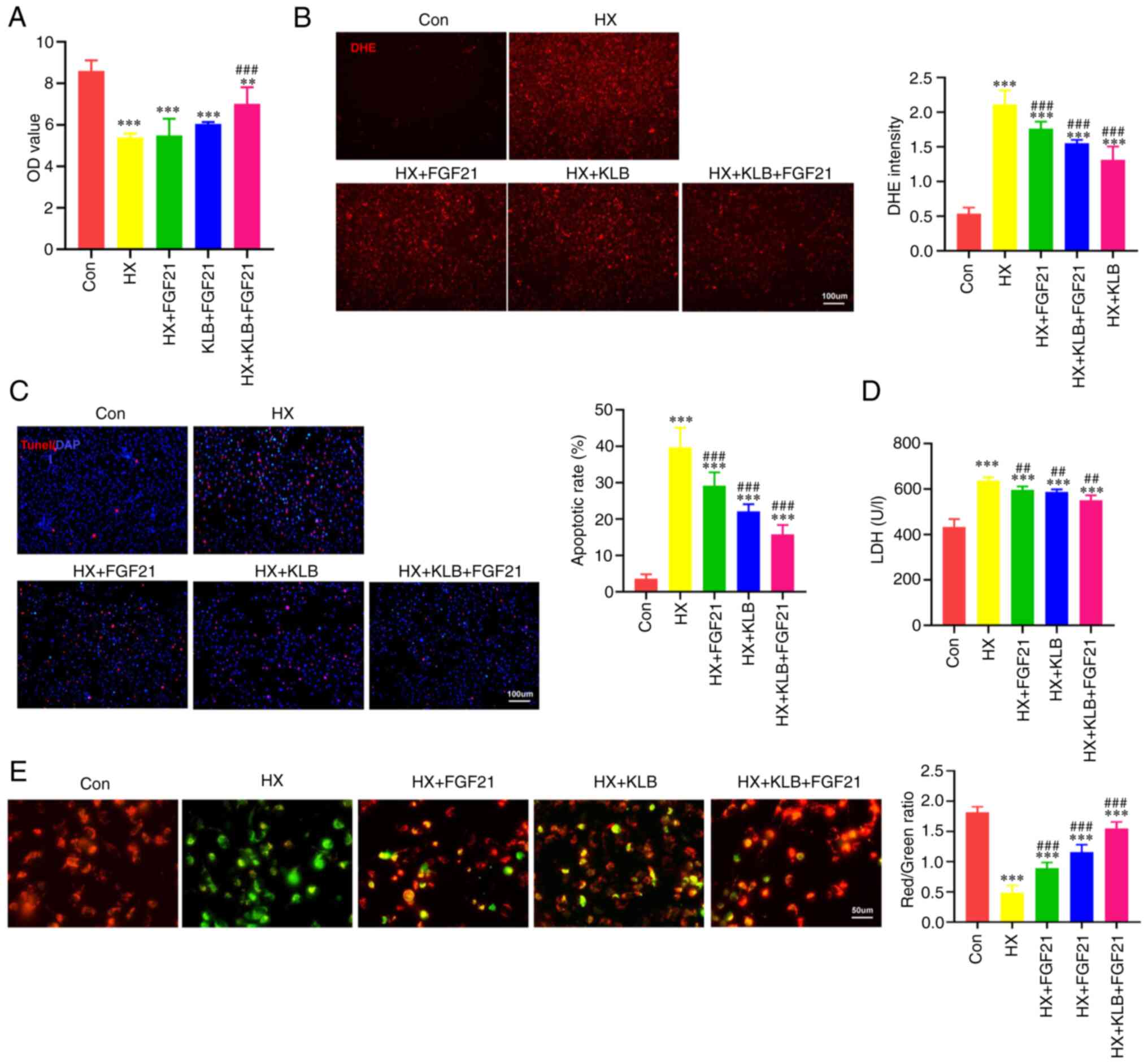 | Figure 2KLB overexpression enhances the
protective effects of FGF21 treatment on hypoxia-induced
cardiomyocyte injury in vitro. (A) Viability of H9C2 cells
exposed to hypoxia (HX), FGF21 and KLB overexpression (n=5). (B)
Representative images of DHE staining (scale bar, 100 μm),
and DHE intensity was quantified to reflect ROS levels. (C)
Representative TUNEL staining images of KLB-overexpressing H9C2
cells exposed to hypoxia and treated with FGF21 (n=4; scale bar,
100 μm). (D) LDH leak from KLB-overexpressing H9C2 cells
exposed to hypoxia and treated with FGF21 (n=8). (E) JC-1 staining
of H9C2 cells (scale bar, 50 μm). Mitochondrial membrane
potential was estimated by the ratio of JC-1 aggregates (red,
healthy mitochondria) and JC-1 monomers (green, depolarized
mitochondria, n=5). **P<0.01 and
***P<0.001 vs. the control (Con) group;
##P<0.01 and ###P<0.001 vs. hypoxia
(HX). KLB, β-klotho; FGF21, fibroblast growth factor 21; AMI, acute
myocardial infarction; LDH, lactate dehydrogenase. |
Generation and characterization of
KLB@CMBs
CMBs were synthesized to carry plasmids expressing
the KLB gene according to published procedures (10) (Fig. 3A). Dynamic light scattering
revealed that he CMBs had a particle diameter of 1,482±303 nm and a
zeta potential of +24.2±4.5 mV (Fig.
3B). The carrying capacity of the CMBs for the plasmids was
estimated by the addition of 40 μg plasmid DNA to the CMBs,
revealing the maximized carrying capacity of
0.5×109/CBMs (Fig.
3C). Fluorescence microscopy revealed the stabilized
KLB@CMBs with the KLB-expressing plasmid labeled by
propidium iodide (Fig. 3D).
According to the results of flow cytometric analysis, the binding
rate of CMBs to the plasmid reached 55.2% (Fig. 3E). The synthesized KLB@
CMBs that were prepared had a good stability within at least 6 h
under room temperature (Fig.
S2). The distribution of CMBs was visualized in the heart with
echocardiography, demonstrating that the UTMD-triggered CMBs burst
and released the KLB-expressing plasmid in myocardial tissue
(Fig. 3F). At 1 week after the
UTMD-mediated KLB delivery, GFP fluorescence was detected in
the heart tissue (Fig. 3G).
These data confirm that the UTMD delivery of the KLB gene to
the heart is feasible and appropriate for use in following
experiments.
