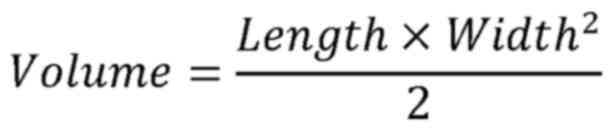Introduction
Esophageal cancer (EC) is a notable global health
burden and a leading cause of cancer mortality worldwide. In 2020,
an estimated 544,076 deaths were attributed to E (1). Esophageal squamous cell carcinoma
(ESCC) accounts for the majority of EC cases, representing >50%
of diagnoses in China (2).
Despite considerable research efforts, treatment outcomes for ESCC
remain poor, with median survival ~11 months, largely due to
late-stage diagnosis and the lack of effective targeted therapies
(3,4). Thus, identifying molecular markers
to enable early diagnosis and prognosis prediction, as well as
discovering novel therapeutic targets, is key for improving patient
outcomes.
Aberrant energy metabolism is a hallmark of cancer
that supports tumor growth and progression (5). Increasing evidence suggests that
overactivity of one-carbon metabolism drives tumorigenesis and is
associated with epigenetic regulation of cells (6,7).
Methylenetetrahydrofolate dehydrogenase 2 (MTHFD2), a mitochondrial
enzyme involved in one-carbon metabolism, serves an important role
in physiology and pathology (8).
As a bifunctional enzyme, MTHFD2 catalyzes interconversion of
folate metabolites to support biosynthetic processes, including
purine synthesis. MTHFD2 expression is induced in cancer to meet
increased metabolic demands, and high MTHFD2 levels promote
occurrence and development of tumors (9,10). While MTHFD2 dysregulation is
implicated in ovarian cancer (11), glioma (12) and lung (13) and colorectal cancer (14), its functional role and mechanisms
in ESCC remain unexplored.
N6-methyladenosine (m6A) RNA methylation is a
prevalent post-transcriptional modification by methyltransferase
complexes, recognized by RNA-binding proteins, and removed by
demethylases (15). m6A
signaling regulates cancer progression (16,17). In ESCC, m6A has been shown to
control processes such as proliferation, invasion and metastasis
(18-20). However, different m6A regulators
may confer distinct regulatory outcomes in ESCC (21).
The present study investigated the clinical value
and functional role of one-carbon metabolism-associated genes in
ESCC, with a particular focus on MTHFD2. To elucidate the
regulatory mechanisms involved, the effects of key m6A regulators,
methyltransferase-like 3 (METTL3) and insulin-like growth factor 2
mRNA binding protein 2 (IGF2BP2), on MTHFD2 expression were
assessed. Additionally, downstream signaling pathways influenced by
MTHFD2 and the impact on the phosphatidylethanolamine-binding
protein 1 (PEBP1) and raf-1 proto-oncogene (RAF1) interaction was
assessed. PEBP1 inhibits the RAF1/MEK/ERK pathway, which regulates
key cellular processes such as survival, proliferation and
apoptosis (22,23).
Materials and methods
Data acquisition and preprocessing
The Cancer Genome Atlas (TCGA)biolinks R package
(24) was used to retrieve mRNA
expression data (RNA-seq V2) and corresponding clinical information
for 80 patients with ESCC in the TCGA-ESCA cohort from the Genomic
Data Commons portal (portal.gdc.cancer.gov/) using the GDCquery function.
Patients were selected based on the disease type information
classified as 'Squamous Cell Neoplasms'. GSE130078, GSE53622, and
GSE53624 were downloaded from the Gene Expression Omnibus (GEO)
database (https://www.ncbi.nlm.nih.gov/geo/). TCGA-ESCA cohort
includes patients of Asian, White and Black or African American
descent. The GSE130078 cohort consists of South Korean patients,
while the GSE53622 and GSE53624 cohorts include patients from
China.
Differentially expressed genes and
clinical relevance analyses
MTHFD2 expression levels across pan-cancer were
evaluated using the TIMER 2.0 database (25). BEST tool (rookieutopia.
com/app_direct/BEST/) was used to compare MTHFD2 mRNA levels
between tumor and normal tissue in ESCC datasets from GEO database.
BEST also analyzed the correlation between MTHFD2 expression and
clinical features in ESCC using Spearman's method. Kaplan-Meier
plotter website (26) was used
to assess the prognostic value of MTHFD2 in ESCC.
Gene set enrichment analysis (GSEA)
GSEA was performed to assess enrichment of one
carbon pool by folate and PI3K/AKT signaling pathway gene sets
using MSigDB databases (https://www.gsea-msigdb.org/gsea/msigdb).
Online prediction
The sequence-based RNA adenosine methylation site
predictor (SRAMP; cuilab.cn/sramp) was used to identify potential
m6A modification sites in MTHFD2 mRNA. Potential protein
interactions of MTHFD2 were predicted using Biological General
Repository for Interaction Datasets (BioGRID) (https://thebiogrid.org/).
Cell culture
ESCC cell lines, including TE1 (CVCL_1759), KYSE30,
KYSE410, KYSE150 and KYSE510, and immortalized esophageal
epithelial cell Het-1A, were acquired from Wuhan Pricella
Biotechnology Co., Ltd. Cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Procell Life Science
& Technology Co., Ltd.) in a humidified incubator at 37°C with
5% CO2.
RNA interference, lentivirus production
and infection
Lentiviral vectors expressing short hairpin RNAs
(shRNAs) targeting MTHFD2 (shRNA#1, 5′-GCC TCT TCC AGA GCA TAT
TGA-3′; shRNA#2, 5′-GCA GTC ATT GAT GTG GGA ATA-3′) and scrambled
negative control (NC; 5′-TTC TCC GAA CGT GTC ACG T-3′) were
designed and synthesized by Shanghai GeneChem Co., Ltd.. Small
interfering (si)PEBP1 (5′-GTG GTC AAC ATG AAG GGC AAT-3′) and
control siNC (Table SI) were
also synthesized by GeneChem. The lentivirus production and
infection and siRNA transfection were performed as previously
described (27). Briefly, KYSE30
and KYSE410 cells were infected with lentivirus expressing shMTHFD2
and shNC for 48 h. Following selection with puromycin (2
µg/ml) for 72 h, knockdown efficiency was confirmed by qPCR
and WB analysis as aforementioned. As for siRNA transfection, a 500
µl mixture containing 10 µl of 20 µM
siPEBP1/siNC and 10 µl Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) was added to KYSE30 and KYSE410 cells that had
been seeded in 6-well plates 24 h earlier. The medium was replaced
with fresh complete medium after 6 h. The efficiency of
interference was detected by WB and qPCR after 48 h.
Reverse transcription quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1 µg RNA
was reverse-transcribed to cDNA using PrimeScript RT reagent kit
(Takara Biotechnology Co., Ltd.). RT-qPCR was performed with SYBR
Premix Ex Taq II (Takara Biotechnology Co., Ltd.) on a 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and annealing/extension at 60°C for
30 sec. The relative expression of target genes was calculated
using the 2-ΔΔCq method with GAPDH as the reference gene (28). The primers are shown in Table SI.
Western blot (WB) analysis
Western blot was performed as described previously
(29) using primary antibodies
against MTHFD2 (Proteintech Group, Inc.; cat. no. #12270-1-AP),
METTL14 (Proteintech Group, Inc.; #26158-1-AP) and IGF2BP2
(Proteintech Group, # 11601-1-AP; all 1:1,000) followed by
HRP-conjugated secondary antibodies (1:2,000; Cell Signaling
Technology, Inc.).
Immunohistochemistry (IHC)
A total of 80 paired clinical ESCC and adjacent
normal tissue samples collected from February 2018 to September
2019 (median age, 61 years; age range, 43-76 years; 5 female and 75
male; distance, 3 cm) were obtained from the tissue bank of the
First Xiangya Hospital of Central South University (Changsha,
China). IHC staining was performed to evaluate the expression of
MTHFD2. Tissues were fixed in 4% paraformaldehyde at room
temperature for 24 h and embedded in paraffin. Then, 5-µm
sections were cut and mounted on slides. Antigen retrieval was
performed using citrate buffer at 95°C for 20 min. Slides were then
washed with xylene and rehydrated in a descending alcohol series.
Blocking was performed with 5% BSA(cat. no. #37520, Thermo Fisher
Scientific, Inc.) at 25°C for 1 hour. Endogenous peroxidase
activity was quenched with 3% hydrogen peroxide for 10 min at 25°C.
The primary antibody against MTHFD2 (#12270-1-AP; Proteintech
Group, Inc.) was (1:100) and incubated at 4°C overnight. The
secondary antibody (#SA00004-2; Proteintech Group, Inc.;
Biotin-conjugated) was diluted (1:200) and incubated at room
temperature for 1 h. Protein localization and staining intensity
were visualized using diaminobenzidine (DAB) and scored as
described previously (30).
Images were captured using a light microscope at 100×
magnification. Image analysis was performed using ImageJ (Java 8
version, National Institutes of Health).
Methylated RNA immunoprecipitation
(MeRIP)-Qpcr
MeRIP was conducted using Protein A/G magnetic beads
(cat. no. #Bes5203-1, BersinBio,) according to the manufacturer's
protocol. In each reaction, 50-100 µg of RNA were sonicated
to produce small fragments of about 100 nt in length. After
precipitation and washing, the RNA fragments were dissolved in 400
microliters of IP buffer containing RNase inhibitors. The m6A
antibody (1 mg/ml) was used for immunoprecipitation, with IgG (1
mg/ml) as a negative control. Immunoprecipitated complexes were
washed three times with IP buffer 3 at 4°C for 5 min each. RNA
extraction involved centrifugation at 13,000 g, 4°C for 10 min,
followed by ethanol precipitation and collection at 16,000 g, 4°C
for 30 min. The complexes were isolated using a magnetic rack, and
RNA was eluted with Elution Buffer and Proteinase K at 55°C for 30
min. Immunoprecipitated RNA was purified and reverse-transcribed to
cDNA, and MTHFD2 enrichment was measured using qPCR and normalized
to input controls as aforementioned. Knockdown of METTL3 and
IGF2BP2 was verified to assess specificity of m6A modification.
