Introduction
Diabetic foot ulcer (DFU) is a common, highly morbid
consequence of longstanding and poorly-managed diabetes, exhibiting
a complex underlying pathophysiology. Among the estimated 537
million individuals with diabetes worldwide (1), 19-34% will develop a DFU in their
lifetime and 17% of patients with complications associated with DFU
undergo lower-extremity, minor (below the ankle), major (above the
ankle) or minor and major amputation (2). Additionally, the severity of DFU
causes death in 10% of these patients within 1 year of their first
diagnosis (3). Therefore,
researchers have been investigating clinical treatments and
underlying mechanisms that can facilitate DFU wound healing and
enhance patient survival rates.
Normal skin wound healing has four consecutive and
overlapping stages: i) The 'hemostasis' stage; ii) the
'inflammation' stage; iii) the 'proliferation' stage; and iv) the
'reshaping' stage (4). In the
diabetes wound, tissue ischemia, hypoxia and the high glucose
microenvironment interfere with the progress of these programmed
healing stages, resulting in delayed or absent healing of the wound
and some clinical complications (5). Re-epithelialization and dermal
repair play a crucial role in the DFU healing process. Research has
shown that during the re-epithelialization stage of wounds,
keratinocytes can migrate to the wound site and proliferate and
differentiate into different structures to restore the integrity of
the epidermal structure and function (6). However, the high glucose
environment and the chronic inflammatory state of the diabetes
wound damage the normal function of keratinocytes, resulting in
delayed re-epithelialization of the wound (7). Therefore, exploring therapeutic
targets to improve the function of diabetes skin keratinocytes to
promote chronic wound healing has become one of the research
hotspots in the DFU field in recent years.
RNA-binding proteins (RBPs) play a key role in
inflammation and immune regulation by affecting the metabolism and
stability of mRNA. The fused in sarcoma (FUS) and interleukin
enhancer-binding factor 2 (ILF2) proteins are RBPs that interact
with RNA molecules, exerting pivotal biological functions and
regulating processes such as metabolism, transport, stability and
translation (8). FUS is an RBP
containing 526 amino acids in the FET-binding protein family namely
FUS, EWS RNA binding protein 1 and TATA-box binding protein
associated factor 15. FUS is a widely expressed protein that can
shuttle between the nucleus and the cytoplasm (9). A study has shown that the
expression of RBP genes, such as FUS, in retinal microvascular
endothelial cells of high glucose-induced diabetic mice is markedly
different from that in the normal control group (10). Another study has shown that FUS
can bind to paired box 3 mRNA and positively regulate its
expression, increasing cardiac fibroblast fibrosis (11). In addition, a study has shown
that circular (circ)FNDC3B participates in the regulation of
angiogenesis through interaction with FUS (12). Although the research on FUS has
made progress in some diseases, its role in diabetic skin wound
healing is rarely reported. ILF2, also known as nuclear factor 45
(NF45), is essential for cell growth and inflammatory responses,
participating in DNA damage repair and cell division as well as
affecting cyclin expression (13). Numerous studies have shown that
ILF2 plays a key role in promoting the proliferation of cancer
cells (14,15). In addition, Jin et al
(16) found that ILF2 inhibited
NLR family pyrin domain containing 3 inflammasome activation in
macrophages. However, the role of ILF2 in diabetic skin
keratinocytes should be investigated further.
Negative pressure wound therapy (NPWT) is a widely
adopted strategy in contemporary wound care, particularly
recommended for managing complex wounds, such as foot wounds in
individuals with diabetes mellitus (DM). Evidence shows that NPWT
reduces rehospitalizations, associated surgical procedures,
dressing changes, personnel commitments, hospitalization, treatment
time and costs (17,18). A number of findings suggest that
treating DFU with NPWT reduces ulcer size, enhances granulation
tissue formation, shortens hospital stay and confers complete wound
healing (19-21). This therapy also demonstrates
promising improvements in healing rates without a notable increase
in wound complications (21).
However, the specific mechanism through which NPWT promotes DFU
wound healing remains unclear and deserves further
investigation.
RNA sequencing (RNA-seq) is a powerful genomic
technique used to study the transcriptome, which involves capturing
the sequence information of RNA molecules using high-throughput
sequencing (HTS) technologies. Hence, it enables the identification
of upregulated (i.e., activated) or downregulated (i.e., repressed)
genes under specific biological conditions, allowing crucial
insights into the mechanisms of various diseases and in biomarker
discovery. As such, the aim of the present study was to identify
key genes that promote wound healing in patients with DFU by
analyzing transcriptome sequencing data before and after NPWT, and
to explore the clinical significance of these genes in promoting
wound healing.
Materials and methods
Study participants and grouping
The present study included 27 patients with DFU who
were hospitalized in the Department of Endocrinology at The First
Affiliated Hospital of Anhui Medical University (Hefei, China) from
October, 2022 to March, 2023, and received NPWT for the first time.
The inclusion criteria for all participants were as follows: i)
Type 2 DM (T2DM) diagnosis; ii) age range, 18-80 years old; iii)
ulcer duration ≥4 weeks; iv) ulcer area range, 2-20 cm2
with a Wagner grade 2-3 (22);
and v) ankle-brachial index (ABI) range, 0.7-1.3. The exclusion
criteria were as follows: i) Severe kidney, liver and cardiac
dysfunctions; ii) a history of malignant tumors; iii) autoimmune
diseases; iv) recent use of glucocorticoids, immunosuppressants or
exogenous cytokines within the last 6 months; and v) severe sepsis.
All participants received a standard systemic treatment, including
lipid regulation, nerve-nutrition, hypoproteinemia improvement,
enhancement of blood supply of a lower limb wound and
anti-infection and antihypertensive treatments. In addition, the
patients received an appropriate glycemic control treatment. The
participants underwent wound debridement to remove blackened and
necrotic soft and bone tissues, followed by NPWT. For NPWT, a
vacuum assisted closure negative pressure-assisted healing therapy
system (Kinetic Concepts, Inc.; 3M) was used according to published
protocols (23), at a dose of
−125 mmHg (1 mmHg=0.133 kPa) for 1 week. Full-thickness skin tissue
was collected within 0.5 cm of the wound edge before and after 1
week of NPWT, only after removing the negative pressure device. The
collected granulation tissue was stored at −80°C for further
examination.
The experimental grouping was as follows: i) RNA-seq
was performed on the granulation tissue of 3 patients with DFU
before and after NPWT; and ii) 24 patients with DFU receiving NPWT
were defined as the pre-NPWT group and the post-NPWT group based on
their pre-treatment and post-treatment status. Fig. 1 shows the flowchart of the study
procedure.
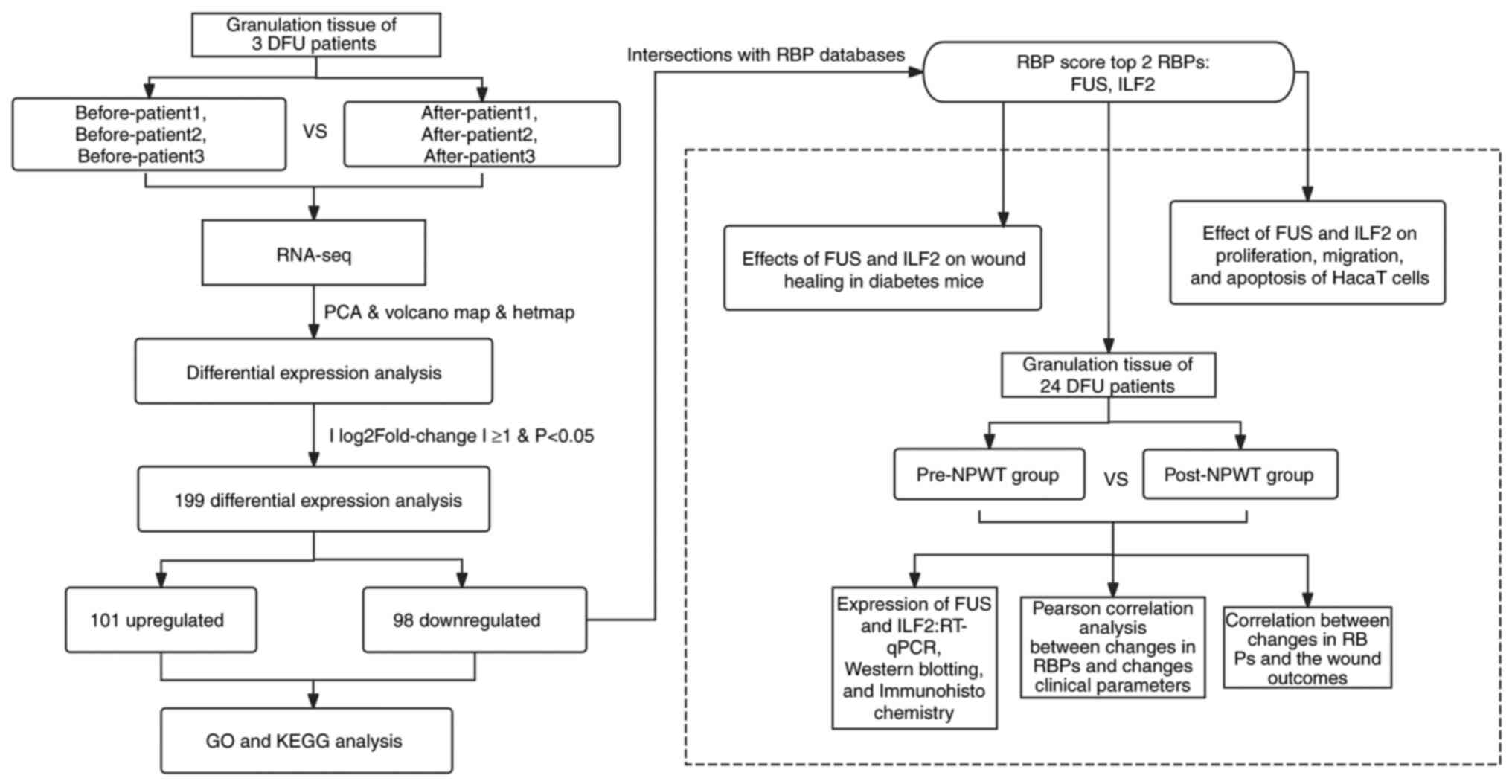 | Figure 1Flow chart of the study design. DFU,
diabetic foot ulcer; NPWT, negative pressure wound therapy; PCA,
principal component analysis; GO, Gene Ontology; KEGG, Kyoto
Encyclopedia of Genes and Genomes; RT-qPCR, reverse
transcription-quantitative PCR; FUS, fused in sarcoma; ILF2,
interleukin enhancer binding factor 2; RBP, RNA-binding protein;
RNA-seq, RNA sequencing. |
All procedures involving human participants in the
present study complied with the 1964 Helsinki Declaration and
subsequent amendments or similar ethical standards. The present
study was approved by the Medical Ethics Committee of The First
Affiliated Hospital of Anhui Medical University (approval no.
CDEC000004982), and written informed consent was obtained from the
subjects.
RNA extraction, library construction and
RNA-seq
Total RNA was extracted from the granulation tissue
of full-thickness wounds collected from 3 patients before and after
NPWT using TRIzol Reagent (cat. no. 15596026CN; Thermo Fisher
Scientific, Inc.). DNA digestion was carried out after RNA
extraction by DNaseI. RNA quality was determined by examining the
A260/A280 with a Nanodrop™ OneCspectrophotometer (Thermo Fisher
Scientific, Inc.). RNA Integrity was confirmed by 1.5% agarose gel
electrophoresis. Next, 2 μg total RNA was used for stranded
RNA sequencing library preparation using a KC™ Stranded mRNA
Library Prep kit for Illumina® (cat. no. DR08402; Wuhan
Seqhealth Co., Ltd.; Wuhan Kangee Technology) following the
manufacturer's instruction. After the library construction was
completed, RNA quantification was performed using the Qubit3.0 with
Qubit™ RNA Broad Range Assay kit (cat. no. Q10210; Thermo Fisher
Scientific, Inc.) and diluted to 4 nM. The Illumina NovaSeq 6000
sequencer was for sequencing according to the instructions of the
sequencing kit (cat. no. 12310ES96; Shanghai Yeason Biotechnology
Co., Ltd.) and generated a paired-end reading of 150 bp. The
RNA-seq data for every sample are shown in Table SI.
