Introduction
Aberrant glycosylation expressed in
glycosphingolipids and glycoproteins in tumor cells has been
implicated as an essential mechanism in defining stage and fate of
tumor progression. In ovarian cancer, this abnormality mainly
focuses on the type II sugar chain such as changes in type II H
antigen, Lewis Y (LeY) and LeX blood group antigen. LeY is mainly
expressed during embryogenesis and limits to epithelium and
granulocytes in adults under physiological conditions. However, LeY
is expressed in most epithelial cancers and elevated expression of
LeY has been found in 70–90% of the human carcinomas of epithelial
cell origin, including colon, lung, ovarian and breast cancer and
the elevated expression level is closely associated with poor
prognosis (1–4).
Previously, we transfected the ovarian cancer cell
line RMG-I with α1,2-fucosyltransferase (α1,2-FT) gene to obtain
stable transfectants, RMG-I-H, that highly express LeY (5,6). Our
studies showed that, compared with cells without transfection,
RMG-I-H cells have enhanced malignant behavior, a shorter cell
cycle, and increased resistance to 5-fluorouracil (5,7,8). In
addition, LeY mAb dramatically inhibits cell proliferation and cell
adhesion of RMG-I-H cells in vitro, and the size and weight
of tumors derived from RMG-I-H cells in vivo are reduced
significantly by preincubation of RMG-I-H cells with anti-LeY mAb
(8,9). All these suggest that LeY is involved
in the changes in biological behavior of the RMG-I cells.
TGF-β (transforming growth factor-β) belongs to the
TGF-β superfamily of growth factors and exerts a diverse range of
biological functions including differentiation, proliferation,
angiogenesis and immunosuppression. Wang et al (10) reported that α1,6-fucosyltransferase
gene (FUT8) deficient mice have dysregulation of TGF-β
receptor activation and downstream signaling and show
emphysema-like changes in the lung. By reintroducing FUT8,
the TGF-β receptor signaling abnormality was rescued. We found
previously that 88 genes were changed in RMG-I-H cells by gene chip
technique and the altered genes were involved in protein
phosphorylation, cell signaling and transcription. Among the genes
with modified expression, TGFBI (GenBank ID: BC000097) was
significantly up-regulated (11).
By immunohistochemical staining and Western blot analysis, we
examined the expression of TGF-β1 in nude mouse xenograft tumors
and found an increased expression in RMG-I-H cells (9). Because LeY is present on cell surface
and may modify the growth factor receptor (12,13),
we therefore hypothesized that LeY may be involved in the
regulation of TGF-β mediated cell growth as part of TGF-β receptors
(TβRs).
In the present investigation, TβRs was selected to
study the effects of α1,2-FT on its expression and LeY content.
Furthermore, Smad2/3, Smad7, Akt, ERK1/2 and MEK were analyzed as
the signaling molecules involved in TβRs signaling. Mock cells
transfected with the vector were used as controls. We report for
the first time that as an important part of TβRI and TβRII, LeY
antigen regulates Smad, ERK1/2 and PI3K pathways though TβRs to
participate in development of ovarian cancer.
Materials and methods
Cell lines and reagents
The human ovarian cancer cell line, RMG-I, which was
originated from the tissues of human ovarian clear cell carcinoma,
was donated by Professor M. Iwamori of Tokyo University of Japan.
RMG-I-H cell line was established as previously reported (6,7). The
RMG-I-C cells transfected with the vector alone were used as
controls.
Recombinant human TGF-β1 was from peprotech.
Anti-TGFβ RI, anti-TGFβ RII, anti-ERK1, anti-dually phosphorylated
ERK (Thr202/Tyr204), anti-MEK-1/2, anti-p-MEK-1/2, anti-Akt,
anti-p-Akt, anti-Smad7 antibody, horseradish peroxidase
(HRP)-labeled secondary antibodies and protein A/G Plus-Agarose
were from Santa Cruz Biotechnology. Anti-Smad2/3 and anti-p-Smad2/3
antibodies were from Abzoom. TRIzol, PrimeScript™ RT reagent kit,
SYBR® Premix Ex Taq™ and GAPDH primer (D3702) from
Takara. Mouse anti-human LeY mAb was produced by immunization of
female BALB/c weanling mice with LeY purified from a SK-LU-3 lung
cancer cell line. Antigen preparation was mixed with complete
Freunds adjuvant and mice were injected intraperitoneally with 0.2
ml of this preparation on Day 0 and intravenously on Day 21
(14). Harvested immune spleen
cells were fused with myeloma cells 4 days after the second
immunization to produced hybridomas as described previously
(15). When the cells had reached
50% confluency in the majority of wells showing cell growth, the
supernatants were collected and assayed by enzyme-linked
immunosorbent assay for reaction with synthetic LeY and
additionally with a panel of related A, B, H blood group antigens
and Lewis antigens (Isosep AB, Tullinge, Sweden and Dextra
Laboratories, Reading, UK) as described previously (16). The specific anti-LeY antibody was
purified from culture supernatants using conventional
ultrafiltration techniques. Protein concentration was determined
spectrophotometrically by absorption at 280 nm. Immunoglobulin
isotype was identified as of the IgM class by using a MonoAb-ID
immunoassay kit (Invitrogen) and electrophoretic analysis (14). The mouse mAb was suspended in
buffer containing 0.01 M phosphate-buffered saline, 0.1% sodium
azide and 1% bovine serum albumin.
Cell culture and treatment
The method for cell culture has been described
previously (6). For Western blot
assays, subconfluent cell layers were rendered quiescent by serum
starvation for 12–24 h. Cells were stimulated subsequently by
addition of medium containing or lacking TGF-β1 (5 ng/ml) for the
specified time period. For inhibition assay, LeY mAb (10 μg/ml) was
added for different times (1, 10 and 30 min) before stimulation
with TGF-β1.
Real-time PCR
Total RNA was extracted using trizol reagent. cDNA
was synthesized using Takara PrimeScript™ RT reagent Kit. Primers
used for amplification: TβRI (158 bp) forward,
AGTGTTCTGGCTCCAAATGGTAGT; reverse, GGCCCATGGGTATTCCAGTAATC. TβRII
(75 bp) forward, GCAGGTGGGAACTGCAAGAT; reverse, GAAGGACTCA
ACATTCTCCAAATTC. Reaction conditions were 37°C for 15 min, 85°C for
5 sec, 4°C for 5 min. The real-time PCR reaction conditions were
denature at 94°C for 20 sec, 45 cycles of 94°C for 20 sec and 60 or
58°C for 20 sec in a 20-μl reaction mixture containing
SYBR® Premix Ex TaqTM (2X) 10 μl, forward
primer (5 μmol/l) 1 μl, reverse primer (5 μmol/l) 1 μl, cDNA 2 μl,
dH2O 6 μl. GAPDH was used as the endogenous control. The
Light Cycler PCR and detection system (Roche Diagnostics, Mannheim,
Germany) was used for real-time PCR amplification and Ct value
calculation. Once the amplification was completed, the melting
curve was analyzed. The change of target gene expression level was
calculated using the 2−ΔΔCT method (17).
