Introduction
Colorectal cancer is the fourth most common cancer
in men and the third in women worldwide (1). In countries of Latin America, Asia
and Africa, previously exhibiting a low frequency of colorectal
cancer, incidence is now rapidly increasing (2), probably due to a rising prevalence of
obesity in combination with decreased physical activity and longer
life-span (3).
The insulin-like growth factor-1 receptor (IGF-1R)
is a transmembrane tyrosine kinase receptor composed of two α
subunits and two β subunits. As the ligand (mainly IGF-1 or IGF-2)
binds to the IGF-1R α-subunit, tyrosine residues in the
intracellular part of the membrane-bound β-subunit become
autophosphorylated (4). Subsequent
phosphorylation of a chain of intracellular proteins then enables
the activation of the phosphatidylinositol 3-kinase (PI3K)/AKT and
the mitogen-activated protein kinase (MAPK) pathways leading to
proliferation, cell survival and differentiation (5). Several studies have demonstrated
increased expression of the IGF-1R in different cancers (6–8),
where it facilitates anchorage-independent growth, migration and
chemoresistance (9). The
cyclolignan picropodophyllin (PPP) has been launched as a potent
and selective inhibitor of IGF-1R inhibiting malignant cell growth
with low or no toxicity on normal cells (10–14).
In preclinical models, PPP exhibits anti-tumor activity in several
malignancies, e.g., multiple myeloma, uveal melanoma and
glioblastoma (11,14,15)
and is currently tested in an open label combined phase I/II
clinical study against advanced, solid tumors which have progressed
despite several lines of treatment. Encouragingly, stabilized
disease has been demonstrated in four PPP-treated squamous
non-small cell lung cancer patients (16).
The prognosis of colon cancer after curative-aiming
surgery depends almost completely on the presence of metastases, in
particular liver metastases (17).
The IGF axis seems to play a critical role for the development of
these, since colon cancer cells manipulated to express a
dominant-negative IGF-1R failed to produce liver metastases
although the malignant cells were injected directly into the organ
(18). Moreover, increased serum
level of IGF-1 in colon cancer patients was demonstrated to
correlate with more severe disease (19).
Matrix metalloproteinases (MMPs) play important
roles in the process of cancer invasion and metastasis by their
capacity to process growth factors, growth factor binding proteins,
cell surface proteins and degrade extracellular matrix (ECM)
components (20). Mainly MMP-2
(21,22), -7 (23) and -9 (24) have been implicated in colon cancer,
where MMP-2, together with MMP-3 and -11, have been suggested to
facilitate late-stage tumor invasion and metastasis (21). MMP-7 is widely expressed in colon
adenocarcinomas, but has also been proposed to play a role in the
early events of tumor progression since low levels have been
detected in 50% of benign adenomas (21). Additionally, MMP-7 expression seems
to correlate with the metastatic potential of colon cancer cells
(23). MMP-9 has been demonstrated
in neutrophils and macrophages of the tumor tissue, whereas the
tumor cells themselves were negative, suggesting that MMP-9 might
be an important part of the inflammatory response elicited by the
malignant cells (24).
In this study, we demonstrate a significant
overexpression of IGF-1R, IGF-2 and MMP-7 mRNA in colorectal cancer
tissues. The treatment of colon cancer cell lines with the IGF-1R
inhibitor PPP strongly impaired proliferation and survival, and
also negatively affected cell attachment and migration. However, a
net decrease of cells seemed to occur only when downregulation of
IGF-1R (together with AKT and ERK) expression/phosphorylation was
achieved. In addition, PPP showed capability of inhibiting
expression of MMP-7 and -9, suggesting multiple targets for this
compound, which, together with its good tolerability in
vivo, might be favorable from a therapeutic point of view.
Patients and methods
Patient material
The study subjects comprised 48 histopathologically
confirmed colorectal cancer patients who underwent surgery at the
Second Affiliated Hospital of Dalian Medical University between
June 2005 and February 2006. Patients with previous chemotherapy
and radiation therapy, patients with diabetes or hyperthyroidism as
well as patients taking glucocorticoids were excluded from the
study, which was approved by the local ethics committee according
to the Declaration of Helsinki. After informed consent, paired
colorectal specimens of tumor and normal control tissues (>10 cm
from the tumor) were surgically obtained and placed in liquid
nitrogen within 20 min post-excision and processed as described
(25).
