Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most common malignant cancers of the oral cavity. OSCC, being one
of the most disfiguring type of cancer, often causes a lack of
quality of life in patients, including a loss of general cognitive,
social, emotional or physical functions and prompting isolation
(1). It is suggested that the
primary treatment of OSCC is surgical removal, but radiation and/or
chemotherapy are alternative treatment regimens against OSCC
(2,3). Characteristically, OSCC cells, at the
late stage of malignancies, are very resistant to cancer
therapy-mediated apoptosis. Thus, research towards not only
understanding and overcoming resistance mechanism but also
identification of new drugs or substances with better preventive
and/or therapeutic efficacy against OSCC is needed.
Many therapeutic and chemopreventive agents
eliminate cancer cells by inducing apoptosis, a form of cell death.
Apoptosis is well characterized with distinct morphological
changes, including plasma membrane blebbing, depolarization of
mitochondria and DNA fragmentation (4). Induction of apoptosis is mediated by
a variety of cellular proteins and/or factors. Caspases are the
essential proteases for the execution of cell death by apoptotic
stimuli (5). The members of B cell
lymphoma (Bcl-2) family or inhibitor of apoptosis (IAP) family are
anti-apoptotic proteins, and their expressions are critical for
cell survival and/or death (6–8).
Oxidative stress is another key factor linked to induction of
apoptosis (9,10). A role of endoplasmic reticulum (ER)
stress in induction of apoptosis also has been reported (11,12).
There is also substantial evidence to indicate involvement of
activities of some intracellular signaling proteins, such as the
family of mitogen-activated protein kinase (MAPK), in induction of
apoptosis (13,14).
Essential oil is a volatile, natural and complex
compound present in a variety of aromatic plants and mostly
extracted by steam or hydro-distillation from the plants. In
nature, plant-derived essential oil, due to its anti-bacterial,
anti-viral, anti-fungal and/or insecticidal activities, play a role
in the protection of the plants (15). It is now well accepted that some
plants-derived essential oils are commercially important for food,
sanitary, cosmetic and perfume industries (15–18),
while others have potential to be developed as medicinal purposes,
such as anticancer drugs. For example, it is shown that essential
oil from Myrica gale L., a native plant from Canada used in
traditional medicine, has strong cytotoxic effects on human lung
(A549) and colon (DLD-1) cancer cell lines (19) and that essential oil from Ocimum
basilicum L. and Psidium guajava L., Thai medicinal
plants, inhibits proliferation of murine leukemia (P388) and human
mouth epidermal carcinoma (KB) cell lines, respectively (20). Moreover, a recent study
demonstrates that essential oil from pine needle inhibits growth
and induces apoptosis in human liver carcinoma cells by
down-regulation of Bcl-2 expression and telomerase activity
(21).
The leaf of Pinus (P.) densiflora, a pine
tree widely distributed in Asian mountains, has been used as a
traditional medicine (22). Little
is known about the relationship between pine leaf-derived essential
oil and oral malignancies. In this study, we evaluated the
anti-proliferative, anti-survival and/or pro-apoptotic effects of
essential oil extracted by steam distillation from the leaf of
P. densiflora on human OSCC cells and determined the
possible molecular and cellular mechanisms.
Materials and methods
Materials
Materials were purchased as follows: RPMI-1640
medium, fetal bovine serum (FBS) and penicillin-streptomycin were
from WelGene (Daegu, Korea). Antibody of procaspase-9 was from Enzo
Life Science (Farmingdale, NY, USA). Antibody of XIAP was from
R&D Systems (Minneapolis, MN, USA). Antibodies of Bcl-2, Bax
and glucose-regulated protein 78 (GRP78) were from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Antibodies against
phosphorylated forms of ERK-1/2 (p-ERK-1/2), total forms (both
phosphorylated and non-phosphorylated) of ERK-1/2 (T-ERK-1/2),
p-JNK-1/2 and T-JNK-1/2 were from Cell Signaling Technology
(Danvers, MA, USA). Antibodies against poly (ADP-ribose) polymerase
(PARP) were from Roche Diagnostics (Mannheim, Germany). Antibodies
against goat anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP were
from Santa Cruz Biotechnology. Enzyme-linked chemiluminescence
(ECL) Western detection reagents were from Thermo Scientific
(Waltham, MA, USA).
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) reagent was from Promega (Madison, WI, USA).
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) and
proteinase inhibitor cocktail (x100) were from Calbiochem (Madison,
WI, USA). Bradford reagent was from Bio-Rad (Hercules, CA, USA).
Plasticware: 6-, 24- and 96-well plates and 60-mm cell culture dish
was from SPL Life Sciences (Gyeonggi-do, Korea). Other reagents,
including actin antibody, was from Sigma (St. Louis, MO, USA).
Cell culture
Three human OSCC cell lines YD-8, YD-10B and YD-38
were purchased from Korean Cell Line Bank (Seoul, Korea). They all
were grown at 37°C in a humidified condition of 95% air and 5%
CO2 in RPMI-1640 supplemented with 10% heat-inactivated
FBS, 100 units/ml penicillin and 100 μg/ml streptomycin.
Cell proliferation assay
YD-8 cells (0.4×104/100 μl/well) were
seeded into 96-well plates overnight. Cells were then treated
without or with different concentrations of PLEO for 8 h and
incubated with MTS (20 μl/well) for 1.5 h at 37°C. The absorbance
was measured at 595 nm using a microplate reader.
Cell count analysis
Briefly, YD-8, YD-10B or YD-38 cells were seeded in
24-well plates (1×105/500 μl/well) overnight. Respective
cells were then treated without or with PLEO for 8 h. The number of
surviving cells, which cannot be stained with trypan blue dye, was
counted under microscope. Approximately, <100 cells were counted
for the analysis.
Measurement of DNA fragmentation
YD-8 cells were seeded in 6-well plates at a density
of 0.5×106 cells per well in 2 ml volume the day before
PLEO treatment. Cells were incubated without or with different
concentrations of PLEO for 8 h. Control or PLEO-treated cells were
then harvested, washed and lysed in a buffer [50 mM Tris (pH 8.0),
0.5% sarkosyl, 0.5 mg/ml proteinase K and 1 mM EDTA] at 55°C for 3
h, followed addition of RNase A (0.5 μg/ml) and further incubation
at 55°C for 18 h. The lysates were centrifuged at 10,000 × g for 20
min. The genomic DNA in the supernatant was extracted with equal
volume of neutral phenol-chloroform-isoamyl alcohol mixture
(25:24:1) and analyzed by electrophoresis on 1.7% agarose gel. The
DNA was visualized and photographed under UV illumination after
staining with ethidium bromide (0.1 μg/ml).
Preparation of whole cell lysates
To see the effect of PLEO on total expression levels
of cellular proteins, including procaspase-9, PARP, Bcl-2, Bax,
XIAP, GRP78 and actin, YD-8 cells (0.5×106/2 ml/well)
were seeded in 6-well plates the day before PLEO treatment. Cells
were treated without or with different concentrations of PLEO for
2, 4 or 8 h. Control or PLEO-treated cells were then washed twice
with PBS and exposed to cell lysis buffer [50 mM Tris-Cl (pH 7.4),
150 mM NaCl, 0.1% sodium dodecyl sulfate, 0.25% sodium
deoxycholate, 1% Triton X-100, 1% Nonidet P-40, 1 mM EDTA, 1 mM
EGTA, proteinase inhibitor cocktail (x1)]. The cell lysates were
collected in a 1.5-ml tube and centrifuged for 20 min at 4°C at
12,000 rpm. The supernatant was saved and protein concentrations
were determined with Bradford reagent.
