Introduction
The fruit of Schisandra chinensis has been
used traditionally to alleviate suffering from chronic cough and
asthma and also to promote the production of body fluid to quench
thirst and arrest sweating in East Asian countries. S.
chinensis has also been employed in the treatment and
prevention of some chronic diseases, such as inflammation,
hepatitis and cancer. The major bioactive constituents of S.
chinensis are lignans, including gomisins A, B, C, E, F, G, K,
N and J, schisandrol B, and schisandrin C (1,2).
Pharmacological studies revealed that lignans isolated from S.
chinensis show anticancer, anti-hepatotoxic, anti-oxidative and
anti-inflammatory activities (3–5).
Gomisins A and N are dibenzo[a,c]cyclooctadiene lignans with R- and
S-biphenyl configurations, respectively (6–8).
Gomisin A shows anti-apoptotic activity and protects the liver from
hepatotoxic chemicals (9). In
contrast, gomisin N induces apoptosis of human hepatic carcinoma
cells (10), and we have recently
reported that gomisin N enhances TNF-α-induced apoptosis via
inhibition of the NF-κB and EGFR survival pathways (11).
On the other hand, tumor necrosis factor
(TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the
TNF superfamily that can initiate apoptosis via the activation of
death receptor 4 (DR4) and DR5 (12,13).
Since TRAIL induces apoptosis in transformed or tumor cells but not
in normal cells, it is considered to be a promising cancer
therapeutic agent, better than other TNF superfamily members, such
as TNF and Fas ligand (14–17),
which have no selectivity for normal and cancer cells. However,
many types of cancer cells are resistant to TRAIL-induced apoptosis
(18), therefore, it is important
to overcome this resistance to expand the ability of TRAIL in
cancer therapy. In this study, we focused on whether gomisin N was
able to enhance TRAIL-induced apoptosis in HeLa cells and tried to
explore the underlying molecular mechanisms.
Materials and methods
Antibodies and reagents
Anti-Bcl-xL, XIAP, Poly (ADP-ribose) polymerase-1
(PARP-1), caspase-8 and caspase-3 antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies
against Bcl-2, caspase-9, cytochrome-c and β-Actin (C-11) were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Recombinant human TRAIL Apo II ligand was obtained from PeproTech,
Inc. (Rocky Hill, NJ, USA). Gomisins A and N were purchased from
Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Annexin V was
purchased from BioLegend, Inc. (San Diego, CA, USA). Anti-DR4 and
anti-DR5 antibodies used for receptor blockage and z-VAD-FMK were
obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA).
Cell culture and cytotoxicity assay
HeLa cells were maintained in Dulbecco’s modified
Eagle’s medium (high glucose) supplemented with 10% fetal calf
serum, 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C
in 5% CO2. Cell viability was quantified using the cell
proliferation reagent WST-1
{4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene
disulfonate} (Dojindo, Kumamoto, Japan). HeLa cells were plated in
96-well microplates at 6×103 cells/wells and then
incubated for 24 h. Gomisin N-containing medium was added to the
wells, and cells were incubated for 30 min and then stimulated with
TRAIL. After 24-h incubation, 10 μl of WST-1 solution was added and
absorbance was measured at 450 nm.
Immunoblotting
Cells were treated with gomisin A, gomisin N and
TRAIL, and whole-cell lysates were prepared with lysis buffer [25
mM HEPES pH 7.7, 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA,
10% Triton X-100, 20 mM β-glycerophosphate, 1 mM sodium
orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM
dithiothreitol (DTT), 10 μg/ml aprotinin and 10 μg/ml leupeptin].
Cell lysates were collected from the supernatant after
centrifugation at 14,000 rpm for 10 min. Cell lysates were resolved
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred to an Immobilon-P-nylon membrane (Millipore). The
membrane was treated with Block Ace (Dainippon Pharmaceutical Co.,
Ltd., Suita, Japan) and probed with primary antibodies. The
antibodies were detected using horseradish peroxidase-conjugated
anti-rabbit, anti-mouse and anti-goat immunoglobulin G (Dako) and
visualized with an enhanced chemiluminescence system (Amersham
Biosciences). Some antibody reactions were carried out in Can Get
Signal solution (Toyobo).
Analyses of apoptotic cells by Annexin
V-FITC
Cells pretreated with gomisin N (100 μM) for 30 min
were treated with TRAIL (100 ng/ml) for 6 h. After harvesting, the
cells were washed twice with 1,000 μl FACS buffer and resuspended
in 500 μl FACS buffer containing 2.5 mM CaCl2 and 1 μg
Annexin V-FITC for 15 min in the dark on ice. The samples were
analyzed with the FACSCalibur System (BD Biosciences).
Real-time RT-PCR
Total RNAs were prepared using the RNeasy Mini kit
(Qiagen). First-strand cDNAs were synthesized by SuperScript II
reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The cDNAs
were amplified quantitatively using SYBR Premix Ex Taq (Takara).