RNA isolation and semi-quantitative
RT-PCR analysis
Total RNA was extracted from 80–100 mg frozen cancer
or normal control tissue from colon/rectum using the TRIzol Reagent
(Invitrogen, San Diego, CA) according to instructions provided by
the manufacturer. The concentration and the purity of the isolated
RNA were analyzed by the spectrophotometer DU-640 (Beckman Coulter,
Brea, CA). RNA (1 μg) was used to produce 20 μl of cDNA with TaKaRa
RNA LA PCR™ Kit (TaKaRa Biotechnology, Dalian, China). PCR was
conducted with TaKaRa Ex Taq HS DNA polymerase in 50 μl reaction
volumes. Primers (TaKaRa Biotechnology) used were as follows: IGF-1
(sense 5′-GAAGGTGAAGATGCACACCA-3′, antisense 5′-AGC
GAGCTGACTTGGCAGGCTTGA-3′) with a product length of 299 bp, IGF-2
(sense 5′-GGAATCCCAATGGGGAAGT-3′, antisense
5′-TGGGTGGGTAGAGCAATCAGG-3′) with a product length of 488 bp,
IGF-1R (sense 5′-ACCCGGAGTACT TCAGAGCT-3′, antisense
5′-CACAGAAGCTTCGTTGAG AA-3′) with a product length of 229 bp, MMP-7
(sense 5′-GAG TGCCAGATGTTGCAGAA-3′, antisense 5′-TGGGGATCTC
CATTTCCATA-3′) with a product length of 463 bp and, as internal
control, β-actin (sense 5′-GCATGGAGTCCTGTGG CAT-3′, antisense
5′-CTAGAAGCATTTGCGGTGG-3′) with a product length of 326 bp. Using a
Thermocycler (Eppendorf, Hamburg, Germany) 35, 35, 35, 32 and 30
PCR cycles were carried out for analysis of IGF-1, IGF-2, IGF-1R,
MMP-7 and β-actin mRNA expression, respectively. Each cycle
consisted of 30-sec denaturation at 94°C, 40 sec at 63°C, 61°C or
57°C, 30-sec annealing at 59°C or 58°C for IGF-1, IGF-2, IGF-1R,
MMP-7 and β-actin primer, and 60-sec extension at 72°C. After
amplification, the products were electrophoresed in 2% agarose-1X
TAE gels containing 0.5 μg/ml ethidium bromide, the band
intensities and the relative ratio between target band and control
band were quantified by Labworks Analysis Software (UV, Upland,
CA).
Cell culture
The human colon adenocarcinoma cell lines HT-29,
HCT-116, DLD-1 and CaCO-2 were kindly provided by Professor Lars
Holmberg and Professor Klas Wiman at CCK, Karolinska Institutet.
The cell lines were routinely cultured in complete medium, i.e.,
DMEM (1 mM pyruvate and 1 g/l D-glucose) supplemented with 10%
fetal bovine serum, glutamine and antibiotics (Sigma, St. Louis,
MO) at 37°C in a humidified incubator with a 5% CO2
in-air atmosphere. All cell lines were confirmed
mycoplasma-negative by MycoAlert® (Lonza, Copenhagen,
Denmark).
Analysis of proliferation/viability and
surface IGF-1R expression
PPP, synthesized as described (10), was dissolved in DMSO at 10 mM and
prediluted in the solvent before addition to cell cultures, where
the final concentration of DMSO was always 0.1%.
Proliferation/viability was analyzed by using the resazurin assay
(14) and by counting using trypan
blue exclusion. Surface IGF-1R expression was quantified by flow
cytometry using phycoerythrin-conjugated anti-IGF-1Rα and isotype
control antibodies (BD Biosciences, San José, CA) as described
(14).
Cell migration analysis
Cell migration was assessed by using the scratch
assay (26), where near-confluent
cell monolayers were scratched with a plastic micropipette tip,
carefully rinsed with PBS and then treated with IGF-1 (R&D
Systems, Abingdon, UK) or PPP in complete medium. The degree of
closure was photographed at 24 and 48 h through a Plan Fluor
10x/0.30 objective lens (Nikon Instruments Europe BV, Amstelveen,
The Netherlands).
Western blotting
Subconfluent layers of the cell lines were treated
with 0.5 μM PPP for different times in complete medium followed by
5-min IGF-1 stimulation before wash in cold PBS and lysis on ice
using modified RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1
mM NaF, 1 mM EDTA, 1% Igepal CA-630, 0.25% sodium deoxycholate),
centrifugation at 14,000 × g for 10 min at 4°C and immunoblotting.
Primary antibodies were specific for phospho-IGF-1R
(Tyr1135/Tyr1136) (R&D Systems), IGF-1Rβ (C20), GAPDH (Santa
Cruz Biotechnology, Santa Cruz, CA), phospho-AKT (Ser473),
total-AKT, ERK1/2 (Thr202/Tyr204), total-ERK1/2, MMP-2, MMP-7 and
MMP-9 (Cell Signaling Technology, Danvers, MA).
