Introduction
Pancreatic cancer is an aggressive malignant tumor
and pancreatic ductal adenocarcinoma (PDAC) is the most common
form, representing ~90% of all types of pancreatic cancer (1). PDAC is one of the most lethal types
of cancer, with a 5-year survival rate of <10%. Of patients with
PDAC, ~50% are diagnosed with locally advanced disease, but 85-90%
are unsuitable for surgical resection; so the majority receive
systemic treatment (2). Despite
recent advances in combination chemotherapy regimens based on
gemcitabine or 5-FU, the median overall survival for patients with
metastatic PDAC is <1 year (3). This underscores the urgent need for
novel and effective treatments for PDAC. The standard therapeutic
approach for newly diagnosed PDAC involves a combination of surgery
and platinum-based chemotherapy.
Cisplatin, a potent platinum-based
chemotherapeutics, has been used to treat various solid tumors,
including lung, breast, ovarian and PDAC (4). Cisplatin interferes with DNA
replication and transcription, leading to cell death, which is
particularly effective against rapidly dividing cells such as
cancer cells (5). Cisplatin- and
platinum-based therapies are commonly employed to treat PDAC,
either alone or in combination with other chemotherapeutic agents
such as gemcitabine (6).
However, despite its clinical efficacy, cisplatin often causes
severe side effects, including peripheral neuropathy, hearing loss
and kidney damage (7).
Therefore, there is an urgent need to develop new pancreatic cancer
treatment strategies that do not cause these side effects.
Drug repositioning is a promising strategy for
developing new cancer treatments. This approach involves
identifying new therapeutic applications for drugs already used in
clinical practice, including those approved for other diseases or
those unsuccessful for their original indications (8). Repositioned drugs have undergone
extensive testing, including safety, toxicity and pharmacokinetic
evaluations, which can expedite their clinical use in cancer
therapy at a lower discovery cost (9). More importantly, they can be used
in combination with existing cancer therapies to enhance efficacy,
overcome drug resistance and improve patient outcomes, thereby
providing a route for optimizing and personalizing cancer treatment
(10). The synergistic use of
repositioned drugs with current treatment regimens offers an avenue
to target rare and neglected cancers, providing new hope to
patients with limited treatment options.
Markedly, a lower incidence of cancer has been noted
in patients with schizophrenia who are prescribed neuroleptics.
Some antipsychotic drugs including chlorpromazine and aripiprazole,
have demonstrated anticancer effects in preclinical studies
(11). Aripiprazole, the
second-generation antipsychotic drug for mental disorders, exhibits
unique pharmacological activities as a serotonin 5-HT1A and 5-HT2A
antagonist. Studies have shown that aripiprazole possesses
anticancer properties and enhances radiosensitizing effects in
various cancer cells (12-18). It also inhibits the growth of
cancer stem cells and overcame the chemoresistance (12). Given the recent emphasis on the
importance of sensitization and improving chemotherapeutic
resistance in cancer treatment (19), along with the low toxicity of
aripiprazole and previous studies suggesting its role in enhancing
resistance, it was hypothesized that it could reduce cisplatin
toxicity and exert synergistic anticancer effects when combined
with cisplatin. To test this hypothesis, the present study
investigated the effects of aripiprazole combined with cisplatin on
apoptotic cell death and proliferation in pancreatic cancer cells
and elucidated its molecular mechanisms involved.
Materials and methods
Ethics statement
Animal protocols were approved by the INHA
Institutional Animal Care and Use Committee (INHA IACUC) at the
College of Medicine, Inha University (Incheon, Korea; approval nos.
INHA 2211124-848 and INHA 230731-883).
Cell culture
MIA PaCa-2 (cat. no. CRL-1420) and Capan-1 cell
lines (cat. no. HTB-79) were obtained from the American Type
Culture Collection. MIA PaCa-2 cell lines were cultured in
Dulbecco's Modified Eagle's Medium (DMEM; Welgene, Inc.), while
Capan-1 cell lines were cultured in Roswell Park Memorial Institute
1640 (RPMI-1640) medium (Gibco; Thermo Fisher Scientific, Inc.).
The medium for MIA PaCa-2 was supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin. The medium for Capan-1 was supplemented
with 15% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
All cell culture reagents, including FBS and
penicillin/streptomycin, were purchased from Gibco (Thermo Fisher
Scientific, Inc.). The cells were maintained in a CO2
incubator with 95% air and 5% CO2 at 37°C.
Chemicals
Aripiprazole and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from MilliporeSigma. For in vitro studies,
aripiprazole was dissolved in dimethyl sulfoxide (DMSO) at a 20 mM
stock concentration and freshly diluted to working concentrations.
For in vivo experiments, the desired doses were prepared by
dissolving in a DMSO:Cremophor:DW (1:2:7) mixture. Cisplatin was
purchased from Selleck Chemicals.
Cell viability and proliferation
assay
Cell viability was assessed using the MTT assay.
Briefly, MIA PaCa-2 and Capan-1 cells were seeded at densities of
2,500 cells and 5,000 cells per well, respectively, in 96-well
plates and treated with aripiprazole and/or cisplatin. After 48 h
of incubation, 20 µl of MTT solution (2 mg/ml) was added and
incubated for an additional 4 h at 37°C. The plates were read using
a microplate reader at a wavelength of 540 nm. The combination
index for cisplatin and aripiprazole was calculated using CompuSyn
v1.0 (Biosoft), where combination index (CI)<1, CI=1 and CI>1
indicate synergistic, additive and antagonistic effects,
respectively (10). Cell
proliferation was monitored using the JULI Stage real-time image
recording system (NanoEntek).
Colony formation assay
MIA PaCa-2 and Capan-1 cells (1×106) were
treated with cisplatin (1 or 10 mM) and/or aripiprazole (10 mM) for
48 h. The treated cells were seeded in six-well plates at densities
of 1×103 and 3×103 cells per well with a
monolayer and incubated for 14 days. Colonies were washed twice
with Dulbecco's phosphate-buffered saline (DPBS), fixed with 4%
paraformaldehyde for 15 min at 4°C and stained with 1% crystal
violet for 15 min at room temperature.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
The TUNEL assay was conducted using the ApopTag
peroxidase in situ apoptosis detection kit (MilliporeSigma).
MIA PaCa-2 and Capan-1 cells were treated with cisplatin (1 or 10
µM) and aripiprazole (10 µM) for 48 h, fixed and
washed three times with phosphate-buffered saline (PBS). After the
terminal deoxynucleotidyl transferase enzyme was activated, the
reaction was stopped, and the digoxigenin-conjugated antibody was
attached overnight. After washing, 10% 3,3′-diaminobenzidine
staining was performed to visualize apoptotic cells and stained for
15 min at room temperature.
Immunofluorescence
The cells were fixed in an acetic acid solution
(1:2) for 10 min at 4°C and permeabilized with 0.5% Triton X-100
for 10 min and incubated in CAS block solution (Life Technologies)
for 1 h at room temperature. Next, the cells were incubated
overnight at 4°C with a primary antibody against cleaved caspase-3
(1:50; cat. no. 9661; Cell Signaling Technology). After several
washes with PBS, the cells were incubated with fluorescently
labeled secondary antibodies (1:60; cat. no. 31460; Invitrogen) for
2 h at room temperature and stained with
4,6-diamidino-2-phenylindole (Antifade mounting medium with DAPI;
Vectashield; Vector Laboratories, Inc.) at a 1:100 dilution for 1 h
at room temperature. The cells were viewed using a confocal laser
scanning microscope (FluoView 1000; Olympus Corporation) at
wavelengths of 488 and 568 nm.
