Introduction
Interleukin-6 (IL-6) is a pleiotropic cytokine that
plays an important role in many chronic inflammatory diseases.
Recent studies have shown that IL-6 plays a primary role in the
pathophysiology of cancer (1,2). In
breast cancer, tumor tissue exhibits high expression levels of IL-6
compared with matched normal breast tissue samples, which also
correlate with more advanced tumor grades (3). Furthermore, elevated serum IL-6
levels correlate with advanced breast tumor stage (4), increased number of metastatic sites
(4), and poor survival in patients
with breast cancer (5).
Cancer stem-like cells (CSCs) are a highly
tumorigenic cell type. CSCs exist as a small subset within tumors
and are hypothesized to be critical initiators of cancers, as well
as sustaining tumor growth and producing metastases (6–8).
They also mediate resistance to conventional anti-tumor therapies
(9). Breast cancer is the first
human tumor for which a putative CSC subpopulation has been
isolated as CD44+CD24−/low cells (10). These breast CSCs (BrCSCs) exhibit
high tumorigenicity when injected into immunocompromized mice, and
possess characteristics that are associated with normal stem cells;
specifically, they have the ability to give rise to all cell types
found in a particular cancer sample.
In view of the important role of IL-6 in the
malignant features of cancer, we were interested to explore the
relationship between IL-6 and BrCSCs. Recent studies have indicated
that IL-6 is capable of inducing an epithelial-mesenchymal
transition (EMT) phenotype in human breast cancer cells (11). The induction of EMT in immortalized
human mammary epithelial cells (HMLEs) results in the generation of
cells with stem cell properties (12,13).
We hypothesized that IL-6 promotes the generation of cancer cells
with stem-like properties by induction of EMT. We also proposed
that the mammosphere culture is able to enrich CSCs from tumor
samples as a result of cytokines in the mammosphere media inducing
EMT in cancer cells, in a similar manner to IL-6.
Herein, we provide evidence that the inflammatory
cytokine, IL-6, is capable of generating CD44+ cells
with stem-like properties through inducing EMT in the T47D breast
cancer cells. We also show that mammosphere culture consistently
generated stem-like cancer cells solely as a result of the EGF and
bFGF cytokines in the mammosphere media mediating EMT. Thus, EMT
appears to be an important mechanism for the induction of cancer
cells with stem-like properties, and EMT of non-stem cancer cells
could be a source of CSCs.
Materials and methods
Cell culture
Human breast cancer cell lines, including T47D,
MCF7, ZR-75-1, and MDA-MB-453, were obtained from the Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China). T47D, ZR-75-1, and MDA-MB-453 cells were routinely
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS; Hyclone, Logan, UT, USA). MCF7 cells were cultured in
DMEM medium containing 10% FBS. IL-6 (Peprotech, Rocky Hill, NJ,
USA) was added to all cultures at final concentrations of 50 ng/ml
in RPMI-1640 containing 10% FBS.
For the mammosphere culture, cells were suspended at
50,000 cells/ml in DMEM/F12 (1:1) containing 5 μg/ml bovine insulin
(Sigma, St. Louis, MO, USA), 0.4% bovine serum albumin (BSA;
Sigma), 2% B27 (Invitrogen, Carlsbad, CA, USA), 20 ng/ml basic
fibroblast growth factor (bFGF; Peprotech) and 20 ng/ml epidermal
growth factor (EGF; Sigma); cells were then seeded into 6-well
plates (3 ml per well). As they differentiated during culture,
cells were reseeded to standard adherent culture conditions in
serum-supplemented media.
Flow cytometric analysis
Cells were trypsinized, suspended into single-cell
mixtures, washed with phosphate buffered saline (PBS), and
incubated on ice for 30 min with monoclonal antibodies specific for
human cell surface markers CD44-FITC (eBioscience, San Diego, CA,
USA) or CD24-PE (eBioscience). In negative control experiments,
cells were incubated with fluorescence-labeled isotype-matched
pre-immune IgG instead. Cells were washed and analyzed using a flow
cytometer (BD FACS Aria, San Jose, CA, USA).
Real-time quantitative PCR
Cells were harvested, and RNA was extracted using
TRIzol (Invitrogen) following the manufacturer's protocol. One
microgram of total RNA was reverse transcribed into cDNA using the
SuperScript First-Strand Synthesis System (Invitrogen). Real-time
polymerase chain reactions (PCRs) using the SYBR Green PCR Master
Mix were performed using an ABI PRISM 7500 Sequence Detection
System (Perkin-Elmer/Applied Biosystems, Rotkreuz, Switzerland).
Data are shown after normalization to 18S expression. Primer
sequences were as follows: E-cadherin, forward, 5′-CCCACCACGTAC
AAGGGTC-3′, reverse, 5′-CTGGGGTATTGGGGGCATC-3′; vimentin, forward,
5′-CGCCAGATGCGTGAAATGG-3′, reverse, 5′-ACCAGAGGGAGTGAATCCAGA-3′;
twist, forward, 5′-GTCCGCAGTCTTACGAGGAG-3′, reverse,
5′-GCTTGAGGGTCTGAATCTTGCT-3′; CD44: forward,
5′-CAGCAACCCTACTGATGATGACG-3′, reverse, 5′-GCCA AG
AGGGATGCCAAGATGA-3′; 18S, forward, 5′-CCTGGA TACCGCAGCTAGGA-3′
reverse, 5′-GCGGCGCAATA CGAATGCCCC-3′.
Mouse injections
Animal maintenance and experiments were performed in
accordance with the animal care guidelines of the Southern Medical
University, Guangzhou, China. Cells were resuspended in a 1:1 (v/v)
mixture of culture media and Matrigel (BD Biosciences, San Jose,
CA, USA), and 106 CD44+ or CD44−
cells were injected subcutaneously into the mammary fat pads of
4-week old female NOD/SCID mice. Tumor growth was monitored twice a
week with callipers at the site of injection for 40 days. Animals
were sacrificed as soon as tumor size reached 1.0 cm in
diameter.