Statistical analysis
The mRNA data were analyzed statistically by SPSS
software version 10.0 (SPSS, Chicago, IL). Student's t-test was
used for the analysis of differences in mRNA expression, where
P<0.05 were considered statistically significant.
Results
IGF-1R, IGF-2 and MMP-7 mRNA are
overexpressed in colorectal cancer tissues
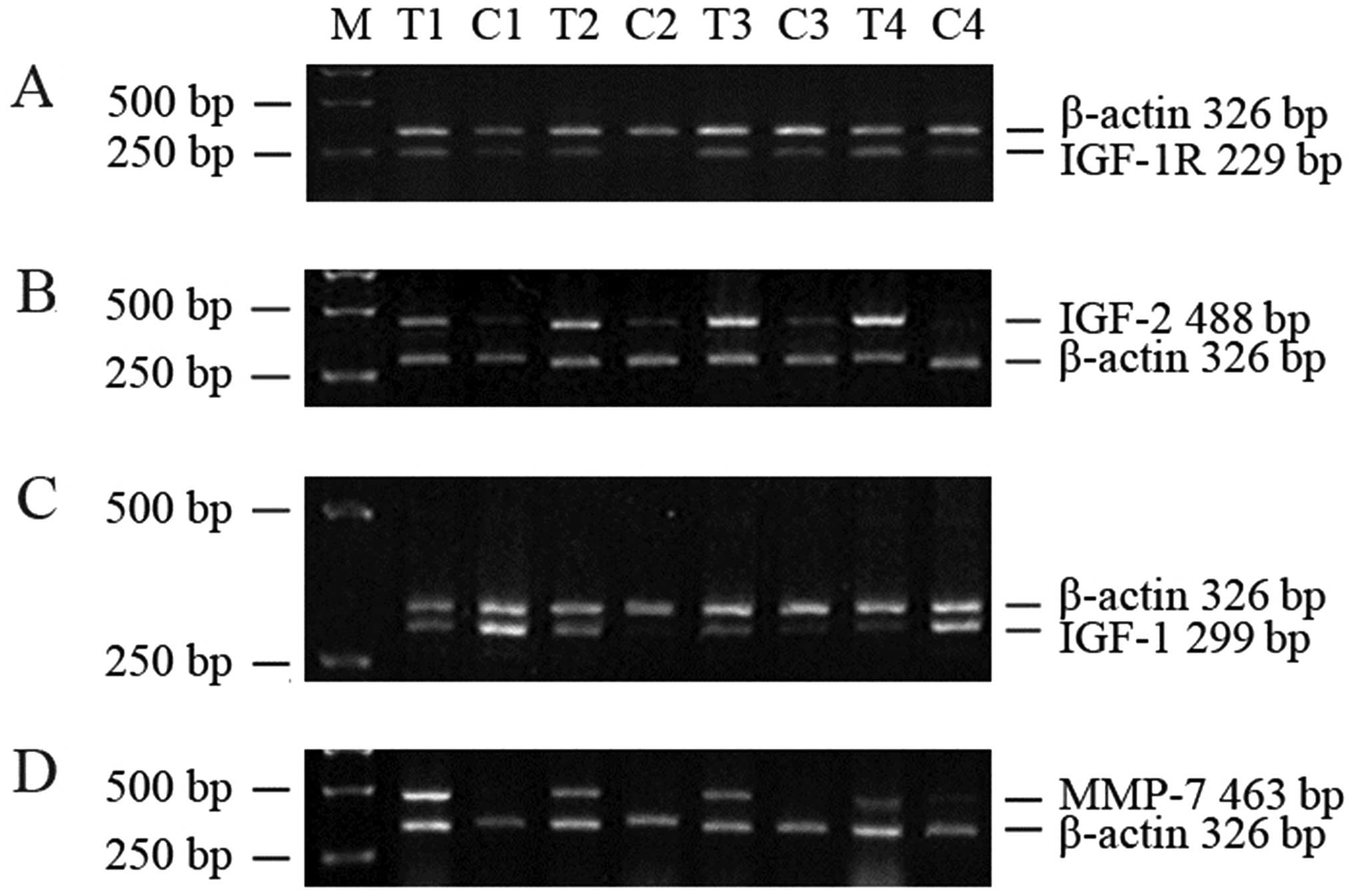
Tumor IGF-1R and IGF-2 mRNA expression were clearly
elevated in 48 paired samples (Table
I) as demonstrated in tissues from four representative patients
(Fig. 1A and B). However, there
was no significant difference in IGF-1 mRNA expression between
tumor and normal control tissue (Table
1 and Fig. 1C). MMP-7 mRNA was
present in tumor tissues, but could not be detected in normal
control tissues (Fig. 1D).