Western blotting
The cells were washed three times with ice-cold DPBS
before the lysis. Total cellular proteins were extracted using
sterile RIPA lysis buffer (Biosesang; 150 mM sodium chloride, 1%
triton X-100, 1% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH
7.5, and 2 mM EDTA), 1X Phosphatase inhibitor (GenDEPOT, LLC;
sodium fluoride, sodium orthovanadate, sodium pyrophosphate and
sodium glycerophosphate) and 1X Protease inhibitor (GenDEPOT, LLC;
PMSF; pepstatin A, leupeptin, benzamidine, bestatin). Equal amounts
of proteins (50 µg) were separated by 8% or 12% SDS gel
electrophoresis using BCA assay and transferred to PVDF membranes
(MilliporeSigma). The membranes were blocked with PBS containing 5%
skimmed milk for 1 h at room temperature and incubated overnight at
4°C with primary antibodies. After washing, the membranes were
incubated with secondary antibodies (1:2,000, Mouse (cat. no.
7076S), Rabbit (cat. no. 7074S); Cell signaling Technology, Inc.)
for 1 h at room temperature. Proteins were visualized using Clarity
Western ECL Substrate (Amersham Biosciences; Cytiva). Primary
antibodies from Cell Signaling Technology, Inc. included cleaved
caspase-3 (1:1,000; cat. no. 9661), AKT (1:1,000; cat. no. 9272),
phosphorylated (p)-AKT (1:2,000; cat. no. 4060), p-SRC (1:1,000;
cat. no. 6943), STAT3 (1:1,000; cat. no. 9139), p-STAT3 (1:1,000;
cat. no. 94994) and β-actin (1:10,000; cat. no. 4967). The primary
antibody for myeloid cell leukemia-1 (MCL-1, 1:500; cat. no.
sc-819) and XIAP (1:500; cat. no. sc-55550) was obtained from Santa
Cruz Biotechnology, Inc. The densitometry was quantified using
ImageJ software (version 1.54, National Institutes of Health).
Measurement of mitochondrial membrane
potential by JC-1 Staining
The cells were treated with cisplatin (1 or 10
µM) and/or aripiprazole (10 µM) for 48 h at 37°C,
followed by incubation with JC-1 solution (Cayman Chemical) for 45
min at 37°C. Following staining with DAPI, the slides were washed
twice with DPBS, mounted with Antifade Mounting Medium with DAPI
(Vectashield; Vector Laboratories, Inc.) and viewed using confocal
laser-scanning microscopy (FluoView 1000; Olympus Corporation).
Two-chamber migration and invasion
assay
Cell invasion was analyzed using Transwell permeable
support systems (Corning Life Sciences). The inserts were coated
with 10% Matrigel for 30 min at 37°C before cell seeding. Cells
were seeded at a density of 2×104 cells/well in a
serum-free medium. The lower chambers were treated with cisplatin
(1 or 10 µM) and/or aripiprazole (10 µM) for 48 h at
37°C. After 48 h, the cells that invaded the PET membrane were
stained with 0.5% crystal violet for 15 min at room temperature.
The migration assay followed a similar protocol, except Matrigel
was not used. The purple regions were quantified using ImageJ
software (version 1.54; National Institutes of Health).
Fluid shear stress assay
The cells were treated with various concentrations
of cisplatin and aripiprazole for 48 h. Cells were then trypsinized
and resuspended to a concentration of 1×106 cells/ml in
culture medium and subjected to three repeated exposures to shear
stress through a 30-gauge needle, followed by a constant flow of
100 µl/s. Briefly, 1×106 cells were resuspended
in 4 ml PBS and loaded into a 5 ml plastic syringe. A Luer lock
fitting was attached to the end of the syringe and connected to a
polyether ether ketone tubing (inner diameter, 125 µm) which
was connected to a 50 ml centrifuge tube. The syringe plunger was
pushed by a syringe pump to flow the cell solution at flow rates
corresponding to wall shear stresses of 5, 20, or 60
dyn/cm2. The syringe was regularly agitated to maintain
cell suspension. After shearing, the cells were centrifuged at 300
× g for 3 min at 4°C and resuspended in DMEM/10% FBS. The cells
were then incubated for 11 days at 37°C in 96-well ultra-low
cluster round-bottom plates (Costar; Corning, Inc.) in a complement
medium supplemented with basic fibroblast growth factor, human
epidermal growth factor, N-2 and B-27 at 37°C to form
three-dimensional (3D) tumor spheroids. After 11 days, the cells
were observed under a phase-contrast microscope and captured
images. The size and shape of the 3D tumor spheroids were recorded
using an inverted light microscope from day 2 to day 11.
Additionally, the cells treated for 48 h were seeded at
8×104 cells per well in collagen-coated 48-well plates,
incubated for 1 h at 37°C, washed twice with DPBS, fixed with 4%
paraformaldehyde and incubated for 1 h at room temperature with a
1:100 dilution of DAPI.
Phospho-kinase array
Relative phosphorylation levels of 43 human protein
kinases were determined using a human phospho-kinase array kit
(R&D Systems, Inc.). MIA PaCa-2 cells were treated with
aripiprazole for 12 h at 37°C and then lysed. After blocking for 1
h at 37°C with Array Buffer 1 (R&D systems, Inc), the membranes
were incubated overnight at 4°C with 600 µg of protein
lysates, washed and then incubated with a streptavidin-HRP
detection antibody for 30 min rocking at room temperature.
Membranes were developed using ECL Western blotting detection
reagents and protein expression levels were quantified using ImageJ
software (version 1.54; National Institutes of Health).
Xenograft animal model
Male BALB/c nude mice (five-weeks-old; 18-20 g) were
purchased from Orient Bio, Inc. All animal experiments were
conducted in accordance with the guidelines of the INHA IACUC
(approval no. INHA 2211124-848). The mice were acclimated for one
week and then injected with 3×106 MIA PaCa-2 cells into
the flank. When the tumor size reached 50-100 mm3, the
mice were randomly assigned to four groups (control, cisplatin,
aripiprazole, cisplatin and aripiprazole; n=9). The treatment group
received cisplatin [2 mg/kg, intraperitoneally (i.p.)] once a week
and/or aripiprazole [10 mg/kg, orally (p.o.)] three times a week.
Tumor size was calculated using Vernier calipers with the formula:
0.5x length × (width)2. After 28 days, the tumors were
carefully dissected out and fixed in 10% paraformaldehyde at 4°C
overnight, embedded in paraffin and sectioned at 3 µm. All
animals had ad libitum access to food and water and were
maintained in a stable environment at 25±1°C, 60±5% humidity and a
12-h light/dark cycle. The health of the mice was monitored by
observing the temperature, humidity, noise and lighting conditions
in the animal room by monitoring the weight and general condition
of mice to assess the health and behavioral status of the mice. At
the end of study, the mice were then anesthetized using
Ketamin:Rompun (9:1) mixture and sacrificed by collecting blood
sample from the heart. The mice were confirmed dead by no
spontaneous breathing for 2-3 min and no blink reflex. The blood,
lung, liver, pancreas, spleen and tumors of mice were collected for
sample preparation. During the experiment, all mice were
anesthetized and sacrificed according to the experimental plan and
all measures were taken to decrease the pain of the experimental
animals. If the mice showed persistent pain behavior, severe
dehydration, inability to eat, extreme fatigue or even severe
infection during the research process, they were sacrificed;
however, no animals in the present study reached these humane
endpoints.
Orthotopic mouse model
Male C57BL/6 mice aged 5 weeks (18-20 g) were
purchased from Orient Bio Animal, Inc. All animal experiments were
conducted in accordance with the guidelines of the INHA IACUC
(approval no. INHA 230731-883). The mice (n=7) were injected with
1×104 KPC cells into the pancreas. After two weeks, the
treatment groups received cisplatin (2 mg/kg, i.p.) once a week
and/or aripiprazole (10 mg/kg, p.o.) three times a week. After 25
days, the tumors were carefully dissected to avoid contamination
from surrounding tissues. At the end of study, the mice were then
anesthetized using Ketamin:Rompun (9:1) mixture and sacrificed by
collecting blood sample from the heart. The mice were confirmed
dead by no spontaneous breathing for 2-3 min and no blink reflex.