The primer sequences are summarized in Table I (19). Real-time quantitative RT-PCR was
performed using an ABI PRISM 7300 sequence detection system
(Applied Biosystems). All data were normalized to GAPDH mRNA.
 | Table ISequences of RT-PCR primers. |
Table I
Sequences of RT-PCR primers.
| Genes | Forward | Reverse |
|---|
| GAPDH |
GCACCGTCAAGGTGAGAAC |
ATGGTGGTGAAGACGCCAGT |
| DR4 |
ACAGCAATGGGAACATAG |
GTCACTCCAGGGCGTACAAT |
| DR5 | GCACCACGACCAGAAA | CACCGACCTTGACCAT |
| DcR1 |
GTTTGTTTGAAAGACTTCACTGTG |
GCAGGCGTTTCTGTCTGTGGGAAC |
| DcR2 |
CTTCAGGAAACCAGAGCTTCCCTC |
TTCTCCCGTTTGCTTATCACACGC |
Measurement of intracellular ROS
Reactive oxygen species (ROS) generation was
measured by flow cytometry following staining with a chloromethyl
derivative of dichloro dihydro-fluorescein diacetate
(CMH2DCFDA; Invitrogen). Briefly, HeLa cells pretreated
with gomisin N (100 μM) for 30 min were treated with TRAIL (100
ng/ml) for 2.5 h. The cells were stained with 5 μM
CMH2DCFDA for 30 min at 37°C and harvested, and
fluorescence intensity was analyzed using the FACSCalibur System
(BD Biosciences).
Statistical analysis
All values are presented as the mean ± SD and were
analyzed by one-way analysis of variance (ANOVA) followed by the
Tukey-Kramer test. P<0.05 was accepted as significant.
Results
Gomisin N augments TRAIL-induced
apoptosis in HeLa cells
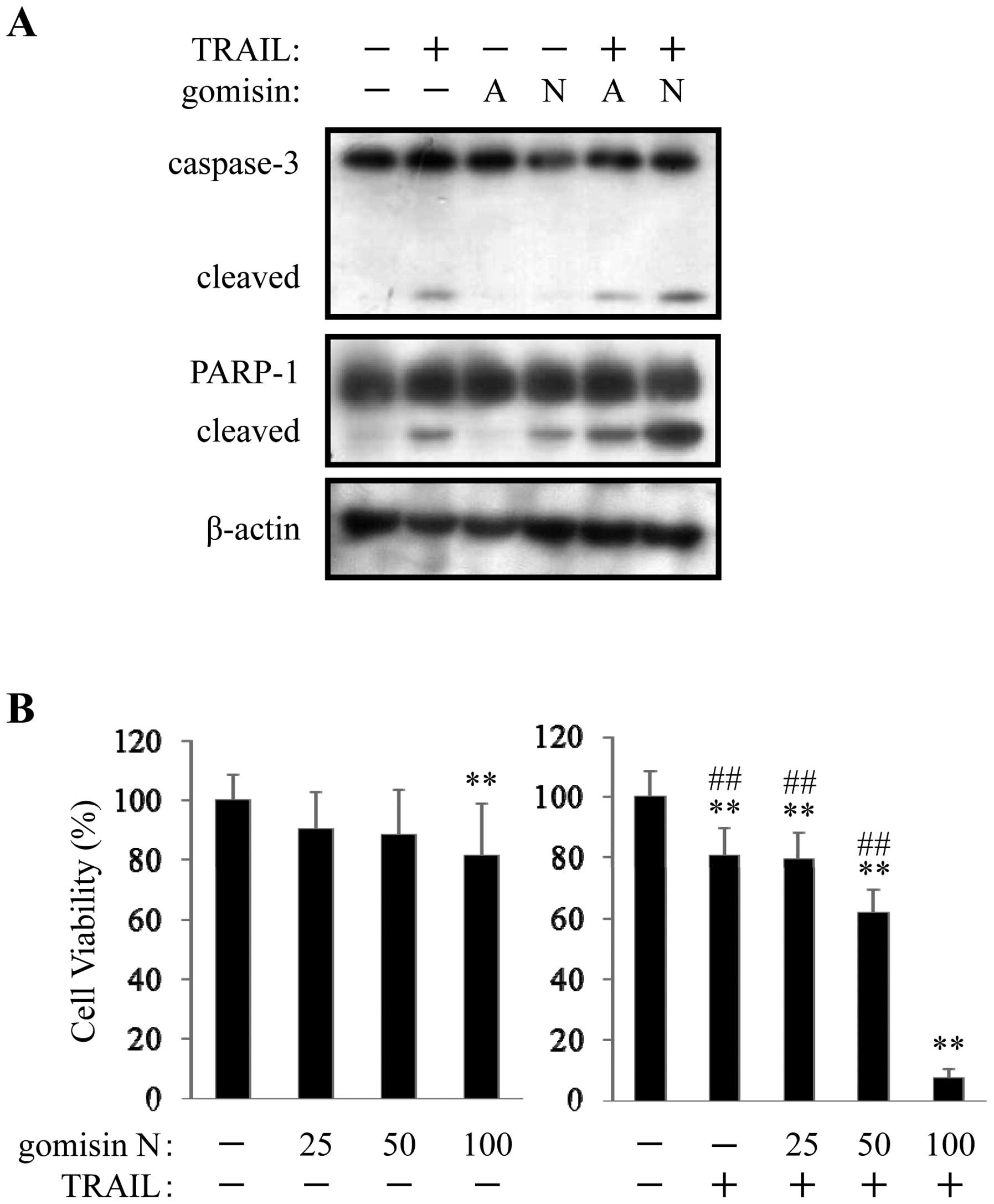
Gomisins have been shown to induce apoptosis in
cancer cells. We first confirmed the effects of gomisins A and N on
TRAIL-induced apoptosis in HeLa cells. Gomisin A alone did not
cause evident degradation of PARP-1, an important marker of
apoptosis, and gomisin N and TRAIL alone induced cleavage of PARP-1
weakly, however, gomisin N, but not gomisin A, significantly
enhanced TRAIL-induced cleavage of caspase-3 and PARP-1 (Fig. 1A). As shown in Fig. 1B, although gomisin N showed slight
inhibition at a concentration of 100 μM, gomisin N enhanced
TRAIL-induced cell death in a concentration-dependent manner. When
treated with TRAIL alone at 100 ng/ml, cleavage of PARP-1 was
detected in a time-dependent manner, and pretreatment with gomisin
