Proprotein convertase subtilisin kexin type 9
(PCSK9) influences lipid metabolism by accelerating the degradation
of low-density lipoprotein (LDL) receptor (LDLR) (1). The clearance of human
LDL-cholesterol (LDL-C) depends on the circulation of LDLR in the
liver (2). PCSK9, synthesised in
hepatocytes, is subsequently secreted and binds to specific LDLRs
on hepatocytes, thus forming a trimolecular complex with LDLR and
LDL. The PCSK9-LDLR-LDL complexes prevent the recycling of LDLR to
the surface of hepatocytes, resulting in elevated plasma levels of
LDL-C (3,4). LDL-C is a crucial factor in
atherosclerosis and cardiovascular events. In the subendothelial
space, LDL-C undergoes oxidation and aggregation, and its small and
dense metabolites enter the arterial wall, leading to the
progression of atherogenesis and major adverse cardiovascular
events (MACEs) (5).
In the early stages, PCSK9 research predominantly
centred on abnormal lipid metabolism. In 2000, a locus for
autosomal dominant hypercholesterolaemia was found within the
PCSK9 gene (human chromosome 1p33-p34.3) (6), later established as the causative
gene for familial hypercholesterolaemia in 2003 (7). Subsequently, naturally occurring
PCSK9 nonsense mutations were detected and associated with
significantly low LDL-C levels and the risk of developing
cardiovascular disease shortly thereafter. Currently, over a
hundred mutations influencing PCSK9 function have been identified
(8). Since the launch of the
first PCSK9 inhibitor in 2015, unexpected effects have been
progressively unveiled. In 2020, patients undergoing treatment with
PCSK9 inhibitor were found to exhibit a reduced platelet reactivity
and increased sensitivity to aspirin suppression (9). Later that year, it was first
confirmed that short-term treatment with evolocumab significantly
ameliorated coronary endothelial dysfunction (10). Mechanistic studies on the impact
of PCSK9 on thrombosis and inflammation have also garnered
attention during this period (please see section 4 below). Beyond
atherosclerotic heart disease, the connection between PCSK9 and
heart failure, atrial fibrillation and calcific aortic valve
disease has come to light (11).
The association between PCSK9 and cardiovascular events appears to
transcend lipid levels, yet critical pieces of the puzzle are still
missing to explain these findings.
Thrombosis and haemostasis are the leading causes of
premature deaths. Apart from increasing plasma levels of LDL-C,
PCSK9 regulates the hypercoagulable state and thrombosis processes
in disease states. Subsequent studies have underscored this point,
indicating that PCSK9 levels in diabetic patients following
percutaneous coronary intervention (PCI) were independently
associated with MACEs, including thromboembolism risk, but no such
association was found in non-diabetic patients (12). The mechanism behind this
phenomenon has yet to be elucidated, thereby hindering the
tailoring of personalized therapies for patients with concurrent
metabolic disorders to achieve further mitigation of residual
cardiovascular risk. PCSK9 inhibitors are expected to reach US$
15,140.3 million by the year 2034, growing at an annual rate of
18.7% (13). The population of
patients receiving PCSK9 inhibitors with multiple diagnoses is
rapidly increasing. Hence, comprehending the role of PCSK9 in
disease states is paramount for enhancing the cost-effectiveness
and quality of life for hyperlipidaemia patients globally.
The present review focuses on the effects of changes
in PCSK9 levels on thrombosis and haemostasis, and the related
modulatory mechanisms of platelet function, coagulation factor
activity, inflammatory response and endothelial function in
pathological conditions. The aim of the present review was not to
comprehensively cover all information regarding the effects of
PCSK9 on thrombosis and haemostasis, but rather to focus on disease
states, as it was considered that these warrant further extensive
research and may have a potential influence on the drug market,
regarding the expansion of indications, dose adjustment and drug
interactions.
The prodomain is not only essential for the correct
folding PCSK9 in the endoplasmic reticulum, but is also related to
the lack of activity against exogenous substrates (15). The C-terminus Gln152 of the
prodomain binds to His226, filling the oxyanion hole between Asn317
and Ser386 and shielding the active site cleft, thus inhibiting
interaction with other proteins and peptides (16). Consequently, the activity of
PCSK9 is manifested through its binding to target substrates and
escorting the complexes into intracellular degradation
compartments, rather than as a protein hydrolase. The prodomain
remains in the catalytic groove after self-cleavage, hindering the
binding of PCSK9 to other proteins. Removal of the prodomain
increases the affinity of PCSK9 for LDLR by 10-fold (17). The PCSK9 Arg93Cys variant is
associated with a reduced risk of premature myocardial infarction
(18).
The catalytic domain of PCSK9 (aa 153-421) contains
essential active sites of PCSK9 and comes into contact with the
epidermal growth factor-A (EGF-A) domain of the LDLR, resulting in
a ~1,000 Å2 interface (19). As the complex PCSK9-LDLR
transitions from extracellular (high pH) to endosomes/lysosomes
(low pH), the conformation of PCSK9 due to the pH changes. The
difference between the closed and extended conformations revolves
around the residue Ser376, which serves as a hinge connecting LDLR.
It has been observed that PCSK9 Asp374 forms an extra salt bridge
with the EGF-A His306 side chain at a low pH (20). The binding affinity of PCSK9 for
LDLR exhibits an increase, from Kd=750±80 nM at pH 7.5 to Kd=8±1 nM
at pH 5.5, thus hindering the recycling of LDLR to the cell surface
(20). However, the structure of
CD36, an important ligand of PCSK9, does not exhibit the EGF-A-like
domain, thus the respective binding sequences have yet to be
confirmed (14). Variants in the
catalytic domain lead to PCSK9 loss or gain of function (21,22).
The hinge region (aa 422-452) links the CHRD. The
absence of the CHRD does not affect the binding of PCSK9 to LDLR at
a neutral pH (20). However,
there is an interaction between CHRD and ligand-binding domain of
LDLR at low pH, assisting in the formation of the complex (23). The CHRD comprises three similar
modules (M1: aa 453-529, M2: aa 530-603, and M3: aa 604-692), each
forming a six-stranded two-sheet β-sandwich. Asn533 in the M2
module is the only glycosylation site in PCSK9, but it is not
necessary for PCSK9 activity (16). The three modules exhibit a
structural homology to the homotrimeric form of resistin (24). Resistin is an adipocyte-specific
hormone associated with hypercoagulative, hypofibrinolytic
activities and insulin resistance (25,26).
In addition to reducing cardiovascular events by
decreasing LDL-C in a dose-dependent manner, PCSK9 inhibition
exerts a cardiovascular protective effect beyond lipid management.
For decades, statins were known as first-line lipid-lowering agents
and for the protection of cardiovascular health in hyperlipidaemia.
However, the benefits of statins in patients with resistance or
intolerance are limited (40).
Researchers have observed the plaque stabilization and regression
of atherosclerosis using CT angiography and intravascular
ultrasound in patients treated with PCSK9 inhibitors (41,42). With the advent of inclisiran, a
siRNA PCSK9 inhibitor, appearing on the market, the rate of major
adverse cardiovascular events is expected to decrease by as much as
24-30% (43). The effects of
PCSK9 on oxidative stress, endothelial injury, inflammation and
immunity have been widely described (44-46). The improvement in MACEs due to
PCSK9 inhibition may be attributed to various reasons; primarily of
interest are the potential direct and indirect roles of PCSK9 in
thrombosis and haemostasis.
A high level of PCSK9 has been found to increase
platelet reactivity and significantly increase the risk of
ischaemic events (47). The
results of the Further Cardiovascular Outcomes Research with PCSK9
Inhibition in Subjects with Elevated Risk (FOURIER) study (34) demonstrated that the incidence of
venous thromboembolism (VTE) was decreased in patients treated with
PCSK9 inhibitors, which cannot be completely explained by
lipid-lowering (48,49). In another study, several platelet
activation markers were observed to be significantly reduced after
12 months of PCSK9 monoclonal antibody therapy in patients with
hypercholesterolaemia (9),
indicating a potential impact of PCSK9 inhibition on platelet
function. Additionally, circulating platelets store PCSK9 and
release it upon activation, which may promote platelet aggregation
and thrombus formation (50).
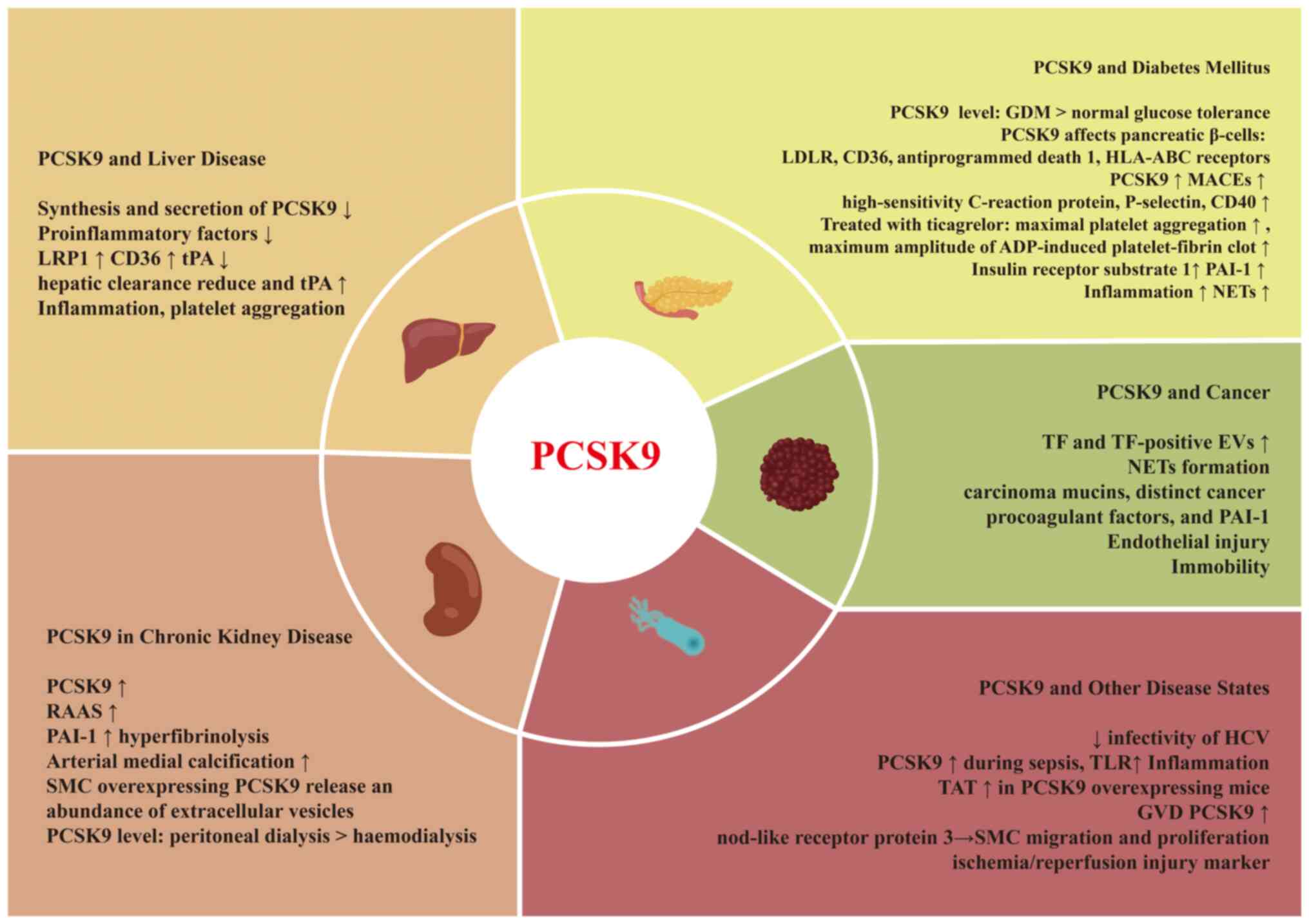
Abnormal PCSK9 levels are present in several disease
states. Patients on maintenance dialysis and following successful
renal transplantation have been shown to have higher serum levels
of PCSK9 than subjects without chronic kidney disease (51). Patients with liver cirrhosis
exhibit lower serum PCSK9 levels, while these levels are increased
in patients with hepatitis C virus (HCV)-induced liver cirrhosis
(52). PCSK9 levels increase
with the severity of breast cancer (53). These specific pathological
conditions may lead to changes in thrombosis or haemostasis.