Analysis of the relationship between MMP-7 mRNA expression and
histopathological parameters demonstrated a significant correlation
with depth of infiltration and lymph node metastasis (Table II). No correlation was found
between MMP-7 and tumor size.
 | Table IIGF-1R, IGF-2 and IGF-1 mRNA
expression in tumor (T) and normal control (C) tissue of 48
colorectal carcinoma patients.a |
Table I
IGF-1R, IGF-2 and IGF-1 mRNA
expression in tumor (T) and normal control (C) tissue of 48
colorectal carcinoma patients.a
| mRNA | T (mRNA/β-actin) | C (mRNA/β-actin) | P-value |
|---|
| IGF-1R | 0.607±0.327 | 0.333±0.22 | 0.027 |
| IGF-2 | 0.979±0.436 | 0.293±0.261 | 0.000 |
| IGF-1 | 0.529±0.546 | 0.329±0.194 | 0.246 |
 | Table IIMMP-7 mRNA expression in tumor tissue
and histopathological characteristics of 48 colorectal carcinoma
patients.a |
Table II
MMP-7 mRNA expression in tumor tissue
and histopathological characteristics of 48 colorectal carcinoma
patients.a
| Parameter | No. of cases | MMP-7
mRNA/β-actin | P-value |
|---|
| Serosal
involvement |
| With | 29 | 1.310±0.402 | |
| Without | 19 | 0.872±0.376 | 0.030 |
| Lymph node
metastasis |
| With | 27 | 1.390±0.381 | |
| Without | 21 | 0.942±0.376 | 0.010 |
| Tumor size
(cm) |
| ≥5 | 31 | 1.332±0.361 | |
| <5 | 17 | 1.019±0.469 | 0.190 |
PPP reduces proliferation and survival in
human colon cancer cell lines
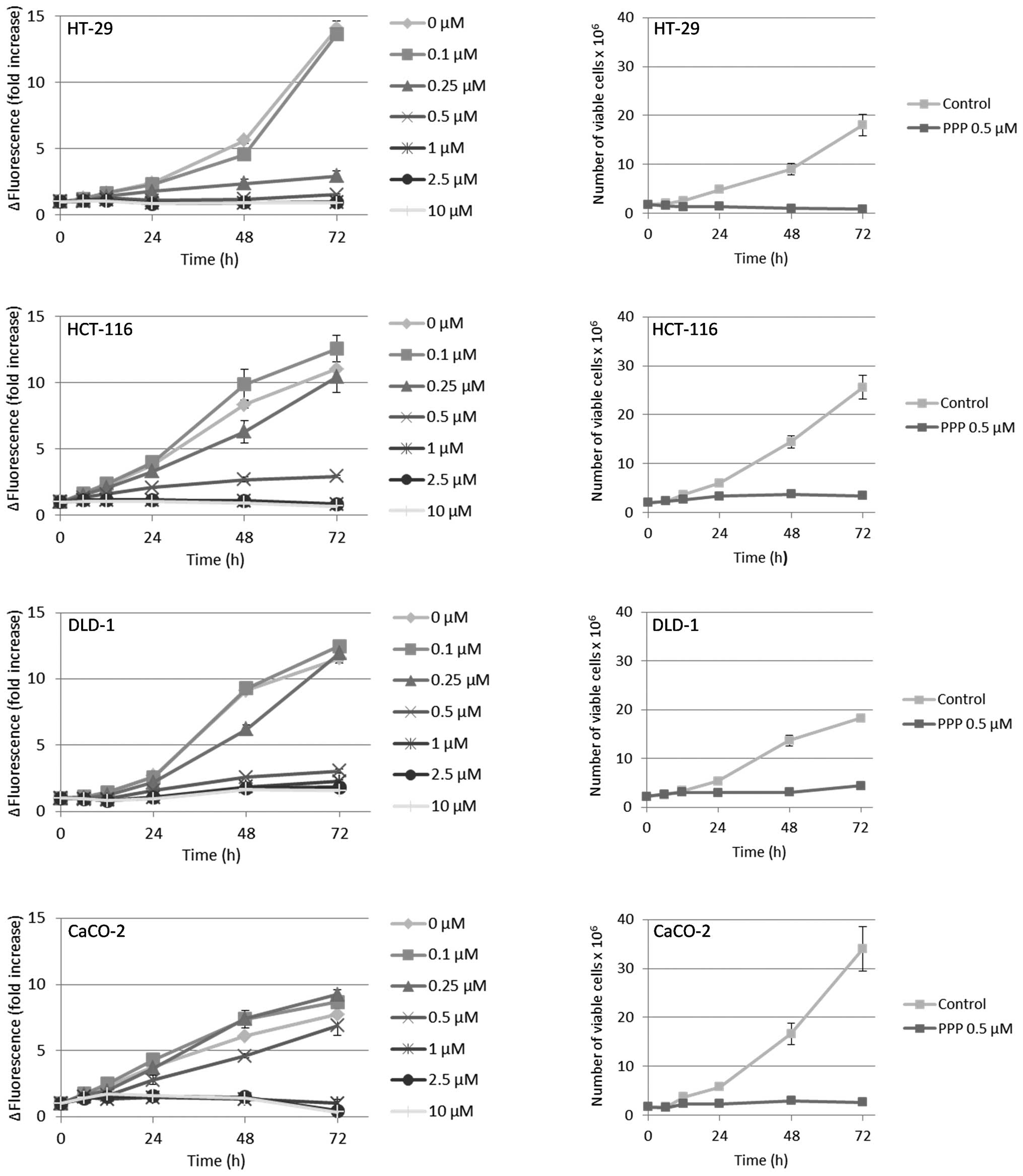
The results from the resazurin assay indicated that
all cell lines except the DLD-1, when compared with the
fluorescence at the beginning of the experiment (0 h), exhibited a
net reduction of viable cells when treated with PPP at
concentrations ≥1 μM (Fig. 2, left
panel). However, since the resazurin assay only provides a relative
estimation of viable cell number, we also counted cells by trypan
blue exclusion, then using the intermediate PPP concentration 0.5
μM. Furthermore, adherent and suspension cells were collected and
counted separately. In these experiments, only the HT-29 cell line
exhibited a net reduction of viable cells as compared with the cell
number at the beginning of the experiment (Fig. 2, right panel). The HCT-116, DLD-1
and CaCO-2 cell lines continued to proliferate, although at a lower
rate, reaching a plateau at 48–72 h. Moreover, the HT-29, HCT-116
and DLD-1 cell lines demonstrated a substantial detachment of
viable cells in response to PPP, an effect that occurred already at
6–12 h (Fig. 3, left panel) and in