N accelerated TRAIL-induced cleavage of PARP-1 (Fig. 2A). Morphological changes were also
observed after treatment with gomisin N and TRAIL together
(Fig. 2B). The flow cytogram of
Annexin V analysis is shown in Fig.
2C. For the control group, the percentage of apoptotic cells
was 4.4%. After treatment with TRAIL or gomisin N alone, the
percentage of Annexin V-positive cells was 14.7 or 10.1%,
respectively, however, after co-treatment with gomisin N and TRAIL,
the percentage of apoptotic cells markedly increased to 66.1%.
These results indicated that gomisin N enhanced TRAIL-induced
apoptosis.
TRAIL-induced apoptosis is promoted by
gomisin N through the caspase cascade
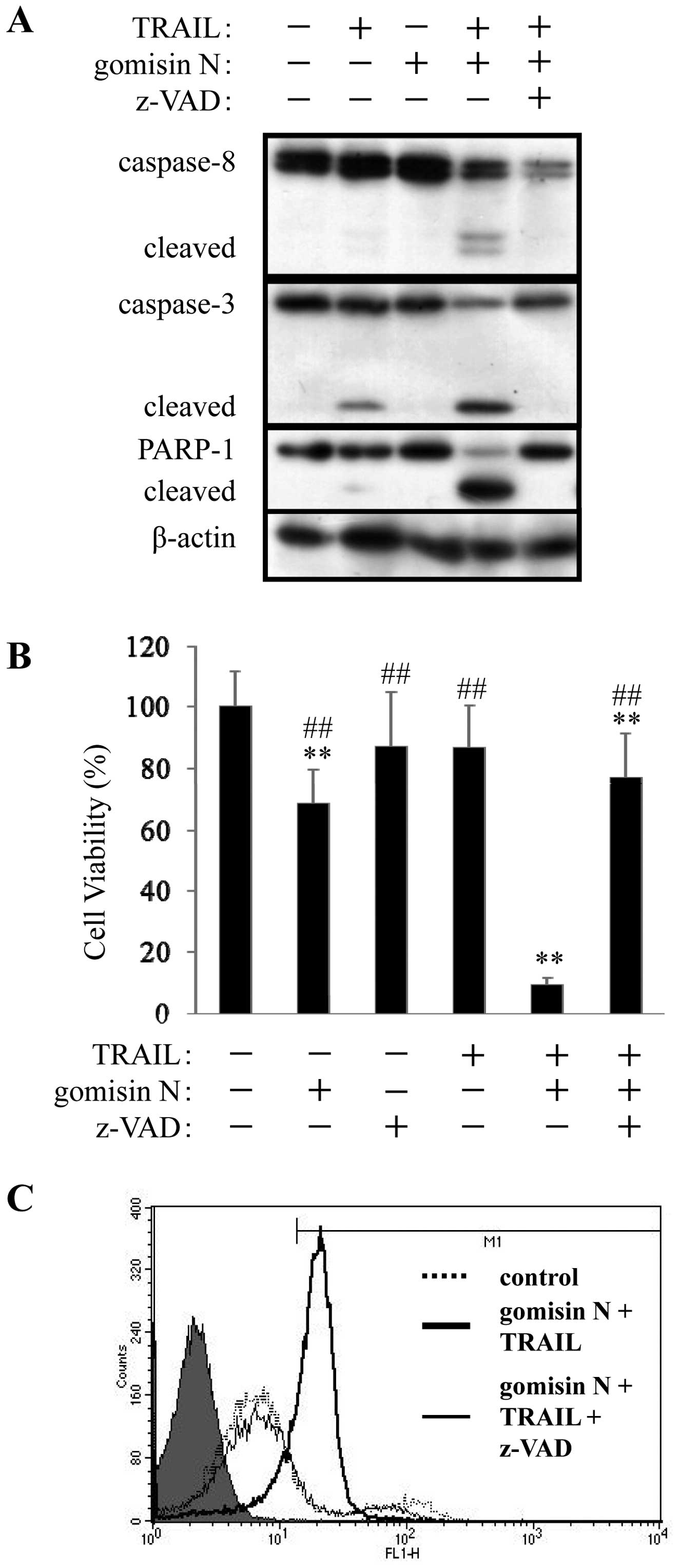
Previous reports indicate that TRAIL-induced
apoptosis is mainly executed through the extrinsic apoptosis
pathway, involving caspase-8 and caspase-3 (20). We first examined the effect of
gomisin N on TRAIL-induced activation of the caspase cascade.
Fig. 3A shows that gomisin N alone
had no effect on the degradation of caspase-8, caspase-3 and
PARP-1, however, pretreatment with gomisin N significantly enhanced
TRAIL-induced cleavage of caspase-8, caspase-3 and PARP-1.
Moreover, pretreatment with z-VAD-FMK, a pan-caspase inhibitor,
completely inhibited cleavage of caspase-8, caspase-3 and PARP-1
(Fig. 3A). In addition, WST-1 and
Annexin V analyses showed that z-VAD-FMK also inhibited apoptosis
induced by the combined treatment with gomisin N and TRAIL
(Fig. 3B and C). These results
suggested that gomisin N was able to promote TRAIL-induced
apoptosis through the caspase cascade involving caspase-8 and
caspase-3.
Gomisin N enhances TRAIL-induced
apoptosis via up-regulation of DR4 and DR5
It is well documented that decreased expression of
TRAIL receptors DR4 and DR5 or increased expression of the decoy
receptors DcR1 and DcR2 are responsible for TRAIL resistance in
several cancer cell lines (13,20–22).
To explore the underlying mechanism by which gomisin N enhanced
TRAIL-induced apoptosis, we first detected the mRNA levels of DR4