Western blot analysis
Cells were rinsed with PBS and 1% of Triton X-100
lysis buffer (20 mM Tris-HCl, pH 7.4, 10 mM EGTA, 10 mM
MgCl2, 1 mM benzamidine, 60 mM β-glycerophosphate, 1 mM
Na3VO4, 20 mM NaF, 2 μg/ml aprotinin, 5 μg/ml
leupeptin, 0.1 mM phenylmethylsulfonyl fluoride) was added. Then
centrifuged, and the supernatants were collected. Protein content
was measured using the protein assay BCA kit (Beyotime
Biotechnology, China) and equal amounts of protein were loaded on
SDS-PAGE gels. Subsequently, proteins were transferred to PVDF
membranes (Millipore, Beaford, MA) and were probed with antibodies
(1:1000). Immunoreactive bands were visualized by chemiluminescence
(ECL; Pierce) using a secondary antibodies (1:8000).
Membrane protein isolation and
immunoprecipitation
Membrane proteins were extracted and concentrated
with Mem-PER® eukaryote membrane protein extraction kit
and Pierce® SDS-PAGE Sample Prep Kit (Pierce, Rockford,
USA). Membrane proteins were then incubated at 4°C for 2 h with
TβRI or TβRII antibody. The immune complexes were isolated by
stirring the mixture at 4°C overnight with Protein A/G
Plus-Agarose. Thereafter, the samples were loaded onto 10% SDS-PAGE
for Western blotting with the procedure described above. The LeY
antibody (1:2000) was used to detect the expression of LeY in TβRI
and TβRII. TβRI and TβRII antibodies (1:1000) were used to detect
the expression of TβRI and TβRII, respectively.
Immunofluorescence-staining
procedure
Cells were fixed with 4% paraformaldehyde. After
blocking with normal goat serum, cells were incubated with LeY and
TβRI (or TβRII) antibodies (1:100) for 1 h at RT. Cells were then
incubated with goat anti-mouse tetramethylrhodamine isothiocyanate
(TRITC) conjugated antibody and goat anti-rabbit fluorescein
isothiocyanate (FITC) labeled antibody (1:200) (Zhongshan Biotech,
Beijing, China) for 1 h at RT in dark. 4,6-Diamidino-2-phenylindole
(DAPI) was used to stain the nuclei at RT for 1 min. Stained slide
was observed with a laser confocal microscope (C1-SI; Nikon, Tokyo,
Japan). Data were collected using a computer and the digital images
were generated.
Statistical analysis
The SPSS 12.0 statistical analysis software was
used, while the analysis of variance was employed. p<0.05 was
regarded as with statistical significance.
Results
TβRI expression does not change, while
TβRII expression is elevated
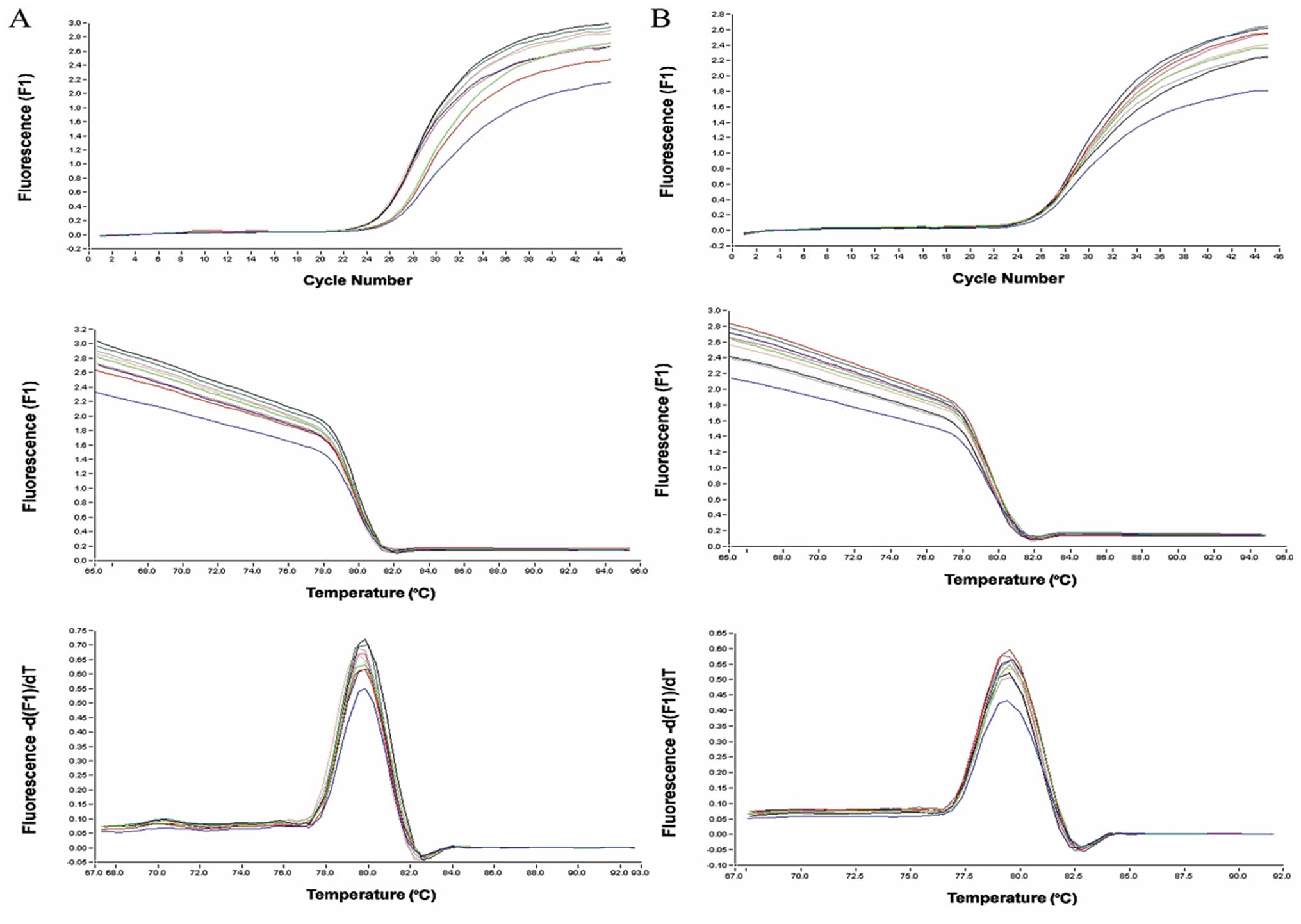
Cells were subjected to real-time PCR analyses to
assess the mRNA levels of TβRI and TβRII. The results show that the
TβRII mRNA levels in RMG-I-H cells were 1.69- and 1.74-fold
(>1.3-fold) higher than that in the RMG-I and RMG-I-C cells,
respectively. However, TβRI mRNA level in RMG-I-H cells was 1.03-
and 1.00-fold compared to that in the RMG-I and RMG-I-C cells,
respectively (Fig. 1).