Combined with the regulatory role of PCSK9 in thrombosis and the
difference in the level of the disease states, thrombosis in
metabolism-related diseases may also be regulated by PCSK9, and its
regulatory pathway may differ from that in the physiological
state.
Platelet reactivity, coagulation factor activity and
endothelial function are crucial factors affecting thrombosis and
haemostasis. Inflammation has been found to contribute to
thrombosis mechanisms over the past decade (54). Several studies have proven that
PCSK9 has an essential effect on these processes, which may provide
a new direction for studying the regulatory mechanisms of
thrombosis or bleeding in particular disease states.
Compared to other LDL-C-lowering drugs, where a
reduction of 1 mmol/l is associated with a relative risk (RR) of
0.77 [95% confidence interval (CI), 0.75-0.79] for major vascular
events, the estimated RR for PCSK9 inhibitors is lower at 0.49 (95%
CI, 0.34-0.71) (63). This
suggests that although the LDL-C-lowering effect is undoubtedly a
key contributor to the haemostasis and thrombosis benefits of PCSK9
inhibition, PCSK9 has also been found to stimulate platelet
activation beyond lipids (Fig.
2). First, scavenger receptor CD36, a major platelet
glycoprotein, plays a crucial role in dyslipidaemia and the
prothrombotic phenotype (64).
PCSK9 in the plasma directly combines with CD36 on platelets,
thereby enhancing Src kinase phosphorylation. This activation
triggers NAD phosphate oxidase-2 (NOX-2), leading to the increased
generation of reactive oxygen species (ROS) and the subsequent
activation of MAPK-ERK5, ultimately promoting platelet
hyperactivity (65,66). Moreover, NOX-2 induces the
generation of 8-ISO-prostaglandin F2α, which forms platelet
aggregation via GP-IIb/IIIa activation (67). Second, PCSK9 binding to CD36 also
activates the JNK and p38 MAPK pathways. The activation of p38 MAPK
stimulates cytosolic phospholipase A2, which releases arachidonic
acid from phospholipid membranes. Arachidonic acid is then
converted into thromboxane A2 (TXA2) through the
cyclooxygenase (COX)-1 and Tx synthase pathways (66). TXA2 acts
synergistically with platelet agonists to enhance platelet
aggregation by activating GP-IIb/IIIa receptors. TXA2 is
rapidly metabolised to TXB2, which can be detected in
urine as 11-dehydro-TxB2 by liver metabolism, indicating
platelet activation in patients (68). Third, positive feedback interplay
was found between PCSK9 expression and oxidised LDL (oxLDL)
generation (69). Elevated
levels of oxLDL combined with CD36 and lectin-like oxLDL receptor 1
(LOX-1) follow a pattern similar to that of PCSK9, leading to
platelet aggregation and secretion (70). A recent study demonstrated that
inclisiran inhibited human umbilical vein endothelial cell death
induced by oxLDL, suggesting a potential role in the treatment of
atherosclerosis (71). Finally,
the aforementioned mechanism enhances integrin αIIbβ3 activation,
P-selectin release, agonist-induced platelet aggregation and clot
retraction (65). The PCSK9
regulation of platelets may partly influence the haemostasis
process in liver disease.
Another mechanism by which the haemostasis process
is impacted by PCSK9 involves blood coagulation factors.
Coagulation factor VIII (FVIII) is unequivocally a vital
participant in the intrinsic pathway, where the lack of FVIII may
cause haemophilia (72).
Together with LDLR, LDLR-related protein (LRP) modulates FVIII
levels (73). Although there are
still no experimental results in support of this premise, it is
possible that PCSK9 indirectly increases the circulation of FVIII,
given its considerable effect on LDLR reduction (73). Additionally, PCSK9 augments
plasma FVIII levels in vivo by decreasing LRP-1 expression
(74). LRP-1 is also associated
with TF levels on the cell surface. TF downregulation occurs
through rapid internalization when the factor VII (FVII)/TF complex
bridges LRP-1 (75).
Furthermore, PCSK9 induces the de novo synthesis of TF,
attributed to the TF/FVIII interaction via the Toll-like receptor 4
(TLR4)/nuclear factor κB (NF-κB) signalling pathway (76), thereby initiating the extrinsic
pathway, where abnormal TF levels may contribute to coagulation
initiation.
The increased expression of PCSK9 has been reported
to contribute to lower a plasma prothrombin time (PT) and to also
be associated with a higher risk of MACEs (77). It is known that PT is crucial for
exposing the activation of the extrinsic pathway; thus, a low PT
suggests that TF and FVII could be in abnormal states (77). It is possible that PCSK9 could
promote the formation of the TF-FVII complex.
PCSK9 affects fibrinolysis through plasminogen
activator inhibitor-1 (PAI-1). Levine et al (78) performed a series of experiments
to support this view. RNA sequencing revealed that TM5614, a PAI-1
inhibitor, led to an 86% gene transcript downregulation of PCSK9 in
mice. Similar results were also found in vivo in mice with
hyperlipidaemia. Furthermore, the authors of that study observed
that patients with heart failure treated with PCSK9 monoclonal
antibodies exhibited a substantial increase in plasma PCSK9 and a
reduction in plasma PAI-1 levels (78).
In addition, fibrinogen is also known as coagulation
factor I. Candidate gene analysis detected significant associations
between total fibrinogen and polymorphisms of PCSK9 and LDLR genes
(79). PCSK9 levels were found
to be associated with fibrinogen in patients with isolated
hyperlipidaemia and stable coronary atherosclerosis (80,81). However, studies on familial
hypercholesterolaemia found that PCSK9 inhibitors did not affect
fibrinogen and D-dimer levels (82). Conflicting evidence suggests that
the association between PCSK9 and fibrinogen should be further
investigated for detailed analysis.
Inflammation plays a cruical role in atherosclerotic
plaque and thrombosis. As a result, persistent inflammation is
strongly linked to higher risk of ischaemic events (83). Anti-inflammatory therapy can
significantly reduce the risk of recurrent cardiovascular events
without affecting lipid levels in patients with previous myocardial
infarction (84). The PCSK9
pathway promotes the inflammatory response and plays a role in in
the thrombosis and atherosclerosis processes (85,86).
PCSK9 is associated with key inflammatory markers,
such as white blood cells, fibrinogen and high-sensitivity
C-reactive protein (45).
However, the underlying mechanisms remain unclear. The increased
levels of adhesion molecules and cytokines (e.g., PF4) expressed by
endothelial cells under atherosclerosis lead to the attachment of
monocytes and lymphocytes at the intima and further stimulate
monocytes to differentiate into macrophages (87,88). PF4 is a protein synthesised from
platelet particles. Recent research has found that PF4 interacts
with von Willebrand factor (VWF) to form immune complexes. The
PF4-VWF complex stimulates platelet activation and inflammation,
and promotes the formation of immune-associated thrombosis by
suppressing a disintegrin and metalloproteinase with a
thrombospondin type 1 motif, member 13 in a concentration-dependent
manner (89). A cohort study
supported the assumption that after 12 months of PCSK9 inhibition
therapy, the plasma level of PF4 decreased, which may play a role
in cardiovascular event reduction (9). VWF participates in the coagulation
process as a carrier and protector of FVIII. Since plasma FVIII
levels are increased by PCSK9 (74), it can be hypothesized that low
PCSK9 levels may further become a barrier to thrombosis. In
summary, the PCSK9-FVIII-PF4 pathway in thrombosis is a direction
worthy of further research. This may be helpful to explain VTE
reduction in patients with PCSK9 inhibitors. Yurtseven et al
(90) observed a significant
increase in Ly6C (hi) monocytes, which are the inflammatory
monocytes, following treatment with PCSK9, followed by an
enhancement of their recruitment to the arterial wall. Furthermore,
oxLDL and PCSK9 potently bind to LDLR, CD36, LOX-1, TLR4 and
scavenger receptor class A on macrophages, thereby stimulating the
NF-κB, MAPK and COX-2 pathways. This induction leads to an
increased oxLDL uptake by macrophages, as well as to the increased
expression of cytokines and mRNA of adhesion molecules, including
interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF)-α,
chemokine C-X-C motif ligand 2 and monocyte chemotactic protein 1
(MCP1) (70,91,92). In a previous study, compared with
the atherosclerotic plaques of untreated ApoE-null mice, mice
treated with PCSK9 small hairpin RNA were found to have less
macrophage infiltration. The mice in which PCSK9 was suppressed had
lower levels of vascular inflammatory regulators, such as TNF-α,
IL-1β, MCP1, TLR4 and NF-κB (93). Macrophages turn into foam cells
(94). Foam cells, which
participate in atherosclerotic plaque rupture, mediate the
sustained inflammatory response, resulting in vascular remodelling.
After the plaque is disrupted, tissue factor, collagen and other
plaque components are exposed, which leads to platelet activation
and aggregation, and thrombus formation (95). Clinical research has confirmed
that circulating PCSK9 levels are significantly associated with an
increased risk of developing myocardial infarction (96).
