Introduction
Ulcerative colitis (UC) is a chronic inflammatory
bowel disease (IBD), the etiology of which is not completely
understood. Patients with UC face an increased risk of developing
colorectal cancer (CRC) (1,2).
Recent advances in IBD research have provided genetic insights into
its pathogenesis. The innate and adaptive immune system (3–5),
autophagy pathway (6–8), and epithelial barrier (9,10)
participate in fighting pathogens involved in IBD. Interaction
between host and pathogens leads to persistent or severe
inflammation, whereas insufficient interaction may result in
failure to prevent cancer development.
A genome-wide association study previously
identified genetic variants in Disks large homolog 5
(DLG5) gene associated with IBD (10). DLG5 regulates cell shape,
polarity (11) and cell-cell
contact (12), disruption of which
interferes with the epithelial barrier function in the colon. In
our previous study, genome-wide analysis of methylation alterations
using a methylation-sensitive representational difference analysis
identified the Pleckstrin and Sec7 domain-containing (PSD)
gene (13), which has similar
roles to DLG5, such as coordination of cell shape and
polarity PSD was more frequently methylated in both
UC-associated colorectal cancer tissues (71.4%) and matched normal
epithelia (57.1%) than in non-neoplastic UC epithelia (27.3%) and
sporadic colorectal cancer tissue (18.8%). In addition, silencing
of PSD inhibited apoptosis in a fibroblast cell line and in
tissue specimens from UC patients harboring PSD methylation.
These findings led us to address the potential roles of PSD
methylation in the mechanisms underlying UC-associated
carcinogenesis.
PSD regulates Ras-related C3 botulinum toxin
substrate 1 (Rac1), a Rho GTPase. Rac1 is implicated in the
regulation of neutrophil functions in response to inflammatory
signals, including actin remodeling, chemotaxis and production of
NADPH oxidase. Rac1 is reported to induce apoptosis in response to
UV light (14) and other damaging
agents such as Fas (15) and TNF-α
(16). These findings indirectly
support our data that previously reported PSD silencing and
methylation inhibited apoptosis in vitro and in tissue
specimens, respectively. In this study, we elucidated the effect of
PSD methylation on Rac1, which governs neutrophil chemotaxis
and apoptosis signaling, in a normal human fibroblast cell line
(HNDF) and a human promyelocytic leukemia cell line (HL-60), which
look and behave like neutrophils (17–19),
and in tissue sections from UC patients with and without CRC.
Materials and methods
Patients and tissues
Six samples of UC-associated colorectal cancer
tissue (UCT) with matched normal epithelial tissue (UCN), and 15
samples of non-neoplastic UC epithelial tissue (UCI) were obtained
from patients who had undergone surgery at Jichi Medical University
Saitama Medical Center and Jichi Medical University Hospital
between November 2000 and September 2006. Matched normal epithelia
were taken from lesions harboring colitis adjacent to tumors. This
study was approved by the Ethics Committee of Jichi Medical
University, and informed consent was obtained from each
participant.
Cell lines
A human skin fibroblast cell line, NHDF, was
obtained from Kurabo (Osaka, Japan) and was maintained in medium
106S supplemented with low-serum growth supplement. A human
promyelocytic leukemia cell line, HL-60, which orient their
polarity in response to a gradient and migrate towards the stimulus
(17–19), were obtained from the Japanese
Collection of Research Bioresources (JCRB, Osaka, Japan). HL-60
cells were maintained in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal bovine serum.
Quantitative reverse
transcription-PCR
Tissue specimens were immediately added to RNA later
(Ambion, Austin, TX, USA) and stored at −80°C until DNA or RNA
extraction. Total RNA was immediately treated with DNase I
(Invitrogen, Carisbad, CA, USA) and reverse-transcribed using a
Superscript II reverse trans-criptase kit (Invitrogen) to prepare
first-strand cDNA. The primer sequences for PSD were
5′-CCATAGACGAGGAGGAGCTG-3′ (forward) and 5′-TCTTCCTGCAGTCAGGGTCT-3′
(reverse). Thermal cycling conditions were 42°C for 60 min (cDNA
synthesis), 95°C for 10 sec (hot start), and then 40 cycles of 95°C
for 5 sec, 58°C for 10 sec, and 72°C for 30 sec. The expression
level of PSD was determined using the fluorescence intensity
measurements from the ABI 7900HT Real-Time PCR System Data Analysis
Software. A GAPDH fragment was amplified as an internal
control.
Knockdown of PSD in HNDF and HL-60
cells
PSD-specific siRNA (siPSD) was purchased from
Invitrogen. RNA oligonucleotides were resuspended in 10 μM
Tris-HCl, pH 8.0, 20 mM NaCl, and 1 mM EDTA to make a 20 μM siRNA
solution. The final siRNA concentration was 30 nM in Opti-MEM I
without serum. HNDF and HL-60 cells were cultured in dishes at
30–50% confluency without antibiotics, and transfection was
performed with Lipofectamine 2000 (Invitrogen) according to the
manufacturer’s instructions. The Block-iT™ Fluorescent Oligo
(Invitrogen), a fluorescently labeled double-stranded RNA duplex
with the same length, charge, and configuration, was used for the
assessment of transfection efficiency, and Scrambled Stealth™ RNA
molecule was used as the control siRNA (siControl). Cells were
incubated for 48 h after transfection at 37°C in a CO2
incubator, and used for subsequent experiments.
Actin cytoskeleton analysis
siPSD-treated and siControl-treated cells
were stained with fluorescent rhodamine-labeled phalloidin using a
F-actin Visualization BiochemKit™ (Cytoskelton, Denver, USA)
according to the manufacturer’s instructions. Cells were stimulated
with EGF (10 ng/ml for 1, 2 and 30 min) or calpeptin (0.1 mg/ml for
30 min) after treatment with siPSD or siControl for 48 h and
subsequently stained with rhodamine-labeled phalloidin. Hoechst
33342 was used to observe nuclear morphology. Signals were observed
by a fluorescence microscope (Fluoview FV500; Olympus) with
excitation at 535 nm and emission at 585 nm for the detection of
rhodamine, and with excitation at 365 nm and emission at 480 nm for
the detection of Hoechst 33342. EGF was purchased from Wako (Tokyo,
Japan) and Hoechst 33342 was included in the Total ROS/Superoxide
Detection kit™ (Enzo, PA, USA).