Expression of LeY in TβRI and TβRII on
the cell membrane is elevated
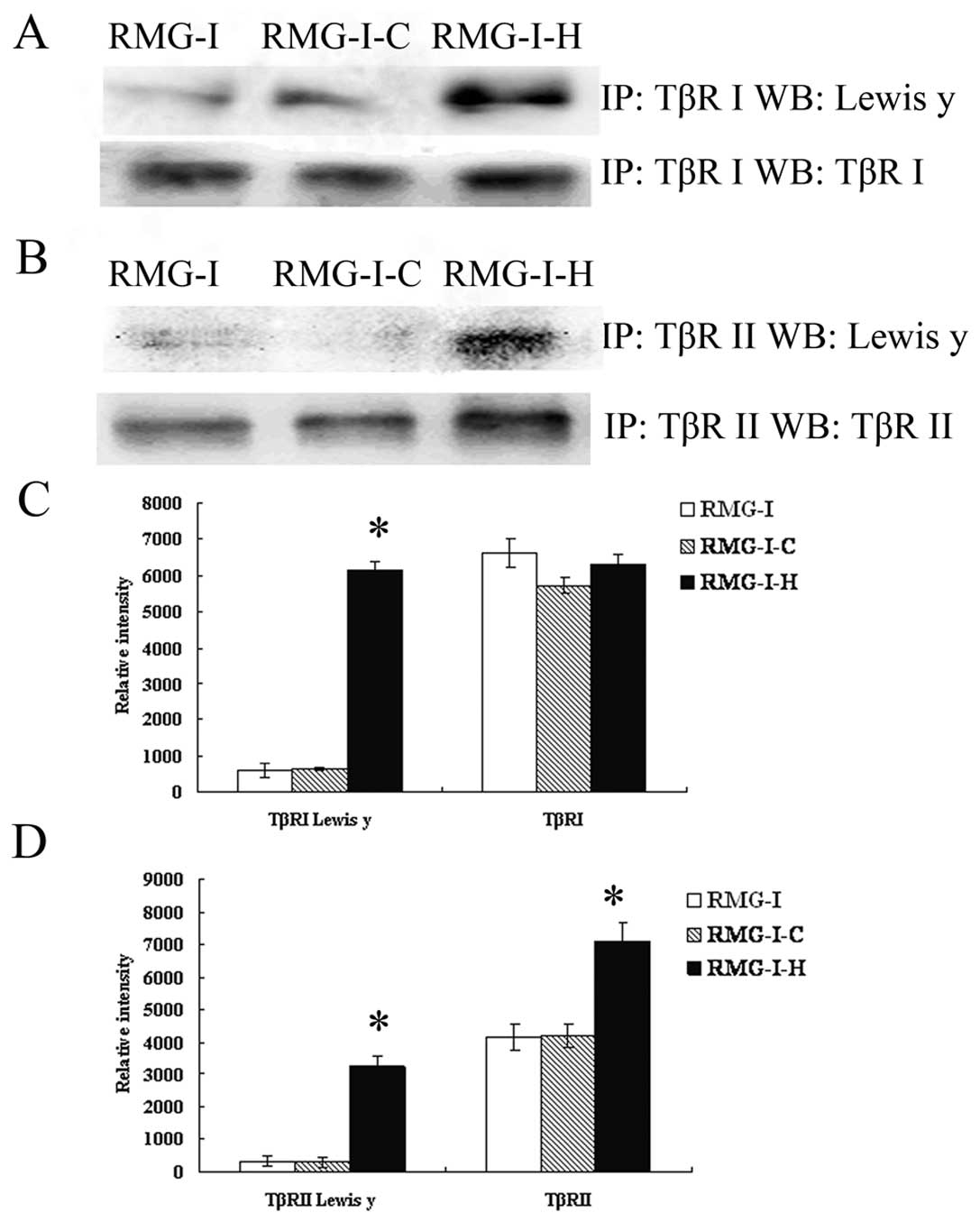
To estimate the expression of the LeY
oligosaccharide in TGF-β receptors, we performed a series of
immunoprecipitation experiments to determine whether LeY mAb would
bind to membrane extracts precipitated by TβRI and TβRII antibody.
After SDS-PAGE, followed by immunoblotting, anti-LeY mAb stained
the 53/70 kDa protein bands precipitated by TβRI and TβRII
antibody, respectively. The results showed that both TβRI and TβRII
contained LeY structures, and TβRI and TβRII showed absolute or
relative increase in the content of LeY in the RMG-I-H cells,
respectively (Fig. 2).