Bioinformatics analysis
The sequenced fragments were transformed into
sequence data with CASAVA (version1.6; https://gaow.github.io/genetic-analysis-software/c/casava/)
base recognition using image data measured by HTS machines.
Differential expression analysis was conducted to identify
differentially expressed genes (DEGs) between samples using DESeq2
(version 1.19.40; https://github.com/thelovelab/DESeq2), and the
significance was inferred by P-value and false discovery rate
(FDR). In the present study, the screening criteria for DEGs were
FDR<0.05 and the absolute value of log2FoldChange (FC)
>1.
Principal component analysis (PCA) was utilized to
examine the grouping of samples based on gene expression data from
the granulation tissue of full-thickness wounds collected from 3
patients before and after NPWT. The PCA, conducted using the prcomp
function in the stats package of R software (version 4.4.1;
https://cran.r-project.org), revealed
distinct clusters of gene expression patterns.
Gene Ontology (GO) enrichment analysis was performed
on the DEGs using GOSeq (version 1.0; https://bioconductor.org/packages/release/bioc/html/goseq.html)
and GO terms in DEGs with a corrected P<0.05 were deemed
significantly enriched. A Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analysis (http://www.genome.jp/kegg/) was conducted to assess
the pathways enriched in the DEGs. Gene Set Enrichment Analysis
(GSEA) was conducted using the clusterProfiler package (version
4.12.0; https://github.com/YuLab-SMU/clusterProfiler) under
the R software with P<0.05. Gene Set Variation Analysis (GSVA)
analysis was used to explore the differences in biological pathways
between different pattern clusters based on the enrichment scores.
The GSVA (version 1.52.3; https://github.com/rcastelo/GSVA) R software package
was used to perform functional enrichment analysis on DFU wound
granulation tissue samples before and after NPWT to obtain
enrichment pathways. A corrected P<0.05 was considered to
indicate a statistically significant difference.
Reverse transcription-qPCR (RT-qPCR)
Total RNA was extracted from the wound granulation
tissue of patients with DFU, HaCaT cells and the skin tissue of
mice using TRIzol reagent (cat. no. 15596026CN; Thermo Fisher
Scientific, Inc.), which was reverse transcribed into cDNA using a
PrimeScript RT kit (Takara Biotechnology Co., Ltd.) as per the
provided protocol. qPCR was performed with 2X Q3 SYBR qPCR Master
Mix (Universal) (cat. no. 22204; Tolo Biotech Co., Ltd.) and the
PCR procedure was as follows: Initial denaturation at 94°C for 30
sec, followed by 40 cycles of 94°C denaturation for 5 sec, 56°C
annealing for 30 sec and 72°C extensions for 10 sec. The
2−ΔΔCq method (24)
was used to calculate the relative expression level of mRNA, with
β-actin or 18S ribosomal RNA as the internal reference. All primers
used in the present study are listed in Table SII.
Collection of general information and
laboratory parameters
The laboratory parameter data were collected from
medical records. The demographic information including sex and age
were obtained from the patients. The area of the wound ulcers was
quantified using digital photography and ImageJ (version 1.8.0;
National Institutes of Health). The ABI was determined using a
doppler blood flow detector (DPL-03; Shanghai Hanfei Medical
Equipment Co., Ltd.). The transcutaneous oxygen pressure (TcPO2)
near the ulcer was measured using a TCM 400 monitoring device
(Radiometer).
After fasting for ≥8 h, venous blood samples from
the elbow vein were collected between 6 and 7 am. The collected
venous blood samples were used to determine various laboratory
indicators, including C-reactive protein (CRP), white blood cell
(WBC) count and neutrophil percentage (NEUT). Other parameters were
tested using the following methods: Fasting plasma glucose (FPG)
was measured using the glucose oxidase method; glycosylated
hemoglobin A1c (HbA1c) was measured using high-pressure liquid
chromatography; triglyceride (TG), total cholesterol (TC),
high-density lipoprotein cholesterol (HDL-C) and low-density
lipoprotein cholesterol (LDL-C) were measured using oxidase-linked
colorimetry; and renal function was measured using the estimated
glomerular filtration rate (eGFR).
Detection of related biomarkers in
granulation tissue
Granulation tissue frozen at −80°C was reduced to a
fine powder in a nitrogen-chilled mortar and transferred to a
liquid nitrogen pre-cooled centrifuge tube. The tube was
centrifuged at 9,659.5 × g for 10 min, 4°C, followed by aspirating
the supernatants. The reactive oxygen species (ROS) levels were
assessed using a dichloro-dihydro-fuorescein diacetate assay (cat.
no. KGA7501-1000; Jiangsu Kaiji Biotechnology Co., Ltd.) and the
malondialdehyde (MDA) levels were assessed using a thiobarbituric
acid assay (cat. no. KGA7101-100; Jiangsu Kaiji Biotechnology Co.,
Ltd.), following the manufacturer's instructions. The tumor
necrosis factor-α (TNF-α; cat. no. E-EL-H0109; Elabscience
Biotechnology Co., Ltd.; Wuhan Eliorite Biotech Co., Ltd.) and IL-4
(cat. no. E-EL-H0101; Elabscience Biotechnology Co., Ltd.; Wuhan
Eliorite Biotech Co., Ltd.) levels were determined using ELISA kits
following the manufacturer's instructions. The activities of the
matrix metalloproteinase (MMP) 2 and MMP9 enzymes were determined
with a gelatin zymogram kit (Cosmo Bio Co., Ltd.) according to
kit's instructions.
Western blotting
Total protein was extracted from cells and the
granulation tissue from patients using radioimmunoprecipitation
assay buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology), and protein concentrations were determined by
bicinchoninic acid protein assay (cat. no. P0010; Beyotime
Institute of Biotechnology). Equal amounts (20 μg) of
protein were loaded per lane and separated by 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis, and transferred to
polyvinylidene fluoride membranes, which were blocked with 5%
skimmed milk at room temperature for 2 h. The membranes were probed
overnight at 4°C with the following primary antibodies: Rabbit
anti-FUS (1:5,000; cat. no. 11570-1-AP; Proteintech Group, Inc.),
rabbit anti-ILF2 (1:1,000; cat. no. 14714-1-AP; Proteintech Group,
Inc.) and mouse β-actin (1:1,000; cat. no. TA-09; ZSGB BIO; OriGene
Technologies, Inc.). Subsequently, the membranes were incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit
secondary antibody (1:2,000; cat. no. ab205718; Abcam) or
anti-mouse secondary antibody (1:5,000; cat. no. 550010; Chengdu
Zen-Bioscience Co., Ltd.) at room temperature for 2 h. Enhanced
chemiluminescence substrates (cat. no. SB-WB004; ShareBio; Shanghai
Shenger Biotechnology Co., Ltd.) were used for signal detection,
and ImageJ was used to identify the grayscale value of the target
protein compared with the internal reference grayscale ratio.
Immunohistochemistry
The granulation tissue obtained from patients was
fixed in 4% paraformaldehyde at room temperature for 24-36 h. The
tissue was embedded in paraffin, cut into 5-μm-thick slices
and placed on a glass slide. The slices were immersed in xylene for
5 min 3 times for dewaxing, then in anhydrous alcohol, 95% alcohol
and 80% alcohol for 5 min each. After removal, the slices were
washed several times with tap water. Subsequently, the slices were
completely immersed in 40 ml EDTA repair solution (pH 8.0; cat. no.
ZLI-90667; ZSGB-BIO; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd.), and the boiling state of the pressure cooker was maintained
for 2.5 min. Then, the pressure cooker was depressurized and washed
with cold water, followed by further drip cooling. The slices were
removed, cooled and placed in a reaction chamber. Then, the slices
were washed with PBS for 3 min 3 times, and the endogenous
peroxidase activity was blocked with 3% H2O2
solution at room temperature for 10 min, before again washing with
PBS buffer for 3 min 3 times. The FUS (1:200; cat. no. 11570-1-AP;
Proteintech Group, Inc.) and ILF2 (1:100; cat. no. 14714-1-AP;
Proteintech Group, Inc.) primary antibodies were added to the
slices dropwise and incubated at 37°C for 60 min, before washing
with PBS for 3 min 3 times. The HRP-conjugated secondary antibody
(1:2,000; cat. no. ab205718; Abcam) was added dropwise and
incubated at room temperature for 10 min, before washing with PBS
for 3 min 3 time. Next, the slices were incubated with DAB color
reagent kit at room temperature for 5-8 min, then washed with tap
water to terminate staining after the color development completed.
The slices were counterstained with hematoxylin for 1 min, washed
with warm water and differentiated with hydrochloric acid and
alcohol. The slices were finally dehydrated, treated with xylene
for transparency and sealed with neutral resin. Images were
obtained using a light microscope and further analyzed using
ImageJ.
Cell culture and transfection
Human epidermal keratinocyte (HaCaT) cells were
purchased from Wuhan Pricella Biotechnology Co., Ltd. (cat. no.
CP-H113), which detected the quality of the cell line by PCK
immunofluorescence, with a purity of >90%. The cell line used in
the present study was also confirmed to be the HaCaT cell line by
the STR analysis method. The HaCaT cells were cultured in
low-glucose Minimum Essential Medium (MEM) containing NEAA (cat.
no. MP150410; Procell Life Science & Technology Co., Ltd.)
supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin and cultured at 37°C in a cell culture
incubator maintained at 5% CO2. When the cell growth
reached >90% confluency, trypsin digestion and passage culture
were carried out.
After cell passage, the HaCaT cells were cultured in
a 6-well plate and randomly divided into three groups: i) The
normal glucose (NG) group (5.5 mM D-glucose); and ii) the high
glucose (HG) groups (25 mM or 50 mM D-glucose). Subsequently, FUS
short-interfering RNA (siRNA) and ILF2 siRNA (Guangzhou RiboBio
Co., Ltd.) were transfected into HaCaT cells in the NG (5.5 mM) and
HG (50 mM) groups. Briefly, 0.1 nM siRNA/NC was prepared in in 200
μl Opti-MEM (cat. no. 31985070; Thermo Fisher Scientific,
Inc.), then incubated with Hieff Trans (12 μl) in
vitro siRNA transfection reagent (cat. no. 40806ES03; Shanghai
Yeason Biotechnology Co., Ltd.) at room temperature for 10 min.
Then, the siRNA mixture was added dropwise to each well and
incubate for 48 h before subsequent experiments. After
transfection, the successful knockdown using siRNA in HaCaT cells
was confirmed using RT-qPCR (Fig.
S1A). The siRNA sequences used in the present study are shown
in Table SIII. In addition, to
confirm whether the results were affected by osmotic pressure, a
supplementary experiment comparing the high osmotic pressure (HO)
group (5.5 mM D-glucose + 44.5 mM mannitol) with the HG group (50
mM) was conducted.
Cell viability assay
Cell viability was measured using the Cell Counting
Kit-8 (CCK-8) assay (Biosharp Life Sciences). The normal digested
resuspension of each group of cells was added to the wells of a
96-well plate at a density of ~1×105 cells/100
μl/well. The edge wells were filled with sterile
phosphate-buffered saline (PBS) and the inoculated cell culture
plate was placed in the incubator (37°C, 5% CO2). After
48 h of culture, the CCK-8 reagent was added to each well for 30-60
min according to the manufacturer's instructions. The absorbance
values of each well were measured at a wavelength of 450 nm using
an enzyme-linked immunosorbent assay plate reader.
Wound healing assay
Logarithmic cells were inoculated at a density of
1×106 cells/well into a 6-well plate, with three
independent wells set up for each group. When cell monomers adhered
to the wall and proliferated to a cell density of 90-100%, a
200-μl pipette tip was used to vertically scratch the plate.
The suspended cells were washed three times with PBS to remove the
scraped cells, then serum-free MEM was added, and the plate was
cultured in an incubator (37°C, 5% CO2). Images were
obtained using an optical microscope at 0 and 48 h and the
experiment was repeated three times. ImageJ (version 1.8.0;
National Institutes of Health) was used to calculate the wound
healing rate. The wound healing rate was calculated as a percentage
using the following formula: Wound healing rate (%)=[(0 h scratch
area-48 h scratch area)/0 h scratch area] ×100%.
