Introduction
Recent statistics show that colorectal cancer (CRC)
is the third most commonly diagnosed cancer in men and the second
in women worldwide, with millions of new cancer cases being
reported annually. CRC is also the third most fatal cancer, causing
~600,000 deaths annually (1–4).
However, the precise mechanisms of immune suppression in CRC are
not yet completely understood.
Natural killer (NK) cells play a critical role in
innate immunity against viral infections and tumors. The functions
of NK cells are regulated by the integration of signals from
inhibitory and activating receptors (5). When activating signals are
predominant, NK cells are activated, and they show cytotoxic
activity and secrete cytokines. However, when inhibitory signals
are predominant, NK cells are not activated and do not show
antitumor immune responses.
Natural-killer group 2 member A (NKG2A) and
natural-killer group 2 member D (NKG2D), both of which belong to
the C-type lectin superfamily, are a pair of vital inhibitory and
activating receptors, respectively, in NK cells. Previous studies
have suggested that these receptors may be expressed in other
immune cells such as T and NKT cells (6–9). The
interaction between NKG2A/NKG2D and its ligands has been linked to
a wide variety of physiologic and pathologic functions (10–26).
Much effort has been devoted to understanding the roles of these
proteins in the regulation of activities of immune cells and
importance in immune responses. However, little is known about the
role of NKG2A/NKG2D in colorectal cancer. In this study, we
examined the expression of NKG2A/NKG2D in NK cells and determined
the role of the NKG2 pathway in the regulation of NK cytotoxicity
in patients with CRC.
We examined the expression of NKG2A and NKG2D in
peripheral blood mononuclear cells (PBMCs) and NK cells from
patients with CRC by using real-time PCR and flow cytometry.
Furthermore, we assessed the functions of NK cells by using
cytotoxicity assay and CD107a degranulation assay. We found that
the NKG2D expression levels were significantly lower in the CRC
patients than in the healthy controls, whereas NKG2A expression
levels in the CRC patients were similar to those in the healthy
controls. We also found that NK cell activity declined when NKG2D
signaling was blocked with anti-NKG2D antibodies. Therefore, we
concluded that the decrease in NKG2D expression may be connected to
NK cell suppression in patients with CRC. Thus, our study provides
a basis for the mechanism underlying the escape of tumor cells from
immune surveillance in vitro.
Materials and methods
Patients and controls
Sixty-two patients (34 men and 28 women) with
primary CRC were recruited from the gastrointestinal surgery ward
of Shandong Provincial Hospital, China. These patients were
diagnosed with CRC on the basis of colorectal cancer diagnosis
standard (2010) issued by Ministry of Health, China. The patients
had no history of other diseases such as heart disease, diabetes,
kidney disease, or autoimmune disease. Thirty-two healthy subjects
(18 men and 14 women) from the physical examination centre of
Shandong Provincial Hospital. Clinical characteristics of the
enrolled subjects are summarized in Table I. All individuals included in this
study were unrelated and randomly selected. The study was approved
by Shandong University Ethics Committee, and informed consent was
acquired from each individual.
 | Table IClinical characteristics of enrolled
subjects. |
Table I
Clinical characteristics of enrolled
subjects.
| Group | CRC | Healthy controls |
|---|
| Case | 62 | 32 |
| Gender (male) | 34 (54.8%) | 18 (56.3%) |
| Age (years)a | 54±11.2 | 50±8.5 |
Cells and cell culture
PBMCs were isolated from venous blood obtained from
CRC patients before surgery and from healthy subjects by using
Ficoll-Hypaque density gradient centrifugation (Tianjin Haoyang Bio
Co. Ltd., China).
NK cells were isolated from PBMCs by positive
selection by using magnetic cell separation (Miltenyi Biotec,
Germany) according to the manufacturer’s instructions. Flow
cytometry revealed the purity of CD3-CD56+ NK
cells to be >95%. For functional assays, the obtained NK cells
were cultured in RPMI-1640 medium (Invitrogen, China) with 10%
heat-inactivated fetal calf serum (FCS) (Invitrogen) and 100 U/ml
recombinant human interleukin 2 (rIL-2) (Sangon Bio Co. Ltd.,
China).