Luciferase reporter assay
A dual-luciferase reporter system assay was
performed as previously described (27). For luciferase reporter
construction, the MTHFD2 sequence 5′-AGC TTG GGG TAG CCA CTA ATT
AAC TAC TGT GTC TTC TGT GTC ACA-3′) containing predicted m6A sites
was amplified by PCR and cloned into the pmirGLO reporter plasmids
(Promega Corporation) downstream of the luciferase reporter gene.
The empty pmirGLO vector served as a control. KYSE30 and KYSE410
cells were seeded in 24-well plates (5×104 cells/well).
After 24 h, cells were co-transfected at 37°C with pmirGLO-MTHFD2
or empty vector (500 ng), Renilla luciferase plasmid (50 ng)
for normalization and siMETTL3 or siNC (50 nM). At 48 h
post-transfection, luciferase activity was measured using the
Dual-Luciferase Reporter Assay System (Promega Corporation). Each
experiment was performed in triplicate.
RNA stability assay
RNA stability assays were conducted as previously
described (27). Briefly, ESCC
cells were treated with 10 µg/ml actinomycin D
(MedChemExpress; #HY-17559) for 0, 2, and 4 h. Following RNA
isolation, qRT-PCR was used to assess MTHFD2 relative expression,
normalized to GAPDH. mRNA half-life was then determined using
linear regression analysis.
Co-immunoprecipitation (Co-IP) assay
Co-IP assays were performed to investigate
protein-protein interactions. Briefly, protein was extracted from
lysed ESCC cells (Pierce IP Lysis Buffer, Thermo Fisher Scientific,
Inc.). Magnetic beads were added to the protein solution and
incubated at 4°C for 30 min. Following magnetic separation, the
supernatant was transferred to a new tube and divided into two
groups: an IgG control group and a target antibody group. The
corresponding antibodies were added overnight at 4°C with shaking.
Magnetic beads (pre-washed twice with cold PBS) were then added to
each protein-antibody complex and incubated for 4-6 h at 4°C. After
another magnetic separation, the supernatant was collected and
heated to 100°C for 10 min to denature the proteins. Following
centrifugation and a final magnetic bead separation, the resulting
supernatant was collected for WB analysis.
T7 biotin labeled RNA synthesis and RNA
pulldown assay
RNA pull-down assay was performed as previously
described (27). Briefly,
biotin-labeled RNA probes specific to MTHFD2 mRNA were synthesized
using the Ribo RNAmax-T7 Biotin Labeled RNA Synthesis kit
(#C11002-2, RiboBio) following the manufacturer's protocol. An RNA
pulldown experiment was then carried out with the BersinBio RNA
pulldown Kit (#Bes5102, BersinBio) according to the standard method
(27). Biotin-labeled RNA probes
were attached to streptavidin-coated magnetic beads for 30 min at
room temperature. RNA-bead conjugates were then incubated with
nucleic acid-depleted lysates (Pierce IP Lysis Buffer, Thermo
Fisher Scientific, Inc.) from ESCC cells for 2 h at room
temperature to capture interacting proteins. The amount of lysate
used per IP reaction was 0.8 ml. The amount of magnetic beads was
used according to manufacturer's protocol. After incubation, the
complexes were washed with protein elution buffer provided in the
kit. The centrifugation steps were performed at 5,000 g for 1 min
at room temperature. The completes were captured by the magnetic
beads, then eluted and identified via WB as aforementioned.
Specifically, the biotin-labeled RNA probes were
attached to streptavidin-coated magnetic beads at room temperature
for 30 min. These RNA-bead conjugates were incubated with nucleic
acid-depleted lysates from ESCC cells at room temperature for 2 h
to capture interacting proteins. The captured proteins were
subsequently eluted and identified via western blot as
aforementioned.
Cell Counting Kit (CCK)-8
CCK-8 assay was employed to assess cell viability.
Cells were at a density of 2,000 cells per well seeded into a
96-well plate and cultured at 37°C. After 24, 48, 72, 96 and 120 h,
CCK-8 solution (10 µl/well, Biosharp) was added, and the
plate was incubated at 37°C for 2 h. Absorbance was measured at 450
nm.
Colony formation assay
ESCC cells were seeded into 6-well plates (600
cells/well) and cultured at 37°C for 14 days. The culture medium
was changed every 3 days. Cells were fixed with 4% paraformaldehyde
at 25°C for 29 min and stained with 0.1% crystal violet at 25°C for
20 min. Colonies (≥50 cells) were counted manually under a light
microscope at x4 magnification. The experiment was repeated ≥3
times.
Transwell invasion assay
ESCC cells were seeded (1×105) in
FBS-free DMEM (Thermo Fisher Scientific, Inc.) at 37°C in the upper
chamber of Matrigel-coated inserts (BD Biosciences). The Matrigel
was pre-coated onto the inserts at 37°C for 30 min. DMEM containing
15% FBS (Thermo Fisher Scientific, Inc.) was added to the lower
chamber. After 24 h at 37°C, non-invading cells on the upper
membrane were removed and invading cells on the lower surface were
fixed with 4% paraformaldehyde at 25°C, stained with 0.5% crystal
violet at 25°C for 20 min and counted in five random fields of view
from three independent experiments (magnification, x100).
Subcutaneous tumor formation in nude
mice
KYSE30 cells with stable MTHFD2 knockdown and
control cells were suspended in a 1:1 mixture of PBS and Matrigel.
Six-week-old female BALB/c nude mice (n=6; Silek Jingda; weighing
20-25 g at the start of the study were subcutaneously injected with
5×106 cells/mouse in the dorsal flank as previously
described (31). Mice were
housed in individually ventilated cages under standard conditions:
22-25°C temperature, 50-60% humidity, 12-h light/12-h dark cycle
with ad libitum access to sterile food and water. Tumor growth was
monitored every 2 days and caliper measurements were taken to
assess tumor volume using the formula:
The longest diameter and maximum tumor volume
measured were 1.25 cm and 0.488 cm3 respectively. After
six weeks, mice were euthanized by cervical dislocation following
injection of sodium pentobarbital at a dose of 50 mg/kg (32). Humane endpoints were body
temperature below 33°C, 3 h of inactivity, and/or a weight loss
exceeding 20% of the initial body weight. Death was confirmed by
cessation of both respiratory and cardiac activity. Tumors were
harvested for further analysis. Tumor tissues underwent IHC
staining to evaluate expression levels of MTHFD2 and Ki67 as
aforementioned. The present study was approved by Experimental
Animal Ethics Committee of Xiangya Hospital, Central South
University (approval no. XY20240407002).
Statistical analysis
All statistical analyses were conducted using R
4.2.1 (r-project.org/) or GraphPad Prism 8
(graphpad. com/). Significant differences were determined by paired
Student's t-test or Wilcoxon test. One-way ANOVA followed by
Tukey's post hoc test was used to evaluate differences between
>2 groups. χ2 test was used to analyze association
between MTHFD2 expression and clinicopathological variables.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were performed with ≥3 biological
replicates. Data are presented as mean ± SD.
Results
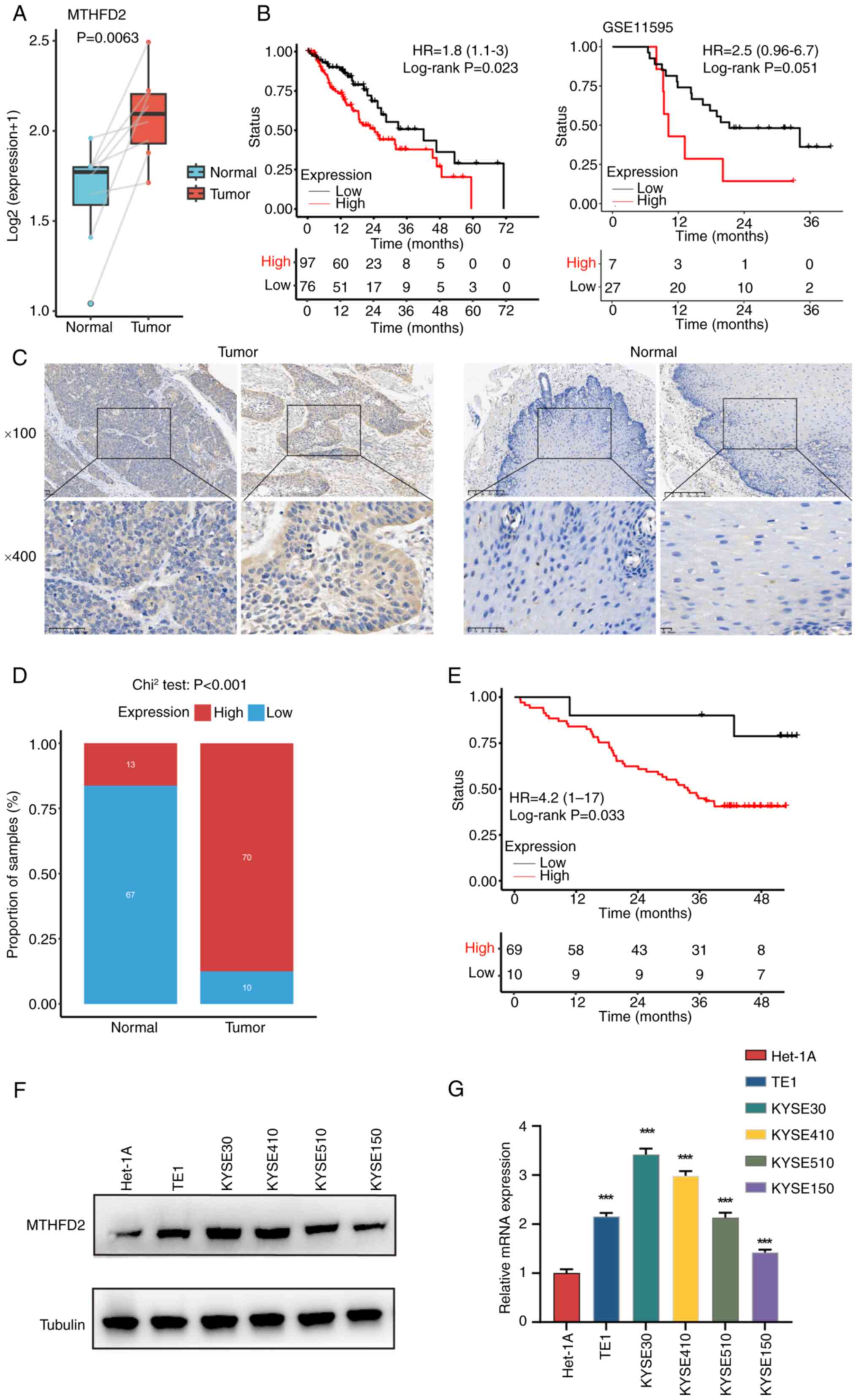
MTHFD2 upregulation in ESCC is associated
with malignant progression and poor prognosis
Activity of the one-carbon metabolism pathway was
significantly enriched in tumor tissue (Fig. S1A). Differential expression
analysis identified nine upand three downregulated genes in ESCC
(Fig. S1B). MTHFD2, MTHFD1L,
MTHFD1 and thymidylate synthase were significantly upregulated in
tumors compared with normal tissue (Figs. 1A and S1C). MTHFD2 was consistently
upregulated across three independent ESCC datasets and its
expression was higher in patients with higher tumor grades and
metastasis (Fig. S1D-F). MTHFD2
expression across pan-cancer was upregulated in multiple tumor
types (including bladder urothelial carcinoma, breast invasive
carcinoma, CESC: Cervical squamous cell carcinoma and endocervical
adenocarcinoma, CHOL: Cholangiocarcinoma, CRC: colorectal cancer,
ESCA: Esophageal carcinoma, HNSC: Head and neck squamous cell
carcinoma, kidney renal clear cell carcinoma, LIHC: Liver
hepatocellular carcinoma, LUAD: Lung adenocarcinoma, LUSC: Lung
squamous cell carcinoma, PCPG: Pheochromocytoma and paraganglioma,
PRAD: Prostate adenocarcinoma, STAD: Stomach adenocarcinoma, THCA:
Thyroid carcinoma, and UCEC: Uterine corpus endometrial carcinoma),
reinforcing its role as a potential oncogene (Fig. S1F and G). Survival analysis
demonstrated that high MTHFD2 expression was associated with poor
recurrence-free survival (Fig.