Cell apoptosis assay
Cell apoptosis was evaluated using the Annexin
V-FITC/PI apoptosis detection kit (Beijing Solarbio Science &
Technology Co., Ltd.). HaCaT cells from each independent sample
were resuspended in 100 μl binding buffer and the membrane
was stained with annexin V-FITC (5 μl) and PI (5 μl)
in the dark for 15 min. Flow cytometry (CytoFLEX LX; Beckman
Coulter Inc.) was performed and cell apoptosis images were analyzed
using CytExpert (version 2.4.0.28; Beckman Coulter Inc.).
Animal experiments
All animal experiments were approved and performed
in accordance with the guidelines of the Animal Care and Use
Committee of Anhui Medical University (Hefei, China; approval no.
LLSC20201040). Male C57BL/6 mice (6 weeks old, 18-20 g) were
purchased from GemPharmatech Co., Ltd., raised in controlled
habitats and provided with water and food. A total of 20 mice (n=5
per group) were placed in a stainless steel cage covered with wood
shavings, at a temperature of ~22°C, with a light/dark cycle of
12:12 h and a humidity of ~ 50%. The health of the mice was
monitored by observing the temperature, humidity, noise and
lighting conditions in the animal room, and monitoring the weight
and general status of mice, such as mental state, activity, hair
loss, excretion, food intake and water intake, to evaluate the
health and behavioral status of mice. After the mice became
familiar with their habitat for 1 week, 15 mice were randomly
selected and were intraperitoneally injected with streptozotocin
(STZ) to create a diabetes model (50 mg/kg for 5 consecutive days).
Starting from day 12 after the first injection of STZ, the fasting
blood-glucose was measured using the tail cut blood sampling method
twice a week for 2 weeks. When the blood glucose was ≥250 mg/dl
(16.7 mmol/l) twice in a row, diabetes was diagnosed. All mice met
the diagnostic criteria for diabetes.
The adeno-associated virus (AAV) short-hairpin
(sh)FUS or shILF2 vectors (Shanghai GenePharma Co., Ltd.) were
generated by replacing the FUS/ILF2 transcriptional sequence with
the shRNA target of FUS/ILF2 (shFUS, 5′-CTTCAAGCAGATTGGAATTAT-3′;
shILF2, 5′-CCGGCAGGTAGGATCATATAA-3′). AAV-shRNA NC was used as the
negative control (shRNA NC, 5′-ACTACCGTTGTTATAGGTG-3′). The back
hair of the mice was shaved off and AAV-shFUS, AAV-shILF2 or
AAV-shRNA NC vector was subcutaneously injected into the back of
the mice (1.0×1011 vector genomes/mouse, 100 μl),
and the knockout efficiency in mouse skin tissue was confirmed
through RT-qPCR after 4 weeks.
Mice were anesthetized by intraperitoneal injection
of 1% sodium pentobarbital (50 mg/kg) (25), then wiped with 5% iodophor
disinfectant. After drying, the skin was disinfected with 75%
alcohol by rotating outward extension, which was repeated twice.
Under anesthesia, a 6-mm biopsy punch was used to symmetrically
form two full-thickness skin excision wounds near the midline of
the back. The mice were divided into the normal control (Ctrl), the
DM-Ctrl, the DM FUS knockdown (DM-shFUS) and the DM ILF2 knockdown
(DM-shILF2) groups. Images were collected and the size of the wound
area was measured every other day. A ruler was placed around the
wound as a reference to correct the distance between the camera and
the animal. After the study was completed, mice were euthanized by
intraperitoneal injection of 3% pentobarbital sodium (100 mg/kg).
The breathing, heartbeat and consciousness of the mice were
observed for >3 min until their death was confirmed (26). If the mice exhibited persistent
pain behavior, severe dehydration, inability to eat, extreme
fatigue or even severe infection during the research process, they
were euthanized; however, no animals in the present study reached
these humane endpoints. ImageJ (version 1.8.0; National Institutes
of Health) was used to calculate the wound area in pixels. The
wound closure rate was calculated as a percentage using the
following formula: Wound healing (%)=[(initial wound area-final
wound area)/initial wound area] ×100%.
Statistical analysis
Data analysis was conducted using SPSS (version
22.0; IBM Corp.). The analysis software used to intersect DEGs with
the RBP2GO dataset was the Venny tool (version 2.1; https://bioinfogp.cnb.csic.es/tools/venny/index.html)
(27). Measured data with a
normal distribution are presented as the mean ± standard deviation,
and those with a non-normal distribution are presented as the
median [interquartile range: P25, P75]. Comparisons before and
after NPWT were performed using paired t-test or the Wilcoxon
rank-sum test. Ranked data were analyzed using the Fisher's exact
test or Kruskal Wallis test followed by the Dunn's post hoc test.
One-way analysis of variance were used to determine the statistical
significance between multiple groups followed by the Bonferroni
post hoc test. Changes before and after NPWT were calculated based
on the values of various indicators measured before and after the
therapy (Δ value). The associations between the expression changes
of target proteins and other clinical variables were evaluated with
Pearson's correlation coefficient. P<0.05 was considered to
indicate a statistically significant difference.
Results
General information on the 3
subjects
A total of 3 patients with DFU were subjected to
RNA-seq (Table I; patients 1, 2
and 3). Of the 3 patients, 1 was female and 2 were male, all with
T2DM and aged 47-64. The patients had a long disease course, poor
blood-glucose control, a long DFU time and normal blood perfusion.
Generally, their wounds had similar infection statuses and blood
supplies before NPWT. In addition, after 1 week of NPWT and a
systemic standard treatment, laboratory results demonstrated that
the WBC, NEUT and CRP levels in the blood decreased, indicating
that 1 week of NPWT improved the wound infection of these 3
patients with DFU (Table I).
 | Table IClinical characteristics of 3
participants with a diabetic foot ulcer subjected to RNA
sequencing. |
Table I
Clinical characteristics of 3
participants with a diabetic foot ulcer subjected to RNA
sequencing.
| Clinical
feature | Patient 1 | Patient 2 | Patient 3 |
|---|
| Sex | Male | Male | Female |
| Age, years | 58 | 64 | 47 |
| BMI,
kg/m2 | 22.89 | 24.52 | 21.94 |
| Ulcer duration,
weeks | 5 | 9 | 7 |
| Ulcer area,
cm2 | 8.7 | 12.5 | 10.9 |
| Diabetes duration,
years | 11.0 | 19.0 | 9.0 |
| Diabetes type | 2 | 2 | 2 |
| Wagner grade | 3 | 3 | 3 |
| ABI | 1.03 | 0.91 | 0.98 |
| TcPO2, mmHg | | | |
| Right | 70.2 | 73.6 | 71.4 |
| Left | 68.9 | 72.5 | 70.7 |
| FPG, mmol/l | 7.9 | 9.0 | 8.4 |
| HbA1c, % | 8.0 | 8.8 | 8.6 |
| WBC,
×109 | | | |
| Before NPWT | 11.8 | 13.2 | 12.8 |
| After NPWT | 8.5 | 9.0 | 8.7 |
| NEUT | | | |
| Before NPWT | 80.3 | 88.9 | 85.5 |
| After NPWT | 69.7 | 73.5 | 71.0 |
| CRP, mg/dl | | | |
| Before NPWT | 19.3 | 22.1 | 20.4 |
| After NPWT | 10.9 | 13.2 | 11.3 |
Transcriptome sequencing analysis of
granulation tissue
To explore the biological significance of the DFU
wound tissue samples before and after NPWT, GSEA was performed to
assess the association between gene sets from the GO and KEGG
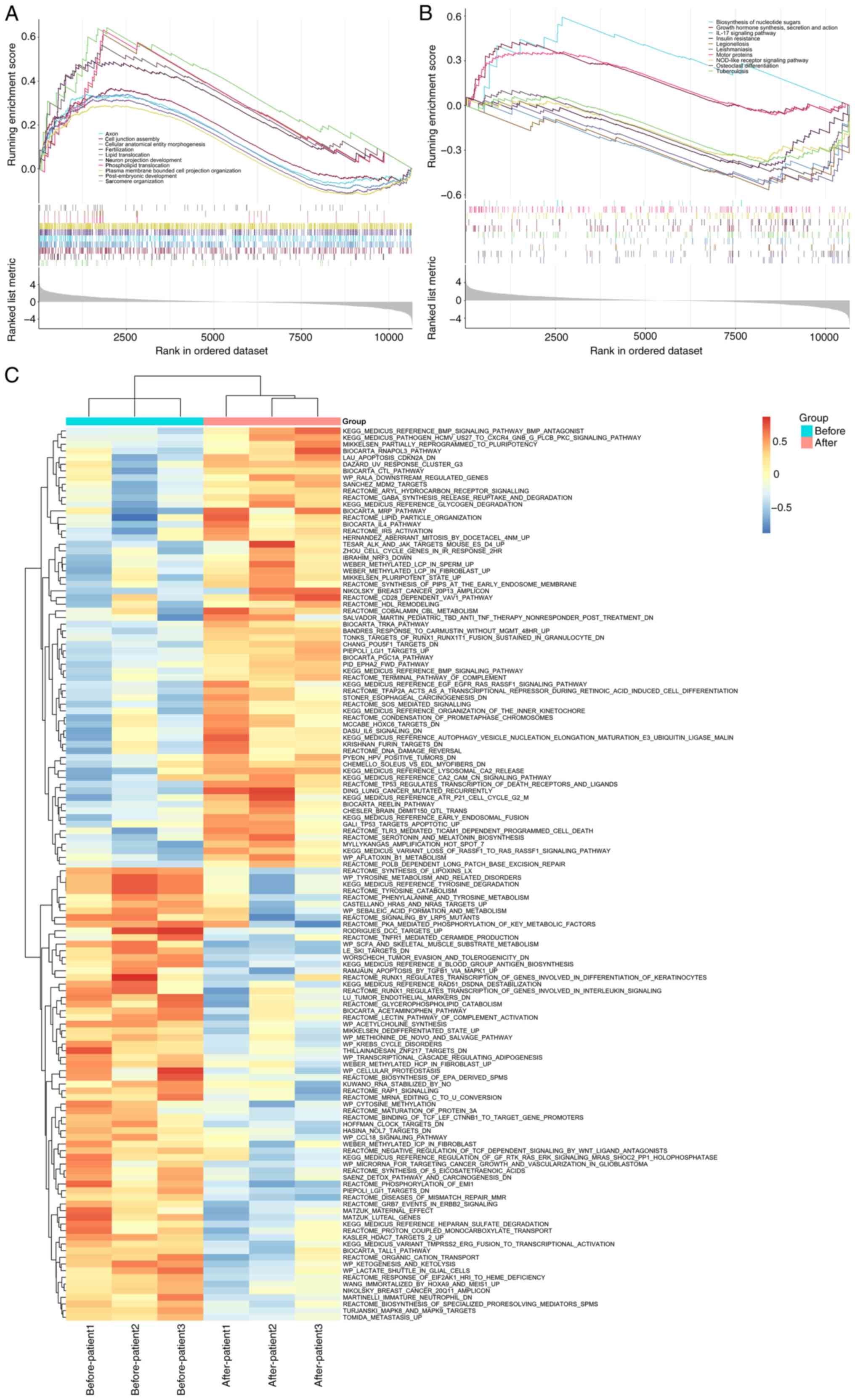
databases and the transcriptome. The enrichment analysis of the top
10 GO gene sets was mainly related to cell tissue formation and
lipid transport (P<0.05; Fig.
2A). The enrichment analysis of the top 10 KEGG gene sets was
mainly related to the 'IL-17 signaling pathway' and 'Insulin
resistance' (P<0.05; Fig.
2B). In addition, GSVA was also conducted and it was found that
before NPWT, the genes were mainly enriched in amino acid
metabolism-related signaling pathways and after NPWT, the genes
were mainly enriched in signaling pathways related to cell
apoptosis (Fig. 2C).