parallel with reduced proliferation (Fig. 2, right panel). The vast majority of
the detached, viable cells were accumulated in the
G2/M-phase of the cell cycle (data not shown). With the
exception of the CaCO-2 cell line, there was a general,
time-dependent decline in cell viability, an effect that was
exclusively due to dying suspension cells (Fig. 3, right panel). However, cells that
despite PPP-treatment remained attached retained high viability
(Fig. 3, right panel). The degree
of growth inhibition induced by 0.5 μM PPP (Fig. 2, right panel) exhibited a linear
correlation to the cell line population doubling times (Table III) of HCT-116, DLD-1 and CaCO-2
(r2=0.94). The expression of surface IGF-1R was highly
variable, i.e. CaCO-2 cells expressed nearly ten times more IGF-1R
than the DLD-1 cells, whereas the HT-29 and HCT-116 cell lines
showed intermediate levels (Table
III).
 | Table IIIIGF-1R expression and population
doubling times of colon carcinoma cell lines.a |
Table III
IGF-1R expression and population
doubling times of colon carcinoma cell lines.a
| HT-29 | HCT-116 | DLD-1 | CaCO-2 |
|---|
| Population doubling
time (h) | 37–45 | 30–32 | 36–42 | 22–24 |
| Surface IGF-1Rα
expression (RFI) | 11.1±1.3 | 5.7±0.3 | 3.3±0.6 | 30.0±5.9 |
PPP inhibits cell migration
We then examined the effects of PPP on migration by
using the scratch assay (26). As
compared with control and IGF-1 treated cells, scratch closure was
clearly reduced by PPP in a dose-dependent manner in all four cell
lines (Fig. 3), this effect being
least pronounced in the CaCO-2 cells.
PPP downregulates
phosphorylation/expression of IGF-1R, AKT and ERK in HT-29
cells
Next, the effects of PPP on IGF-1R, AKT and ERK and
their phosphorylated forms were examined. As expected, treatment of
HT-29 and HCT-116 cells with IGF-1 increased phosphorylation of the
IGF-1R, AKT and ERK (Fig. 4A).
This was also true for the DLD-1 and CaCO-2 cell lines (data not
shown). Pretreatment of HT-29 cells with 0.5 μM PPP decreased the
IGF-1-induced phosphorylation of IGF-1R, AKT and ERK, effects that
were time-dependent starting at 8 h for IGF-1R and AKT and at 12 h
for ERK (Fig. 4A, left panel). In
HCT-116 cells, only AKT phosphorylation was reduced (Fig. 4A, right panel). Moreover, this
effect occurred at a later time-point (12 h) than in the HT-29
cells. Total-IGF-1R showed a marked, time-dependent downregulation
in the HT-29 cell line beginning already at 4-h treatment with PPP.