Measurement of Rac1 activity
The activity of Rac1 was measured by G-LISA™ Rac
Activation Assay Biochem kit™ (Cytoskelton) according to the
manufacturer’s instructions. Cells were stimulated with EGF (10
ng/ml for 1, 2 and 30 min) or calpeptin (0.1 mg/ml for 30 min)
after treatment with siPSD or siControl for 48 h and then
collected in 100 μl of ice-cold lysis buffer provided by G-LISA.
Lysates were centrifuged to remove cellular debris. From each
supernatant, 10 μl was removed to measure protein content using the
Precision Red™ Advanced Protein Assay Reagent included in the
G-LISA Rac Activation Assay Biochem kit™, and 50 μl was used for
the G-LISA Rac activation assay, followed by dilution with 50 μl of
cold binding buffer. Lysate (50 μl) was added into respective wells
in the Rac1-binding plate for duplicate assays, and then the plate
was put on a cold orbital microplate shaker at 4°C for exactly 30
min. Next, the wells were washed twice with wash buffer. Antigen
(200 μl) presenting buffer was immediately added into each well and
the plate was incubated at room temperature for 2 min. Wells were
then washed three times with wash buffer. Next, 50 μl of diluted
anti-Rac1 primary antibody was added to each well and the plate was
placed on an orbital microplate shaker at room temperature for 45
min. Next, 50 μl of diluted anti-Rac1 secondary antibody was added
to each well and the plate was incubated for 45 min, followed by
incubation with 50 μl of HRP detection reagent for 20 min.
Immediately after addition of 50 μl of HRP Stop Buffer, the
absorbance was read using a microplate spectrophotometer.
Detection of reactive oxygen species and
active caspase-3/7
Reactive oxygen species (ROS) and active caspase-3/7
were detected by Total ROS/Superoxide Detection kit (Enzo) and
CaspaTag™ Caspase-3/7 Assay In Situ Assay Kit (Chemicon),
respectively, according to the manufacturer’s instructions. After
treatment with siPSD or siControl for 48 h, NHDF cells were
exposed to lipopolysaccharide (20 ng/ml; Wako) and were co-cultured
with HL-60 cells for an additional 48-h period. Then, HL-60 cells
were removed and NHDF cells were subjected to the ROS detection
assay or the caspase-3/7 activity assay. Fluorescence signals were
subsequently detected by a fluorescence microscope (Fluoview FV500;
Olympus), with excitation at 490 nm and emission at 525 nm for the
detection of ROS, and with excitation at 550 nm and emission at 580
nm for the detection of caspase-3/7, respectively. Average number
of cells inducing ROS or expressing caspase-3/7 in three random
fields was calculated.
Migration assay
Neutrophil chemotaxis in response to inflammation
in vitro was assessed by migration assays using a BD Falcon
companion plate and cell culture insert (Becton-Dickinson) with a
3-μm pore size. After NHDF cells were treated with siPSD or
siControl for 48 h in the bottom wells (24-well companion plate,
Becton-Dickinson), they were exposed to LPS and cultured with HL-60
cells seeded in culture inserts for an additional 48 h. Then,
non-migrating cells were removed from the insert membranes by
cotton swabs. The membrane was mounted onto a slide glass and the
nuclei of migrated cells were then stained with Hoechst 33342. The
number of migrated cells was counted in three random fields using
an inverted microscope.
Histological grades of neutrophil
infiltration in tissue sections
Histological grades of neutrophil infiltration were
determined using a scoring system as previously described (20): 0, normal (no inflammatory cells);
1, mild active; 2, moderate active (with cryptitis). The average
grading of three regions of the colorectum (rectum, descending
colon and ascending colon) were calculated where samples were
available.
Detection of filamentous actin and PSD in
tissue specimens
Detection of filamentous actin (F-actin) and PSD in
paraffin-embedded tissue sections was performed by
immunohistochemical analysis using the I-View DAB Universal kit
(Roche, Rotkreuz, Switzerland). Sections were de-waxed in xylene
and rehydrated with distilled water before analysis, then treated
with a heat-induced epitope retrieval technique using an EDTA
buffer at pH 9.0, and blocked for endogenous peroxidase activity
before addition of the primary antibody. NH3 and ab5962 (Abcam,
Tokyo, Japan) were used as primary antibodies for F-actin and
PSD, respectively. Incubation with primary antibody was
performed overnight at 37°C. Cells displaying slight staining of
the cytoplasm were determined to be positive. A grading system was
applied to the assessment of accumulation of F-actin and PSD
expression in tissue sections. The F-actin and PSD index were
calculated based on percentage of staining cell, with 0, +1, and +2
when <−5% of cells, 5–20% cells, and >20% cells demonstrated
cytoplasm reactivity, respectively. The average grading of three
regions of the colorectum (rectum, descending colon and ascending
colon) was calculated when samples were available.
Statistical analysis
Values are shown as mean ± SE. Statistical
differences between variables were determined by use of an unpaired
t-test or an analysis of variance, as appropriate. Simple
regression coefficient analysis was used to examine associations
between two categorical variables. Values of P<0.05 were
considered significant.
Results
Clinicopathological features
The clinicopathological features of patients
recruited for this study are shown in Tables I and II. There was no significant difference
in the average age. The disease duration of UCI patients (8.0±5.0
years) was significantly shorter than that of the UCT patients
(14.8±7.0 years, P<0.05). Aberrant methylation of PSD was
observed in 4 of 6 UCT patients (71.4%), 4 of 6 UCN patients
(57.1%) and 5 of 15 UCI patients (27.3%), respectively, as
previously reported.
 | Table IClinicopathological characteristics
of tumor specimens from UC patients with and without colorectal
cancer. |
Table I
Clinicopathological characteristics
of tumor specimens from UC patients with and without colorectal
cancer.
| UCT | UCN | UCI |
|---|
| Number | N=6 | N=6 | N=15 |
| Average age
(years) | 54.8±17.1 | 54.8±17.1 | 40.8±14.0 |
| Gender |
| Male | 4 | 4 | 9 |
| Female | 2 | 2 | 6 |
| Disease duration
(years) | 14.8±7.0 | 14.8±7.0 | 8.0±5.0a |
| PSD
methylation (%) | 71.4% | 57.1% | 27.3% |
 | Table IIClinicopathological characteristics
of tumor specimens from patients with UC-associated colorectal
cancer. |
Table II
Clinicopathological characteristics
of tumor specimens from patients with UC-associated colorectal
cancer.