Neutrophil extracellular traps (NETs) composed of
DNA and histones released by neutrophils play a crucial role in
trapping bacteria and driving bactericidal activity. However, NETs
also play a role in the initiation, formation and propagation of
thrombosis. Lipopolysaccharide activates platelets and neutrophils
via TLR4, inducing the release of PF4, the expression of P-selectin
and NETosis. The formation of NETs results in the release of TF via
autophagy and the activation of FVII (97). An analysis of the inferior vena
cava conducted in mice suggested that in comparison with
PCSK9-deficient mice, wild-type mice displayed an inferior vena
cava thrombus with greater weight, length, myeloid cell recruitment
and a greater number of NETs (98). Therefore, PCSK9 promotes
thrombosis by affecting leukocyte mobilisation and NET formation.
These mechanisms may be of particular importance for patients with
diabetes.
Vascular endothelial injury is closely associated
with thrombosis. Elevated levels of cholesterol expose the vascular
walls to oxidative stress, leading to endothelial dysfunction
(99). Inflammation induced by
endothelial dysfunction abnormally induces platelet attachment to
endothelial cells in the arterial walls (100), increasing the cardiovascular
risk. Even with short-term treatment, the PCSK9 inhibitors,
evolocumab and alirocumab, have been found to improve endothelial
function (101,102). Damage to the vascular wall by
PCSK9 can occur via a variety of mechanisms.
First, a study on a cohort of 26 patients with
cardiovascular disease found that reducing PCSK9 levels
significantly increased circulating endothelial progenitor cells,
suggesting the promotion of vascular repair (103). Second, the expression of PCSK9
in smooth muscle cells (SMCs) and endothelial cells was found to be
highest at proper endothelial shear stress (3-6
dynes/cm2), and the levels of ROS followed the same
pattern as PCSK9 (104).
Haemodynamic factors stimulate local lipid accumulation and
oxidative stress, accelerating vascular remodelling (105). It has been shown that
anti-PCSK9 therapy with alirocumab ameliorates oxidative stress in
rats with biliary cirrhosis, without affecting the plasma levels of
oxLDL (106). Third, the Bcl-2
protein family and caspases play an essential role in apoptosis.
Breaking the balance between the apoptosis inhibitor, B-cell
lymphoma-2 (Bcl-2), and the apoptosis inducer, Bcl2-associated X
(Bax), can activate caspase-9 and caspase-3 sequentially by
regulating the release of cytochrome c from the mitochondria
to induce cell apoptosis. Studies using human umbilical
vein-derived endothelial cells have demonstrated that Bax
phosphorylation under oxLDL pressure decreases the Bcl-2/Bax ratio
and activates the apoptotic process (107). Following the silencing of PCSK9
with specific small hairpin RNA, the Bcl-2/Bax ratio increases, and
the phosphorylation levels of the p38 and JNK signalling pathways
are significantly decreased. The programmed death process of
endothelial cells is inhibited (108). In addition, PCSK9 affects the
metabolism of SMC. It promotes age-related atherosclerosis by
downregulating the expression of ApoER2 on SMCs (109). The dysfunction of vascular
endothelial cells may be related to abnormal plasma and local PCSK9
levels in patients with and without renal disease undergoing
dialysis.
Some pathological states may alter the PCSK9 levels,
activity and downstream pathways (Fig. 3). Moreover, this may lead to
abnormal LDL-C levels and an increased risk of bleeding and
thrombosis.
The liver is the predominant synthesis site for
multiple proteins involved in coagulation and liver diseases may
lead to coagulation disorders presenting as either the impairment
or promotion of haemostasis (110). Bleeding was historically
considered to be a frequent and fatal complication of liver
disease; however, a significant rate of VTE was also observed in
patients with chronic liver disease (CLD) (111). The effects of liver disease on
haemostasis involve a several mechanisms, and some of the
haemostatic changes counteract each other. In patients with liver
dysfunction, the reduction of fibrinogen and coagulation factors,
such as FII, FV, FVII, FIX, FX and FXI increase the risk of
bleeding, while elevated FVIII levels, decreased levels of protein
C/S and antithrombin, together with VWF compensation, promote
clotting (112). Mild to
moderate thrombocytopenia and platelet function defects have been
described in patients with CLD, and this can be more severe in
those with cirrhosis and splenomegaly (113). However, enhanced in vivo
platelet activation in patients with liver cirrhosis may contribute
to the thrombosis of large vessels (114). There are also abnormalities in
the fibrinolytic system in the condition of liver disease, the most
prominent of which is the increased level of tissue-type
plasminogen activator (tPA) due to reduced hepatic clearance. This
may explain the high rate of hyperfibrinolysis (30 to 50%) that
occurs in patients with end-stage liver disease (115). However, a causal role of
hyperfibrinolysis in bleeding is uncertain due to concomitant
changes in haemostasis.
PCSK9 is synthesized mainly in the liver. Impaired
hepatic function in patients with liver cirrhosis affects the
synthesis and secretion of PCSK9. Feder et al (116) reported that patients with liver
cirrhosis had lower plasma levels of PCSK9 compared to
non-cirrhotic patients. Moreover, the association between serum
cholesterol and PCSK9 levels is lacking, which may be due to the
overriding effect of PCSK9 on cholesterol metabolism. Lower PCSK9
levels in patients with liver disease are associated with reduced
levels of pro-inflammatory factors, suggesting a reduction in the
occurrence of atherosclerotic events (117). In a previous study, serum PCSK9
levels and the international normalized ratio were shown to be
negatively associated with each other, suggesting a higher risk of
bleeding (118). In that study,
low systemic levels of PCSK9 were associated with decreased albumin
levels and an increased mortality rate in patients suffering from
end-stage liver disease.
PCSK9 mediates the degradation of LDLR homologous
and non-homologous receptors, including CD36 (119) and LRP1 (120), to regulate inflammation and
vascular function. CD36 is a multifunctional signalling molecule
inducing inflammation through JNK activation (121). Since CD36 is a key receptor for
platelet activation, decreased levels of PCSK9 in patients with
liver disease may inhibit CD36 degradation, thereby enhancing the
stimulation of the CD36 pathway and promoting platelet aggregation
(122). In addition, low levels
of PCSK9 have a potential effect on fibrinolysis. tPA is the major
intravascular activator of fibrinolysis and is cleared by LRP1
(115). Low PCSK9 levels in
liver disease may decrease LRP1 degradation, thereby promoting
LRP1-mediated tPA clearance and alleviating hyperfibrinolysis to a
certain extent (120).
In a previous prospective study involving 5,138
individuals with chronic kidney disease, elevated levels of PCSK9
were shown to be associated with an increased risk of
cardiovascular disease (123).
Despite an increase in PCSK9 levels in cases of nephrotic syndrome,
no association was found with the estimated glomerular filtration
rate (124). While some studies
have reported an association between PCSK9 and proteinuria, others
have revealed opposite results (124,125). PCSK9 is expressed in the
kidneys to a lesser extent. The collecting duct is a major source
of increased PCSK9 expression in the kidneys, which may be a new
driver of hyperlipidaemia in nephrotic syndrome (126).
Chronic kidney disease affects the haemostatic
system due to complex alterations in platelets, vessel walls,
microparticles and even drugs (127). The changes in PCSK9 levels
among patients with nephropathy add a further layer of complexity
to haemostasis. The renin-angiotensin-aldosterone system (RAAS) is
abnormally active in patients with renal failure, and is associated
with increased levels of PAI-1 (127). Given the high level of PCSK9,
the interaction between PCSK9 and PAI-1 may promote
hyperfibrinolysis (as aforementioned in the sub-section entitled
'PCSK9 and coagulation factors'). Intracellular PCSK9 promotes
arterial medial calcification. SMCs overexpressing PCSK9 release an
abundance of extracellular vesicles (EVs) that are rich in
Ca2+ and alkaline phosphatase (128). EVs increase pro-calcific
markers, which results in renal impairment by regulating the
calcium-phosphorus balance, and eventually promoting calcification
of SMCs (129). Arterial medial
calcifications promote the progression of atherosclerotic lesions
and affect platelet-vessel wall interactions (130).
Zymogen PCSK9 (73 kDa) undergoes processing in the
endoplasmic reticulum and Golgi body and is secreted as mature
PCSK9 (63 kDa) (131). Dialysis
may influence PCSK9 kinetics, although available literature on this
topic is limited. A prevoius small cohort study indicated increased
PCSK9 levels in patients receiving peritoneal dialysis as compared
to those on haemodialysis and in control subjects (124). Notably, abnormal platelet
activation occurs in patients treated with peritoneal dialysis
(127). Therefore, the
potential benefits of anti-PCSK9 therapy in these patients are
worthy of investigation.
Cardiovascular disease tends to develop in patients
with either type 1 (T1DM) or type 2 (T2DM) diabetes mellitus. The
lipid profile of patients with well-controlled T1DM is similar to
that of the general population (132). However, patients with T2DM
frequently exhibit elevated levels of triglycerides and
non-high-density lipoprotein-cholesterol, even with good glycaemic
control (133). Patients with
T2DM are considered to be in a hypercoagulable state induced by
insulin resistance and endothelial dysfunction, which causes
microvascular and macrovascular complications by augmenting
atherosclerosis (134). Due to
the increased risk of MACEs, clinical guidelines the stricter lipid
targets for individuals with diabetes (135). Hyperglycaemia leads to platelet
hyperactivity through various mechanisms, including the glycation
of platelet surface proteins, the expression of GPⅡb and
P-selectin, and PKC activation (136). Moreover, T2DM is associated
with chronic inflammatory disorders (137). Neutrophils isolated from
diabetic individuals are more prone to forming NETs, and the
interaction between NETs and macrophages contributes to persistent
macrophage inflammation (138).
Hypofibrinolysis is also widely observed in T2DM, partly due to the
elevated concentration and activity of PAI-1 (139). Platelet hyperactivity,
fibrinolytic inhibition, oxidative stress and inflammation further
promote thrombosis (136).
Circulating levels of PCSK9 are significantly higher
in pregnant women with gestational diabetes mellitus (GDM) compared
to those with a normal glucose tolerance. PCSK9 level is positively
associated with fasting plasma glucose, glycosylated haemoglobin,
total cholesterol, and LDL-C levels in patients with GDM (140). PCSK9 deficiency leads to an
increased expression of LDLR in pancreatic β-cells (141). PCSK9 knockdown also promotes
intracellular cholesterol accumulation, islet function and insulin
secretion (141,142). Specifically, PCSK9 interacts
with human pancreatic β-cells through LDLR and an intracellular
signalling mechanism that regulates the expression of CD36,
anti-programmed death 1 and HLA-ABC receptors (141). It remains unclear whether the
use of PCSK9 inhibitors increases the risk of developing diabetes
(143,144). Considering the inconsistent
results, further investigations are required to elucidate the
association between PCSK9 levels and diabetes.