and DR5 in HeLa cells treated with gomisin N. As shown in Fig. 4A, gomisin N significantly
up-regulated the expression of DR4 and DR5 mRNA, and the
combination of gomisin N and TRAIL accelerated the expression of
DR4 and DR5. Furthermore, although TRAIL did not up-regulate mRNA
levels of DR4 and DR5, gomisin N up-regulated DR4 and DR5
expression in a time-dependent manner until 6 h (Fig. 4B). Next to confirm the roles of DR4
and DR5 in TRAIL-induced apoptosis, TRAIL receptors were
neutralized by anti-DR4 and DR5 blocking antibodies. As shown in
Fig. 4C, WST-1 analysis
demonstrated that DR4 blocking antibody was not able to inhibit
TRAIL-induced cell death, however, after the neutralization of DR5,
the percentage of cell viability increased to 23%. Moreover, after
neutralizing both DR4 and DR5, the percentage increased to 37%.
Western blot analysis showed that DR4 and DR5 blocking antibodies
could inhibit the degradation of caspase-8, caspase-3 and PARP-1
similarly (Fig. 4D). These results
suggested that gomisin N up-regulated DR4 and DR5 expression at the
transcriptional level, and not only up-regulation of DR5 but also
that of DR4 might be one of the mechanisms in the sensitization of
TRAIL-induced apoptosis by gomisin N.
Gomisin N up-regulates DR4 and DR5
expression by increasing ROS
It has been reported that several natural products
have ROS-generating activity and ROS are related to the
up-regulation of TRAIL receptors (23–25).
Here, we measured intracellular ROS levels treated with gomisin N.
As shown in Fig. 5A, after
treatment with TRAIL alone, the intracellular ROS level was almost
the same as that of control cells without stimulation; however,
after treatment with gomisin N, the ROS level increased
significantly and co-treatment with gomisin N and TRAIL accelerated
ROS production. Moreover, pretreatment with N-acetyl cysteine
(NAC), an antioxidant, markedly reduced ROS production (Fig. 5B) and also significantly inhibited
up-regulation of DR4 and DR5 induced by gomisin N (Fig. 5C). These findings indicated that
up-regulation of DR4 and DR5 induced by gomisin N was dependent on
ROS generation.