| Group | PSD | Age | Gender | Duration | Onset | Loc | Type | Dukes | INF | Ope |
|---|
| UCT1 | M | 77 | M | 13 | 64 | R | Well | A | 1 | Total |
| UCT2 | M | 40 | M | 8 | 32 | A | Muc | A | 0 | Total |
| UCT3 | M | 64 | F | 9 | 55 | D | Well | A | 1 | Total |
| UCT4 | U | 35 | M | 15 | 20 | D | Poor | D | 0 | Partial |
| UCT5 | M | 68 | F | 24 | 44 | R | Well | B | 0 | Total |
| UCT6 | U | 45 | M | 25 | 20 | D | Well | B | 0 | Total |
Knockdown of PSD inhibited membrane
ruffling and reduced Rac1 activity in NHDF and HL-60 cells
To determine the inhibitory effect of PSD
silencing on the activation of Rac1, NHDF and HL-60 cells were
treated with PSD-specific siRNA (siPSD) or siControl.
Transfection efficiency was 89.1% in NHDF cells and 73.0% in HL-60
cells, which reduced the mRNA levels of PSD by 90.1% in
siPSD-transfected NHDF cells and 61.3% in siPSD-transfected HL-60
cells, respectively. PSD promotes numerous F-actin-rich
membrane extensions (21), which
leads to the activation of Rac1. The accumulation of F-actin by
stimulation with EGF was visualized by staining with fluorescent
rhodamine-labeled phalloidin. EGF-stimulated membrane ruffling was
observed in both siControl-treated NHDF and HL-60 cells, whereas no
morphological changes of the membrane were found in either
siPSD treated-NHDF or HL-60 cells (Fig. 1A). Likewise, Rac1 activity was
increased in both siControl-treated NHDF and HL-60 cells after
stimulation with EGF (Fig. 1B1 and
B2), whereas activation was hindered in either
siPSD-treated NHDF or HL-60 cells. Stimulation with
calpeptin for 30 min also resulted in increased levels of Rac1 in
both siControl-treated NHDF and HL-60 cells (data not shown). Rac1
activity declined to basal levels within half an hour after
stimulation with EGF (Fig. 1B3),
which was consistent with the results reported by Kurokawa et
al (22).
Knockdown of PSD in NHDF cells prevented
induction of ROS and ROS-induced caspase-3/7 activity in the
presence of HL-60 cells
Our previous study showed that NHDF cells were
stimulated to release reactive oxygen species (ROS) by
pyocyanin-harboring redox reactions, while lipopolysaccharide
(LPS), which mediates the activation of NADPH oxidation in
neutrophils, did not stimulate NHDF cells (13). In the present study, we attempted
to elucidate whether LPS stimulates NHDF cells to release ROS when
co-cultured with HL-60 cells, and if ROS induction is inhibited by
PSD silencing in NHDF cells because they co-exist and
interact in the body. LPS stimulated siControl-treated NHDF cells
to release ROS in the presence of HL-60 cells, whereas LPS did not
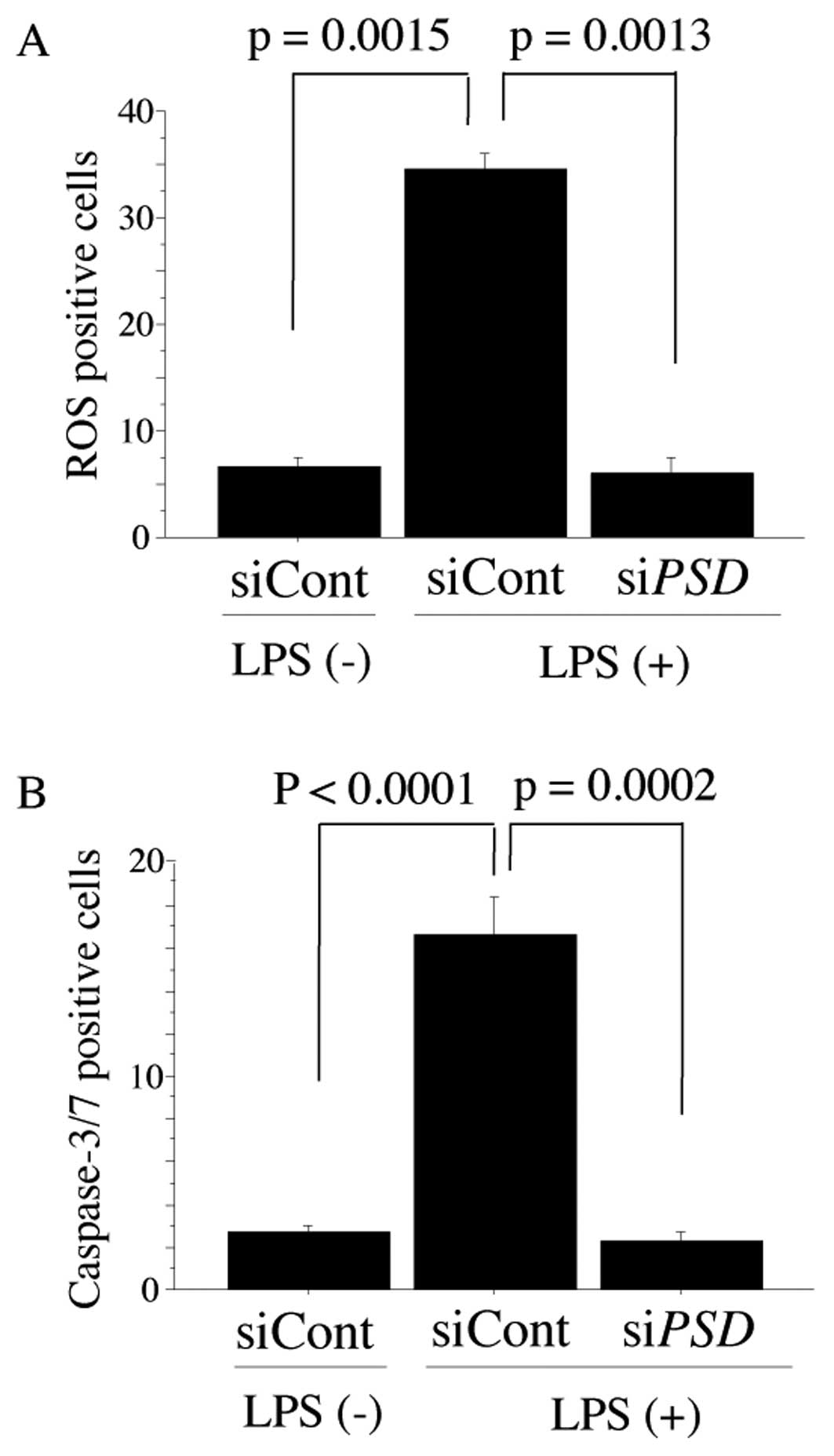
stimulate siPSD-treated NHDF cells to release ROS (Fig. 2A; 16.67±3.06 in siControl vs.