Invasion assay
Transwell insert chambers with an 8-μm porous
membrane (Corning Costar, Cambridge, MA, USA) were used for the
assay. Transwell insert chambers were pre-coated with a 1:5 (v/v)
mixture of Matrigel (BD Biosciences) and RPMI-1640 medium. The
following day, cells were washed three times with PBS and
1×105 cells were added to the top chamber in serum-free
media. The bottom chamber was filled with media containing 10% FBS.
Cells were incubated for 24 h at 37°C in a 5% CO2
humidified incubator. To quantify the number of invasive cells,
cells on the top chamber were removed with a cotton-tipped swab,
and migrated cells were fixed in methanol and stained with 1%
crystal violet. Five random fields were counted.
Western blot analysis
Primary antibodies included mouse anti-E-cadherin
(1:5,000; BD Biosciences), mouse anti-vimentin (1:500; Clone V9,
Dako, Glostrup, Denmark), and rabbit anti-CD44 (1:5,000; GeneTex
Inc., Irvine, CA, USA). Secondary antibodies included rabbit
anti-mouse IgG-HRP (1:1,000; Santa Cruz Biotechnology, Santa Cruz,
CA, USA) and goat anti-rabbit IgG-HRP (1:1,000; GE Healthcare,
Chalfont St Giles, UK). HRP-conjugated monoclonal mouse anti-GAPDH
(Kangchen, Shanghai, China) was used as an internal parameter. All
antibodies were diluted with 5% milk in PBS containing 0.1%
Tween-20 (PBS-T) and incubated for either 1 h at room temperature
or overnight at 4°C. All Western blots were visualized with ECL
Western blotting substrate (Pierce, Rockford, IL, USA).
Immunofluorescence
A total of 1×105 cells per chamber were
plated into Lab-Tek two-chamber slides overnight. The next day,
when cells were 50–70% confluent, they were washed with PBS twice,
fixed in 3% paraformaldehyde (Sigma) and permeabilized in 0.1%
Triton X-100 (Sigma) in PBS buffer at 4°C for 30 min. The cells
were then washed 3 times with PBS and incubated with blocking
solution (10% horse serum in PBS). The cells were then incubated
with primary antibodies for anti-E-cadherin (BD Biosciences) or
anti-vimentin V9 (Clone V9, Dako) overnight at 4°C. The cells were
washed three times in PBS and incubated with the secondary
antibody, goat anti-mouse-Alexa Fluor 488 (1:1,000; Molecular
Probes, Invitrogen) in blocking buffer for 1 h at room temperature
in the dark. Finally, the cells were washed three times in PBS and
incubated with 0.25 mg/ml DAPI (Roche) for 1 min at room
temperature in the dark. The slides were washed extensively with
PBS and mounted with Fluoromount-G (Southern Biotech, Birmingham,
AL, USA). All matched samples were photographed (controls and
tests) using immunofluorescence microscope (Olympus BX51, Tokyo,
Japan) with identical exposure times.
Irradiation and clonogenic assay
Cells were dissociated by trypsinization and
mechanical agitation with a Pasteur pipette into single cell
suspensions in RPMI-1640 medium supplemented with 10% FBS. The
cells were seeded in 6-well plates at the indicated densities, and
then incubated overnight before the irradiation treatment. Cells
were irradiated from a vertical direction at a dose rate of 400
cGy/min with 6-MV X-rays produced by a Varian 2100C linear
accelerator at the Southern Medical University. Cells were
irradiated for the time required to receive a total dose of 0, 2,
4, 6, or 8 Gy. Negative control cells were sham-irradiated.
Following the irradiation, the cells were incubated for 15 days at
37°C in a 5% CO2 environment to allow the formation of
colonies. The resulting colonies were fixed with 100% ethanol and
stained with 1% crystal violet. Colonies containing >50 cells
were counted as clonogenic survivors. Three independent experiments
were performed, each in triplicate. The surviving fraction was
calculated as described previously (14). Using GraphPad Prism 5 software
(GraphPad, La Jolla, CA, USA), the data were fitted into the
following single-hit multitarget formula: S=1-(1-e-D/D0)N.
Assessment of proliferation and
doxorubicin resistance
Cell proliferation potential and the relative
resistance to doxorubicin were evaluated by cell proliferation
assays using a Cell Counting Kit-8 (CCK8, Yiyuan Biotechnologies,
Guangzhou, China). Cells were plated at a concentration of
1×103 cells per well (for growth advantage assays) or
1×104 cells per well (for doxorubicin resistance) into
96-well culture plates. For the cell proliferation potential assay,
10 μl CCK-8 solution was added on days 1–5. For the doxorubicin
resistance assay, 10 μl of CCK-8 solution was added to each well of
the plate 4 h, and 1–5 days after treatment with doxorubicin at a
final concentration of 10 μg/ml. After the addition of CCK-8
solution, plates were incubated for 4 h, and absorbance was then
measured at 450 nm using a microplate reader (SpectraMax M5,
Sunnyvale, CA, USA).
Statistical analysis
In all experiments, differences among groups were
analyzed by ANOVA or Student's t-test using SPSS version 13.0
(SPSS, Chicago, IL, USA). A p=0.05 was considered to be
statistically significant.