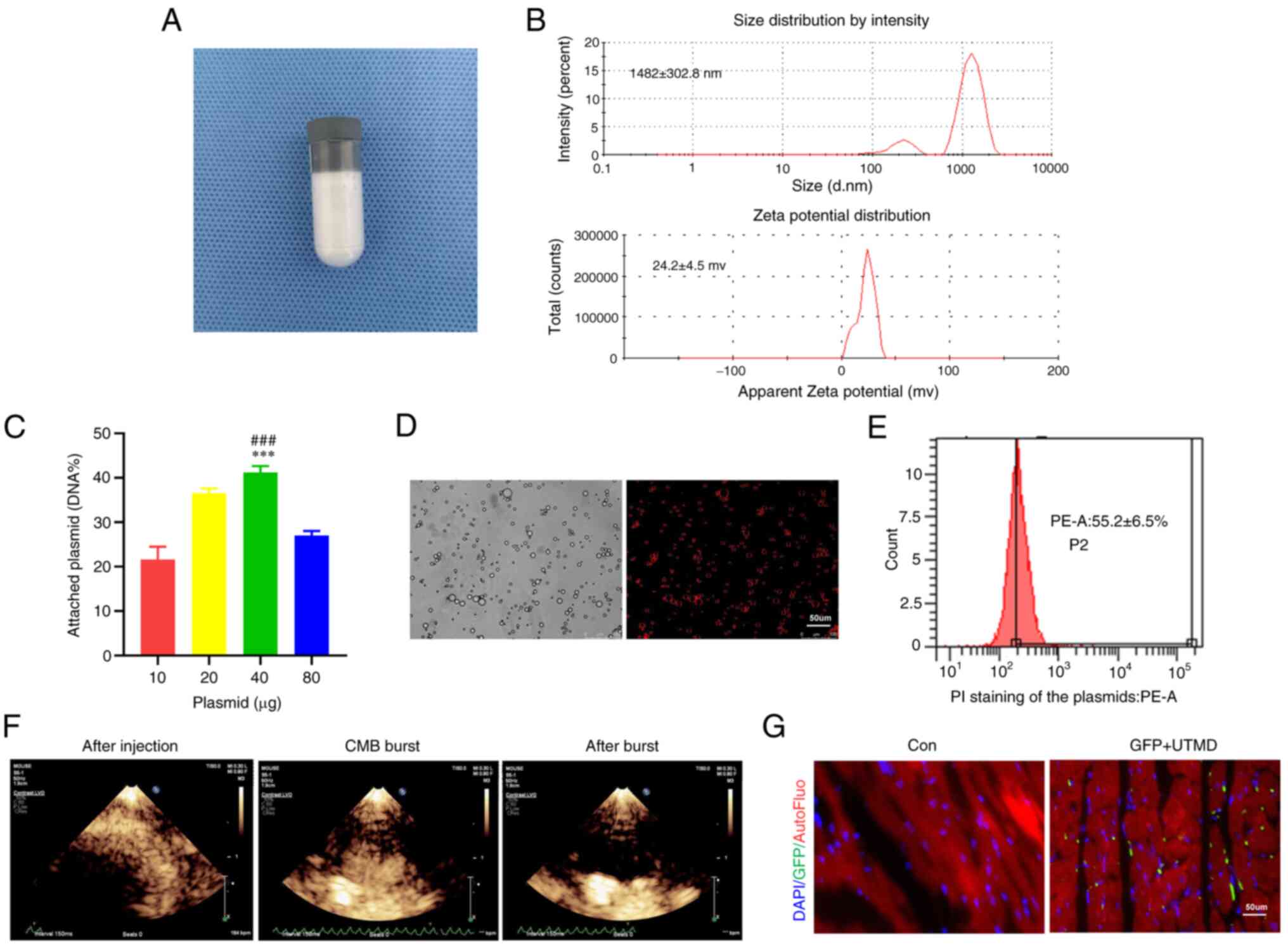 | Figure 3Assessment of plasmid@CMB preparation
and delivery efficiency via UTMD technology. (A) The preparation of
CMB particles. (B) Size and potential distribution of the CMBs. (C)
The binding rates of several doses of plasmid on CMBs (n=4). (D)
Representative images of KLB@CMBs. KLB plasmid labeled by PI
staining (red) and the outline of CMB (bright) are shown (scale
bar, 50 μm). (E) The binding rates of KLB@CMBs were assessed
using flow cytometry (n=4). (F) Typical ultrasound contrast images
of KLB@CMBs in the heart before injection, at ultrasound-targeted
CMB blast, and after the CMB blast. After the CMB injection,
microbubbles with high-echo intensity filled the ventricle chambers
and wall tissue. The second harmonic mode with an
electrocardiograph-mediated trigger was applied to activate
microbubble bursting, and then only a small number of microbubbles
remained and showed a low echo shadow. (G) Fluorescence images of
GFP expression after plasmid containing GFP gene delivered by UTMD
technique (DAPI for nucleus; green for GFP; scale bar, 50
μm). and ***P<0.001 vs. the control (Con)
group; and ###P<0.001 vs. 20 μg plasmid. CMB,
cationic microbubble; UTMD, ultrasound-targeted microbubble
destruction; KLB, β-klotho; FGF21, fibroblast growth factor 21. |
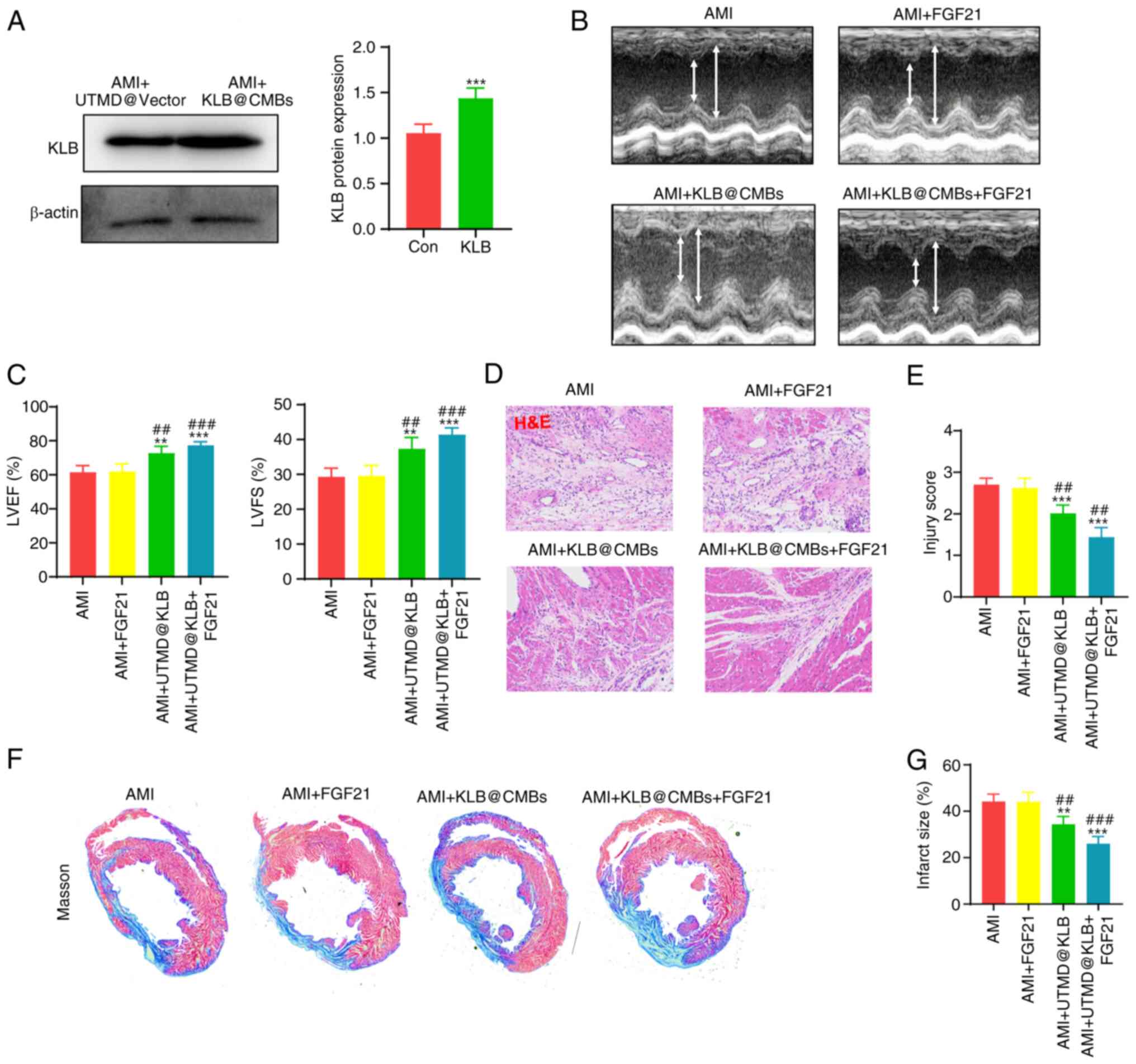
Combined cardiac delivery of KLB@CMBs and
FGF21 administration attenuate heart remodeling
At 1 week after establishing the rat model of MI,
the UTMD-mediated KLB delivery was repeated to the heart
tissues three times. Subsequently, cardiac KLB expression was
assessed, revealing that the UTMD technique significantly increased
the KLB protein levels in myocardial tissue (Fig. 4A). In addition, cardiac structure
and function were examined using echocardiography at 4 weeks