2.67±10.58 in siPSD, P=0.0013). Representative data for
siControl-treated and siPSD-treated NHDF cells are shown in
Fig. 3A. Next, we accessed
ROS-mediated caspase-3/7 activation. Activation of caspase-3/7 was
observed in siControl-treated NHDF cells, but was not noted in
siPSD-treated NHDF cells (Fig.
2B; 34.67±2.51 in siControl vs. 6.00±2.65 in siPSD,
P=0.0002). Representative caspase-3/7 positive cells detected by
the CaspaTag Caspase-3/7 Assay In Situ Assay Kit (Chemicon) and
cell nucleus stained with Hoechst 33342 in NHDF cells treated with
siPSDor siControl are shown in Fig. 3B.
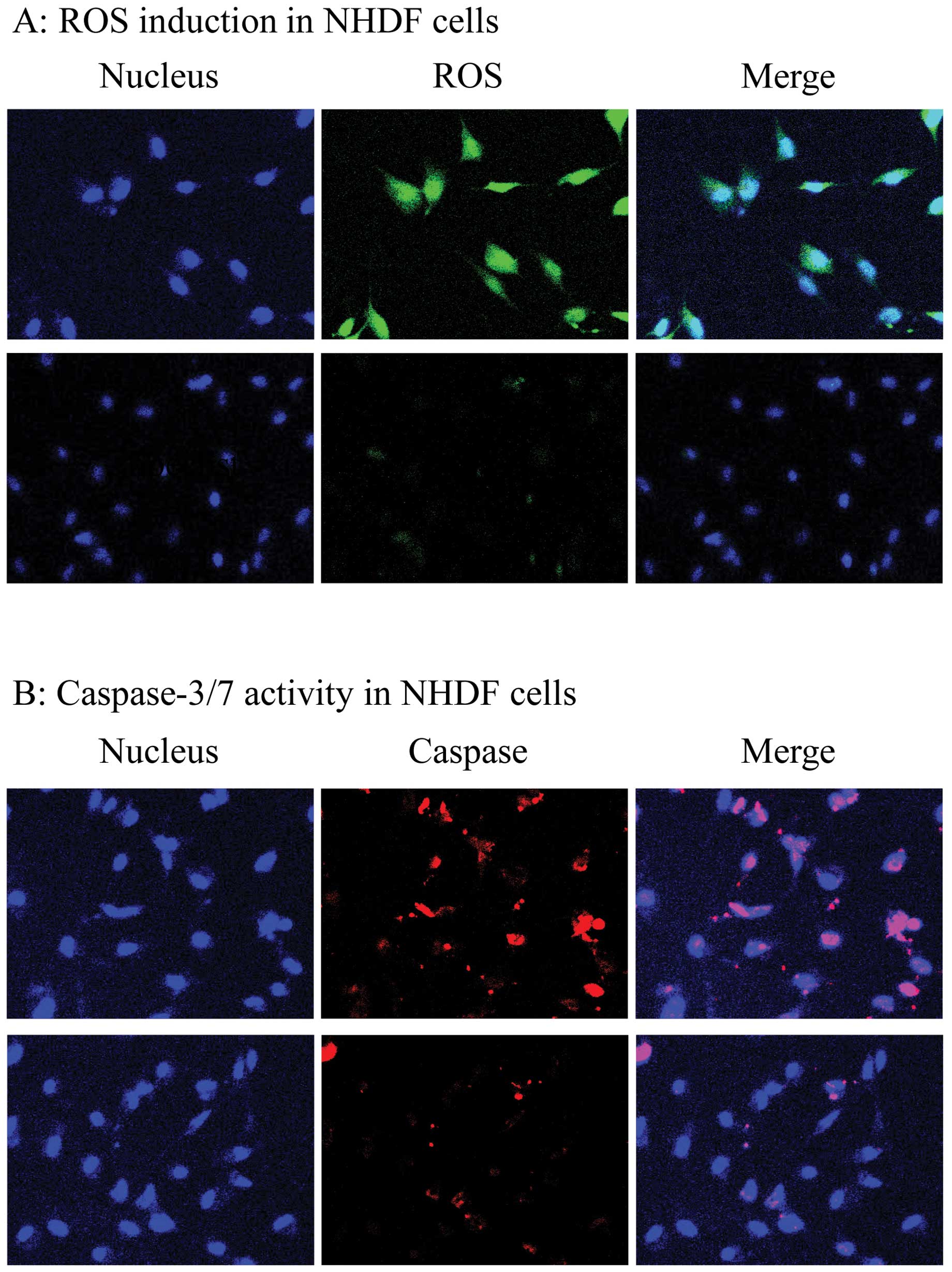 | Figure 3(A) Cells inducing reactive oxygen
species (ROS) in siControl-treated (top panel) and
siPSD-treated NHDF cells (bottom panel). Nuclear morphology
of cells stained with Hoechst 33342 (blue, left), cells inducing
ROS stained with Total ROS/Superoxide Detection kit Reagent (green,
middle), and merged staining with both reagents (white green,
bottom). (B) Cells expressing active caspase-3/7 in
siControl-treated (top panel) and siPSD-treated NHDF cells
(bottom panel). Nuclear morphology of cells stained with Hoechst
33342 (blue, left), cells expressing active caspase-3/7 stained
with CaspaTag Reagent (red, middle), and merged staining with both
reagents (pink, right). |
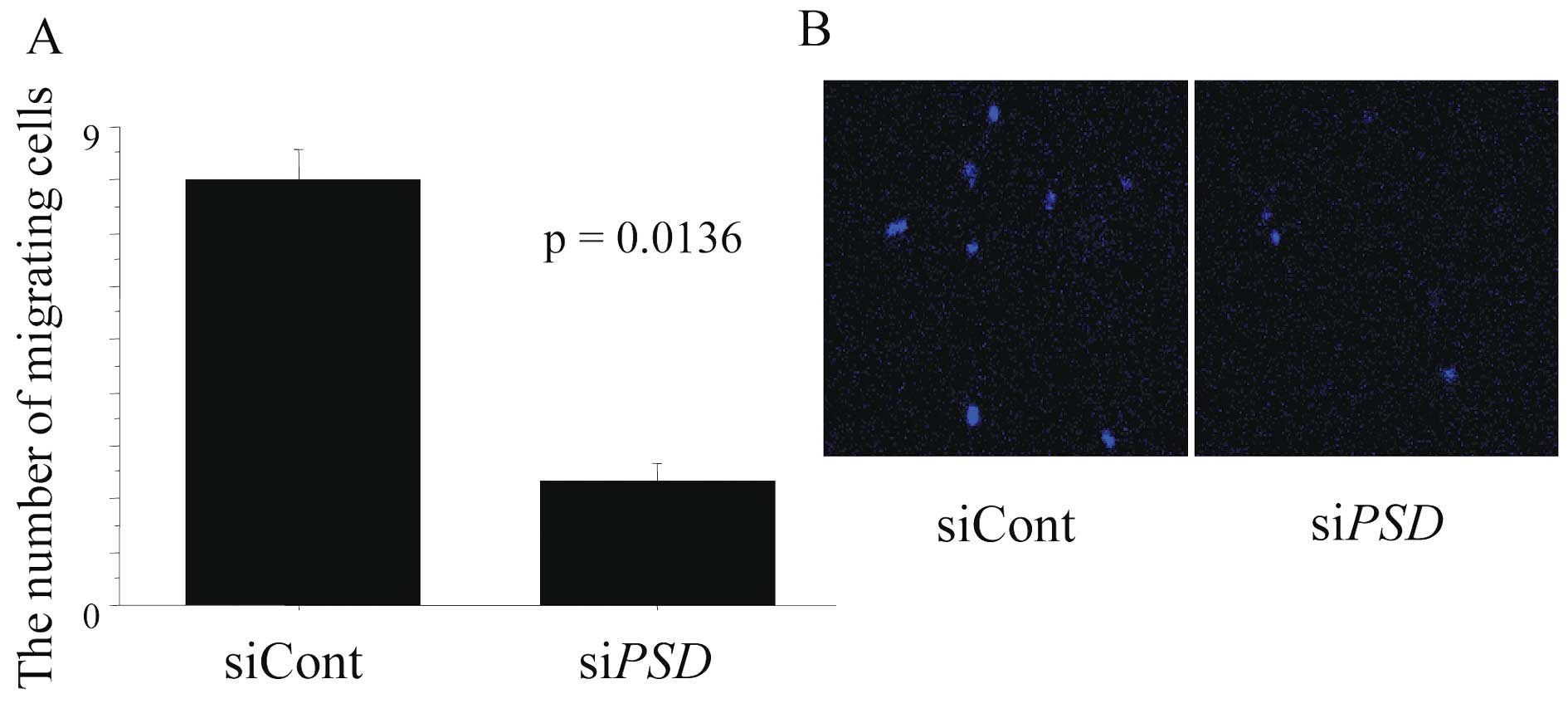
Chemotaxis of HL-60 cells was disturbed
by PSD silencing in NHDF cells
NHDF cells exhibited induction of LPS-mediated ROS
and activation of caspase-3/7 when cultured with HL-60 cells, which