1B).
To validate these bioinformatics findings, 80 ESCC
samples were collected. IHC confirmed the elevated expression of
MTHFD2 in tumor tissues (Fig. 1C and
D). Kaplan-Meier analysis showed that patients with high MTHFD2
expression had worse survival outcomes (Fig. 1E). MTHFD2 expression was assessed
in normal esophageal and ESCC cell lines. WB and qPCR results
indicated that KYSE410 and KYSE30 cells exhibited higher MTHFD2
levels (Fig. 1F and G) and were
selected for subsequent experiments.
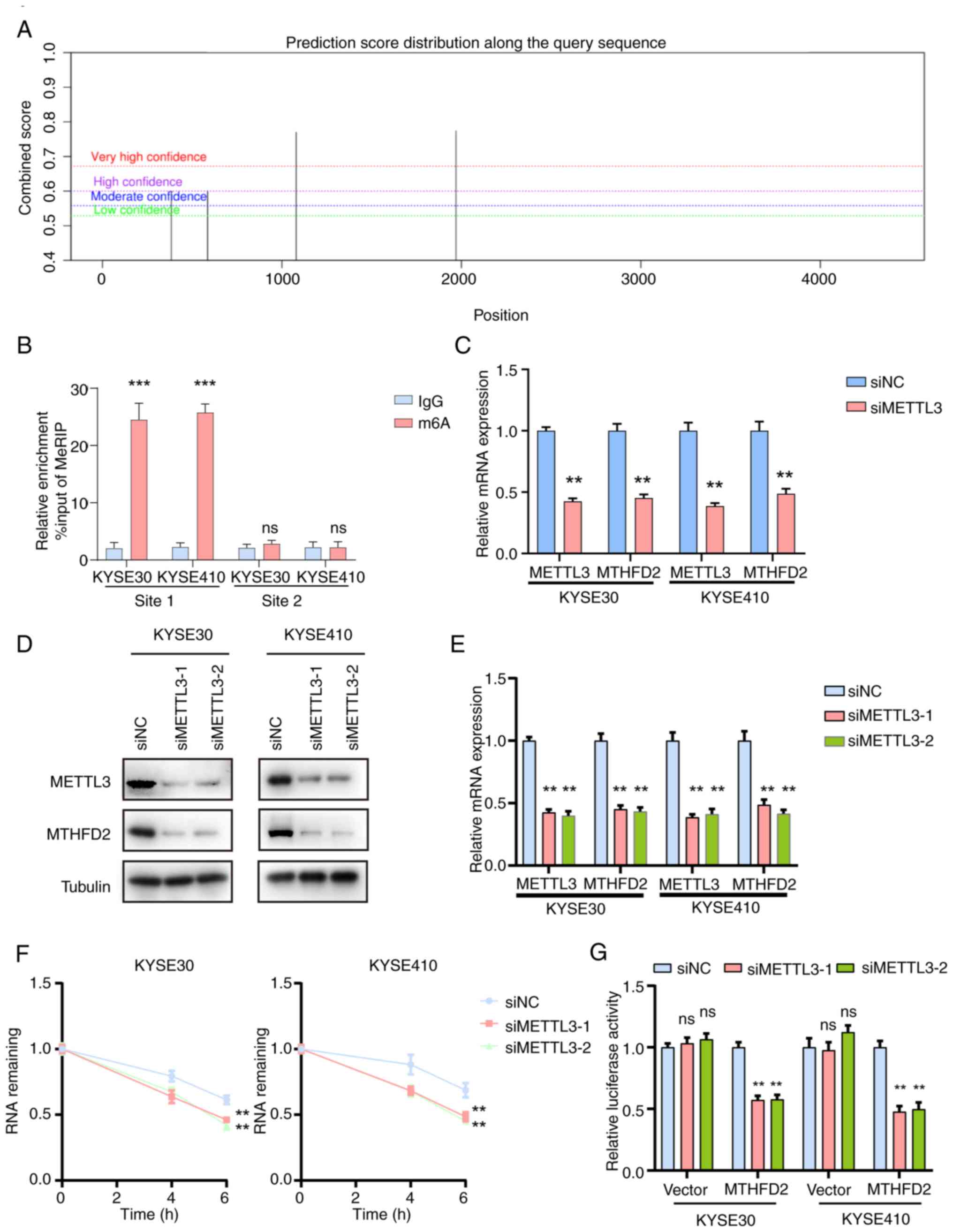
m6A modification regulates MTHFD2
expression via METTL3-mediated mRNA stability
Considering the importance of m6A modification in
the regulation of gene expression (33), it was hypothesized that
upregulation of MTHFD2 is regulated by m6A modification. SRAMP
database (34) identified two
high-confidence m6A modification sites on MTHFD2 (Fig. 2A). MeRIP confirmed that site 1
was modified by m6A (Fig. 2B).
Further analysis revealed an association between MTHFD2 expression
and 30 known m6A regulators; there were positive associations
between MTHFD2 and most regulatory factors, supporting a potential
regulatory role of m6A modification in MTHFD2 expression (Fig. S2A).
To explore the mechanism of m6A modification on
MTHFD2, common m6A writers (METTL3, METTL16 and WTAP) were knocked
down in KYSE30 and KYSE410 cells. qPCR showed that METTL3 knockdown
resulted in a significant downregulation of MTHFD2 mRNA, while
METTL16 and WTAP knockdown did not have a similar effect (Figs. 2C and S2B). To validate this, cells were
transfected with additional METTL3-targeting sequence. WB and qPCR
confirmed effective knockdown of METTL3 expression with the two
sequences. (Fig. 2D and E). The
RNA stability assay revealed that METTL3 knockdown led to decreased
stability of MTHFD2 mRNA (Fig.
2F). Dual-luciferase reporter assay demonstrated a significant
reduction in luciferase activity upon METTL3 knockdown (Fig. 2G). These findings suggested that
METTL3-mediated m6A modification serves a key role in regulating
MTHFD2 expression by enhancing stability of its mRNA.