Western blot analysis
Proteins (50 μg) were separated by SDS-PAGE (10%)
and transferred onto nitrocellulose membranes (Millipore). The
membranes were washed with TBS (10 mM Tris, 150 mM NaCl)
supplemented with 0.05% (vol/vol) Tween-20 (TBST) followed by
blocking with TBST containing 5% (wt/vol) non-fat dried milk. The
membranes were incubated overnight with antibodies specific for
procaspase-9 (1:2,000), PARP (1:2,000), Bcl-2 (1:1,000), Bax
(1:2,000), XIAP (1:1,000), GRP78 (1:1,000), p-ERK-1/2 (1:1,000),
T-ERK-1/2 (1:2,000), p-JNK-1/2 (1:1,000), T-JNK-1/2 (1:1,000) or
actin (1:5,000) at 4°C. The membranes were then exposed to
secondary antibodies coupled to horseradish peroxidase for 2 h at
room temperature. The membranes were washed three times with TBST
at room temperature. Immunoreactivities were detected by ECL
reagents. Equal protein loading was assessed by the expression
level of actin protein.
Measurement of intracellular ROS
The generation of ROS was measured by a flow
cytometry analysis using 2′,7′-dichlorfluorescein-diacetate
(DCFH-DA) as a substrate. Briefly, YD-8 cells were grown in 60-mm
cell culture dish at a density of 0.8×106 cells in 2 ml
volume overnight. YD-8 cells were loaded with DCFH-DA to a final
concentration of 20 μM for 20 min and then treated without or with
PLEO (60 μg/ml) for 10 min to 8 h. YD-8 cells were harvested,
washed twice with PBS and suspended in PBS. The ROS generation was
measured by the DCF fluorescence intensity (FL-1, 530 nm) from
10,000 cells with a FACS Caliber flow cytometer
(Becton-Dickinson).
Statistical analysis
MTS or cell count analysis was done in triplicates
and repeated three times. Data are expressed as mean ± standard
error (SE). The significance of difference was determined by
One-Way ANOVA. All significance testing was based on upon a P-value
of <0.05.
Results
Treatment with P. densiflora leaf
essential oil (PLEO) inhibits proliferation and survival and
induces apoptosis in YD-8 cells
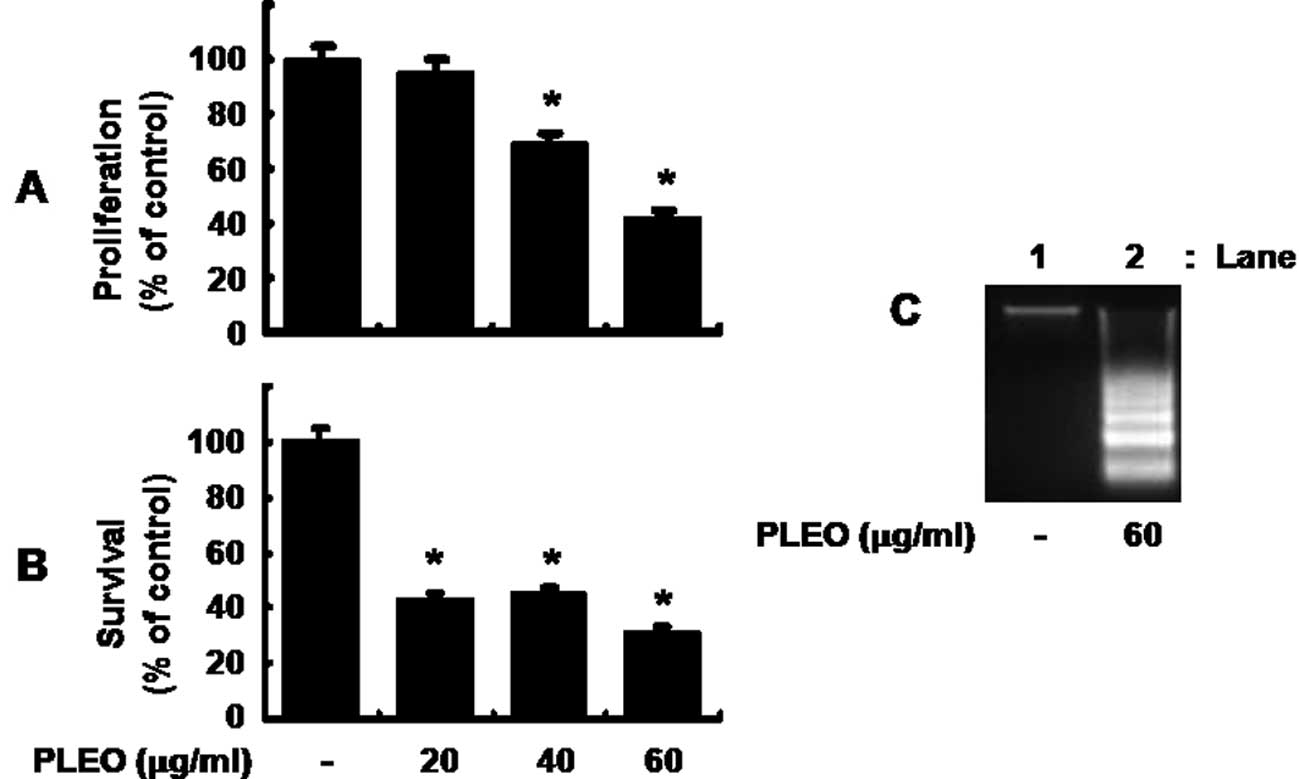
Initially, we measured the effect of PLEO in
different concentrations on proliferation of YD-8 cells by MTS
assay. As shown in Fig. 1A, while
treatment with PLEO at 20 μg/ml for 8 h did not affect YD-8 cell
proliferation, that with PLEO at 40 and 60 μg/ml inhibited the cell
proliferation by 30 and 60%, respectively. Cell count analysis was
next carried out to test the effect of PLEO on survival of YD-8
cells. As shown in Fig. 1B,
treatment with PLEO at 20 or 40 μg/ml decreased survival of YD-8
cells by about 60%. PLEO treatment at 60 μg/ml further increased
reduction of the cell survival by 70%. Due to strongest inhibitory
effects on both proliferation and survival of YD-8 cells, we
selected 60 μg/ml concentration of PLEO for further studies.
Whether PLEO treatment induces apoptosis in YD-8 cells was next
investigated by measuring nuclear DNA fragmentation, an apoptotic
marker. As shown in Fig. 1C,
compared with control (lane 1), there was strong induction of
nuclear DNA fragmentation in YD-8 cells treated with PLEO at 60
μg/ml for 8 h (lane 2).