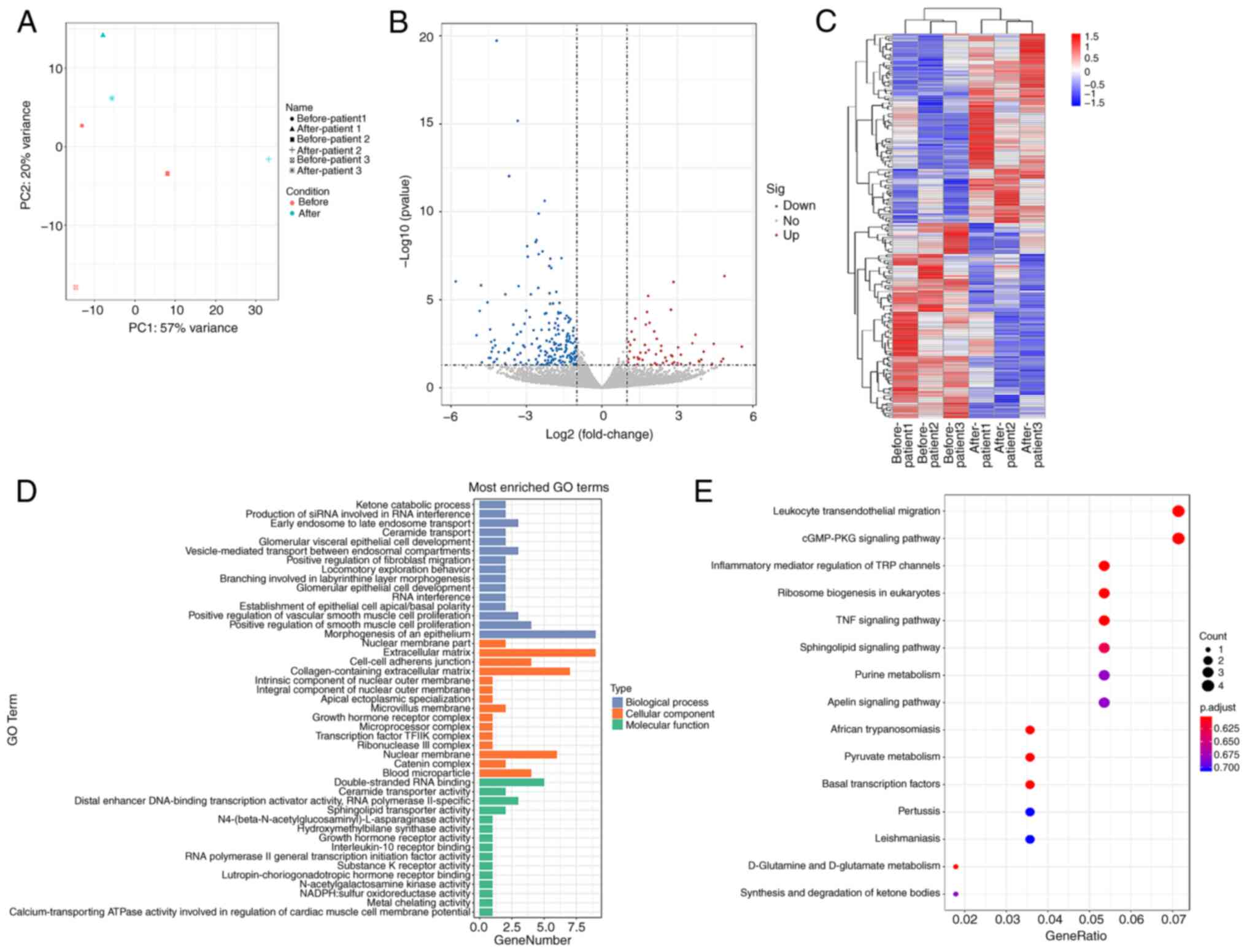
Clusters of distinct groups were evident in the
principal component analysis (Fig.
3A). Using |log2FC|≥1 and adjusted P<0.05 as the screening
threshold, 199 DEGs were identified, of which 101 were upregulated
and 98 were downregulated genes (Fig. 3B and C). To elucidate the
biological characteristics of the DEGs, GO and KEGG pathway
analyses were used to demonstrated that that DEGs have notable
roles in cell proliferation and extracellular matrix (ECM)
synthesis, mainly involving pathways in inflammation and immune
cell signaling, amino acid metabolism and lipid metabolism
(Fig. 3D and E; Tables SIV and SV).
Validation of FUS and ILF2 expression in
24 patients with DFU
Previous studies have shown that some RNA-encoded
proteins are RBPs (28,29). The DEGs before and after NPWT
described in the aforementioned results were intersected with the
human RBP dataset (https://rbp2go.dkfz.de/), and it was shown that a
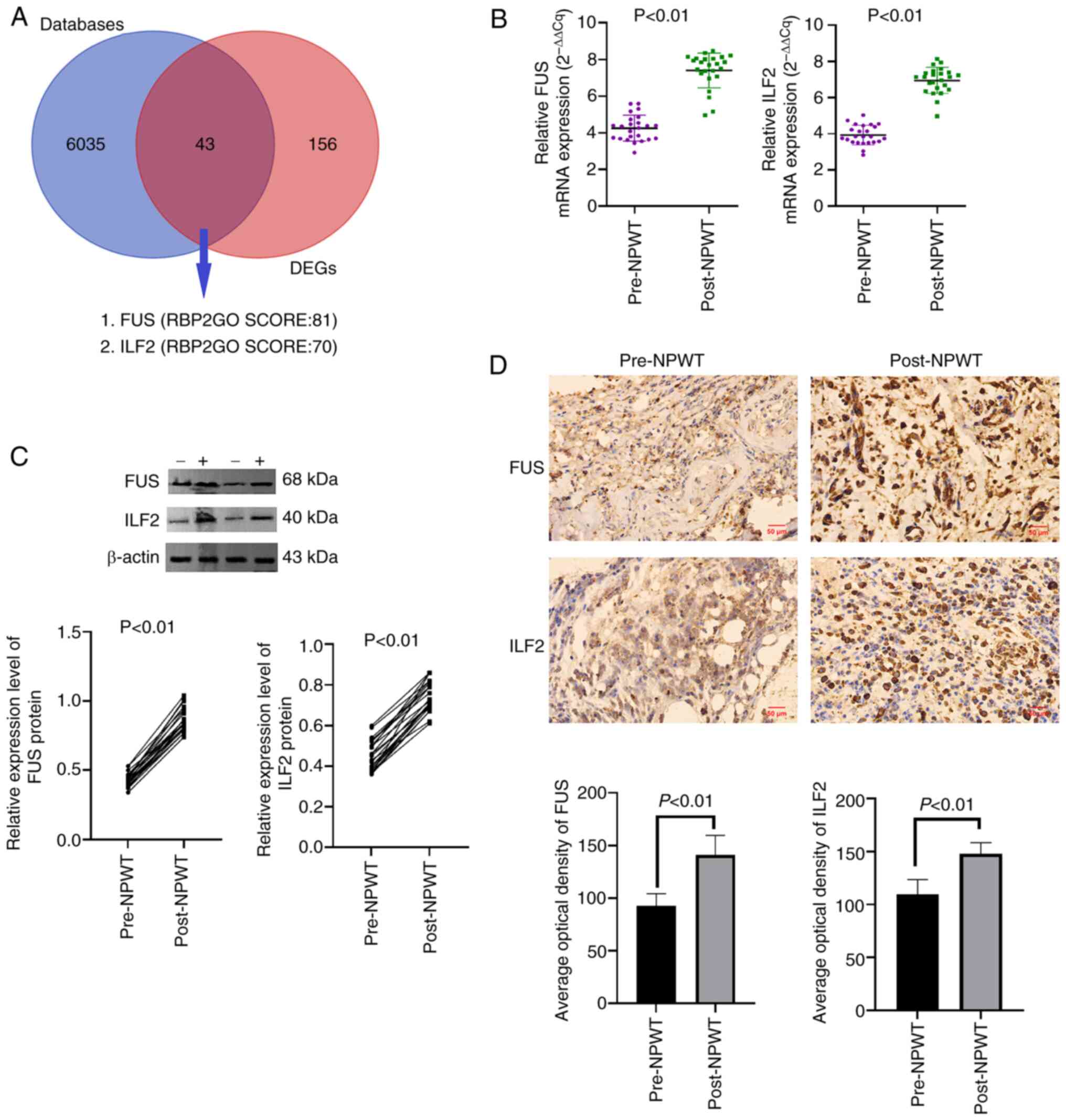
total of 43 DEGs encoded RBPs. Among them, the top two with the
highest RBP2GO score were FUS and ILF2 (Fig. 4A). Subsequently, the expression
levels of FUS and ILF2 in granulation tissue of skin wound margin
collected from 24 patients with DFU before and after NPWT were
detected by RT-qPCR, western blotting and immunohistochemistry As
anticipated, the mRNA and protein [tissue (T)-FUS; T-ILF2]
expression levels of FUS and ILF2 in granulation tissue were
markedly upregulated after 1 week of NPWT (Fig. 4B and C). Moreover,
immunohistochemical staining showed that the expression of FUS and
ILF2 in the granulation tissue of the wound margin increased
significantly after 1 week of NPWT (Fig. 4D), confirming the effectiveness
of RNA-seq and the accuracy of the results.
Comparison of primary parameters among 24
patients before and 1 week after NPWT
After 1 week of NPWT and 1 week systemic standard
treatment, the FPG (P<0.05) and the inflammatory markers, WBC,
NEUT and CRP, of patients significantly decreased (P<0.05)
(Table II). In addition, the
levels of ROS, lipid peroxides (MDA) and the pro-inflammatory
factor, TNF-α, significantly decreased, and those of the
anti-inflammatory factor, IL-4, significantly increased in the
wound granulation tissue after NPWT (P<0.05). Notably, the MMP2
and MMP9 levels also significantly decreased (P<0.05). Except
for the aforementioned indicators, no significant change was
observed in other indicators before and after NPWT (P>0.05).
 | Table IIComparison of the primary parameters
among the 24 participants before and 1 week after NPWT. |
Table II
Comparison of the primary parameters
among the 24 participants before and 1 week after NPWT.
| Variables | Pre-NPWT | Post-NPWT | P-value |
|---|
| Sex,
male/female | 18/6 | 18/6 | - |
| Age, years | 49.2±11.9 | 49.2±11.9 | - |
| BMI,
kg/m2 | 24.22±2.13 | 24.09±2.26 | 0.086 |
| Ulcer duration,
weeks | 6.7±0.7 | 7.75±1.29 | 0.010 |
| Ulcer area,
cm2 | 9.5±1.3 | 8.00±1.12 | <0.001 |
| ABI | 0.89±0.04 | 0.89±0.03 | 0.663 |
| FPG, mmol/l | 9.50±3.37 | 6.14±0.89 | <0.001 |
| HbA1c, % | 8.11±1.19 | 8.08±1.27 | 0.565 |
| TG, mmol/l | 1.70±0.41 | 1.70±0.40 | 0.435 |
| TC, mmol/l | 5.52 (4.32,
6.01) | 5.51 (4.36,
6.05) | 0.156 |
| LDL-C, mmol/l | 3.05 (2.63,
3.21) | 3.06 (2.56,
3.25) | 0.435 |
| HDL-C, mmol/l | 1.26±0.17 | 1.26±0.17 | 0.784 |
| eGFR, ml/min/1.73
m2 | 94.88±13.48 | 93.75±11.40 | 0.200 |
| WBC,
×109 | 15.78 (11.40,
25.53) | 8.32 (6.92,
12.40) | <0.001 |
| NEUT | 78.00 (71.2,
83.8) | 64.66 (59.25,
71.25) | <0.001 |
| CRP, mg/dl | 35.85 (17.53,
47.28) | 9.06 (7.74,
15.07) | <0.001 |
| TNF-α, pg/ml | 97.70±3.02 | 65.85±3.27 | <0.001 |
| IL-4, ng/l | 45.75±3.28 | 75.72±3.57 | <0.001 |
| ROS, U/ml | 8.58±0.48 | 4.16±0.47 | <0.001 |
| MDA, nmol/mg | 3.71±0.33 | 1.62±0.36 | <0.001 |
| MMP2, ng/ml | 204.79±5.58 | 152.09±5.70 | <0.001 |
| MMP9, ng/ml | 145.99±3.49 | 106.80±4.96 | <0.001 |
| T-FUS | 0.44 (0.38,
0.46) | 0.84 (0.80,
0.94) | <0.001 |
| T-ILF2 | 0.45±0.08 | 0.74±0.07 | <0.001 |
Correlation between ΔT-FUS and ΔT-ILF2
and the Δvalues of clinical parameters before and after NPWT
The changes in the T-FUS and T-ILF2 (ΔT-FUS and
ΔT-ILF2) before and after NPWT were negatively correlated with
ΔFPG, ΔWBC, ΔNEUT, ΔCRP, ΔTNF-α, ΔROS, ΔMDA, ΔMMP2 and ΔMMP9
(P<0.05), but positively correlated with ΔIL-4 (P<0.05)
(Fig. 5). In addition, no
significant correlation with other clinical indicators was noted
(P>0.05).