Results
The enrichment of CD44-positive cells by
IL-6 exposure
Human breast cancer T47D cells, which are
characterized as estrogen and progesterone receptor (ER/PR)
positive and Her-2/neu (Her2) negative, were cultured in RPMI-1640
medium containing 10% FBS. T47D cells exhibit epithelial-like
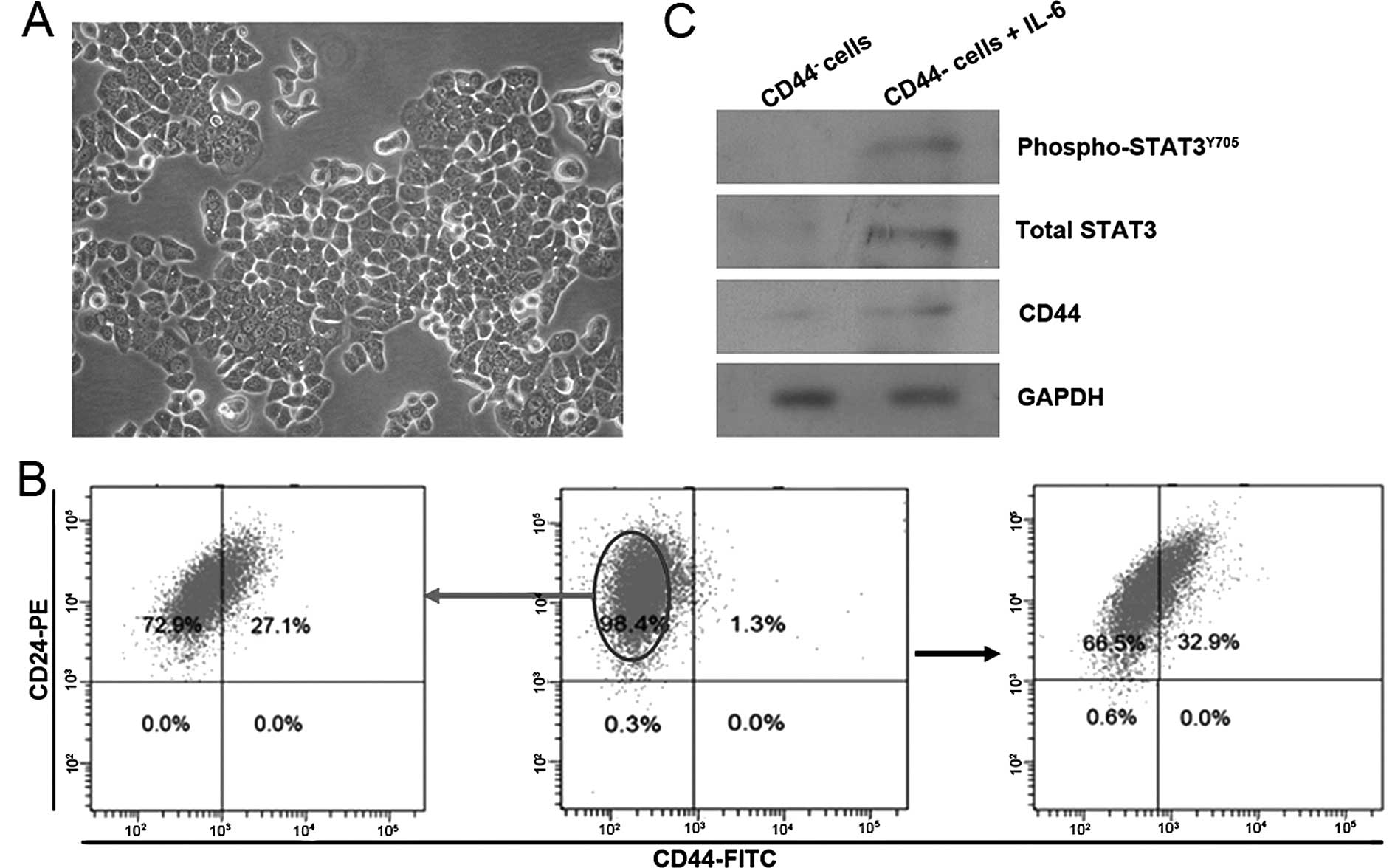
features (Fig. 1A), and contain a
very low proportion of CD44+ cells (Fig. 1B middle). After 10 days of exposure
to 50 ng/ml IL-6, the number of CD44+ cells had
increased by ~30-fold, as shown by the FACS analysis (Fig. 1B right). To determine whether this
enrichment of CD44+ cells was because CD44+
cells that already existed in T47D cell cultures prior to the
exposure to IL-6 that acquired a growth advantage in
IL-6-containing media, CD44− cells were isolated from
T47D cells and cultured in IL-6-containing media. The results
showed a similarly significant enrichment of CD44+ cells
(Fig. 1B left). Western blot
analysis showed IL-6-treated cells expressed elevated active
STAT3Y705 (phospho-STAT3Y705), a main
downstream molecular of IL-6 signaling, and total STAT3, which
coincided with induction of CD44 (Fig.
1C). These data showed that IL-6 exposure activated IL-6/STAT3
signaling in CD44− T47D cells, and induced up-regulation
of CD44 protein expression, resulting to the enrichment of
CD44+ cell population.
CD44+ cells induced by IL-6
exposure exhibit many properties of CSCs
CD44 is an important CSC marker in breast cancer
cells, especially in epithelial-like breast cancer cells. We
therefore speculated that the CD44+ cells resulting from
IL-6 exposure might exhibit phenotypes similar to those reported
previously for CSCs. To test this hypothesis, IL-6-induced cells
were fractionated based on their CD44/CD24 antigen marker profile.