following the induction of MI to determine whether the
UTMD-mediated KLB delivery affects cardiac remodeling.
Indeed, while the intravenous administration of FGF21 did not
significantly improve LVEF and LVFS in the rats post-MI, the
UTMD-mediated cardiac delivery of KLB significantly enhanced
these parameters, amplifying the effects of FGF21 on heart
dysfunction (Fig. 4B and C).
Moreover, H&E staining revealed that the infarct border zone
exhibited an improved tissue structure and lower injury scores in
the FGF21-treated rats with KLB@CMBs delivered by UTMD
(Fig. 4D and E). Masson's
staining was also performed on the cardiac sections to evaluate the
effects of combined treatment with FGF21 and the UTMD-mediated
KLB delivery on the degree of fibrosis. The administration
of FGF21 alone did not significantly reduce the infarct size, while
the cardiac-specific KLB delivery partially improved
collagen fiber alignment in the infarct area (Fig. 4F and G). These data indicated
that the overexpression of KLB in the heart using the UTMD
technique increased FGF21 sensitivity and attenuated adverse
ventricular remodeling following MI.
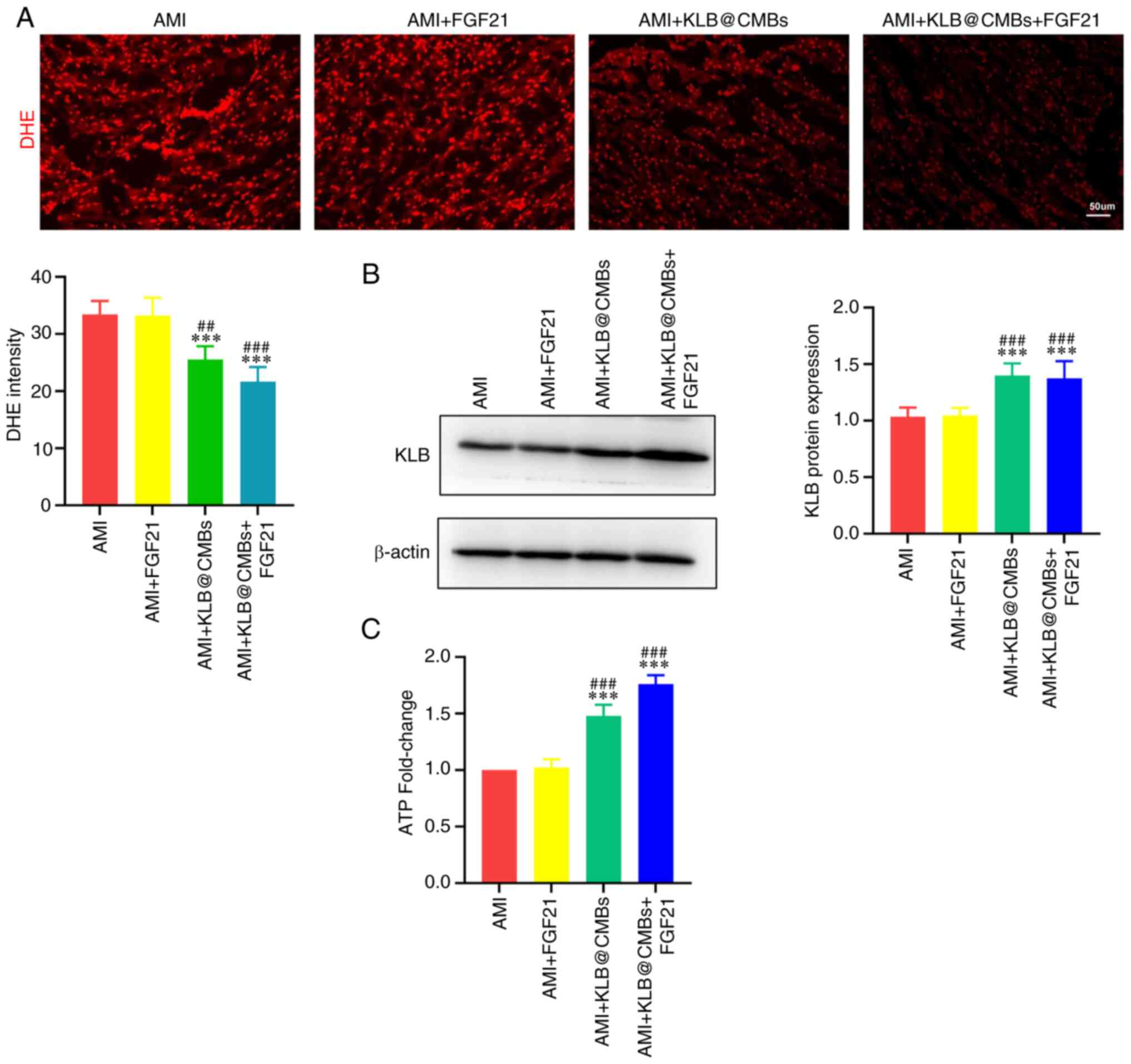
Combined KLB@CMB delivery and FGF21
administration reduce oxidative stress and mitochondrial
dysfunction in heart tissue post-infarction
Previous research has demonstrated that FGF21
regulates mitophagy, mitochondrial dynamics and mitochondrial
metabolism to alleviate mitochondrial stress injury (4). Therefore, the present study
examined whether the FGF21 regulation of mitochondrial function is
dependent on KLB in myocardial tissue. As shown by DHE staining,
whereas the administration of FGF21 did not significantly decrease
myocardial ROS generation, the KLB gene delivery to the
heart markedly reinforced the effects of FGF21 on myocardial ROS
production post-infarction (Fig.