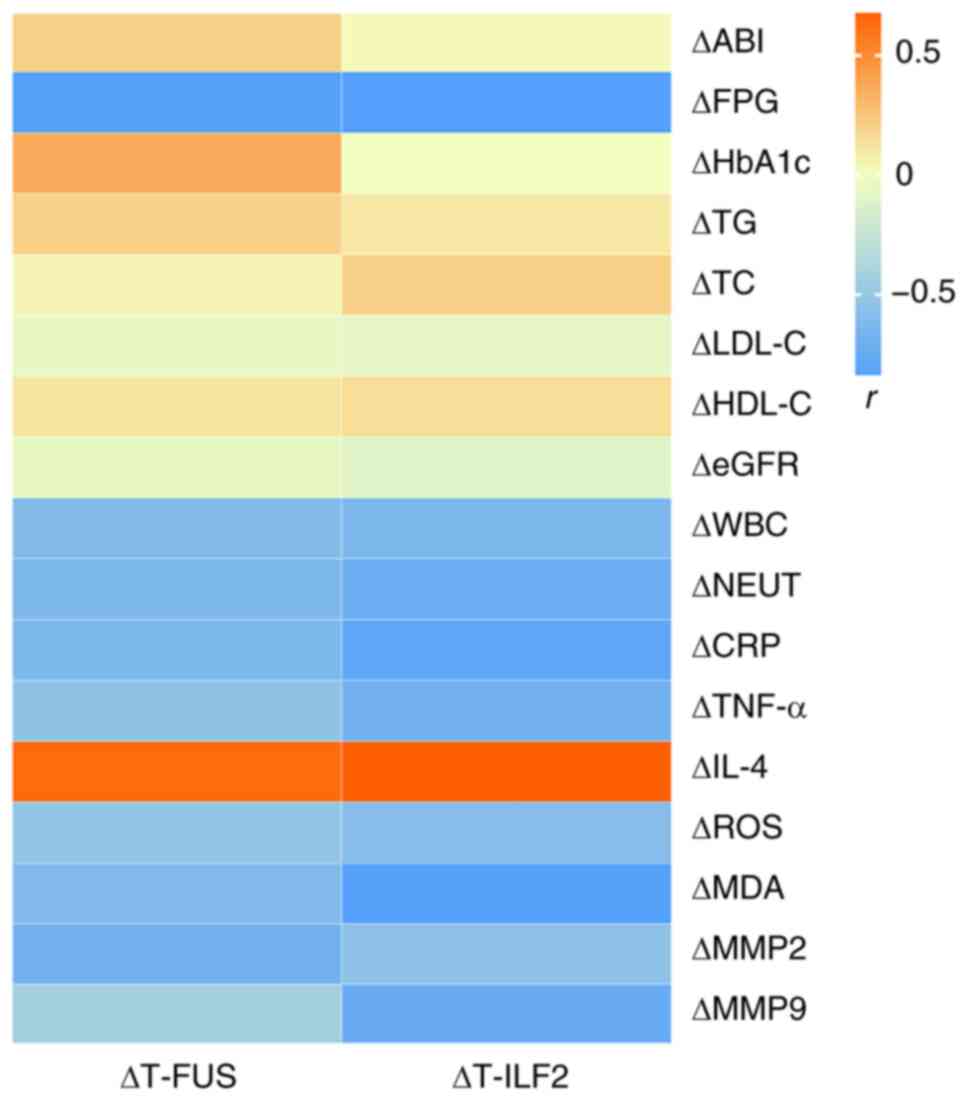 | Figure 5Pearson's correlation coefficient
between changes in T-FUS and T-ILF2 and changes in other clinical
parameters (r). T-FUS, the protein expression levels of FUS in the
granulation tissue; T-ILF2, the protein expression levels of ILF2
in the granulation tissue; NPWT, negative pressure wound therapy;
ABI, ankle brachial index; FPG, fasting plasma glucose; HbA1c,
glycated hemoglobin A1c; TG, triacylglycerol; TC, total
cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C,
high density lipoprotein cholesterol; eGFR, estimated glomerular
filtration rate; WBC, white blood cell; NEUT, neutrophil
percentage; CRP, C-reactive protein; TNF-α, tumor necrosis
factor-α; IL-4, interleukin-4; ROS, reactive oxygen species; MDA,
malondialdehyde; MMP2, matrix metalloproteinase 2; MMP9, matrix
metalloproteinase 9; FUS, fused in sarcoma; ILF2, interleukin
enhancer binding factor 2. |
Correlation between ΔT-FUS and ΔT-ILF2,
and the wound outcomes
The median values of ΔT-FUS and ΔT-ILF2 were used as
the cut-off values to investigate the relationship between ΔT-FUS
and ΔT-ILF2 and the wound healing rate after 4 weeks of NPWT.
Patients with values below the median were classified as the low
change group (LCG), while those with values equal to or greater
than the median were designated the high change group (HCG). It was
observed that ΔT-FUS and ΔT-ILF2 were positively correlated with
the wound healing rate 4 weeks after NPWT (ΔT-FUS, P=0.043;
ΔT-ILF2, P=0.011; Table III).
In addition, the wound healing rate of patients with DFU after
stopping NPWT for 4 weeks was 41.67% (n=10/24).
 | Table IIIAssociation between ΔT-FUS and
ΔT-ILF2 and wound outcomes. |
Table III
Association between ΔT-FUS and
ΔT-ILF2 and wound outcomes.
| ΔT-FUS
| ΔT-ILF2
|
|---|
| HCG, n=12 | LCG, n=12 | P-value | HCG, n=11 | LCG, n=13 | P-value |
|---|
| Mean age ± SD,
years | 49.7±13.0 | 48.7±11.1 | 0.842 | 49.0±13.1 | 49.3±11.3 | 0.951 |
| Sex, n (%) | | | 1.000 | | | 0.166 |
| Male | 9 (75.0) | 9 (75.0) | | 10 (90.9) | 8 (61.5) | |
| Female | 3 (25.0) | 3 (25.0) | | 1 (9.1) | 5 (38.5) | |
| Ulcer duration, n
(%) | | | 0.742 | | | 0.860 |
| ≤6 weeks | 4 (33.3) | 5 (41.7) | | 4 (36.4) | 5 (38.5) | |
| 6-10 weeks | 7 (58.3) | 5 (41.7) | | 5 (45.5) | 7 (53.8) | |
| >10 weeks | 1 (8.4) | 2 (16.6) | | 2 (18.1) | 1 (7.7) | |
| Ulcer area, n
(%) | | | 0.688 | | | 0.440 |
| ≤10
cm2 | 6 (50.0) | 5 (41.7) | | 6 (54.5) | 5 (38.5) | |
| >10
cm2 | 6 (50.0) | 7 (58.3) | | 5 (45.5) | 8 (61.5) | |
| Wagner, n (%) | | | 0.680 | | | 0.918 |
| Ⅱ | 4 (33.3) | 5 (41.7) | | 4 (36.4) | 5 (38.5) | |
| Ⅲ | 8 (66.7) | 7 (58.3) | | 7 (63.6) | 8 (61.5) | |
| Ulcer healing rate
after | | | 0.043 | | | 0.011 |
| 4 weeks, n (%) | | | | | | |
| Healing | 8 (66.7) | 2 (16.7) | | 8 (72.7) | 2 (15.4) | |
| Non-healing | 4 (33.3) | 10 (83.3) | | 3 (27.3) | 11 (84.6) | |
Effect of FUS and ILF2 on HaCaT cell
function
To investigate the effect of FUS and ILF2 on HaCaT
cell function, the following experiment was conducted: HaCaT cells
were cultured in 5.5, 25 and 50 mM glucose for 72 h, and it was
found that the mRNA and protein levels of FUS and ILF2 in the HG
group (50 mM) were significantly reduced compared with the NG group
(Fig. 6A). In addition, it was
confirmed that the effect of high sugar on target genes was not
related to the effect of osmotic pressure (Fig. S2). Therefore, a high glucose
concentration of 50 mM was selected as the concentration for the HG
group for subsequent experiments. After transfecting HaCaT cells of
the NG and HG groups with FUS and ILF2 siRNA (Fig. 6B), the expression of FUS and ILF2
was significantly reduced (P<0.05), indicating successful
transfection.
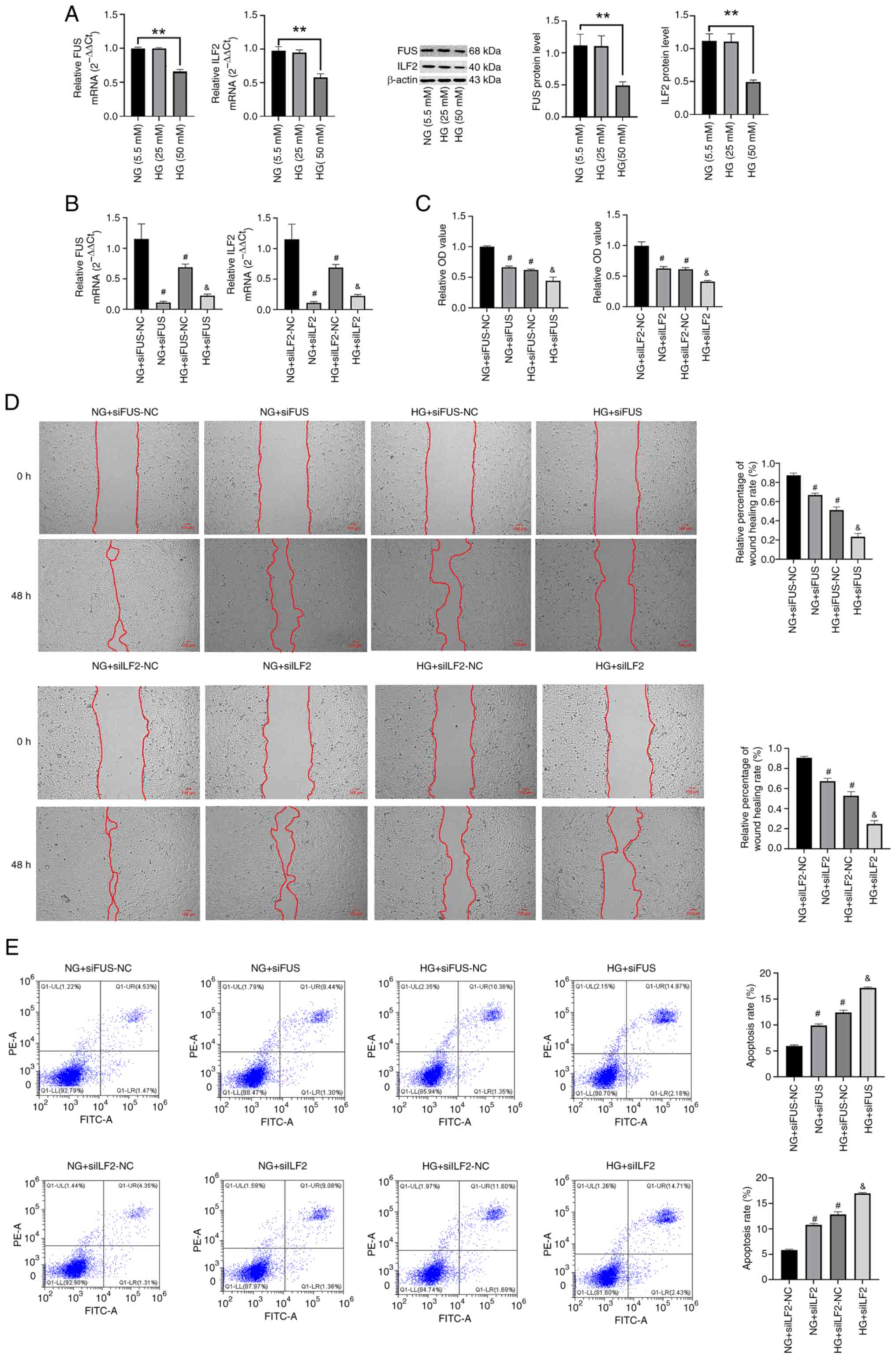 | Figure 6Effects of FUS and ILF2 on the
proliferation, migration and apoptosis of HaCaT cells. (A) mRNA and
protein expression levels of FUS and ILF2 in HaCaT cells under
different glucose concentration were measured by RT-qPCR and
western blotting, respectively. (B) mRNA expression levels of FUS
and ILF2 in HaCaT cells under different conditions were measured by
RT-qPCR. (C) Cell viability was monitored by the Cell Counting
Kit-8 assay. (D) Cell migratory capability was measured by wound
healing assay. (E) Cell apoptosis was measured by flow cytometry
(Annexin V-PI). Data are presented as the mean ± SEM. One-way
analysis of variance was used to determine the statistical
significance between groups followed by the Bonferroni post hoc
test. vs. NG, **P<0.01; vs. NG + si(FUS/ILF2)-NC,
#P<0.05; vs. HG + si(FUS/ILF2)-NC,
&P<0.05. FUS, fused in sarcoma; ILF2, interleukin
enhancer binding factor 2; RT-qPCR, reverse
transcription-quantitative PCR; NG, normal glucose; HG, high
glucose; siRNA, short-interfering RNA; NC, negative control. |
The CCK-8 assay results indicated that the cell
viability of the HG-siFUS/ILF2-NC group was significantly reduced
compared with the NG-siFUS/ILF2-NC group (P<0.05). In addition,
under different glucose concentrations, knockdown of the FUS and
ILF2 genes significantly reduced the cell viability (P<0.05;
Fig. 6C).