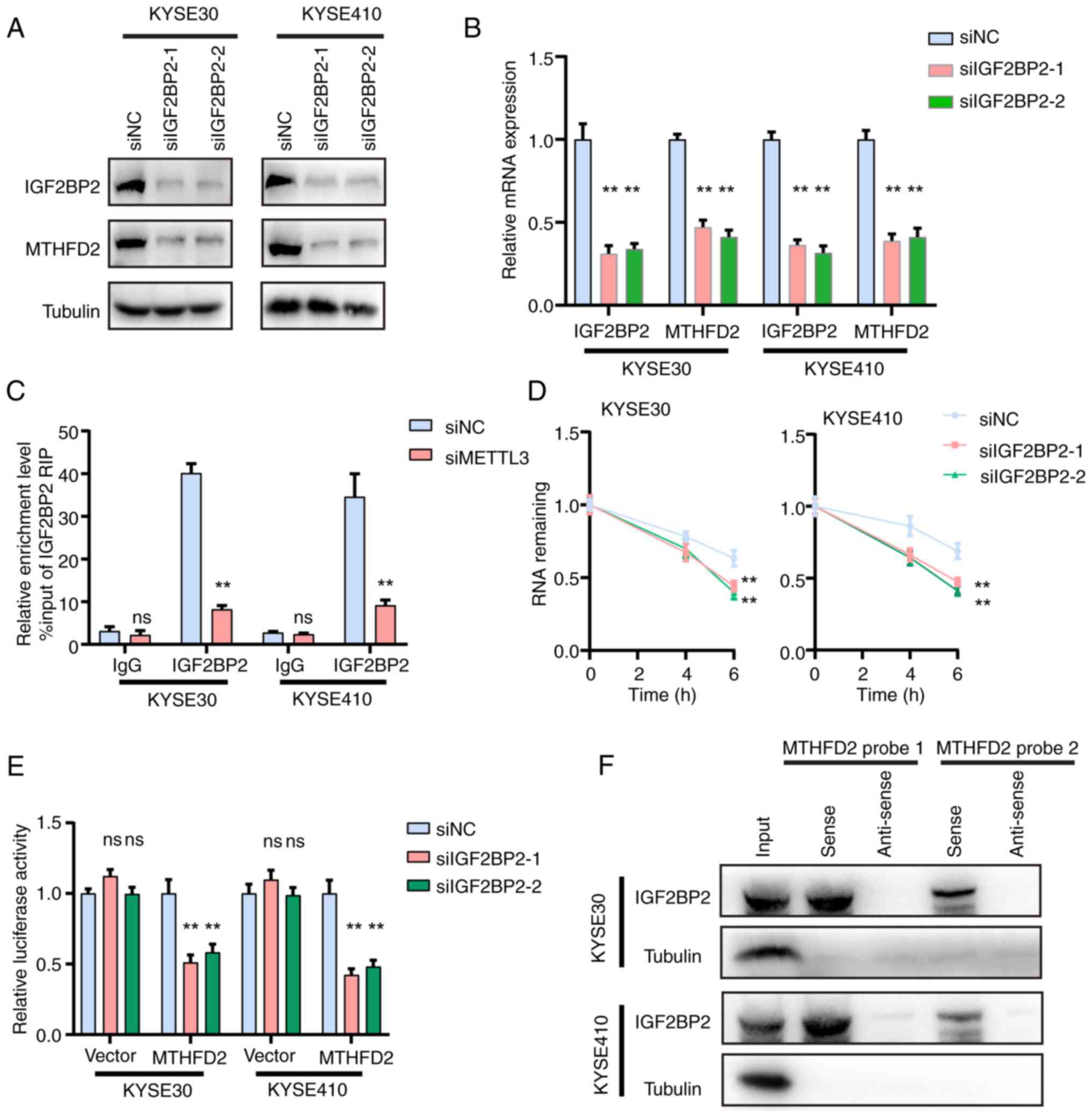
IGF2BP2 recognizes m6A of MTHFD2 mRNA and
enhances its stability in ESCC
In addition to writers, the m6A methylation process
involves readers, the main function of which is to recognize bases
where m6A modification occurs (15). To identify the readers of MTHFD2,
readers (IGF2BP1, IGF2BP2 and IGF2BP3) with a high association with
MTHFD2 were selected. qPCR results indicated that IGF2BP1 and
IGF2BP3 knockdown did not lead to a significant downregulation of
MTHFD2 mRNA (Fig. S3).
Furthermore, knockdown sequences for IGF2BP2 effectively decreased
METTL3 expression, as confirmed by WB and qPCR (Fig. 3A and B). MeRIP-qPCR demonstrated
that the m6A antibody enriched MTHFD2 mRNA and this enrichment was
markedly diminished upon METTL3 knockdown (Fig. 3C). RNA stability assay revealed
that IGF2BP2 knockdown reduced the stability of MTHFD2 mRNA in both
KYSE30 and KYSE410 cells (Fig.
3D). Dual-luciferase reporter experiments confirmed that MTHFD2
mRNA stability decreased following IGF2BP2 knockdown (Fig. 3E). Moreover, the RNA pull-down
assay showed that the biotin-labeled MTHFD2 mRNA probe specifically
precipitated IGF2BP2, whereas the control antisense probe did not
(Fig. 3F). These findings
indicated that METTL3 and IGF2BP2 jointly regulate stability of
MTHFD2 mRNA via m6A modification in ESCC.
To investigate the role of m6A modification in
regulating MTHFD2, adenine at the 1,080th position of the MTHFD2
mRNA was mutated to thymine to create a mutant construct (Fig. S4A). qPCR and WB analyses
revealed that after knocking down MTHFD2, overexpression of
wild-type MTHFD2 restored protein expression to baseline levels,
while overexpression of m6A-mutant MTHFD2 partially restored the
expression (Fig. S4B and C).
MeRIP-qPCR revealed significantly reduced m6A modification in
mutant compared with wild-type MTHFD2 (Fig. S4D). RIP-qPCR demonstrated that
this mutation substantially impaired the binding between MTHFD2 and
IGF2BP2 (Fig. S4E), which was
further validated by RNA pull-down experiments (Fig. S4F). mRNA stability assay showed
that the mutant MTHFD2 exhibited significantly decreased stability
compared with wild-type (Fig.
S4G). These findings indicated that m6A modification plays a
critical role in stabilizing MTHFD2 mRNA and promoting its
interaction with IGF2BP2.
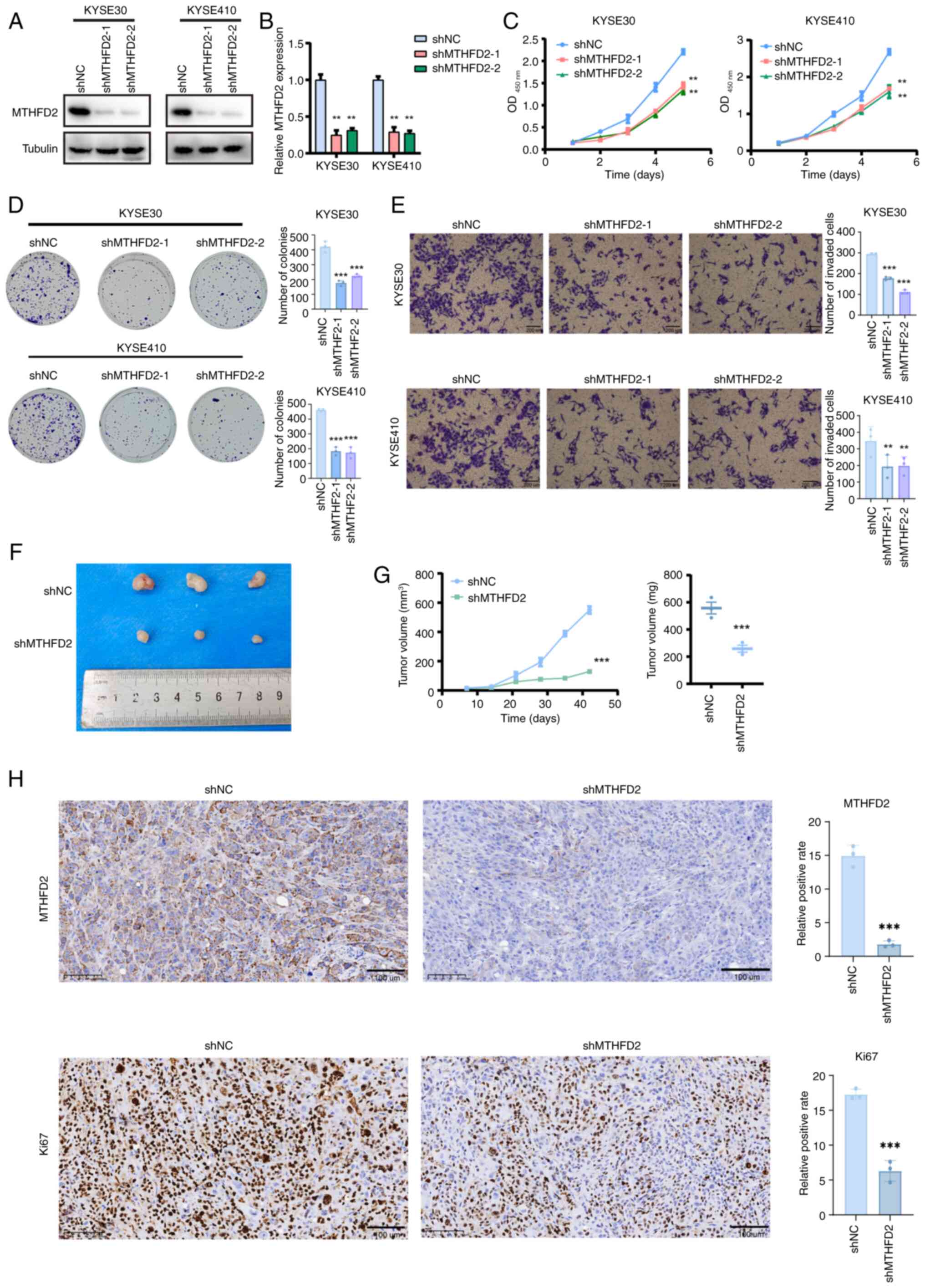
Knockdown of MTHFD2 inhibits ESCC
progression in vitro and tumor growth in vivo
To elucidate the functional role of MTHFD2 in ESCC,
stable knockdown of MTHFD2 was induced in two cell lines, KYSE30
and KYSE410. qPCR and WB experiments confirmed significant
downregulation of MTHFD2 expression (Fig. 4A and B). CCK-8 assay indicated
that the viability of the MTHFD2 knockdown group was notably
decreased compared with that of the control group (Fig. 4C). Plate colony formation assay
supported these findings, revealing a significant reduction in
proliferative capacity of ESCC cells following MTHFD2 knockdown
(Fig. 4E). To assess the in
vivo implications, a nude mouse subcutaneous tumor model was
constructed. Tumor growth and weight monitoring revealed smaller
tumors in the MTHFD2 knockdown group compared with controls
(Fig. 4F and G). IHC results
corroborated these observations, indicating significant
downregulation of MTHFD2 and Ki67 levels in subcutaneous tumor
tissues of the shMTHFD2 compared with shNC group (Fig. 4H). These results collectively
suggest an oncogenic role of MTHFD2 in ESCC progression.