PCSK9 monoclonal antibody therapy is safe and
effective for patients with diabetes (145). Plasma PCSK9 levels appear to
affect inflammation and coagulation in patients with diabetes
mellitus. A previous observational study reported that patients
with diabetes and PCSK9 levels of ≤43.5 ng/ml had the highest
survival from MACEs (12). High
PCSK9 levels were found to be associated with MACEs in diabetic
patients undergoing primary PCI, possibly due to their strong
association with inflammation and platelet activation markers, such
as high-sensitivity C-reactive protein, P-selectin and CD40L
(12). In diabetic patients
treated with ticagrelor, PCSK9 levels were positively associated
with maximal platelet aggregation and maximum amplitude of
ADP-induced platelet-fibrin clots, while no association was found
in non-diabetic patients (12).
In addition, PCSK9 levels have been shown to be independently
associated with the platelet count in patients with T2DM (146).
It appears that patients with DM experience
additional anticoagulation benefits from PCSK9 inhibitors. On the
one hand, diabetes is associated with chronic low-grade
inflammation, suggesting that the inhibition of PCSK9 in diabetic
patients may yield greater cardiovascular benefits through its
anti-inflammatory effects than in non-diabetic patients. On the
other hand, PCSK9 may also interact with fibrinolytic processes.
Patients with T2DM exhibit impaired fibrinolysis due to the
downregulation of insulin receptor substrate 1 in endothelial
cells. The level of PAI-1 is increased by 25-280% compared to that
in non-diabetic patients (139). Since PCSK9 levels positively
correlate with PAI-1 levels (78), diabetic patients are more likely
to have high PCSK9 levels and hypercoagulable states. Although
there is a lack of evidence on the effect of PCSK9 levels on the
fibrinolytic state, the role of PCSK9 in the regulation of the
fibrinolytic process should be further explored.
Thromboembolism is the second leading cause of
mortality among patients with cancer undergoing outpatient
chemotherapy, immediately following the progression of cancer
(147). To the best of our
knowledge, to date, no large-scale clinical studies have evaluated
the effects of anti-PCSK9 therapy on thrombosis in patients with
cancer, although it is likely a promising research area. It is
well-known that hypercoagulability, endothelial injury and abnormal
haemodynamics contribute to thrombosis, and cancer-associated
thrombosis (CAT) presents peculiarities in pathophysiological
mechanisms. First, host and malignant cells abnormally synthesize
excessive TF and TF-positive EVs, which initiate the extrinsic
pathway (148). TF-positive EVs
generated from endothelial cells, monocytes and macrophages play a
role in thrombosis, while platelet-derived TF and TF-positive EVs
are controversial. The exposed phosphatidylserine on TF-positive
EVs appears to promote the binding of coagulation factors, rather
than TF. NETs serve as a scaffold for various procoagulants and act
as mediators of CAT (149). It
has been shown that 4T1-derived exosome range EVs induce NET
formation in the neutrophils of granulocyte colony-stimulating
factor-treated mice (150).
Human studies have shown that blood and tissue samples from
patients with gastric cancer were more likely to form NETs,
promoting the conversion of thrombin and fibrin to develop
thrombosis compared with healthy individuals (151). Moreover, other substances
produced by cancer cells also exacerbate the hypercoagulable state,
such as carcinoma mucins, distinct cancer procoagulant factors and
PAI-1 (152). Second, surgical
procedures, catheters and chemotherapy often lead to endothelial
injury (153). Third, oncologic
pain causes immobility, which aggravates circulatory stasis
(154).
Changes in immune-related factors can lead to
alterations in PCSK9 levels and functions, which can also affect
clotting functions. Herein, the changes in the levels of PCSK9 in
infection and transplantation are described. Research on the marine
fish, Epinephelus coioides, has demonstrated that the
upregulation of PCSK9 expression increased the activities of NF-κB
promoter, Singapore grouper iridovirus-induced apoptosis, and
pro-inflammatory factors (155). These findings highlight the
role of PCSK9 in modulating innate immunity in pathogen infections.
Moreover, PCSK9 reduces the infectivity of HCV by acting on LDLR,
very LDLR, scavenger receptor class B type 1 and CD81 receptors on
hepatocytes (156). PCSK9
expression is upregulated during sepsis, increasing cholesterol
levels and promoting cholesterol accumulation in immune cells
(157). These factors improve
TLR function and promote inflammation (157,158). Thrombin-antithrombin complex
levels have been found to be elevated in PCSK9-overexpressing mice,
which indicates that PCSK9 exacerbates the hypercoagulation state
in early sepsis (159).
Therefore, although PCSK9 promotes pathogen clearance, it is
possible that PCSK9 also exacerbates the procoagulant state of the
body. A number of organ transplant patients suffer from graft
vascular disease (GVD). Previous studies have reported that PCSK9
levels are upregulated in wild-type GVD mice, while PCSK9 knockout
mice have a reduced vascular stenosis, intimal hyperplasia area and
collagen deposition (160).
Furthermore, the lack of PCSK9 probably inhibits the inflammasome
through the nod-like receptor protein 3 pathway and suppresses the
migration and proliferation of vascular SMCs (161). Previous studies on patients who
have undergone a heart transplant suggested that PCSK9 inhibitors
can stabilise coronary intimal hyperplasia (161). Additionally, donor-specific
antibodies do not increase after 1 month of treatment (162). PCSK9 is a potential marker of
ischemia/reperfusion injury during visceral vascular surgery or
myocardial infarction (163).
Ischemia/reperfusion triggers the upregulation of PCSK9 and
increased the levels of autophagy regulatory proteins light chain 3
and beclin-1, at the same time, activating the Bcl-2/adenovirus E1B
19-kDa interacting protein pathway aggravated ischemia/reperfusion
injury (164).
Based on their superior safety profile, current
PCSK9 inhibitors have been approved for use in the majority of
patients with hyperlipidaemia, including those with liver disease,
chronic kidney disease, diabetes and cancer. Nevertheless, both
clinical evidence and in vitro data suggest that during
pathological conditions, the concentration and effects of PCSK9
could differ from those in general patients under pathological
conditions. The effect of PCSK9 on thrombosis and haemostasis could
also be intricate. Generally, PCSK9 levels are elevated in several
metabolic states, primarily inciting thrombophilia through platelet
activation and inflammation pathways. Nonetheless, in certain
cases, reduced levels of PCSK9 may alleviate hyperfibrinolysis by
mediating the PAI-1 pathway.
To date, numerous key questions remain to be
addressed. The PAI-1/PCSK9 pathways regulate the fibrinolysis
process; however, the specific mechanism that dominates
fibrinolysis in patients with liver damage remains to be
determined. It also remains unclear whether the concurrent
inhibition of the RAAS and PCSK9 inhibitors may be beneficial in
patients with chronic kidney disease. Future research is thus
required to elucidate the following: i) The mechanisms through
which different disease states alter PCSK9 function and its impact
on the clotting process beyond serum concentrations; ii) the
specific roles of PCSK9 in local coagulation within particular
tissues; and iii) provide further evidence of the extent of the
inhibitory effects of PCSK9 on haemostasis and thrombosis, and
determine the associated clinical outcomes. Therefore, the use of
anti-PCSK9 therapy in specific patients may alter the course of
haemostasis and thrombosis, presenting a double-edged sword. On the
one hand, the incidence of bleeding events may be increased, while
on the other hand, patients with thrombotic tendencies may benefit.
At the same time, drug interactions can be reshuffled. It is
expected that more high-quality evidence will emerge, as it may
change medication regimens for certain patients.
Not applicable.
SC, GM and QX were involved in the investigative
aspects of the review, such as the literature search for related
studies. SC, GM and XC were involved in visualization (preparation
of the figures). SC and GM were involved in the writing of the
original draft of the manuscript. XC, QX and YC were involved in
the writing, reviewing and editing of the manuscript. GM, QX and YC
were involved in the conceptualization and supervision of the
study, and in funding acquisition. All authors have read and agreed
to the published version of the manuscript. Data authentication is
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors would like to thank Professor Yan Zhang
(Department of Cardiology, Peking University First Hospital) for
his professional guidance of this review during the revision
process.
The present study was supported by the National Key Research and
Development Program of China (grant nos. 2021YFC2500500 and
2021YFC2500503), the National High Level Hospital Clinical Research
Funding (Scientific Research Fund of Peking University First
Hospital (grant no. 2022SF15), and the National Natural Science
Foundation of China General Program (grant nos. 82073935 and
82373950).
|
1
|
Mu G, Xiang Q, Zhou S, Liu Z, Qi L, Jiang
J, Gong Y, Xie Q, Wang Z, Zhang H, et al: Efficacy and safety of
PCSK9 monoclonal antibodies in patients at high cardiovascular
risk: An updated systematic review and meta-analysis of 32
randomized controlled trial. Adv Ther. 37:1496–1521. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang DW, Garuti R, Tang WJ, Cohen JC and
Hobbs HH: Structural requirements for PCSK9-mediated degradation of
the low-density lipoprotein receptor. Proc Natl Acad Sci USA.
105:13045–13050. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paciullo F, Momi S and Gresele P: PCSK9 in
haemostasis and thrombosis: Possible pleiotropic effects of PCSK9
inhibitors in cardiovascular prevention. Thromb Haemost.
119:359–367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grundy SM, Stone NJ, Bailey AL, Beam C,
Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S,
Faiella-Tommasino J, Forman DE, et al: 2018
AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline
on the management of blood cholesterol: Executive summary: A report
of the American College of Cardiology/American Heart Association
Task Force on clinical practice guidelines. J Am Coll Cardiol.
73:3168–3209. 2019. View Article : Google Scholar
|
|
5
|
Henein MY, Vancheri S, Longo G and
Vancheri F: The role of inflammation in cardiovascular disease. Int
J Mol Sci. 23:129062022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunt SC, Hopkins PN, Bulka K, McDermott
MT, Thorne TL, Wardell BB, Bowen BR, Ballinger DG, Skolnick MH,
Samuels ME, et al: Genetic localization to chromosome 1p32 of the
third locus for familial hypercholesterolemia in a Utah kindred.