The blood, lung, liver, pancreas, spleen and tumors of mice were
collected for sample preparation. During the experiment, all mice
were anesthetized and sacrificed according to the experimental plan
and all measures were taken to decrease the pain of the
experimental animals. If the mice showed persistent pain behavior,
severe dehydration, inability to eat, extreme fatigue or even
severe infection during the research process, they were sacrificed;
however, no animals in the present study reached these humane
endpoints.
Immunohistochemistry
Tumor samples were fixed in 10% buffered
formaldehyde at 4°C overnight, embedded in paraffin, and sectioned,
Immunostaining was performed on 3-µm sections of tumor
samples after deparaffinization. Antigen retrieval was performed by
incubation of 0.2 mg/ml Proteinase K (Thermo Fisher Scientific,
Inc.) in PBS for 15 min at room temperature. After gently washing
twice with PBS, tissue sections were permeabilized with 0.5% Triton
X-100 for 10 min and endogenous peroxidase was blocked with 0.3%
H2O2 in distilled water for 15 min at room
temperature, followed by incubation in CAS block solution (Life
Technologies) for 1 h at room temperature. The tissue sections were
incubated with primary antibodies (1:50 dilution) following antigen
retrieval at 4°C overnight. Subsequently, sections were incubated
with a biotinylated secondary antibody (1:60 dilution; cat. no.
31460) for 1 h at room temperature. Primary antibodies from Cell
Signaling Technology, Inc. included cleaved caspase-3 (1:50; cat.
no. 9661), BCL-2 (1:50; cat. no. 15071), p-STAT3 (1:50; cat. no.
94994). Primary antibody from Abcam included Ki-67 (1:50; cat. no.
ab16667). Immunoreactive proteins were detected by incubating
sections with an avidin-biotin peroxidase complex solution ABC kit
(Vector Laboratories, Inc.). After washing with PBS, proteins were
visualized by incubating sections with DAB for 15 min at room
temperature, followed by counterstaining with hematoxylin solution
for 40 sec at room temperature. At least three random fields of
each section were examined at ×400 magnification and analyzed using
a computer image analysis system (Olympus Corporation).
Statistical Analysis
All experiments were repeated three times. The data
were analyzed using one-way ANOVA with Tukey's post hoc tests for
multiple comparisons. Results were presented as mean ± standard
deviation (SD). All analyses were performed with GraphPad Prism
(version 8.00, GraphPad Software, Inc.; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
Aripiprazole enhances the anticancer
efficacy of cisplatin in PDAC cells
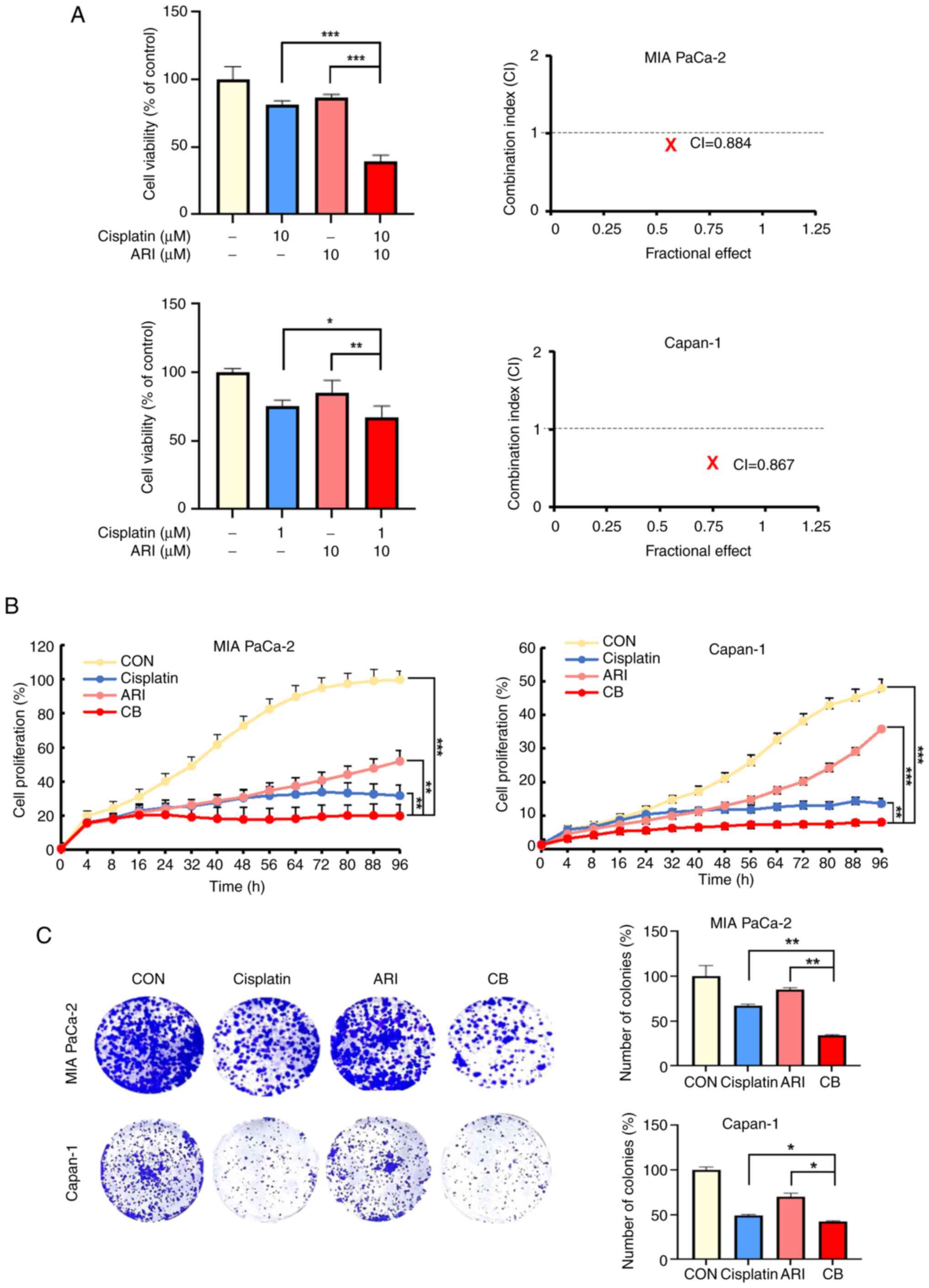
To identify the synergistic effects of cisplatin and
aripiprazole, PDAC cells (MIA PaCa-2 and Capan-1) were treated with
both drugs for 48 h. The co-treatment exhibited significant
synergistic effects, with CI<1 for the combination of 10
µM cisplatin and 10 µM aripiprazole in MIA PaCa-2
cells (CI=0.884) and 1 µM cisplatin and 10 µM
aripiprazole in Capan-1 cells (CI=0.867; Fig. 1A). The cell proliferation curves
showed similar trends (Fig. 1B).
Additionally, the clonogenic survival of PDAC cells treated with
this combination was inhibited in a dose-dependent manner compared
with single-agent treatments (Fig.
1C). These findings indicated that aripiprazole enhanced the
anticancer efficacy of cisplatin and synergistically inhibits the
proliferation of PDAC cells.