The results of the wound healing experiment showed
that, compared with the NG-siFUS/ILF2-NC group, the
HG-siFUS/ILF2-NC group exhibited a significant decrease in cell
migration ability (P<0.05). In addition, HaCaT cells under
different glucose concentration conditions that were transfected
with FUS or ILF2 siRNA showed a significant decrease in the cell
migration rate (P<0.05; Fig.
6D).
Flow cytometry analysis was used to evaluate the
apoptosis of HaCaT cells, and the results revealed a significant
increase in cell apoptosis rate under HG-siFUS/ILF2-NC conditions
compared with NG-siFUS/ILF2-NC conditions. Moreover, under both NG
and HG conditions, the apoptosis rate of HaCaT cells transfected
with FUS or ILF2 siRNA was significantly increased (P<0.05;
Fig. 6E). In summary, HG culture
conditions caused a degree of damage to the function of HaCaT
cells, while FUS and ILF2 enhances the proliferation and migration
ability of HaCaT cells, while reducing cell apoptosis.
Effects of FUS and ILF2 on wound healing
in diabetic mice
After subcutaneously injecting AAV-shFUS, AAV-ILF2
or AAV-shCtrl vector into the backs of diabetic mice for 4 weeks,
the knockout efficiency was verified by RT-qPCR (Fig. S1B). As shown in Fig. 7A, compared with DM-Ctrl, the
expression level of the target genes in the DM-shFUS and DM-shILF2
groups significantly decreased (P<0.05). Additionally, compared
with healthy mice, wound healing in diabetic mice was significantly
delayed. Furthermore, compared with the DM-Ctrl mice, wound healing
in diabetic mice was slower after the administration of AAV-shFUS
(P<0.05) or AAV-shILF2 (P<0.05) (Fig. 7B and C).
Discussion
DFUs affect ~18.6 million individuals worldwide
annually, and these ulcers are associated with impaired physical
function and reduced quality of life (30). If not treated promptly, foot
ulcers can progress to soft tissue infections, gangrene and limb
loss (31). Therefore,
identifying therapeutic targets for the healing of DFU wounds is
particularly important.
There are numerous clinical adjuvant treatments for
DFU, including wound dressings such as hydrogels and alginates,
local treatments, placenta-derived treatments, hyperbaric oxygen
therapy and NPWT (32). NPWT has
been widely used as an adjuvant treatment technique for complex
wounds in DFU, mainly for wounds with soft tissue infection, bone
and tendon exposure, osteomyelitis, skin graft or flap graft after
surgery and amputation/toe surgery (33-35). A retrospective study of 75
patients with chronic diabetic ulcers treated with surgical
debridement, mesh skin grafts and NPWT for biofilm-related
infections showed that all 75 wounds healed successfully, with an
average complete wound healing time of 3.5±1.8 weeks (36). A 16-week-long, 18-center
randomized clinical trial conducted in the United States included
162 diabetic foot amputees, and the results showed that more
patients in the NPWT group healed compared with the control group,
and the wound healing speed was faster than that in the control
group (37). As has been
previously reported, NPWT can markedly increase the number of
endothelial progenitor cells in the peripheral blood of patients
with DFU, thereby promoting wound healing (23). In addition, NPWT can promote
wound healing in DFUs by affecting the expression of microRNAs
(miRs) (38). Although previous
studies have shown that NPWT has high clinical application value in
promoting wound healing in DFU (39,40), its potential mechanism of action
is still unclear. The present study used rigorous RNA-seq
technology to accurately and systematically describe the
transcriptome differences in wound granulation tissue before and
after 1 week of NPWT in patients with DFU. In addition, the
mechanisms of action of the top two genes encoding the RBPs, FUS
and ILF2, were explored in wound healing in DFU through in
vitro and in vivo experiments.
Previous studies have shown that the incidence rate
of DFU is higher among male than female patients with diabetes
(41). Seghieri et al
(42) conducted a study based on
the diabetes population in Italy and showed that of the 4,589
patients with diabetic foot (DF), 3,119 were male and 1,470 were
female. In addition, a retrospective study by Iacopi et al
(43) showed that 615 male
patients and 227 female patients were hospitalized for DF from
January, 2011 to December, 2015. Sex differences may arise from
potential risk factors, access to care, differences in screening
and treatment compliance (44).
Additionally, the prevalence of peripheral neuropathy and
peripheral vascular disease is higher in male patients with
diabetes (), and the occurrence and development of DFU are
typically related to poor blood-glucose control, blood circulation
disorders, peripheral neuropathy and secondary infection wounds in
patients with diabetes (45).
This explains the sex difference in the number of male and female
patients with DFU included in the present study and highlights the
importance of comprehensive treatment for patients with DFU, with
optimizing blood-glucose levels being the first priority. High
blood-glucose can have a notable impact on endothelial cell
function, and reduced endothelial cell numbers and dysfunction can
lead to the development of macrovascular and microvascular
complications in diabetes (46).
Multiple observational studies have found a positive correlation
between blood-glucose control and wound healing (47-49). In addition, a retrospective study
found that intensive control of blood-glucose reduced the risk of
lower limb amputation in patients by 35% (50). Similarly, the in vitro
experiments of the present study showed that high glucose
significantly inhibited the proliferation and migration of
keratinocytes, while promoting their apoptosis. Further animal
experiments showed that high glucose significantly inhibited skin
wound healing.
Dyslipidemia can lead to the formation of
atherosclerotic plaques and arteriosclerosis, ultimately resulting
in vascular stenosis and occlusion. Peripheral blood vessels are
some of the main blood vessels that are often present in
atherosclerosis. In addition, vascular inflammation is one of the
mechanisms of vascular disease formation (51). Therefore, effective lipid
regulation is essential for the treatment of DFU. It is noteworthy
that strict blood-glucose control and lipid-lowering therapy can
also markedly improve diabetic peripheral neuropathy (52). More notably, antibiotic treatment
is needed in cases of DFU complicated by infection. The Infectious
Diseases Society of America recommends a 1-2-week course of
antibiotics for mild infections and a 2-3-week course of
antibiotics for moderate-to-severe infections, but antibiotics can
typically be stopped once the clinical symptoms and signs of
infection have resolved (53).
As demonstrated in the present study, the hematological
inflammatory indicators (WBC, CRP and NEUT) in patients with DFU
after NPWT were significantly decreased compared with those before
treatment, which may be due to the rational use of antibiotics
during the entire treatment period, although it cannot be ruled out
that NPWT use may have also contributed. Previous studies have
shown that NPWT can inhibit bacterial growth in tissues, reduce
inflammation and alleviate oxidative stress (54). This was also reflected in the
results of the present study, where the markers of oxidative stress
(ROS and MDA) were significantly decreased after NPWT.
RBPs are associated with numerous diseases,
particularly metabolic disorders such as hyperuricemia,
hyperlipidemia, hypertension, non-alcoholic fatty liver and
diabetes (7), but there are
relatively fewer studies on their role in wound healing. There has
been a study reporting that long non-coding RNA (lncRNA) TINCR
binds directly to the RBP, staphylococcal nuclease and tudor domain
containing 1, and mediates TGF-β1 expression to promote excessive
proliferation and inflammation in burn-induced skin fibroblasts
(55). Meder et al
(56) reported that
overexpression of the RBP, lin-28 homolog B (LIN28B), upregulates
VEGFA mRNA and miR-21 expression, thereby enhancing angiogenesis
and accelerating wound healing. Guo et al (57) reported that the RBP, LIN28A,
promotes proliferation and ECM synthesis in human skin fibroblasts
after thermal injury. ECM is a complex three-dimensional network of
fibronectin and matrix, which supports and connects tissue
structures, regulates tissue growth and cell physiological
activities and plays an important role in organism development,
tissue dynamic equilibrium and wound healing (58). MMPs, as a family of
Zn2+-dependent metalloproteinases, degrade ECM
components and participate in wound healing (59). A study has found that MMP2 and
MMP9 are expressed at higher levels in diabetic tissue and high
blood-glucose levels can increase the activity of MMP2 in vascular
cells, stimulating the degradation of ECM and causing imbalance in
diabetes (60). Furthermore,
certain studies have shown that excessive MMP9 is a predictor of
poor wound healing in diabetic skin lesions (61,62). Cui et al (63) showed that the RBP, HuR, binds to
MMP9 mRNA, enhancing its stability and promoting wound healing. The
results of the present study indicated that expression of MMP2 and
MMP9 in the DFU wound tissue was significantly reduced after NPWT.
Conversely, expression of FUS and ILF2 was significantly increased,
and the changes in these proteins were negatively correlated with
the changes in MMPs. Therefore, the promotion of DFU wound healing
by NPWT may be related to a significant downregulation in MMPs, and
this downregulation may be related to the changes in FUS or ILF2,
which warrants further investigation in the future.
FUS is an RBP with multiple functions and domains.
Previous studies have shown that lncRNA GAS6-AS1 promotes
tripartite motif containing 14-mediated cell proliferation,
migration and invasion of colorectal cancer through competing
endogenous RNA networks and FUS-dependent pathways (64). Another study has shown that
FUS-mediated circRHOBTB3, a tumor activator, promotes proliferation
of pancreatic ductal adenocarcinoma cells by regulating autophagy
regulated by the miR-600/nucleus accumbens associated 1/Akt/mTOR
axis (65). In addition, Wang
et al (66) found that
FUS can inhibit the proliferation and migration of human umbilical
vein endothelial cells and reduce inflammation in atherosclerosis.
It can therefore be inferred that FUS plays an important role in
promoting cell proliferation and reducing inflammation in certain
diseases. This is consistent with the findings of the present
study, as it was shown that the upregulation of FUS in the
granulation tissue of DFU wounds after NPWT was positively
correlated with the anti-inflammatory cytokine, IL-4, and wound
outcomes. By contrast, FUS was negatively correlated with
inflammation-related (WBC, NEUT and CRP) and oxidative stress (ROS
and MDA) markers. The in vitro experiments in the present
study further demonstrated that knockdown of FUS inhibited the
proliferation and migration of HaCaT cells in different glucose
environments, while it promoted cell apoptosis. The in vivo
experiments in the present study confirmed that knockdown of FUS
delayed the skin wound healing in diabetic mice. On the contrary, a
study has shown that FUS can reduce the expression of proliferation
factors such as cyclin D1, thereby preventing the growth of
prostate cancer cells (67).