Arterioscler Thromb Vasc Biol. 20:1089–1093. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abifadel M, Varret M, Rabès JP, Allard D,
Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich
D, et al: Mutations in PCSK9 cause autosomal dominant
hypercholesterolemia. Nat Genet. 34:154–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hummelgaard S, Vilstrup JP, Gustafsen C,
Glerup S and Weyer K: Targeting PCSK9 to tackle cardiovascular
disease. Pharmacol Ther. 249:1084802023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barale C, Bonomo K, Frascaroli C, Morotti
A, Guerrasio A, Cavalot F and Russo I: Platelet function and
activation markers in primary hypercholesterolemia treated with
anti-PCSK9 monoclonal antibody: A 12-month follow-up. Nutr Metab
Cardiovasc Dis. 30:282–291. 2020. View Article : Google Scholar
|
|
10
|
Andreadou I, Schulz R, Badimon L, Adameová
A, Kleinbongard P, Lecour S, Nikolaou PE, Falcão-Pires I, Vilahur
G, Woudberg N, et al: Hyperlipidaemia and cardioprotection: Animal
models for translational studies. Br J Pharmacol. 177:5287–5311.
2020. View Article : Google Scholar :
|
|
11
|
Guo Y, Yan B, Tai S, Zhou S and Zheng XL:
CSK9: Associated with cardiac diseases and their risk factors? Arch
Biochem Biophys. 704:1087172021. View Article : Google Scholar
|
|
12
|
Song L, Zhao X, Chen R, Li J, Zhou J, Liu
C, Zhou P, Wang Y, Chen Y, Zhao H and Yan H: Association of PCSK9
with inflammation and platelet activation markers and recurrent
car-diovascular risks in STEMI patients undergoing primary PCI with
or without diabetes. Cardiovasc Diabetol. 21:802022. View Article : Google Scholar
|
|
13
|
INC., F.M.I: PCSK9 inhibitor market
outlook from 2024 to 2034. 2023, (cited 2023 March 31st); Available
from: https://www.futuremarketinsights.com/reports/pcsk9-inhibitors-market.
|
|
14
|
Seidah NG, Awan Z, Chrétien M and Mbikay
M: PCSK9: A key modulator of cardiovascular health. Circ Res.
114:1022–1036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garvie CW, Fraley CV, Elowe NH, Culyba EK,
Lemke CT, Hubbard BK, Kaushik VK and Daniels DS: Point mutations at
the catalytic site of PCSK9 inhibit folding, autoprocessing, and
interaction with the LDL receptor. Protein Sci. 25:2018–2027. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cunningham D, Danley DE, Geoghegan KF,
Griffor MC, Hawkins JL, Subashi TA, Varghese AH, Ammirati MJ, Culp
JS, Hoth LR, et al: Structural and biophysical studies of PCSK9 and
its mutants linked to familial hypercholesterolemia. Nat Struct Mol
Biol. 14:413–419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wiciński M, Żak J, Malinowski B, Popek G
and Grześk G: PCSK9 signaling pathways and their potential
importance in clinical practice. EPMA J. 8:391–402. 2017.
View Article : Google Scholar
|
|
18
|
Yang L, Pu T, Zhang Y, Yan H, Yu H and Gao
W: The R93C variant of PCSK9 reduces the risk of premature mi in a
Chinese Han population. Front Genet. 13:8752692022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwon HJ, Lagace TA, McNutt MC, Horton JD
and Deisenhofer J: Molecular basis for LDL receptor recognition by
PCSK9. Proc Natl Acad Sci USA. 105:1820–1825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lo Surdo P, Bottomley MJ, Calzetta A,
Settembre EC, Cirillo A, Pandit S, Ni YG, Hubbard B, Sitlani A and
Carfí A: Mechanistic implications for LDL receptor degradation from
the PCSK9/LDLR structure at neutral pH. EMBO Rep. 12:1300–1305.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cameron J, Holla OL, Laerdahl JK, Kulseth
MA, Ranheim T, Rognes T, Berge KE and Leren TP: Characterization of
novel mutations in the catalytic domain of the PCSK9 gene. J Intern
Med. 263:420–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Timms KM, Wagner S, Samuels ME, Forbey K,
Goldfine H, Jammulapati S, Skolnick MH, Hopkins PN, Hunt SC and
Shattuck DM: A mutation in PCSK9 causing autosomaldominant
hypercholesterolemia in a Utah pedigree. Hum Genet. 114:349–353.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto T, Lu C and Ryan R: A Two-step
binding model of PCSK9 interaction with the low density lipoprotein
receptor. J Biol Chem. 286:5464–5470. 2011. View Article : Google Scholar :
|
|
24
|
Hampton EN, Knuth MW, Li J, Harris JL,
Lesley SA and Spraggon G: The self-inhibited structure of
full-length PCSK9 at 1.9 A reveals structural homology with
resistin within the C-terminal domain. Proc Natl Acad Sci USA.
104:14604–14609. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang WQ, Zhang Q, Peng YB, Chen M, Lin XP,
Wu JH, Cai CH, Mei YF and Jin H: Resistin level is positively
correlated with thrombotic complications in Southern Chinese
metabolic syndrome patients. J Endocrinol Invest. 34:e36–e42. 2011.
View Article : Google Scholar
|
|
26
|
Jamaluddin MS, Weakley SM, Yao Q and Chen
C: Resistin: Functional roles and therapeutic considerations for
cardiovascular disease. Br J Pharmacol. 165:622–632. 2012.
View Article : Google Scholar :
|
|
27
|
Jeong HJ, Lee HS, Kim KS, Kim YK, Yoon D
and Park SW: Sterol-dependent regulation of proprotein Convertase
Subtilisin/Kexin type 9 expression by sterol-regulatory element
binding protein-2. J Lipid Res. 49:399–409. 2008. View Article : Google Scholar
|
|
28
|
Costet P, Cariou B, Lambert G, Lalanne F,
Lardeux B, Jarnoux AL, Grefhorst A, Staels B and Krempf M: Hepatic
PCSK9 expression is regulated by nutritional status via insulin and
sterol regulatory element-binding protein 1c. J Biol Chem.
281:6211–6218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Dong B, Park SW, Lee HS, Chen W and
Liu J: Hepatocyte nuclear factor 1alpha plays a critical role in
PCSK9 gene transcription and regulation by the natural
hypocholesterolemic compound berberine. J Biol Chem.
284:28885–28895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raal F, Panz V, Immelman A and Pilcher G:
Elevated PCSK9 levels in untreated patients with heterozygous or
homozygous familial hypercholesterolemia and the response to
high-dose statin therapy. J Am Heart Assoc. 2:e0000282013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, McCabe T, Condra JH, Ni YG,
Peterson LB, Wang W, Strack AM, Wang F, Pandit S, Hammond H, et al:
An anti-PCSK9 antibody reduces LDL-cholesterol on top of a statin
and suppresses hepatocyte SREBP-regulated genes. Int J Biol Sci.
8:310–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong B, Singh AB, Shende VR and Liu J:
Hepatic HNF1 transcription factors control the induction of PCSK9
mediated by rosuvastatin in normolipidemic hamsters. Int J Mol Med.
39:749–756. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robinson JG, Farnier M, Krempf M, Bergeron
J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M,
et al: Efficacy and safety of alirocumab in reducing lipids and
cardiovascular events. N Engl J Med. 372:1489–1499. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sabatine MS, Giugliano RP, Keech AC,
Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T,
Wasserman SM, et al: Evolocumab and clinical outcomes in patients
with cardiovascular disease. N Engl J Med. 376:1713–1722. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ray KK, Wright RS, Kallend D, Koenig W,
Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ,
et al: Two phase 3 trials of inclisiran in patients with elevated
LDL cholesterol. N Engl J Med. 382:1507–1519. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Björkegren JLM and Lusis AJ:
Atherosclerosis: Recent developments. Cell. 185:1630–1645. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Santos RD, Gidding SS, Hegele RA, Cuchel
MA, Barter PJ, Watts GF, Baum SJ, Catapano AL, Chapman MJ, Defesche
JC, et al: Defining severe familial hypercholesterolaemia and the
implications for clinical management: A consensus statement from
the international atherosclerosis society severe familial
hypercholesterolemia panel. Lancet Diabetes Endocrinol. 4:850–861.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Raal F, Fourie N, Scott R, Blom D, De
Vries Basson M, Kayikcioglu M, Caldwell K, Kallend D and Stein E;
LIBerate-HeFH Investigators: Long-term efficacy and safety of
lerodalcibep in heterozygous familial hypercholesterolaemia: The
LIBerate-HeFH trial. Eur Heart J. 44:4272–4280. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Agarwala A, Asim R and Ballantyne CM: Oral
PCSK9 inhibitors. Curr Atheroscler Rep. Mar 27–2024. View Article : Google Scholar : Epub ahead of
print. PubMed/NCBI
|
|
40
|
Gallego-Colon E, Daum A and Yosefy C:
Statins and PCSK9 inhibitors: A new lipid-lowering therapy. Eur J
Pharmacol. 878:1731142020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baumann S, Kettel L, Stach K, Özdemir GH,
Renker M, Tesche C, Becher T, Hetjens S, Schoepf UJ, Akin I, et al:
Serial changes in coronary plaque formation using CT angiography in
patients undergoing PCSK9-Inhibitor therapy with 1-year Follow-up.
J Thorac Imaging. 37:285–291. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ota H, Omori H, Kawasaki M, Hirakawa A and
Matsuo H: Clinical impact of PCSK9 inhibitor on stabilization and
regression of lipid-rich coronary plaques: A near-infrared
spectroscopy study. Eur Heart J Cardiovasc Imaging. 23:217–228.
2022. View Article : Google Scholar
|
|
43
|
Nishikido T: Clinical potential of
inclisiran for patients with a high risk of atherosclerotic
cardiovascular disease. Cardiovasc Diabetol. 22:202023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cammisotto V, Baratta F, Simeone PG,
Barale C, Lupia E, Galardo G, Santilli F, Russo I and Pignatelli P:
Proprotein convertase subtilisin Kexin type 9 (PCSK9) beyond
lipids: The role in oxidative stress and thrombosis. Antioxidants
(Basel). 11:5692022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Momtazi-Borojeni AA, Sabouri-Rad S, Gotto
AM, Pirro M, Banach M, Awan Z, Barreto GE and Sahebkar A: PCSK9 and
inflammation: A review of experimental and clinical evidence. Eur
Heart J Cardiovasc Pharmacother. 5:237–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luquero A, Badimon L and Borrell-Pages M:
PCSK9 functions in atherosclerosis are not limited to plasmatic
LDL-Cholesterol regulation. Front Cardiovasc Med. 8:6397272021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Navarese EP, Kolodziejczak M, Winter MP,
Alimohammadi A, Lang IM, Buffon A, Lip GY and Siller-Matula JM:
Association of PCSK9 with platelet reactivity in patients with
acute coronary syndrome treated with prasugrel or ticagrelor: The
PCSK9-REACT study. Int J Cardiol. 227:644–649. 2017. View Article : Google Scholar
|
|
48
|
Marston NA, Gurmu Y, Melloni GEM, Bonaca
M, Gencer B, Sever PS, Pedersen TR, Keech AC, Roselli C, Lubitz SA,
et al: The effect of PCSK9 (Proprotein Convertase Subtilisin/Kexin
type 9) inhibition on the risk of venous thromboembolism.