Induction of apoptosis in PDAC cells by
combination treatment of cisplatin and aripiprazole
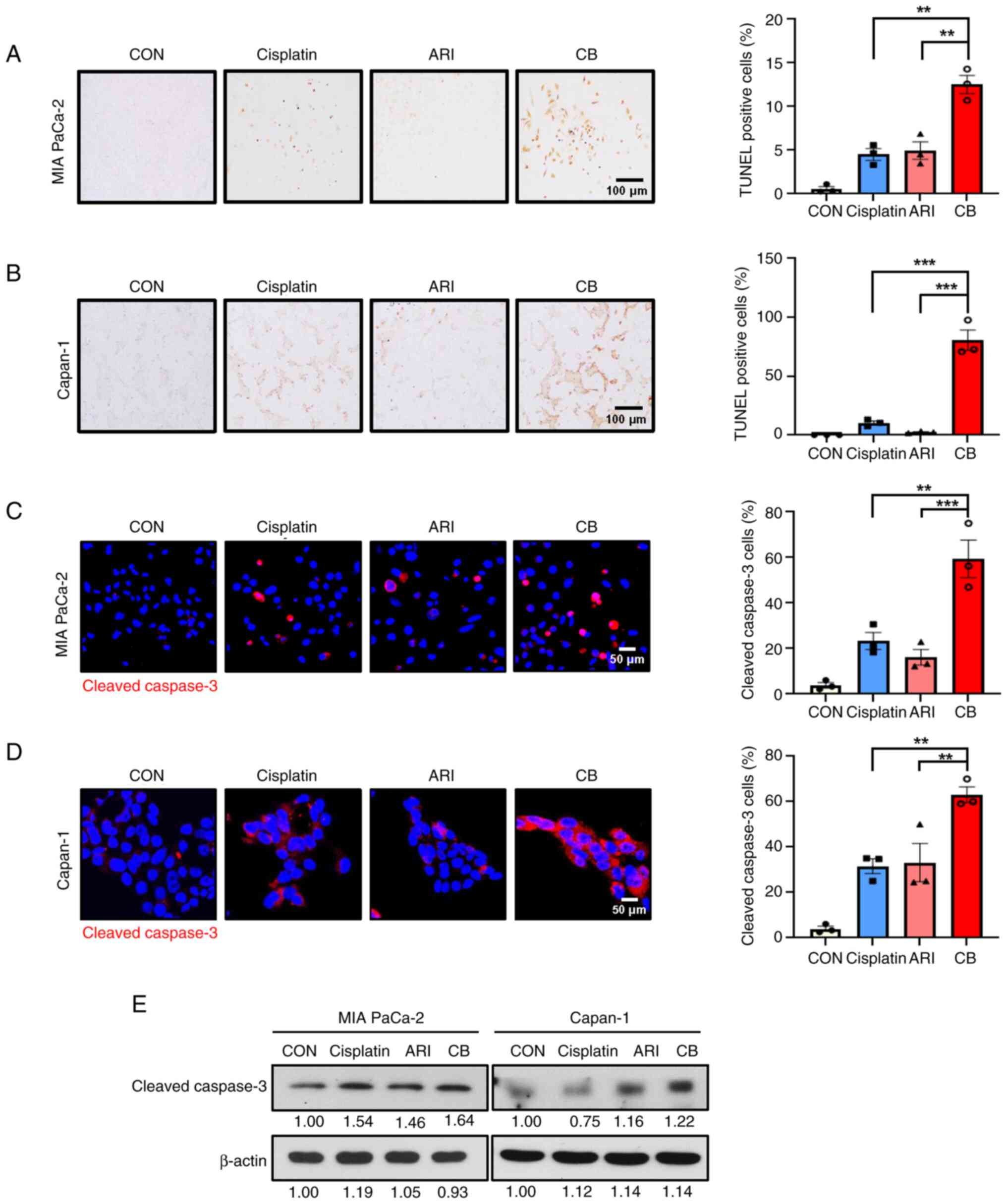
Since the co-treatment significantly reduced cell
proliferation, it was investigated whether it could induce
apoptosis in PDAC cells. TUNEL staining revealed an increase in the
percentage of TUNEL-positive cells following treatment (Fig. 2A and B). Furthermore, the
combination treatment significantly increased the expression levels
of cleaved caspase-3 (Fig. 2C and
D). These apoptotic effects were confirmed by the increase in
cleaved caspase-3 compared with single-agent treatments, as
demonstrated by western blotting (Fig. 2E). Taken together, these results
indicated that the combination of cisplatin and aripiprazole
synergistically induced apoptosis in pancreatic cancer cells.
Combining of cisplatin and aripiprazole
synergistically induces mitochondria-mediated apoptosis
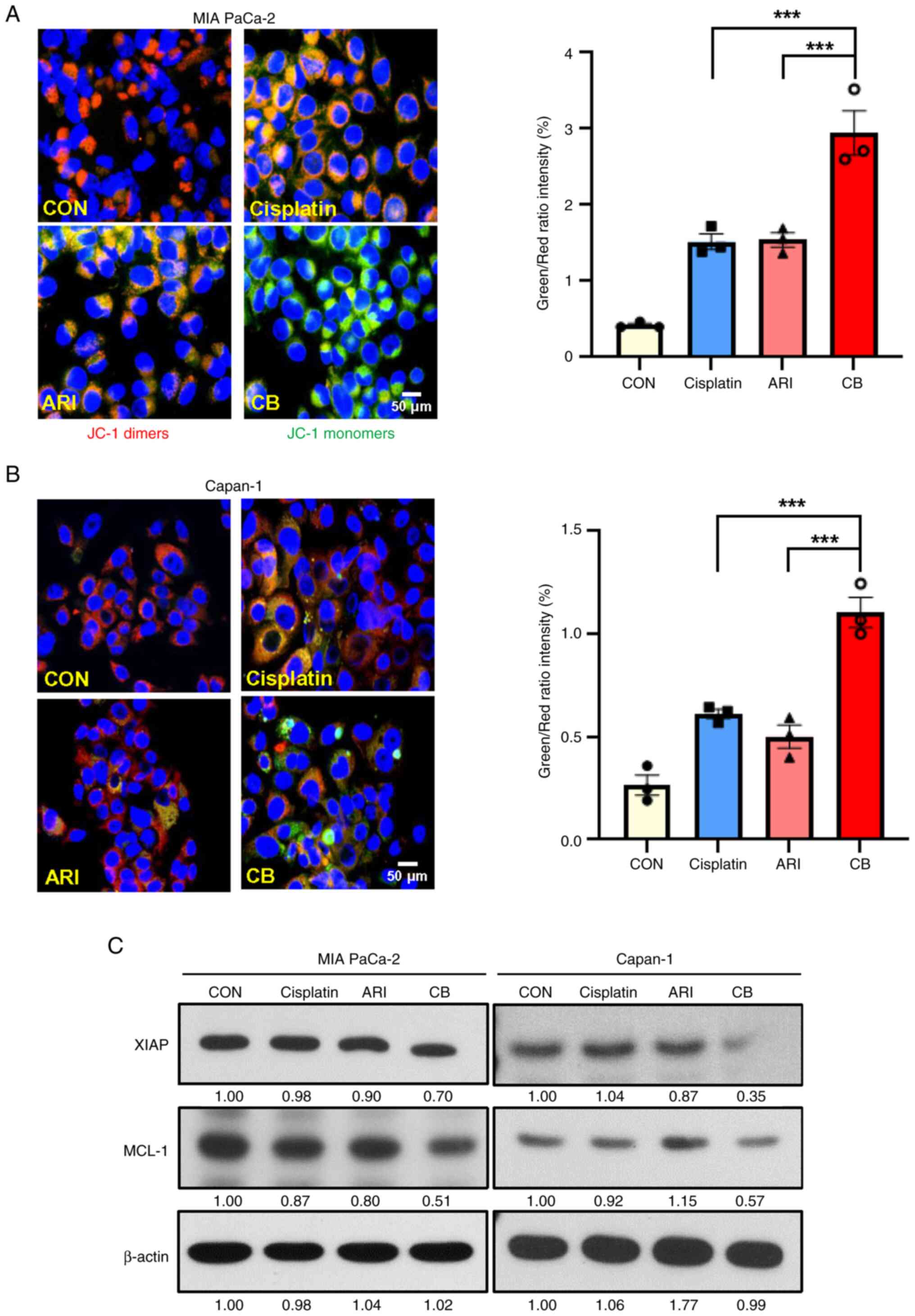
Given that the combined treatment of cisplatin and
aripiprazole markedly increased apoptotic cell death, the present
study next investigated whether it affects the mitochondrial
membrane potential (MMP), which is associated with apoptosis. MMP
was examined using JC-1 staining. The control cells exhibited
strong red fluorescence in the cytoplasm, whereas the combined
treatment showed a clear decrease in red fluorescence and an
increase in green fluorescence, indicating changes in MMP
associated with apoptosis (Fig. 3A
and B). Additionally, the combination treatment decreased the
expression of XIAP and MCL-1, prominent anti-apoptotic proteins
(Fig. 3C). The results suggested
that the synergistic effects of aripiprazole and cisplatin are
mediated by mitochondria-mediated apoptosis in PDAC cells.