Co-localization of TGF-β receptors and
LeY on RMG-I-H cell surface
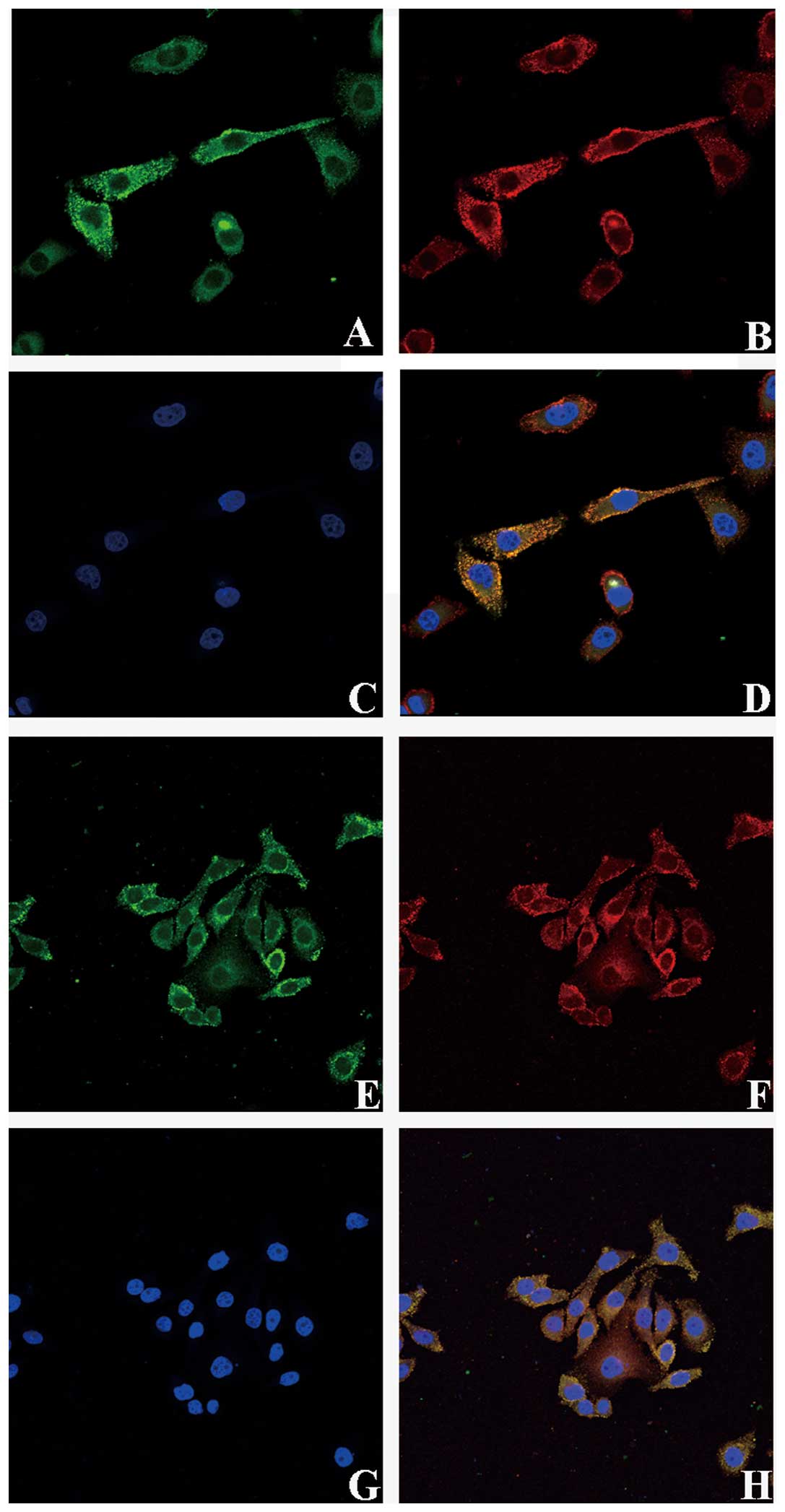
Immunoprecipitation assay showed that TβRI and TβRII
contain LeY structure, we verified the spatial orientation of TGF-β
receptors and LeY by immunofluorescence double staining. TβRI and
TβRII were labeled with FITC and LeY antigen was labeled with
TRITC. Images were scanned using a confocal microscope in serial
Z-sections and then overlaid. The results clearly showed that TβRI
and TβRII in green fluorescence were mainly localized on the cell
membrane with a small amount localized in the cytoplasm (Fig. 3A and E); LeY antigen in red
fluorescence was mainly on the cell membrane (Fig. 3B and F). As shown in the merged
figures, spatial co-localization of TGF-β receptors and LeY
exhibited yellow fluorescence (Fig. 3D
and H, white arrow).
LeY down-regulates TGF-β/Smad
pathways
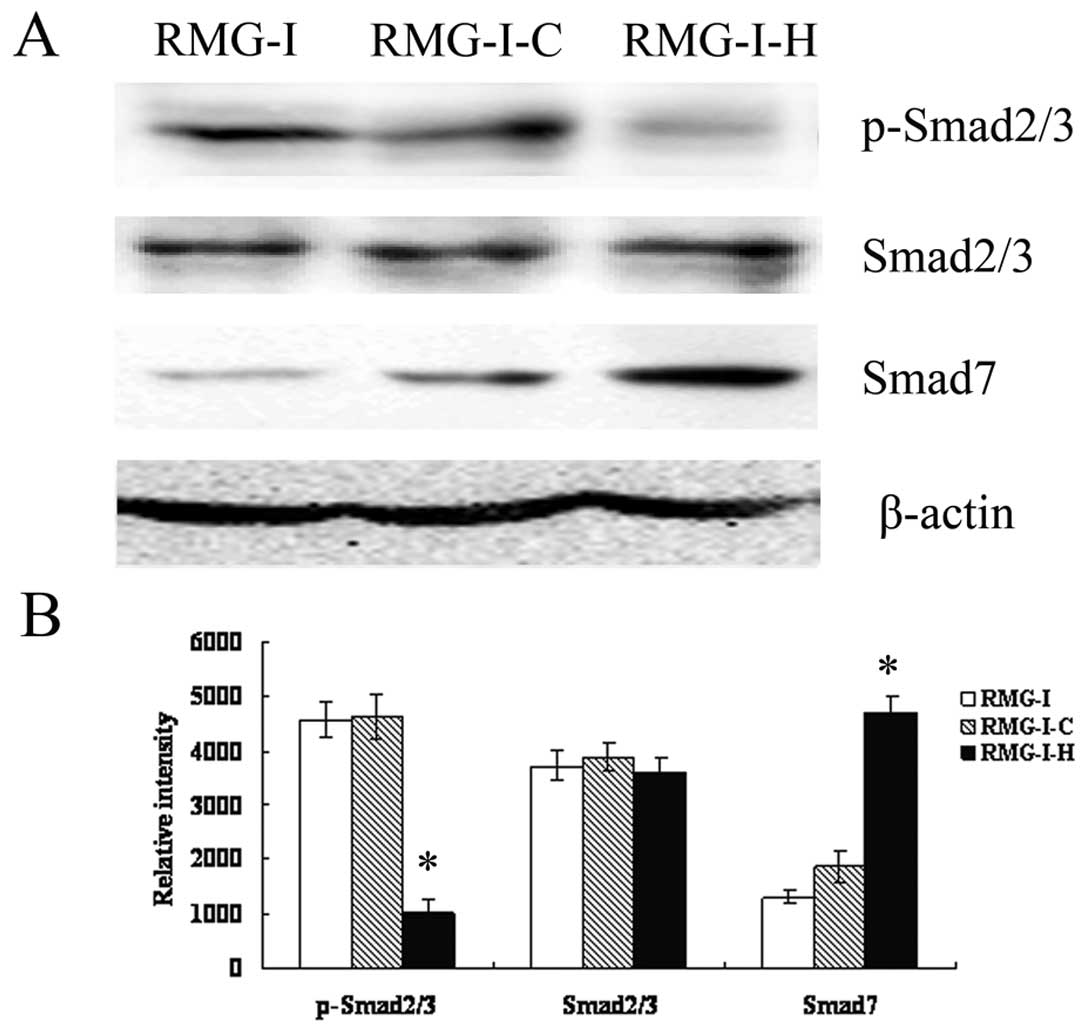
To further characterize the effect of LeY on
TGF-β/Smad pathway, Western blot analysis was performed to analyze
the expression of Smad2/3, p-Smad2/3 and Smad7 (inhibitory Smad),
which are key members of TGF-β1/Smad, in cells before and after
α1,2-FT gene transfection after TGF-β1 stimulation (5 ng/ml). The
results showed that total protein levels of Smad2/3 did not change
significantly (p>0.05), however, p-Smad2/3 level was
significantly decreased in RMG-I-H cells compared to RMG-I and
RMG-I-C cells. The expression of Smad7 was up-regulated in RMG-I-H
cells (Fig. 4). The results
indicated that LeY down-regulated the activation of TGF-β1/Smad
pathway.