5A). In agreement with this finding, KLB expression was not
affected by FGF21 administration alone (Fig. 5B). Enhanced ATP generation
reflects the re-establishment of mitochondrial energy homeostasis
impaired by mitochondrial dysfunction post-infarction. As was
expected, treatment with FGF21 slightly recovered the ATP levels in
the heart tissues, while the UTMD-mediated KLB delivery
significantly increased the ATP generation post-infarction
(Fig. 5C). Hence, KLB
overexpression, but not FGF21 administration, recovered cardiac ATP
production following AMI, and the combined interventions largely
improved mitochondrial energy. On the whole, these data indicated
that the UTMD-mediated cardiac delivery of KLB promoted
FGF21 sensitivity, recovering mitochondrial homeostasis and
alleviating adverse cardiac remodeling.
FGF21 improves mitochondrial quality via
KLB/NRF2 signals to prevent the progression of heart failure
following AMI
The present study then aimed to investigate how the
combined FGF21 and KLB interventions mitigate oxidative stress and
safeguard mitochondria post-infarction. Nrf2 antioxidant signaling
is a critical protective pathway against intracellular oxidative
injury (10). Thus, the present
study assessed whether combined FGF21 and KLB@CMBs activate
the Nrf2-mediated antioxidant pathway to improve heart dysfunction.
Indeed, FGF21 administration and KLB delivery significantly
sensitized the heart to FGF21 treatment and increased Nrf2
expression, which was inhibited by ML385, an NRF2 inhibitor
(Fig. 6A). By contrast, the
protein expression of kelch-like ECH-associated protein 1 (KEAP1),
an inhibitor of Nrf2 binding, did not significantly decrease after
the combined FGF21 and KLB interventions (Fig. 6A). Therefore, the combined
interventions may prevent heart failure post-infarction by
promoting Nrf2 expression and not reducing KEAP1 expression
(Fig. 6A). As was expected,
KLB delivery and FGF21 treatment increased the expression of
the Nrf2 target proteins, heme oxygenase 1 (HO-1), NAD(P) H quinone
dehydrogenase 1 (NQO1) and glutamate-cysteine ligase modifier
subunit (GCLM) (Fig. 6B).
Conversely, the Nrf2 inhibitor, ML385, significantly abolished
these effects and reduced the expression of these antioxidant
enzymes (Fig. 6B). Furthermore,
it was verified that the ML385 injection significantly reduced the
protective effects of combined FGF21 and KLB@CMB treatment
on cardiac dysfunction in rats post-infarction (Fig. 6C-E). Finally, DHE staining was
performed to assess superoxide anion free radicals, demonstrating
that oxidative stress was moderately attenuated by KLB
cardiac delivery, and the Nrf2 inhibitor compromised the
antioxidant effect of KLB in the heart post-infarction (Fig. 6F and G). Moreover, the total