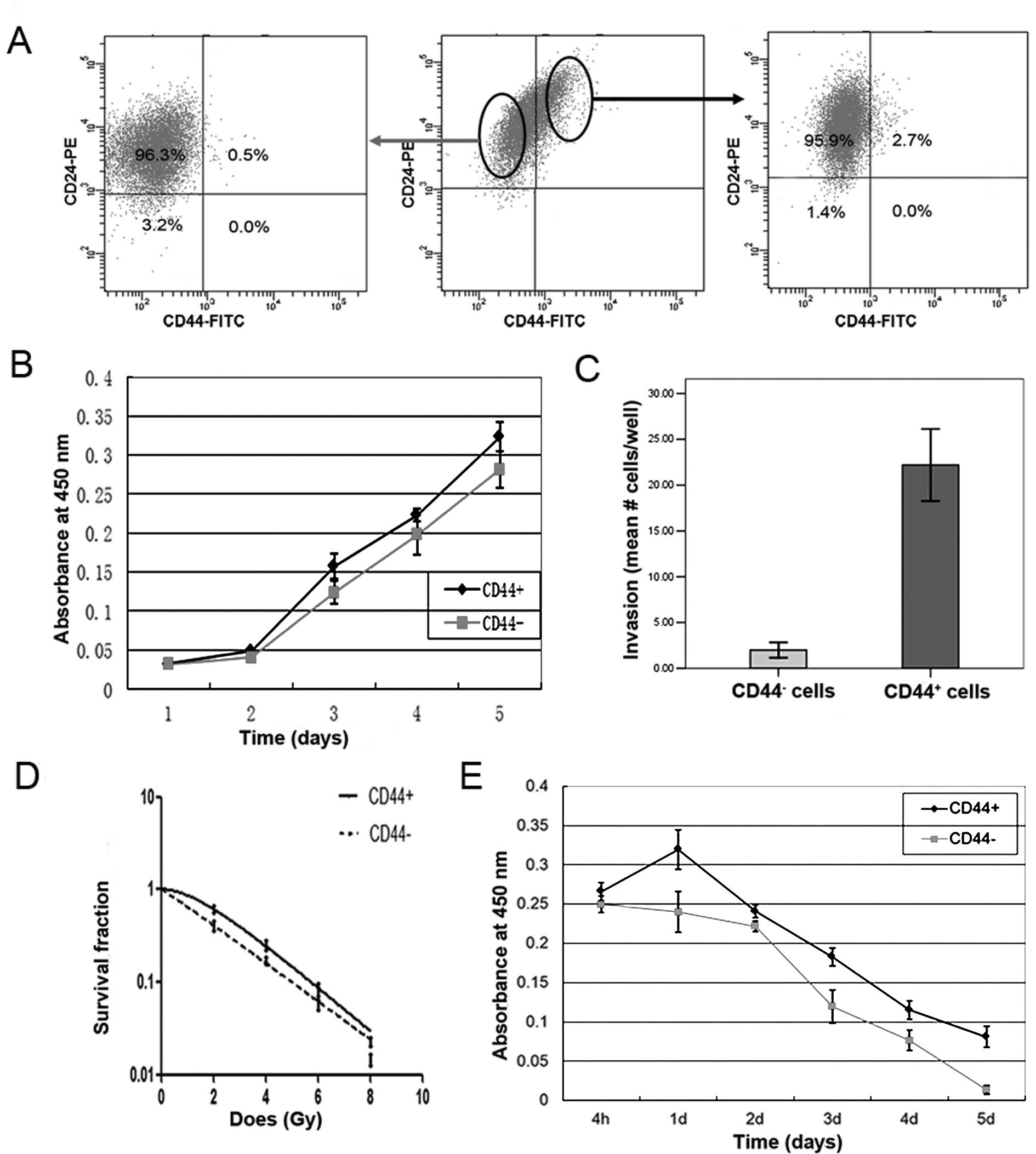
Tumorigenic potential was evaluated by injecting CD44+
cells and CD44− cells into the mammary fat pads of
NOD/SCID mice. As anticipated, the CD44+ cells displayed
enhanced tumorigenic potential compared with CD44− cells
(2/5 for CD44+ versus 0/5 for CD44−cells).
Furthermore, these isolated CD44+ cells differentiated
into a variety of cell types, as did parental T47D cells, after 10
days of culture in standard serum-supplemented culture conditions
(Fig. 2A right). In contrast, no
CD44+ cells were generated by culturing isolated
CD44− cells in standard culture conditions for the same
period of time (Fig. 2A left). We
confirmed and extended these findings by purifying single-cell
clones from CD44+ and CD44− cell populations,
and observed similar behavior in vitro (data not
shown).
To investigate further whether they exhibited other
enhanced malignant features, the proliferation and invasive
potential, and the response to radiation and doxorubicin, of
CD44+ cells induced by IL-6 exposure were determined.
The IL-6-induced CD44+ cells displayed significantly
enhanced proliferation potential (Fig.
2B) and increased invasive potential (Fig. 2C) compared with CD44−
cells, as demonstrated by the CCK-8 and transwell assays,
respectively. Additionally, the IL-6-induced CD44+ cells
exhibited increased resistance to radiation (Fig. 2D), and reduced cell death after
doxorubicin treatment (Fig. 2E)
compared with CD44− cells, which was consistent with the
characteristics of breast cancer stem cells (BrCSCs) reported in
previous studies (15,16). These data suggest that
CD44+ cells induced by IL-6 exposure exhibit the
characteristics of CSCs.
