Introduction
Bladder cancer is one of the most common urogenital
malignancies. More than 2/3 of bladder cancer presents as
superficial (pTa or pT1), which means the tumor is confined to the
epithelium or lamina propria. Noninvasive bladder cancer can be
completely resected by transurethral resection of bladder tumors.
However, 50–70% of bladder cancer recurs and 15–30% progresses to
muscle invasive disease, despite a complete transurethral operation
(1). Therefore, bladder cancer is
a highly recurrent disease and intravesical instillation of
anti-cancer agents after transurethral operation is an attractive
strategy to prevent the cancer cell dissemination and recurrence.
Intravesical chemotherapy and/or immunotherapy are often added
after a transurethral operation to prevent intravesical cancer
recurrence and progression. However, the tumor recurrence rates are
still high and reported to be 30–44% with the adjuvant intravesical
treatment (2). Therefore, novel
therapeutic agents for the treatment of superficial lesion and
floating/disseminated cancer cells are necessary.
The reduced expression in immortalized cell (REIC)
gene is identical to Dickkopf-3 (Dkk-3), which is a member of the
Dickkopf gene family. Expression of REIC/Dkk-3 gene is
significantly downregulated in a broad range of human cancer cells,
but typically expressed in non-malignant cells (3–10).
The REIC/Dkk-3 is thought to be a tumor suppressor gene and to
provide a possible means of gene therapy for human malignant
tumors. An adenovirus vector carrying REIC/Dkk-3 (Ad-REIC) induces
apoptosis in prostate cancer and testicular cancer, but not in
normal cells (5,9,10).
Overexpression of REIC/Dkk-3 protein in cancer cells by Ad-REIC
treatment leads to endoplasmic reticulum (ER) stress and activation
of c-Jun-NH2-kinase (JNK), which induce cancer specific cell
apoptosis (9).
The aim of present study was to investigate the
potential of Ad-REIC as a therapeutic agent for bladder cancer.
Recent study showed that some human bladder cancer cell lines are
resistant to Ad-REIC treatment for apoptosis induction (11). This study used an in vitro
cancer cell floating condition to assess the possibility of Ad-REIC
intravesical treatment and re-evaluated the efficacy of Ad-REIC in
these resistant bladder cancer cell lines. In addition, the
appearance of cancer cells resistant to multiple chemotherapeutic
agents is a serious obstacle. Doxorubicin (adriamycin) is a major
intravesical chemotherapeutic agent that is used immediately after
a transurethral operation to prevent cancer cell dissemination
(12). However, doxorubicin
resistant bladder cancer has become a clinical problem. The
multidrug resistance phenotype is often associated with increased
expression of adenosine triphosphate (ATP) binding cassette (ABC)
superfamily proteins, P-glycoprotein (P-gp) and multidrug
resistance-associated protein1 (MRP1) (13,14).
The enhancement of the JNK pathway downregulates P-gp and reverses
P-gp mediated multidrug resistance in cancer cells (15). These findings led to the hypothesis
that the JNK activation by Ad-REIC treatment might be able to
downregulate P-gp and overcome multidrug resistance. Therefore,
this study also investigated the potential of Ad-REIC as a
sensitizer of chemotherapeutic agents in the adriamycin resistant
KK47 bladder cancer cells.
Materials and methods
Cells and cell culture
Human bladder cancer cell lines, KK47 (KK47/Wt),
RT4, T24, J82 and TccSup were obtained from the American Type
Culture Collection (Rockville, MD, USA). The adriamycin resistant
human bladder cancer cell line KK47/ADM was kindly provided by
Professor S. Naito (Department of Urology, University of Kyushu,
Fukuoka, Japan) (16). KK47/ADM
cells were grown in RPMI-1640 medium (Sigma, St. Louis, MO, USA)
supplemented with 10% (v/v) fetal bovine serum, penicillin (100
IU/ml), streptomycin (100 μg/ml), and 1 μM doxorubicin (Adriacin™,
Kyowa Hakkoh Co., Tokyo, Japan). Other bladder cell lines were
grown in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine
serum, penicillin (100 IU/ml) and streptomycin (100 μg/ml).