Circulation. 141:1600–1607. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zaccardi F, Kunutsor SK, Seidu S, Davies
MJ and Khunti K: Is the lower risk of venous thromboembolism with
statins related to low-density-lipoprotein reduction? A network
metaanalysis and meta-regression of randomised controlled trials.
Atherosclerosis. 271:223–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Petersen-Uribe Á, Kremser M, Rohlfing AK,
Castor T, Kolb K, Dicenta V, Emschermann F, Li B, Borst O, Rath D,
et al: Platelet-derived PCSK9 is associated with LDL metabolism and
modulates atherothrombotic mechanisms in coronary artery disease.
Int J Mol Sci. 22:111792021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Konarzewski M, Szolkiewicz M,
Sucajtys-Szulc E, Blaszak J, Lizakowski S, Swierczynski J and
Rutkowski B: Elevated circulating PCSK-9 concentration in renal
failure patients is corrected by renal replacement therapy. Am J
Nephrol. 40:157–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Grimm J, Peschel G, Müller M, Schacherer
D, Wiest R, Weigand K and Buechler C: Rapid decline of serum
proprotein convertase Subtilisin/Kexin 9 (PCSK9) in Non-cirrhotic
patients with chronic Hepatitis C infection receiving direct-acting
antiviral therapy. J Clin Med. 10:16212021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wong Chong E, Joncas FH, Seidah NG, Calon
F, Diorio C and Gangloff A: Circulating levels of PCSK9, ANGPTL3
and Lp(a) in stage III breast cancers. BMC Cancer. 22:10492022.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Stark K and Massberg S: Interplay between
inflammation and thrombosis in cardiovascular pathology. Nat Rev
Cardiol. 18:666–682. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Camera M, Rossetti L, Barbieri SS, Zanotti
I, Canciani B, Trabattoni D, Ruscica M, Tremoli E and Ferri N:
PCSK9 as a positive modulator of platelet activation. J Am Coll
Cardiol. 71:952–954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Panes O, González C, Hidalgo P, Valderas
JP, Acevedo M, Contreras S, Sánchez X, Pereira J, Rigotti A and
Mezzano D: Platelet tissue factor activity and membrane cholesterol
are increased in hypercholesterolemia and normalized by
rosuvastatin, but not by atorvastatin. Atherosclerosis.
257:164–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schwartz GG, Szarek M, Bittner VA, Diaz R,
Goodman SG, Jukema JW, Landmesser U, López-Jaramillo P, Manvelian
G, Pordy R, et al: Lipoprotein(a) and benefit of PCSK9 inhibition
in patients with nominally controlled LDL cholesterol. J Am Coll
Cardiol. 78:421–433. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schwartz GG, Steg PG, Szarek M, Bittner
VA, Diaz R, Goodman SG, Kim YU, Jukema JW, Pordy R, Roe MT, et al:
Peripheral artery disease and venous thromboembolic events after
acute coronary syndrome: Role of Lipoprotein(a) and modification by
Alirocumab: Prespecified analysis of the ODYSSEY OUTCOMES
randomized clinical trial. Circulation. 141:1608–1617. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Boffa MB: Beyond fibrinolysis: The
confounding role of Lp(a) in thrombosis. Atherosclerosis.
349:72–81. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsimikas S, Tsironis LD and Tselepis AD:
New insights into the role of lipoprotein(a)-associated
lipoprotein-associated phospholipase A2 in atherosclerosis and
cardiovascular disease. Arterioscler Thromb Vasc Biol.
27:2094–2099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Assinger A, Wang Y, Butler LM, Hansson GK,
Yan ZQ, Söderberg-Nauclér C and Ketelhuth DF: Apolipoprotein B100
danger-associated signal 1 (ApoBDS-1) triggers platelet activation
and boosts platelet-leukocyte proinflammatory responses. Thromb
Haemost. 112:332–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hagström E, Steg PG, Szarek M, Bhatt DL,
Bittner VA, Danchin N, Diaz R, Goodman SG, Harrington RA, Jukema
JW, et al: Apolipoprotein B, residual cardiovascular risk after
acute coronary syndrome, and effects of alirocumab. Circulation.
146:657–672. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Silverman MG, Ference BA, Im K, Wiviott
SD, Giugliano RP, Grundy SM, Braunwald E and Sabatine MS:
Association between lowering LDL-C and cardiovascular risk
reduction among different therapeutic interventions: A systematic
review and Meta-analysis. JAMA. 316:1289–1297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Podrez EA, Byzova TV, Febbraio M, Salomon
RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR,
et al: Platelet CD36 links hyperlipidemia, oxidant stress and a
prothrombotic phenotype. Nat Med. 13:1086–1095. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Magwenzi S, Woodward C, Wraith KS, Aburima
A, Raslan Z, Jones H, McNeil C, Wheatcroft S, Yuldasheva N,
Febbriao M, et al: Oxidized LDL activates blood platelets through
CD36/NOX2-mediated inhibition of the cGMP/protein kinase G
signaling cascade. Blood. 125:2693–2703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qi Z, Hu L, Zhang J, Yang W, Liu X, Jia D,
Yao Z, Chang L, Pan G, Zhong H, et al: PCSK9 (Proprotein Convertase
Subtilisin/Kexin 9) enhances platelet activation, thrombosis, and
myocardial infarct expansion by binding to platelet CD36.
Circulation. 143:45–61. 2021. View Article : Google Scholar
|
|
67
|
Pignatelli P, Carnevale R, Di Santo S,
Bartimoccia S, Sanguigni V, Lenti L, Finocchi A, Mendolicchio L,
Soresina AR, Plebani A and Violi F: Inherited human gp91phox
deficiency is associated with impaired isoprostane formation and
platelet dysfunction. Arterioscler Thromb Vasc Biol. 31:423–434.
2011. View Article : Google Scholar
|
|
68
|
Pastori D, Nocella C, Farcomeni A,
Bartimoccia S, Santulli M, Vasaturo F, Carnevale R, Menichelli D,
Violi F and Pignatelli P; ATHERO-AF Study Group: Relationship of
PCSK9 and urinary thromboxane excretion to cardiovascular events in
patients with atrial fibrillation. J Am Coll Cardiol. 70:1455–1462.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Schlüter KD, Wolf A, Weber M,
Schreckenberg R and Schulz R: Oxidized low-density lipoprotein
(oxLDL) affects load-free cell shortening of cardiomyocytes in a
proprotein convertase Subtilisin/Kexin 9 (PCSK9)-dependent way.
Basic Res Cardiol. 112:632017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gurbel PA, Navarese EP and Tantry US:
Exploration of PCSK9 as a cardiovascular risk factor: Is there a
link to the platelet? J Am Coll Cardiol. 70:1463–1466. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kong N, Xu Q, Cui W, Feng X and Gao H:
PCSK9 inhibitor inclisiran for treating atherosclerosis via
regulation of endothelial cell pyroptosis. Ann Transl Med.
10:12052022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang H and Bai X: Mechanisms of bone
remodeling disorder in hemophilia. Semin Thromb Hemost. 47:43–52.
2021. View Article : Google Scholar
|
|
73
|
Bovenschen N, Mertens K, Hu L, Havekes LM
and van Vlijmen BJ: LDL receptor cooperates with LDL
receptor-related protein in regulating plasma levels of coagulation
factor VIII in vivo. Blood. 106:906–912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Paciullo F, Petito E, Falcinelli E,
Gresele P and Momi S: Pleiotropic effects of PCSK9-inhibition on
hemostasis: Anti-PCSK9 reduce FVIII levels by enhancing LRP1
expression. Thromb Res. 213:170–172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Strickland DK, Au DT, Cunfer P and
Muratoglu SC: Low-density lipoprotein receptor-related protein-1:
Role in the regulation of vascular integrity. Arterioscler Thromb
Vasc Biol. 34:487–498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Scalise V, Sanguinetti C, Neri T,
Cianchetti S, Lai M, Carnicelli V, Celi A and Pedrinelli R: PCSK9
induces tissue factor expression by activation of TLR4/NFkB
signaling. Int J Mol Sci. 22:126402021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Peng J, Liu MM, Liu HH, Guo YL, Wu NQ,
Dong Q, Qian J, Dou KF, Zhu CG and Li JJ: Association of
circulating proprotein convertase Subtilisin/Kexin type 9
concentration, pro-thrombin time and cardiovascular outcomes: A
prospective cohort study. Thromb J. 19:902021. View Article : Google Scholar
|
|
78
|
Levine JA, Oleaga C, Eren M, Amaral AP,
Shang M, Lux E, Khan SS, Shah SJ, Omura Y, Pamir N, et al: Role of
PAI-1 in hepatic steatosis and dyslipidemia. Sci Rep. 11:4302021.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cronjé HT, Nienaber-Rousseau C, Zandberg
L, Chikowore T, de Lange Z, van Zyl T and Pieters M: Candidate gene
analysis of the fibrinogen phenotype reveals the importance of
polygenic co-regulation. Matrix Biol. 60-61:16–26. 2017. View Article : Google Scholar
|
|
80
|
Zhang Y, Zhu CG, Xu RX, Li S, Guo YL, Sun
J and Li JJ: Relation of circulating PCSK9 concentration to
fibrinogen in patients with stable coronary artery disease. J Clin
Lipidol. 8:494–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Basiak M, Hachula M, Kosowski M and
Okopien B: Effect of PCSK9 inhibitors on hemostasis in patients
with isolated hypercholesterolemia. J Clin Med. 11:25422022.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Schol-Gelok S, Galema-Boers JAMH, van
Gelder T, Kruip MJHA, Roeters van Lennep JE and Versmissen J: No
effect of PCSK9 inhibitors on D-dimer and fibrinogen levels in
patients with familial hypercholesterolemia. Biomed Pharmacother.
108:1412–1414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ahn JH, Tantry US, Kang MG, Park HW, Koh
JS, Bae JS, Cho SY, Kim KH, Jang JY, Park JR, et al: Residual
inflammatory risk and its association with events in east asian
patients after coronary intervention. JACC Asia. 2:323–337. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ridker PM, Everett BM, Thuren T, MacFadyen
JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker
SD, et al: Antiinflammatory therapy with canakinumab for
atherosclerotic disease. N Engl J Med. 377:1119–1131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Leung AKK, Xue YC, de Guzman A,
Grzelkovski G, Kong HJ, Genga KR, Russell JA, Boyd JH, Francis GA
and Walley KR: Modulation of vascular endothelial inflammatory
response by proprotein convertase Subtilisin-Kexin type 9.