Treatment with PLEO leads to activation
of caspase-9, PARP cleavage and down-regulation of Bcl-2 in YD-8
cells
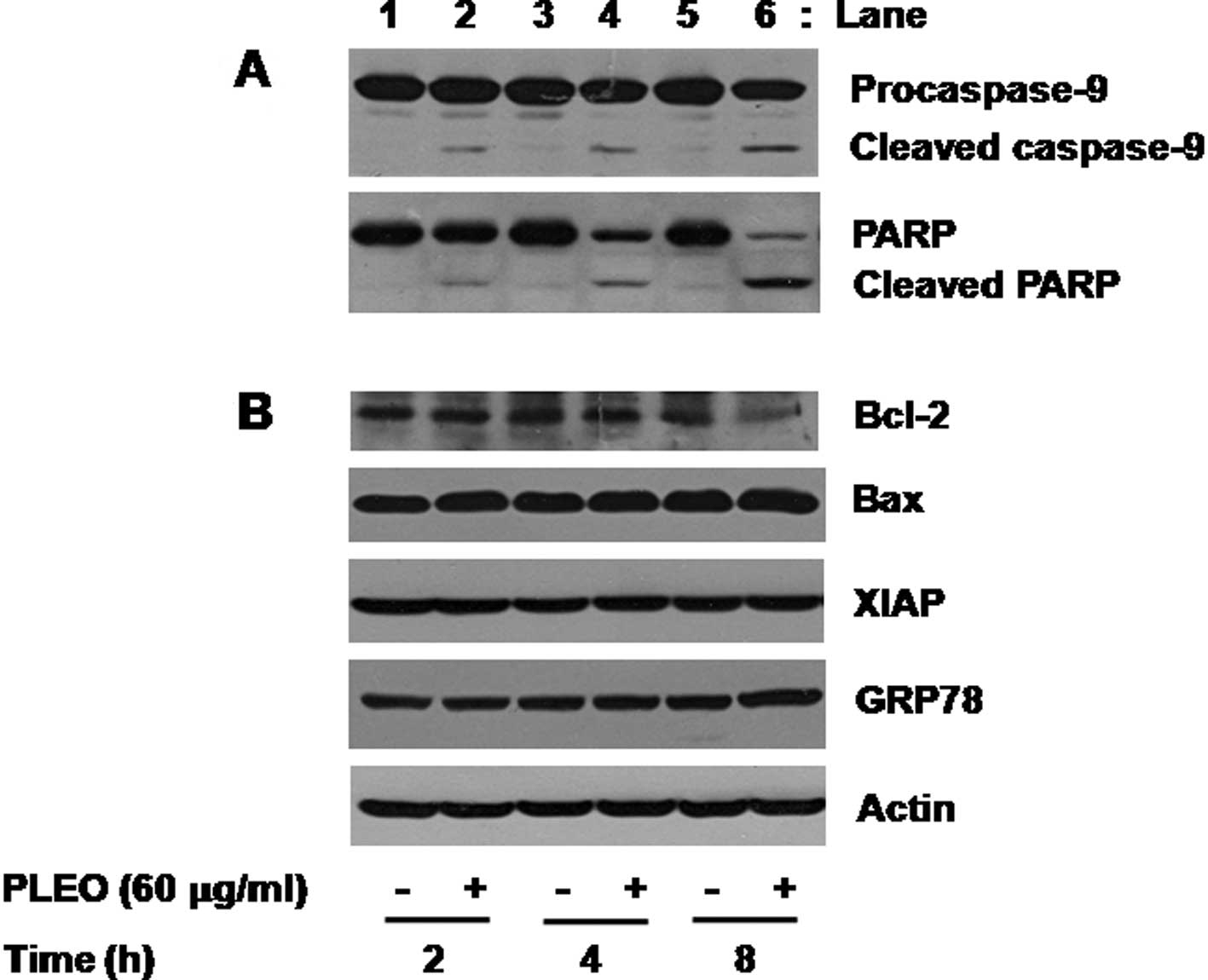
We next investigated the molecular and cellular
mechanisms and/or factors leading to the PLEO-induced apoptosis and
growth inhibition in YD-8 cells. We primarily determined the effect
of PLEO on activities of caspases, a family of proteases involved
in apoptosis, in YD-8 cells using Western blot analysis. Activation
of caspases, herein caspase-9, was assessed by measuring the
amounts of inactive proform of caspase-9 (procaspase-9) and of
cleaved form (active) of caspase-9 in YD-8 cells. As shown in
Fig. 2A (panel 1), compared with
control (lanes 1, 3 and 5), treatment of YD-8 cells with PLEO (60
μg/ml) at 2 h induced generation of cleaved caspase-9 (lane 2). The
PLEO-induced generation of cleaved caspase-9 was further detected
by the time of 4 or 8 h (lane 4 or 6). To confirm the PLEO-induced
activation of the caspase pathway, we next determined the effect of
PLEO on expression of PARP, a known substrate of caspases, in YD-8
cells. As shown in Fig. 2A (panel
2), treatment with PLEO resulted in a time-dependent generation of
cleaved PARP (lanes 2, 4 and 6). Next, the effect of PLEO on
expression of anti-apoptotic proteins, such as Bcl-2, Bax and XIAP,
in YD-8 cells was investigated. As shown in Fig. 2B (panel 1), compared with control
(lanes 1, 3 and 5), treatment with PLEO at 2 or 4 h had little
effect on expression of Bcl-2 (lane 2 or 4), but PLEO treatment at
8 h strongly repressed Bcl-2 expression (lane 6). As shown in
Fig. 2B (panels 2 and 3), however,
expressions of Bax and XIAP were not changed in YD-8 cells treated
without or with PLEO at the times tested (lanes 1–6). We also
examined whether PLEO induces ER stress in YD-8 cells by measuring
expression of GRP78, an ER stress-inducible protein. PLEO treatment
did not modulate expression of GRP78 in YD-8 cells (Fig. 2B, panel 4, lanes 1–6). Expression
of control actin protein was not affected in YD-8 cells treated
without or with PLEO at the times tested (Fig. 2B, panel 5, lanes 1–6).