LeY up-regulates TGF-β1-dependent ERK and
PI3K pathways
Given that ERK/MAPK and PI3K are two important
pathways that can also be activated by TGF-β in some cell types
(18–21), we first analyzed the expression of
ERK1/2 and Akt in TGF-β1 stimulated RMG-I-H cells following serum
starvation by Western blot analysis to determine whether TGF-β can
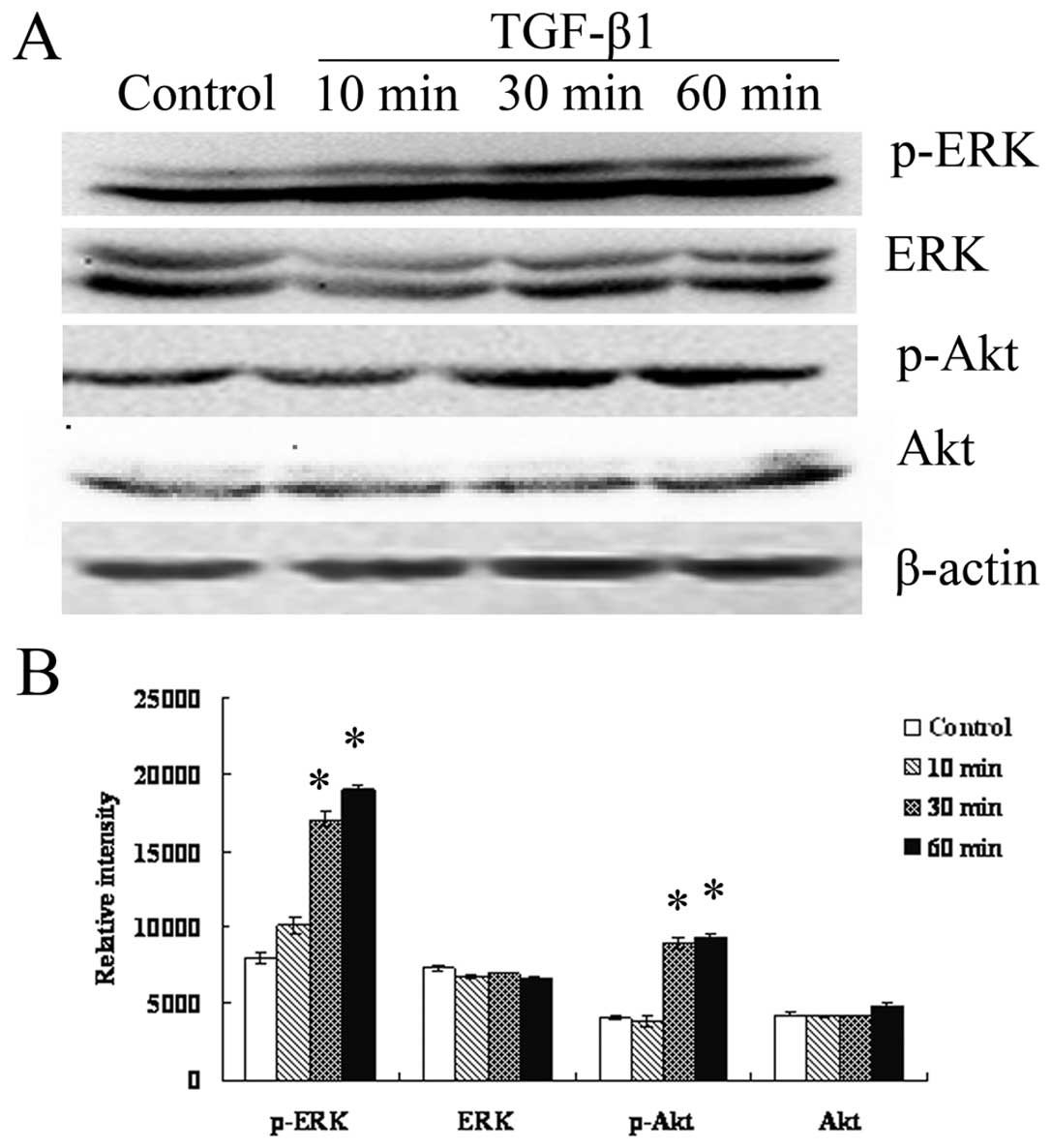
activate ERK/MAPK and PI3K in RMG-I-H cells. As shown in Fig. 5, expression of EKR1/2 and Akt did
not change (p>0.05), however, p-EKR1/2, p-Akt increased over
time, suggesting TGF-β1 indeed activate the ERK and PI3K pathways
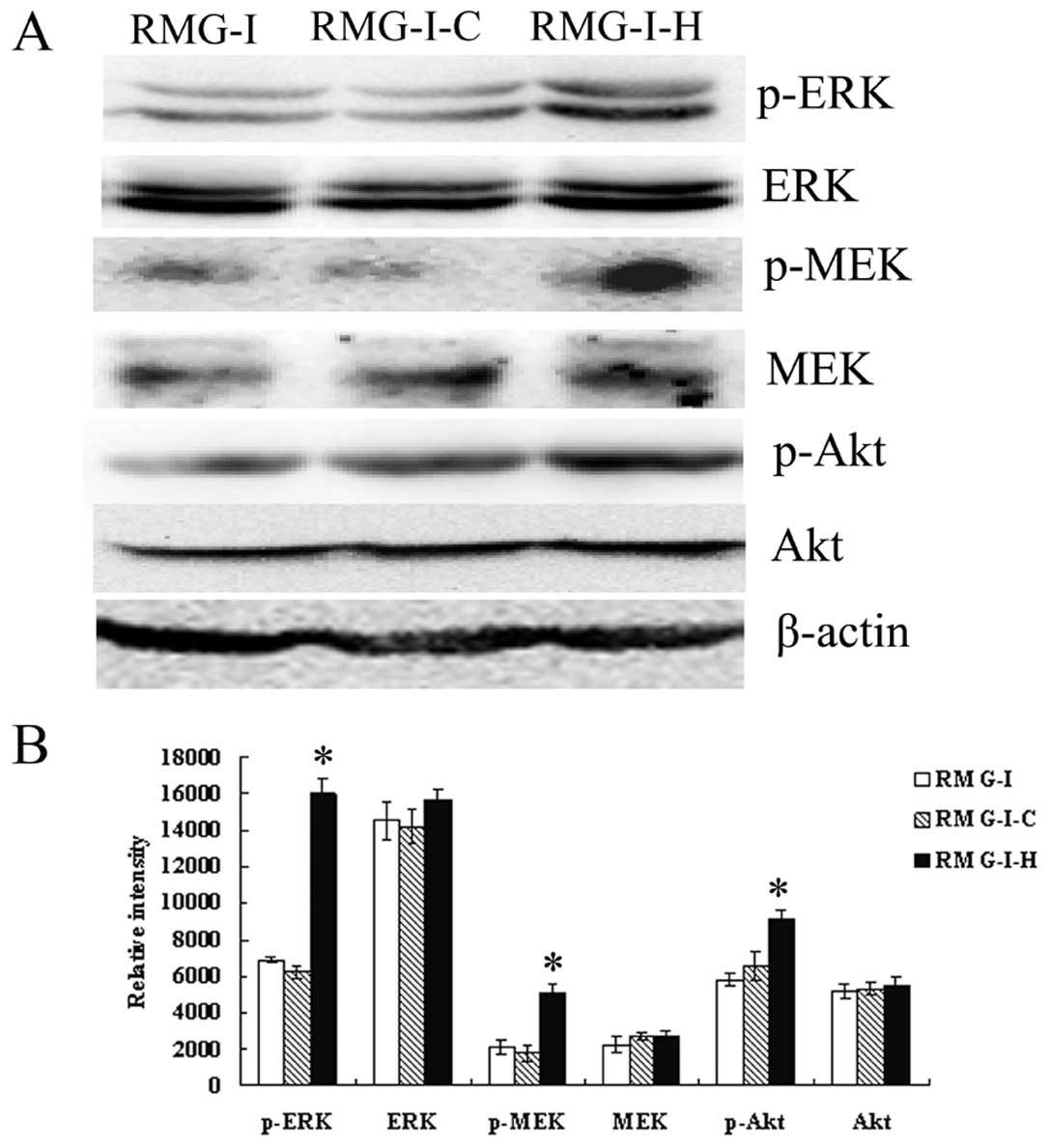
in RMG-I-H cells. Then we compared the expression of ERK1/2, MEK1/2
and Akt in cells before and after α1,2-FT gene transfection, the
results showed that the total protein levels of ERK1/2, MEK1/2 and
Akt did not change significantly among cells (p>0.05), but the
p-ERK1/2, p-MEK1/2 and p-Akt levels were significantly increased in
RMG-I-H cells than those in RMG-I and RMG-I-C cells (Fig. 6). These results demonstrate that
LeY up-regulated the activation of TGF-β1 mediated ERK and PI3K
pathways.
LeY mAb inhibits TGF-β1-dependent
activation of Smad, ERK and PI3K pathways
The expression of phosphorylated ERK1/2, Smad2/3 and
Akt in RMG-I-H cells at different time-points (1, 10 and 30 min)
after treatment with LeY mAb (10 μg/ml) was analyzed by Western
blotting. The results show that the expression levels of p-Smad2/3,
p-ERK1/2 and p-Akt decreased over the time of antibody treatment
(Fig. 7), indicating LeY antigen
involvement in the TGF-β1-dependent Smad, ERK and PI3K
pathways.
Discussion
TGF-β is a member of the growth factor superfamily
that has multiple biological functions. During the early stage of