Human colon carcinoma cell line HT29 was kindly
provided by Professor Zhang Jian, School of Pharmaceutical Science,
Shandong University. HT29 cells were cultured in RPMI-1640
supplemented with 10% FCS and were used as target cells. The medium
was regularly changed, and the cells were always washed twice
before use.
Neutralizing antibodies against NKG2D (R&D
Systems, USA) were used in antibody-blocking experiments. Purified
NK cells were pre-incubated with 10 μg/ml anti-NKG2D antibodies for
30 min before they were cultured with the target cells. Then, the
cytotoxicity and CD107a degranulation assays were performed.
Real-time PCR
Total cellular RNA was extracted from PBMCs by using
TRIzol reagent (Invitron, USA) according to the manufacturer’s
instructions. Concentration and quality of the extracted total RNA
were determined by measuring its light absorbance at 260 nm (A260)
and the ratio of (A260/A280). A 1 μg of total RNA was reverse
transcribed in a 20-μl reaction mixture containing 2 μl of Maxima
enzyme mix (Fermentas, Canada) and 4 μl of 5X PCR mix (Fermentas).
The procedure for reverse transcription was performed as follows:
10 min at 25°C, 30 min at 55°C, and then 5 min at 85°C. All these
procedures were performed using the GeneAmp PCR system 2720
(Applied Biosystems, USA). The obtained cDNA was diluted 1:10
before PCR analysis.
The primers used for CD94, NKG2A, NKG2D, and β-actin
are shown in Table II. All the
primers were synthesized and validated by Sangon Bio Co. Ltd.
Reactions were performed in a total volume of 20 μl, which included
5 μl of cDNA sample, 5 μl of 0.8 μM primer pair, and 10 μl of 2X
PCR mix (Takara, Japan). PCR was performed as follows: 5 min at
95°C and 45 cycles of 15 sec at 95°C, 30 sec at 60°C, and 15 sec at
72°C. Incubation and on-line detection of the PCR products were
carried out using optical 96-well plates and the LightCycler 480
sequence detection system (Roche, Germany). Finally, the PCR
products were subjected to electrophoresis to determine whether the
required products were formed.
 | Table IIPrimer pairs used in real-time PCR
analysis. |
Table II
Primer pairs used in real-time PCR
analysis.
| Primer | Sequence |
|---|
| CD94 |
| Forward | 5′-TTG ATG GCT ACG
TTG GGA ATT-3′ |
| Reverse | 5′-TTG GCA AGA ACA
GCA GTC AGA-3′ |
| NKG2A |
| Forward | 5′-TTG CTG GCC TGT
ACT TCG A-3′ |
| Reverse | 5′-CCA AAC CAT TCA
TTG TCA CCC-3′ |
| NKG2D |
| Forward | 5′-TTC AAC ACG ATG
GCA AAA GC-3′ |
| Reverse | 5′-CTA CAG CGA TGA
AGC AGC AGA-3′ |
| β-actin |
| Forward | 5′-ACA GAG CCT CGC
CTT TGC C-3′ |
| Reverse | 5′-ACA TGC CGG AGC
CGT TGT C-3′ |
Flow cytometry
PBMCs were washed with phosphate-buffered saline
(PBS) containing 2% FCS and stained with anti-CD3-PC5 (Beckman
Coulter, USA), anti-CD56-FITC (Beckman Coulter), anti-NKG2A-PE
(Becton-Dickinson, USA), and anti-NKG2D-PE (Becton-Dickinson)
antibodies. At least 10,000 cells were analyzed using a 3-color
EPICS XL flow cytometer (Beckman Coulter).