Treatment with PLEO leads to activation
of ERK-1/2 and JNK-1/2 in YD-8 cells
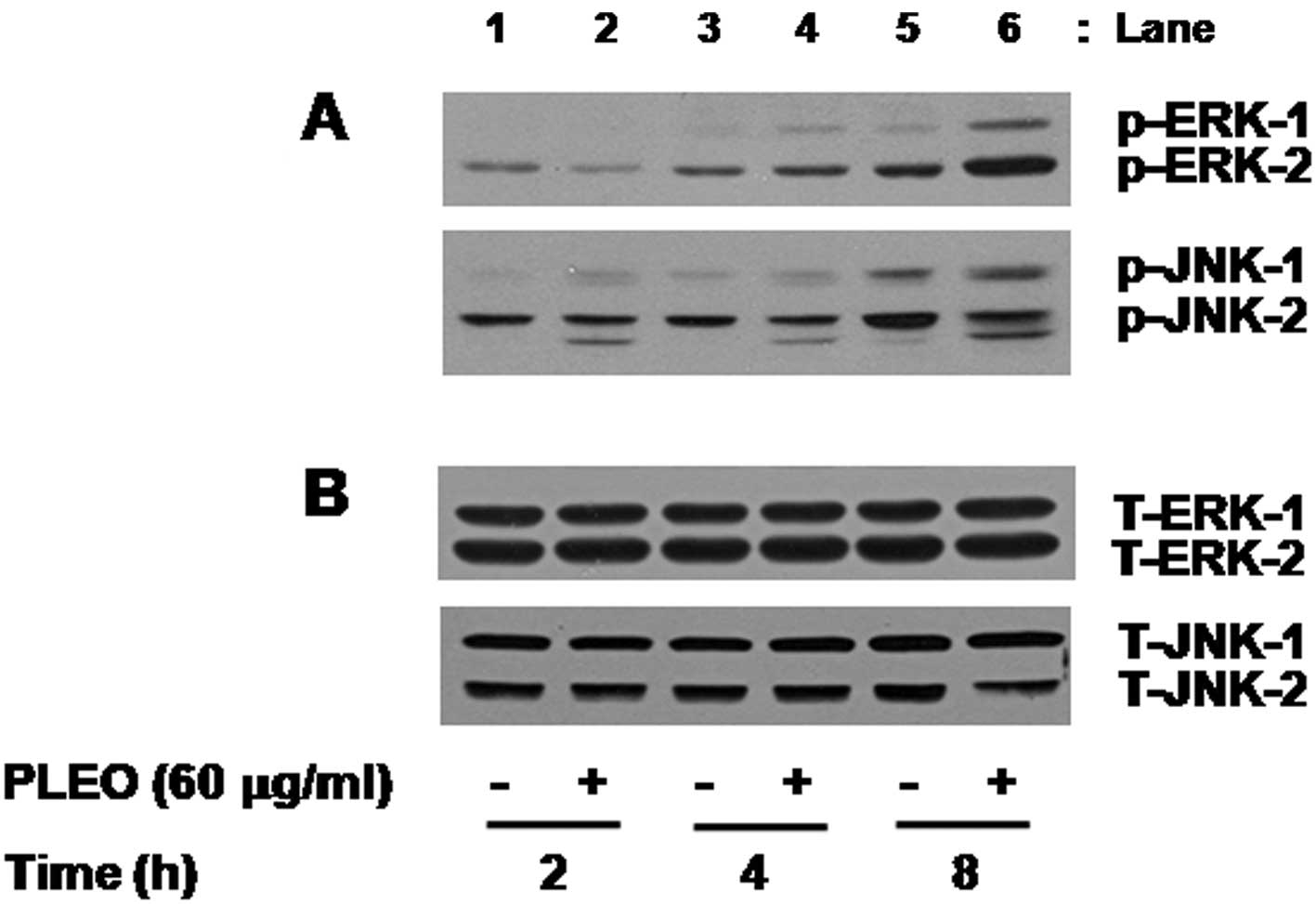
We also determined the effect of PLEO on activities
of the family of MAPK in YD-8 cells. In this study, activation of
the MAPK family, including ERK-1/2, JNK-1/2 and p38 MAPK, was
assessed by measuring phosphorylation level of each protein in YD-8
cells treated without or with PLEO (60 μg/ml). As shown in Fig. 3A (panel 1), compared with control
(lane 1 or 3), there was little effect on phosphorylation level of
ERK-1/2 by treatment with PLEO at 2 or 4 h (lane 2 or 4). However,
PLEO treatment at 8 h largely increased phosphorylation level of
ERK-1/2 in YD-8 cells (lane 6) compared with control (lane 5).
Notably, as shown in Fig. 3B
(panel 2), compared with control (lanes 1, 3 and 5), treatment with
PLEO at 2 h slightly enhanced phosphorylation level of JNK-1/2
(lane 2) and the enhanced JNK-1/2 phosphorylation sustained by the
time of 4 or 8 h (lane 4 or 6). However, there was no detection of
phosphorylated forms of p38 MAPK in control YD-8 cells and PLEO
treatment at the times tested did not modulate phosphorylation of
p38 MAPK in YD-8 cells (data not shown). As shown in Fig. 3B, Western blot analysis applying an
antibody which recognizes total expression levels of ERK-1/2 or
JNK-1/2 into the stripped immunoblot used in Fig. 3A demonstrated no change of total
expression levels of ERK-1/2 (lanes 1–6, panel 1) or JNK-1/2 (lanes
1–6, panel 2) in YD-8 cells treated without or with PLEO at the
times tested, suggesting the ability of PLEO to increase
phosphorylation levels of pre-existed ERK-1/2 and JNK-1/2 in YD-8
cells without de novo protein synthesis of these
proteins.
Treatment with PLEO leads to generation
of intracellular ROS in YD-8 cells
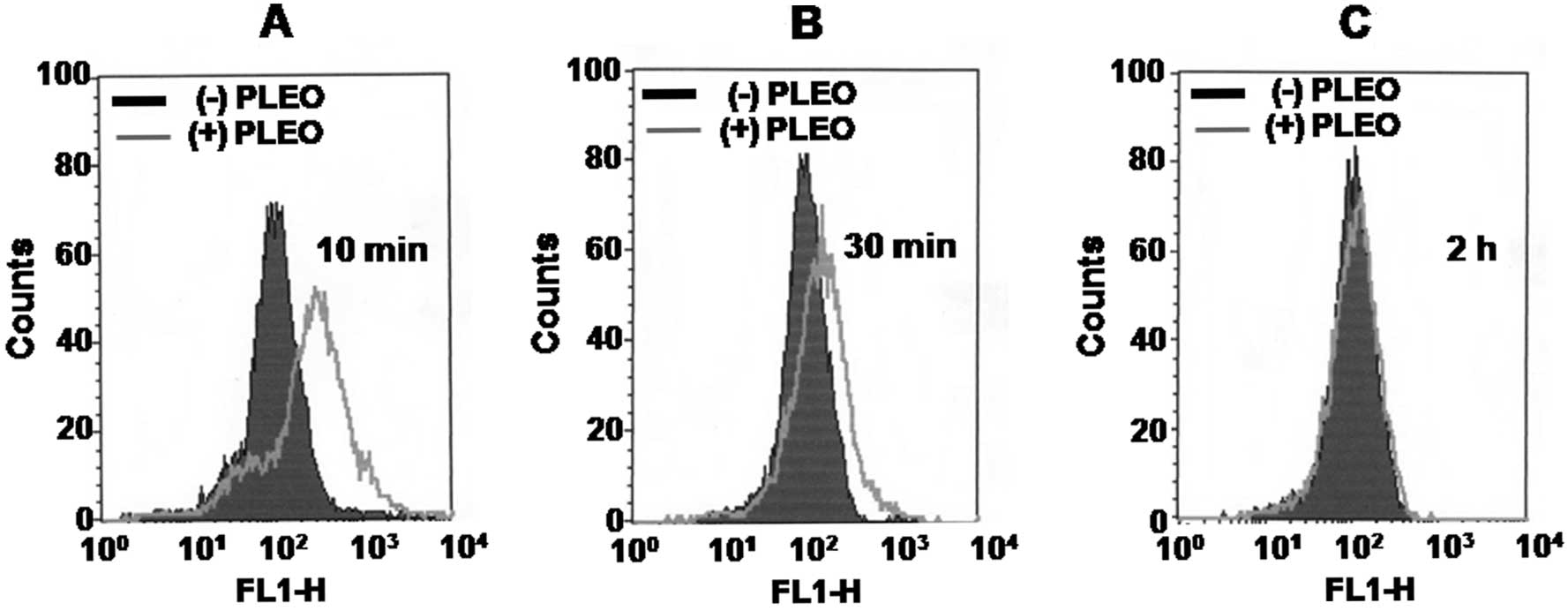
Whether PLEO treatment alters intracellular levels
of ROS in YD-8 cells was next determined by flow cytometry
analysis. As shown in Fig. 4A and
B, compared with control (dark line), treatment of YD-8 cells
with PLEO at 10 min led to generation of intracellular ROS (grey
line). The PLEO-induced ROS generation was further detected at the
time of 30 min. However, there was no detection of intracellular
ROS in YD-8 cells after treatment with PLEO at 2 h (Fig. 4C) and thereafter (data not
shown).