Gomisin N does not markedly affect the
mRNA expression of TRAIL decoy receptors, and protein expression of
the intrinsic apoptosis pathway
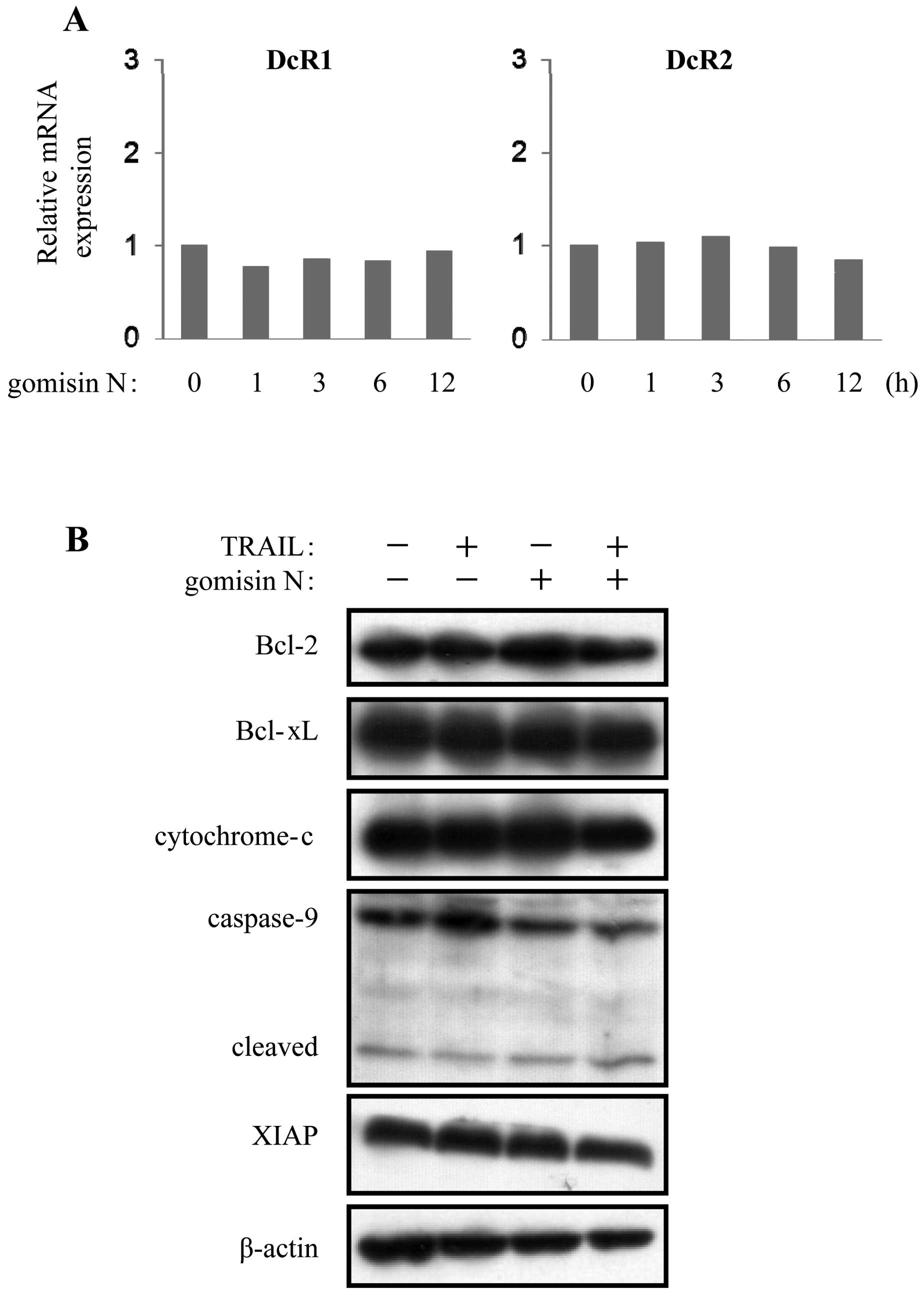
To investigate whether TRAIL decoy receptors were
relevant to the sensitization of TRAIL-induced apoptosis by gomisin
N, we examined mRNA expression of DcR1 and DcR2. As shown in
Fig. 6A, gomisin N did not change
the expression of DcR1 and DcR2. We also examined the protein
levels of the intrinsic apoptosis pathway. Bcl-2, Bcl-xL, XIAP,
cytochrome c and caspase-9 were not significantly changed by
gomisin N treatment in HeLa cells (Fig. 6B). These findings suggested that
gomisin N did not affect the mRNA expression of TRAIL decoy
receptors and the intrinsic apoptosis pathway.