For CD107a degranulation assays, NK cells and HT29
cells were co-cultured for 4 h at 37°C. Then, they were washed with
PBS and stained with anti-CD3-PC5, anti-CD56-FITC, and
anti-CD107a-PE antibodies (Becton-Dickinson). Expression of CD107a
on CD3-CD56+ NK cells was analyzed by using the EPICS XL
flow cytometer.
Cytotoxicity assays
HT29 cells were used as target cells, and the
purified NK cells were used as effector cells in the
51Cr release assay. The HT29 cells were labeled by
incubating them with the 51Cr isotope for 1 h at 37°C.
The labeled targets were then co-incubated with NK cells at
different ratios for 4 h. The supernatant was harvested and
analyzed using a γ-counter (Packard Cobra II 5002, USA). The
percentage of 51Cr released (counts per min, c.p.m.) was
calculated as follows: [(experimental release - spontaneous
release)/(maximum release - spontaneous release)] ×100%.
Spontaneous release was <15% of the maximum release. All
experiments were performed in triplicates.
Statistical analysis
A t-test was used for comparing the findings of the
patient group and the control group. A one-way Anova was used for
comparing the data of three or four groups. Probability values were
considered significant at p<0.05. Statistical analyses were
performed by using the GraphPad Prism 4 (GraphPad Software,
USA).
Results
Expression levels of NKG2A mRNA in the
CRC patients were similar to those in the healthy controls, whereas
those of NKG2D mRNA in the PBMCs of the CRC patients were lower
than those in the healthy controls
To evaluate the expression levels of NKG2A and NKG2D
mRNA in PBMCs, we performed real-time PCR analysis for 32 CRC
patients and 20 healthy subjects. There was no difference in NKG2A
expression levels in PBMCs in the CRC patients and in the healthy
controls [mean ± SD, 1.02±0.47 (CRC patients) vs. 1.25±0.52
(healthy controls)]; p>0.05] (Fig.
1B). Instead, there was a significant decline in NKG2D
expression in the CRC group [1.11±0.60 (CRC patients) vs. 1.65±0.71
(healthy controls); p<0.01] (Fig.
1C). Taken together, these results indicated that the
expression of inhibitory receptor NKG2A mRNA did not change,
whereas that of the activating receptor NKG2D mRNA decreased in CRC
patients.
Expression levels of NKG2A protein were
similar in the CRC patients and healthy controls, whereas those of
NKG2D protein in the PBMCs were lower in the CRC patients than in
the healthy controls
Flow cytometry studies in 30 CRC patients and 12
healthy subjects showed that NKG2A protein levels were similar in
both the groups [10.83%±3.11% (CRC patients) vs. 10.15%±2.20%
(healthy group); p>0.05] (Fig. 2A
and C). However, the NKG2D protein levels were significantly
lower in the CRC patients than in the healthy controls
[42.70%±7.35% (CRC group) vs. 50.06%±7.13% (healthy groups);
p<0.01] (Fig. 2B and D). These
results indicated that the expression levels of NKG2A protein were
similar in both the groups, whereas those of NKG2D protein were
lower in the CRC patients than in the healthy controls.
Although NKG2A expression levels were
similar in the CRC patients and healthy controls, NKG2D expression
levels in NK cells were lower in the CRC patients than in the
healthy controls
NKG2A expression levels in NK cells were similar in
both the groups [42.34%±13.20% (CRC group) vs. 44.91%±13.77%
(healthy group); p>0.05] (Fig. 3A
and C). However, the number of NKG2D+ NK cells in
the CRC patients was significantly lower than that in the healthy
controls [71.23%±8.31% (CRC group) vs. 79.39%±5.58% (healthy
group); p<0.01] (Fig. 3B and
C). These results indicated that, although the expression of
NKG2A in NK cells was similar in both groups, those of NKG2D in NK
cells were lower in the CRC than in the healthy controls.