tumorigenesis, TGF-β1 functions as an important tumor suppressor to
inhibit tumor cell proliferation. However, after the cells become
resistant to TGF-β induced inhibition, TGF-β promotes tumor
development (22–24). It has been reported that all
epithelial tumors (>85% of human cancers) can become resistant
to TGF-β mediated growth inhibition (23,25,26)
including ovarian cancer (27,28).
In gastric cancer, colon cancer and pancreatic cancer, loss of the
sensitivity to TGF-β inhibition has mainly been attributed to
mutations in TβRII (29,30) and in downstream molecules such as
Smad2 and Smad4 (31–33). However, in ovarian cancers,
mutations in the TGF-β receptor and Smad are rare (34–36).
Therefore, it remains likely that there are other mechanisms
underlying the interference of the TGF-β signaling pathway.
LeY antigen is carried by glycoconjugates on cell
surface, this result in the modification of cell surface receptors
by the LeY. We examined the structural relationship of LeY and TβRs
and found that both TβRI and TβRII contain LeY structures (Figs. 2 and 3). Meanwhile, we found that the
expression of LeY was significantly increased in RMG-I-H cells.
Since it is known that the carbohydrate moieties on the cell
surface can be changed by altering the expression of
glycosyltransferase, which in turn affect the receptor’s
functionality (37–39), we examined the effect of LeY on
TGF-β/Smad pathway. We found that although the expression of
Smad2/3 did not change significantly in LeY overexpressing cells,
the level of p-Smad2/3 was down-regulated and Smad7 expression
levels were significantly elevated (Fig. 4), suggestting that activation of
TGF-β/Smad pathway was inhibited in LeY overexpressing cells.