Adenovirus vector carrying REIC/Dkk-3
(Ad-REIC)
A full-length cDNA of REIC/Dkk-3 was integrated into
the cosmid vector, pAxCAwt, and then it was transferred into an
adenovirus vector by the COS-TPC method (Takara Bio, Shiga, Japan)
(17). An adenovirus vector
carrying the LacZ gene (Ad-LacZ) was used as a control, as
described previously (5).
Apoptosis assay
A sample of 5.0×105 cells were seeded in
flat-bottom 6-well plates and incubated for 24 h. The cells were
then treated with Ad-LacZ and Ad-REIC at the indicated MOI in 0.5
ml of complete medium for 1 h, and 1.5 ml of fresh medium was added
and the cells were incubated for 72 h. The apoptotic cells were
visualized by Hoechst 33342 staining. Hoechst 33342 is an
intercalating dye that can help to determine the total chromatin
quantity variations and the degree of chromatin condensation
(18,19). The dye solution was added into the
medium and incubated in the dark for 10 min. The cells were
directly observed with phase contrast and fluorescence microscopy.
The apoptotic cells were identified by the presence of highly
condensed or fragmented nuclei. Apoptotic cells were counted in
five different fields using microscopic observations. One hundred
cells were judged under one field.
Cell viability assay
The cells were detached by trypsin and
5.0×105 cells in 1 ml complete medium were put into 15
ml tube to assess the anti-proliferative effect of Ad-REIC in the
floating cell condition. These floating cancer cells were treated
with Ad-LacZ or with Ad-REIC at the indicated MOI with constant
agitation for 1 h. These cells were then seeded in flat-bottom
6-well plates and incubated for 72 h. The cell viability was
determined using the MTS assay (CellTiter 96® Aqueous
One Solution Cell Proliferation Assay, Promega Corp., Madison, WI,
USA), according to the manufacturer’s instructions.
The cells were seeded in flat-bottom 96-well
microplates at a concentration of 1,000 cells per well to carry out
the cell viability assay in the Ad-REIC and doxorubicin combined
treatment. The cells were incubated for 24 h and treated with
Ad-LacZ and Ad-REIC at 100 MOI in the complete medium for 1 h. The
medium was exchanged to the fresh medium and the cells were
incubated for 24 h. The floating dead cells were removed and
attached cells were treated with doxorubicin at the indicated
concentration for 48 h. The cell viability was determined using the
MTS assay.
Western blot analysis
The cells were treated with Ad-LacZ and Ad-REIC at
100 MOI, and cultured for 24 h. The floating dead cells were
removed and attached cells were lysed for the sample. The cells
were lysed with ice-cold lysis buffer to extract proteins.
Insoluble fragments were removed by centrifugation, and the
supernatants were adjusted to equal protein concentration in each
experiment. Samples (10 μg of protein) were separated on a 7.5%
SDS-PAGE gel and transferred onto a polyvinylidene fluoride
membrane (PVDF membranes; Millipore, Billerica, MA, USA) for
western blotting. Following the transfer, the membranes were
blocked for 1 h with 5% nonfat milk powder, 6% glycine and 0.1%
Tween-20 in Tris buffered saline (TBS) at room temperature. The
membranes were incubated for 1 h at room temperature with the
primary antibodies; T5168 (1:8,000) for tubulin (Sigma), rabbit
anti-human REIC/Dkk-3 antibody raised in this laboratory (1:1,000)
and C219 (1:250) for P-gp (Calbiochem, San Diego, CA, USA). After 3
washes in TBS supplemented with 0.1% Tween-20, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
for 1 h at room temperature. The bound antibodies were visualized
by the enhanced chemiluminescence detection method (ECL kit,
Amersham Pharmacia Biotech, Chandler, AZ, USA) using medical X-ray
film. JNK inhibitor (1 μM) (SP600125, A.G. Scientific Inc., San
Diego, CA, USA) was added to inhibit the kinase activity of JNK in
some experiments.