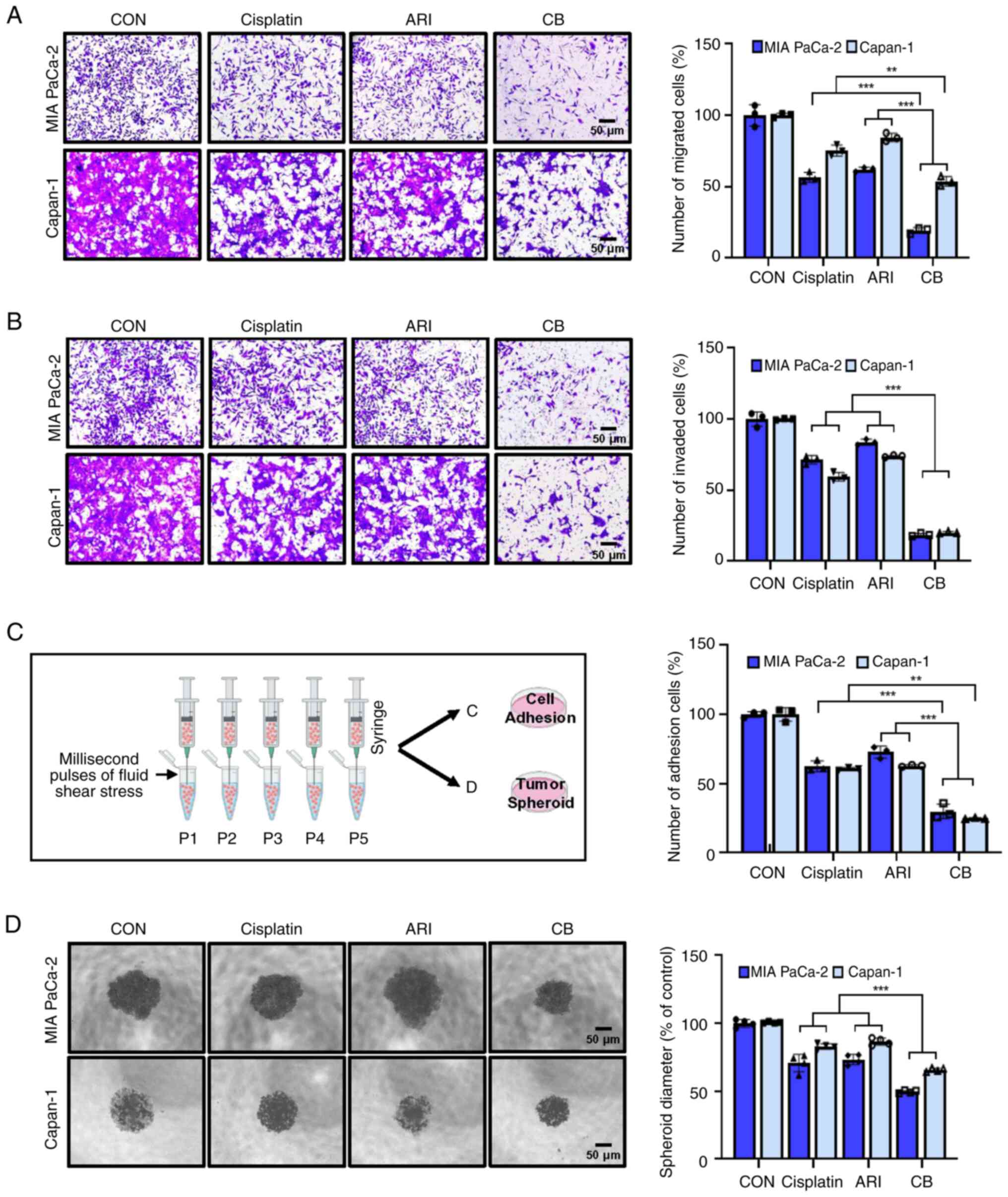
Combining cisplatin and aripiprazole
inhibits cell migration and invasion
Cancer cell migration and invasion are critical
steps in metastasis. To investigate the potential inhibitory
effects of co-treatment with aripiprazole and cisplatin on these
processes, migration and invasion assays were conducted using MIA
PaCa-2 and Capan-1 cell lines. Transwell assays demonstrated that
the combination of aripiprazole and cisplatin synergistically
inhibited both cell migration and invasion compared with the
effects of either agent alone (Fig.
4A and B). Additionally, cell spheroid growth and attachment to
collagen-coated plates were significantly diminished by the
combined treatment, in contrast to the effects of single-agent
treatments (Fig. 4C and D).
Aripiprazole inhibits the STAT3
pathway
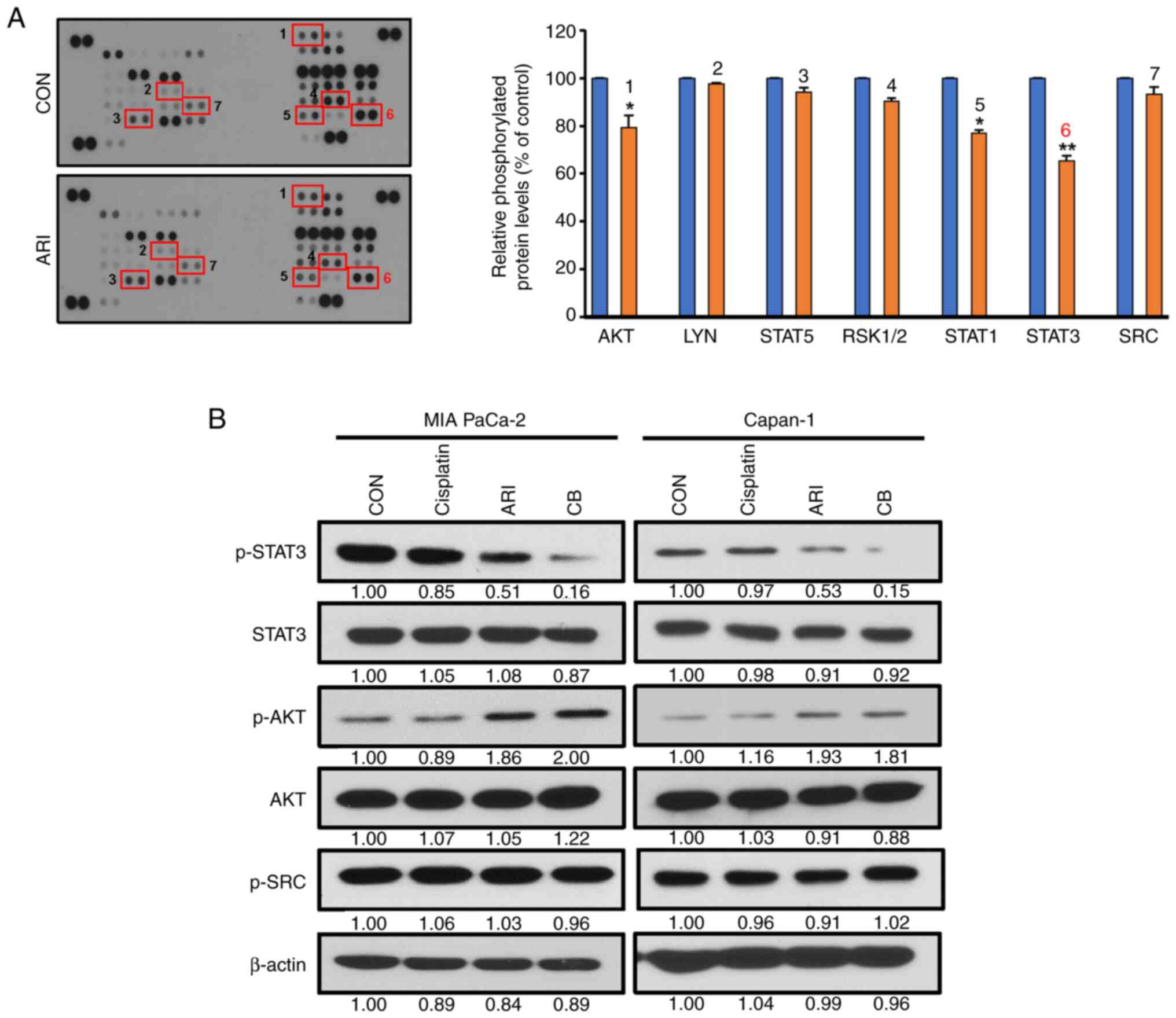
Among the 43 kinases analyzed, aripiprazole
inhibited STAT3 expression by 40% (Fig. 5A). To validate these findings,
cells were treated with aripiprazole (10 µM) for 12 h,
followed by western blot analysis to examine the expression of
p-STAT3, p-AKT and p-SRC. As shown in Fig. 5B, aripiprazole decreased p-STAT3
expression, and the combination of aripiprazole and cisplatin
almost completely abolished its expression in MIA PaCa-2 cells.