Atherosclerosis. 362:29–37. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Raheem Lateef Al-Awsi G, Hadi Lafta M,
Hashim Kzar H, Samieva G, Alsaikhan F, Ahmad I, Mahmood Saleh M,
Alamin Altoum A, Aravindhan S, Fakri Mustafa Y, et al: PCSK9
pathway-noncoding RNAs crosstalk: Emerging opportunities for novel
therapeutic approaches in inflammatory atherosclerosis. Int
Immunopharmacol. 113:1093182022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kattoor AJ, Goel A and Mehta JL: LOX-1:
Regulation, signaling and its role in atherosclerosis. Antioxidants
(Basel). 8:2182019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Theofilis P, Sagris M, Antonopoulos AS,
Oikonomou E, Tsioufis C and Tousoulis D: Inflammatory mediators of
platelet activation: Focus on atherosclerosis and COVID-19. Int J
Mol Sci. 22:111702021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu ZY, Sun MX, Hua MQ, Zhang HX, Mu GY,
Zhou S, Wang Z, Xiang Q and Cui YM: New perspectives on the
induction and acceleration of immune-associated thrombosis by PF4
and VWF. Front Immunol. 14:10986652023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yurtseven E, Ural D, Baysal K and
Tokgözoğlu L: An update on the role of PCSK9 in atherosclerosis. J
Atheroscler Thromb. 27:909–918. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ding Z, Liu S, Wang X, Theus S, Deng X,
Fan Y, Zhou S and Mehta JL: PCSK9 regulates expression of scavenger
receptors and ox-LDL uptake in macrophages. Cardiovasc Res.
114:1145–1153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ricci C, Ruscica M, Camera M, Rossetti L,
Macchi C, Colciago A, Zanotti I, Lupo MG, Adorni MP, Cicero AFG, et
al: PCSK9 induces a pro-inflammatory response in macrophages. Sci
Rep. 8:22672018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tang ZH, Peng J, Ren Z, Yang J, Li TT, Li
TH, Wang Z, Wei DH, Liu LS, Zheng XL and Jiang ZS: New role of
PCSK9 in atherosclerotic inflammation promotion involving the
TLR4/NF-κB pathway. Atherosclerosis. 262:113–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Puteri MU, Azmi NU, Kato M and Saputri FC:
PCSK9 promotes cardiovascular diseases: Recent evidence about its
association with platelet Activation-Induced myocardial infarction.
Life (Basel). 12:1902022.PubMed/NCBI
|
|
95
|
Vergallo R and Crea F: Atherosclerotic
plaque healing. N Engl J Med. 383:846–857. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Laugsand LE, Åsvold BO, Vatten LJ, Janszky
I, Platou CG, Michelsen AE, Damås JK, Aukrust P and Ueland T:
Circulating PCSK9 and risk of myocardial infarction: The HUNT study
in Norway. JACC Basic Transl Sci. 1:568–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kimball AS, Obi AT, Diaz JA and Henke PK:
The emerging role of NETs in venous thrombosis and
immunothrombosis. Front Immunol. 7:2362016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang H, Wang Q, Wang J, Guo C, Kleiman K,
Meng H, Knight JS and Eitzman DT: Proprotein convertase
Subtilisin/Kexin type 9 (PCSK9) deficiency is protective against
venous thrombosis in mice. Sci Rep. 7:143602017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Heitzer T, Schlinzig T, Krohn K, Meinertz
T and Münzel T: Endothelial dysfunction, oxidative stress, and risk
of cardiovascular events in patients with coronary artery disease.
Circulation. 104:2673–2678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Massberg S, Brand K, Grüner S, Page S,
Müller E, Müller I, Bergmeier W, Richter T, Lorenz M, Konrad I, et
al: A critical role of platelet adhesion in the initiation of
atherosclerotic lesion formation. J Exp Med. 196:887–896. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Di Minno A, Gentile M, Iannuzzo G,
Calcaterra I, Tripaldella M, Porro B, Cavalca V, Di Taranto MD,
Tremoli E, Fortunato G, et al: Endothelial function improvement in
patients with familial hypercholesterolemia receiving PCSK-9
inhibitors on top of maximally tolerated lipid lowering therapy.
Thromb Res. 194:229–236. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Metzner T, Leitner DR, Dimsity G, Gunzer
F, Opriessnig P, Mellitzer K, Beck A, Sourij H, Stojakovic T,
Deutschmann H, et al: Short-Term treatment with alirocumab,
flow-dependent dilatation of the brachial artery and use of
magnetic resonance diffusion tensor imaging to evaluate vascular
structure: An exploratory pilot study. Biomedicines. 10:1522022.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Itzhaki Ben Zadok O, Mager A, Leshem-Lev
D, Lev E, Kornowski R and Eisen A: The effect of proprotein
convertase Subtilisin Kexin type 9 inhibitors on circulating
endothelial progenitor cells in patients with cardiovascular
disease. Cardiovasc Drugs Ther. 36:85–92. 2022. View Article : Google Scholar
|
|
104
|
Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun
C, Wang Y and Mehta JL: Hemodynamic shear stress via ROS modulates
PCSK9 expression in human vascular endothelial and smooth muscle
cells and along the mouse aorta. Antioxid Redox Signal. 22:760–771.
2015. View Article : Google Scholar :
|
|
105
|
Chatzizisis YS, Coskun AU, Jonas M,
Edelman ER, Feldman CL and Stone PH: Role of endothelial shear
stress in the natural history of coronary atherosclerosis and
vascular remodeling: Molecular, cellular, and vascular behavior. J
Am Coll Cardiol. 49:2379–2393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Huang HC, Hsu SJ, Chang CC, Chuang CL, Hou
MC and Lee FY: Effects of PCSK-9 Inhibition by alirocumab
treatments on biliary cirrhotic rats. Int J Mol Sci. 23:73782022.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wu CY, Tang ZH, Jiang L, Li XF, Jiang ZS
and Liu LS: PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL
via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem.
359:347–358. 2012. View Article : Google Scholar
|
|
108
|
Li J, Liang X, Wang Y, Xu Z and Li G:
Investigation of highly expressed PCSK9 in atherosclerotic plaques
and ox-LDL-induced En-Dothelial cell apoptosis. Mol Med Rep.
16:1817–1825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Guo Y, Tang Z, Yan B, Yin H, Tai S, Peng
J, Cui Y, Gui Y, Belke D, Zhou S and Zheng XL: PCSK9 (Proprotein
Convertase Subtilisin/Kexin Type 9) triggers vascular smooth muscle
cell senescence and apoptosis: Implication of its direct role in
degenerative vascular disease. Arterioscler Thromb Vasc Biol.
42:67–86. 2022. View Article : Google Scholar
|
|
110
|
O'Leary JG, Greenberg CS, Patton HM and
Caldwell SH: AGA clinical practice update: Coagulation in
cirrhosis. Gastroenterology. 157:34–43.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Subhani M, Sheth A, Ahmed J, Wijayasiri P,
Gardezi SA, Enki D, Morling JR, Aithal GP, Ryder SD and Aravinthan
AD: Incidence and prevalence of venous thromboembolism in chronic
liver disease: A Sys-Tematic review and meta-analysis. Thromb Res.
215:19–29. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pant A, Kopec AK and Luyendyk JP: Role of
the blood coagulation cascade in hepatic fibrosis. Am J Physiol
Gastrointest Liver Physiol. 315:G171–G176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Roberts LN, Patel RK and Arya R:
Haemostasis and thrombosis in liver disease. Br J Haematol.
148:507–521. 2010. View Article : Google Scholar
|
|
114
|
Northup PG, Garcia-Pagan JC, Garcia-Tsao
G, Intagliata NM, Superina RA, Roberts LN, Lisman T and Valla DC:
Vascular liver disorders, portal vein thrombosis, and procedural
bleeding in patients with liver disease: 2020 practice guidance by
the American association for the study of liver diseases.
Hepatology. 73:366–413. 2021. View Article : Google Scholar
|
|
115
|
Leebeek FW and Rijken DC: The fibrinolytic
status in liver diseases. Semin Thromb Hemost. 41:474–480. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Feder S, Wiest R, Weiss TS, Aslanidis C,
Schacherer D, Krautbauer S, Liebisch G and Buechler C: Proprotein
convertase Subtilisin/Kexin type 9 (PCSK9) levels are not
associated with severity of liver disease and are inversely related
to cholesterol in a cohort of thirty eight patients with liver
cirrhosis. Lipids Health Dis. 20:62021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Grewal T and Buechler C: Emerging insights
on the diverse roles of proprotein convertase Subtilisin/Kexin type
9 (PCSK9) in chronic liver diseases: Cholesterol metabolism and
beyond. Int J Mol Sci. 23:10702022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Schlegel V, Treuner-Kaueroff T, Seehofer
D, Berg T, Becker S, Ceglarek U, Thiery J and Kaiser T: Low PCSK9
levels are correlated with mortality in patients with end-stage
liver disease. PLoS One. 12:e01815402017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Demers A, Samami S, Lauzier B, Des Rosiers
C, Ngo Sock ET, Ong H and Mayer G: PCSK9 induces CD36 degradation
and affects long-chain fatty acid uptake and tri-glyceride
metabolism in adipocytes and in mouse liver. Arterioscler Thromb
Vasc Biol. 35:2517–2525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Canuel M, Sun X, Asselin MC, Paramithiotis
E, Prat A and Seidah NG: Proprotein convertase Subtilisin/Kexin
type 9 (PCSK9) can mediate degradation of the low-density
lipoprotein receptor-related protein 1 (LRP-1). PLoS One.
8:e641452013. View Article : Google Scholar
|
|
121
|
Zhang C, Shi X, Su Z, Hu C, Mu X, Pan J,
Li M, Teng F, Ling T, Zhao T, et al: CD36 deficiency ameliorates
drug-induced acute liver injury in mice. Mol Med. 27:572021.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Rada P, González-Rodríguez Á,
García-Monzón C and Valverde ÁM: Understanding lipotoxicity in
NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis.
11:8022020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kheirkhah A, Lamina C, Kollerits B,
Schachtl-Riess JF, Schultheiss UT, Forer L, Sekula P, Kotsis F,
Eckardt KU and Kronenberg F; GCKD Investigators: PCSK9 and
cardiovascular disease in individuals with moderately decreased
kidney function. Clin J Am Soc Nephrol. 17:809–818. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Pavlakou P, Liberopoulos E, Dounousi E and
Elisaf M: PCSK9 in chronic kidney disease. Int Urol Nephrol.