Discussion
The effectiveness of chemotherapeutic drugs in
cancer treatment has been limited by systemic toxicity and drug
resistance. The distinct ability of triggering apoptosis in many
types of human cancer cells while sparing normal cells makes TRAIL
an attractive agent for cancer therapy, however, resistance to
TRAIL-mediated apoptosis in cancer cells is a limitation in its
clinical application as a cancer therapeutic agent. Thus,
researchers are currently seeking TRAIL sensitizers to overcome
resistance to TRAIL in cancer cells (18). A number of chemical compounds,
including some natural products, have been identified as effective
sensitizing agents to TRAIL-induced apoptosis (23,26–36).
Gomisin N, a dibenzocyclooctadiene lignan isolated from the fruit
of S. chinensis, has been reported as an anticancer drug
candidate. Recent study demonstrated that gomisin N inhibited
proliferation and induced apoptosis in human hepatic carcinomas
(10). We have shown that gomisin
N could sensitize TNF-α-induced apoptosis in HeLa cells (11), and the findings from this study
provided substantial evidence that gomisin N was also capable of
sensitizing TRAIL-induced apoptosis in HeLa cells. Thus, this study
presents a novel anticancer effect of gomisin N and enhances the
possibility of TRAIL in clinical application.
In the present study, to explore TRAIL sensitivity
in human cervical cancer cells, HeLa cells were treated with 100
ng/ml TRAIL for 24 h. As shown in Fig.
1B, the viability of HeLa cells treated with TRAIL alone was
81%, but when treated with gomisin N (100 μM) and TRAIL, the
percentage of viability decreased significantly to 7%. Analyses of
apoptotic cells by Annexin V-FITC are shown in Fig. 2C. It was revealed that cells
induced to undergo apoptosis by TRAIL alone were only 14.7%, but
after treatment with gomisin N and TRAIL, the percentage of
apoptotic cells increased to 66.1%. These results suggested that
HeLa cells were resistant to TRAIL-induced apoptosis and gomisin N
could promote TRAIL-induced apoptosis. To clarify the signaling
pathway of apoptosis induced by TRAIL, we characterized the
caspase-dependent pathway. The activation of caspase-8, caspase-3
and PARP-1 confirmed that the cell death induced by gomisin N and
TRAIL was caspase-dependent apoptosis. As shown in Fig. 3, the pan-caspase inhibitor
(z-VAD-FMK) was able to prevent PARP-1 cleavage and apoptosis
induced by combined treatment with gomisin N and TRAIL, therefore,
it was clarified that the apoptosis induced by gomisin N and TRAIL
was caspase-dependent. Gomisin A enhanced the cleavage of PARP-1
induced by TRAIL slightly, but did not augment the cleavage of
caspase-3 induced by TRAIL (Fig.
1A), therefore it was necessary to investigate the molecular
mechanisms of gomisin A in enhancing the cleavage of PARP-1.
The expression level of death receptors (DR4 or DR5)
plays a key role in determining the cell fate in response to TRAIL
(20–22). There are numerous reports that the
up-regulation of DR4 or DR5 could sensitize TRAIL-resistant cells
to TRAIL-induced cell death (37,38).
In this study, we showed for the first time that gomisin N
increased DR4 and DR5 expression in HeLa cells (Fig. 4A and B). When we neutralized only
DR4 by using a blocking antibody, the percentage of cell viability
was not recovered, as compared with that of the combination of
gomisin N and TRAIL. However, pretreatment with both DR4 and DR5
blocking antibodies inhibited the cell death induced by gomisin N
and TRAIL more strongly than that of only DR5 (Fig. 4C), so we believed that both DR4 and
DR5 up-regulated by gomisin N played key roles in sensitizing HeLa
cells to TRAIL-induced apoptosis.
ROS generation has been proposed to be involved in
the up-regulation of TRAIL receptors (23–25).
The present study revealed that the mechanism by which gomisin N
induced up-regulation of DR4 and DR5 was through the production of
ROS. The antioxidant (NAC) could abolish the up-regulation of TRAIL
receptors by gomisin N (Fig. 5C),
therefore ROS production led to the up-regulation of DR4 and DR5,
caspase cascade and eventually enhanced cell death.
In summary, we showed that gomisin N overcame TRAIL
resistance through ROS-mediated up-regulation of DR4 and DR5
expression. Gomisin N might be useful to increase TRAIL efficacy in
the treatment of malignant tumors.
Acknowledgements
This study was supported in part by Grants-in-Aid
for Challenging Exploratory Research (No. 09002374) and Scientific
Research (C) (No. 23590071) from the Ministry of Education,
Culture, Sports, Science and Technology (MEXT), Japan and a grant
from the First Bank of Toyama Foundation.