CD44+ T47D cells induced by
IL-6 exposure undergo EMT
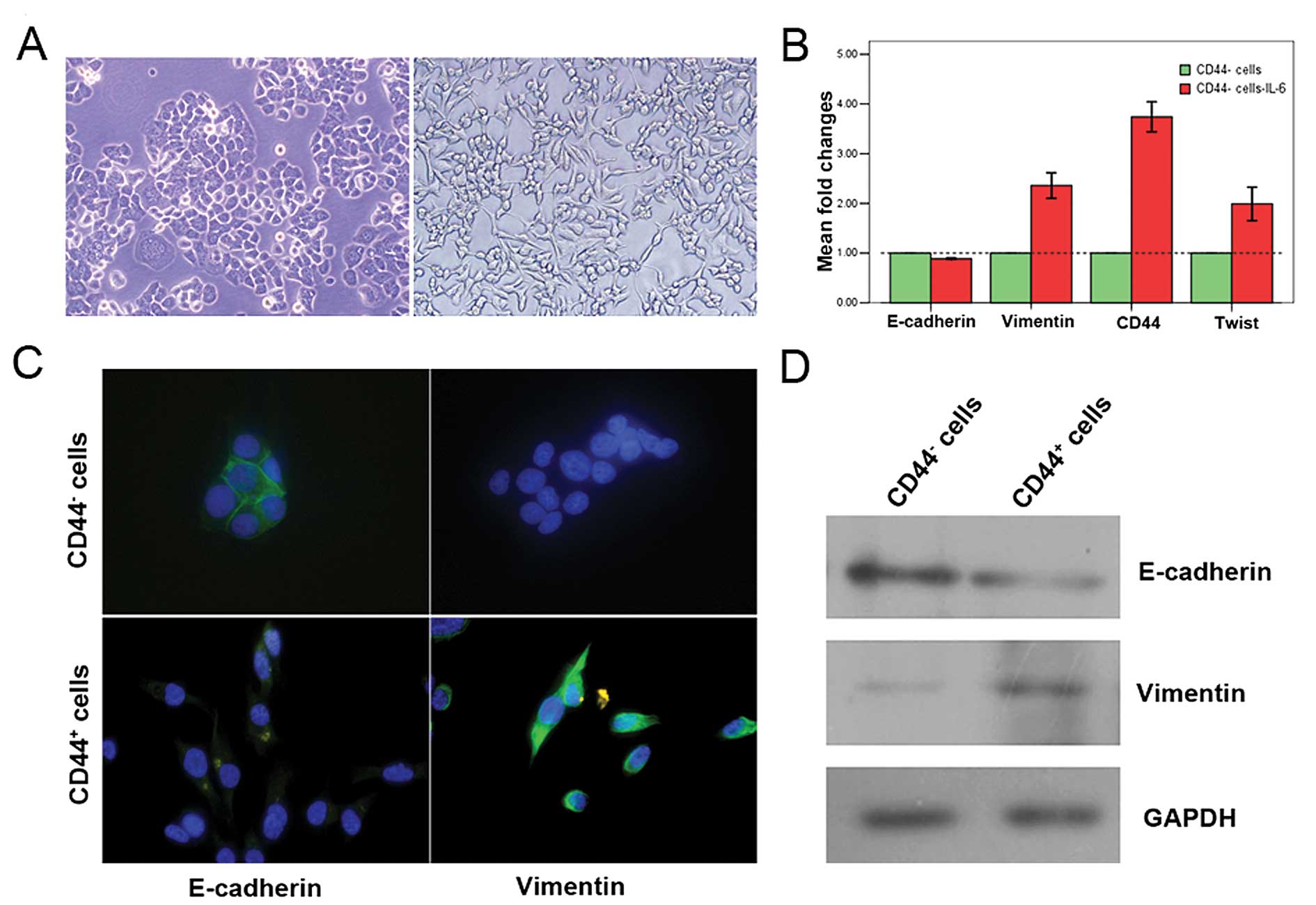
A previous study has shown that IL-6 can induce the
EMT phenotype in human breast cancer epithelial-like cell lines
(11). To explore whether the
enrichment of CD44+ cells by exposure to IL-6 is
associated with EMT phenotypes, the CD44− sub-population
was isolated from T47D cells and subjected to induction with 50
ng/ml IL-6. Morphological changes from a cobblestone to a
spindle-like morphology, a classical marker of EMT induction, were
seen 10 days after IL-6 exposure (Fig.
3A). Quantitative real-time PCR analysis showed a gene
expression pattern that was consistent with EMT, including
E-cadherin repression and the concomitant induction of vimentin and
twist, which was accompanied by the induction of CD44 (Fig. 3B). Immunofluorescence microscopy
was utilized to compare the immunostaining of E-cadherin and
vimentin in CD44− versus CD44+ cells.
CD44− cells showed epithelial homophilic adhesion and
prominent levels of E-cadherin, and lacked the expression of
vimentin. CD44+ cells displayed decreased E-cadherin and
prominent vimentin expression (Fig.
3C). Western blot analyses also showed the down-regulation of
E-cadherin expression and the induction of vimentin (Fig. 3D).
CD44+ cells induced by IL-6
exposure resemble CD44+ cells enriched by mammosphere
culture
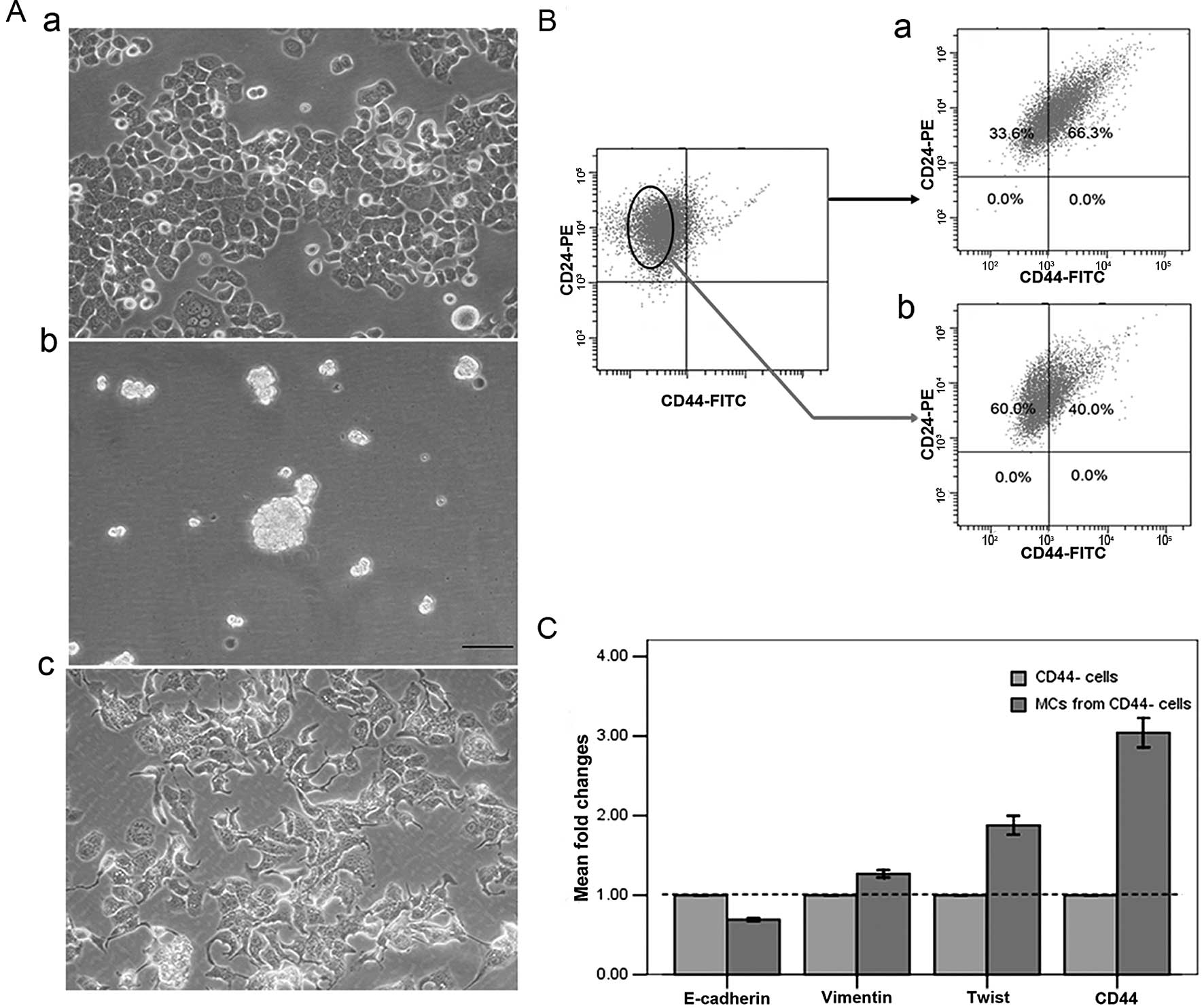
The non-adherent mammosphere culture system, in
which stem-like cells are capable of forming suspended spheres, has
been extensively utilized to enrich cultures for BrCSCs with the
CD44+CD24−/low phenotype (17). Similarly, the mammosphere culture
of T47D cells resulted in the formation of suspended spheres
(Fig. 4A-b). Interestingly, these
formed mammospheres were significantly enriched for
CD44+ cells but not for
CD44+CD24−/low cells (Fig. 4B-a). This finding was consistent
with the induction of CD44+ cells by IL-6 exposure,
which were also enriched for CD44+ cells but not for
CD44+CD24−/low cells. These data indicated