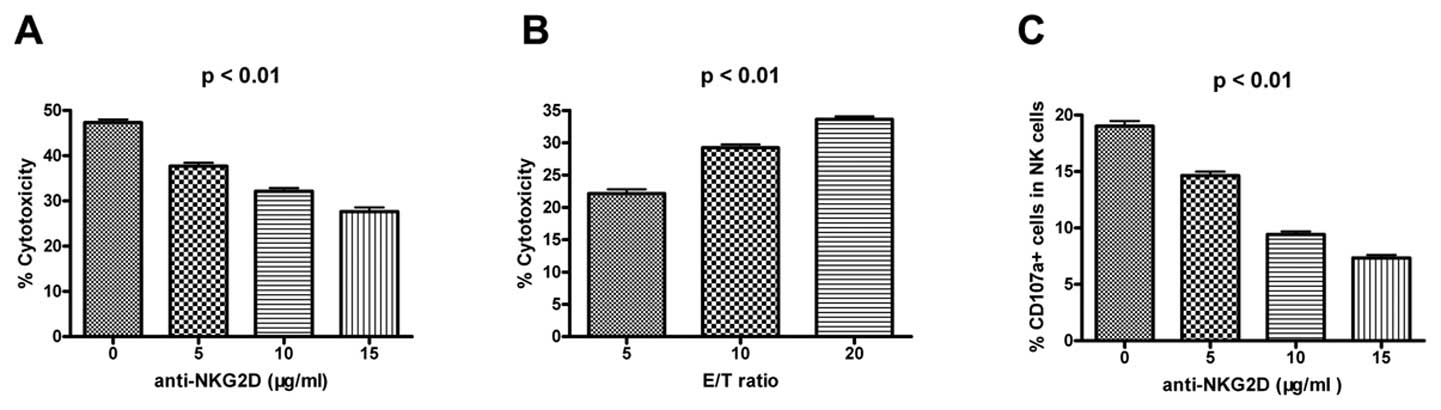
Blocking NKG2D expression reduces NK
cytotoxicity and CD107a degranulation
In order to investigate the role of NKG2D in NK
cells, we used anti-NKG2D antibodies to block the NKG2D pathway in
an NK cell-mediated cytotoxicity assay. NK cytotoxicity (Fig. 4A and B) and CD107a degranulation
(Fig. 4C) decreased with an
increase in the concentration of anti-NKG2D antibody. These results
suggested that NKG2D could activate NK cell functions. Therefore, a
decrease in NKG2D expression levels would result in a decrease in
the level of NK cell activity.
Expression of NKG2A and NKG2D in NK
cells, NKT cells, and T cells
We also found that NKG2A and NKG2D were expressed on
the membranes of CD3+CD56− T and
CD3+CD56+ NKT cells. However, NKG2A
expression levels decreased successively in NK cells, NKT cells,
and T cells (Fig. 5A; p<0.01).
The NKG2D expression levels in T cells were lower than the
corresponding levels in NK cells and NKT cells (Fig. 5B; p<0.01).
Discussion
In this study, we first detected the expression of
NKG2A and NKG2D by using real-time PCR. We found that NKG2A
expression levels were similar in both groups, whereas NKG2D
expression levels were significantly lower in the CRC patients than
in the healthy controls. These findings suggest an imbalance in
NKG2A/NKG2D expression at the transcription level in CRC
patients.
Next, we detected NKG2A and NKG2D expression in
PBMCs by flow cytometry and found that NKG2A expression levels in
PBMCs and NK cells in the CRC patients were similar to the
corresponding levels in the healthy controls. However, NKG2D
expression levels in not only PBMCs but also NK cells were
significantly lower in CRC patients than in the healthy controls. A
similar kind of decrease in NKG2D expression levels was observed in
patients with hepatic carcinoma (27). Therefore, these findings suggest
that NKG2D expression may be altered at the translation level in
CRC patients. Taken together, the balance of inhibitory NKG2A and
activating NKG2D receptors shifted in CRC patients. Once the NKG2
receptors recognize and combine with their corresponding ligands on
tumor cells, inhibitory signals are generated predominantly. NKG2D
ligands MICA, MICB, and ULBPs and NKG2A ligand HLA-E may be
expressed in CRC patients (13,15,23,27).