Such a response could not be detected in the HCT-116 cell line
(Fig. 4A). In addition, total-AKT
and total-ERK was downregulated in the HT-29 cells at 12 and 24 h,
effects that could not be clearly established in the HCT-116 cell
line (Fig. 4A). The DLD-1 and
CaCO-2 cells showed no downregulation of phosphorylated or total
forms of IGF-1R, AKT and ERK in response to PPP (data not
shown).
PPP reduces the expression of MMP-7 and
MMP-9
Since MMP-2, -7 and -9 have been associated with
different aspects of colon cancer (21–24),
we assessed whether IGF-1 and PPP could affect their protein
expression in the HT-29 cell line. IGF-1 increased the expression
of MMP-7, whereas pretreatment with 0.5 μM PPP for 8 h inhibited
this effect (Fig. 4B). IGF-1 was
unable to affect the expression of MMP-9, however, when combined
with PPP, MMP-9 was downregulated. MMP-2 expression showed no
regulation in response to IGF-1 alone or in combination with PPP
(Fig. 4B).
Discussion
Accumulated evidence during the last decades
indicate a pivotal role for the IGF-1R signaling in cancer cells,
suggesting this receptor to be a promising molecular target in
cancer treatment (27). However,
very few attempts have been made to interfere with its function in
colon cancer (18,28). Therefore, in parallel to analyses
of IGF-1R, IGF-1/2 and MMP-7 mRNA expression in tissue from
colorectal cancer patients, we assessed the effects of the
IGF-1R-inhibitory compound PPP in four colon carcinoma cell
lines.
Tumor tissue extracted from 48 colon cancer patients
significantly overexpressed IGF-1R and IGF-2 mRNA, but not IGF-1
mRNA. These findings are basically in accordance with previous
studies (29–32), thus strongly emphasizing the
potential impact of the IGF axis in the development and progression
of colon cancer. The expression of MMP-7 mRNA in all colon tumors
and its correlation with depth of invasion/serosal involvement and
lymph node metastasis were as well confirmatory (21,23).
Besides that elevated MMP-7 expression seems to correlate with the
metastatic potential of colon cancer, it should be noted that the
capability of this MMP to locally increase the IGF-1/2
bioavailability by cleavage of IGF-1 binding protein(s) (32) might contribute to increased
proliferation/survival and metastatic potential of the malignant
cells.
Multiple approaches have been utilized to block
IGF-1R signaling in vitro and in vivo. We used the
small molecule inhibitor PPP, which inhibited
proliferation/survival of the four colon cancer cell lines in a
dose-dependent manner as determined by the resazurin assay.
However, the DLD-1 cell line was the only one that did not exhibit
a net decrease of relative number of viable cells although it was
treated with 10 μM PPP as analyzed by the resazurin assay. In
contrast, the other cell lines showed diminished relative cell
number when treated with ≥1 μM PPP. A more detailed investigation
by manual cell counting, here exposing the cells to 0.5 μM PPP
revealed that all cell lines except the HT-29, despite
PPP-treatment, continued to slowly proliferate until they reached a
plateau. The HT-29 cell line instead showed a net decrease of
viable cells as compared with the cell number at the beginning of
the experiment. This finding is also supported by the linear
correlation between the population doubling times and PPP-mediated
growth inhibition in the colon cancer cell lines that could be
established only when the HT-29 cell line was omitted.
Interestingly, only in this cell line 0.5 μM PPP was able to
downregulate the IGF-1R, which would fit with the hypothesis that
the IGF-1R has to be downregulated/degraded for extensive tumor
cell kill (33,34). Downregulation of IGF-1R in the
HT-29 cell line seemed to precede PPP-mediated inhibition of
IGF-1-induced phosphorylation of IGF-1R and AKT/ERK. Although these
findings differ from some other investigations (10,35),
a similar PPP-induced downregulation of both IGF-1R/AKT and their
phosphorylated forms has been shown in a multi-drug resistant
osteosarcoma cell line (36). The
relatively late downregulation of ERK1/2 and its phosphorylated
form in the HT-29 cell line, however, represents a novel finding as
compared with published results obtained using other cell lines
(10,34). A similar, more or less simultaneous
downregulation of various intracellular signaling proteins along
with their phosphorylated forms has also been observed in multiple
myeloma cell lines after 48 h of exposure to PPP (unpublished
data). However, the explanation behind this phenomenon and its
potential clinical relevance for anti-cancer treatment remains
elusive.
Inhibition of proliferation in the colon carcinoma
cell lines was detected already at 6–12 h of PPP-incubation and
paralleled detachment of viable cells in the cell lines. In
contrast, PPP-induced cell death occurred later (~24 h) and
exclusively affected the detached cell population. Thus, the CaCO-2
cells, exhibiting only small amounts of detached cells, showed
pronounced decrease in proliferation but negligible cell death when
exposed to 0.5 μM PPP. Possibly, the high expression of IGF-1R in
this cell line might provide survival advantage(s) as suggested for
glioblastoma cell lines (14).