antioxidant capacity in the heart tissues increased by the
combination of FGF21 and KLB@CMB treatment was attenuated by
the inhibition of Nrf2 (Fig.
S3).
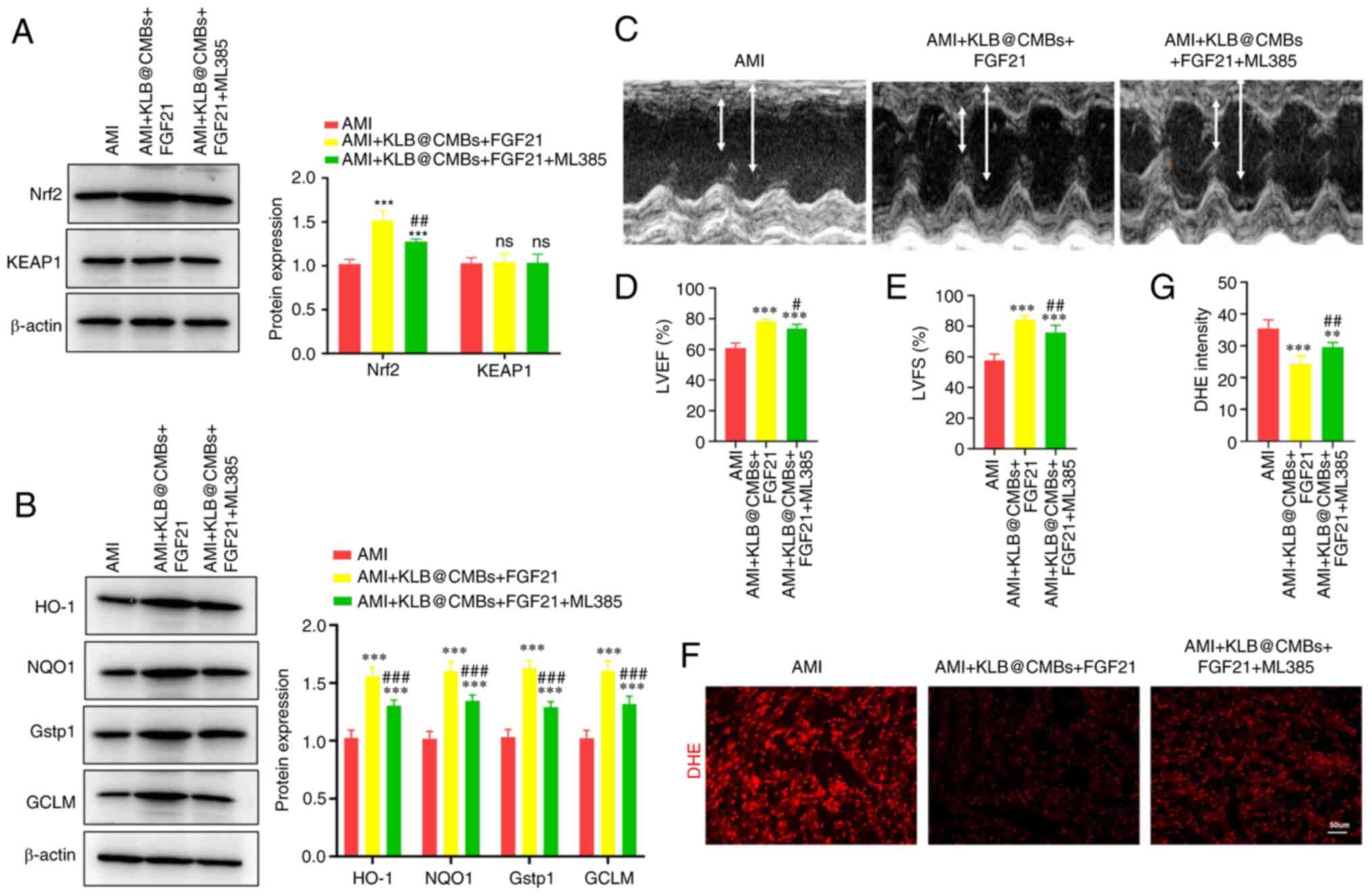 | Figure 6Cardiac delivery of KLB enhances the
antioxidant effects of FGF21 in the heart following AMI. (A)
Western analysis and quantification of Nrf2 and KEAP1 expression in
H9C2 cells (n=4). (B) The protein expression of HO-1, NQO1, Gstp1
and GCLM was assessed using western blot analysis (n=4). (C)
Representative images of echocardiography at 4 weeks following AMI
surgery. (D and E) The LVEF and LVFS were calculated (n=5). (F and
G) DHE staining of the heart section (scale bar, 50 μm) and
quantification (n=5). **P<0.01 and
***P<0.001 vs. AMI; #P<0.05,
##P<0.01 and ###P<0.001 vs. the AMI +
KLB@cMBs + FGF21 group; ns, not significant. AMI, acute myocardial
infarction; KLB, β-klotho; FGF21, fibroblast growth factor 21; CMB,
cationic microbubble; LVEF, left ventricular ejection fraction;
LVFS, left ventricular fractional shortening; Nrf2, nuclear factor
erythroid 2-related factor 2; KEAP1, kelch-like ECH-associated
protein 1; HO-1, heme oxygenase 1; NQO1, NAD(P)H quinone
dehydrogenase 1; Gstp1, glutathione S-transferase pi-1; GCLM,
glutamate-cysteine ligase modifier subunit. |
The present study further evaluated the effects of
Nrf2 inhibition and the FGF21-KLB-mediated activation on
mitochondrial quality. The combined interventions improved the loss
of mitochondrial membrane potential in the myocardial tissues
post-infarction, and this effect was attenuated by the inhibition
of Nrf2 (Fig. 7A and B). The
mitochondrial transmembrane potential reflects mitochondrial
function and impaired mitochondria are the primary source of ROS
production. The mitochondrial oxygen consumption rate (OCR)
revealed that FGF21-KLB boosted the basal respiration level, partly
reduced by Nrf2 inhibitor treatment in the H9C2 cardiomyoblasts
(Fig. 7C). In addition, the
synthetic compound, FCCP, uncoupled mitochondria respiration from
oxidative phosphorylation, indicating a similar difference in the
measurement of maximal OCR (Fig.
7C-G). These results imply that combined GFG21-KLB
interventions improve mitochondrial function in H9C2 cardiomyoblast
cells under hypoxic conditions, and this effect is compromised by
Nrf2 inhibition.
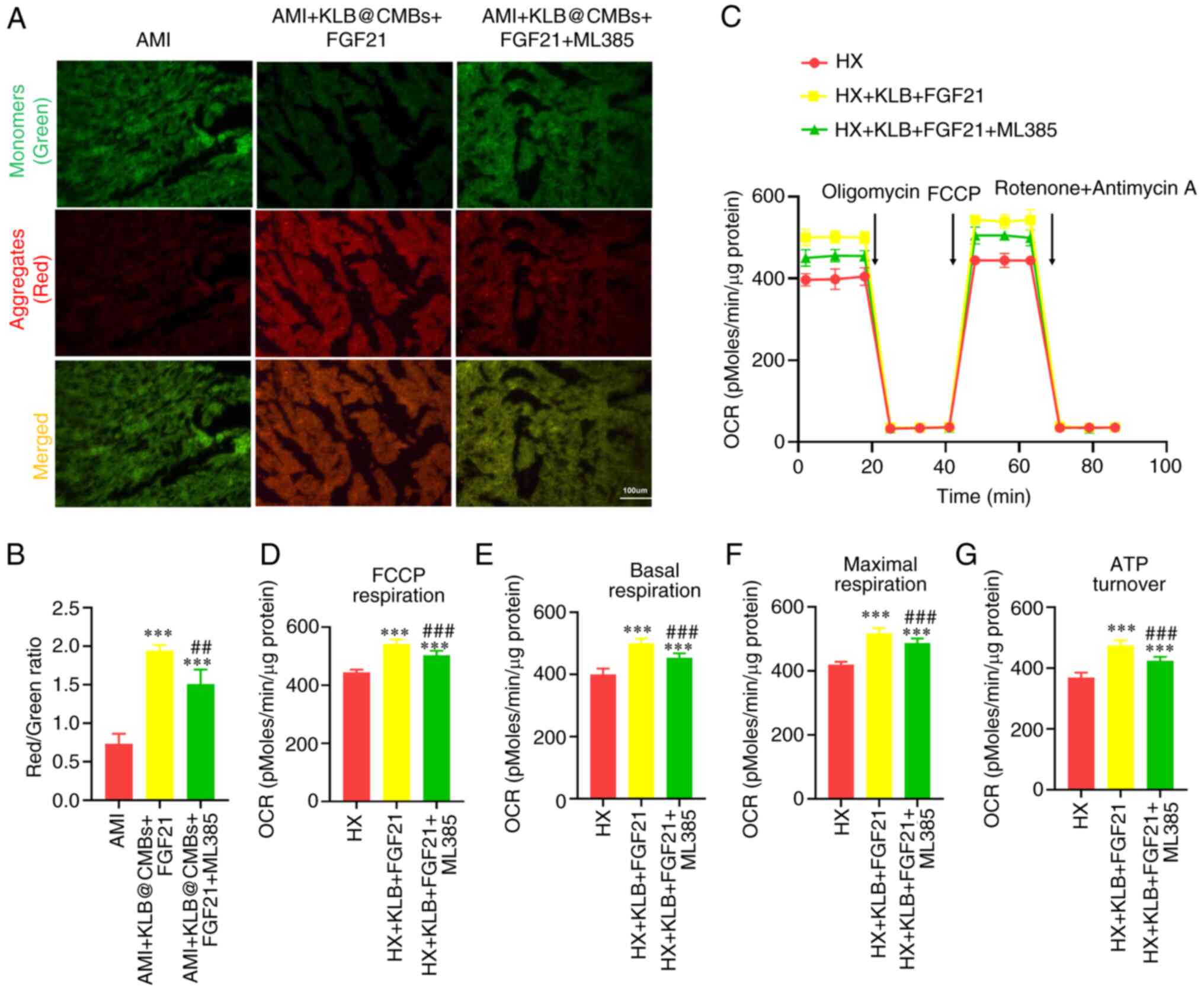 | Figure 7Mitochondrial quality in the heart is
improved by the UTMD-mediated KLB delivery and FGF21 treatment in
rats post-infarction. (A) JC-1 staining of heart section
post-infarction (scale bar, 100 μm). (B) The ratio of JC-1
aggregates (red) and monomers (green) was used to assess
mitochondrial membrane potential (n=5-6). (C) Mitochondrial OCR
profile in H9C2 cells using an XF24 Extracellular Flux Analyzer.