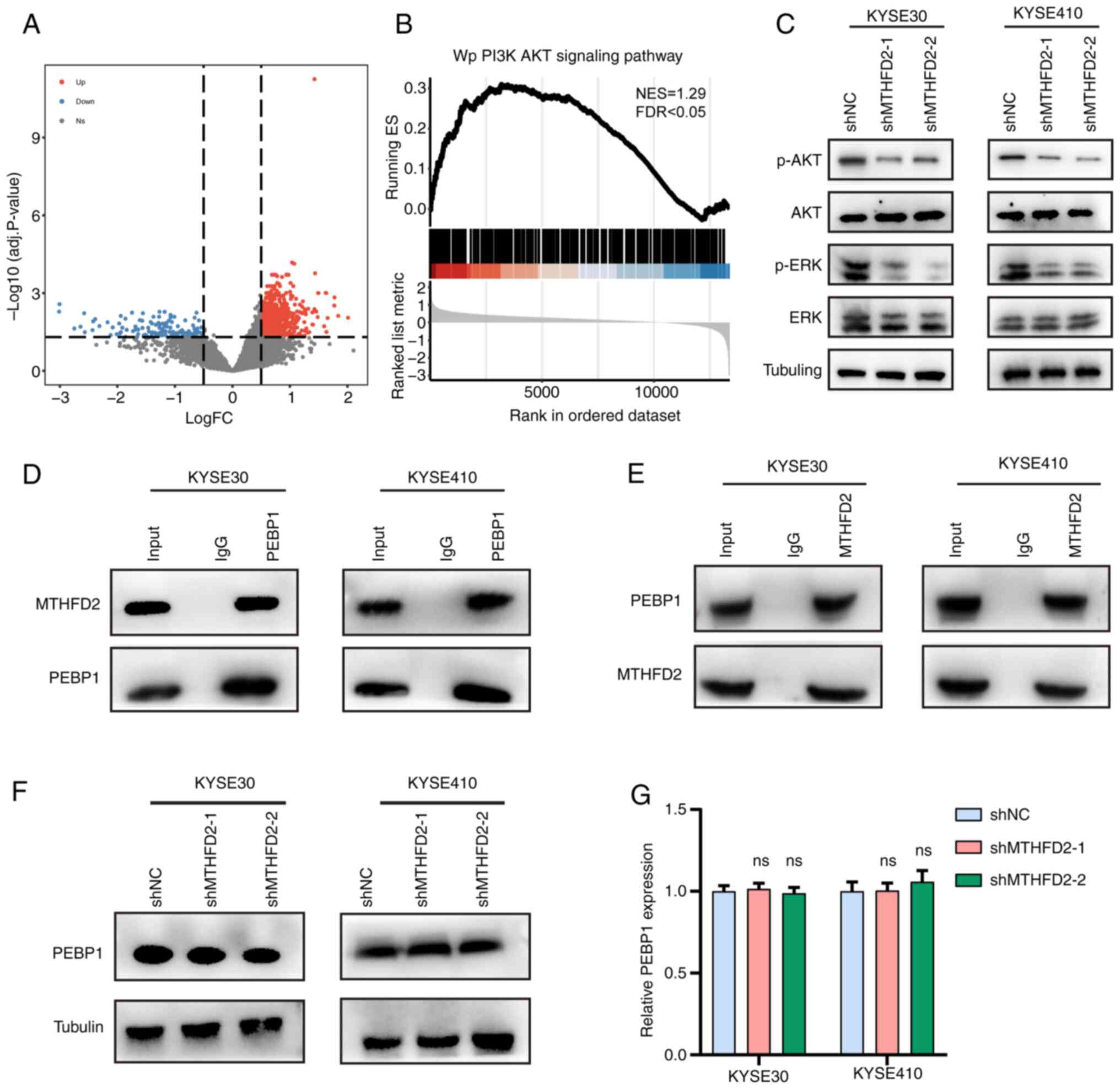
MTHFD2 promotes AKT and ERK signaling
pathway activation in ESCC
To analyze the potential biological behaviors of
MTHFD2 in ESCC, patients in TCGA-ESCC were divided into high and
low expression groups based on median MTHFD2 expression levels
(cutoff value=19.03). Differential analysis identified 124 downand
1,073 upregulated genes (Fig.
5A). Gene Set Enrichment Analysis revealed significant
enrichment of the PI3K/AKT signaling pathway in the high expression
group (Fig. 5B). WB experiments
showed that knockdown of MTHFD2 in KYSE30 and KYSE410 cells led to
a significant decrease in levels of phosphorylated AKT (p-AKT) and
p-ERK, while the total levels of AKT and ERK remained unchanged
(Fig. 5C).
BioGRID database (35) identified a potential interaction
between MTHFD2 PEBP1 (Fig. S5).
Co-immunoprecipitation (co-IP) experiments in ESCC cells confirmed
that PEBP1 and MTHFD2 bind each other (Fig. 5D and E). However, MTHFD2
knockdown did not alter the protein or mRNA levels of PEBP1, as
demonstrated by WB and qPCR (Fig. 5F
and G). These findings suggested that MTHFD2 promoted
activation of the PI3K/AKT signaling pathway in ESCC, potentially
via interaction with PEBP1, without affecting PEBP1 expression.
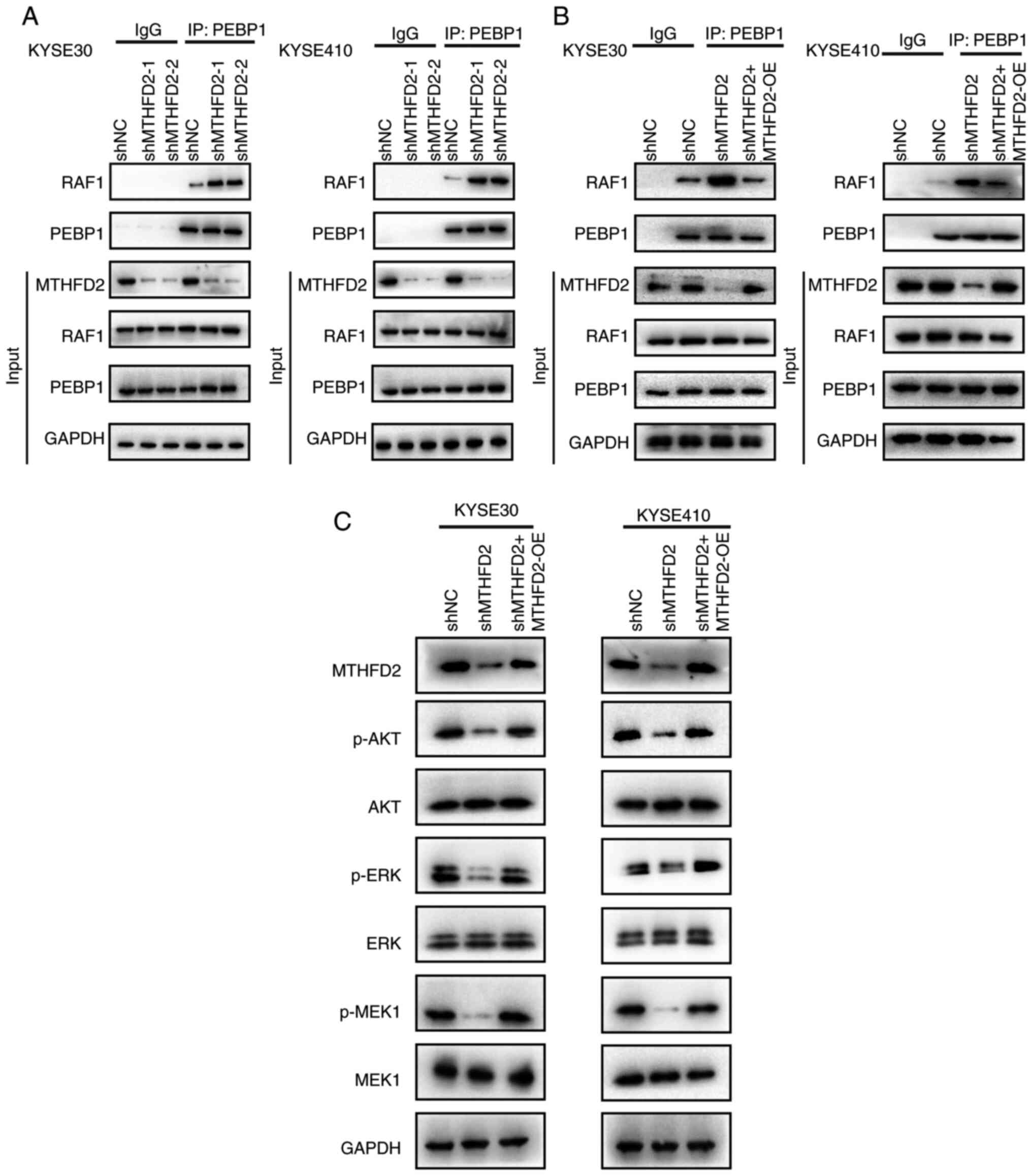
MTHFD2 modulates PEBP1-RAF1 interaction
and PI3K/AKT pathway activation
PEBP1 binds RAF1 and inhibits the RAF1/MAPK pathway
(36). It was hypothesized that
MTHFD2 may inhibit binding of PEBP1 to RAF1 by interacting with
PEBP1. Interaction between PEBP1 and RAF1 was assessed after
knocking down MTHFD2 in KYSE30 and KYSE410 cells. Co-IP showed that
the interaction between PEBP1 and RAF1 was enhanced following
MTHFD2 knockdown (Fig. 6A).
Knocking down and subsequently overexpressing MTHFD2 eliminated the
enhanced interaction between PEBP1 and RAF1 induced by MTHFD2
knockdown (Fig. 6B) and restored
the levels of p-AKT, p-ERK and p-MEK1 to wild-type levels, while
the total levels of AKT, ERK, and MEK1 remained largely unchanged
(Fig. 6C). These findings
suggested that MTHFD2 modulated the interaction between PEBP1 and
RAF1, thereby influencing activation of the PI3K/AKT signaling
pathway in ESCC.