References
|
1
|
Sterbova H, Sevcikova P, Kvasnickova L,
Glatz Z and Slanina J: Determination of lignans in Schisandra
chinensis using micellar electrokinetic capillary
chromatography. Electrophoresis. 23:253–258. 2002.
|
|
2
|
He X, Lian L and Lin L: Analysis of lignan
constituents from Schisandra chinensis by liquid
chromatography-electrospray mass spectrometry. J Chromatogr A.
757:81–87. 1997.
|
|
3
|
Min HY, Park EJ, Hong JY, Kang YJ, Kim SJ,
Chung HJ, Woo ER, Hung TM, Youn UJ, Kim YS, Kang SS, Bae K and Lee
SK: Antiproliferative effects of dibenzocyclooctadiene lignans
isolated from Schisandra chinensis in human cancer cells.
Bioorg Med Chem Lett. 18:523–526. 2008.
|
|
4
|
Choi MS, Kwon KJ, Jeon SJ, Go HS, Kim KC,
Ryu JR, Lee JM, Han SH, Cheong JH, Ryu JH, Shin KH and Ko CY:
Schizandra chinensis alkaloids inhibit
lipopolysaccharide-induced inflammatory responses in BV2 microglial
cells. J Biomol Tech. 17:47–56. 2009.
|
|
5
|
Oh SY, Kim YH, Bae DS, Um BH, Pan CH, Kim
CY, Lee HJ and Lee JK: Anti-inflammatory effects of gomisin N,
gomisin J, and schisandrin C isolated from the fruit of
Schisandra chinensis. Biosci Biotechnol Biochem. 74:285–291.
2010.
|
|
6
|
Marek J and Slanina J: Gomisin N. Acta
Cryst. 54:1548–1550. 1998.
|
|
7
|
Opletal L, Sovova H and Bartlova M:
Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra:
importance, isolation and determination. J Chromatogr B Analyt
Technol Biomed Life Sci. 812:357–371. 2004.
|
|
8
|
Choi YW, Takamatsu S, Khan SI, Srinivas
PV, Ferreira D, Zhao J and Khan IA: Schisandrene, a
dibenzocyclooctadiene lignan from Schisandra chinensis:
structure-antioxidant activity relationships of
dibenzocyclooctadiene lignans. J Nat Prod. 69:356–359. 2006.
|
|
9
|
Kim SH, Kim YS, Kang SS, Bae K, Hung TM
and Lee SM: Anti-apoptotic and hepatoprotective effects of gomisin
A on fulminant hepatic failure induced by D-galactosamine and
lipopolysaccharide in mice. J Pharmacol Sci. 106:225–233. 2008.
|
|
10
|
Yim SY, Lee YJ, Lee YK, Jung SE, Kim JH,
Kim HJ, Son BG, Park YH, Lee YG, Choi YW and Hwang DY: Gomisin N
isolated from Schisandra chinensis significantly induces
antiproliferative and pro-apoptotic effects in hepatic carcinoma.
Mol Med Rep. 2:725–732. 2009.
|
|
11
|
Waiwut P, Shin MS, Inujima A, Zhou Y,
Koizumi K, Saiki I and Sakurai H: Gomisin N enhances TNF-α-induced
apoptosis via inhibition of the NF-κB and EGFR survival pathways.
Mol Cell Biochem. 350:169–175. 2011.
|
|
12
|
Mahmood Z and Shukla Y: Death receptors:
targets for cancer therapy. Exp Cell Res. 316:887–899. 2010.
|
|
13
|
Wu GS: TRAIL as a target in anti-cancer
therapy. Cancer Lett. 285:1–5. 2009.
|
|
14
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen
H, Shahrokh Z and Schwall RH: Safety and antitumor activity of
recombinant soluble Apo2 ligand. J Clin Invest. 104:155–162.
1999.
|
|
15
|
Shankar S and Srivastava RK: Enhancement
of therapeutic potential of TRAIL by cancer chemotherapy and
irradiation: mechanisms and clinical implications. Drug Resist
Updat. 7:139–156. 2004.
|
|
16
|
Sheridan JP, Marsters SA, Pitti RM, Gurney
A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood
WI, Goddard AD, Godowski P and Ashkenazi A: Control of
TRAIL-induced apoptosis by a family of signaling and decoy
receptors. Science. 277:818–821. 1997.
|
|
17
|
MacFarlane M: TRAIL-induced signalling and
apoptosis. Toxicol Lett. 139:89–97. 2003.
|
|
18
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005.
|
|
19
|
Fandy TE and Srivastava RK: Trichostatin A
sensitizes TRAIL-resistant myeloma cells by downregulation of the
antiapoptotic Bcl-2 proteins. Cancer Chemother Pharmacol.
58:471–477. 2006.
|
|
20
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003.
|
|
21
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003.
|
|
22
|
Takeda K, Stagg J, Yagita H, Okumura K and
Smyth MJ: Targeting death-inducing receptors in cancer therapy.