Oligomycin (1 μmol/l), FCCP (4 μmol/l) and rotenone
(0.5 μmol/l) plus antimycin A (0.5 μmol/l) were added
sequentially. (D) FCCP-related respiration, (E) basal respiration,
(F) maximal respiration and (G) ATP turnover were calculated (n=4).
***P<0.001 vs. AMI; ##P<0.01 and
###P<0.001 vs. the AMI + KLB@CMBs + FGF21 group. CMB,
cationic microbubble; UTMD, ultrasound-targeted microbubble
destruction; KLB, β-klotho; FGF21, fibroblast growth factor 21;
OCR, oxygen consumption rate; FCCP, carbonyl cyanide
4-(trifluoromethoxy) phenylhydrazone; AMI, acute myocardial
infarction; HX, hypoxia. |
Discussion
The FGF21 signaling pathway improves insulin
resistance and mitigates mitochondrial stress, exerting a
protective effect on cardiometabolism (8). However, little is known about how
to translate FGF21-targeted interventions into clinical practice
for preventing adverse remodeling. In the present study, it was
discovered that serum FGF21 levels increased, but cardiac KLB
levels decreased following MI infarction in rats. The co-receptor
KLB-dependent pathway was essential for the protective effects of
FGF21 on cardiac adverse remodeling post-infarction. Cardiac KLB
delivery using UTMD technology markedly upregulated KLB protein
expression in heart tissues, sensitizing the myocardium to FGF21
and optimizing its therapeutic effect in rats with AMI beyond FGF21
intervention alone. Our findings offer novel insights into the
translation of FGF21 therapy in cardiac remodeling. Moreover, the
FGF21-KLB pathway was identified as a promising target with which
to prevent HF following MI by alleviating mitochondrial dysfunction
and oxidative stress via Nrf2 activation.
There is evidence to indicate the promising
potential of FGF21 administration in addressing cardiometabolic
disorders, such as obesity, diabetes and ageing (5,6,8,31). Herein, significantly elevated
serum levels of FGF21 were observed in rats post-infarction,
consistent with previous reports that serum FGF21 levels
paradoxically increase in diabetes and its cardiovascular
complications (9,32). Hence, the increased serum levels
of FGF21 and the decreased expression of KLB following MI may
reflect a compensatory response to FGF21 resistance (5). Similarly, cardiac remodeling or HF
is accompanied by the elevated expression of natriuretic peptides,
suggesting that the intravenous injection of natriuretic peptides
could also provide additional benefits to improve HF symptoms
(33). The findings of the
present study and those of other studies suggest that the targeted
restoration of cardiac KLB expression significantly promotes the
activation of FGF21 receptor-dependent pathways in rats post-MI,
closely associated with improvements in cardiometabolic parameters,
suggesting an increase in FGF21 sensitivity (5,34). Treadmill exercise substantially
induces cardiac KLB expression, enhancing cardiomyocyte
responsiveness to FGF21 and reinforcing its therapeutic effect on
the diabetic heart in rat models (5). The present study demonstrated that
FGF21 treatment alone had limited preventive effects on HF
post-infarction, as FGF21 supplementation failed to reverse
mitochondrial dysfunction and cardiac remodeling. Notably, the
therapeutic effectiveness of FGF21 against cardiac dysfunction was
markedly augmented by the UTMD-mediated cardiac KLB
delivery. As patients in the recovery phase post-infarction may not
endure high-intensity exercise (35), the impact of moderate- to
low-intensity exercise on cardiac KLB expression remains unclear.
Nonetheless, the present study provides a feasible approach for
preventing HF progression post-infarction. The findings indicate
that the cardiac-targeted KLB delivery may enhance the FGF21
pathway, conferring the protective effects of FGF21 administration
on cardiomyocytes. In summary, following AMI, UTMD-mediated
delivery to the heart reprograms it into a target organ responsive
to FGF21 by strengthening its receptor-dependent pathway (8), promoting the protective effects of
FGF21 administration on myocardial tissues.
Dysfunctional mitochondria are the primary sites of
ROS generation and oxidative stress in the heart post-infarction,
playing a crucial role in the pathological progression of adverse
cardiac remodeling (30).
Mitochondrial dysfunction and structural abnormalities are observed
in several common types of heart diseases, including hypertrophic
cardiomyopathy, diabetic cardiomyopathy and tumor-related cardiac
injury (36). These disorders
are strongly associated with abnormalities in cardiac energy
metabolism, a decreased ATP production and deteriorating cardiac
function (1,5). Mitochondrial structural anomalies
and elevated levels of oxidative stress have been observed in
cardiac biopsies from patients with MI (1). In the present study, in rat hears
post-MI, significant mitochondrial functional abnormalities,
excessive ROS production and a disrupted energy homeostasis were
observed. These features were reversed in the rats with AMI exposed
to the combined FGF21-KLB interventions, but not in the rats
stimulated only with FGF21. Hence, the UTMD-mediated cardiac
delivery of KLB helps restore FGF21 signaling, mitigating
the impact of oxidative stress and mitochondrial dysfunction in
myocardium post-MI. In agreement with previous research, targeting
mitochondrial homeostasis is a potential therapeutic approach with
which to attenuate adverse myocardial remodeling (3).
The Nrf2 protein is a primary transcription factor
controlling the expression of various genes encoding antioxidant
factors, detoxifying enzymes, drug transporters and other
cytoprotective proteins (37).
It potentially enhances the antioxidant signals against
accumulative oxidative stress and regulates various aspects of
energy metabolism and mitochondrial function (38). Specifically, Nrf2 enhances
mitochondrial metabolism and the efficiency of ATP production by
improving oxidative phosphorylation (38). In the present study, the combined
FGF21-KLB interventions promoted the balance between oxidant and
antioxidant signals by upregulating Nrf2 expression and downstream
signals. When Nrf2 was inhibited, the protective effects of FGF21
administration and KLB delivery on cardiac function
following infarction were significantly attenuated. Thus, these
findings provide new insight into the role and mechanisms of the
FGF21-KLB pathway in the heart post-infarction. The
cardiac-targeted delivery of KLB assists FGF21
administration, significantly elevating antioxidant signals and
inhibiting mitochondrial injury, which may be a potential strategy
with which to prevent HF following AMI (8).