Therefore, the functions of FUS are diverse and worth exploring in
depth.
In addition, ILF2 is also crucial for cell growth
and the inflammatory response (13). As a previous study has shown,
circ406961 inhibits the activation of the STAT3/c-Jun N-terminal
kinase pathway by interacting with ILF2 protein, thereby inhibiting
the PM2.5-induced inflammatory response (68). In addition, lncRNA LINC00470 can
promote cell proliferation by binding to NF45/NF90 complexes
(39). Previous studies have
also confirmed that ILF2 promotes the proliferation of tumor cells
(70,71). However, other studies have
demonstrated that ILF2 promotes keratinocyte proliferation and
inflammatory response in a long non-coding RNA KLHDC7B-DT-dependent
manner (72). In the present
study, it was shown through the analysis of clinical samples that
the expression of ILF2 was significantly upregulated after NPWT.
This change had the opposite trend to the proinflammatory factors
and oxidative stress markers (ROS and MDA) and was positively
correlated with wound outcomes. In addition, it was observed that
knocking down ILF2 inhibited the proliferation and migration of
HaCaT cells and caused a significant delay in the healing of skin
wounds in diabetic mice.
According to the KEGG pathway analysis, the DEGs of
DFU before and after NPWT were associated with the TNF signaling
pathway. Previous studies have shown that ginsenoside combined with
bone marrow mesenchymal stem cells can reduce the inflammatory
reaction and promote the healing of diabetic skin ulcers by
downregulating the expression of TNF-α, a key component of the TNF
signaling pathway (73). In
addition, curcumin treatment of wounds in diabetic mice can inhibit
the expression of TNF-α and MMP-9 and accelerate the healing of
diabetic skin wounds (74). In
the present study, a significant decrease in TNF-α expression in
the clinical samples after NPWT compared with before treatment was
observed, and this change was significantly negatively correlated
with the upregulation of FUS and ILF2. Although a series of studies
have explored the mechanism by which FUS and ILF2 may promote the
proliferation and migration of keratinocytes by regulating the TNF
signaling pathway, reducing inflammatory reaction and oxidative
stress and thus promoting the healing of diabetic skin wounds from
different aspects, additional studies are warranted to further
clarify the mechanism in the future.
When reviewing the present study, certain
limitations were identified. First, although the present study
revealed the crucial roles of the RBPs, FUS and ILF2, in promoting
DFU wound healing, the downstream targets of their effects were not
thoroughly identified. To comprehensively understand the mechanisms
of FUS and ILF2 in promoting DFU wound healing, high-throughput
studies such as RNA immunoprecipitation and transcriptome
sequencing will be conducted in the future to identify and validate
key genes that interact with FUS or ILF2. Second, the present study
mainly focused on the effects of FUS and ILF2 on skin
keratinocytes, but it is not yet clear how they affect other key
cells in skin wound healing, such as dermal microvascular
endothelial cells and skin fibroblasts, which requires further
exploration. Third, in terms of signaling pathway research,
although the present study focused on the importance of the TNF
signaling pathway in promoting DFU wound healing by NPWT, there is
a lack of direct experimental evidence to intervene in this pathway
clinically. Finally, the present study is a single-center study
with a limited clinical sample size, which may lead to slight
selection bias. To more reliably evaluate the potential role of
these DEGs in promoting DFU wound healing, data collection from
multicenter clinical samples will be carried out in the future.
Additionally, in vitro and in vivo experiments will
be carried out to explore the molecular mechanisms involved,
including inflammation and immune related and amino acid metabolism
related-mechanisms, providing a more scientific basis for future
clinical applications.
In summary, to the best of our knowledge, the
present study revealed for the first time the genomic changes in
the granulation tissue of DFU wounds before and after NPWT. The
present study further demonstrated that the RBPs, FUS and ILF2,
accelerate DFU wound healing by promoting proliferation and
migration of skin keratinocytes, inhibiting inflammation and
oxidative stress. These findings provide new insights for the early
diagnosis, treatment monitoring and prognostic evaluation of
DFUs.
Supplementary Data
Availability of data and materials
The RNA sequencing data generated in the present
study may be found in the Gene Expression Omnibus database under
accession no. GSE272918 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE272918.
All other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
YT contributed to the conception and design of the
study, carried out the high-throughput sequencing experiments,
performed the bioinformatics analysis and was a major contributor
in writing the manuscript. HJ performed the collection and
detection of granulation tissue. YY and DH completed the cell
culture and cell function experiments. MuX and MiX conducted the
data integration and analysis and participated in manuscript
writing. XZ and MC analyzed and interpreted the data and reviewed
the manuscript. XZ and MC confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The patient study was approved by the Medical Ethics
Committee of The First Affiliated Hospital of Anhui Medical
University (approval no. CDEC000004982). Informed consent was
obtained from all individual participants included in the study.
All animal experiments were approved and performed in accordance
with the guidelines of the Animal Care and Use Committee of Anhui
Medical University (Hefei, China; approval no. LLSC20201040).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of
Anhui Province in China (grant no. 2108085MH269), the Anhui
Provincial Health Research Project (grant no. AHWJ2023BAc10012) and
the Postgraduate Innovation Research and Practice Program of Anhui
Medical University (grant no. YJS20230124).
References
|
1
|
Sun H, Saeedi P, Karuranga S, Pinkepank M,
Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et
al: IDF diabetes atlas: Global, regional and country-level diabetes
prevalence estimates for 2021 and projections for 2045. Diabetes
Res Clin Pract. 183:1091192022. View Article : Google Scholar
|
|
2
|
Senneville É, Albalawi Z, van Asten SA,
Abbas ZG, Allison G, Aragón-Sánchez J, Embil JM, Lavery LA, Alhasan
M, Oz O, et al: IWGDF/IDSA guidelines on the diagnosis and
treatment of diabetes-related foot infections (IWGDF/IDSA 2023).
Diabetes Metab Res Rev. 40:e36872024. View Article : Google Scholar
|
|
3
|
Meloni M, Izzo V, Giurato L,
Lázaro-Martínez JL and Uccioli L: Prevalence, clinical aspects and
outcomes in a large cohort of persons with diabetic foot disease:
Comparison between neuropathic and ischemic ulcers. J Clin Med.
9:17802020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hassanshahi A, Moradzad M, Ghalamkari S,
Fadaei M, Cowin AJ and Hassanshahi M: Macrophage-mediated
inflammation in skin wound healing. Cells. 11:29532022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis FM, Kimball A, Boniakowski A and
Gallagher K: Dysfunctional wound healing in diabetic foot ulcers:
New crossroads. Curr Diab Rep. 18:22018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X, Xu M, Tang Y, Xie D, Deng L, Chen
M and Wang Y: Decreased expression of miR-204-3p in peripheral
blood and wound margin tissue associated with the onset and poor
wound healing of diabetic foot ulcers. Int Wound J. 20:413–429.
2023. View Article : Google Scholar
|
|
7
|
Hu SCS and Lan CEE: High-glucose
environment disturbs the physiologic functions of keratinocytes:
Focusing on diabetic wound healing. J Dermatol Sci. 84:121–127.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salem ESB, Vonberg AD, Borra VJ, Gill RK
and Nakamura T: RNAs and RNA-binding proteins in immuno-metabolic
homeostasis and diseases. Front Cardiovasc Med. 6:1062019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Assoni AF, Foijer F and Zatz M:
Amyotrophic lateral sclerosis, FUS and protein synthesis defects.
Stem Cell Rev Rep. 19:625–638. 2023. View Article : Google Scholar
|
|
10
|
Zhao H, Kong H, Wang B, Wu S, Chen T and
Cui Y: RNA-binding proteins and alternative splicing genes are
coregulated in human retinal endothelial cells treated with high
glucose. J Diabetes Res. 2022:76805132022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang G, Wu H, Liang P, He X and Liu D: Fus
knockdown inhibits the profibrogenic effect of cardiac fibroblasts
induced by angiotensin II through targeting Pax3 thereby regulating
TGF-β1/Smad pathway. Bioengineered. 12:1415–1425. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garikipati VNS, Verma SK, Cheng Z, Liang
D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, et
al: Circular RNA CircFndc3b modulates cardiac repair after
myocardial infarction via FUS/VEGF-A axis. Nat Commun. 10:43172019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du H, Le Y, Sun F, Li K and Xu Y: ILF2
directly binds and stabilizes CREB to stimulate malignant
phenotypes of liver cancer cells. Anal Cell Pathol (Amst).
2019:15750312019.PubMed/NCBI
|
|
14
|
Zhao M, Liu Y, Chang J, Qi J, Liu R, Hou
Y, Wang Y, Zhang X, Qiao L and Ren L: ILF2 cooperates with E2F1 to
maintain mitochondrial homeostasis and promote small cell lung
cancer progression. Cancer Biol Med. 16:771–783. 2019. View Article : Google Scholar
|
|
15
|
Xi Z, Huang H, Hu J, Yu Y, Ma X, Xu M,
Ming J, Li L, Zhang H, Chen H and Huang T: LINC00571 drives
tricarboxylic acid cycle metabolism in triple-negative breast
cancer through HNRNPK/ILF2/IDH2 axis. J Exp Clin Cancer Res.
43:222024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin J, Li A, Wang W and Wu J:
Interleukin-enhanced binding factor 2 interacts with NLRP3 to
inhibit the NLRP3 inflammasome activation. Biochem Biophys Res
Commun. 500:398–404. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji S, Liu X, Huang J, Bao J, Chen Z, Han
C, Hao D, Hong J, Hu D, Jiang Y, et al: Consensus on the
application of negative pressure wound therapy of diabetic foot
wounds. Burns Trauma. 9:tkab0182021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saraiya HA and Shah MN: Use of
indigenously made negative-pressure wound therapy system for
patients with diabetic foot. Adv Skin Wound Care. 26:74–77. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maranna H, Lal P, Mishra A, Bains L,
Sawant G, Bhatia R, Kumar P and Beg MY: Negative pressure wound
therapy in grade 1 and 2 diabetic foot ulcers: A randomized
controlled study. Diabetes Metab Syndr. 15:365–371. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang N, Liu Y, Yan W and Liu F: The
effect of negative pressure wound therapy on the outcome of
diabetic foot ulcers: A meta-analysis. Int Wound J. 21:e148862024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Campitiello F, Mancone M, Corte AD,
Guerniero R and Canonico S: Expanded negative pressure wound
therapy in healing diabetic foot ulcers: A prospective randomised
study. J Wound Care. 30:121–129. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeon BJ, Choi HJ, Kang JS, Tak MS and Park
ES: Comparison of five systems of classification of diabetic foot
ulcers and predictive factors for amputation. Int Wound J.
14:537–545. 2017. View Article : Google Scholar
|
|
23
|
Mu S, Hua Q, Jia Y, Chen MW, Tang Y, Deng
D, He Y, Zuo C, Dai F and Hu H: Effect of negative-pressure wound
therapy on the circulating number of peripheral endothelial
progenitor cells in diabetic patients with mild to moderate degrees
of ischaemic foot ulcer. Vascular. 27:381–389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Oh SS and Narver HL: Mouse and rat
anesthesia and analgesia. Curr Protoc. 4:e9952024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dutton JW II, Artwohl JE, Huang X and
Fortman JD: Assessment of pain associated with the injection of
sodium pentobarbital in laboratory mice (Mus musculus). J Am Assoc
Lab Anim Sci. 58:373–379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caudron-Herger M, Jansen RE, Wassmer E and
Diederichs S: RBP2GO: A comprehensive pan-species database on
RNA-binding proteins, their interactions and functions. Nucleic
Acids Res. 49(D1): D425–D436. 2021. View Article : Google Scholar :
|
|
28
|
Smith MR and Costa G: RNA-binding proteins
and translation control in angiogenesis. FEBS J. 289:7788–7809.
2022. View Article : Google Scholar
|
|
29
|
Gerstberger S, Hafner M and Tuschl T: A
census of human RNA-binding proteins. Nat Rev Genet. 15:829–845.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Lazzarini PA, McPhail SM, van
Netten JJ, Armstrong DG and Pacella RE: Global disability burdens
of diabetes-related lower-extremity complications in 1990 and 2016.