MTHFD2 exerts oncogenic functions through
PEBP1 in ESCC
To confirm that MTHFD2 exerts oncogenic functions
through PEBP1, MTHFD2 was knocked down followed by PEBP1. WB
analysis showed that after knocking down PEBP1, the knockdown of
MTHFD2 did not decrease levels of p-AKT, p-ERK and p-MEK1 (Fig. 7A). Cell viability assays using
CCK-8 demonstrated that the decrease in cell viability caused by
MTHFD2 knockdown was attenuated when PEBP1 was also knocked down
(Fig. 7B). This was further
supported by colony formation and Transwell assays, which confirmed
that the decrease in cell viability induced by MTHFD2 knockdown was
mitigated by PEBP1 knockdown (Fig.
7C and D).
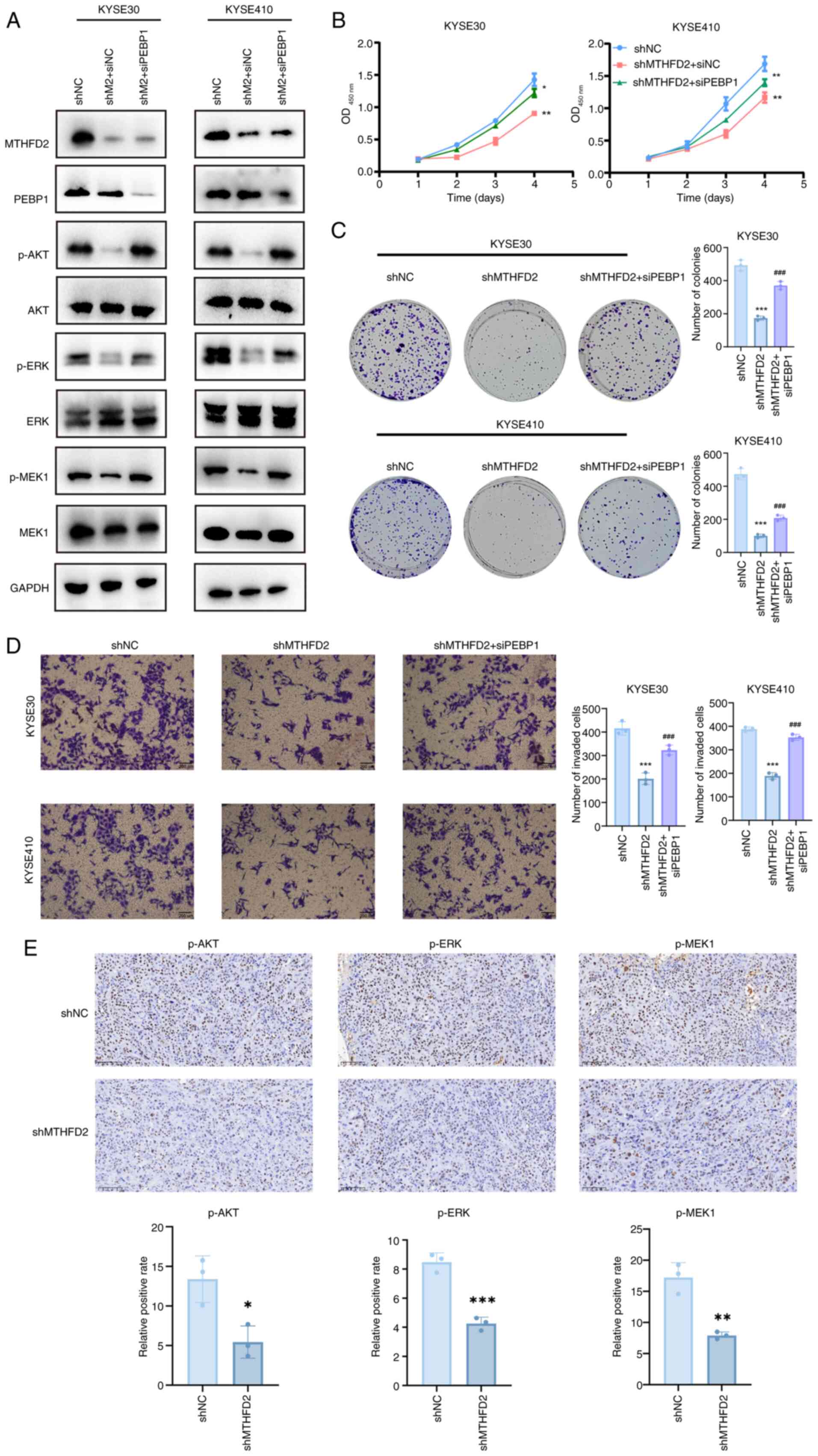 | Figure 7MTHFD2 exerts oncogenic functions via
PEBP1 in esophageal squamous cell carcinoma. (A) Western blot
analysis of AKT/ERK/MEK signaling pathway following MTHFD2 and
PEBP1 knockdown. (B) Cell Counting Kit-8, (C) colony formation and
(D) Transwell invasion assay following MTHFD2 and PEBP1 knockdown.
(E) Immunohistochemical staining of p-AKT, p-ERK and p-MEK1 in
mouse tumors. Scale bar, 100 µm. *P<0.05,
**P<0.01, ***P<0.001 vs. shNC.
###P<0.001 vs. shMTHFD2. MTHFD,
methylenetetrahydrofolate dehydrogenase; PEBP,
phosphatidylethanolamine-binding protein; p-, phosphorylated; sh,
short hairpin; NC, negative control; si, small interfering; OD,
optical density. |
As aforementioned, knockdown of MTHFD2 selectively
decreased levels of p-AKT, ERK and MEK-1 without affecting the
total protein levels of AKT, ERK and MEK-1. In vivo, IHC
revealed significantly decreased levels of p-AKT, p-ERK and p-MEK1
in the MTHFD2 knockdown compared with the control group, indicating
suppression of the AKT/ERK/MEK pathway in mice (Fig. 7E).
Discussion
The present study demonstrated significant
upregulation of MTHFD2 in ESCC tumor tissue, which was associated
with advanced pathological grade. MTHFD2 was associated with poor
recurrence-free survival in patients with ESCC; this was validated
in clinical samples. The proposed mechanism of action for
MTHFD2-induced carcinogenic effects is summarized in Fig. 8.
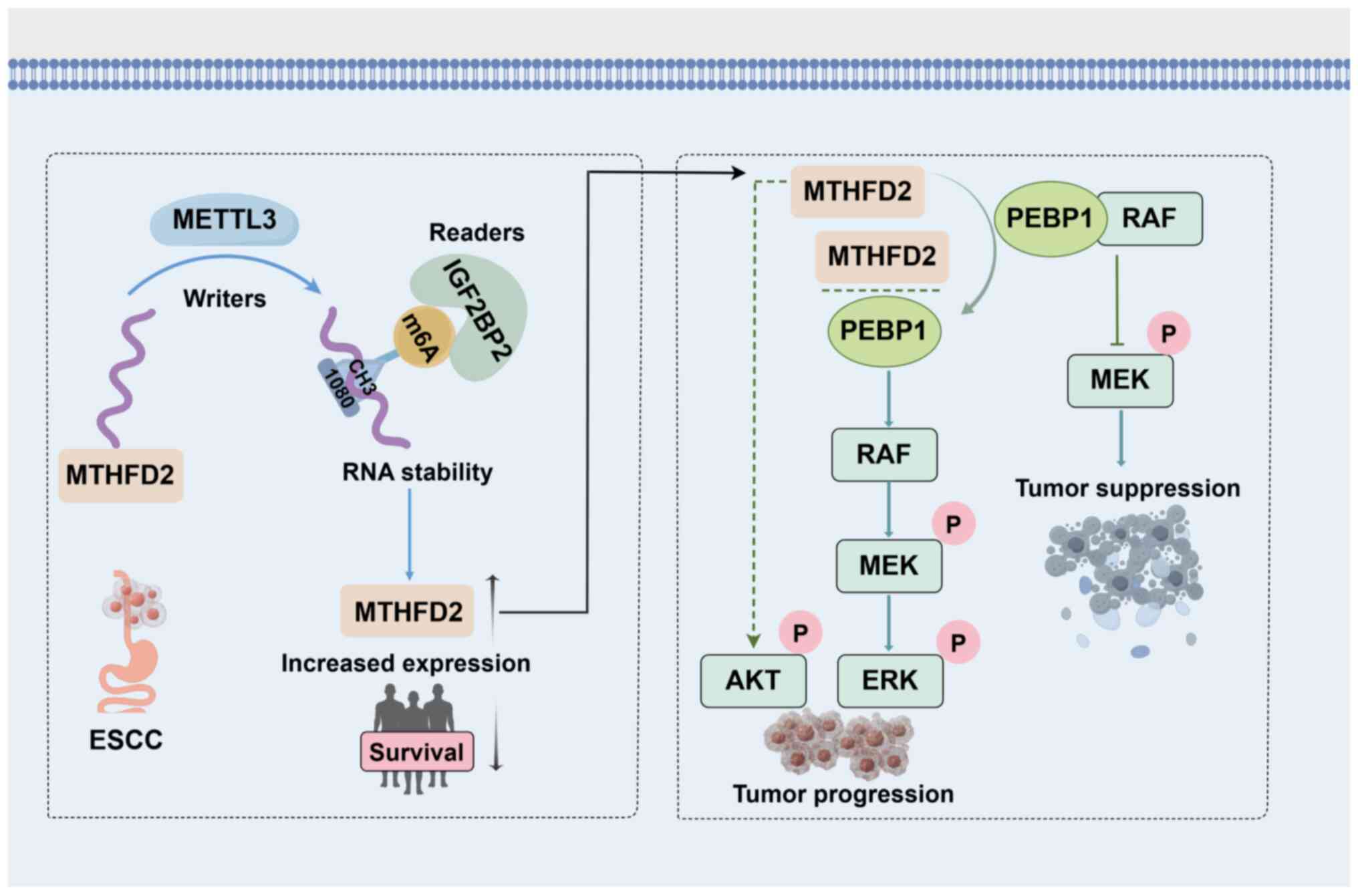 | Figure 8Mechanism by which m6A
modification-mediated MTHFD2 upregulation drives tumor-promoting
effects via the PEBP1-RAF1/MEK/ERK signaling cascade. METTL3 and
IGF2BP2, which collaboratively induce m6A modification at position
1,080 of MTHFD2 mRNA, enhancing its stability and expression.