These findings suggested that the combination treatment could
induce pancreatic cancer cell death by inhibiting STAT3
signaling.
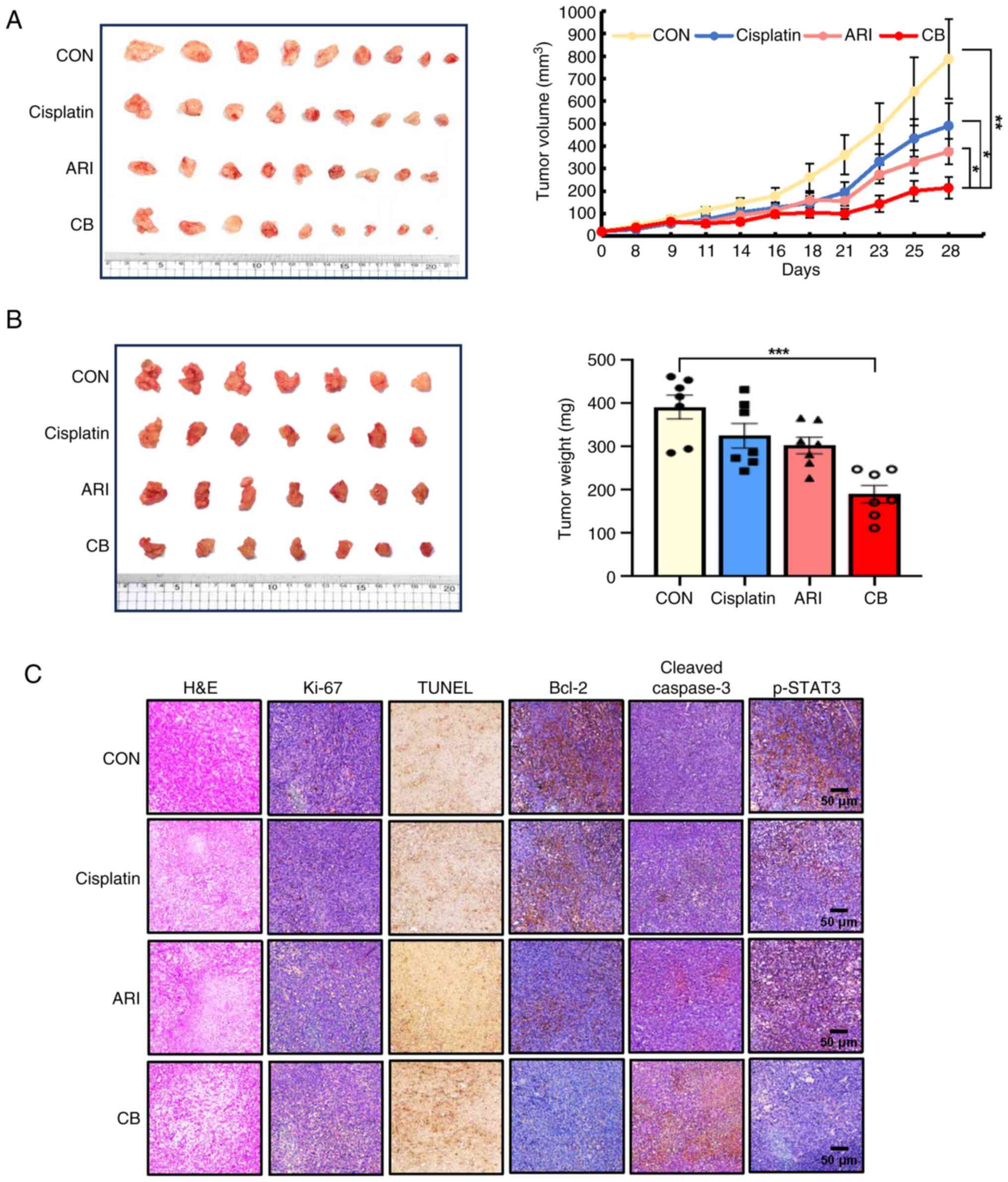
Aripiprazole inhibits tumor growth in
mouse pancreatic cancer models
Tumor growth was delayed in mice treated with either
aripiprazole or cisplatin alone compared with the control group.
Notably, the combination treatment significantly reduced tumor
volume by 78% relative to the control (Fig. 6A). These results were
corroborated using an orthotopic pancreatic cancer model (Fig. 6B). All treatments were
well-tolerated, with no significant differences between groups.
Tumor formation incidence was >95%. Treatment with aripiprazole
or cisplatin inhibited primary pancreatic tumor growth by 33 and
23%, respectively, compared with the control. The combination
treatment markedly enhanced the anti-tumor effect, with a 73%
growth inhibition observed compared with control. Furthermore, the
combined treatment reduced the expression of Ki-67 and Bcl-2,
markers of cell proliferation and apoptosis, respectively and
increased the expression of cleaved caspase-3 and the number of
TUNEL-positive cells. The combination treatment also significantly
reduced p-STAT3 expression (Fig.
6C).
Discussion
Of patients with pancreatic cancer, ~80% are
diagnosed at a locally advanced or metastatic stage, where
combination chemotherapy is generally preferred over single-agent
chemotherapy. However, the effectiveness of these treatments
remains limited, prompting increased interest in combination
therapies with fewer side effects and higher efficacy. Currently,
cisplatin is used to treat various solid tumors, including PDAC.
Despite its usefulness, cisplatin has shown severe side effects
drug resistance, potentially contributing to treatment failure in
PDAC (4,20). Nevertheless, cisplatin continues
to be evaluated in clinical trials for its potential in combination
chemotherapy for PDAC. Therefore, combination treatment with
sensitizing agents that have fewer side effects may be an effective
strategy for improving anticancer efficacy and overcoming cisplatin
resistance. The present study found that the combination of
aripiprazole and cisplatin synergistically induced apoptosis and
inhibited cell growth by blocking STAT3 signaling. Moreover, this
combination significantly reduced tumor growth in animal models
without notable side effects, suggesting that the combination of
aripiprazole and cisplatin could enhance PDAC treatment
outcomes.