Oncogene. 26:3745–3757. 2007.
|
|
23
|
Zhou J, Lu GD, Ong CS, Ong CN and Shen HM:
Andrographolide sensitizes cancer cells to TRAIL-induced apoptosis
via p53-mediated death receptor 4 up-regulation. Mol Cancer Ther.
7:2170–2180. 2008.
|
|
24
|
Taniguchi H, Yoshida T, Horinaka M, Yasuda
T, Goda AE, Konishi M, Wakada M, Kataoka K, Yoshikawa T and Sakai
T: Baicalein overcomes tumor necrosis factor-related
apoptosis-inducing ligand resistance via two different
cell-specific pathways in cancer cells but not in normal cells.
Cancer Res. 68:8918–8927. 2008.
|
|
25
|
Prasad S, Ravindran J, Sung B, Pandey MK
and Aggarwal BB: Garcinol potentiates TRAIL-induced apoptosis
through modulation of death receptors and antiapoptotic proteins.
Mol Cancer Ther. 9:856–868. 2010.
|
|
26
|
Li X, Wang JN, Huang JM, Xiong XK, Chen
MF, Ong CN, Shen HM and Yang XF: Chrysin promotes tumor necrosis
factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced
apoptosis in human cancer cell lines. Toxicol In Vitro. 25:630–635.
2011.
|
|
27
|
Lee DH, Rhee JG and Lee YJ: Reactive
oxygen species up-regulate p53 and Puma; a possible mechanism for
apoptosis during combined treatment with TRAIL and wogonin. Br J
Pharmacol. 157:1189–1202. 2009.
|
|
28
|
Ishiguro K, Ando T, Maeda O, Ohmiya N,
Niwa Y, Kadomatsu K and Goto H: Ginger ingredients reduce viability
of gastric cancer cells via distinct mechanisms. Biochem Biophys
Res Commun. 362:218–223. 2007.
|
|
29
|
Frese S, Pirnia F, Miescher D, Krajewski
S, Borner MM, Reed JC and Schmid RA: PG490-mediated sensitization
of lung cancer cells to Apo2L/TRAIL-induced apoptosis requires
activation of ERK2. Oncogene. 22:5427–5435. 2003.
|
|
30
|
Lee KY, Park JS, Jee YK and Rosen GD:
Triptolide sensitizes lung cancer cells to TNF-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition
of NF-kappaB activation. Exp Mol Med. 34:462–468. 2002.
|
|
31
|
Inoue S, MacFarlane M, Harper N, Wheat LM,
Dyer MJ and Cohen GM: Histone deacetylase inhibitors potentiate
TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in
lymphoid malignancies. Cell Death Differ. 11(Suppl 2): S193–S206.
2004.
|
|
32
|
Palacios C, Yerbes R and Lopez-Rivas A:
Flavopiridol induces cellular FLICE-inhibitory protein degradation
by the proteasome and promotes TRAIL-induced early signaling and
apoptosis in breast tumor cells. Cancer Res. 66:8858–8869.
2006.
|
|
33
|
Ganten TM, Koschny R, Haas TL, Sykora J,
Li-Weber M, Herzer K and Walczak H: Proteasome inhibition
sensitizes hepatocellular carcinoma cells, but not human
hepatocytes, to TRAIL. Hepatology. 42:588–597. 2005.
|
|
34
|
Shi RX, Ong CN and Shen HM: Protein kinase
C inhibition and x-linked inhibitor of apoptosis protein
degradation contribute to the sensitization effect of luteolin on
tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis in cancer cells. Cancer Res. 65:7815–7823. 2005.
|
|
35
|
Zhang S, Shen HM and Ong CN:
Down-regulation of c-FLIP contributes to the sensitization effect
of 3,3′-diindolylmethane on TRAIL-induced apoptosis in cancer
cells. Mol Cancer Ther. 4:1972–1981. 2005.
|
|
36
|
Son YG, Kim EH, Kim JY, Kim SU, Kwon TK,
Yoon AR, Yun CO and Choi KS: Silibinin sensitizes human glioma
cells to TRAIL-mediated apoptosis via DR5 up-regulation and
down-regulation of c-FLIP and survivin. Cancer Res. 67:8274–8284.
2007.
|
|
37
|
Galligan L, Longley DB, McEwan M, Wilson
TR, McLaughlin K and Johnston PG: Chemotherapy and TRAIL-mediated
colon cancer cell death: the roles of p53, TRAIL receptors, and
c-FLIP. Mol Cancer Ther. 4:2026–2036. 2005.
|
|
38
|
Jin Z, McDonald ER III, Dicker DT and
El-Deiry WS: Deficient tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) death receptor transport to the
cell surface in human colon cancer cells selected for resistance to
TRAIL-induced apoptosis. J Biol Chem. 279:35829–35839. 2004.
|