Using the scratch assay (26), we investigated colon cancer cell
migration during PPP-treatment. Migration was dose-dependently
inhibited, suggesting that PPP might reduce invasion as well as the
process of metastasis of colon cancer cells. Since this effect,
which has not been reported previously, was evident in all cell
lines, we propose mechanism(s) unrelated to signaling via
IGF-1R/AKT/ERK as responsible. In this context, it is interesting
to note that we could not detect PPP-mediated inhibition of MMP-2
expression as previously shown in uveal melanoma cell lines
(15). Instead, we demonstrated
downregulation of MMP-7 and -9.
In conclusion, PPP strongly decreases proliferation,
survival and migration and also promotes detachment in colon cancer
cell lines. Although a net reduction of tumor cells seems to
correlate with PPP-induced downregulation of IGF-1R, AKT and ERK,
the effects described above, including the downregulation of MMP-7
and -9, may partly be independent of IGF-1R inhibition. Further
investigations are required to disclose such, possibly IGF-1R
unrelated mechanism(s). However, the multiple inhibitory effects of
PPP in colon carcinoma cells combined with the promising results
from the open label combined phase I/II clinical study against
advanced solid tumors (16),
suggest a rationale for the therapeutic use of PPP in colorectal
carcinoma.
Acknowledgements
This work was supported by Swedish Cancer
Foundation, Swedish Research Council, European Commission Marie
Curie Fellowship (EA), Cancer Society in Stockholm, Children Cancer
Society, Lundberg's Research Foundation in Gothenburg, Stockholm
County Council and Karolinska Institutet.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Sandhu MS, Dunger DB and Giovannucci EL:
Insulin, insulin-like growth factor-I (IGF-I), IGF binding
proteins, their biologic interactions, and colorectal cancer. J
Natl Cancer Inst. 94:972–980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ullrich A, Gray A, Tam AW, et al:
Insulin-like growth factor I receptor primary structure: comparison
with insulin receptor suggests structural determinants that define
functional specificity. EMBO J. 5:2503–2512. 1986.
|
|
5
|
Navarro M and Baserga R: Limited
redundancy of survival signals from the type 1 insulin-like growth
factor receptor. Endocrinology. 142:1073–1081. 2001.PubMed/NCBI
|
|
6
|
Belfiore A, Pandini G, Vella V, Squatrito
S and Vigneri R: Insulin/IGF-I hybrid receptors play a major role
in IGF-I signaling in thyroid cancer. Biochimie. 81:403–407. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
All-Ericsson C, Girnita L, Seregard S,
Bartolazzi A, Jager MJ and Larsson O: Insulin-like growth factor-1
receptor in uveal melanoma: a predictor for metastatic disease and
a potential therapeutic target. Invest Ophthalmol Vis Sci. 43:1–8.
2002.PubMed/NCBI
|
|
8
|
Xie Y, Skytting B, Nilsson G, Brodin B and
Larsson O: Expression of insulin-like growth factor-1 receptor in
synovial sarcoma: association with an aggressive phenotype. Cancer
Res. 59:3588–3591. 1999.PubMed/NCBI
|
|
9
|
Sachdev D and Yee D: Disrupting
insulin-like growth factor signaling as a potential cancer therapy.
Mol Cancer Ther. 6:1–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Girnita A, Girnita L, del Prete F,
Bartolazzi A, Larsson O and Axelson M: Cyclolignans as inhibitors
of the insulin-like growth factor-1 receptor and malignant cell
growth. Cancer Res. 64:236–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stromberg T, Ekman S, Girnita L, et al:
IGF-1 receptor tyrosine kinase inhibition by the cyclolignan PPP
induces G2/M-phase accumulation and apoptosis in multiple myeloma
cells. Blood. 107:669–678. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Menu E, Jernberg-Wiklund H, Stromberg T,
et al: Inhibiting the IGF-1 receptor tyrosine kinase with the
cyclolignan PPP: an in vitro and in vivo study in the 5T33MM mouse
model. Blood. 107:655–660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menu E, Jernberg-Wiklund H, De Raeve H, et
al: Targeting the IGF-1R using picropodophyllin in the
therapeutical 5T2MM mouse model of multiple myeloma: beneficial
effects on tumor growth, angiogenesis, bone disease and survival.
Int J Cancer. 121:1857–1861. 2007. View Article : Google Scholar
|
|
14
|
Yin S, Girnita A, Stromberg T, et al:
Targeting the insulin-like growth factor-1 receptor by
picropodophyllin as a treatment option for glioblastoma.