UTMD is an emerging technique for target-specific
gene delivery with possible clinical utility (15). Compared to traditional MBs, CMBs
exhibit a strong affinity for negatively charged plasmids,
significantly enhancing the efficiency of gene delivery (10). The energy of low-frequency
ultrasonic-mediated blasting is unlikely to cause cell oxidative
injury (10). The present study
combined the favorable features of different interdisciplinary
patterns to effectively and safely carry genes to the heart. The
findings presented herein confirm that the UTMD-mediated KLB
delivery to the heart is a promising translational approach to
optimize the effect of FGF21 administration. Consistent with
previous research (8), the
cardiac expression of KLB was significantly increased by
target the blasting of CMBs, indicating that targeted gene delivery
with CMBs and UTMD has promising prospects for clinical translation
(13). Indeed, UTMD-mediated
gene delivery is validated in translational studies involving islet
regeneration in primates (39).
Although other studies have found increased levels of circulating
FGF21 following MI (9), the
cardiac benefit of additional FGF21 supplementation is limited,
raising concerns regarding the clinical translation of FGF21
therapy. The findings presented herein offer a feasible approach
for addressing the undesirable effects of FGF21 therapy through
UTMD-mediated receptor delivery. The combined KLB@CMB and
FGF21 interventions effectively atteniated myocardial oxidative
stress and mitochondrial damage induced post-infarction,
significantly improving cardiac dysfunction and adverse
remodeling.
In conclusion, the findings of the present study
demonstrate that KLB co-receptor expression is significantly
reduced in the myocardium following AMI, explaining the negligible
effect of FGF21 in cardiometabolic disorders. A UTMD delivery
system with CMBs carrying the KLB gene to the heart assists
FGF21 treatment against cardiac adverse remodeling following AMI.
As the targeted KLB delivery promotes the protective effect
of FGF21 administration on the heart post-infarction, these results
may provide distinctive perspectives on the translation of FGF21
administration into clinical; practice. Considering the excellent
efficiency and biosafety of the UTMD system, the synergistic effect
of FGF21 and KLB@CMBs appears to be a promising
interdisciplinary approach for the prevention of heart dysfunction
following AMI.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
CY, RLi and MY substantially contributed to the
experimental conception and design of the study. CL, DZ and SL
contributed to the acquisition of clinical data. CY, YY and TY
contributed to the acquisition of data and analysis from in
vitro experiments. CY, RLi, XH, YL, RLe, QY and QL contributed
to the animal experiment. RLi and CL contributed to statistical
analysis and visualization. CY and RLi were responsible for
manuscript writing. All authors have read and approved the final
manuscript. MY supervised all experiments. All authors took the
responsibility for the integrity and accuracy. CY and RLi confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The clinical study protocols were approved by the
Ethics Review Board of Qujing First People's Hospital, Qujing,
China (approval no. QJFH2021-017). Written informed consent was
obtained from all participants prior to enrollment. Ethical
approval for the animal experiments was obtained from the Ethical
Committee of Second Hospital of Kunming Medical University
(approval no. kmmu 20211022 and kmmu 20221592). All animal
experiments conducted in the present study followed the guidelines
and regulations specified in this ethical approval.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Special Foundation for
Basic Research Program of Yunnan Province Science and Technology
Department and Kunming Medical University (grant no.
202201AY070001-217) and the Innovation Project of Qujing First
People's Hospital (grant no. 2022YJKTY05).
References
|
1
|
Ramachandra C, Hernandez-Resendiz S,
Crespo-Avilan GE, Lin YH and Hausenloy DJ: Mitochondria in acute
myocardial infarction and cardioprotection. EBioMedicine.
57:1028842020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang S, Duan Y, Feng X, Liu L, Shi Z, Wang
B, Xia C, Man W, Wang H, Zhao Z and Sun D: Sustained nicorandil
administration reduces the infarct size in ST-segment elevation
myocardial infarction patients with primary percutaneous coronary
intervention. Anatol J Cardiol. 21:163–171. 2019.PubMed/NCBI
|
|
3
|
Wang S, Zhao Z, Fan Y, Zhang M, Feng X,
Lin J, Hu J, Cheng Z, Sun C, Liu T, et al: Mst1 inhibits Sirt3
expression and contributes to diabetic cardiomyopathy through
inhibiting Parkin-dependent mitophagy. Biochim Biophys Acta Mol
Basis Dis. 1865:1905–1914. 2019. View Article : Google Scholar
|
|
4
|
Chen S, Guan S, Yan Z, Ouyang F, Li S, Liu
L and Zhong J: Role of RIPK3-CaMKII-mPTP signaling pathway-mediated
necroptosis in cardiovascular diseases (review). Int J Mol Med.
52:982023. View Article : Google Scholar :
|
|
5
|
Jin L, Geng L, Ying L, Shu L, Ye K, Yang
R, Liu Y, Wang Y, Cai Y, Jiang X, et al: FGF21-sirtuin 3 axis
confers the protective effects of exercise against diabetic
cardiomyopathy by governing mitochondrial integrity. Circulation.
146:1537–1557. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao Z, Cui Y, Xu X and Han T: FGFR
redundancy limits the efficacy of FGFR4-selective inhibitors in
hepatocellular carcinoma. Proc Natl Acad Sci USA.
119:e22088441192022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romanello V: The interplay between
mitochondrial morphology and myomitokines in aging sarcopenia. Int
J Mol Sci. 22:912020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jimenez V, Jambrina C, Casana E, Sacristan
V, Munoz S, Darriba S, Rodó J, Mallol C, Garcia M, León X, et al:
FGF21 gene therapy as treatment for obesity and insulin resistance.
EMBO Mol Med. 10:e87912018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Chu S, Ding W and Wang F: Serum
level of fibroblast growth factor 21 is independently associated
with acute myocardial infarction. PLoS One. 10:e01297912015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Chen K, Wang Y, Wang Z, Li Z, Guo
J, Chen J, Liu W, Guo X, Yan G, et al: Cardiac-targeted delivery of
nuclear receptor RORα via ultrasound targeted microbubble
destruction optimizes the benefits of regular dose of melatonin on
sepsis-induced cardiomyopathy. Biomater Res. 27:412023. View Article : Google Scholar
|
|
11
|
Li H, Zhang Y, Shu H, Lv W, Su C and Nie
F: Highlights in ultrasound-targeted microbubble
destruction-mediated gene/drug delivery strategy for treatment of
malignancies. Int J Pharm. 613:1214122022. View Article : Google Scholar
|
|
12
|
Zheng J, Huang J, Zhang L, Wang M, Xu L,
Dou X, Leng X, Fang M, Sun Y and Wang Z: Drug-loaded microbubble
delivery system to enhance PD-L1 blockade immunotherapy with
remodeling immune microenvironment. Biomater Res. 27:92023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omata D, Unga J, Suzuki R and Maruyama K:
Lipid-based microbubbles and ultrasound for therapeutic
application. Adv Drug Deliv Rev. 154-155:236–244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beccaria K, Sabbagh A, de Groot J, Canney
M, Carpentier A and Heimberger AB: Blood-brain barrier opening with
low intensity pulsed ultrasound for immune modulation and immune
therapeutic delivery to CNS tumors. J Neurooncol. 151:65–73. 2021.