was not observed in NHDF cells cultured alone or siPSD-treated NHDF
cells co-cultured with HL-60 cells, suggesting that the interaction
between activated NHDF cells and HL-60 cells was involved in this
process. To investigate whether LPS-mediated PSD activation
in NHDF cells affects the chemotaxis of HL-60 cells, migration
assays were performed. Fig. 4
shows microscopy images of cells in a migration assay for each
experimental setting. The number of migrated HL-60 cells
co-cultured with siControl-treated NHDF cells was 4.6 times greater
than HL-60 cells co-cultured with siPSD-treated HL-60 cells
(Fig. 4A; 2.667±0.882 in siControl
vs. 12.333±1.453 in siPSD, P=0.0047). Microscopic images of
migrated NHDF cells are shown in Fig.
4B.
The level of neutrophil infiltration was
significantly decreased in specimens from patients with PSD
methylation
To verify if PSD methylation affected
neutrophil chemotaxis in tissue specimens, the infiltration of
neutrophils was evaluated by histological assessment. Neutrophil
infiltration was significantly decreased in specimens from patients
with PSD methylation than in those without (Fig. 5A; 0.51±0.55 with vs. 1.01±0.55
without, P=0.0166). Regardless of cancer status, the level of
neutrophil infiltration was significantly decreased in both UCT and
UCN when compared with UCI (0.29±0.49 in UCT vs. 1.1±0.55 in UCI,
P=0.0018; 0.39±0.32 in UCN vs. 1.1±0.55 in UCI, P=0.0034).
Representative data from tissue specimens from UC patients without
and with PSD methylation are shown in Fig. 5B.