Generation of ROS and activities of
caspases are important for the PLEO-induced growth inhibition and
apoptosis in YD-8 cells
Whether ROS generation and/or activation of
caspases, ERK-1/2 and JNK-1/2 is necessary for the PLEO-induced
growth inhibition and/or apoptosis in YD-8 cells was next
investigated using different pharmacological inhibitors, including
z-VAD-fmk (z-VAD, a pan-caspase inhibitor), PD98059 (an inhibitor
of ERK-1/2), SP600125 (an inhibitor of JNK-1/2) or vitamin E (an
anti-oxidant). As shown in Fig.
5A, the PLEO-induced nuclear DNA fragmentation (apoptosis)
(lane 2) was strongly inhibited by treatment with z-VAD-fmk (lane
3) or vitamin E (lane 4), but not with PD98059 (lane 5) or SP600125
(lane 6); rather the JNK-1/2 inhibitor enhanced the stimulating
effect of PLEO on nuclear DNA fragmentation. Moreover, as shown in
Fig. 5B, the PLEO-induced
reduction of YD-8 cell survival (column 2) was effectively blocked
by the caspase inhibitor (column 3) or the anti-oxidant (column 4),
but not with the ERK-1/2 inhibitor (column 5) or the JNK-1/2
inhibitor (column 6).
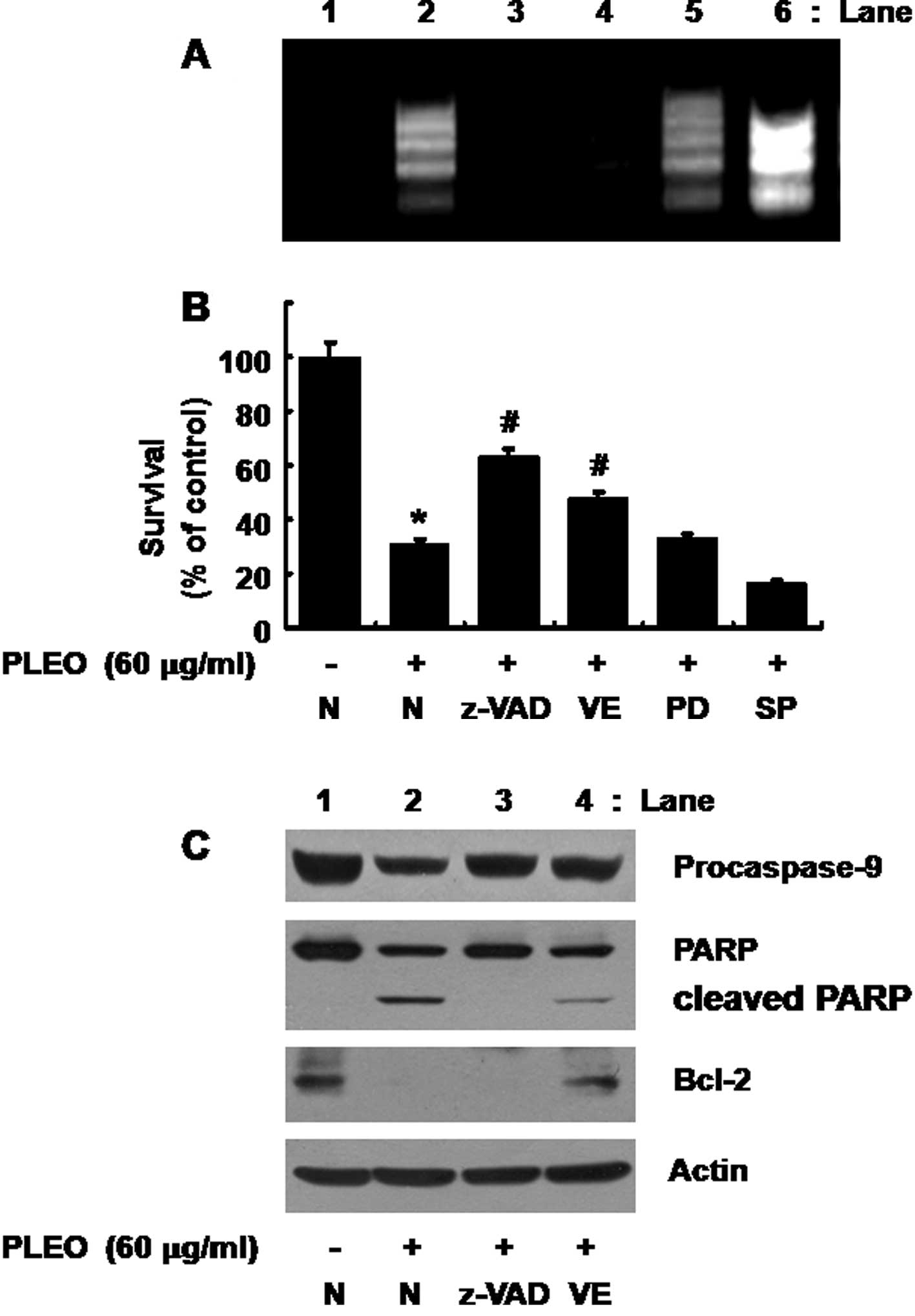 | Figure 5Effects of z-VAD-fmk, vitamin E,
PD98059 or SP600125 on DNA fragmentation, reduction of survival,
activation of caspase-9, PARP cleavage and/or Bcl-2 down-regulation
induced by PLEO in YD-8 cells. (A, B) YD-8 cells were pretreated
without or with a pan-caspase inhibitor z-VAD-fmk (z-VAD, 100 μM),
an anti-oxidant vitamin E (VE, 100 μM), an ERK-1/2 inhibitor
PD98059 (PD, 50 μM) or a JNK-1/2 inhibitor SP600125 (SP, 25 μM) for
1 h and treated without or with PLEO (60 μg/ml) for additional 8 h.
(A) Nuclear DNA was then extracted from the conditioned cells and
analyzed on a 1.7% agarose gel. The image is a representative of
three independent experiments. (B) The number of surviving cells
was counted under a microscope. Data are mean ± SE of three
independent experiments. *P<0.05 compared to the
value of control (no PLEO); #P<0.05 compared to the
value of PLEO treatment in the absence of z-VAD, VE, PD or SP. (C)
YD-8 cells were pretreated without or with z-VAD-fmk (z-VAD, 100
μM) or vitamin E (VE, 100 μM) for 1 h and treated without or with
PLEO (60 μg/ml) for additional 8 h. Whole cell lysates were
prepared and analyzed by Western blotting. The image is a
representative of three independent experiments. |
Evidence that ROS generation lies
upstream of activation of caspases, PARP cleavage and Bcl-2
down-regulation in response to PLEO exposure
We further tested any crosstalk among ROS
generation, activation of caspases and/or Bcl-2 down-regulation
induced by PLEO in YD-8 cells. As shown in Fig. 5C (panels 1 and 2), as expected, the
PLEO-induced activation of caspase-9 and PARP cleavage in YD-8
cells (lane 2) was suppressed by treatment with z-VAD-fmk (lane 3).