Aripiprazole, an atypical antipsychotic, which is
effective in treating schizophrenia and schizoaffective disorders,
exhibits unique pharmacological activities as a serotonin 5-HT1A
and 5-HT2A antagonist. During tumor development, cancer not only
evades the body's regulatory mechanisms, but also affects local and
systemic homeostasis. It has been shown in human and animal cancer
models that tumors affect the production of classic
neurotransmitters such as hypothalamic and pituitary hormones
(21). Indeed, the
neurotransmitter regulator aripiprazole shows anti-cancer effects
across different types of cancer cells (13,14). This drug repositioning strategy
has recently demonstrated potent radiosensitizing activity in
various cancer cells (18,22). Therefore, it was hypothesized
that combining cisplatin with aripiprazole could result in
synergistic effects in PDAC cells. The combined treatment
significantly inhibited PDAC cell growth compared with individual
agent treatments. Cotreatment with cisplatin and aripiprazole
exhibited the highest synergistic effects in PDAC cells, as
indicated by the CI values. The present study investigated whether
this combination induced synergistic apoptotic effects. Indeed, it
led to significant apoptosis, evidenced by increased TUNEL-positive
nuclear fragmentation and elevated cleaved caspase-3 expression.
While studies have confirmed the apoptotic effect of aripiprazole
in combination with other antipsychotic drugs such as fluphenazine,
halaven and thioridazine in cancer (14,18), few have explored its combination
with anticancer drugs for clinical use. Therefore, the apoptotic
effect observed with aripiprazole combined with cisplatin appears
to be substantial.
Apoptosis occurs through two distinct pathways: The
extrinsic pathway, activated by death receptors, and the intrinsic
pathway, involving mitochondrial factors. Given reports that the
combination of cisplatin with other agents induces the intrinsic
mitochondrial apoptotic pathway (23,24), the present study extended its
analysis to investigate whether aripiprazole affected mitochondrial
membrane potential when combined with cisplatin, using JC-1
staining. As expected, the combined treatment synergistically
reduced mitochondrial membrane potential. To validate these
findings, the expression of XIAP and MCL-1, members of the
inhibitor of apoptosis proteins (IAPs) family crucial in
mitochondria-mediated apoptosis, were examined. The analyses
demonstrated a significant reduction in XIAP and MCL-1 expression
alongside increased levels of cleaved caspase-3 in PDAC cells
following combined treatment. Overall, the results suggested that
the synergistic apoptotic effects of this combination therapy may
operate through the mitochondria-mediated apoptotic pathway in PDAC
cells, potentially contributing to the inhibition of tumor growth
in animal models.
To date, aripiprazole has been reported to modulate
the cAMP/PKA, ERK/C-Fos and AKT/GSK3β signaling pathways in brain
diseases (14,25,26). Studies have indicated that
aripiprazole enhances cancer cell sensitivity to ionizing radiation
by increasing reactive oxygen species production (17) and that SRC serves as a primary
target for aripiprazole's anti-tumor activity in glioma cells
(13). However, there has been
limited research exploring the precise mechanisms by which
aripiprazole affects cancer signaling pathways. To further
investigate the potential mechanism underlying the anticancer
effects of aripiprazole, a phosphokinase array analysis was
conducted. The present study demonstrated that aripiprazole
effectively inhibited STAT3 phosphorylation. Notably, contrary to
previous reports (13,27), increased expression of AKT and
SRC was observed in response to aripiprazole in pancreatic cancer
cells, suggesting differential effects on AKT and SRC signaling
pathways. Given that STAT3 is frequently overexpressed in tumors
and its increased signaling promotes cancer cell survival and
chemoresistance, the inhibition of p-STAT3 expression by the
combination of aripiprazole and cisplatin, mediated by
aripiprazole, holds significant therapeutic promise.
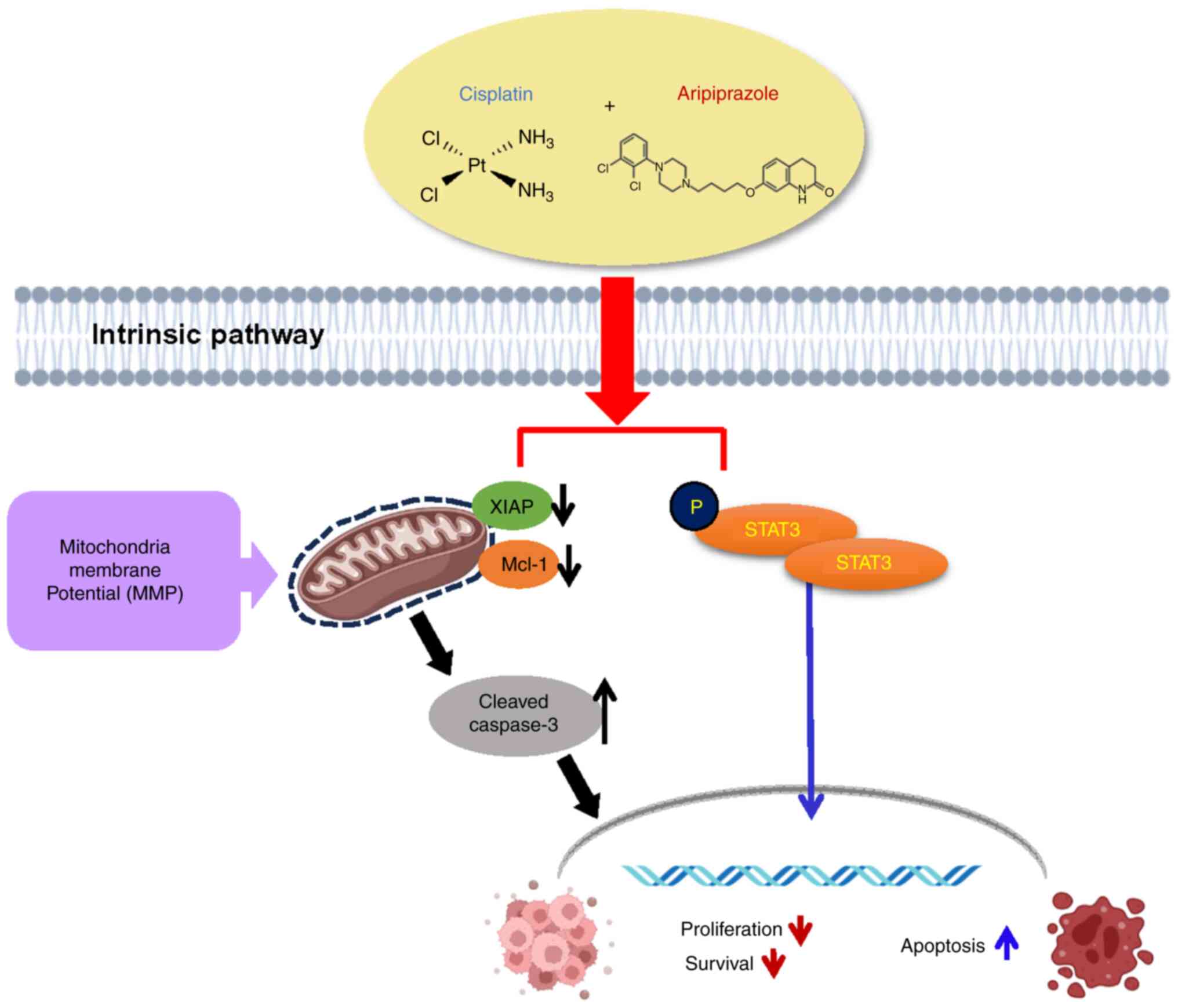
In conclusion, the present study is the first, to
the best of the authors' knowledge, to demonstrate that combined
treatment of aripiprazole and cisplatin markedly inhibited PDAC
cell growth and synergistically exhibited anticancer activities by
suppressing cell proliferation and inducing apoptosis both in
vitro and in vivo via STAT3 pathway inhibition (Fig. 7). Aripiprazole is an FDA approved
antidepressant currently in clinical use; therefore, it is expected
to be useful as an excellent adjuvant that can markedly increase
the efficacy of cisplatin therapy for pancreatic cancer. These
findings suggested that the combination of aripiprazole and
cisplatin represents an innovative therapeutic approach for
pancreatic cancer in humans.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YJC performed all the experiments with the
assistance of BSH, SK, MSP, YJL, SEK, PL and HGG. HL, SK, SP and
ERP interpreted the results. YJC, KHJ and SH wrote the manuscript.