Diabetes Care. 43:964–974. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Armstrong DG, Boulton AJM and Bus SA:
Diabetic foot ulcers and their recurrence. N Engl J Med.
376:2367–2375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Armstrong DG, Tan TW, Boulton AJM and Bus
SA: Diabetic foot ulcers: A review. JAMA. 330:62–75. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tricco AC, Antony J, Vafaei A, Khan PA,
Harrington A, Cogo E, Wilson C, Perrier L, Hui W and Straus SE:
Seeking effective interventions to treat complex wounds: An
overview of systematic reviews. BMC Med. 13:892015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eneroth M and van Houtum WH: The value of
debridement and vacuum-assisted closure (V.A.C.) therapy in
diabetic foot ulcers. Diabetes Metab Res Rev. 24(Suppl 1): S76–S80.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raphael A and Gonzales J: Use of
cryopreserved umbilical cord with negative pressure wound therapy
for complex diabetic ulcers with osteomyelitis. J Wound Care.
26(Sup10): S38–S44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Namgoong S, Jung SY, Han SK, Kim AR and
Dhong ES: Clinical experience with surgical debridement and
simultaneous meshed skin grafts in treating biofilm-associated
infection: An exploratory retrospective pilot study. J Plast Surg
Hand Surg. 54:47–54. 2020. View Article : Google Scholar
|
|
37
|
Armstrong DG and Lavery LA; Diabetic Foot
Study Consortium: Negative pressure wound therapy after partial
diabetic foot amputation: A multicentre, randomised controlled
trial. Lancet. 366:1704–1710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu L, Chen R, Jia Z, Li X, Tang Y, Zhao
X, Zhang S, Luo L, Fang Z, Zhang Y and Chen M: Downregulation of
hsa-miR-203 in peripheral blood and wound margin tissue by negative
pressure wound therapy contributes to wound healing of diabetic
foot ulcers. Microvasc Res. 139:1042752022. View Article : Google Scholar
|
|
39
|
Liu Z, Dumville JC, Hinchliffe RJ, Cullum
N, Game F, Stubbs N, Sweeting M and Peinemann F: Negative pressure
wound therapy for treating foot wounds in people with diabetes
mellitus. Cochrane Database Syst Rev. 10:CD0103182018.PubMed/NCBI
|
|
40
|
Chen L, Zhang S, Da J, Wu W, Ma F, Tang C,
Li G, Zhong D and Liao B: A systematic review and meta-analysis of
efficacy and safety of negative pressure wound therapy in the
treatment of diabetic foot ulcer. Ann Palliat Med. 10:10830–10839.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McDermott K, Fang M, Boulton AJM, Selvin E
and Hicks CW: Etiology, Epidemiology, and disparities in the burden
of diabetic foot ulcers. Diabetes Care. 46:209–221. 2023.
View Article : Google Scholar :
|
|
42
|
Seghieri G, Policardo L, Gualdani E,
Anichini R and Francesconi P: Gender difference in the risk for
cardiovascular events or mortality of patients with diabetic foot
syndrome. Acta Diabetol. 56:561–567. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Iacopi E, Pieruzzi L, Riitano N,
Abbruzzese L, Goretti C and Piaggesi A: The weakness of the strong
sex: Differences between men and women affected by diabetic foot
disease. Int J Low Extrem Wounds. 22:19–26. 2023. View Article : Google Scholar
|
|
44
|
Jarl G, Alnemo J, Tranberg R and Lundqvist
LO: Gender differences in attitudes and attributes of people using
therapeutic shoes for diabetic foot complications. J Foot Ankle
Res. 12:212019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sorber R and Abularrage CJ: Diabetic foot
ulcers: Epidemiology and the role of multidisciplinary care teams.
Semin Vasc Surg. 34:47–53. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kotlinowski J, Dulak J and Józkowicz A:
Type 2 diabetes mellitus impairs endothelial progenitor cells
functions. Postepy Biochem. 59:257–266. 2013.In Polish.
|
|
47
|
Fernando ME, Seneviratne RM, Tan YM,
Lazzarini PA, Sangla KS, Cunningham M, Buttner PG and Golledge J:
Intensive versus conventional glycaemic control for treating
diabetic foot ulcers. Cochrane Database Syst Rev.
2016:CD0107642016.PubMed/NCBI
|
|
48
|
Markuson M, Hanson D, Anderson J, Langemo
D, Hunter S, Thompson P, Paulson R and Rustvang D: The relationship
between hemoglobin A(1c) values and healing time for lower
extremity ulcers in individuals with diabetes. Adv Skin Wound Care.
22:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Christman AL, Selvin E, Margolis DJ,
Lazarus GS and Garza LA: Hemoglobin A1c predicts healing rate in
diabetic wounds. J Invest Dermatol. 131:2121–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hemmingsen B, Lund SS, Gluud C, Vaag A,
Almdal TP, Hemmingsen C and Wetterslev J: Targeting intensive
glycaemic control versus targeting conventional glycaemic control
for type 2 diabetes mellitus. Cochrane Database Syst Rev.
CD0081432013.PubMed/NCBI
|
|
51
|
Zhang WL, Yan WJ, Sun B and Zou ZP:
Synergistic effects of atorvastatin and rosiglitazone on
endothelium protection in rats with dyslipidemia. Lipids Health
Dis. 13:1682014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zangiabadi N, Shafiee K, Alavi KH, Assadi
AR and Damavandi M: Atorvastatin treatment improves diabetic
polyneuropathy electrophysiological changes in non-insulin
dependent diabetic patients: A double blind, randomized clinical
trial. Minerva Endocrinol. 37:195–200. 2012.PubMed/NCBI
|
|
53
|
Xie X, Bao Y, Ni L, Liu D, Niu S, Lin H,
Li H, Duan C, Yan L, Huang S and Luo Z: Bacterial profile and
antibiotic resistance in patients with diabetic foot ulcer in
Guangzhou, Southern China: Focus on the differences among different
Wagner's grades, IDSA/IWGDF grades, and ulcer types. Int J
Endocrinol. 2017:86949032017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang Y, Liu L, Jie R, Tang Y, Zhao X, Xu M
and Chen M: Negative pressure wound therapy promotes wound healing
of diabetic foot ulcers by up-regulating PRDX2 in wound margin
tissue. Sci Rep. 13:161922023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qin G, Song Y, Guo Y, Sun Y and Zeng W:
LincRNA TINCR facilitates excessive proliferation and inflammation
in post-burn skin fibroblasts by directly binding with SND1 protein
and inducing SND1-mediated TGF-β1 expression. Biochem Biophys Res
Commun. 509:903–910. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Meder L, König K, Dietlein F, Macheleidt
I, Florin A, Ercanoglu MS, Rommerscheidt-Fuss U, Koker M, Schön G,
Odenthal M, et al: LIN28B enhanced tumorigenesis in an
autochthonous KRASG12V-driven lung carcinoma mouse model. Oncogene.
37:2746–2756. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo L and Huang X, Liang P, Zhang P, Zhang
M, Ren L, Zeng J, Cui X and Huang X: Role of XIST/miR-29a/LIN28A
pathway in denatured dermis and human skin fibroblasts (HSFs) after
thermal injury. J Cell Biochem. 119:1463–1474. 2018. View Article : Google Scholar
|
|
58
|
Ayerst BI, Merry CLR and Day AJ: The good
the bad and the ugly of glycosaminoglycans in tissue engineering
applications. Pharmaceuticals (Basel). 10:542017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rohani MG and Parks WC: Matrix remodeling
by MMPs during wound repair. Matrix Biol. 44-46:113–121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lobmann R, Ambrosch A, Schultz G, Waldmann
K, Schiweck S and Lehnert H: Expression of
matrix-metalloproteinases and their inhibitors in the wounds of
diabetic and non-diabetic patients. Diabetologia. 45:1011–1016.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu Y, Min D, Bolton T, Nubé V, Twigg SM,
Yue DK and McLennan SV: Increased matrix metalloproteinase-9
predicts poor wound healing in diabetic foot ulcers: Response to
Muller et al. Diabetes Care. 32:e1372009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chang M: Restructuring of the
extracellular matrix in diabetic wounds and healing: A perspective.
Pharmacol Res. 107:243–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cui YH, Feng QY, Liu Q, Li HY, Song XL, Hu
ZX, Xu ZY, Li JH, Li MJ, Zheng WL, et al: Posttranscriptional
regulation of MMP-9 by HuR contributes to IL-1β-induced pterygium
fibroblast migration and invasion. J Cell Physiol. 235:5130–5140.
2020. View Article : Google Scholar
|
|
64
|
Chen Q, Zhou L, Ma D, Hou J, Lin Y, Wu J
and Tao M: LncRNA GAS6-AS1 facilitates tumorigenesis and metastasis
of colorectal cancer by regulating TRIM14 through
miR-370-3p/miR-1296-5p and FUS. J Transl Med. 20:3562022.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang T, Shen P, Chen Q, Wu P, Yuan H, Ge
W, Meng L, Huang X, Fu Y, Zhang Y, et al: FUS-induced circRHOBTB3
facilitates cell proliferation via miR-600/NACC1 mediated autophagy
response in pancreatic ductal adenocarcinoma. J Exp Clin Cancer
Res. 40:2612021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang Q, Yang Y, Fu X, Wang Z, Liu Y, Li M,
Zhang Y, Li Y, Li PF, Yu T and Chu XM: Long noncoding RNA
XXYLT1-AS2 regulates proliferation and adhesion by targeting the
RNA binding protein FUS in HUVEC. Atherosclerosis. 298:58–69. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ghanbarpanah E, Kohanpour MA,
Hosseini-Beheshti F, Yari L and Keshvari M: Structure and function
of FUS gene in prostate cancer. Bratisl Lek Listy. 119:660–663.
2018.PubMed/NCBI
|
|
68
|
Jia Y, Li X, Nan A, Zhang N, Chen L, Zhou
H, Zhang H, Qiu M, Zhu J, Ling Y and Jiang Y: Circular RNA 406961
interacts with ILF2 to regulate PM2.5-induced
inflammatory responses in human bronchial epithelial cells via
activation of STAT3/JNK pathways. Environ Int. 141:1057552020.
View Article : Google Scholar
|
|
69
|
Huang W, Liu J, Yan J, Huang Z, Zhang X,
Mao Y and Huang X: LncRNA LINC00470 promotes proliferation through
association with NF45/NF90 complex in hepatocellular carcinoma. Hum
Cell. 33:131–139. 2020. View Article : Google Scholar
|
|
70
|
Li Y, Wang M, Yang M, Xiao Y, Jian Y, Shi
D, Chen X, Ouyang Y, Kong L, Huang X, et al: Nicotine-induced ILF2
facilitates nuclear mRNA export of pluripotency factors to promote
stemness and chemoresistance in human esophageal cancer. Cancer
Res. 81:3525–3538. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chiu CL, Li CG, Verschueren E, Wen RM,
Zhang D, Gordon CA, Zhao H, Giaccia AJ and Brooks JD: NUSAP1 binds
ILF2 to modulate R-loop accumulation and DNA damage in prostate
cancer. Int J Mol Sci. 24:62582023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yin X, Yang Z, Zhu M, Chen C, Huang S, Li
X, Zhong H, Wen H, Sun Q, Yu X, et al: ILF2 contributes to
hyperproliferation of keratinocytes and skin inflammation in a
KLHDC7B-DT-dependent manner in psoriasis. Front Genet.
13:8906242022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Du Y, Chen W, Li Y, Liang D and Liu G:
Study on the regulatory effect of Panax notoginseng saponins
combined with bone mesenchymal stem cell transplantation on
IRAK1/TRAF6-NF-κB pathway in patients with diabetic cutaneous
ulcers. J Orthop Surg Res. 18:802023. View Article : Google Scholar
|
|
74
|
Yen YH, Pu CM, Liu CW, Chen YC, Chen YC,
Liang CJ, Hsieh JH, Huang HF and Chen YL: Curcumin accelerates
cutaneous wound healing via multiple biological actions: The
involvement of TNF-α, MMP-9, α-SMA, and collagen. Int Wound J.
15:605–617. 2018. View Article : Google Scholar : PubMed/NCBI
|