We further tested the activation of MAPK and PI3K
pathway to judge whether TGF-β/Smad pathway was inhibited by
excessive activation of MAPK and PI3K. The results showed that the
ERK/MAPK and PI3K pathways were not only activated in RMG-I-H
cells, but also up-regulated in RMG-I-H cells compared to RMG-I and
RMG-I-C cells. Using LeY mAb to block the function of LeY, we found
that phospho-Smad2/3, phospho-ERK1/2 and phospho-Akt levels all
decreased over time in RMG-I-H cells (Fig. 7). These results prove that LeY
antigen is involved in the regulation of TGF-β mediated activation
of Smad, MAPK and PI3K pathway. Therefore, we came to the
conclusion that LeY is involved in regulating Smad, MAPK and PI3K
signaling pathways as a key structure on TGF-β receptor. The ways
LeY possibly regulates signal transduction are: i) LeY alters the
amount of TGF-β that binds to TGF-β receptors and/or the affinity
between the TGF-β receptors and TGF-β, leading to the change of
activation level of TGF-β signaling; or ii) LeY changes the 3-D
conformation of TβRI and TβRII, which results in a relatively high
number of TGF-β receptors on cell surface by weakening the receptor
endocytosis in cells, and also increases the sensitivity of cells
to TGF-β stimulation; iii) i) and/or ii) lead to excessive
activation of MAPK and PI3K, which inhibited the activation of
TGF-β/Smad pathway.
The interaction of Smad and non-Smad signaling
determines the ultimate response of cells to TGF-β. The mechanism
underlying the interaction and regulation between the ERK (and/or
PI3K) pathways and the Smad pathway warrants further study. Due to
the important negative regulatory function of Smad7 in Smad
pathway, its role in regulation of MAPK and PI3K pathways has
gained much attention. Dowdy et al (40) found that TGF-β-activated protein
kinase TAK1 activates ER81 via the p-38MAPK pathway and modulates
Smad7 transcription. Ohashi et al reported that TGF-β
activates the Smurf2 (regulates the Smad pathway by degrading Smad2
and Smad7) promoter through Smad-independent PI3K/Akt pathway and
up-regulates Smurf2 expression (41). However, in PC-3U prostate cancer
cells, Smad7, by promoting the interaction between receptor and
MKK3 and p38, is involved in the activation of p38 by TGF-β1
(42,43). Mazars et al also verified
that both transient and stable transfected Smad7 can induce strong
and durable activation of JNK, and speculated that Smad7 activates
JNK by direct interaction with JNK upstream molecules (44).
In conclusion, this study is the first to
demonstrate that LeY antigen, as an important component in TβRI and
TβRII, participates the development of ovarian cancer by regulating
TGF-β1-dependent Smad, ERK and PI3K pathways. It provides the
rational support for targeted treatment of LeY, and opens a new
avenue for exploring the mechanism underlying the resistance of
cancer cells to TGF-β induced cell inhibition. Moreover, an
interesting suggestion raised by the studies is that LeY antigen
may exist in most growth factor receptors in many kinds of cancers
and affect cell development via receptor signaling, which will make
LeY an attractive candidate for cancer diagnosis and treatment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (30170980, 30571958, 30872757,
81072118); Liaoning Natural Science Foundation (20052107); the
Scientific and Technical Project of the Educational Department of
Liaoning Province (05L492); the Educational Department Doctor
Projects Fund (20070159023); the Key Laboratory Project of Liaoning
Province Education Office (2008S247); the Free Researchers Plan of
Shengjing Hospital (200807); Programs of Science and Technology
Commission of Shenyang (F10-14-9-52).
References
|
1
|
Baldus SE, Mönig SP, Zirbes TK, et al:
Lewis(y) antigen (CD174) and apoptosis in gastric and colorectal
carcinomas: correlations with clinical and prognostic parameters.
Histol Histopathol. 21:503–510. 2006.PubMed/NCBI
|
|
2
|
Kuemmel A, Single K, Bittinger F, et al:
The prognostic impact of blood group-related antigen Lewis Y and
the ABH blood groups in resected non-small cell lung cancer. Tumour
Biol. 28:340–349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chhieng DC, Rodriguez-Burford C, Talley
LI, et al: Expression of CEA, Tag-72, and Lewis-Y antigen in
primary and metastatic lesions of ovarian carcinoma. Hum Pathol.
34:1016–1021. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madjd Z, Parsons T, Watson NF, Spendlove
I, Ellis I and Durrant LG: High expression of Lewis y/b antigens is
associated with decreased survival in lymph node negative breast
carcinomas. Breast Cancer Res. 7:R780–R787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwamori M, Tanaka K, Kubushiro K, et al:
Alterations in the glycolipid composition and cellular properties
of ovarian carcinoma-derived RMG-1 cells on transfection of the
α1,2-fucosyltransferase gene. Cancer Sci. 96:26–30. 2005.PubMed/NCBI
|
|
6
|
Lin B, Hao YY, Wang DD, Zhu LC, Zhang SL,
Saito M and Iwamori M: Transfection of α1,2-fucosyltransferase gene
increases the antigenic expression of Lewis y in ovarian cancer
cell line RMG-I. Acta Acad Med Sin. 30:284–289. 2008.
|
|
7
|
Hao YY, Lin B, Zhao Y, et al:
Alpha1,2-fucosyltransferase gene transfection influences on
biological behavior of ovarian carcinoma-derived RMG-I cells. Fen
Zi Xi Bao Sheng Wu Xue Bao. 41:435–442. 2008.
|
|
8
|
Liu J, Lin B, Hao Y, et al: Lewis y
antigen promotes the proliferation of ovarian carcinoma-derived
RMG-I cells through the PI3K/Akt signaling pathway. J Exp Clin
Cancer Res. 28:1542009. View Article : Google Scholar
|
|
9
|
Li F, Lin B, Hao Y, et al: Lewis y
promotes growth and adhesion of ovarian carcinoma-derived RMG-I
cells by upregulating growth factors. Int J Mol Sci. 11:3748–3759.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Inoue S, Gu J, et al:
Dysregulation of TGF-β1 receptor activation leads to abnormal lung
development and emphysema-like phenotype in core fucose-deficient
mice. Proc Natl Acad Sci USA. 102:15791–15796. 2005.
|
|
11
|
Zhu LC, Lin B, Hao YY, Li FF, Diao B and
Zhang SL: Impact of α1,2-fucosyltransferase gene transfection on
cancer-related gene expression profile of human ovarian cancer cell
line RMG-1. Ai Zheng. 27:934–941. 2008.
|
|
12
|
Basu A, Murthy U, Rodeck U, Herlyn M,
Mattes L and Das M: Presence of tumor-associated antigens in
epidermal growth factor receptors from different human carcinomas.
Cancer Res. 47:2531–2536. 1987.PubMed/NCBI
|
|
13
|
Klinger M, Farhan H, Just H, et al:
Antibodies directed against Lewis-Y antigen inhibit signaling of
Lewis-Y modified ErbB receptors. Cancer Res. 64:1087–1093. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kosunen TU, Bång BE and Hurme M: Analysis
of Campylobacter jejuni antigens with monoclonal antibodies.