HLA-E expression on the surface of tumor cells may allow the tumor
cells to escape the immune surveillance by T and NK cells (13,15,22).
How does this shift affect the functions of NK cells
in CRC patients? We assessed NK cell-mediated cytotoxicity and
CD107a degranulation and found NK cytotoxicity and CD107a
degranulation dropped to a lower degree when NKG2D expression was
blocked in CRC patients. These results are in accordance with the
results of previous studies on other tumors (18,28,29).
Therefore, we concluded that NKG2D plays an important role in
activating NK cells, and the decrease in NKG2D expression level may
result in decline of the activity of NK cells in CRC patients.
Thus, NK cell-mediated antitumor immune responses may be inhibited
via the NKG2 pathway in CRC patients. Recent studies have tried to
illustrate the role of this pathway in spontaneous malignancy in
NKG2D-deficent animal models (17).
Previous studies have stated that tumor cells may
escape immune surveillance through a variety of mechanisms, despite
innate and adaptive immunity in humans. Our study shows that the
imbalance of NKG2A and NKG2D expression may mediate the suppression
of NK cell activity in CRC patients, thereby contributing to the
escape of tumor cells from NK-mediated lysis. It has been reported
that some cytokines, for instance, IL-2, IL-12, IL-15, and IFN-α,
may change NKG2 expression levels and enhance NK cell-mediated
cytotoxicity (27,28,30).
These studies provide a promising prospect for immunotherapy for
carcinomas.
In conclusion, our results showed that decrease of
NKG2D expression level in CRC patients may be associated with
suppression of NK cell activity. We inferred that tumor cells may
escape NK cell surveillance via the NKG2 pathway in CRC patients;
however, the underlying mechanism needs to be investigated further
in detail. The current study is a starting point in our continuous
efforts to understand the NKG2 immune escape pathway and the
pathogenesis of CRC. Our results may provide important insights for
the development of anticancer strategies.
Acknowledgements
This work was supported by grants from Shandong
Medicine and Health Technology Development Program (No. 2009Q2022)
and Shandong Outstanding Young and Middle-aged Scientists Research
Award Fund (No. 2008BS03032). We thank the participants in the
study for making this work possible.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
5
|
Lanier LL: NK cell receptors. Annu Rev
Immunol. 16:359–393. 1998. View Article : Google Scholar
|
|
6
|
Bauer S, Groh V, Wu J, et al: Activation
of NK cells and T cells by NKG2D, a receptor for stress-inducible
MICA. Science. 285:727–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eleme K, Taner SB, Onfelt B, et al: Cell
surface organization of stress-inducible proteins ULBP and MICA
that stimulate human NK cells and T cells via NKG2D. J Exp Med.
199:1005–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ota T, Takeda K, Akiba H, et al:
IFN-gamma-mediated negative feedback regulation of NKT-cell
function by CD94/NKG2. Blood. 106:184–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheu BC, Chiou SH, Lin HH, et al:
Up-regulation of inhibitory natural killer receptors CD94/NKG2A
with suppressed intracellular perforin expression of
tumor-infiltrating CD8+ T lymphocytes in human cervical
carcinoma. Cancer Res. 65:2921–2929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lanier LL: Follow the leader: NK cell
receptors for classical and nonclassical MHC class I. Cell.
92:705–707. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Groh V, Rhinehart R, Secrist H, Bauer S,
Grabstein KH and Spies T: Broad tumor-associated expression and
recognition by tumor-derived gamma delta T cells of MICA and MICB.
Proc Natl Acad Sci USA. 96:6879–6884. 1999. View Article : Google Scholar
|
|
12
|
Wu J, Song Y, Bakker AB, et al: An
activating immunoreceptor complex formed by NKG2D and DAP10.