However, as shown in Fig. 5C
(panel 3), the PLEO-induced Bcl-2 down-regulation in YD-8 cells
(lane 2) was not affected by z-VAD-fmk (lane 3). Of note, as shown
in Fig. 5C (panels 1–3), the
PLEO-induced activation of caspase-9, PARP cleavage and Bcl-2
down-regulation (lane 2) was effectively blocked by vitamin E (lane
4). Expression of control actin protein remained constant in YD-8
cells treated without or with PLEO in the absence or presence of
z-VAD or vitamin E (Fig. 5C, panel
4, lanes 1–4).
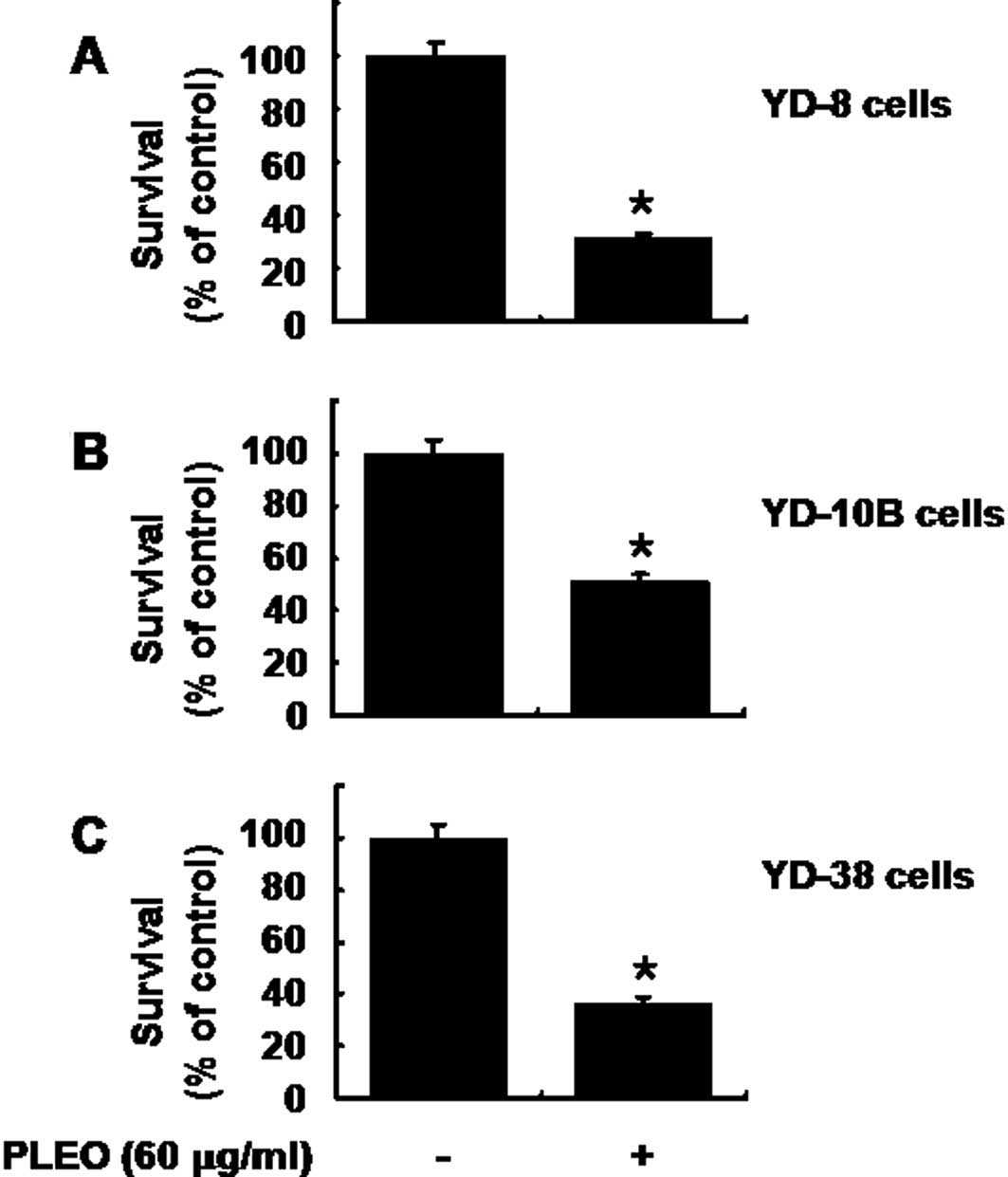
Treatment with PLEO also has strong
anti-survival effect on other types of human OSCC cells
We carried out additional cell culture experiments
to see whether PLEO treatment affects growth of other human OSCC
cell lines, including YD-10B and YD-38. As expected, treatment of
YD-8 cells with PLEO (60 μg/ml, 8 h) decreased the cell survival by
68% (Fig. 6A). Treatment with PLEO
(60 μg/ml, 8 h) also decreased survival of YD-10B and YD-38 cells
by about 50 and 60%, respectively. These results suggest that the
growth suppressive effect of PLEO is not limited to YD-8 cells.
Discussion
In the present study, we investigated the anticancer
activity of essential oil extracted from the leaf of P.
densiflora, a pine tree widely distributed in Asian countries
that has been used as a traditional medicine. Our data show that
P. densiflora leaf essential oil (PLEO) has
anti-proliferative, anti-survival and pro-apoptotic effects on YD-8
cells and the effects are associated with ROS generation,
activation of caspases and Bcl-2 down-regulation.
In initial experiments, we showed that treatment
with PLEO (60 μg/ml, 8 h) inhibits proliferation and survival of
YD-8 cells (Fig. 1A and B). It is
well understood that cells undergoing apoptosis have distinct
biochemical and morphological characteristics, such as nuclear DNA
fragmentation (4,23). Thus, considering that PLEO
treatment leads to strong nuclear DNA fragmentation in YD-8 cells
(Fig. 1C), it is obvious that PLEO
treatment induces death of YD-8 cells by apoptosis.
Induction of apoptosis is largely influenced by a
variety of cellular factors and proteins. Oxidative stress, which
often occurs due to imbalance of intracellular levels of oxidants
(e.g., ROS) and reducing agents (e.g., glutathione), is one of key
cellular factors involved in induction of apoptosis. The role of
ROS in apoptosis induction and/or cell death signaling has been
reported (9,10). Interestingly, a recent study has
shown that essential oil from Zanthoxylium schinifolium, an
aromatic plant induces ROS-dependent apoptosis in human liver
cancer cells (24). In this study,
we have demonstrated that PLEO treatment even at 10 min is able to
increase intracellular levels of ROS in YD-8 cells (Fig. 3), suggesting that PLEO treatment
rapidly induces oxidative stress by ROS generation in YD-8 cells.