Statistical analysis
The data are presented as the mean ± SE. Student’s
unpaired t-test was performed for the statistical analysis between
the two groups and the difference was considered to be significant
at p<0.05. The analyses were carried out using the StatView 4.5
software package (Abacus Concepts, Berkeley, CA, USA).
Results
Apoptosis induction by Ad-REIC treatment
in various human bladder cancer cell lines
Significant apoptotic induction was observed in KK47
and RT4 human bladder cancer cells after Ad-REIC treatment, but not
in T24, J82 and TccSup cells (Fig. 1A
and B). The incidence of apoptosis by Ad-REIC at 100 MOI was
75.6% in KK47 and 78.0% in RT4 and a significant difference was
observed in comparison to the control Ad-LacZ treatment at 100 MOI.
The apoptotic cell rate by Ad-REIC in T24, J82 and TccSup human
bladder cancer cells was 4.2%, 2.0% and 1.8%, respectively.
Therefore, KK47 and RT4 cells are sensitive to Ad-REIC treatment,
however, T24, J82 and TccSup cells are resistant under these
conditions.
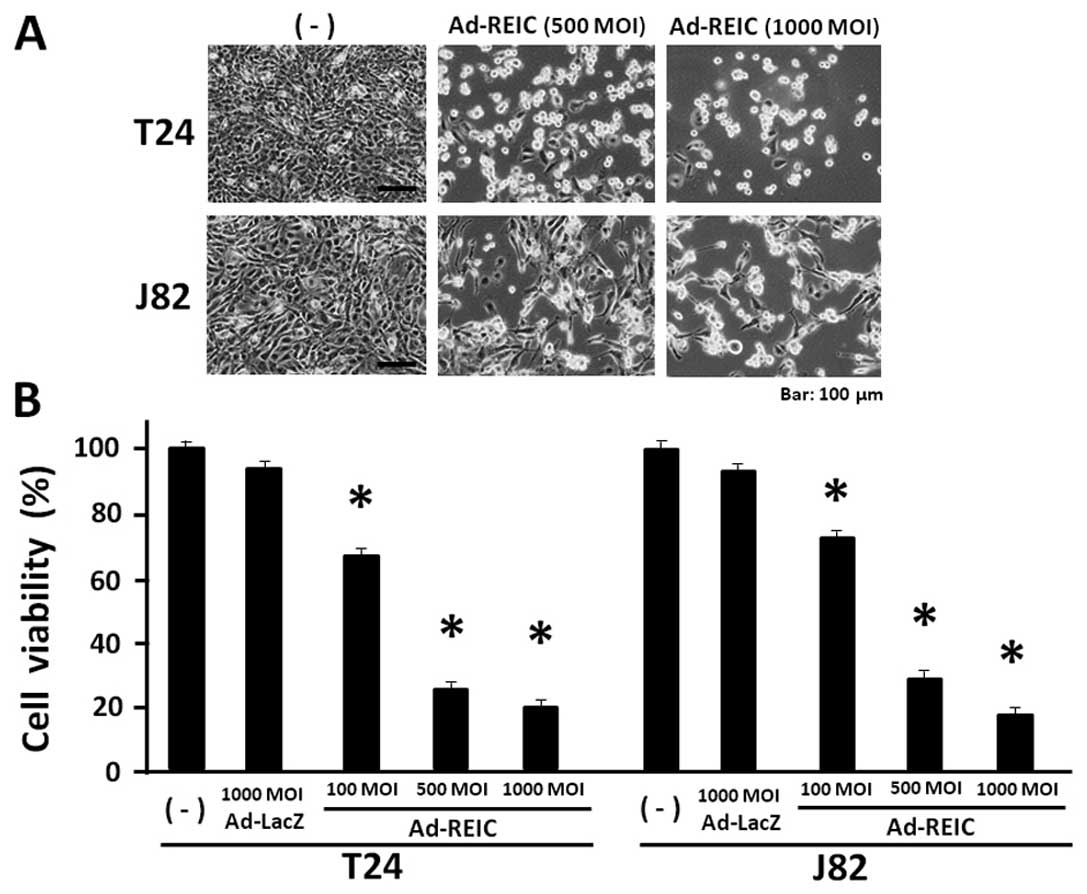
Ad-REIC significantly reduces bladder
cancer cell viability in the floating cell condition
T24, J82 and TccSup cancer cells were resistant to
the Ad-REIC treatment for apoptosis induction under standard cell
culture conditions. These resistant cancer cell lines were
re-evaluated for Ad-REIC treatment in a floating cell condition,
which is clinically observed after transurethral operation and
becomes a cause of the intravesical cancer dissemination. The
resistant cancer cell lines, T24 and J82 were treated with Ad-REIC
at 100, 500 and 1,000 MOI in a floating culture for 1 h and then
cultured for 72 h to access the anti-proliferative effect of
Ad-REIC. Significant cell growth inhibition was observed in both
T24 and J82 bladder cancer cell lines (Fig. 2A). The MTS assay showed that the
cell viability at Ad-REIC 1,000 MOI was 20.0% in T24 and 18.2% in
J82 cells and a significant reduction in cell viability was
observed in comparison to that of the control Ad-LacZ treatment at
1,000 MOI (Fig. 2B). Therefore,
significant cell growth inhibition was observed in T24 and J28
cells by the floating cell culture with Ad-REIC at 100, 500 and
1,000 MOI. The TccSup cancer cells also showed significant growth
suppression following the Ad-REIC treatment at 1,000 MOI in