Neurooncology. 12:19–27. 2010.PubMed/NCBI
|
|
15
|
Girnita A, All-Ericsson C, Economou MA, et
al: The insulin-like growth factor-I receptor inhibitor
picropodophyllin causes tumor regression and attenuates mechanisms
involved in invasion of uveal melanoma cells. Clin Cancer Res.
12:1383–1391. 2006. View Article : Google Scholar
|
|
16
|
Ekman S, Frodin JE, Harmenberg J, et al:
Clinical phase I study with an Insulin-like growth factor-1
receptor inhibitor: experiences in patients with squamous non-small
cell lung carcinoma. Acta Oncol. 50:441–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Midgley R and Kerr D: Colorectal cancer.
Lancet. 353:391–399. 1999. View Article : Google Scholar
|
|
18
|
Reinmuth N, Fan F, Liu W, et al: Impact of
insulin-like growth factor receptor-I function on angiogenesis,
growth, and metastasis of colon cancer. Lab Invest. 82:1377–1389.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Yakar S, Zhao L, Hennighausen L and
LeRoith D: Circulating insulin-like growth factor-I levels regulate
colon cancer growth and metastasis. Cancer Res. 62:1030–1035.
2002.PubMed/NCBI
|
|
20
|
Sounni NE and Noel A: Membrane type-matrix
metalloproteinases and tumor progression. Biochimie. 87:329–342.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newell KJ, Witty JP, Rodgers WH and
Matrisian LM: Expression and localization of matrix-degrading
metalloproteinases during colorectal tumorigenesis. Mol Carcinog.
10:199–206. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barozzi C, Ravaioli M, D'Errico A, et al:
Relevance of biologic markers in colorectal carcinoma: a
comparative study of a broad panel. Cancer. 94:647–657. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adachi Y, Yamamoto H, Itoh F, Hinoda Y,
Okada Y and Imai K: Contribution of matrilysin (MMP-7) to the
metastatic pathway of human colorectal cancers. Gut. 45:252–258.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nielsen BS, Timshel S, Kjeldsen L, et al:
92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and
macrophages but not in malignant epithelial cells in human colon
cancer. Int J Cancer. 65:57–62. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horiuchi S, Yamamoto H, Min Y, Adachi Y,
Itoh F and Imai K: Association of ets-related transcriptional
factor E1AF expression with tumour progression and overexpression
of MMP-1 and matrilysin in human colorectal cancer. J Pathol.
200:568–576. 2003. View Article : Google Scholar
|
|
26
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buck E and Mulvihill M: Small molecule
inhibitors of the IGF-1R/IR axis for the treatment of cancer.
Expert Opin Investig Drugs. 20:605–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reinmuth N, Liu W, Fan F, et al: Blockade
of insulin-like growth factor I receptor function inhibits growth
and angiogenesis of colon cancer. Clin Cancer Res. 8:3259–3269.
2002.PubMed/NCBI
|
|
29
|
Hakam A, Yeatman TJ, Lu L, et al:
Expression of insulin-like growth factor-1 receptor in human
colorectal cancer. Hum Pathol. 30:1128–1133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weber MM, Fottner C, Liu SB, Jung MC,
Engelhardt D and Baretton GB: Overexpression of the insulin-like
growth factor I receptor in human colon carcinomas. Cancer.
95:2086–2095. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peters G, Gongoll S, Langner C, et al:
IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and
predictive markers in colorectal-cancer. Virchows Arch.
443:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakamura M, Miyamoto S, Maeda H, et al:
Matrix metalloproteinase-7 degrades all insulin-like growth factor
binding proteins and facilitates insulin-like growth factor
bioavailability. Biochem Biophys Res Commun. 333:1011–1016. 2005.
View Article : Google Scholar
|
|
33
|
Baserga R: Targeting the IGF-1 receptor:
from rags to riches. Eur J Cancer. 40:2013–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vasilcanu R, Vasilcanu D, Sehat B, et al:
Insulin-like growth factor type-I receptor-dependent
phosphorylation of extracellular signal-regulated kinase 1/2 but
not Akt (protein kinase B) can be induced by picropodophyllin. Mol
Pharmacol. 73:930–939. 2008. View Article : Google Scholar
|
|
35
|
Vasilcanu R, Vasilcanu D, Rosengren L, et
al: Picropodophyllin induces downregulation of the insulin-like
growth factor 1 receptor: potential mechanistic involvement of Mdm2
and beta-arrestin1. Oncogene. 27:1629–1638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duan Z, Choy E, Harmon D, et al:
Insulin-like growth factor-I receptor tyrosine kinase inhibitor
cyclolignan picropodophyllin inhibits proliferation and induces
apoptosis in multidrug resistant osteosarcoma cell lines. Mol
Cancer Ther. 8:2122–2130. 2009. View Article : Google Scholar
|