View Article : Google Scholar
|
|
15
|
Carpentier A, Canney M, Vignot A, Reina V,
Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon
JY, et al: Clinical trial of blood-brain barrier disruption by
pulsed ultrasound. Sci Transl Med. 8:343re22016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen K, Bai L, Lu J, Chen W, Liu C, Guo E,
Qin X, Jiao X, Huang M and Tian H: Human decidual mesenchymal stem
cells obtained from early pregnancy improve cardiac
revascularization postinfarction by activating ornithine
metabolism. Front Cardiovasc Med. 9:8377802022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Zhou J, Luo Y, Li J, Shang L, Zhou
F and Yang S: Honokiol alleviates LPS-induced acute lung injury by
inhibiting NLRP3 inflammasome-mediated pyroptosis via Nrf2
activation in vitro and in vivo. Chin Med. 16:1272021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Z, Ju F, Du L and Abbott GW:
Empagliflozin protects the heart against
ischemia/reperfusion-induced sudden cardiac death. Cardiovasc
Diabetol. 20:1992021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Safari F, Hajizadeh S, Shekarforoush S,
Bayat G, Foadoddini M and Khoshbaten A: Influence of ramiprilat and
losartan on ischemia reperfusion injury in rat hearts. J Renin
Angiotensin Aldosterone Syst. 13:29–35. 2012. View Article : Google Scholar
|
|
20
|
Tang MN, Hu JL and Yan Y: The efficacy and
mechanism of renal denervation (RD) versus drugs in treating
post-acute myocardial infarction (AMI) heart failure (HF) in rats.
Fudan Univ J Med Sci. 46:584–591. 2019.
|
|
21
|
Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen
M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, et al:
Fibroblast growth factor 21 reverses hepatic steatosis, increases
energy expenditure, and improves insulin sensitivity in
diet-induced obese mice. Diabetes. 58:250–259. 2009. View Article : Google Scholar :
|
|
22
|
Du GQ, Shao ZB, Wu J, Yin WJ, Li SH, Wu J,
Weisel RD, Tian JW and Li RK: Targeted myocardial delivery of GDF11
gene rejuvenates the aged mouse heart and enhances myocardial
regeneration after ischemia-reperfusion injury. Basic Res Cardiol.
112:72017. View Article : Google Scholar
|
|
23
|
Muskula PR and Main ML: Safety with
echocardiographic contrast agents. Circ Cardiovasc Imaging.
10:e0054592017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borrelli MJ, O'Brien WJ Jr, Hamilton E,
Oelze ML, Wu J, Bernock LJ, Tung S, Rokadia H and Culp WC:
Influences of microbubble diameter and ultrasonic parameters on in
vitro sonothrombolysis efficacy. J Vasc Interv Radiol.
23:1677–1684.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Jiang S, Li S, Yu W, Chen J, Yu D,
Zhao C, Li Y, Kang K, Wang R, et al: Targeted galectin-7 inhibition
with ultrasound microbubble targeted gene therapy as a sole therapy
to prevent acute rejection following heart transplantation in a
rodent model. Biomaterials. 263:1203662020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang S, Zhao Z, Feng X, Cheng Z, Xiong Z,
Wang T, Lin J, Zhang M, Hu J, Fan Y, et al: Melatonin activates
Parkin translocation and rescues the impaired mitophagy activity of
diabetic cardiomyopathy through Mst1 inhibition. J Cell Mol Med.
22:5132–5144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aishwarya R, Alam S, Abdullah CS, Morshed
M, Nitu SS, Panchatcharam M, Miriyala S, Kevil CG and Bhuiyan MS:
Pleiotropic effects of mdivi-1 in altering mitochondrial dynamics,
respiration, and autophagy in cardiomyocytes. Redox Biol.
36:1016602020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Wang S, Fan Y, Feng X, Sun C, Shi Z, Li T,
Lv J, Yang Z, Zhao Z and Sun D: Nicorandil alleviates myocardial
injury and post-infarction cardiac remodeling by inhibiting Mst1.
Biochem Biophys Res Commun. 495:292–299. 2018. View Article : Google Scholar
|
|
31
|
Burtscher J, Soltany A, Visavadiya NP,
Burtscher M, Millet GP, Khoramipour K and Khamoui AV: Mitochondrial
stress and mitokines in aging. Aging Cell. 22:e137702023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang M, Liu C, Jiang N, Liu Y, Luo S, Li
C, Zhao H, Han Y, Chen W, Li L, et al: Fibroblast growth factor 21
in metabolic syndrome. Front Endocrinol (Lausanne). 14:12204262023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsutsui H, Albert NM, Coats A, Anker SD,
Bayes-Genis A, Butler J, Chioncel O, Defilippi CR, Drazner MH,
Felker GM, et al: Natriuretic peptides: Role in the diagnosis and
management of heart failure: A scientific statement from the heart
failure association of the European society of cardiology, heart
failure society of America and Japanese heart failure society. Eur
J Heart Fail. 25:616–631. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei YJ, Wang JF, Cheng F, Xu HJ, Chen JJ,
Xiong J and Wang J: miR-124-3p targeted SIRT1 to regulate cell
apoptosis, inflammatory response, and oxidative stress in acute
myocardial infarction in rats via modulation of the
FGF21/CREB/PGC1α pathway. J Physiol Biochem. 77:577–587. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bäck M, Caldenius V, Svensson L and
Lundberg M: Perceptions of kinesiophobia in relation to physical
activity and exercise after myocardial infarction: A qualitative
study. Phys Ther. 100:2110–2119. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang S, Liu Y, Liu J, Tian W, Zhang X, Cai
H, Fang S and Yu B: Mitochondria-derived methylmalonic acid, a
surrogate biomarker of mitochondrial dysfunction and oxidative
stress, predicts all-cause and cardiovascular mortality in the
general population. Redox Biol. 37:1017412020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Silva-Palacios A, Ostolga-Chavarria M,
Zazueta C and Königsberg M: Nrf2: Molecular and epigenetic
regulation during aging. Ageing Res Rev. 47:31–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Esteras N and Abramov AY: Nrf2 as a
regulator of mitochondrial function: Energy metabolism and beyond.
Free Radic Biol Med. 189:136–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang C, Chen S, Li Q, Wu J, Qiu F, Chen
Z, Sun Y, Luo J, Bastarrachea RA, Grayburn PA, et al:
Ultrasound-targeted microbubble destruction mediates gene
transfection for beta-cell regeneration and glucose regulation.
Small. 17:e20081772021. View Article : Google Scholar : PubMed/NCBI
|