J Clin Microbiol. 19:129–133. 1984.
|
|
15
|
Bång BE, Hurme M, Juntunen K and Mäkelä O:
Studies of monoclonal and polyclonal anti-digoxin antibodies for
serum digoxin radioimmunoassay. Scand J Clin Lab Invest. 41:75–78.
1981.PubMed/NCBI
|
|
16
|
Moran AP, Knirel YA, Senchenkova SN,
Widmalm G, Hynes SO and Jansson PE: Phenotypic variation in
molecular mimicry between Helicobacter pylori
lipopolysaccharides and human gastric epithelial cell surface
glycoforms. Acid-induced phase variation in Lewisx and Lewisy
expression by H pylori lipopolysaccharides. J Biol Chem.
277:5785–5795. 2002.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu L, Hébert MC and Zhang YE: TGF-β
receptor-activated p38 MAP kinase mediates Smad-independent TGF-β
responses. EMBO J. 21:3749–3759. 2002.
|
|
19
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-β family signaling. Nature.
425:577–584. 2003.
|
|
20
|
Wilkes MC, Mitchell H, Penheiter SG, et
al: Transforming growth factor-β activation of phosphatidylinositol
3-kinase is independent of Smad2 and Smad3 and regulates fibroblast
responses via p21-activated kinase-2. Cancer Res. 65:10431–10440.
2005.
|
|
21
|
Zhang YE: Non-Smad pathways in TGF-β
signaling. Cell Res. 19:128–139. 2009.
|
|
22
|
Pasche B: Role of transforming growth
factor beta in cancer. J Cell Physiol. 186:153–168. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elliott RL and Blobe GC: Role of
transforming growth factor beta in human cancer. J Clin Oncol.
23:2078–2093. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian M and Schiemann WP: The TGF-β paradox
in human cancer: an update. Future Oncol. 5:259–271. 2009.
|
|
25
|
Fynan TM and Reiss M: Resistance to
inhibition of cell growth by transforming growth factor-beta and
its role in oncogenesis. Crit Rev Oncol. 4:493–540. 1993.PubMed/NCBI
|
|
26
|
Massagué J, Blain SW and Lo RS: TGFβ
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000.
|
|
27
|
Yamada SD, Baldwin RL and Karlan BY:
Ovarian carcinoma cell cultures are resistant to TGF-β1-mediated
growth inhibition despite expression of functional receptors.
Gynecol Oncol. 75:72–77. 1999.PubMed/NCBI
|
|
28
|
Hu W, Wu W, Nash MA, Freedman RS, Kavanagh
JJ and Verschraegen CF: Anomalies of the TGF-beta postreceptor
signaling pathway in ovarian cancer cell lines. Anticancer Res.
20:729–733. 2000.PubMed/NCBI
|
|
29
|
Chang J, Park K, Bang YJ, Kim WS, Kim D
and Kim SJ: Expression of transforming growth factor β type II
receptor reduces tumorigenicity in human gastric cancer cells.
Cancer Res. 57:2856–2859. 1997.
|
|
30
|
Grady WM, Rajput A, Myeroff L, Liu DF,
Kwon K, Willis J and Markowitz S: Mutation of the type II
transforming growth factor-β receptor is coincident with the
transformation of human colon adenomas to malignant carcinomas.
Cancer Res. 58:3101–3104. 1998.
|
|
31
|
Hahn SA, Schutte M, Hoque AT, et al: DPC4,
a candidate tumor suppressor gene at human chromosome 18q21.1.
Science. 271:350–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyaki M, Iijima T, Konishi M, et al:
Higher frequency of Smad4 gene mutation in human colorectal cancer
with distant metastasis. Oncogene. 18:3098–3103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eppert K, Scherer SW, Ozcelik H, et al:
MADR2 maps to 18q21 and encodes a TGFβ-regulated MAD-related
protein that is functionally mutated in colorectal carcinoma. Cell.
86:543–552. 1996.PubMed/NCBI
|
|
34
|
Wang D, Kanuma T, Takama F, et al:
Mutation analysis of the smad3 gene in human ovarian cancers. Int J
Oncol. 15:949–953. 1999.PubMed/NCBI
|
|
35
|
Vincent F, Nagashima M, Takenoshita S,
Khan MA, Gemma A, Hagiwara K and Bennett WP: Mutation analysis of
the transforming growth factor-β type II receptor in human cell
lines resistant to growth inhibition by transforming growth
factor-β. Oncogene. 15:117–122. 1997.
|
|
36
|
Wang D, Kanuma T, Mizumuma H, Ibuki Y and
Takenoshita S: Mutation analysis of the Smad6 and Smad7 gene in
human ovarian cancers. Int J Oncol. 17:1087–1091. 2000.PubMed/NCBI
|
|
37
|
Dettke M, Pálfi G and Loibner H:
Activation-dependent expression of the blood group-related lewis Y
antigen on peripheral blood granulocytes. J Leukoc Biol.
68:511–514. 2000.PubMed/NCBI
|
|
38
|
Farhan H, Schuster C, Klinger M, et al:
Inhibition of xenograft tumor growth and down-regulation of ErbB
receptors by an antibody directed against Lewis Y antigen. J
Pharmacol Exp Ther. 319:1459–1466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang QY, Zhang Y, Chen HJ, Shen ZH and
Chen HL: Alpha 1,3-fucosyltransferase-VII regulates the signaling
molecules of the insulin receptor pathway. FEBS J. 274:526–538.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dowdy SC, Mariani A and Janknecht R:
HER2/Neu- and TAK1-mediated up-regulation of the transforming
growth factor β inhibitor Smad7 via the ETS protein ER81. J Biol
Chem. 278:44377–44384. 2003.PubMed/NCBI
|
|
41
|
Ohashi N, Yamamoto T, Uchida C, et al:
Transcriptional induction of Smurf2 ubiquitin ligase by TGF-β. FEBS
Lett. 579:2557–2563. 2005.
|
|
42
|
Edlund S, Bu S, Schuster N, et al:
Transforming growth factor-β1 (TGF-β)-induced apoptosis of prostate
cancer cells involves Smad7-dependent activation of p38 by
TGF-β-activated kinase 1 and mitogen-activated protein kinase
kinase 3. Mol Biol Cell. 14:529–544. 2003.
|
|
43
|
Iwai T, Murai J, Yoshikawa H and Tsumaki
N: Smad7 inhibits chondrocyte differentiation at multiple steps
during endochondral bone formation and down-regulates p38 MAPK
pathways. J Biol Chem. 283:27154–27164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mazars A, Lallemand F, Prunier C, et al:
Evidence for a role of the JNK cascade in Smad7-mediated apoptosis.
J Biol Chem. 276:36797–36803. 2001. View Article : Google Scholar : PubMed/NCBI
|