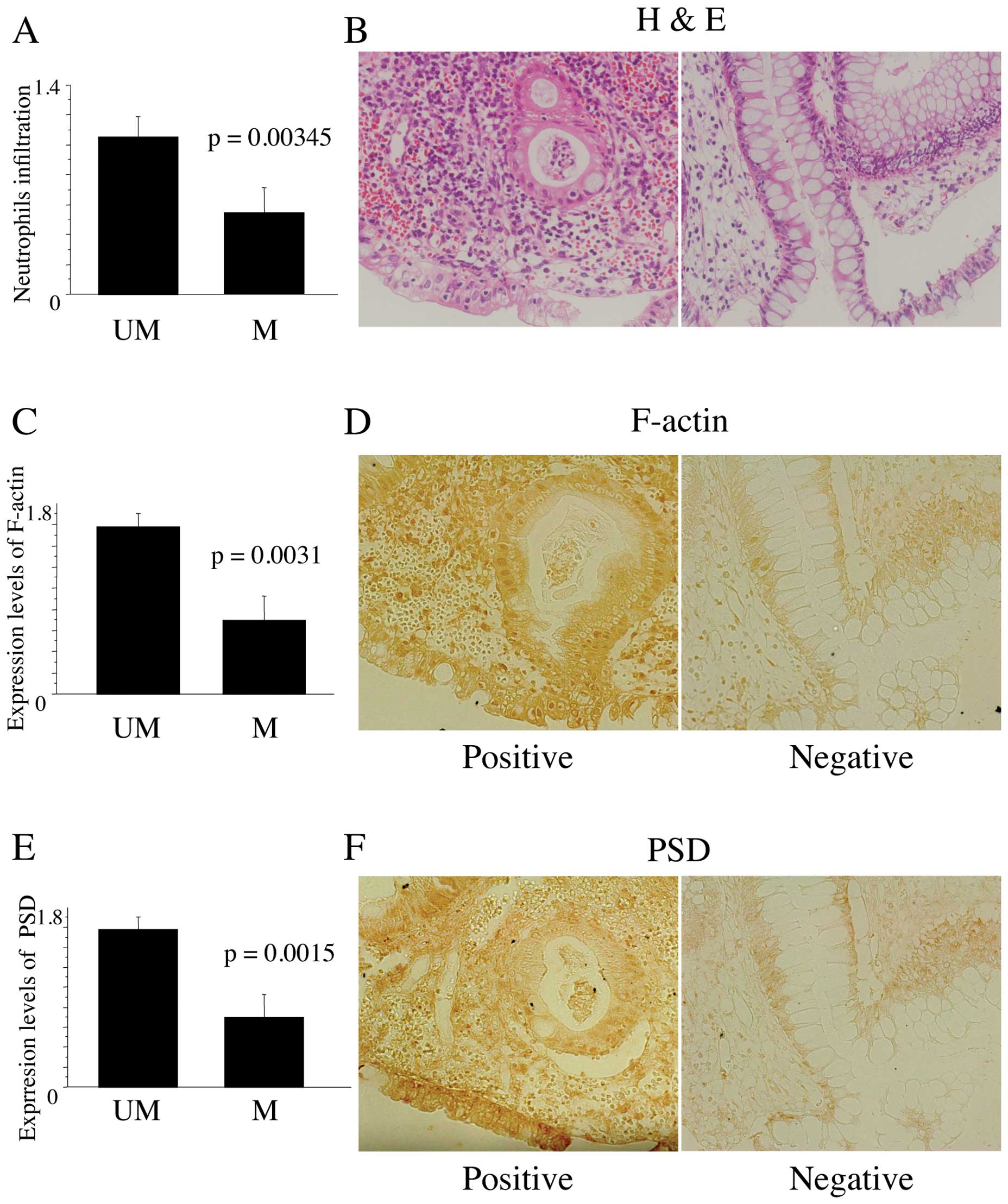 | Figure 5(A) Histological grades of neutrophil
infiltration in tissue specimens from UC patients with (M) and
without PSD methylation (UM). For evaluation, histological
grades of inflammation were determined using a scoring system based
on epithelial neutrophils as previously described (19): 0, normal (no inflammatory cells);
1, mild active; 2, moderate active (with cryptitis). The average
grading from three regions of the colorectum was calculated. (B)
Representative neutrophil infiltration in tissue specimens from UC
patients without (left) and with PSD methylation (right).
(C) The F-actin index in tissue specimens from UC patients with (M)
and without PSD methylation (UM). For evaluation, three
grades were determined as 0, +1, and +2 when <5% of cells, 5–20%
cells, and >20% cells demonstrated cytoplasm reactivity,
respectively. The average grading from three regions of the
colorectum was calculated. (D) Representative positive (right) and
negative cells for F-actin (left) in tissue specimens from UC
patients without and with PSD methylation, respectively. (E)
The PSD index in tissue specimens from UC patients with (M)
and without PSD methylation (UM). For evaluation, three
grades were determined as 0, +1, and +2 when <5% of cells, 5–20%
cells, and >20% cells demonstrated cytoplasm reactivity,
respectively. The average grading from three regions of the
colorectum was calculated. (F) Representative positive (right) and
negative cells for PSD in tissue specimens from UC patients
without and with PSD methylation, respectively. |
Accumulation of F-actin was decreased in
tissue specimens from UC patients with PSD methylation
To determine the distribution of PSD promoting
accumulation of F-actin in tissue specimens, immunohistochemistry
was performed. F-actin levels were significantly decreased in
specimens from UC patients with PSD methylation compared to
those without (Fig. 5C; 0.69±0.86
with vs. 1.57±0.51 without, P=0.0031), suggesting that accumulation
of F-actin was inhibited by PSD methylation. This change was
seen not only in colorectal mucosa but also in infiltrating cells.
Representative F-actin-positive and -negative cells in tissue
specimens are shown in Fig.
5D.
PSD expression was decreased in tissue
specimens from UC patients with PSD methylation, and was
preferentially suppressed in epithelial cells rather than
neutrophils
To clarify the distribution of PSD expression in
tissue specimens, immunohistochemical analysis was performed. The
expression level of PSD was significantly decreased in
specimens from UC patients with PSD methylation compared
with those without (Fig. 5E;
0.727±0.141 with vs. 1.462±0.144 without, P=0.0015), and was
significantly correlated with the methylation status of PSD.
In addition, immunohistochemical analysis revealed that PSD
expression was inhibited in colorectal mucosa with methylated
PSD, whereas PSD was rarely expressed in infiltrating cells
regardless of PSD methylation status. These results
indicated that aberrant methylation of PSD in UC-associated
colorectal mucosa circumvented neutrophil chemotaxis, which
resulted in the disturbance of neutrophil-regulated apoptosis.
Representative PSD-positive and -negative cells are shown in
Fig. 5F.
Discussion
The present study demonstrated the crucial role of
PSD silencing in NHDF cells through its inhibitory effect on
Rac1 activation, which disturbed membrane ruffling and chemotaxis
of HL-60 cells and consequently hampered the apoptotic machinery.