comparison to the control Ad-LacZ treatment (data not shown).
Apoptosis induction in KK47/Wt and
KK47/ADM cells by Ad-REIC treatment
Apoptosis induction by Ad-REIC was assayed in
adriamycin resistant KK47 bladder cancer cells (KK47/ADM) which
also presents multidrug resistance (16). The incidence of apoptosis by
Ad-REIC at 100 MOI was 75.6% in KK47/Wt and 65.2% in KK47/ADM and
significant apoptosis induction was observed in comparison to the
control Ad-LacZ treatment (Fig.
3). The incidence of apoptosis by Ad-REIC at 10 MOI was not
significant in either KK47/Wt or KK47/ADM cells in comparison to
the control Ad-LacZ treatment.
Ad-REIC treatment sensitizes multidrug
resistant KK47/ADM cells to doxorubicin
The multidrug resistant KK47/ADM cells were used to
determine the effect of combined treatment with Ad-REIC and
doxorubicin (adriamycin). KK47/Wt and KK47/ADM cells were divided
into three groups of no treatment, Ad-LacZ and Ad-REIC at 100 MOI.
The ability of Ad-REIC to reverse drug resistance was evaluated by
exposing the cells to increasing concentrations of doxorubicin.
KK47/Wt cells showed no significant difference between the
treatment groups (Fig. 4).
However, Ad-REIC treatment significantly shifted the dose-response
curve for doxorubicin toxicity to lower concentrations in KK47/ADM
cells (Fig. 4). The cell viability
at 2.5 μM doxorubicin was 80.4%, 85.3% and 42.3% in the no
treatment, Ad-LacZ and Ad-REIC groups, respectively. Therefore,
Ad-REIC treatment partially restored the sensitivity of KK47/ADM
cells to doxorubicin.
Ad-REIC treatment downregulates P-gp
expression in KK47/ADM cells in a JNK-dependent manner
The expression level of REIC/Dkk-3 and P-gp after
Ad-REIC treatment was examined using western blot analysis
(Fig. 5). REIC/Dkk-3 protein was
strongly expressed by Ad-REIC treatment in both KK47/Wt and
KK47/ADM cells. P-gp, the representative multidrug resistant
protein, was expressed in KK47/ADM cells with no treatment or
Ad-LacZ treatment. Ad-REIC treatment significantly down-regulated
the expression of P-gp in KK47/ADM cells. The JNK pathway is a
crucial factor for the cancer specific apoptosis in Ad-REIC
treatment (5). SP600125, a JNK
inhibitor, inhibits the kinase activity of JNK (20). Combined treatments with Ad-REIC and
SP600125 reversed the expression level of P-gp in KK47/ADM cells.