that IL-6 exposure resembles mammosphere culture, in that it can
induce cancer stem-like cells in cultures of T47D cells. We found
that the mammosphere culture of CD44− cells isolated
from T47D cells identically enriched for CD44+ cells
(Fig. 4B-b), which was coincident
with the result that CD44+ cells were induced from
CD44− cells in T47D cells by IL-6 exposure. Cells
exhibited a spindle-like morphology and mesenchymal appearance
(Fig. 4A-c) after 24 h culture in
mammosphere media supplemented with 3% FBS which should promote
cell adhesion.
To determine whether mammosphere culture also
triggers EMT, we examined the expression levels of markers
associated with EMT. CD44+ cells generated by
mammosphere culture consistently downregulated the expression of
mRNAs encoding epithelial markers, such as E-cadherin, and
upregulated mRNAs encoding mesenchymal markers, such as vimentin
and twist, even at 12 h after culture in mammosphere media
(Fig. 4C). These results suggest
that CD44+ cells generated by mammosphere culture are
also associated with EMT.
To determine further whether cytokines (EGF and
bFGF) present in mammosphere culture medium promote EMT, and thus
result in the enrichment of CD44+ cells,
CD44− cells were cultured in standard adherent culture
conditions supplemented with 20 ng/ml EGF and 20 ng/ml bFGF.
Similarly, cells exhibited a spindle-like morphology after 24 h
culture in standard media containing EGF and bFGF (Fig. 4D-a). The significantly upregulated
expression at mRNA levels of mesenchymal markers and CD44 was
detectable, even at 12 h after culture in the media (Fig. 4D-b). Accordingly, CD44+
cells were significantly enriched after a 1-week culture, as shown
by flow cytometric analysis (Fig.
4D-c). Conversely, when cultured in mammosphere media without
EGF and bFGF, CD44+ cells were not enriched and the
majority of CD44− cells underwent apoptosis (data not
shown). All of these results suggest that as in the enrichment of
CD44+ cells by IL-6, the enrichment of CD44+
cells by mammosphere culture is the result of cytokine-mediated EMT
by EGF and bFGF.