In tissue specimens from UC patients, aberrant methylation of
PSD interfered with actin rearrangement, neutrophil
infiltration and apoptosis. Taken together, these data indicate
that aberrant methylation of PSD in UC-associated colorectal
mucosa circumvents Rac1-mediated immune responses, neutrophil
chemotaxis and the apoptotic machinery, and thus likely plays a
pivotal role in the mechanisms underlying UC-associated
carcinogenesis.
The small GTPases have been implicated in diverse
biological functions such as cytoskeleton rearrangement, cell
growth, transformation, cell motility, migration, metastasis, and
response to stress. One of these GTPases, Rac1, is reported to play
a crucial role in inducing apoptosis in response to several stimuli
such as UV light (14), and other
damaging agents such as Fas (15)
and TNF-α (16). These findings
strongly support our data showing that Rac1-mediated apoptosis was
inhibited by PSD silencing in siPSD-treated cells and
was decreased in tissue specimens from UC patients harboring
PSD methylation. Some reports, however, have presented
conflicting results (23–25), raising the possibility that Rac1
has a complex role, and is involved in both inhibition and
stimulation of apoptosis. This dual role in cell proliferation and
apoptosis has been observed for other Rho proteins such as
oncogenic vav (16) and
R-ras (26,27). Esteve et al, explain that a
dual role of Rho proteins in the regulation of cell apoptosis comes
from the evidence that Rho proteins induce activation of the
pathway leading to the JNK/SPAK cascade in several cell systems as
well as nuclear factor κB (16).
The fate of cells as determined by these molecules still remains to
be explored.
Rho GTPases coordinate many cellular responses,
often by regulating formation of different actin assemblies.
PSD promotes numerous F-actin-rich membrane extensions
(21), which leads to the
activation of Rac1. Our results revealed that the accumulation of
F-actin was significantly decreased in siPSD-treated NHDF
and HL-60 cells, and in tissue specimens from UC patients harboring
methylated PSD, which indicated that PSD methylation
abolished F-actin-induced membrane extensions and consequently
inactivated Rac1. Our findings concur with the report by Srinivasan
et al, that a dominant-negative Rac1 mutant inhibits
chemoattachment-stimulated accumulation of F-actin and polarization
(28). A significant correlation
between a decreased level of apoptosis and the accumulation of
F-actin in specimens from UC patients with PSD methylation
indicated that PSD methylation inactivated Rac1 and
consequently abrogated Rac1-mediated apoptosis.
In considering alternative participants in the
immune system underlying inflammatory bowel disease, neutrophils
should be taken into consideration. Neutrophils are recruited from
the circulation to take part in the defense against infectious
agents but they may also cause tissue destruction in the host by
secretion of toxic granule proteins and ROS (29). In this machinery, Rac1 also plays a
crucial role in neutrophil migration and oxygen radical generation
through NADPH oxidase. Glogauer et al demonstrated that
infiltration of neutrophils was decreased in Rac1-null mice
compared to wild-type mice (30).
Delayed accumulation of neutrophils was also observed in Rac1-null
mice. In addition, Srinivasan et al (28) demonstrated that a dominant-negative
Rac1 mutant inhibited chemoattachment-stimulation. In accordance
with these observations, our results revealed that the accumulation
of neutrophils was significantly decreased in specimens from
patients with PSD methylation. The inactivation of Rac1
resulting from PSD methylation may lead to delayed
recruitment of neutrophils or disturbance of neutrophil chemotaxis.
In the present study, we demonstrated that PSD-promoted
accumulation of F-actin was decreased in colorectal mucosa, as well
as infiltrating cells from ulcerative colitis patients with
PSD methylation. PSD expression was preferentially inhibited
in colorectal mucosa by PSD methylation, whereas PSD
expression was rarely observed in infiltrating cells regardless of
PSD methylation status. Considering the short half-life of
neutrophils, it is unlikely that they would be methylated,
indicating that colorectal mucosa could be targeted for aberrant
methylation under circumstances of chronic inflammation such as
ulcerative colitis, leading to neutrophil dysfunction.
Oxidative stress occurs in connection with
inflammatory bowel disease. Neutrophils participate in this
mechanism to release ROS, leading to protein damage, lipid
peroxidation, and DNA damage. This results in genetic and
epigenetic alterations, which pave the way for increasing grades of
dysplasia and carcinoma. Furthermore, ROS have important functions
in intracellular signaling, partly with anti-carcinogenic effects
such as triggering apoptosis (31). Inadequate interaction between
neutrophils and colorectal mucosa by PSD methylation could
result in the disruption of the immune system in inflammatory bowel
disease, which would be implicated in the mechanisms underlying
UC-associated carcinogenesis. However, apoptotic pathways mediated
by PSD signaling still remain to be considered as direct
effectors on UC-associated colorectal mucosa to induce
apoptosis.
Although the number of samples included in this
study was limited and further investigations are required to draw
definitive conclusions, the present study demonstrates that the
inter-action between colorectal mucosa and neutrophils that governs
neutrophil chemotaxis and apoptosis is disturbed by aberrant
methylation of PSD, which may suppress the host immune
system and result in UC-associated carcinogenesis.
Acknowledgements
This work was supported in part by a grant-in-aid
for the post graduate student from Jichi Medical University, a
grant-in-aid from the Ministry of Education, Culture, Sports,
Science and Technology, and the JKA Foundation through its
promotion funds from Keirin Racing.
References
|
1
|
Ekbom A, Helmick C, Zack M and Adami HO:
Ulcerative colitis and colorectal cancer. a population-based study.
N Engl J Med. 323:1228–1233. 1990.
|
|
2
|
Lashner BA, Silverstein MD and Hanauer SB:
Hazard rates for dysplasia and cancer in ulcerative colitis.
Results from a surveillance program. Dig Dis Sci. 34:1536–1541.
1989.
|
|
3
|
Duerr RH, Taylor KD, Brant SR, et al: A
genome-wide association study identifies IL23R as an inflammatory
bowel disease gene. Science. 314:1461–1463. 2006.
|
|
4
|
Franke A, Balschun T, Karlsen TH, et al:
Replication of signals from recent studies of Crohn’s disease
identifies previously unknown disease loci for ulcerative colitis.