Therefore, Ad-REIC treatment suppressed the P-gp expression in the
multidrug resistant KK47/ADM cells in a JNK-dependent manner.
Discussion
This study showed that KK47 and RT4 cells are
sensitive to Ad-REIC treatment for apoptosis induction and three
human bladder cancer cell lines, T24, J82, and TccSup, are
resistant. However, the efficacy of Ad-REIC treatment must not be
evaluated in the standard cell culture conditions but in floating
cell conditions in order to demonstrate the utility of Ad-REIC as
intravesical therapeutic agent to prevent the recurrence of bladder
cancer. Floating cancer cells are clinically observed after
transurethral operation and are a cause of the intravesical cancer
dissemination and recurrence. We herein demonstrated that Ad-REIC
treatment significantly inhibited cell proliferation of the
resistant bladder cancer cell lines in the floating cell condition.
Since the Ad-REIC treatment did not inhibit proliferation of T24
and J82 cancer cells under the standard cell culture condition
(data not shown), the floating cancer cells are more sensitive to
Ad-REIC treatment. It is conceivable that Ad-REIC could transfect
more efficiently to the floating cancer cells than the attached
cells on the dish and therefore indicated the significant
anti-cancer effect in the floating cell condition. The influence of
adenovirus itself on the cells can be denied, because there was no
significant difference between no treatment and Ad-LacZ treatment.
Finally, Ad-REIC has anti-cancer effect in 6 human bladder cancer
cell lines, RT4, KK47/Wt, KK47/ADM, T24, J82 and TccSup. This
result suggests that the intravesical instillation with Ad-REIC
could be an attractive therapeutic strategy to treat superficial
lesion and floating/disseminated cells of human bladder cancer.
Bladder cancer often recurs after the transurethral
resection of bladder tumors and such recurrent tumors arise at
different sites in the urothelium. Even though the tumor is
completely resected macroscopically by the operation, microscopic
floating or disseminated cells will implant in the bladder
epithelium and the spread of the original clone forms multifocal
tumors (21). The intravesical
instillation of chemotherapeutic agents immediately after
transurethral operation is clinically performed to prevent tumor
recurrence (22,23) and it is important for the
intravesical chemotherapy to kill the floating or disseminated
malignant cells in the bladder. Representative anti-cancer agents
for intravesical chemotherapy are epirubicin, mitomycin C and
doxorubicin. However, multidrug resistant cancer cells or multidrug
resistant cancer cells newly emerge during repeated doxorubicin
treatments. The present study showed that combination therapy with
Ad-REIC and doxorubicin significantly suppressed the growth of the
multidrug resistant cell line KK47/ADM by restoring the sensitivity
to doxorubicin.
Multidrug resistance is mainly attributed to the
overexpression of efflux transporters such as P-gp and MRP1. P-gp
and MRP1 are members of the ATP binding cassette (ABC) superfamily
of transporters and are capable of effluxing many chemotherapeutics
out of cancer cells, and allowing them to survive the toxic insult
(13,14). There is a positive correlation
between the expression of P-gp and multidrug resistant phenotypes
in transitional cell carcinoma (24). On the other hand, the relationship
between P-gp expression and JNK pathway was highlighted in a
previous study showing that enhancement of the JNK pathway
downregulates P-gp and reverses P-gp mediated multidrug resistance
in cancer cells (15).
Overexpression of REIC/Dkk-3 by Ad-REIC in cancer cells gives rise
to endoplasmic stress and induces cancer cell specific apoptosis
through the activation of JNK and c-Jun, whereas apoptosis is not
induced in normal cells (5,25).