Importantly, the present data with strong blockage of the
PNEO-induced apoptosis in YD-8 cells and reduction of their
survival by vitamin E, an anti-oxidant (Fig. 5A and B) further imply that ROS
generation is critical for the PLEO-induced apoptosis and growth
inhibition in YD-8 cells.
Previously, the potential role of caspases in
certain plant-derived essential oil-induced apoptosis in human
cancer cells has been suggested. For example, it is shown that
caspases are involved in induction of apoptosis by essential oil of
Curcuma wenyujin, a perennial herbal plant in human liver
cancer cells (25). Of interest,
it has been reported that activation of caspases is critical for
apoptosis induced by essential oil isolated from Artemisia
iwayomogi, a perennial herbal plant in human oral epidermoid
cancer cells (26). In resting
cells, caspases are expressed in a precursor form (inactive) with
certain molecular weight. However, when cells are exposed to
apoptogenic stimuli, they are processed via partial proteolytic
cleavage and activated. Once activated, caspases cleave many
cellular proteins, including PARP and other vital proteins, leading
to induction and/or execution of apoptosis (27,28).
Therefore, assuming that PLEO treatment induces activation of
caspase-9 in YD-8 cells (Fig. 2)
and treatment with z-VAD-fmk, a pan-caspase inhibitor inhibits the
PLEO-induced DNA fragmentation in YD-8 cells and reduction of their
survival (Fig. 5A and B), it is
likely that activation of the caspase pathway is also important for
the PLEO-induced apoptosis and growth suppression in YD-8
cells.
Substantial evidence suggests that the members of
Bcl-2 family are also involved in apoptosis initiation and caspase
activation by regulating the mitochondrial membrane integrity
(6,29). It was previously shown that lower
expression level of Bcl-2 is associated with mitochondrial
dysfunction, resulting in the release of intermembrane proteins,
such as cytochrome c, that function in the activation and assembly
of caspases, such as caspase-9 (30). Evidence further indicates high
expression level of Bcl-2 protein with poor survival in OSCC
(31–33) and that low expression level of
Bcl-2 correlates with high expression level of pro-apoptotic Bax
protein, which would promote apoptosis of OSCC (32). In view of this, it is interesting
to note recent studies that treatment with pine needle-derived
essential oil induces apoptosis in human liver cancer cells and
Bcl-2 expression is down-regulated in the essential oil-induced
apoptotic cells (21) and the
apoptotic response in human oral epidermoid cancer cells to
essential oil from Artemisia iwayomogi is in part mediated
via Bcl-2 down-regulation (26).
In this study, we have shown that PLEO treatment only at 8 h
decreases expression of Bcl-2 in YD-8 cells, but Bax expression is
not affected by PLEO treatment at the times tested (Fig. 2B). The family of human IAP,
including XIAP, is another suppressor of apoptosis (7) and inhibitor of caspases, including
caspase-9 (34,35). In this study, we have demonstrated
that PLEO treatment does not influence expression of XIAP in YD-8
cells (Fig. 2B). Given that
activation of caspase-9 is inducible at 2 h PLEO treatment in YD-8
cells (Fig. 2A), it is obvious
that early activation of the caspase-9 in the PLEO-treated YD-8
cells is not through modulation of Bcl-2 expression but via other
mechanisms. Importantly, the present study provides experimental
evidence that ROS generation lies upstream of activation of
caspase-9 and Bcl-2 down-regulation in response to PLEO treatment,
as demonstrated by ROS generated at 10 min PLEO treatment in YD-8
cells (Fig. 4A) and that
PLEO-induced both activation of caspase-9 and Bcl-2 down-regulation
is not shown by treatment with vitamin E, an anti-oxidant (Fig. 5C). Thus, it is likely that PLEO
treatment rapidly increased intracellular ROS, which subsequently
leads to activation of caspase-9 and down-regulation of Bcl-2 in
YD-8 cells.
Evidence suggests that activities of the family of
MAPK are also linked to cell proliferation, survival and/or
apoptosis. It is previously shown that ERK-1/2 is phosphorylated
and activated in cells upon exposure to mitogenic stimuli and the
activated ERK-1/2 facilitates cell proliferation and/or
transformation (36). On the other
hand, JNK-1/2 and/or p38 MAPK are activated in cells exposed to
stressful conditions (37) and
often linked to induction of apoptosis (38). In a recent study, it has been
demonstrated that treatment with essential oil from Artemisia
iwayomogi leads to activations of ERK-1/2, JNK-1/2 and p38 MAPK
and the activation is important for the mitochondrial- and
caspase-dependent apoptotic death of human oral epidermoid cancer
cells (26). The present study,
however, demonstrates that though PLEO treatment leads to
activation of ERK-1/2 and JNK-1/2 in YD-8 cells (Fig. 3), their activation is not necessary
for the PLEO-induced apoptosis and growth inhibition in YD-8 cells,
which is deduced from no effect of the PLEO-induced DNA
fragmentation in YD-8 cells and reduction of their survival by
treatment with PD98059 (an ERK-1/2 inhibitor) or SP600125 (a
JNK-1/2 inhibitor) (Fig. 5A and
B).
It is obvious that PLEO has anti-proliferative,
anti-survival and pro-apoptotic effects on YD-8 cells. However, at
present, it remains unclear whether the effects are mediated
through whole extract of PLEO or some component(s) in it. Through
GC and GC-MS analyses, in this study, we have indentified 17
compounds in PLEO, of which 2,2-dimethyl-3-methylenenorbornane
(22.38%), α-pinene (20.58%), α-limonene (20.16%) and bornyl acetate
(9.79%) are the main constituents (data not shown). Interestingly,
studies have recently shown that essential oil Tanacetum
gracile with high contents of α-pinene induces
mitochondrial-dependent apoptosis in human leukemia cells (39) and treatment with α-pinene (150
μg/ml) isolated from Schinus terebinthifolius Raddi is able
to induce apoptosis in a murine melanoma cell line (40). Considering these previous reports
and the present observation of high contents of α-pinene in PLEO,
it is conceivable that α-pinene may be one of the bioactive
compounds in PLEO leading to the cytotoxic and/or apoptotic effect.
However, in this study, we observed that single treatment with
α-pinene has little effect on growth of YD-8 cells (data not
shown). It could be informative to indentify which component(s) in
PLEO, except α-pinene, exerts the cytotoxic and/or apoptotic
effects on YD-8 cells.
Cancer cells and/or tissues may differ in many ways,
such as in their cell of origin, the molecular alterations causing
them and the susceptibility and defenses of the patient, and this
makes the choice of appropriate treatment more difficult. Thus,
drugs or agents which inhibit growth and/or induce apoptosis in
different origins of cancer cells may be good anticancer
strategies. In view of this, it is important to note the ability of
PLEO to inhibit survival of not only YD-8 but also YD-10B and
YD-38, other types of OSCC cells.
In conclusion, we demonstrated for the first time
that essential oil extracted from the leaf of P. densiflora
has strong anti-proliferative, anti-survival and pro-apoptotic
effects on YD-8 cells and the effects are largely due to the
ROS-dependent activation of caspases and Bcl-2 down-regulation. It
is suggested that P. densiflora leaf essential oil has
potential as an anticancer agent against human OSCC.
Acknowledgements
We thank Min Young Kim for great technical
assistance in GC and GC-MS analyses, and Dr Ki-Young Nam for
helpful comments on the manuscript.