SSH and KHJ contributed to the design of the study and assembled
data. SSH and KHJ confirmed the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal protocols were approved by the INHA
Institutional Animal Care and Use Committee (approval no. INHA
IACUC) at the College of Medicine, Inha University (Incheon, Korea;
approval nos. INHA 2211124-848 and INHA 230731-883).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, the authors
used DeepL (www.deepl.com) to assist in translating
the original text into English to check for grammatical errors and
confirm alternative expressions. After using this tool, the authors
reviewed and edited the content as necessary and therefore they
take full responsibility for the ultimate content of the present
manuscript.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Basic Science Research
Program through the National Research Foundation of Korea (grant
nos. 2021R1A5A2031612, 2024M3A9J4006509 and 2022R1A2C1092933) and
the Technology development Program (RS-2022-TI023745) funded by the
Ministry of SMEs and Startups (MSS; Korea).
References
|
1
|
Wang S, Zheng Y, Yang F, Zhu L, Zhu XQ,
Wang ZF, Wu XL, Zhou CH, Yan JY, Hu BY, et al: The molecular
biology of pancreatic adenocarcinoma: Translational challenges and
clinical perspectives. Sig Transduct Target Ther. 6:2492021.
View Article : Google Scholar
|
|
2
|
Muaddi H, Kearse L and Warner S:
Multimodal approaches to patient selection for pancreas cancer
surgery. Curr Oncol. 31:2260–2273. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Awais N, Satnarine T, Ahmed A, Haq A,
Patel D, Hernandez GN, Seffah KD, Zaman MA and Khan S: A systematic
review of chemotherapeutic regimens used in pancreatic cancer.
Cureus. 15:e466302023.PubMed/NCBI
|
|
4
|
Du J, Wang X, Li Y, Ren X, Zhou Y, Hu W,
Zhou C, Jing Q, Yang C, Wang L, et al: DHA exhibits synergistic
therapeutic efficacy with cisplatin to induce ferroptosis in
pancreatic ductal adenocarcinoma via modulation of iron metabolism.
Cell Death Dis. 12:7052021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ouyang G, Liu Z, Huang S, Li Q, Xiong L,
Miao X and Wen Y: Gemcitabine plus cisplatin versus gemcitabine
alone in the treatment of pancreatic cancer: A meta-analysis. World
J Surg Onc. 14:592016. View Article : Google Scholar
|
|
6
|
Principe DR, Underwood PW, Korc M, Trevino
JG, Munshi HG and Rana A: The current treatment paradigm for
pancreatic ductal adenocarcinoma and barriers to therapeutic
efficacy. Front Oncol. 11:6883772021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santos NAGD, Ferreira RS and Santos ACD:
Overview of cisplatin-induced neurotoxicity and ototoxicity, and
the protective agents. Food Chem Toxicol. 136:1110792020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Low ZY, Farouk IA and Lal SK: Drug
repositioning: New approaches and future prospects for
life-debilitating diseases and the COVID-19 pandemic outbreak.
Viruses. 12:10582020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Islam S, Wang S, Bowden N, Martin J and
Head R: Repurposing existing therapeutics, its importance in
oncology drug development: Kinases as a potential target. Br J Clin
Pharmacol. 88:64–74. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shankar E, Subramaniam V and Allimuthu D:
Editorial: Adopting drug repurposing to overcome drug resistance in
cancer. Front Cell Dev Biol. 11:11916822023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stępnicki P, Kondej M and Kaczor AA:
Current concepts and treatments of schizophrenia. Molecules.
23:20872018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki S, Okada M, Kuramoto K, Takeda H,
Sakaki H, Watarai H, Sanomachi T, Seino S, Yoshioka T and Kitanaka
C: Aripiprazole, an antipsychotic and partial dopamine agonist,
inhibits cancer stem cells and reverses chemoresistance. Anticancer
Res. 36:5153–5161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MS, Yoo BC, Yang WS, Han SY, Jeong D,
Song JM, Kim KH, Aravinthan A, Kim JH, Kim JH, et al: Src is the
primary target of aripiprazole, an atypical antipsychotic drug, in
its anti-tumor action. Oncotarget. 9:5979–5992. 2017. View Article : Google Scholar
|
|
14
|
Kim JY, Tae IH, Lee BM, Kim HS and Yoon
SP: Low doses of the anti-psychotic drug aripiprazole have strong
P-gp-inhibitory activity and sensitize anti-mitotic drug-resistant
cancer cells. Anticancer Res. 38:5101–5108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuo C, Xun Z, Hou W, Ji F, Lin X, Tian H,
Zheng W, Chen M, Liu C, Wang W and Chen C: Surprising anticancer
activities of psychiatric medications: Old drugs offer new hope for
patients with brain cancer. Front Pharmacol. 10:12622019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Badran A, Wahab AT, Zafar H, Mohammad N,
Imad R, Khan MA, Baydoun E and Choudhary MI: Antipsychotics drug
aripiprazole as a lead against breast cancer cell line (MCF-7) in
vitro. PLoS One. 15:e02356762020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang C, Lee SH, Park JH, Lee JS, Park JW,
Kim JR, Lee SH, Kim HS and Yoon SP: A low dose of aripiprazole has
the strongest sensitization effect among 19 repositioned bipolar
drugs in P-gp-overexpressing drug-resistant cancer cells.
Anticancer Res. 41:687–697. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee HJ, Kang SM, Sonn JK and Lim YB:
Dopamine receptor D2 activation suppresses the
radiosensitizing effect of aripiprazole via activation of AMPK.
FEBS Open Bio. 9:1580–1588. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh P, Patel M, Bhowmik D, Kumari N,
Prajapati SK and Gupta R: Identification of common biomarkers
affecting patient survival in cancers. World Acad Sci J. 6:1–17.
2024. View Article : Google Scholar
|
|
20
|
de Oliveira G, Freire PP, Cury SS, de
Moraes D, Oliveira JS, Dal-Pai-Silva M, Reis PP and Carvalho RF: An
integrated meta-analysis of secretome and proteome identify
potential biomarkers of pancreatic ductal adenocarcinoma. Cancers
(Basel). 12:7162020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slominski RM, Ramen C, Chen JY and
Slominski AT: How cancer hijacks the body's homeostasis through the
neuroendocrine system. Trends Neurosci. 4:263–275. 2023. View Article : Google Scholar
|
|
22
|
Jeong HJ, Jung CW, Kim HJ, Park BH, Moon
Y, Kim JY and Park MJ: Aripiprazole sensitizes head and neck cancer
cells to ionizing radiation by enhancing the production of reactive
oxygen species. Pharmacol Res Perspect. 10:e009892022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodrigues T and Ferraz L: Therapeutic
potential of targeting mitochondrial dynamics in cancer. Biochem
Pharmacol. 182:1142822020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Guo S, Xiong XK, Peng BY, Huang JM,
Chen MF, Wang FY and Wang JN: Combination of quercetin and
cisplatin enhances apoptosis in OSCC cells by downregulating xIAP
through the NF-κB pathway. J Cancer. 10:4509–4521. 2019. View Article : Google Scholar :
|
|
25
|
Pan B, Chen J, Lian J, Huang XF and Deng
C: Unique effects of acute aripiprazole treatment on the dopamine
D2 receptor downstream cAMP-PKA and Akt-GSK3β signalling pathways
in rats. PLoS One. 10:e01327222015. View Article : Google Scholar
|
|
26
|
Pereira A, Zhang B, Malcolm P,
Sugiharto-Winarno A and Sundram S: Quetiapine and aripiprazole
signal differently to ERK, p90RSK and c-Fos in mouse frontal cortex
and striatum: role of the EGF receptor. BMC Neurosci. 15:302014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan B, Huang XF and Deng C: Aripiprazole
and haloperidol activate GSK3β-dependent signalling pathway
differentially in various brain regions of rats. Int J Mol Sci.
17:4592016. View Article : Google Scholar
|