The Ad-REIC treatment in KK47/ADM cells seemed to downregulate P-gp
expression through JNK activation and reversed the drug resistance
to doxorubicin. Similar findings were also obtained in the
multidrug resistant human breast cancer MCF7/ADR cells. Ad-REIC
treatment upregulates the expression of phosphorylated JNK and
c-Jun in MCF7/ADR cells, and downregulates the level of P-gp
following partial reversal of doxorubicin resistance (26).
The current study demonstrated two therapeutic
aspects of the Ad-REIC agent against human bladder cancer cells,
namely as an apoptosis inducer/cell growth inhibitor and as a
sensitizer to chemotherapeutic agents in the multidrug resistant
cancer cells. Therefore, the intravesical instillation of Ad-REIC
could be an attractive therapeutic method in human bladder cancer
where the treatment of superficial lesions and
floating/disseminated or multidrug resistant cancer cells is
required.
References
|
1
|
Dobruch J and Herr H: Should all patients
receive single chemotherapeutic agent instillation after bladder
tumour resection? BJU Int. 104:170–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurth KH, Bouffioux C, Sylvester R, van
der Meijden AP, Oosterlinck W and Brausi M: Treatment of
superficial bladder tumors: achievements and needs. The EORTC
Genitourinary Group Eur Urol. 37(Suppl 3): 1–9. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows down-regulation in human
immortalized cells and human tumor-derived cell lines. Biochem
Biophys Res Commun. 268:20–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuji T, Nozaki I, Miyazaki M, Sakaguchi
M, Pu H, Hamazaki Y, Iijima O and Namba M: Antiproliferative
activity of REIC/Dkk-3 and its significant down-regulation in
non-small-cell lung carcinomas. Biochem Biophys Res Commun.
289:257–263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abarzua F, Sakaguchi M, Takaishi M, Nasu
Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H and Huh NH:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005. View Article : Google Scholar
|
|
6
|
Abarzua F, Sakaguchi M, Tanimoto R,
Sonegawa H, Li DW, Edamura K, Kobayashi T, Watanabe M, Kashiwakura
Y, Kaku H, Saika T, Nakamura K, Nasu Y, Kumon H and Huh NH: Heat
shock proteins play a crucial role in tumor-specific apoptosis by
REIC/Dkk-3. Int J Mol Med. 20:37–43. 2007.PubMed/NCBI
|
|
7
|
Edamura K, Nasu Y, Takaishi M, Kobayashi
T, Abarzua F, Sakaguchi M, Kashiwakura Y, Ebara S, Saika T,
Watanabe M, Huh NH and Kumon H: Adenovirus-mediated REIC/Dkk-3 gene
transfer inhibits tumor growth and metastasis in an orthotopic
prostate cancer model. Cancer Gene Ther. 14:765–772. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe M, Kashiwakura Y, Huang P, Ochiai
K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH and
Kumon H: Immunological aspects of REIC/Dkk-3 in monocyte
differentiation and tumor regression. Int J Oncol. 34:657–663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kashiwakura Y, Ochiai K, Watanabe M,
Abarzua F, Sakaguchi M, Takaoka M, Tanimoto R, Nasu Y, Huh NH and
Kumon H: Down-regulation of inhibition of differentiation-1 via
activation of activating transcription factor 3 and Smad regulates
REIC/Dickkopf-3-induced apoptosis. Cancer Res. 68:8333–8341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanimoto R, Abarzua F, Sakaguchi M,
Takaishi M, Nasu Y, Kumon H and Huh NH: REIC/Dkk-3 as a potential
gene therapeutic agent against human testicular cancer. Int J Mol
Med. 19:363–368. 2007.PubMed/NCBI
|
|
11
|
Jin Y, Murata H, Sakaguchi M, Kataoka K,
Watanabe M, Nasu Y, Kumon H and Huh NH: Partial sensitization of
human bladder cancer cells to a gene-therapeutic adenovirus
carrying REIC/Dkk-3 by downregulation of BRPK/PINK1. Oncol Rep.