References
|
1
|
Zain RB: Cultural and dietary risk factors
of oral cancer and precancer - a brief overview. Oral Oncol.
37:205–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claus F, Duthoy W, Boterberg T, et al:
Intensity modulated radiation therapy for oropharyngeal and oral
cavity tumors: clinical use and experience. Oral Oncol. 38:597–604.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakazawa M, Ohnishi T, Ohmae M, et al:
Phase II study of a novel oral formation of 5-fluorouracil in
combination with low-dose cisplatin as preoperative chemotherapy of
oral squamous cell carcinoma. Int J Clin Pharmacol Res. 25:115–122.
2005.PubMed/NCBI
|
|
4
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: the significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar
|
|
5
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.
|
|
6
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Themsche C, Chaudhry P, Leblanc V, et
al: XIAP gene expression and function is regulated autocrine and
paracrine TGF-beta signaling. Mol Cancer. 9:2162010.PubMed/NCBI
|
|
9
|
Fleury C, Mignotte B and Vayssiere JL:
Mitochondrial reactive oxygen species in cell death signaling.
Biochimie. 84:131–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Groenendyk J and Michalak M: Endoplasmic
reticulum quality control and apoptosis. Acta Biochim Pol.
52:381–395. 2005.PubMed/NCBI
|
|
12
|
Huang X, Zhang Z, Jia L, et al:
Endoplasmic reticulum stress contributes to vitamin E
succinate-induced apoptosis in human gastric cancer SGC-7901 cells.
Cancer Lett. 296:123–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feuerstein GZ and Young PR: Apoptosis in
cardiac diseases: stress- and mitogen-activated signaling pathways.
Cardiovasc Res. 45:560–569. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lucas M and Sánchez-Margalet V: Protein
kinase C involvement in apoptosis. Gen Pharmacol. 26:881–887. 1995.
View Article : Google Scholar
|
|
15
|
Bakkali F, Averbeck S, Averbeck D, et al:
Biological effects of essential oils - a review. Food Chem Toxicol.
46:446–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hajhashemi V, Ghannadi A and Sharif B:
Anti-inflammatory and analgesic properties of the leaf extracts and
essential oil of Lavandula angustifolia Mill. J
Ethnopharmacol. 89:67–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perry NS, Bollen C, Perry EK, et al:
Salvia for dementia therapy: review of pharmacological activity and
pilot tolerability clinical trial. Pharmacol Biochem Behav.
75:651–659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva J, Abebe W, Sousa SM, et al:
Analgesic and anti-inflammatory effects of essential oils of
Eucalyptus. J Ethnopharmacol. 89:277–283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sylvestre M, Legault J, Dufour D, et al:
Chemical composition and anticancer activity of leaf essential oil
of Myrica gale L. Phytomedicine. 12:299–304. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manosroi J, Dhumtanom P and Manosroi A:
Anti-proliferative activity of essential oil extracted from thai
medicinal plants on KB and P388 cell lines. Cancer Lett.
235:114–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei FX, Li MY, Song YH, et al: Apoptosis
and activity changes of telomerase induced by essential oil from
pine needles in HepG2 cell line. Zhong Yao Cai. 31:1197–1200.
2008.PubMed/NCBI
|
|
22
|
Kim KY and Chung HJ: Flavor compounds of
pine sprout tea and pine needle tea. J Agric Food Chem.
48:1269–1272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allen RT, Hunter WJ III and Agrawal DK:
Morphological and biochemical characterization and analysis of
apoptosis. J Pharmacol Toxicol Methods. 37:215–228. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paik SY, Koh KH, Beak SM, et al: The
essential oils from Zanthoxylum schinifolium pericarp induce
apoptosis of HepG2 human hepatoma cells through increased
production of reactive oxygen species. Biol Pharm Bull. 28:802–807.
2005.PubMed/NCBI
|
|
25
|
Xiao Y, Yang FQ, Li SP, et al: Essential
oil of Curcuma wenyujin induces apoptosis in human hepatoma
cells. World J Gastroenterol. 14:4309–4318. 2008.
|
|
26
|
Cha JD, Jeong MR, Kim HY, et al: MAPK
activation is necessary to the apoptotic death of KB cells induced
by the essential oil isolated from Artemisia iwayomogi. J
Ethnopharmacol. 123:308–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Emoto Y, Manome Y, Meinhardt G, et al:
Proteolytic activation of protein kinase C delta by an ICE-like
protease in apoptotic cells. EMBO J. 14:6148–6156. 1995.PubMed/NCBI
|
|
28
|
Lazebnik YA, Kaufmann SH, Desnoyers S, et
al: Cleavage of poly(ADP-ribose) polymerase by a proteinase with
properties like ICE. Nature. 371:346–347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Festjens N, Mvan Gurp M, van Loo G, et al:
Bcl-2 family members as sentinels of cellular integrity and role of
mitochondrial intermembrane space proteins in apoptotic cell death.
Acta Haematol. 111:7–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borner C: The Bcl-2 protein family: sensor
and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Vincente JC, Olay S,
Lequerica-Fernandez P, et al: Expression of Bcl-2 but not Bax has a
prognostic significance in tongue carcinoma. J Oral Pathol Med.
35:140–145. 2006.
|
|
32
|
Kato K, Kawashiri S, Yoshizawa K, et al:
Apoptosis-associated markers and clinical outcome in human oral
squamous cell carcinomas. J Oral Pathol Med. 37:364–371. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang B, Gojo I and Fenton RG: Myeloid
cell factor-1 is a critical survival factor for multiple myeloma.
Blood. 99:1885–1893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deveraux QL, Takahashi R, Salvesen GS, et
al: X-linked IAP is a direct inhibitor of cell-death proteases.
Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shiozaki EN, Chai J, Rigotti DJ, et al:
Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell.
11:519–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Troppmair J, Bruder JT, Munoz H, et al:
Mitogen-activated protein kinase/extracellular signal-regulated
protein kinase activation by oncogenes, serum, and 12-O
tetradecanoylphorbol-13-acetate requires Raf and is necessary for
transformation. J Biol Chem. 269:7030–7035. 1994.
|
|
37
|
Clerk A, Fuller SJ, Michael A, et al:
Stimulation of ‘stressregulated’ mitogen-activated protein kinases
(stress-activated protein kinases/c-Jun N-terminal kinases and
p38-mitogen-activated protein kinases) in perfused rat hearts by
oxidative and other stresses. J Biol Chem. 273:7228–7234. 1998.
|
|
38
|
Shim HY, Park JH, Paik HD, et al:
Acacetin-induced apoptosis of human breast cancer MCF-7 cells
involves caspase cascade, mitochondria-mediated death signaling and
SAPK/JNK1/2-c-Jun activation. Mol Cell. 24:95–104. 2007.PubMed/NCBI
|
|
39
|
Verma M, Singh SK, Bhushan S, et al:
Induction of mitochondrial-dependent apoptosis by an essential oil
from Tanacetum gracile. Planta Med. 74:515–520. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuo AL, Figueiredo CR, Arruda DC, et
al: α-Pinene isolated from Schinus terebinthifolius Raddi
(Anacardiaceae) induces apoptosis and confers antimetastatic
protection in a melanoma model. Biochem Biophys Res Commun.
411:449–454. 2011.
|