Science. 285:730–732. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto K, Fujiyama Y, Andoh A, Bamba T
and Okabe H: Oxidative stress increases MICA and MICB gene
expression in the human colon carcinoma cell line (CaCo-2). Biochim
Biophys Acta. 1526:10–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gasser S, Orsulic S, Brown EJ and Raulet
DH: The DNA damage pathway regulates innate immune system ligands
of the NKG2D receptor. Nature. 436:1186–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palmisano GL, Contardi E, Morabito A,
Gargaglione V, Ferrara GB and Pistillo MP: HLA-E surface expression
is independent of the availability of HLA class I signal
sequence-derived peptides in human tumor cell lines. Hum Immunol.
66:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao W, Xi X, Wang Z, et al: Four novel
ULBP splice variants are ligands for human NKG2D. Int Immunol.
20:981–991. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guerra N, Tan YX, Joncker NT, et al:
NKG2D-deficient mice are defective in tumor surveillance in models
of spontaneous malignancy. Immunity. 28:571–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ljunggren HG: Cancer immunosurveillance:
NKG2D breaks cover. Immunity. 28:492–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melum E, Buch S, Schafmayer C, et al:
Investigation of cholangiocarcinoma associated NKG2D polymorphisms
in colorectal carcinoma. Int J Cancer. 123:241–242. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nausch N and Cerwenka A: NKG2D ligands in
tumor immunity. Oncogene. 27:5944–5958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barber A and Sentman CL: Chimeric NKG2D T
cells require both T cell-and host-derived cytokine secretion and
perforin expression to increase tumor antigen presentation and
systemic immunity. J Immunol. 183:2365–2372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berezhnoi AE, Chernisheva AD, Zakeeva IR,
et al: HLA-E molecule induction on the surface of tumor cells
protects them from cytotoxic lymphocytes. Vopr Onkol. 55:224–229.
2009.(In Russian).
|
|
23
|
Kopp R, Glas J, Lau-Werner U, Albert ED
and Weiss EH: Association of MICA-TM and MICB C1_2_A microsatellite
polymorphisms with tumor progression in patients with colorectal
cancer. J Clin Immunol. 29:545–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng XH, Sha WH, Li YY, Nie YQ, Li QN and
Liang PZ: Expression of NK cells receptor NKG2D from peripheral
blood in patients with primary hepatic carcinoma and its clinical
significance. Zhonghua Yi Xue Za Zhi. 89:1272–1274. 2009.(In
Chinese).
|
|
25
|
Bjorklund AT, Schaffer M, Fauriat C, et
al: NK cells expressing inhibitory KIR for non-self-ligands remain
tolerant in HLA-matched sibling stem cell transplantation. Blood.
115:2686–2694. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kren L, Slaby O, Muckova K, et al:
Expression of immune-modulatory molecules HLA-G and HLA-E by tumor
cells in glioblastomas: an unexpected prognostic significance?
Neuropathology. 31:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang C, Zhang J, Sun R, Feng J, Wei H and
Tian Z: Opposing effect of IFNgamma and IFNalpha on expression of
NKG2 receptors: negative regulation of IFNgamma on NK cells. Int
Immunopharmacol. 5:1057–1067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Zhang J, Niu J, Zhou Z and Tian
Z: Interleukin-12 improves cytotoxicity of natural killer cells via
upregulated expression of NKG2D. Hum Immunol. 69:490–500. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serrano AE, Menares-Castillo E,
Garrido-Tapia M, et al: Interleukin 10 decreases MICA expression on
melanoma cell surface. Immunol Cell Biol. 89:447–457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Sun R, Wei H and Tian Z:
Characterization of interleukin-15 gene-modified human natural
killer cells: implications for adoptive cellular immunotherapy.
Haematologica. 89:338–347. 2004.PubMed/NCBI
|