Elevated MTHFD2 levels correlate with poor prognosis in ESCC
patients. Mechanistically, MTHFD2 interacts with PEBP1, disrupting
PEBP1's interaction with RAF1 and consequently activating the
RAF1/MEK/ERK pathway. ESCC, esophageal squamous cell carcinoma;
MTHFD2, methylenetetrahydrofolate dehydrogenase 2; METTL3,
methyltransferase-like 3; IGF2BP2, insulin-like growth factor 2
mRNA binding protein 2; PEBP1, phosphatidylethanolamine-binding
protein 1; RAF, Raf-1 proto-oncogene; MEK, mitogen-activated
protein kinase kinase 1; ERK, mitogen-activated protein kinase
1. |
He et al (37) identified high MTHFD2 expression
in ESCC. The present study performed qPCR and WB analyses on
freshly collected patient samples, providing more comprehensive
validation. Furthermore, functional experiments both in
vitro and in vivo suggested the potential of MTHFD2 as a
biomarker in ESCC. However, clinical reliance on a single biomarker
may not always provide optimal diagnostic accuracy. Combining
MTHFD2 with other markers (such as proliferation marker protein
Ki-67, carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9,
IL6 and B-cell lymphoma-3) (38,39) may improve the diagnostic
precision for ESCC in the future.
Based on the role of m6A modification in mRNA
stability and ESCC progression (18,19), it was hypothesized that m6A
modification promotes high MTHFD2 expression. MeRIP-qPCR and RNA
pull-down demonstrated interaction between METTL3, IGF2BP2 and
MTHFD2 mRNA stabilization. Notably, the present findings align with
a prior study linking IGF2BP2 with ESCC progression (40), suggesting that its functional
role may rely on the stabilization of MTHFD2. This contributes to
understanding of the regulatory role of m6A in ESCC.
Although previous studies have established the
connection between MTHFD2 and AKT and ERK signaling pathways
(41-44), the mechanism by which MTHFD2
regulates these pathways remains unclear. After knocking down
MTHFD2 in KYSE30 and KYSE410 cells, Co-IP showed that the
interaction between PEBP1 and RAF1 was enhanced. In addition,
knocking down and subsequently overexpressing MTHFD2 abolished the
enhanced interaction between PEBP1 and RAF1 induced by MTHFD2
knockdown. The levels of downstream key effectors p-AKT, p-ERK and
p-MEK1 were also restored to wild-type levels. The present results
suggested that MTHFD2 may inhibit PEBP1 binding to RAF1 in a
competitive binding manner. However, the specific region of
interaction between MTHFD2 and PEBP1 warrants further investigation
to elucidate how MTHFD2 regulates PEBP1-RAF1 interaction.
Given the role of MTHFD2 in tumorigenesis (41,45-47), it may serve as a promising
therapeutic target in ESCC. Targeting MTHFD2 may allow modulation
of the PEBP1-RAF1 signaling axis, potentially overcoming resistance
to conventional therapy. Preclinical studies have demonstrated the
efficacy of small molecule MTHFD2 inhibitors in disrupting cancer
cell proliferation across various tumor types, including
glioblastoma and non-small cell lung cancer, and breast cancer
(48-50), suggesting a similar therapeutic
potential in ESCC. Further preclinical studies, including in
vivo models of ESCC, and subsequent clinical trials are
necessary to evaluate the safety, efficacy and optimal therapeutic
regimen for MTHFD2-targeted intervention.
Cancer immunotherapy, particularly immune checkpoint
inhibitors, has reshaped the treatment landscape for cancer
(51). However, a limited number
of patients responds to immunotherapy, highlighting the need for
investigation into the tumor microenvironment and development of
alternative immunotherapeutic strategies. Studies have revealed
that MTHFD2 upregulates PD-L1 expression, thereby facilitating
immune evasion (52,53). Furthermore, MTHFD2 is implicated
in regulation of macrophage polarization and T cell function, both
of which are key for the immune response (54,55). As immune checkpoint molecules
such as programmed cell death protein 1 and PD-L1 are increasingly
used in immunotherapy (56,57), MTHFD2 could serve not only as a
prognostic biomarker but also as a potential therapeutic
target.
The present study has limitations. MTHFD2, as a
single biomarker, may not capture the full complexity of ESCC
progression. Integrating MTHFD2 with other biomarkers may enhance
prognostic accuracy. Larger patient cohorts are key for external
validation and future research should investigate additional
regulatory mechanisms affecting MTHFD2 expression in ESCC.
In conclusion, the present study contributes to
understanding ESCC pathogenesis by highlighting the role of MTHFD2
and its regulation in disrupting the suppression of RAF1 by PEBP1.
The present results may facilitate further investigation into the
intricate biology of ESCC and the potential therapeutic
implications of targeting MTHFD2.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ conceived the study, designed the experiments and
revised the manuscript. HZ and HG performed the experiments,
analyzed the data and wrote the manuscript. XZ and CZ performed the
experiments. DL and JL analyzed the data. All authors have read and
approved the final manuscript. HZ and YZ confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was performed according to the
ethical guidelines of the Helsinki Declaration and approved by the
institutional review board of Xiangya Hospital of Central South
University (approval no. 2024111342). All patients provided
informed written consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hunan Provincial Health
High Level Talent Support Program (grant no. 20240304103), Key
Research and Development Program of Hunan Province (grant no.
2024JK2108), Wu Jieping Medical Foundation Research Special Fund
(grant no. 320.6750.2023-19-30), Natural Science Foundation of
Hunan Province, China (grant nos. 2022JJ40251 and 2022JJ30985),
'Love Lung' Tumor Treatment Clinical Scientific Research Innovation
Public Welfare Project (grant no. AF008), Health Research Project
of Hunan Provincial Health Commission (grant no. W20243220).
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DaSilva LL, Aguiar PN Jr and de Lima Lopes
G: Immunotherapy for advanced esophageal squamous cell
carcinoma-renewed enthusiasm and a lingering challenge. JAMA Oncol.
7:1613–1614. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Yang B, Ma Y, Peng X, Wang Z, Sheng
B, Wei Z, Cui Y and Liu Z: Phosphoproteomics reveals therapeutic
targets of esophageal squamous cell carcinoma. Signal Transduct
Target Ther. 6:3812021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang K, Yin X, Zhang Q, Yin J, Tang Q, Xu
M, Wu L, Shen Y, Zhou Z, Yu H and Yan S: STC2 activates PRMT5 to
induce radioresistance through DNA damage repair and ferroptosis
pathways in esophageal squamous cell carcinoma. Redox Biol.
60:1026262023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pavlova NN, Zhu J and Thompson CB: The
hallmarks of cancer metabolism: Still emerging. Cell Metab.
34:355–577. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Locasale JW: Serine, glycine and
one-carbon units: Cancer metabolism in full circle. Nat Rev Cancer.
13:572–583. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan S, Fan M, Liu Z, Li X and Wang H:
Serine, glycine and one-carbon metabolism in cancer (Review). Int J
Oncol. 58:158–170. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ducker GS and Rabinowitz JD: One-carbon
metabolism in health and disease. Cell Metab. 25:27–42. 2017.
View Article : Google Scholar :
|
|
9
|
Nilsson R, Jain M, Madhusudhan N, Sheppard
NG, Strittmatter L, Kampf C, Huang J, Asplund A and Mootha VK:
Metabolic enzyme expression highlights a key role for MTHFD2 and
the mitochondrial folate pathway in cancer. Nat Commun. 5:31282014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Z and Leung GKK: More than a metabolic
enzyme: MTHFD2 as a novel target for anticancer therapy? Front
Oncol. 10:6582020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui X, Su H, Yang J, Wu X, Huo K, Jing X
and Zhang S: Up-regulation of MTHFD2 is associated with
clinicopathological characteristics and poor survival in ovarian
cancer, possibly by regulating MOB1A signaling. J Ovarian Res.
15:232022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi LF, Zhang Q, Shou XY and Niu HJ:
Expression and prognostic value identification of
methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) in brain
low-grade glioma. Int J Gen Med. 14:4517–4527. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geng QQ, Wu QF, Zhang Y, Zhang GJ, Fu JK
and Chen NZ: Clinical significance of circ-MTHFD2 in diagnosis,
pathological staging and prognosis of NSCLC. Eur Rev Med Pharmacol
Sci. 24:9473–9479. PubMed/NCBI
|
|
14
|
Ju HQ, Lu YX, Chen DL, Zuo ZX, Liu ZX, Wu
QN, Mo HY, Wang ZX, Wang DS, Pu HY, et al: Modulation of redox
homeostasis by inhibition of MTHFD2 in colorectal cancer:
Mechanisms and therapeutic implications. J Natl Cancer Inst.
111:584–596. 2019. View Article : Google Scholar :
|
|
15
|
An Y and Duan H: The role of m6A RNA
methylation in cancer metabolism. Mol Cancer. 21:142022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y,
Cheng C, Li L, Pi J, Si Y, et al: The m6A reader YTHDF1 promotes
ovarian cancer progression via augmenting EIF3C translation.
Nucleic Acids Res. 48:3816–3831. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng C, Huang W, Li Y and Weng H: Roles of
METTL3 in cancer: Mechanisms and therapeutic targeting. J Hematol
Oncol. 13:1172020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui Y, Zhang C, Ma S, Li Z, Wang W, Li Y,
Ma Y, Fang J, Wang Y, Cao W and Guan F: RNA m6A demethylase
FTO-mediated epigenetic up-regulation of LINC00022 promotes
tumorigenesis in esophageal squamous cell carcinoma. J Exp Clin
Cancer Res. 40:2942021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Shao F, Yang X, Wang J, Zhu R,
Yang Y, Zhao G, Guo D, Sun Y, Wang J, et al: METTL3 promotes tumour
development by decreasing APC expression mediated by APC mRNA
N(6)-methyladenosine-dependent YTHDF binding. Nat Commun.