EMT generally exists in mammosphere
culture
To determine whether the EMT process occurs
generally in mammosphere culture, we performed homologous
experiments using three other breast cancer cell lines, MCF7,
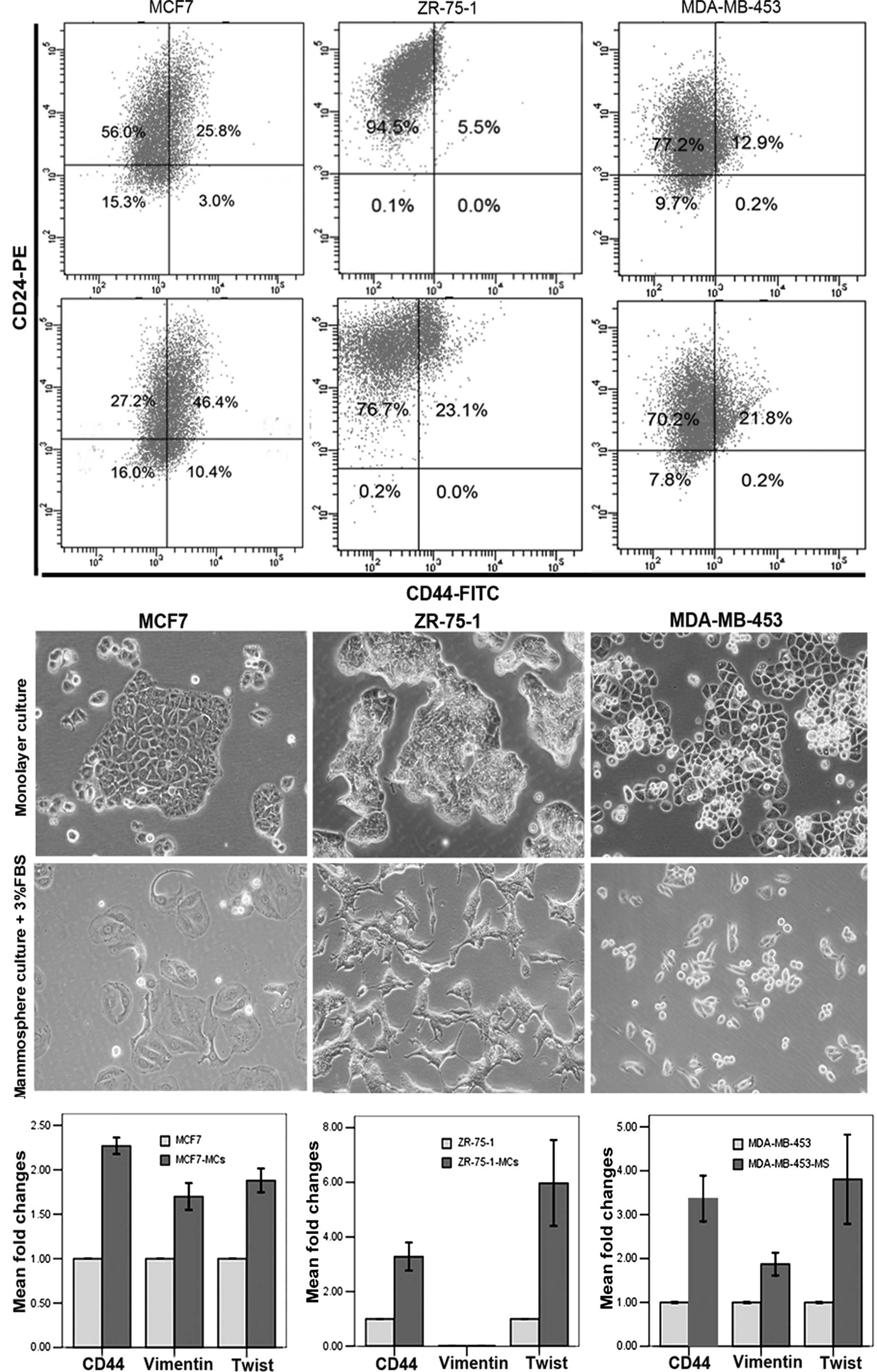
ZR-75-1, and MDA-MB-453. Mammosphere culture of MCF7 significantly
enriched cultures for CD44+CD24−/low cells
(Fig. 5A), which was consistent
with previous studies (17).
However, as with T47D cells, mammosphere culture of ZR-75-1 or
MDA-MB-453 cells enriched for CD44+ cells but not for
CD44+CD24−/low cells (Fig. 5A). All three cell lines exhibited
mesenchymal-like morphology after a 2-day culture in mammosphere
media supplemented with 3% FBS (Fig.
5B). As for T47D cells, MCF7, ZR-75-1 and MDA-MB-453 cells
developed a gene expression pattern consistent with EMT even within
12 h of mammosphere culture, including induction of mesenchymal
markers, vimentin and Twist, along with the induction of CD44
(Fig. 5C). These observations
further support our conclusion that EMT promotes the enrichment of
CSCs in the mammosphere culture system.
Discussion
IL-6 is recognized as a major mediator involved in
the regulation and maintenance of the inflammatory response. IL-6
is elevated in human breast tumors and breast cancer patient sera,
and is associated with a poor prognosis in breast cancer (5). Almost all solid tumors are
characterized by the presence of an inflammatory component in their
microenvironments, including IL-6 (18), yet the link between IL-6 and BrCSCs
remains poorly understood. We now show that the breast cancer cells
T47D, are capable of producing CD44+ cells with
stem-like properties on exposure to IL-6, which suggests that IL-6
promotes the induction of CSCs. CSCs are responsible for tumor
initiation, sustaining tumor growth (19), tumor metastasis (20), and resistance to conventional
anti-tumor therapies (15,21). These data explain, at least in
part, why IL-6 is capable of triggering malignant features in human
breast cancer cells.
EMT has been described over the past decade as a
cellular biological program that is required for the remodeling of
cells and tissues during embryogenesis, during certain types of
wound healing, and during the acquisition of malignant traits by
carcinoma cells (22,23). Many types of cancer cells, except
for primary carcinomas, appear to rely on the EMT program to
facilitate execution of most of the invasion-metastasis cascade
(23). Mani et al (13) demonstrated for the first time that
EMT induces properties of BrCSCs in addition to endowing cells with
migratory and invasive potential. In this study, we have shown that
IL-6 mediates the EMT process and promotes the generation of
CD44+ cells with CSC-like attributes. This supports the
important role of EMT in the generation of cancer stem-like
cells.
Mammosphere culture has been used widely for the
enrichment of mammary epithelial stem cells (24) and breast cancer stem cells
(17). We examined whether
mammosphere culture conditions per se induced EMT in
the epithelial T47D breast cancer cell line. As anticipated, the
mammosphere culture induced EMT within 12 h, involving the
abrogation or induction of EMT-associated markers. Interestingly,
when cultured in mammosphere media supplemented with 3% FBS, cells
consistently displayed a mesenchymal-like morphology. We also
showed here that EGF and bFGF, two important cytokines present in
mammosphere media, consistently induced EMT, showing a spindle-like
morphology and induction of mesenchymal markers, which was
concomitant with the enrichment of CD44+ cells.