27:695–699. 2012.PubMed/NCBI
|
|
12
|
Zincke H, Utz DC, Taylor WF, Myers RP and
Leary FJ: Influence of thiotepa and doxorubicin instillation at
time of transurethral surgical treatment of bladder cancer on tumor
recurrence: a prospective randomized, double blind, controlled
trial. J Urol. 129:505–509. 1983.
|
|
13
|
Cole SP, Bhardwaj G, Gerlach JH, Mackie
JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM and
Deeley RG: Overexpression of a transporter gene in a
multidrug-resistant human lung cancer cell line. Science.
258:1650–1654. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gottesman MM and Pastan I: Biochemistry of
multidrug resistance mediated by the multidrug transporter. Annu
Rev Biochem. 62:385–427. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Liu M, Aneja R, Chandra R, Lage H
and Joshi HC: Reversal of P-glycoprotein-mediated multidrug
resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer
Res. 66:445–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimiya K, Naito S, Soejima T, Sakamoto N,
Kotoh S, Kumazawa J and Tsuruo T: Establishment and
characterization of doxorubicin-resistant human bladder cancer cell
line, KK47/ADM. J Urol. 148:441–445. 1992.PubMed/NCBI
|
|
17
|
Abarzua F, Kashiwakura Y, Takaoka M,
Watanabe M, Ochiai K, Sakaguchi M, Iwawaki T, Tanimoto R, Nasu Y,
Huh NH and Kumon H: An N-terminal 78 amino acid truncation of
REIC/Dkk-3 effectively induces apoptosis. Biochem Biophys Res
Commun. 375:614–618. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belloc F, Dumain P, Boisseau MR,
Jalloustre C, Reiffers J, Bernard P and Lacombe F: A flow
cytometric method using Hoechst 33342 and propidium iodide for
simultaneous cell cycle analysis and apoptosis determination in
unfixed cells. Cytometry. 17:59–65. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maciorowski Z, Delic J, Padoy E,
Klijanienko J, Dubray B, Cosset JM, Dumont J, Magdelénat H and
Vielh P: Comparative analysis of apoptosis measured by Hoechst and
flow cytometry in non-Hodgkin’s lymphomas. Cytometry. 32:44–50.
1998.PubMed/NCBI
|
|
20
|
Bennett BL, Sasaki DT, Murray BW, O’Leary
EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y,
Bhagwat SS, Manning AM and Anderson DW: SP600125, an
anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad
Sci USA. 98:13681–13686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris AL and Neal DE: Bladder cancer -
field versus clonal origin. N Engl J Med. 326:759–761. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oosterlinck W, Kurth KH, Schroder F,
Bultinck J, Hammond B and Sylvester R: A prospective European
Organization for Research and Treatment of Cancer Genitourinary
Group randomized trial comparing transurethral resection followed
by a single intravesical instillation of epirubicin or water in
single stage Ta, T1 papillary carcinoma of the bladder. J Urol.
149:749–752. 1993.
|
|
23
|
Solsona E, Iborra I, Ricos JV, Monros JL,
Casanova J and Dumont R: Effectiveness of a single immediate
mitomycin C instillation in patients with low risk superficial
bladder cancer: short and long-term follow-up. J Urol.
161:1120–1123. 1999. View Article : Google Scholar
|
|
24
|
Naito S, Sakamoto N, Kotoh S, Goto K,
Matsumoto T and Kumazawa J: Correlation between the expression of
P-glycoprotein and multidrug-resistant phenotype in transitional
cell carcinoma of the urinary tract. Eur Urol. 22:158–162.
1992.PubMed/NCBI
|
|
25
|
Sakaguchi M, Kataoka K, Abarzua F,
Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y,
Ochiai K, Nasu Y, Kumon H and Huh NH: Overexpression of REIC/Dkk-3
in normal fibroblasts suppresses tumor growth via induction of
interleukin-7. J Biol Chem. 284:14236–14244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawasaki K, Watanabe M, Sakaguchi M,
Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH,
Kumon H and Date H: REIC/Dkk-3 overexpression downregulates
P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces
apoptosis in breast cancer. Cancer Gene Ther. 16:65–72. 2009.
View Article : Google Scholar : PubMed/NCBI
|