12:38032021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Y, Li Y, Zhu R, Feng R, Cui H, Yu X,
Huang F, Zhang R, Chen X, Li L, et al: RPS15 interacted with
IGF2BP1 to promote esophageal squamous cell carcinoma development
via recognizing m6A modification. Signal Transduct
Target Ther. 8:2242023. View Article : Google Scholar
|
|
21
|
Li Y, Niu C, Wang N, Huang X, Cao S, Cui
S, Chen T, Huo X and Zhou R: The role of m6A
modification and m6A regulators in esophageal cancer.
Cancers (Basel). 14:51392022. View Article : Google Scholar
|
|
22
|
Qi ZH, Xu HX, Zhang SR, Xu JZ, Li S, Gao
HL, Jin W, Wang WQ, Wu CT, Ni QX, et al: RIPK4/PEBP1 axis promotes
pancreatic cancer cell migration and invasion by activating
RAF1/MEK/ERK signaling. Int J Oncol. 52:1105–1116. 2018.PubMed/NCBI
|
|
23
|
Yang X, Wang Y, Lu P, Shen Y, Zhao X, Zhu
Y, Jiang Z, Yang H, Pan H, Zhao L, et al: PEBP1 suppresses HIV
transcription and induces latency by inactivating MAPK/NF-kappaB
signaling. EMBO Rep. 21:e493052020. View Article : Google Scholar
|
|
24
|
Colaprico A, Silva TC, Olsen C, Garofano
L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM,
Castiglioni I, et al: TCGAbiolinks: An R/bioconductor package for
integrative analysis of TCGA data. Nucleic Acids Res. 44:e712016.
View Article : Google Scholar :
|
|
25
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagy A, Munkacsy G and Gyorffy B:
Pancancer survival analysis of cancer hallmark genes. Sci Rep.
11:60472021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou H, Zeng C, Liu J, Luo H and Huang W:
F-box protein 43, stabilized by N6-methyladenosine methylation,
enhances hepatocellular carcinoma cell growth and invasion via
promoting p53 degradation in a ubiquitin conjugating enzyme E2
c-dependent manner. Cancers (Basel). 15:9572023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Yang Q, Zhu W and Gong H: Subtype
classification based on T cell proliferation-related regulator
genes and risk model for predicting outcomes of lung
adenocarcinoma. Front Immunol. 14:11484832023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang P, Li H, Gong H, Tian Y, Chen F, Li
X, Xie C, Tu C, Qian S, Tan Y, et al: c-Myc-XRCC2-FOS axis promotes
the proliferation and the resistance to doxorubicin of NSCLC.
Biomed Pharmacother. 179:1173152024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song L, Gong H, Lin C, Wang C, Liu L, Wu
J, Li M and Li J: Flotillin-1 promotes tumor necrosis factor-alpha
receptor signaling and activation of NF-kappaB in esophageal
squamous cell carcinoma cells. Gastroenterology. 143:995–1005.e12.
2012. View Article : Google Scholar
|
|
32
|
Carbone L, Carbone ET, Yi EM, Bauer DB,
Lindstrom KA, Parker JM, Austin JA, Seo Y, Gandhi AD and Wilkerson
JD: Assessing cervical dislocation as a humane euthanasia method in
mice. J Am Assoc Lab Anim Sci. 51:352–356. 2012.PubMed/NCBI
|
|
33
|
Sendinc E and Shi Y: RNA m6A methylation
across the transcriptome. Mol Cell. 83:428–441. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Zeng P, Li YH, Zhang Z and Cui Q:
SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based
on sequence-derived features. Nucleic Acids Res. 44:e912016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oughtred R, Stark C, Breitkreutz BJ, Rust
J, Boucher L, Chang C, Kolas N, O'Donnell L, Leung G, McAdam R, et
al: The BioGRID interaction database: 2019 update. Nucleic Acids
Res. 47:D529–D41. 2019. View Article : Google Scholar :
|
|
36
|
Abd Alla J and Quitterer U: The RAF kinase
inhibitor protein (RKIP): Good as tumour suppressor, bad for the
heart. Cells. 11:6542022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He H, Li PC, Jia W, Hu B and Ji CS: High
expression of methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) in
esophageal squamous cell carcinoma and its clinical prognostic
significance. Med Sci Monit. 26:e9202592020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soares-Lima SC, Gonzaga IM, Camuzi D,
Nicolau-Neto P, Vieira da Silva R, Guaraldi S, Ferreira MA,
Hernandez-Vargas H, Herceg Z and Ribeiro Pinto LF: IL6 and BCL3
expression are potential biomarkers in esophageal squamous cell
carcinoma. Front Oncol. 11:7224172021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Huang X, Zhou L, Deng T, Ning T,
Liu R, Zhang L, Bai M, Zhang H, Li H and Ba Y: Clinical use of
tumor biomarkers in prediction for prognosis and chemotherapeutic
effect in esophageal squamous cell carcinoma. BMC Cancer.
19:5262019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu F, Chen W, Jiang T, Cheng C, Wang B, Lu
Z, Huang G, Qiu J, Wei W, Yang M and Huang X: Expression profile,
clinical significance and biological functions of IGF2BP2 in
esophageal squamous cell carcinoma. Exp Ther Med. 23:2522022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi Y, Xu Y, Yao J, Yan C, Su H, Zhang X,
Chen E and Ying K: MTHFD2 promotes tumorigenesis and metastasis in
lung adenocarcinoma by regulating AKT/GSK-3beta/beta-catenin
signalling. J Cell Mol Med. 25:7013–7027. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deng X, Liu X, Hu B, Liu J, Fu B and Zhang
W: Upregulation of MTHFD2 is associated with PD-L1 activation in
bladder cancer via the PI3K/AKT pathway. Int J Mol Med. 51:142023.
View Article : Google Scholar :
|
|
43
|
Wu S, Cai W, Shi Z, Ming X, Yang X, Zhou
Y, Chen X and Yang M: Knockdown of MTHFD2 inhibits proliferation
and migration of nasopharyngeal carcinoma cells through the ERK
signaling pathway. Biochem Biophys Res Commun. 614:47–55. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mo X, Liu Q, Liang K and Song Y:
Interference with MTHFD2 induces ferroptosis in ovarian cancer
cells through ERK signaling to suppress tumor malignant
progression. J Bioenerg Biomembr. 56:333–345. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H, Zhu S, Zhou H, Li R, Xia X and
Xiong H: Identification of MTHFD2 as a prognostic biomarker and
ferroptosis regulator in triple-negative breast cancer. Front
Oncol. 13:10983572023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mo HY, Wang RB, Ma MY, Zhang Y, Li XY, Wen
WR, Han Y and Tian T: MTHFD2-mediated redox homeostasis promotes
gastric cancer progression under hypoxic conditions. Redox Rep.
29:23454552024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang J, Yu Z, Jiang Y, Le T, Wu Y, Li Z,
Zhang G, Wu F and Ma H: Downregulation of MTHFD2 inhibits
proliferation and enhances chemosensitivity in hepatocellular
carcinoma via PI3K/AKT pathway. Front Biosci (Landmark Ed).
29:352024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu Z, Kiang KM, Li N, Liu J, Zhang P, Jin
L, He X, Zhang S and Leung GK: Folate enzyme MTHFD2 links
one-carbon metabolism to unfolded protein response in glioblastoma.
Cancer Lett. 549:2159032022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou F, Yuan Z, Gong Y, Li L, Wang Y, Wang
X, Ma C, Yang L, Liu Z, Wang L, et al: Pharmacological targeting of
MTHFD2 suppresses NSCLC via the regulation of ILK signaling
pathway. Biomed Pharmacother. 161:1144122023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ramos L, Henriksson M, Helleday T and
Green AC: Targeting MTHFD2 to exploit cancer-specific metabolism
and the DNA damage response. Cancer Res. 84:9–16. 2024. View Article : Google Scholar
|
|
51
|
Vesely MD, Zhang T and Chen L: Resistance
mechanisms to anti-PD cancer immunotherapy. Annu Rev Immunol.
40:45–74. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shang M, Yang H, Yang R, Chen T, Fu Y, Li
Y, Fang X, Zhang K, Zhang J, Li H, et al: The folate cycle enzyme
MTHFD2 induces cancer immune evasion through PD-L1 up-regulation.
Nat Commun. 12:19402021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li L, Zhang Y, Hu W, Zou F, Ning J, Rao T,
Ruan Y, Yu W and Cheng F: MTHFD2 promotes PD-L1 expression via
activation of the JAK/STAT signalling pathway in bladder cancer. J
Cell Mol Med. 27:2922–2936. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shang M, Ni L, Shan X, Cui Y, Hu P, Ji Z,
Shen L, Zhang Y, Zhou J, Chen B, et al: MTHFD2 reprograms
macrophage polarization by inhibiting PTEN. Cell Rep.
42:1124812023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sugiura A, Andrejeva G, Voss K, Heintzman
DR, Xu X, Madden MZ, Ye X, Beier KL, Chowdhury NU, Wolf MM, et al:
MTHFD2 is a metabolic checkpoint controlling effector and
regulatory T cell fate and function. Immunity. 55:65–81.e9. 2022.
View Article : Google Scholar :
|
|
56
|
Hashimoto K, Nishimura S, Ito T, Kakinoki
R and Akagi M: Immunohistochemical expression and
clinicopathological assessment of PD-1, PD-L1, NY-ESO-1, and
MAGE-A4 expression in highly aggressive soft tissue sarcomas. Eur J
Histochem. 66:33932022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chu X, Tian W, Wang Z, Zhang J and Zhou R:
Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy:
Mechanisms and clinical trials. Mol Cancer. 22:1012023. View Article : Google Scholar
|