Conversely, without EGF and bFGF, CD44+ cells could not
been enriched and a majority of cells underwent apoptosis in
mammosphere media. It seems to be apparent that the enrichment of
CD44+ cells by mammosphere culture occurs as a result of
cytokine-mediated EMT with EGF and bFGF, and is analogous to the
enrichment of CD44+ cells by IL-6 exposure. Using other
three epithelial-like breast cancer cell lines, we further
confirmed that this phenomenon generally existed in mammosphere
culture. Indeed, a similar connection between EGF or bFGF and EMT
was previously shown in human breast carcinoma cell line, PMC42-LA
(25), and tubular cell lines
(26).
Additionally, it was thought that CSCs survive,
self-renew, and propagate in mammosphere media, whereas non-stem
cancer cells undergo cell death, which accordingly leads to the
enrichment of CSCs. In contrast, we showed that mammosphere culture
of CD44− cells enriched for CD44+ cancer
stem-like cells through an EMT process. The data challenge the
traditional view of mammosphere culture as an in vitro
surrogate assay of self-renewal capacity. In view of these results,
we suggest that EMT is an important mechanism for the induction of
cancer cells with stem-like properties.
Collectively, the findings presented here describe a
link between IL-6 and BrCSCs, and an important mechanism of
CSC-formation in mammosphere culture. These findings demonstrate
that induction of EMT in differentiated breast epithelial tumor
cells is sufficient to generate a subpopulation of cancer cells
with stem cell characteristics. Thus, EMT is not only important for
cells to escape from the immediate vicinity of the tumor, but may
also sustain primary tumor growth as well as promoting the
initiation and establishment of secondary tumors.
Acknowledgements
This work was supported by a Natural Science
Foundation of China grant (30670633), key applied and basic
projects of Guangzhou science and technology program (11C22120714),
and Wu Jie Ping Foundation (2011).
References
|
1
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005.
|
|
2
|
Rose-John S, Scheller J, Elson G and Jones
SA: Interleukin-6 biology is coordinated by membrane-bound and
soluble receptors: role in inflammation and cancer. J Leukoc Biol.
80:227–236. 2006.
|
|
3
|
Chavey C, Bibeau F, Gourgou-Bourgade S, et
al: Oestrogen receptor negative breast cancers exhibit high
cytokine content. Breast Cancer Res. 9:R152007.
|
|
4
|
Kozlowski L, Zakrzewska I, Tokajuk P and
Wojtukiewicz MZ: Concentration of interleukin-6 (IL-6),
interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of
breast cancer patients. Rocz Akad Med Bialymst. 48:82–84. 2003.
|
|
5
|
Bachelot T, Ray-Coquard I, Menetrier-Caux
C, Rastkha M, Duc A and Blay JY: Prognostic value of serum levels
of interleukin 6 and of serum and plasma levels of vascular
endothelial growth factor in hormone-refractory metastatic breast
cancer patients. Br J Cancer. 88:1721–1726. 2003.
|
|
6
|
Ailles LE and Weissman IL: Cancer stem
cells in solid tumors. Curr Opin Biotechnol. 18:460–466. 2007.
|
|
7
|
Rosen JM and Jordan CT: The increasing
complexity of the cancer stem cell paradigm. Science.
324:1670–1673. 2009.
|
|
8
|
Shackleton M, Quintana E, Fearon ER and
Morrison SJ: Heterogeneity in cancer: cancer stem cells versus
clonal evolution. Cell. 138:822–829. 2009.
|
|
9
|
Al-Ejeh F, Smart CE, Morrison BJ, et al:
Breast cancer stem cells: treatment resistance and therapeutic
opportunities. Carcinogenesis. 32:650–658. 2011.
|
|
10
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003.
|
|
11
|
Sullivan NJ, Sasser AK, Axel AE, et al:
Interleukin-6 induces an epithelial-mesenchymal transition
phenotype in human breast cancer cells. Oncogene. 28:2940–2947.
2009.
|
|
12
|
Morel AP, Lievre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One.
3:e28882008.
|
|
13
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008.
|
|
14
|
Prevo R, Deutsch E, Sampson O, et al:
Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine
inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res.
68:5915–5923. 2008.
|
|
15
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating
cells to radiation. J Natl Cancer Inst. 98:1777–1785. 2006.
|
|
16
|
Van Phuc P, Nhan PL, Nhung TH, et al:
Downregulation of CD44 reduces doxorubicin resistance of CD44CD24
breast cancer cells. Onco Targets Ther. 4:71–78. 2011.
|
|
17
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005.
|
|
18
|
De Visser KE and Coussens LM: The
inflammatory tumor microenvironment and its impact on cancer
development. Contrib Microbiol. 13:118–137. 2006.
|
|
19
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001.
|
|
20
|
Li F, Tiede B, Massague J and Kang Y:
Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res.
17:3–14. 2007.
|
|
21
|
Yu F, Yao H, Zhu P, et al: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007.
|
|
22
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005.
|
|
23
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003.
|
|
24
|
Dontu G, Abdallah WM, Foley JM, et al: In
vitro propagation and transcriptional profiling of human mammary
stem/progenitor cells. Genes Dev. 17:1253–1270. 2003.
|
|
25
|
Ackland ML, Newgreen DF, Fridman M, et al:
Epidermal growth factor-induced epithelio-mesenchymal transition in
human breast carcinoma cells. Lab Invest. 83:435–448. 2003.
|
|
26
|
Strutz F, Zeisberg M, Ziyadeh FN, et al:
Role of basic fibroblast growth factor-2 in epithelial-mesenchymal
transformation. Kidney Int. 61:1714–1728. 2002.
|