Nat Genet. 40:713–715. 2008.
|
|
5
|
Hugot JP, Chamaillard M, Zouali H, et al:
Association of NOD2 leucine-rich repeat variants with
susceptibility to Crohn’s disease. Nature. 411:599–603. 2001.
|
|
6
|
Barrett JC, Hansoul S, Nicolae DL, et al:
Genome-wide association defines more than 30 distinct
susceptibility loci for Crohn’s disease. Nat Genet. 40:955–962.
2008.
|
|
7
|
Hampe J, Franke A, Rosenstiel P, et al: A
genome-wide association scan of nonsynonymous SNPs identifies a
susceptibility variant for Crohn disease in ATG16L1. Nat Genet.
39:207–211. 2007.
|
|
8
|
Rioux JD, Xavier RJ, Taylor KD, et al:
Genome-wide association study identifies new susceptibility loci
for Crohn disease and implicates autophagy in disease pathogenesis.
Nat Genet. 39:596–604. 2007.
|
|
9
|
Barrett JC, Lee JC, Lees CW, et al:
Genome-wide association study of ulcerative colitis identifies
three new susceptibility loci, including the HNF4A region. Nat
Genet. 41:1330–1334. 2009.
|
|
10
|
Stoll M, Corneliussen B, Costello CM, et
al: Genetic variation in DLG5 is associated with inflammatory bowel
disease. Nat Genet. 36:476–480. 2004.
|
|
11
|
Humbert P, Russell S and Richardson H:
Dlg, Scribble and Lgl in cell polarity, cell proliferation and
cancer. Bioessays. 25:542–553. 2003.
|
|
12
|
Wakabayashi M, Ito T, Mitsushima M, et al:
Interaction of lp-dlg/KIAA0583, a membrane-associated guanylate
kinase family protein, with vinexin and beta-catenin at sites of
cell-cell contact. J Biol Chem. 278:21709–21714. 2003.
|
|
13
|
Okada S, Suzuki K, Kato T, et al: Aberrant
methylation of the Pleckstrin and Sec7 domain-containing gene is
implicated in ulcerative colitis-associated carcinogenesis through
its inhibition of apoptosis. Int J Oncol. DOI:
10.3892/ijo.2011.1231. 2011.
|
|
14
|
Eom YW, Yoo MH, Woo CH, et al: Implication
of the small GTPase Rac1 in the apoptosis induced by UV in Rat-2
fibroblasts. Biochem Biophys Res Commun. 285:825–829. 2001.
|
|
15
|
Gulbins E, Coggeshall KM, Brenner B,
Schlottmann K, Linderkamp O and Lang F: Fas-induced apoptosis is
mediated by activation of a Ras and Rac protein-regulated signaling
pathway. J BiolChem. 271:26389–26394. 1996.
|
|
16
|
Esteve P, Embade N, Perona R, et al:
Rho-regulated signals induce apoptosis in vitro and in vivo by a
p53-independent, but Bcl2 dependent pathway. Oncogene.
17:1855–1869. 1998.
|
|
17
|
Servant G, Weiner OD, Herzmark P, Balla T,
Sedat JW and Bourne HR: Polarization of chemoattractant receptor
signaling during neutrophil chemotaxis. Science. 287:1037–1040.
2000.
|
|
18
|
Servant G, Weiner OD, Neptune ER, Sedat JW
and Bourne HR: Dynamics of a chemoattractant receptor in living
neutrophils during chemotaxis. Mol Biol Cell. 10:1163–1178.
1999.
|
|
19
|
Wang F, Herzmark P, Weiner OD, Srinivasan
S, Servant G and Bourne HR: Lipid products of PI(3)Ks maintain
persistent cell polarity and directed motility in neutrophils. Nat
Cell Biol. 4:513–518. 2002.
|
|
20
|
Rutter M, Saunders B, Wilkinson K, et al:
Severity of inflammation is a risk factor for colorectal neoplasia
in ulcerative colitis. Gastroenterology. 126:451–459. 2004.
|
|
21
|
Franco M, Peters PJ, Boretto J, et al:
EFA6, a sec7 domain-containing exchange factor for ARF6,
coordinates membrane recycling and actin cytoskeleton organization.
EMBO J. 18:1480–1491. 1999.
|
|
22
|
Kurokawa K, Itoh RE, Yoshizaki H, Nakamura
YO and Matsuda M: Coactivation of Rac1 and Cdc42 at lamellipodia
and membrane ruffles induced by epidermal growth factor. Mol Biol
Cell. 15:1003–1010. 2004.
|
|
23
|
Boehm JE, Chaika OV and Lewis RE:
Rac-dependent anti-apoptotic signaling by the insulin receptor
cytoplasmic domain. J BiolChem. 274:28632–28636. 1999.
|
|
24
|
Joneson T and Bar-Sagi D: Suppression of
Ras-induced apoptosis by the RacGTPase. Mol Cell Biol.
19:5892–5901. 1999.
|
|
25
|
Nishida K, Kaziro Y and Satoh T:
Anti-apoptotic function of Rac in hematopoietic cells. Oncogene.
18:407–415. 1999.
|
|
26
|
Saez R, Chan AM, Miki T and Aaronson SA:
Oncogenic activation of human R-ras by point mutations analogous to
those of prototype H-ras oncogenes. Oncogene. 9:2977–2982.
1994.
|
|
27
|
Wang HG, Millan JA, Cox AD, et al: R-Ras
promotes apoptosis caused by growth factor deprivation via a Bcl-2
suppressible mechanism. J Cell Biol. 129:1103–1114. 1995.
|
|
28
|
Srinivasan S, Wang F, Glavas S, et al: Rac
and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and
polarity during neutrophil chemotaxis. J Cell Biol. 160:375–385.
2003.
|
|
29
|
Lampinen M, Ronnblom A, Amin K, et al:
Eosinophil granulocytes are activated during the remission phase of
ulcerative colitis. Gut. 54:1714–1720. 2005.
|
|
30
|
Glogauer M, Marchal CC, Zhu F, et al: Rac1
deletion in mouse neutrophils has selective effects on neutrophil
functions. J Immunol. 170:5652–5657. 2003.
|
|
31
|
Roessner A, Kuester D, Malfertheiner P and
Schneider-Stock R: Oxidative stress in ulcerative
colitis-associated carcinogenesis. Pathol Res Pract. 204:511–524.
2008.
|