49:1015–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Elewa U, Fernández-Fernández B,
Mahillo-Fernández I, Martin-Cleary C, Sanz AB, Sanchez-Niño MD and
Ortiz A: PCSK9 in diabetic kidney disease. Eur J Clin Invest.
46:779–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Artunc F: Kidney-derived PCSK9-a new
driver of hyperlipidemia in nephrotic syndrome? Kidney Int.
98:1393–1395. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lutz J, Menke J, Sollinger D, Schinzel H
and Thürmel K: Haemostasis in chronic kidney disease. Nephrol Dial
Transplant. 29:29–40. 2014. View Article : Google Scholar
|
|
128
|
Lupo MG, Bressan A, Donato M, Canzano P,
Camera M, Poggio P, Greco MF, Garofalo M, De Martin S, Panighel G,
et al: PCSK9 promotes arterial medial calcification.
Atherosclerosis. 346:86–97. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yang W, Zou B, Hou Y, Yan W, Chen T and Qu
S: Extracellular vesicles in vascular calcification. Clin Chim
Acta. 499:118–122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Jinnouchi H, Sato Y, Sakamoto A,
Cornelissen A, Mori M, Kawakami R, Gadhoke NV, Kolodgie FD, Virmani
R and Finn AV: Calcium deposition within coronary atherosclerotic
lesion: Implications for plaque stability. Atherosclerosis.
306:85–95. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ahamad S, Mathew S, Khan WA and Mohanan K:
Development of small-molecule PCSK9 inhibitors for the treatment of
hypercholesterolemia. Drug Discov Today. 27:1332–1349. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
de Ferranti SD, de Boer IH, Fonseca V, Fox
CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ,
et al: Type 1 diabetes mellitus and cardiovascular disease: A
scientific statement from the American Heart Association and
American Diabetes Association. Circulation. 130:1110–1130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Feingold KR: Dyslipidemia in diabetes.
2020 Aug 10. Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder
WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, et al:
Endotext (Internet) South Dartmouth (MA): MDText.com, Inc.;
2000
|
|
134
|
Morel O, Jesel L, Abbas M and Morel N:
Prothrombotic changes in diabetes mellitus. Semin Thromb Hemost.
39:477–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Arnett DK, Blumenthal RS, Albert MA,
Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A,
Lloyd-Jones D, McEvoy JW, et al: 2019 ACC/AHA guideline on the
primary prevention of cardiovascular disease: Executive summary: A
report of the American college of Cardiology/American heart
association task force on clinical practice guidelines.
Circulation. 140:e563–e595. 2019.PubMed/NCBI
|
|
136
|
Kaur R, Kaur M and Singh J: Endothelial
dysfunction and platelet hyperactivity in type 2 diabetes mellitus:
Molecular insights and therapeutic strategies. Cardiovasc Diabetol.
17:1212018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Dregan A, Charlton J, Chowienczyk P and
Gulliford MC: Chronic inflammatory disorders and risk of type 2
diabetes mellitus, coronary heart disease, and stroke: A
population-based cohort study. Circulation. 130:837–844. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Josefs T, Barrett TJ, Brown EJ, Quezada A,
Wu X, Voisin M, Amengual J and Fisher EA: Neutrophil extracellular
traps promote macrophage inflammation and impair atherosclerosis
resolution in diabetic mice. JCI Insight. 5:e1347962020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Bryk-Wiązania AH and Undas A:
Hypofibrinolysis in type 2 diabetes and its clinical implications:
From mechanisms to pharmacological modulation. Cardiovasc Diabetol.
20:1912021. View Article : Google Scholar
|
|
140
|
Wu Y, Shi J, Su Q, Yang Z and Qin L:
Correlation between circulating PCSK9 levels and gestational
diabetes mellitus in a Chinese population. Front Endocrinol
(Lausanne). 13:8267572022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Saitoski K, Ryaboshapkina M, Hamza GM,
Jarnuczak AF, Berthault C, Carlotti F, Armanet M, Sengupta K,
Underwood CR, Andersson S, et al: Proprotein convertase PCSK9
affects expression of key surface proteins in human pancreatic beta
cells via intracellular and extracellular regulatory circuits. J
Biol Chem. 298:1020962022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Da Dalt L, Ruscica M, Bonacina F,
Balzarotti G, Dhyani A, Di Cairano E, Baragetti A, Arnaboldi L, De
Metrio S, Pellegatta F, et al: PCSK9 deficiency reduces insulin
secretion and promotes glucose intolerance: The role of the
low-density lipoprotein receptor. Eur Heart J. 40:357–368. 2019.
View Article : Google Scholar
|
|
143
|
Tchéoubi SER, Akpovi CD, Coppée F,
Declèves AE, Laurent S, Agbangla C and Burtea C: Molecular and
cellular biology of PCSK9: Impact on glucose homeostasis. J Drug
Target. 30:948–960. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Goldman A, Raschi E, Cukierman-Yaffe T,
Dankner R, Shouval R, Shechter M, Ben-Zvi I, Gerstein HC and Maor
E: Hyperglycaemic disorders associated with PCSK9 inhibitors: A
real-world, pharmacovigilance study. Eur J Prev Cardiol.
29:1334–1342. 2022. View Article : Google Scholar
|
|
145
|
Marouf BH, Iqbal Z, Mohamad JB, Bashir B,
Schofield J, Syed A, Kilpatrick ES, Stefanutti C and Soran H:
Efficacy and Safety of PCSK9 monoclonal antibodies in patients with
diabetes. Clin Ther. 44:331–348. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Furuhashi M, Sakuma I, Morimoto T,
Higashiura Y, Sakai A, Matsumoto M, Sakuma M, Shimabukuro M,
Nomiyama T, Arasaki O, et al: Differential effects of DPP-4
inhibitors, anagliptin and sitagliptin, on PCSK9 levels in patients
with type 2 diabetes mellitus who are receiving statin therapy. J
Atheroscler Thromb. 29:24–37. 2022. View Article : Google Scholar :
|
|
147
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Thromboembolism is a leading cause of
death in cancer patients receiving outpatient chemotherapy. J
Thromb Haemost. 5:632–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kim AS, Khorana AA and McCrae KR:
Mechanisms and biomarkers of cancer-associated thrombosis. Transl
Res. 225:33–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wolach O and Martinod K: Casting a NET on
cancer: The multiple roles for neutrophil extracellular traps in
cancer. Curr Opin Hematol. 29:53–62. 2022. View Article : Google Scholar
|
|
150
|
Leal AC, Mizurini DM, Gomes T, Rochael NC,
Saraiva EM, Dias MS, Werneck CC, Sielski MS, Vicente CP and
Monteiro RQ: Tumor-derived exosomes induce the formation of
neutrophil extracellular traps: Implications for the establishment
of cancer-associated thrombosis. Sci Reps. 7:64382017. View Article : Google Scholar
|
|
151
|
Li JC, Zou XM, Yang SF, Jin JQ, Zhu L, Li
CJ, Yang H, Zhang AG, Zhao TQ and Chen CY: Neutrophil extracellular
traps participate in the development of cancer-associated
thrombosis in patients with gastric cancer. World J Gastroenterol.
28:3132–3149. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Fernandes CJ, Morinaga LTK, Alves JL Jr,
Castro MA, Calderaro D, Jardim CVP and Souza R: Cancer-associated
thrombosis: The when, how and why. Eur Respir Rev. 28:1801192019.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Taxbro K, Hammarskjöld F, Thelin B, Lewin
F, Hagman H, Hanberger H and Berg S: Clinical impact of
peripherally inserted central catheters vs implanted port catheters
in patients with cancer: An open-label, randomised, two-centre
trial. Br J Anaesth. 122:734–741. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Falanga A, Russo L, Milesi V and Vignoli
A: Mechanisms and risk factors of thrombosis in cancer. Crit Rev
Oncol Hematol. 118:79–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Cai YJ, Li PH, Wang XA, Xu YM, Yang S,
Tang YN, Zhu Z, Yang XY, He JY, Luo H, et al: Epinephelus coioides
PCSK9 affect the infection of SGIV by regulating the innate immune
response. Fish Shellfish Immunol. 126:113–121. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Pirro M, Bianconi V, Francisci D,
Schiaroli E, Bagaglia F, Sahebkar A and Baldelli F: Hepatitis C
virus and proprotein convertase Subtilisin/Kexin type 9: A
detrimental interaction to increase viral infectivity and disrupt
lipid metabolism. J Cell Mol Med. 21:3150–3161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Paciullo F, Fallarino F, Bianconi V,
Mannarino MR, Sahebkar A and Pirro M: PCSK9 at the crossroad of
cholesterol metabolism and immune function during infections. J
Cell Physiol. 232:2330–2338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Yuan Y, Wu W, Sun S, Zhang Y and Chen Z:
PCSK9: A potential therapeutic target for sepsis. J Immunol Res.
2020:26876922020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Barale C, Melchionda E, Morotti A and
Russo I: PCSK9 biology and its role in atherothrombosis. Int J Mol
Sci. 22:58802021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zou Y, Chen Z, Zhang X, Yu J, Xu H, Cui J,
Li Y, Niu Y, Zhou C, Xia J and Wu J: Targeting PCSK9 ameliorates
graft vascular disease in mice by inhibiting NLRP3 inflam-masome
activation in vascular smooth muscle cells. Front Immunol.
13:8947892022. View Article : Google Scholar
|
|
161
|
Sammour Y, Dezorzi C, Austin BA, Borkon
AM, Everley MP, Fendler TJ, Khumri TM, Lawhorn SL, Nassif ME,
Vodnala D, et al: PCSK9 inhibitors in heart transplant patients:
Safety, efficacy, and angiographic correlates. J Card Fail.
27:812–815. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Jennings DL, Sultan L, Mingov J, Choe J,
Latif F, Restaino S, Clerkin K, Habal MV, Colombo PC, Yuzefpulskaya
M, et al: PCSK9 inhibitors safely and effectively lower LDL after
heart transplantation: A systematic review and meta-analysis. Heart
Fail Rev. 28:149–156. 2023. View Article : Google Scholar
|
|
163
|
Ortona S, Barisione C, Ferrari PF, Palombo
D and Pratesi G: PCSK9 and other metabolic targets to counteract
ischemia/reperfusion injury in acute myocardial infarction and
visceral vascular surgery. J Clin Med. 11:36382022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Huang G, Lu X, Zhou H, Li R, Huang Q,
Xiong X, Luo Z and Li W: PCSK9 inhibition protects against
myocardial ischemia-reperfusion injury via suppressing autophagy.
Microvasc Res. 142:1043712022. View Article : Google Scholar : PubMed/NCBI
|